A Mechanistic Insight into the Molecular Mechanism of the Thermal Decomposition of Nitroalkyl Phosphates: MEDT Computational Study
Abstract
1. Introduction
2. Methods
Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sadowski, M.; Synkiewicz-Musialska, B.; Kula, K. (1E,3E)-1,4-Dinitro-1,3-butadiene—Synthesis, Spectral Characteristics and Computational Study Based on MEDT, ADME and PASS Simulation. Molecules 2024, 29, 542. [Google Scholar] [CrossRef]
- Schales, O.; Graefe, H.A. Arylnitroalkenes: A New Group of Antibacterial Agents. J. Am. Chem. Soc. 1952, 74, 4486–4490. [Google Scholar] [CrossRef]
- Gerogianni, V.-E.; Koutoulogenis, G.S.; Gerokonstantis, D.T.; Kokotos, G. Synthesis of Amino-Acid-Based Nitroalkenes. Organics 2022, 3, 137–149. [Google Scholar] [CrossRef]
- Szumlak, A.; Luchowska-Kocot, D.; Qtaishat, F.; Boguszewska-Czubara, A. Creating a reliable zebrafish model for studying inflammation: Exploring the therapeutic potential of xanthohumol. Sci. Rad. 2024, 3, 266–286. [Google Scholar] [CrossRef]
- Sadowski, M.; Kula, K. Unexpected Course of Reaction Between (1E,3E)-1,4-Dinitro-1,3-butadiene and N-Methyl Azomethine Ylide—A Comprehensive Experimental and Quantum-Chemical Study. Molecules 2024, 29, 5066. [Google Scholar] [CrossRef]
- Janeczko, M.; Masłyk, M.; Demchuk, O.M.; Kurowska-Okoń, A.; Kwaśnik, M.; Górka, K.; Martyna, A.; Foll-Josselin, B.; Ruchaud, S.; Bach, S.; et al. Development of a novel family of antifungal agents based on a quinone methide oxime framework. Sci. Rep. 2025, 15, 13458. [Google Scholar] [CrossRef]
- Rodriguez-Duarte, J.; Galliussi, G.; Dapueto, R.; Rossello, J.; Malacrida, L.; Kamaid, A.; Schopfer, F.J.; Escande, C.; López, G.V.; Batthyány, C. A novel nitroalkene-α-tocopherol analogue inhibits inflammation and ameliorates atherosclerosis in Apo E knockout mice. Br. J. Pharmacol. 2019, 176, 757–772. [Google Scholar] [CrossRef]
- Rodriguez-Duarte, J.; Dapueto, R.; Galliussi, G.; Turell, L.; Kamaid, A.; Khoo, N.K.H.; Schopfer, F.J.; Freeman, B.A.; Escande, C.; Batthyány, C.; et al. Electrophilic nitroalkene-tocopherol derivatives: Synthesis, physicochemical characterization and evaluation of anti-inflammatory signaling responses. Sci. Rep. 2018, 8, 12784. [Google Scholar] [CrossRef]
- Sadowski, M.; Kula, K. Nitro-functionalized analogues of 1,3-Butadiene: An overview of characteristic, synthesis, chemical transformations and biological activity. Curr. Chem. Lett. 2024, 13, 15–30. [Google Scholar] [CrossRef]
