The Effect of Laser Surface Treatment on the Bond Strength of Adhesive Materials to Primary Teeth: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Protocol
2.3. Eligibility Criteria
- Original research articles;
- Laser pretreatment;
- Using all kind of composite materials;
- SBS evaluation studies;
- In vitro studies;
- Examinations performed on primary teeth;
- Studies in English;
- Full-text articles.
- No laser pretreatment;
- No SBS evaluation;
- Studies conducted on permanent teeth;
- Non-English papers;
- Systematic review articles;
- Review articles;
- No full-text accessible;
- Duplicated publications.
2.4. Information Sources, Search Strategy, and Study Selection
2.5. Data Collection and Data Items
2.6. Assessing Risk of Bias in Individual Studies
2.7. Quality Assessment
- Q1 Is it clear in the study what is the ‘cause’ and what is the ‘effect’?
- Q2 Were the participants included in any similar comparisons?
- Q3 Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?
- Q4 Was there a control group?
- Q5 Were there multiple measurements of the outcome both before and after the intervention/exposure?
- Q6 Was a follow up completed, and if not, were differences between groups in terms of their follow up adequately described and analyzed?
- Q7 Were the in vitro results of participants included in any comparisons measured in the same way?
- Q8 Were the outcomes measured in a reliable way?
- Q9 Was an appropriate statistical analysis used?
3. Results
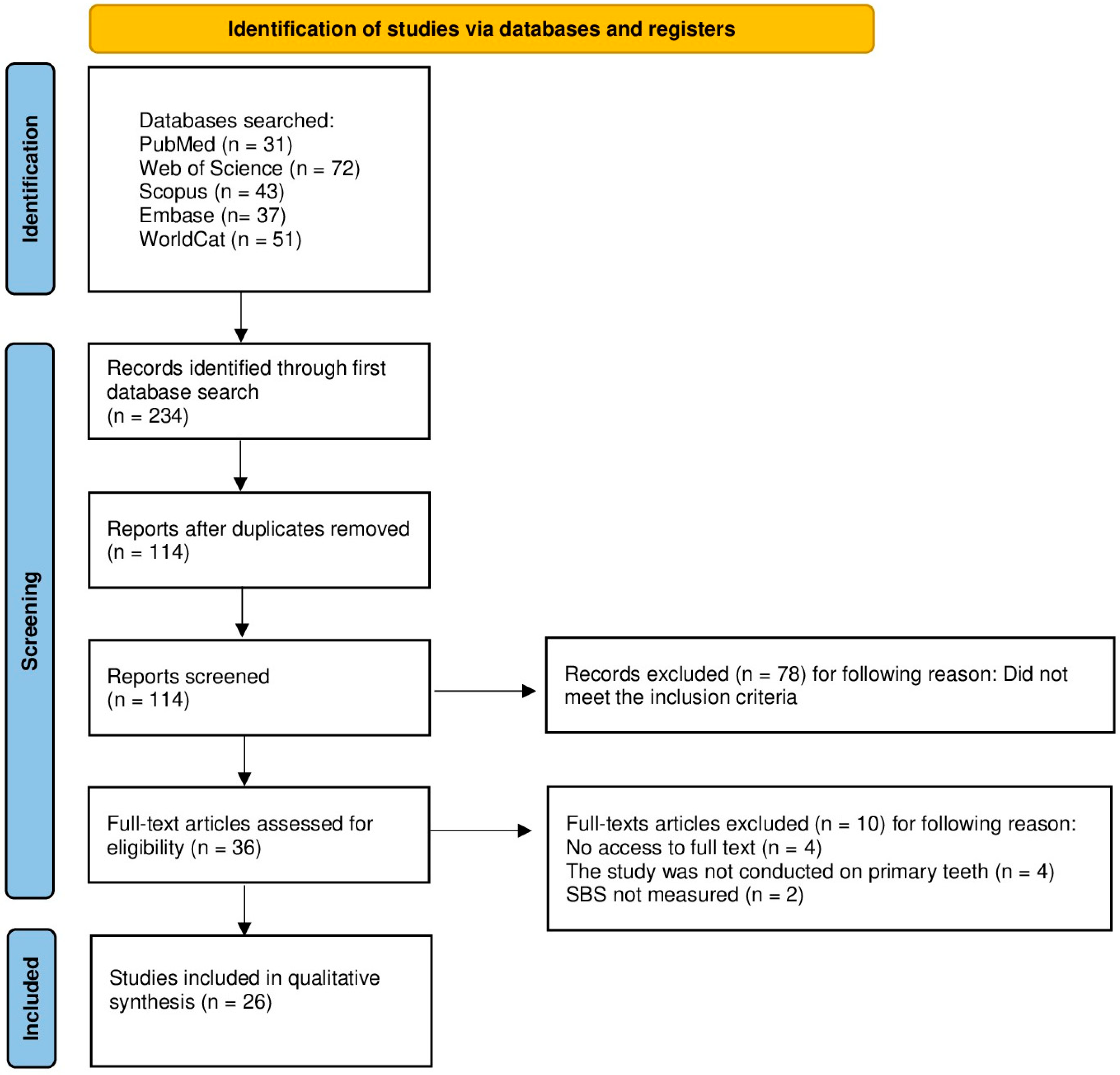
3.1. Study Selection
3.2. General Characteristics of the Included Studies
3.2.1. Shear Bond Strength
3.2.2. Microtensile Bond Strength
3.2.3. Morphological Analysis
3.3. Main Study Outcomes
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| CHX | Chlorhexidinebio |
| CPP–ACP | Casein Phosphopeptide–Amorphous Calcium Phosphate |
| EDX | Energy-Dispersive X-ray Spectroscopy |
| Er:YAG | Erbium-doped Yttrium Aluminum Garnet laser |
| Er,Cr:YSGG | Erbium, Chromium-doped Yttrium Scandium Gallium Garnet laser |
| JBI | Joanna Briggs Institute |
| KTP | Potassium Titanyl Phosphate laser |
| MSP | Micro Short Pulse |
| Nd:YAG | Neodymium-doped Yttrium Aluminum Garnet laser |
| SBS | Shear Bond Strength |
| SEM | Scanning Electron Microscopy |
| µSBS | Microshear Bond Strength |
| µTBS | Microtensile Bond Strength |
| WoS | Web of Science |
References
- Sheiham, A. Dental Caries Affects Body Weight, Growth and Quality of Life in Pre-School Children. Br. Dent. J. 2006, 201, 625–626. [Google Scholar] [CrossRef]
- Abad, M.C.L.; Andrade, M.B.G.; Vidal, T.F.D.L.C. Early Childhood Caries and Its Impact on General Health: Narrative Review. World J. Adv. Res. Rev. 2024, 22, 083–092. [Google Scholar] [CrossRef]
- Wilson, P.R.; Beynon, A.D. Mineralization Differences between Human Deciduous and Permanent Enamel Measured by Quantitative Microradiography. Arch. Oral. Biol. 1989, 34, 85–88. [Google Scholar] [CrossRef]
- Lynch, R.J.M. The Primary and Mixed Dentition, Post-Eruptive Enamel Maturation and Dental Caries: A Review. Int. Dent. J. 2013, 63, 3–13. [Google Scholar] [CrossRef]
- Sønju Clasen, A.B.; Øgaard, B.; Duschner, H.; Ruben, J.; Arends, J.; Sönju, T. Caries Development in Fluoridated and Non-Fluoridated Deciduous and Permanent Enamel in Situ Examined by Microradiography and Confocal Laser Scanning Microscopy. Adv. Dent. Res. 1997, 11, 442–447. [Google Scholar] [CrossRef]
- Sønju Clasen, A.B.; Ruyter, I.E. Quantitative Determination of Type A and Type B Carbonate in Human Deciduous and Permanent Enamel by Means of Fourier Transform Infrared Spectrometry. Adv. Dent. Res. 1997, 11, 523–527. [Google Scholar] [CrossRef]
- Waggoner, W.F.; Nelson, T. Stomatologia Odtwórcza Uzębienia Mlecznego, 6th ed.; Nowak, A.J., Christensen, J.R., Mabry, T.R., Townsend, J.A., Wells, M.H., Eds.; Elsevier: Filadelfia, PA, USA, 2019; Volume 6. [Google Scholar]
- Tichý, A.; Yang, Y.; Sayed, M.; Shimada, Y.; Hosaka, K. The Effect of Bonding Strategy and Aging on Adhesion to Primary Enamel: An In-Vitro Study. J. Adhes. Dent. 2023, 25, 187–194. [Google Scholar] [CrossRef] [PubMed]
- De Menezes Oliveira, M.A.H.; Torres, C.P.; Gomes-Silva, J.M.; Chinelatti, M.A.; De Menezes, F.C.H.; Palma-Dibb, R.G.; Borsatto, M.C. Microstructure and Mineral Composition of Dental Enamel of Permanent and Deciduous Teeth. Microsc. Res. Tech. 2010, 73, 572–577. [Google Scholar] [CrossRef]