- Jovanovic, P.; Jeremic, S.; Djokic, L.; Savic, V.; Radivojevic, J.; Maslak, V.; Ivkovic, B.; Vasiljevic, B.; Nikodinovic-Runic, J. Chemoselective biocatalytic reduction of conjugated nitroalkenes: New application for an Escherichia coli BL21(DE3) expression strain. Enzym. Microb. Technol. 2014, 60, 16–23. [Google Scholar] [CrossRef]
- Kula, K.; Kuś, E. In Silico Study About Substituent Effects, Electronic Properties, and the Biological Potential of 1,3-Butadiene Analogues. Int. J. Mol. Sci. 2025, 26, 8983. [Google Scholar] [CrossRef]
- Fałowska, A.; Grzybowski, S.; Kapuściński, D.; Sambora, K.; Łapczuk, A. Modeling of the General Trends of Reactivity and Regioselectivity in Cyclopentadiene–Nitroalkene Diels–Alder Reactions. Molecules 2025, 30, 2467. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Dresler, E.; Zawadzińska, K.; Wróblewska, A.; Jasiński, R. Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study. Molecules 2024, 29, 4892. [Google Scholar] [CrossRef] [PubMed]
- Kula, L.; Zawadzińska, K. Local nucleophile-electrophile interactions in [3+2] cycloaddition reactions between benzonitrile N-oxide and selected conjugated nitroalkenes in the light of MEDT computational study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Kula, K. Application of β-Phosphorylated Nitroethenes in [3+2] Cycloaddition Reactions Involving Benzonitrile N-Oxide in the Light of a DFT Computational Study. Organics 2021, 2, 26–37. [Google Scholar] [CrossRef]
- Zawadzińska-Wrochniak, K.; Zavecz, I.; Hirka, S. The recent progress in the field of the applications of isoxazoles and their hydrogenated analogs: Mini review. Sci. Rad. 2024, 3, 228–247. [Google Scholar] [CrossRef]
- Łapczuk, A. The [3+2] cycloaddition reaction as an attractive way for the preparation of nicotine analogs (microreview). Chem. Heterocycl. Compd. 2023, 59, 109–111. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kącka-Zych, A.; Kula, K. Recent progress in the field of cycloaddition reactions involving conjugated nitroalkenes. Curr. Chem. Lett. 2019, 8, 13–38. [Google Scholar] [CrossRef]
- Siadati, S.A. Beyond the alternatives that switch the mechanism of the 1,3-dipolar cycloadditions from concerted to stepwise or vice versa: A literature review. Prog. React. Kinet. Mech. 2016, 41, 331–344. [Google Scholar] [CrossRef]
- Jasiński, R. Synthesis of 1,2-oxazine N-oxides via noncatalyzed hetero-Diels–Alder reactions of nitroalkenes (microreview). Chem. Heterocycl. Compd. 2024, 60, 121–123. [Google Scholar] [CrossRef]