- Lenzi, T.L.; Guglielmi, C.D.A.B.; Arana-Chavez, V.E.; Raggio, D.P. Tubule Density and Diameter in Coronal Dentin from Primary and Permanent Human Teeth. Microsc. Microanal. 2013, 19, 1445–1449. [Google Scholar] [CrossRef]
- de Boer, M.; Zimmermann, M.; Attin, T.; Tauböck, T.T.; Hamza, B. Marginal Integrity of Simplified Adhesive Strategies in Primary Teeth. Int. Dent. J. 2023, 73, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Dorri, M.; Martinez-Zapata, M.J.; Walsh, T.; Marinho, V.C.; Sheiham, A.; Zaror, C. Atraumatic Restorative Treatment versus Conventional Restorative Treatment for Managing Dental Caries. Cochrane Database Syst. Rev. 2017, 2018, CD008072. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Chowdhry, S.; Saha, S.; Samadi, F.; Jaiswal, J.N.; Garg, A.; Chowdhry, P. Recent vs Conventional Methods of Caries Removal: A Comparative in Vivo Study in Pediatric Patients. Int. J. Clin. Pediatr. Dent. 2015, 8, 6–11. [Google Scholar] [CrossRef]
- Dhanvijay, A.; Kubde, R.; Shenoi, P.; Badole, G.; Shahu, S. Assessment of Smear Layer Formation After Caries Removal Using Erbium Laser and Papain-Based Chemo-Mechanical Caries Removal Agent: An In Vitro Scanning Electron Microscopy Study. Cureus 2023, 15, e47999. [Google Scholar] [CrossRef]
- Saikaew, P.; Sattabanasuk, V.; Harnirattisai, C.; Chowdhury, A.F.M.A.; Carvalho, R.; Sano, H. Role of the Smear Layer in Adhesive Dentistry and the Clinical Applications to Improve Bonding Performance. Jpn. Dent. Sci. Rev. 2022, 58, 59–66. [Google Scholar] [CrossRef]
- Akşit-Bıçak, D.; Hussein, T.O.; Kumar, G. Pediatric Dentists’ Approaches to Dental Treatment of Children with Dental Fear and Anxiety. Cyprus J. Med. Sci. 2024, 9, 403–410. [Google Scholar] [CrossRef]
- Woś, P.; Kiryk, S.; Dyl, T.; Kiryk, J.; Horodniczy, T.; Szablińska, M.; Dubowik, M.A.; Dobrzyński, W.; Mikulewicz, M.; Matys, J.; et al. Laser Applications in Metal Orthodontic Bracket Debonding: A Systematic Review. Appl. Sci. 2025, 15, 927. [Google Scholar] [CrossRef]
- Michalak, M.; Kiryk, S.; Kotela, A.; Wiśniewska, K.; Kiryk, J.; Zborowski, J.Z.; Matys, J.; Dobrzyński, M. Orthodontic Ceramic Bracket Removal Using Lasers: A Systematic Review. J. Funct. Biomater. 2025, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Zawiślak, I.; Kiryk, S.; Kiryk, J.; Kotela, A.; Kensy, J.; Michalak, M.; Matys, J.; Dobrzyński, M. Toxic Metal Content in Deciduous Teeth: A Systematic Review. Toxics 2025, 13, 556. [Google Scholar] [CrossRef]
- Diéguez-Pérez, M.; Ticona-Flores, J.M. Three-Dimensional Analysis of the Pulp Chamber and Coronal Tooth of Primary Molars: An In Vitro Study. Int. J. Environ. Res. Public Health 2022, 19, 9279. [Google Scholar] [CrossRef]
- Banerjee, A.; Watson, T.F.; Kidd, E.A.M. Dentine Caries Excavation: A Review of Current Clinical Techniques. Br. Dent. J. 2000, 188, 476–482. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Kuropka, P.; Matys, J.; Malecka, M.; Kiryk, J.; Rybak, Z.; Dominiak, M.; Grzech-Lesniak, K.; Wiglusz, K.; et al. Removal of Composite Restoration from the Root Surface in the Cervical Region Using Er: YAG Laser and Drill—In Vitro Study. Materials 2020, 13, 3027. [Google Scholar] [CrossRef]
- Janeczek, M.; Świderski, J.; Czerski, A.; Żywicka, B.; Bujok, J.; Szymonowicz, M.; Bilewicz, E.; Dobrzyński, M.; Korczyński, M.; Chrószcz, A.; et al. Preliminary Evaluation of Thulium Doped Fiber Laser in Pig Model of Liver Surgery. Biomed. Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Kiryk, J.; Matys, J.; Nikodem, A.; Burzyńska, K.; Grzech-Leśniak, K.; Dominiak, M.; Dobrzyński, M. The Effect of Er:YAG Laser on a Shear Bond Strength Value of Orthodontic Brackets to Enamel—A Preliminary Study. Materials 2021, 14, 2093. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Policy on the Use of Lasers for Pediatric Dental Patients. The Reference Manual Pediatric Dentistry; American Academy of Pediatric Dentistry: Las Vegas, NV, USA, 2024. [Google Scholar]
- Sachelarie, L.; Cristea, R.; Burlui, E.; Hurjui, L.L. Laser Technology in Dentistry: From Clinical Applications to Future Innovations. Dent. J. 2024, 12, 420. [Google Scholar] [CrossRef]
- Struzik, N.; Wiśniewska, K.; Piszko, P.J.; Piszko, A.; Kiryk, J.; Matys, J.; Dobrzyński, M. SEM Studies Assessing the Efficacy of Laser Treatment for Primary Teeth: A Systematic Review. Appl. Sci. 2024, 14, 1107. [Google Scholar] [CrossRef]
- Olivi, G.; Caprioglio, C.; Olivi, M.; Genovese, M.D. Paediatric Laser Dentistry. Part 2: Hard Tissue Laser Applications. Eur. J. Paediatr. Dent. 2017, 18, 163–166. [Google Scholar] [CrossRef]
- Milc, A.; Kotuła, J.; Kiryk, J.; Popecki, P.; Grzech-Leśniak, Z.; Kotuła, K.; Dominiak, M.; Matys, J.; Grzech-Leśniak, K. Treatment of Deciduous Teeth in Children Using the Er:YAG Laser Compared to the Traditional Method: A Randomized Clinical Trial. Dent. Med. Probl. 2025, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Kolberg-Babrzyńska, I.; Grzech-Leśniak, K.; Kiryk, J.; Dominiak, M.; Matys, J. Effects of Endodontic Retreatment by Conventional Therapy Compared to Combined Therapy with an Er:YAG Laser and Photobiomodulation: A Randomized Clinical Trial. Dent. Med. Probl. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Kotlow, L.A. Lasers in Pediatric Dentistry. Dent. Clin. N. Am. 2004, 48, 889–922. [Google Scholar] [CrossRef] [PubMed]
- Grzech-Leśniak, K.; Nowicka, J.; Pajączkowska, M.; Matys, J.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Dobrzyński, M.; Dominiak, M. Effects of Nd:YAG Laser Irradiation on the Growth of Candida Albicans and Streptococcus Mutans: In Vitro Study. Lasers Med. Sci. 2019, 34, 129–137. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Shi, H.; Ma, Z.; Lv, B.; Xie, M. Er:YAG Laser Application in Caries Removal and Cavity Preparation in Children: A Meta-Analysis. Lasers Med. Sci. 2019, 34, 273–280. [Google Scholar] [CrossRef]
- Nahas, P.; Nammour, S.; Gerges, E.; Zeinoun, T. Comparison between Shear Bond Strength of Er:YAG and Er,Cr:YSGG Lasers-Assisted Dentinal Adhesion of Self-Adhering Resin Composite: An Ex Vivo Study. Dent. J. 2020, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Montedori, A.; Abraha, I.; Orso, M.; D’Errico, P.G.; Pagano, S.; Lombardo, G. Lasers for Caries Removal in Deciduous and Permanent Teeth. Cochrane Database Syst. Rev. 2016, 2016, CD010229. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Barylyak, A.; Bobitski, Y.; Piotr, K.; Buchwald, T.; Martyła, A.; Zinchenko, V.; Majchrowski, R.; Przekop, R.E.; Dorocka-Bobkowska, B. Electrodeposited Hydroxyapatite Coating on Titanium after Ultrashort-Pulsed Laser Processing for a Novel Surface of Endosseous Implants. Dent. Med. Probl. 2024, 61, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Bommala, M.; Koduganti, R.R.; Panthula, V.N.R.; Jammula, S.P.; Gireddy, H.; Ambati, M.; Ganachari, B. Efficacy of Root Coverage with the Use of the Conventional versus Laser-Assisted Flap Technique with Platelet-Rich Fibrin in Class I and Class II Gingival Recession: A Randomized Clinical Trial. Dent. Med. Probl. 2023, 60, 583–592. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Bourton, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Fleming, P.S.; Koletsi, D.; Pandis, N. Blinded by PRISMA: Are Systematic Reviewers Focusing on PRISMA and Ignoring Other Guidelines? PLoS ONE 2014, 9, e96407. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–671. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenolog 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Borsatto, M.C.; Corona, S.; Souza-Gabriel, A.E. Effect of Er:YAG Laser on Tensile Bond Strength of Sealants in Primary Teeth. J. Dent. Child 2014, 74, 104–108. [Google Scholar]
- Babu, K.L.G.; Hebbar, K.G.; Doddamani, G.M. Shear Bond Strength of a Composite Resin Restoration in Primary Teeth Following Cavity Preparation Using Laser- an in-Vitro Study. Lasers Med. Sci. 2024, 39, 244. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, K.; Zhang, B.; Zhou, Z.; Wang, Z.; Ge, X.; Wang, L.; Chen, Y.; Wang, X. Effects of Er:YAG Laser Pre-Treatment on Dentin Structure and Bonding Strength of Primary Teeth: An in Vitro Study. BMC Oral Heal. 2020, 20, 316. [Google Scholar] [CrossRef] [PubMed]
- Bahrololoomi, Z.; Kabudan, M.; Gholami, L.; Kabudan, M. Effect of Er:YAG Laser on Shear Bond Strength of Composite to Enamel and Dentin of Primary Teeth. J. Dent. 2015, 12, 163–170. [Google Scholar]