- Ziyaei Halimehjani, A.; Namboothiri, I.N.N.; Hooshmand, S.E. Part I: Nitroalkenes in the synthesis of heterocyclic compounds. RSC Adv. 2014, 4, 48022–48084. [Google Scholar] [CrossRef]
- Denmark, S.E.; Thorarensen, A. Tandem [4+2]/[3+2] cycloadditions of nitroalkenes. Chem. Rev. 1996, 96, 137–166. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.G.M.; Graboski, G.G. Conjugated nitroalkenes: Versatile intermediates in organic synthesis. Chem. Rev. 1986, 86, 751–762. [Google Scholar] [CrossRef]
- Zou, D.; Wang, W.; Hu, Y.; Jia, T. Nitroarenes and nitroalkenes as potential amino sources for the synthesis of N-heterocycles. Org. Biomol. Chem. 2023, 21, 2254–2271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mritunjay; Kumar, S.; Gupta, A.; Sharma, S.; Singh, P.; Kumar, A. Advances in the chemical transformations involving nitroalkenes embedded in heterocyclic framework. Asian J. Org. Chem. 2025, 14, e202500140. [Google Scholar] [CrossRef]
- Gupta, A.; Ahmad, F.; Siddiqui, M.S. Nitration of Olefinic and Acetylenic Fatty-Acid Esters. Indian J. Chem. 1987, 26, 870. [Google Scholar]
- Andrews, M.A.; Kelly, K.P. Transition-metal nitro-nitrosyl redox couple: Catalytic oxidation of olefins to ketones. J. Am. Chem. Soc. 1981, 103, 2894–2896. [Google Scholar] [CrossRef]
- Harvey, I.W.; Phillips, E.D.; Whitham, G.H. Free radical addition reactions of allylic sulfones to alkenes. Tetrahedron 1997, 53, 6493–6508. [Google Scholar] [CrossRef]
- Knochel, P.; Seebach, D. Nitroallylation of highly reactive organolithium compounds by 2-nitro-3-pivaloyloxy-1-propene (NPP). Tetrahedron Lett. 1981, 22, 3223–3226. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Ponikiewski, Ł. Single crystal X-ray structure of (Z)-1-bromo-1-nitro-2-phenylethene. Curr. Chem. Lett. 2015, 4, 21–26. [Google Scholar] [CrossRef]
- Miyashita, M.; Yanami, T.; Yoshikoshi, A. 2-nitropropene. Org. Synth. 1981, 60, 101. [Google Scholar] [CrossRef]
- Kącka, A.B.; Jasiński, R.A. A density functional theory mechanistic study of thermal decomposition reactions of nitroethyl carboxylates: Undermine of “pericyclic” insight. Heteroat. Chem. 2016, 27, 279–289. [Google Scholar] [CrossRef]
- Bachman, G.; Bryant, G.; Standish, N.W. Preparation of 1,1,1-trichloro-3-nitro-2-alkenes. J. Org. Chem. 1961, 26, 1474–1477. [Google Scholar] [CrossRef]