- Koyuturk, A.E.; Ozmen, B.; Cortcu, M.; Tokay, U.; Tosun, G.; Erhan Sari, M. Effects of Er: YAG Laser on Bond Strength of Self-Etching Adhesives to Caries-Affected Dentin. Microsc. Res. Tech. 2014, 77, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Paryab, M.; Sharifi, S.; Kharazifard, M.J.; Kumarci, N. Cavity Preparation by Laser in Primary Teeth: Effect 2 Levels of Energy Output on the Shear Bond of Composite Restoration to Dentin. J. Lasers Med. Sci. 2019, 9, 212–218. [Google Scholar] [CrossRef]
- Memarpour, M.; Shafiei, F.; Razmjoei, F.; Kianimanesh, N. Effect of Laser Preparation on Adhesion of a Self-Adhesive Flowable Composite Resin to Primary Teeth. Microsc. Res. Tech. 2016, 79, 334–341. [Google Scholar] [CrossRef]
- Flury, S.; Koch, T.; Peutzfeldt, A.; Lussi, A. Micromorphology and Adhesive Performance of Er:YAG Laser-Treated Dentin of Primary Teeth. Lasers Med. Sci. 2012, 27, 529–535. [Google Scholar] [CrossRef]
- Yildiz, E.; Karaaslan Sirin, E.; Yegin, Z.; Cebe, M.A.; Tosun, G. Effect of Caries Removal on the Bond of adhesives to Caries-Affected Dentin in Vitro. Eur. J. Paediatr. Dent. 2013, 14, 209–214. [Google Scholar]
- Borsatto, M.C.; Martinelli, M.G.; Contente, M.M.M.G.; Mellara, T.D.S.; Pécora, J.D.; Galo, R. Bond Durability of Er:YAG Laser- Prepared Primary Tooth Enamel. Braz. Dent. J. 2013, 24, 330–334. [Google Scholar] [CrossRef]
- Wanderley, R.L.; Monghini, E.M.; Pecora, J.D.; Palma-Dibb, R.G.; Borsatto, M.C. Shear Bond Strength to Enamel of Primary Teeth Irradiated with Varying Er:YAG Laser Energies and SEM Examination of the Surface Morphology: An in Vitro Study. Photomed. Laser Surg. 2005, 23, 260–267. [Google Scholar] [CrossRef]
- Monghini, E.M.; Wanderley, R.L.; Pécora, J.D.; Palma Dibb, R.G.; Corona, S.A.M.; Borsatto, M.C. Bond Strength to Dentin of Primary Teeth Irradiated with Varying Er:YAG Laser Energies and SEM Examination of the Surface Morphology. Lasers Surg. Med. 2004, 34, 254–259. [Google Scholar] [CrossRef]
- Felemban, O.; Abdrabuh, R.; El Meligy, O.; Farsi, N.; Bakry, A.S.; Abu Haimed, T. Micro-Morphological Features of the Er:YAG-Lased Interface in Primary Teeth: 12 Months Randomized Split-Mouth Trial. J. Funct. Biomater. 2024, 15, 17. [Google Scholar] [CrossRef]
- Scatena, C.; Torres, C.P.; Gomes-Silva, J.M.; Contente, M.M.M.G.; Pécora, J.D.; Palma-Dibb, R.G.; Borsatto, M.C. Shear Strength of the Bond to Primary Dentin: Influence of Er:YAG Laser Irradiation Distance. Lasers Med. Sci. 2011, 26, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Bahrololoomi, Z.; Dadkhah, A.; Alemrajabi, M. The Effect of Er: YAG Laser Irradiation and Different Concentrations of Sodium Hypochlorite on Shear Bond Strength of Composite to Primary Teeth’s Dentin. J. Lasers Med. Sci. 2017, 8, 29–35. [Google Scholar] [CrossRef]
- Ersan, Z.; Yazicioğlu, I.; Serin, A.; Doğan, M. The Effects of Disinfection with Er, Cr. Niger. J. Clin. Pract. 2022, 25, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Chikkanarasaiah, N.; Hrishida, P. Shear Bond Strength of Glass Ionomer Cement to Er, Cr:YSGG Laser-Irradiated Dentin in Primary Teeth: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2022, 15, 199–203. [Google Scholar] [CrossRef] [PubMed]
- AlHumaid, J.; Alagl, A.S.; Bedi, S. Effect of Erbium Laser on Microtensile Bond Strength of Fissure Sealant in Primary Teeth: An in Vitro Study. Saudi J. Med. Med. Sci. 2018, 6, 27–31. [Google Scholar] [CrossRef]
- Malekafzali, B.; Fekrazad, R.; Mirfasihi, A.; Saedi, S. Comparison of Microtensile Bond Strength of a Resin Composite to Enamel Conditioned by Acid Etching and Er,Cr:YSGG Laser in Human Primary Teeth. Eur. Arch. Paediatr. Dent. 2015, 16, 57–62. [Google Scholar] [CrossRef]
- Sungurtekin-Ekci, E.; Oztas, N. Microtensile Bond Strength of a Resin-Based Fissure Sealant to Er,Cr:YSGG Laser-Etched Primary Enamel. Odontology 2016, 104, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Faisal Kattan-Bds, H. Adhesive Bond Strength of Er Cr YSGG Laser Treated Deciduous Dentin to Resin Cements. Biosc. Biotech. Res. Comm. 2020, 13, 383–388. [Google Scholar] [CrossRef]
- Öznurhan, F.; Ölmez, A. Morphological Analysis of the Resin-Dentin Interface in Cavities Prepared with Er,Cr:YSGG Laser or Bur in Primary Teeth. Photomed. Laser Surg. 2013, 31, 386–391. [Google Scholar] [CrossRef]