- Wade, P.A.; Murray, J.K.; Shah-Patel, J.S.; Carroll, P.J. Generation and in situ Diels–Alder reactions of activated nitroethylene derivatives. Tetrahedron Lett. 2002, 43, 2585–2588. [Google Scholar] [CrossRef]
- Gold, M.H. Nitroölefins by the vapor phase catalytic cleavage of esters of nitro alcohols. J. Am. Chem. Soc. 1946, 68, 2544–2546. [Google Scholar] [CrossRef]
- Jasiński, R.; Kącka, A. A polar nature of benzoic acids extrusion from nitroalkyl benzoates: DFT mechanistic study. J. Mol. Model. 2015, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Blaha, I.; Leseticky, L. Preparation and Z-E-isomerisation of substitted nitrostyrenes. Coll. Czech. Chem. Commun. 1986, 51, 1094–1099. [Google Scholar] [CrossRef]
- Zawadzińska-Wrochniak, K.; Guarav, G.K.; Demchuk, O.M. Synthesis and physical properties of the (E)-3,3,3-tribromo-1-nitroprop-1-ene. Sci. Radices 2025, 4, 181. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E.; Mikulska, M.; Polewski, D. [3+2] Cycloadditions of 1-halo-1-nitroethenes with (Z)-C-(3,4,5-trimethoxyphenyl)-N-methyl-nitrone as regio- and stereocontrolled source of novel bioactive compounds: Preliminary studies. Curr. Chem. Lett. 2016, 5, 123–128. [Google Scholar] [CrossRef]
- Wilkendorf, R.; Trénel, M. Zur Kenntnis aliphatischer nitro-alkohole (II.). Ber. Dtsch. Chem. Ges. 1924, 57, 306–309. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Ameur, S.; Kącka-Zych, A.; Moussa, Z.; Alsantali, R.I.; Zeroual, A.; Alluhaibi, M.S.; Alsimaree, A.A.; Ahmed, S.A. Study of 1,3-Dipolar Cycloaddition Between 4-Acyl-1H-pyrrole-2,3-diones Fused at the [e]-Side with a Heterocyclic Moiety and Diphenylnitrone: A Comprehensive MEDT, Docking Approach and MD Simulation. Molecules 2025, 30, 3718. [Google Scholar] [CrossRef] [PubMed]
- Karas, A.; Lapczuk, A. Computational model of the formation of novel nitronorbornene analogs via Diels-Alder process. React. Kinet. Mech. Catal. 2025, 138, 2689. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Kącka-Zych, A.; Demchuk, O.M.; Mirosław, B.; Woliński, P.; Jasiński, R. Green synthesis of nitrocyclopropane-type precursors of inhibitors for the maturation of fruits and vegetables via domino reactions of diazoalkanes with 2-nitroprop-1-ene. J. Clean. Prod. 2021, 292, 126079. [Google Scholar] [CrossRef]
- Jasiński, R.; Ziółkowska, M.; Demchuk, O.M.; Maziarka, A. Regio- and stereoselectivity of polar [2+3] cycloaddition reactions between (Z)-C-(3,4,5-trimethoxyphenyl)-N-methylnitrone and selected (E)-2-substituted nitroethenes. Cent. Eur. J. Chem. 2014, 12, 586–593. [Google Scholar] [CrossRef]