- Zarabian, T.; Mood, S.A.; Kiomarsi, N.; Noorollahian, H.; Hakimiha, N. Microshear Bond Strength of a Self-Adhesive Composite to Erbium Laser-Treated Primary Enamel. J. Lasers Med. Sci. 2020, 11, 181–186. [Google Scholar] [CrossRef]
- Kotb, S.A.; Abd Al Gawad, R.Y.; Sabry, S.M. Influence of Diode Laser Irradiation on Shear Bond Strength of Activa and Composite Resin Restorations in Primary Molars: An In-Vitro Study. Adv. Dent. J. 2023, 5, 966–972. [Google Scholar] [CrossRef]
- Bolukbasi, B.; Kucukyilmaz, E. Evaluation of the Bond Strengths of Restorative Materials to Primary Tooth Dentin Treated with Different Pulpotomy Techniques. Microsc. Res. Tech. 2021, 84, 1309–1320. [Google Scholar] [CrossRef]
- Nisar, S.S.; Irfan, F.; Hammad, H.; Abdulla, A.M.; Kamran, M.A.; Barakat, A.; Niazi, F.; Baig, E.A.; Qureshi, A. Disinfection of Caries-Affected Dentin Using Potassium Titanyl Phosphate Laser, Rose Bengal and Ozonated Water on Shear Bond Strength of Deciduous Teeth. Photodiagnosis Photodyn. Ther. 2022, 40, 103044. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
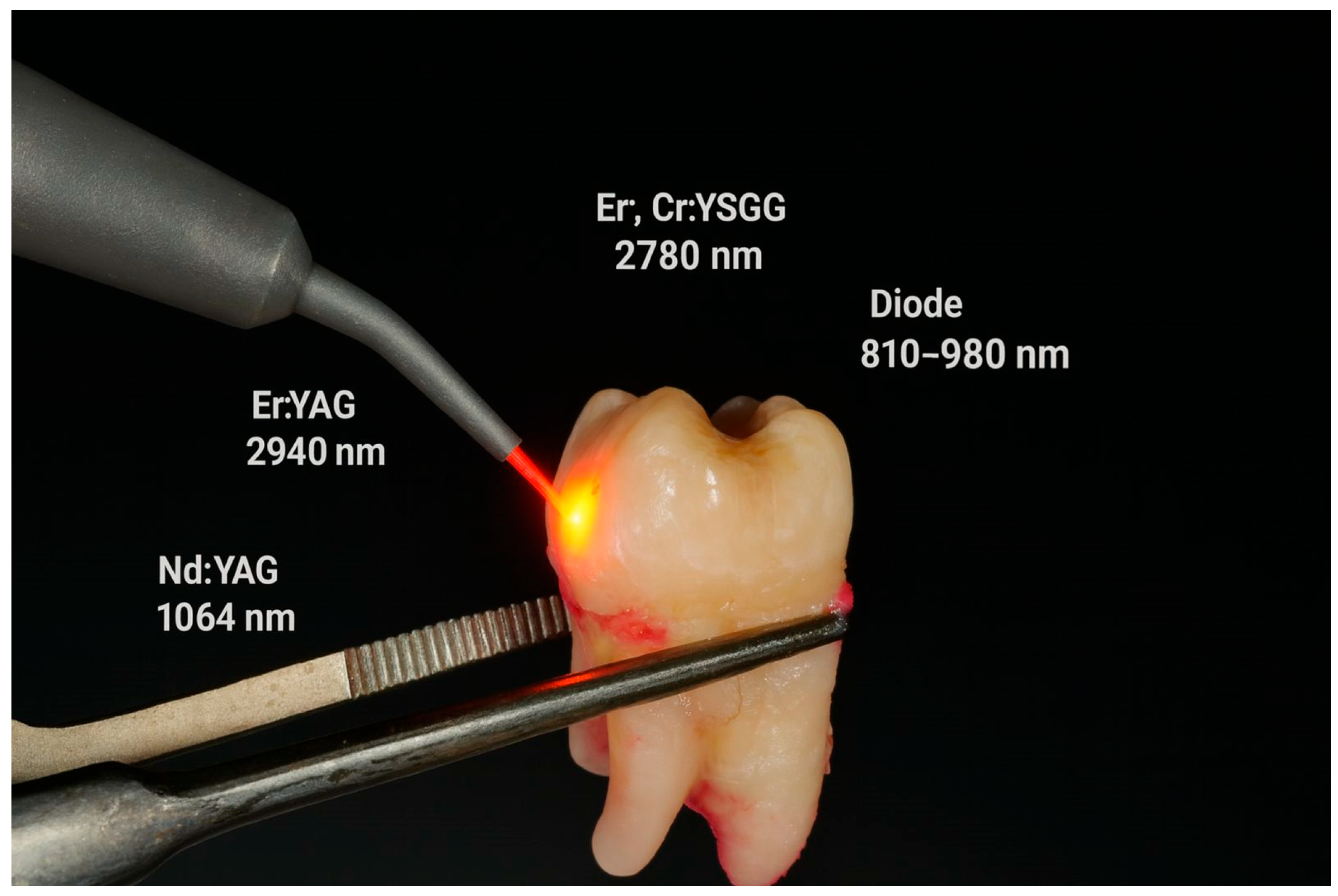
| Study | Aim of the Study | Material and Methods | Results | Conclusions |
|---|---|---|---|---|
| Ersan [61] | To assess how Er,Cr:YSGG laser disinfection, CPP-ACP paste application and NaOCl (alone and combined) affect SBS to primary teeth enamel. | Several pre-application protocols (none, Er,Cr:YSGG, Er,Cr:YSGG+CPP-ACP, NaOCl, NaOCl+CPP-ACP, CPP-ACP) were performed on the polished buccal surfaces of primary teeth, after which a sealant was applied and the SBS and fracture patterns were examined. | The lowest SBS was obtained after NaOCl (alone or with CPP-ACP), with a predominance of adhesive fractures; differences between the other groups were not significant. | Er,Cr:YSGG disinfection and CPP-ACP application may be acceptable alternatives before sealant, but NaOCl weakens the bond. |
| Chikkanarasaiah and Hrishida [62] | Comparison of SBS glass ionomer cement (Fuji IX) to primary dentin after standard conditioning with 10% poly (acrylic acid) vs. after conditioning with Er,Cr:YSGG laser. | 30 primary teeth were prepared for two protocols (PA 10% or Er,Cr:YSGG 0.5 W/25 mJ), GIC restorations, thermocycling and SBS test were performed. | Er,Cr:YSGG conditioning increased SBS GIC relative to standard poly (acrylic acid) conditioning. | Laser dentin conditioning may improve the adhesion of glass ionomer cement in primary teeth. |
| Borsatto [46] | To assess whether enamel conditioning with an Er:YAG laser affects tensile bond strength (TBS) in primary teeth | Thirty primary molars were randomly divided into 3 groups (acid, Er:YAG, Er:YAG+acid), enamel was flattened, sealant was applied, samples were subjected to thermocycling and TBS test. | TBS was similar for “acid” (10.80 ± 3.28 MPa) and “Er:YAG+acid” (12.85 ± 2.14 MPa) and significantly lower for “Er:YAG alone” (4.17 ± 2.31 MPa). | Er:YAG irradiation alone does not provide retention—subsequent etching with phosphoric acid is necessary. |
| Kotb [69] | To assess whether diode laser irradiation (980 nm, 1 W) changes the micro shear bond strength (µSBS) of Activa Bioactive material and composite on enamel and dentin of primary teeth. | 104 primary molars were divided into groups of “laser/no laser” and enamel/dentin and Activa/composite substrates; etching/universal adhesive, laser (or no laser), 500 thermal cycles, and µSBS testing were performed. | The laser significantly reduced the µSBS of Activa (on both enamel and dentin) and did not improve the µSBS of the composite. | Diode laser irradiation before polymerization does not increase the adhesion of the composite in primary teeth and impairs the bonding of Activa. |
| Babu [47] | Compare the shear bond strength (SBS) of the composite after cavity preparation in primary teeth using the classical method and the Er:YAG and Er,Cr:YSGG lasers. | 100 primary molars were randomly assigned to 5 conditioning regimens (phosphate, Er:YAG+acid, Er,Cr:YSGG+acid, Er:YAG alone, Er,Cr:YSGG alone), followed by composite restorations, thermocycling, and SBS testing. | The highest SBS was obtained after classical etching; laser etching alone gave significantly lower values, which were improved by the addition of acid etching. | Phosphoric acid etching remains the most effective, and adding acid to laser-prepared surfaces improves bond strength. |