- Jasiński, R. Molecular mechanism of thermal decomposition of fluoronitroazoxy compounds: DFT computational study. J. Fluor. Chem. 2014, 160, 29–33. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Domingo, L.R. 1999–2024, a quarter century of the Parr’s electrophilicity ω index. Sci. Rad. 2024, 3, 157–186. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Fox, Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Energetic Aspects and Molecular Mechanism of 3-Nitro-substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study. Molecules 2024, 29, 3042. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials 2022, 15, 7584. [Google Scholar] [CrossRef]
- Kula, K.; Jasiński, R. Synthesis of bis(het)aryl systems via domino reaction involving (2E,4E)-2,5-dinitrohexa-2,4-diene: DFT mechanistic considerations. Chem. Heterocycl. Compd. 2024, 60, 600–610. [Google Scholar] [CrossRef]
- Woliński, P.; Dresler, E.; Jasiński, R. A new mechanistic insight into the molecular mechanisms of the addition reactions of 2-aryl-3-nitro-2H-chromenes to pyrazoles and cyclopentadienes. New J. Chem. 2025, 49, 8442–8453. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Version 6.0; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Wróblewska, A.; Sadowski, M.; Jasiński, R. Selectivity and molecular mechanism of the Au(III)-catalyzed [3+2] cycloaddition reaction between (Z)-C,N-diphenylnitrone and nitroethene in the light of the molecular electron density theory computational study. Chem. Heterocycl. Compd. 2024, 60, 639–645. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Olszewska, A.; Zawadzińska, K.; Woliński, P.; Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics 2022, 3, 59–76. [Google Scholar] [CrossRef]
- Ryachi, K.; Mohammad-Salim, H.; Al-Sadoon, M.K.; Zeroual, A.; de Julián-Ortiz, J.V.; El Idrissi, M.; Tounsi, A. Quantum study of the [3+2] cycloaddition of nitrile oxide and carvone oxime: Insights into toxicity, pharmacokinetics, and mechanism. Chem. Heterocycl. Compd. 2024, 60, 646–654. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Wróblewska, A.; Jasiński, R. A New Insight into the Molecular Mechanism of the Reaction between 2-Methoxyfuran and Ethyl (Z)-3-phenyl-2-nitroprop-2-enoate: An Molecular Electron Density Theory (MEDT) Computational Study. Molecules 2024, 29, 4876. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Wróblewska, A.; Wielgus, E.; Dolot, R.; Jasiński, R. Fully Selective Synthesis of Spirocyclic-1,2-oxazine N-Oxides via Non-Catalysed Hetero Diels-Alder Reactions with the Participation of Cyanofunctionalysed Conjugated Nitroalkenes. Molecules 2023, 28, 4586. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Molecular Mechanism of Thermal and LA-Catalysed Diels–Alder Reactions between Cyclopentadiene and Isopropyl 3-Nitroprop-2-Enate. Molecules 2023, 28, 5289. [Google Scholar] [CrossRef]
- Kącka-Zych, A. The Molecular Mechanism of the Formation of Four-Membered Cyclic Nitronates and Their Retro [3 + 2] Cycloaddition: A DFT Mechanistic Study. Molecules 2021, 26, 4786. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, P.; Wang, Y.; Yu, Z.-X. Mechanism and regioselectivity of intramolecular [2+2] cycloaddition of ene–ketenes: A DFT study. Eur. J. Org. Chem. 2020, 2020, 5985–5994. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Krokidis, K.; Noury, S.; Silvi, B. Characterization of elementary chemical processes by catastrophe theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
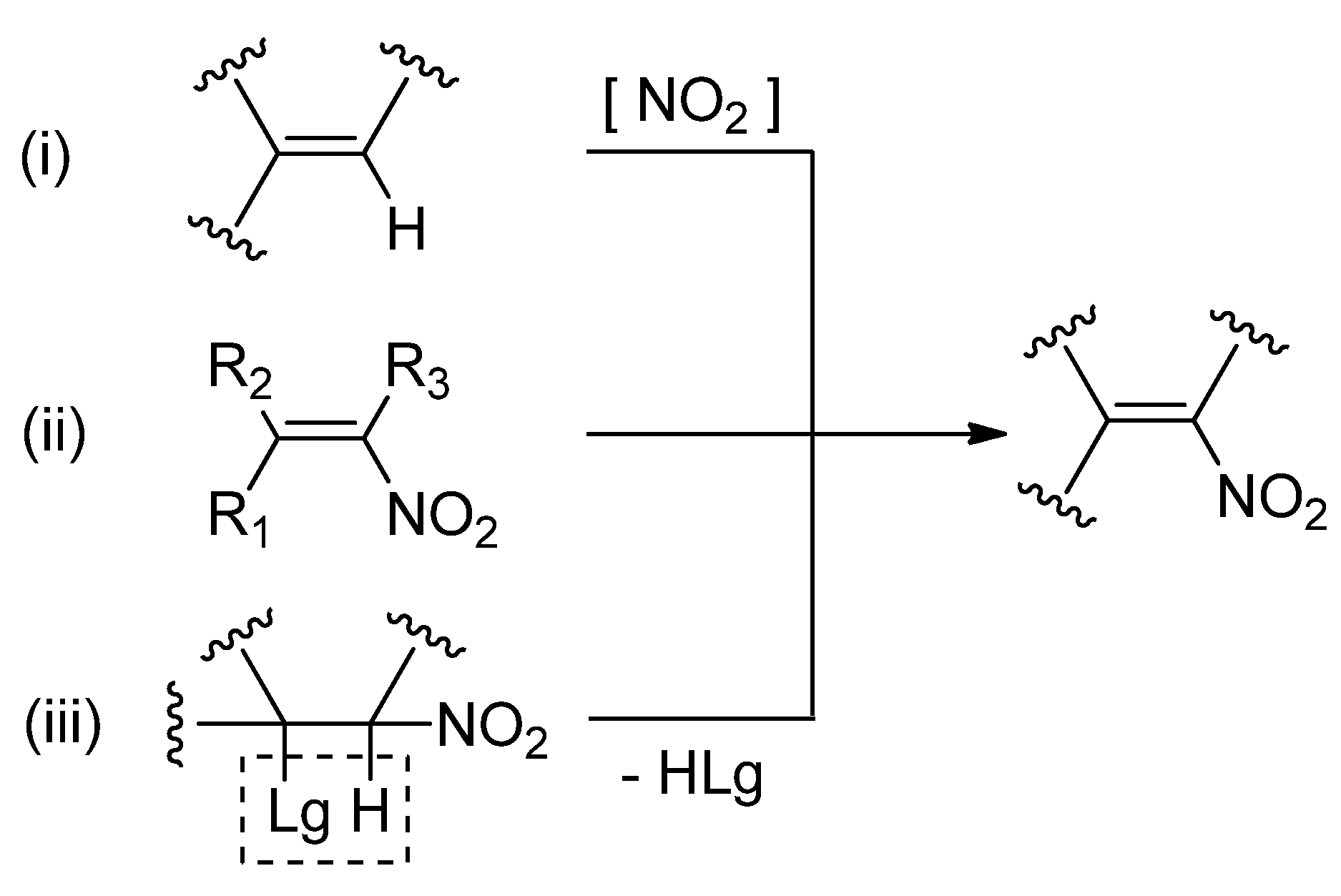
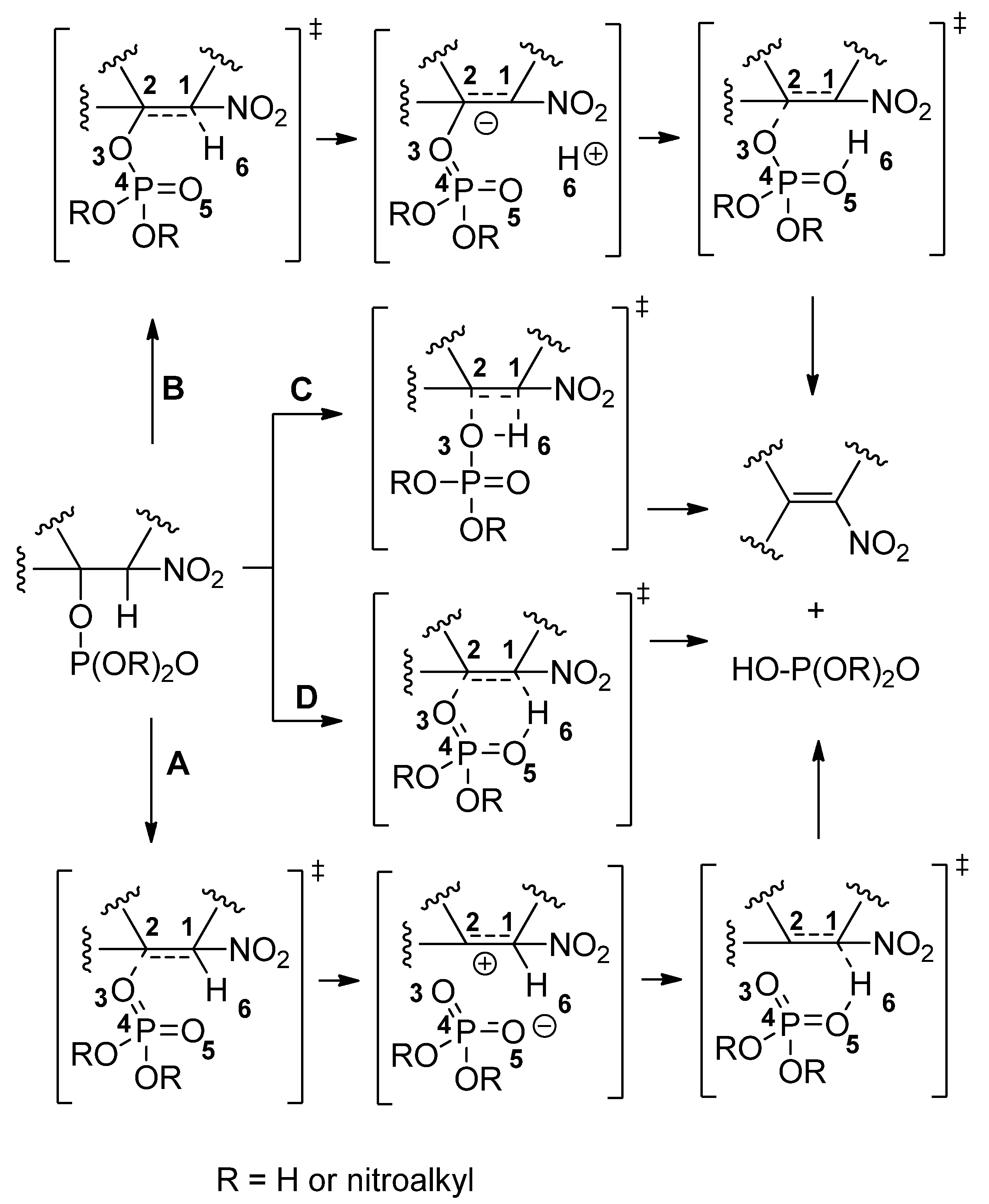


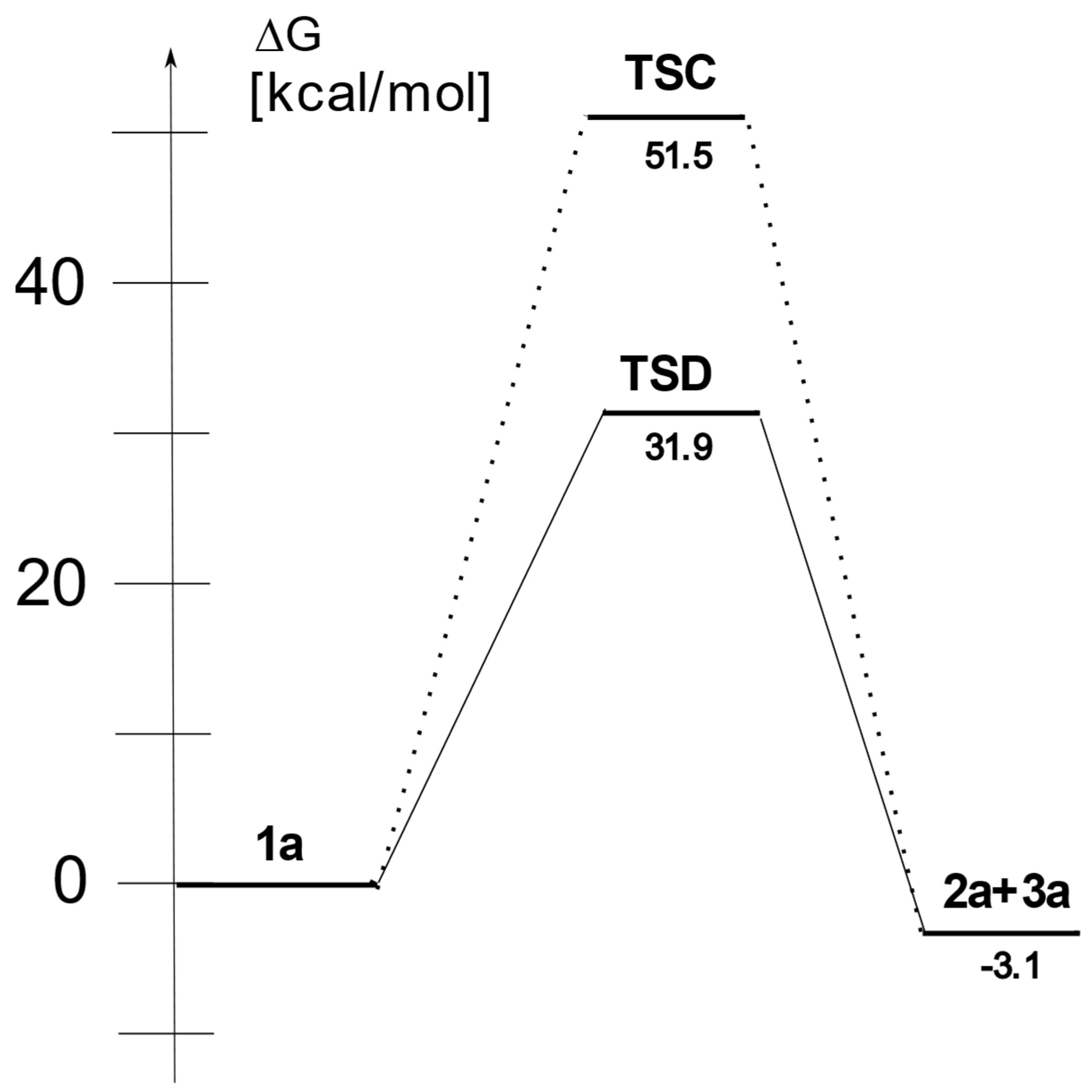
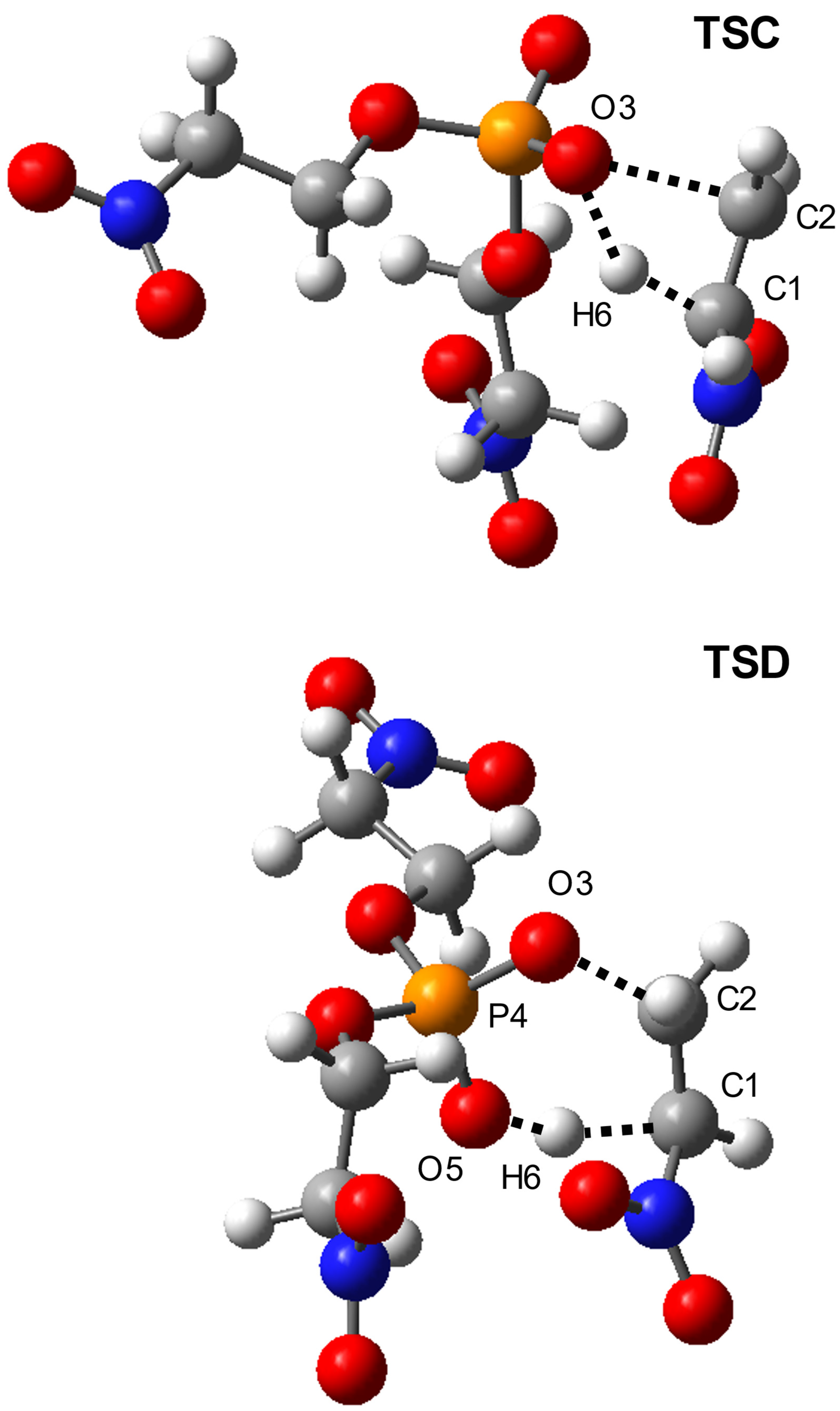

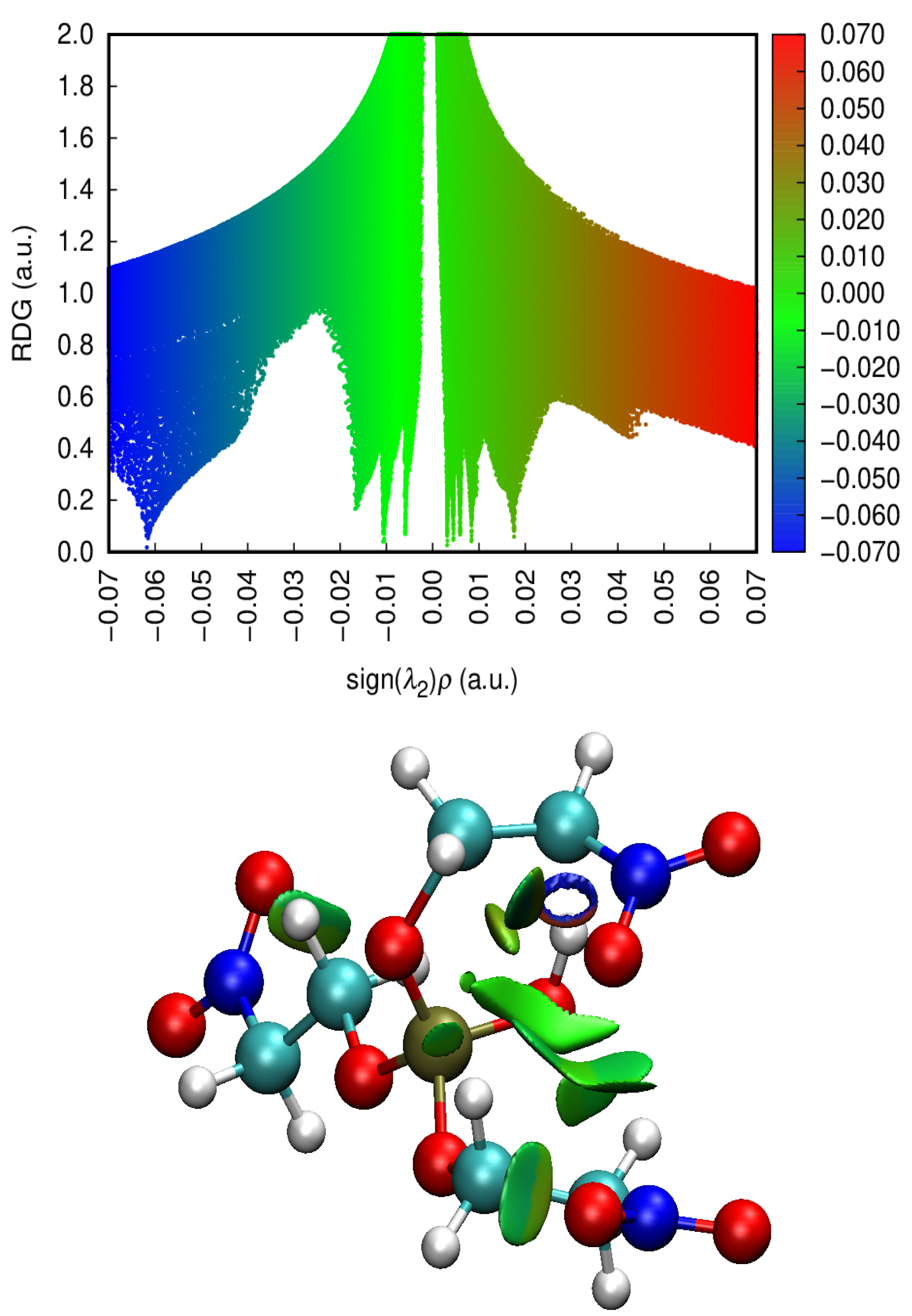
Starting Ester | Transition | Relative Energies | ||||
|---|---|---|---|---|---|---|
| No. | R1 | R2 | ΔH | ΔG | ΔS | |
| 4a | H | H | 4a→TSD | 32.9 | 35.3 | −8.0 |
| 4a→2a + 5 | 12.4 | 1.2 | 37.6 | |||
| 4b | Me | H | 4b→TSD | 36.5 | 37.1 | −2.2 |
| 4b→2b + 5 | 12.3 | −0.5 | 43.2 | |||
| 4c | Cl | H | 4c→TSD | 33.2 | 34.6 | −4.8 |
| 4c→2c + 5 | 13.4 | 1.3 | 40.4 | |||
| 4d | H | Me | 4d→TSD | 32.3 | 33.0 | −2.4 |
| 4d→2d + 5 | 12.4 | −0.4 | 42.9 | |||
| 4e | H | Cl | 4e→TSD | 35.7 | 36.6 | −2.9 |
| 4e→2e + 5 | 14.1 | 1.6 | 41.9 | |||
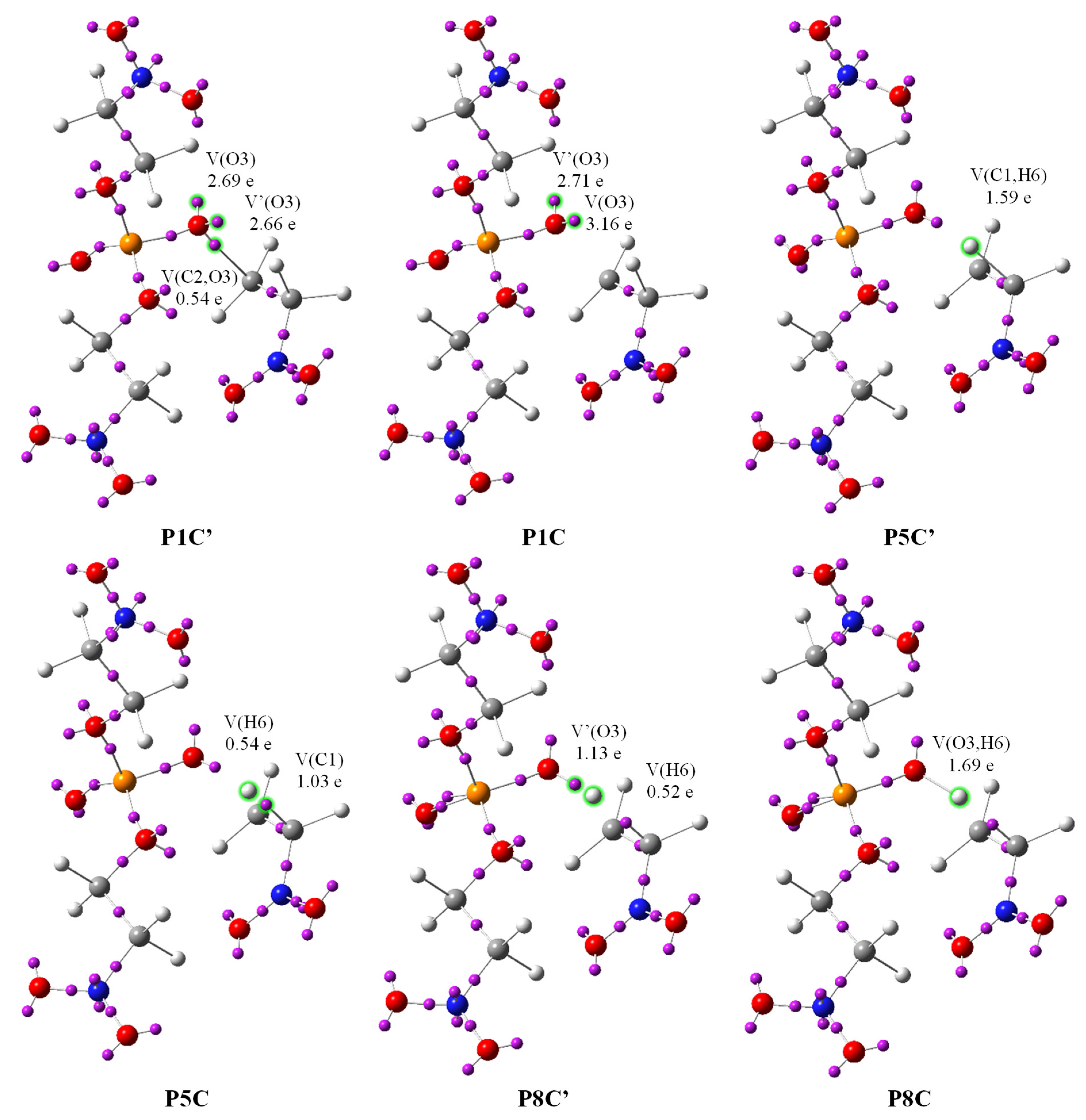
| Structures | 1a | 443 P1C | 515 P2C | 529 P3C | 531 P4C | 534 P5C | 538 P6C | 548 P7C | 562 P8C | 568 P9C | 580 P10C | 606 P11C | 838 P12C | 2a | 3a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phases | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | ||
| d1(C1-H6) | 1.087 | 1.107 | 1.168 | 1.254 | 1.269 | 1.293 | 1.328 | 1.423 | 1.564 | 1.625 | 1.721 | 1.864 | 2.479 | - | - |
| d2(C2-O3) | 1.435 | 1.960 | 2.137 | 2.149 | 2.151 | 2.153 | 2.155 | 2.159 | 2.166 | 2.171 | 2.192 | 2.254 | 2.821 | - | - |
| d3(O3-H6) | 2.616 | 2.032 | 1.599 | 1.457 | 1.435 | 1.402 | 1.358 | 1.245 | 1.091 | 1.032 | 0.976 | 0.971 | 0.978 | - | 0.963 |