| AlHumaid [63] | To evaluate how different Er,Cr:YSGG laser powers affect µTBS to primary enamel. | 50 first primary molars were divided into 5 groups: 3.5 W + acid, 2.5 W + acid, 3.5 W without acid, 2.5 W without acid, acid only; sealant application and µTBS test were performed. | All groups except “2.5 W acid-free” had similar μTBS; 2.5 W ablation alone gave significantly lower values (9.66 MPa). | 3.5 W Er,Cr:YSGG used solo provides µTBS comparable to acid, suggesting that with appropriate power the laser can effectively condition enamel for sealant. |
| Wang [48] | To investigate how Er:YAG laser dentin pretreatment parameters affect dentin morphology and composite bond strength in primary teeth. | Dentin plates from primary molars were subjected to: no treatment, acid etching, Er:YAG etching with different energy (50–300 mJ) or frequency (5–30 Hz); SEM and SBS were assessed after thermocycling. | In the range of 50–200 mJ and 5–20 Hz, the laser opened the tubules without lamellar rupture and increased SBS; outside this range, ruptures/intertubular damage were observed and no further improvement was observed. | Properly selected Er:YAG parameters can improve bonding to dentin in primary teeth by opening the tubules without creating a smear layer. |
| Bahrololoomi [49] | Compare the effect of bur vs. Er:YAG laser preparation (with/without etching) on SBS composite for enamel and dentin of primary teeth. | 75 primary molars were cut mesiodistally (150 samples) and in each tissue (enamel/dentin) three schemes were performed: drill+acid, Er:YAG+acid, Er:YAG only; Single Bond and Z250, thermocycling and SBS test were used. | The highest SBS was obtained with drill+acid, lower with Er:YAG+acid, and lowest with Er:YAG alone; enamel generally had a higher SBS than dentin. | Laser preparation generates lower SBS than mechanical preparation, especially without additional etching. |
| Malekafzali [64] | Comparison of the shear bond strength of composite with primary tooth enamel after acid etching and conditioning with Er,Cr:YSGG laser. | 20 deciduous canines were cut in half, half of the samples were prepared with 37% orthophosphoric acid and half with Er,Cr:YSGG laser and then a microtensile test was performed. | The mean SBS was significantly higher in the acid-etched group (24.62 MPa) than in the laser group (18.55 MPa) | The Er,Cr:YSGG laser cannot replace traditional acid etching in primary teeth. |
| Sungurtekin-Ekci and Oztas [65] | To evaluate the effect of Er,Cr:YSGG laser conditioning alone or in combination with acid etching on the shear bond strength of a fissure sealant for primary tooth enamel. | 25 primary molars were divided into 5 groups (different laser parameters 2.5 W and 3.5 W, acid only, laser + acid), then the micro shear bond strength was measured. | Acid etching and the 3.5 W laser gave comparable results, but the 2.5 W laser had significantly lower bond strength; the best result was achieved by 3.5 W + acid. | A 3.5 W laser can be an alternative to acid, but the weaker parameters (2.5 W) are insufficient to obtain good adhesion. |
| Koyuturk [50] | To investigate the effect of Er:YAG laser on SBS of two self-etching systems for carious dentin in primary teeth. | Ninety primary molars with healthy and carious dentin were prepared using an Er:YAG laser or a drill, two different adhesive systems were used (Clearfil S3, Xeno V) and then microtensile test and SEM analysis were performed. | The highest strength was obtained in the Clearfil S3 + laser group on healthy dentin (24.57 MPa), the lowest in the Xeno V + laser group on healthy dentin (11.01 MPa); Clearfil S3 generally gave better results than Xeno V. | Er:YAG may improve the bonding of some adhesive systems (Clearfil S3), but the effectiveness depends on the type of system used. |
| Paryab [51] | Evaluation of the effect of two Er:YAG laser energy levels on SBS composite for primary dentin compared to bur preparation using total-etch and self-etch systems. | 60 primary molars were divided into 3 groups (drill, 300 mJ laser, 400 mJ laser) and subgroups with total-etch (Single Bond) and self-etch (Clearfil SE Bond) systems; after thermocycling, SBS was measured. | The highest strength was achieved in the drill + Clearfil SE Bond group, the lowest in the 400 mJ laser + Clearfil SE Bond group; the differences between the groups were not statistically significant | Both adhesive systems perform well enough on primary dentin prepared with a 300 and 400 mJ laser, making the laser an alternative to bur preparation. |
| Memarpour [52] | The study aimed to assess how Er:YAG laser preparation affects the shear bond strength (SBS) and micromorphology of a self-adhering flowable composite when applied to primary tooth enamel and dentin, compared to silicon carbide paper (SiC) preparation. | 120 extracted primary canine teeth, divided into enamel and dentin groups. Each group was subdivided based on surface preparation method (SiC or laser) and restorative material: Premise Flowable, Vertise Flow and Vertise Flow combined with adhesive (OptiBond All-In-One). Shear bond strength tests and SEM imaging were performed. |
| Laser pretreatment improved the bond strength of the self-adhering composite on enamel, but not on dentin. In dentin, laser preparation offered no advantage over conventional SiC treatment in terms of SBS. |
| Flury [53] | Comparison of micromorphology and adhesive properties of dentin in primary teeth prepared with a diamond drill and Er:YAG laser in different settings. | A total of 82 extracted jbi primary molars were used. 18 teeth were used for SEM micromorphology analysis and 64 molars were used for μTBS adhesive strength testing. For micromorphological analysis, teeth were treated using either a diamond bur or Er:YAG laser at four different energy settings. SEM imaging was conducted to evaluate surface characteristics. For bond strength tests, 64 teeth were prepared with adhesive (Clearfil SE) and composite (Clearfil Majesty Esthetic), then tested with μTBS. | The μTBS testing showed no statistically significant differences in bond strength between diamond bur-treated teeth and laser-treated groups (p = 0.394). | Er:YAG laser-treated dentin provides bonding strength to resin composite similar to that achieved with conventional bur preparation. However, insufficient laser energy, as applied in Group 2d (50 mJ/35 Hz), resulted in incomplete dentin surface preparation, leading to less favorable bonding performance. |
| Yildiz [54] | Evaluation of the impact of three different caries removal methods affect the microtensile bond strength of adhesives to caries-affected dentin. | 30 extracted primary molars were divided into three groups based on caries removal technique: conventional steel drill, Er:YAG laser and chemomechanical method—Carisolv, then bonded with either etch-and-rinse or self-etch adhesive. Composite was applied and cured. Teeth were cut into 1 mm2 sticks for μTBS testing. Fracture types were examined under a microscope and the dentin surface morphology was analyzed using SEM. | Bond strength was significantly lower in laser-treated groups compared to bur and chemomechanical groups, for both adhesives (p < 0.05). No significant difference was observed between conventional drill and Carisolv groups. Adhesive type (etch-and-rinse vs. self-etch) did not significantly affect results. | The caries removal method significantly affects bond strength in primary dentin. Bur and chemomechanical methods resulted in better adhesion than Er-YAG laser technique. The choice between self-etch or etch-and-rinse adhesives does not significantly influence bond strength. |
| Bolukbasi [70] | To compare how five different pulpotomy techniques affect the shear bond strength of two restorative materials (glass hybrid and composite resin) to primary dentin. | 240 dentin samples from 120 caries-free primary molars were divided into six groups (n = 40) based on the pulpotomy treatment:
| Nd:YAG laser showed the highest SBS in both materials. Biodentine showed the lowest SBS for glass hybrid. Ferric sulfate caused significantly lower SBS for composite resin. SEM showed open dentin tubules with Nd:YAG and occluded tubules with Biodentine and ferric sulfate. Composite resin had consistently higher SBS than glass hybrid across all groups. | Nd:YAG laser treatment enhances adhesion strength, especially with composite resin. Ferric sulfate negatively impacts composite bonding. |