| dE | 0 | 37.4 | 57.4 | 59.4 | 59.4 | 59.2 | 58.6 | 54.6 | 45.0 | 41.1 | 36.3 | 29.6 | 13.9 | - | - |
| V(C1,C2) | 1.97 | 2.09 | 2.23 | 2.33 | 2.35 | 2.38 | 2.42 | 2.56 | 2.82 | 3.48 | 1.79 | 1.76 | 1.77 | 1.76 | - |
| V′(C1,C2) | - | - | - | - | - | - | - | - | - | - | 1.69 | 1.70 | 1.67 | 1.72 | - |
| V(C1,H6) | 2.05 | 1.94 | 1.75 | 1.63 | 1.61 | - | - | - | - | - | - | - | - | - | - |
| V(C1,N7) | 2.24 | 2.20 | 2.21 | 2.23 | 2.23 | 2.23 | 2.24 | 2.39 | 2.44 | 2.45 | 2.45 | 2.51 | 2.58 | 2.58 | - |
| V(C1) | - | - | - | - | - | 1.03 | 1.05 | 0.95 | 0.68 | - | - | - | - | - | - |
| V(C2,O3) | 1.25 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| V(O3,H6) | - | - | - | - | - | - | - | - | 1.69 | 1.74 | 1.78 | 1.75 | 1.73 | - | 1.74 |
| V(O3) | 2.41 | 3.16 | 2.90 | 3.08 | 4.14 | 4.24 | 4.34 | 4.45 | 4.39 | 4.33 | 4.29 | 2.47 | 2.31 | - | 2.24 |
| V′(O3) | 2.32 | 2.71 | 2.23 | 1.68 | 1.62 | 1.50 | 1.39 | 1.25 | - | - | - | 1.91 | 2.17 | - | 2.24 |
| V″(O3) | - | - | 0.68 | 1.02 | - | - | - | - | - | - | - | - | - | - | - |
| V(P4,O3) | 1.67 | 1.71 | 1.77 | 1.78 | 1.80 | 1.81 | 1.81 | 1.81 | 1.80 | 1.79 | 1.77 | 1.72 | 1.67 | - | 1.65 |
| V(P4,O5) | 2.07 | 2.05 | 2.05 | 2.05 | 2.05 | 2.05 | 1.21 | 1.18 | 1.27 | 1.27 | 1.30 | 1.35 | 1.43 | - | 1.05 |
| V′(P4,O5) | - | - | - | - | - | - | 0.79 | 0.83 | 0.81 | 0.82 | 0.80 | 0.77 | 0.69 | - | 0.92 |
| V(O5) | 2.97 | 5.82 | 2.00 | 4.23 | 4.18 | 4.12 | 4.03 | 3.78 | 3.67 | 3.63 | 3.66 | 3.61 | 3.69 | - | 3.49 |
| V′(O5) | 2.85 | - | 1.30 | 1.59 | 1.64 | 1.71 | 1.84 | 2.08 | 2.13 | 2.16 | 2.13 | 2.16 | 2.08 | - | 2.43 |
| V″(O5) | - | - | 2.52 | - | - | - | - | - | - | - | - | - | - | - | - |
| V(H6) | - | - | - | - | - | 0.54 | 0.47 | 0.43 | - | - | - | - | - | - | - |
| V(N7) | - | 0.18 | 0.19 | 0.18 | 0.18 | 0.18 | 0.18 | - | - | - | - | 0.08 | - | - | - |
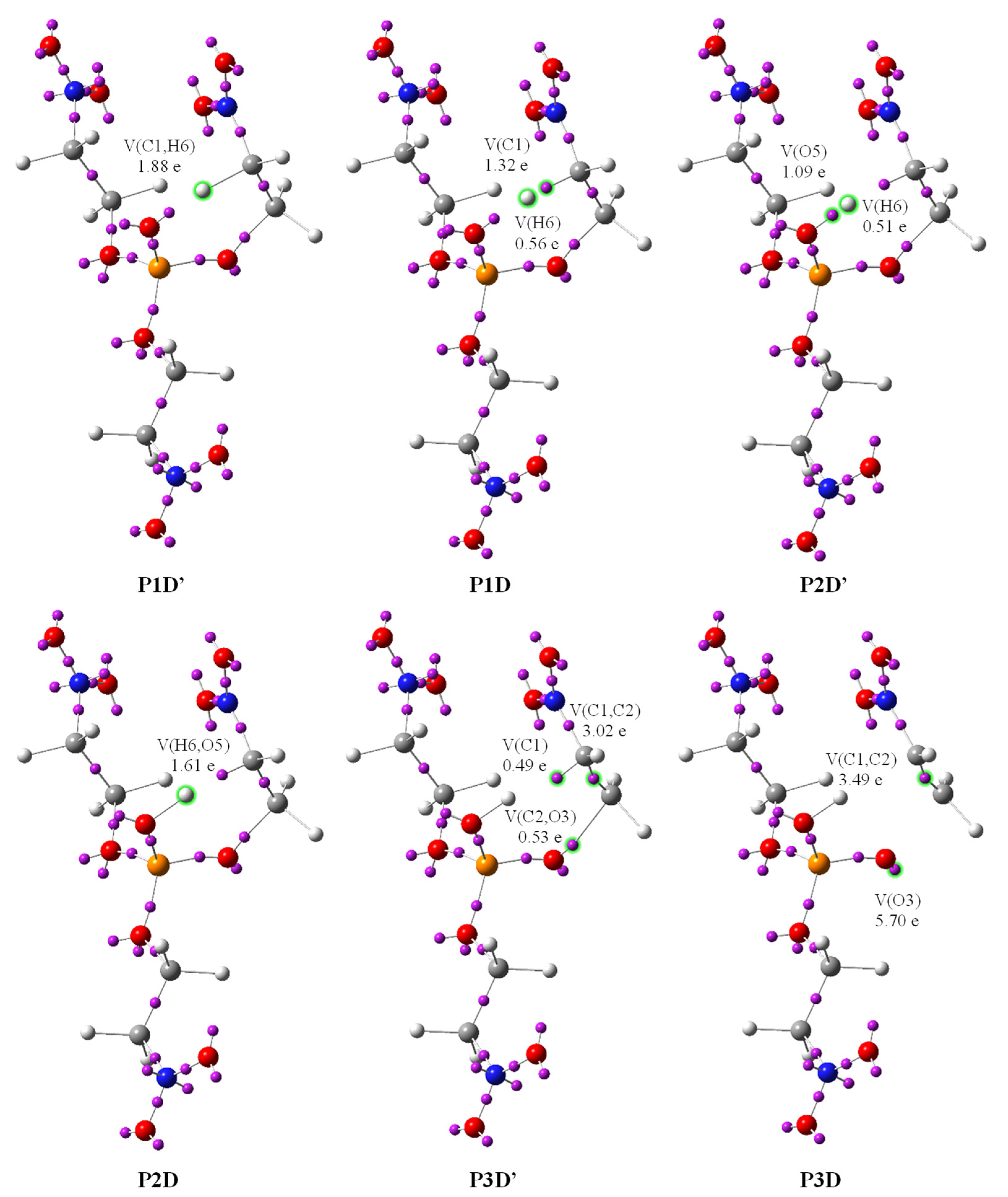
| Structures | 1a | 153 P1D | 168 P2D | 209 P3D | 211 P4D | 223 P5D | 239 P6D | 251 P7D | 254 P8D | 255 P9D | 259 P10D | 2a | 3a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phases | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | ||
| d1(C1-H6) | 1.087 | 1.283 | 1.628 | 1.937 | 1.948 | 2.012 | 2.098 | 2.166 | 2.183 | 2.189 | 2.212 | - | - |
| d2(C2-O3) | 1.435 | 1.587 | 1.636 | 1.949 | 1.965 | 2.057 | 2.171 | 2.250 | 2.269 | 2.275 | 2.300 | - | - |
| d3(O5-H6) | 2.745 | 1.372 | 1.074 | 0.987 | 0.985 | 0.982 | 0.980 | 0.980 | 0.980 | 0.980 | 0.980 | - | 0.962 |
| dE | 0.6 | 25.9 | 34.7 | 29.9 | 29.4 | 26.4 | 22.8 | 20.5 | 20.0 | 19.9 | 19.3 | - | - |
| V(C1,C2) | 2.00 | 2.08 | 2.24 | 3.49 | 3.49 | 1.90 | 1.87 | 1.84 | 1.83 | 1.83 | 1.82 | 1.76 | - |
| V′(C1,C2) | - | - | - | - | - | 1.58 | 1.6 | 1.63 | 1.64 | 1.64 | 1.65 | 1.72 | - |
| V(C1,H6) | 2.04 | - | - | - | - | - | - | - | - | - | - | - | - |
| V(C1,N7) | 2.23 | 2.48 | 2.62 | 2.58 | 2.57 | 2.54 | 2.51 | 2.49 | 2.49 | 2.49 | 2.49 | 2.58 | - |
| V(C1) | - | 1.32 | 1.23 | - | - | - | - | - | - | - | - | - | - |
| V(C2,O3) | 1.28 | 1.05 | 0.99 | - | - | - | - | - | - | - | - | - | - |
| V(O3) | 4.69 | 4.83 | 4.84 | 5.70 | 5.70 | 5.74 | 1.79 | 2.06 | 1.85 | 2.14 | 3.55 | - | 3.20 |
| V′(O3) | - | - | - | - | - | - | 3.99 | 1.27 | 1.32 | 1.35 | 2.24 | - | 2.69 |
| V″(O3) | - | - | - | - | - | - | - | 2.45 | 2.32 | 2.30 | - | - | - |
| V‴(O3) | - | - | - | - | - | - | - | - | 0.30 | - | - | - | - |
| V(P4,O3) | 1.69 | 1.77 | 1.83 | 2.03 | 2.04 | 2.04 | 2.04 | 2.05 | 2.04 | 2.05 | 2.04 | - | 1.99 |
| V(P4,O5) | 2.07 | 1.92 | 1.82 | 1.72 | 1.7 | 1.69 | 1.67 | 1.66 | 1.66 | 1.66 | 1.65 | - | 1.65 |
| V(O5) | 2.97 | 4.90 | 4.54 | 4.46 | 2.36 | 2.34 | 2.25 | 2.19 | 2.17 | 2.17 | 2.15 | - | 2.44 |
| V′(O5) | 2.83 | 0.91 | - | - | 2.13 | 2.15 | 2.24 | 2.31 | 2.32 | 2.32 | 2.35 | - | 2.05 |
| V(O5,H6) | - | - | 1.61 | 1.72 | 1.72 | 1.72 | 1.73 | 1.73 | 1.73 | 1.73 | 1.73 | - | 1.73 |
| V(H6) | - | 0.56 | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woliński, P.; Dresler, E.; Jasiński, R. A Mechanistic Insight into the Molecular Mechanism of the Thermal Decomposition of Nitroalkyl Phosphates: MEDT Computational Study. Materials 2025, 18, 5312. https://doi.org/10.3390/ma18235312
Woliński P, Dresler E, Jasiński R. A Mechanistic Insight into the Molecular Mechanism of the Thermal Decomposition of Nitroalkyl Phosphates: MEDT Computational Study. Materials. 2025; 18(23):5312. https://doi.org/10.3390/ma18235312
Chicago/Turabian StyleWoliński, Przemysław, Ewa Dresler, and Radomir Jasiński. 2025. "A Mechanistic Insight into the Molecular Mechanism of the Thermal Decomposition of Nitroalkyl Phosphates: MEDT Computational Study" Materials 18, no. 23: 5312. https://doi.org/10.3390/ma18235312
APA StyleWoliński, P., Dresler, E., & Jasiński, R. (2025). A Mechanistic Insight into the Molecular Mechanism of the Thermal Decomposition of Nitroalkyl Phosphates: MEDT Computational Study. Materials, 18(23), 5312. https://doi.org/10.3390/ma18235312