| Borsatto [55] | The study aimed to assess how thermocycling and water storage, which simulate long-term oral conditions, influence the shear bond strength of composite resin applied to primary teeth enamel prepared using an Er:YAG laser compared to traditional bur preparation. | 48 primary molars were randomly divided into 2 groups: bur-prepared and Er:YAG laser-prepared cavities. All teeth were restored using an etch-and-rinse adhesive and composite resin. Samples were subjected to aging through water storage (24 h to 6 months) and thermocycling (up to 12,000 cycles). Shear bond strength was measured using a universal testing machine and failure modes were analyzed under a stereomicroscope. | The bur-prepared group exhibited the highest initial bond strength (17.45 MPa after 24 h), but its adhesion significantly decreased after prolonged aging (down to 6.88 MPa after 1 month). Er:YAG laser-prepared enamel showed stable adhesion after 1 month (15.05 MPa), but significant reduction (5.51 MPa) occurred after 6 months. | Significant changes in the adhesion of an etch-and-rinse system to Er:YAG laser-prepared primary enamel were observed only following 6 months of water aging and 12,000 thermal cycles. |
| Wanderley [56] | Evaluation of the effect of Er:YAG laser etching on the shear bond strength (SBS) of an adhesive system. | The study used 48 primary canines. Their surfaces were cleaned. The teeth were divided into four groups: 1. Acid etching only 2. Acid etching with an Er:YAG laser at 60 mJ 3. Acid etching with an Er:YAG laser at 80 mJ 4. Acid etching with an Er:YAG laser at 100 mJ Bonding system was applied to all samples. Filtek Z250 light-cured composite resin blocks were then applied and polymerised. The composite blocks were then loaded until they detached from the teeth. The SBS was measured. Enamel evaluation was performed using SEM. | Samples etched with a laser power of 60 mJ had higher SBS values than samples etched with phosphoric acid only. The higher the laser power, the lower the SBS values. Acid etching results in a smooth surface and etch pattern. Acid etching + laser: uneven surface and etch pattern. Laser etching produced no pattern and increased surface irregularity with higher laser power. | Using an Er:YAG laser as an additional etching agent can increase the shear bond strength (SBS) if the correct parameters are set. The Er:YAG laser affects the morphological structure of enamel. |
| Moghini [57] | Evaluation of the effect of Er:YAG laser etching on the morphology of the dentin and the strength of the bond (SBS). | The study involved 69 primary canines. The teeth were cleaned. Forty-eight of these were used for the SBS study. Group 1 received phosphoric acid etching, while Groups 2, 3 and 4 received acid etching plus 60, 80 and 100 mJ of Er:YAG laser irradiation, respectively. A bonding agent was applied to each sample, which was then irradiated. Composite-polyester resin blocks were then bonded to the teeth. The material was then subjected to loading to determine the SBS. Twenty-one teeth were evaluated using SEM. Seven groups were formed: 1. acid etching, 2. acid etching + laser, 3. acid etching + laser, 4. acid etching + laser, 5. laser etching, 6. laser etching, 7. laser etching. | The acid-only etching group achieved the highest SBS values. The laser-etching groups had lower values. Regardless of power, laser etching produced similar SBS values. Acid etching alone creates a regular dentin surface. Laser etching alone creates an irregular dentin surface. Acid and laser etching together create an irregular surface with open channels. | Using a laser to etch dentin does not improve SBS values. Using a laser in conjunction with etching creates an irregular dentin surface, which may affect the holding strength of composite restorations. |
| Kattan [66] | Assessment of dentin SBS following exposure to an Er,Cr:YSGG laser. | The study used forty primary teeth. The surfaces of the teeth were cleaned. The control group comprised: Ten teeth were etched with phosphoric acid and thirty with an Er,Cr:YSGG laser. Group A of the control group received Rely-X ARC, while groups B, C and D received Rely-X Unicem, Rely-X ARC and glass ionomer cement (GIC), respectively. The samples were loaded and the SBS was measured. | The control group had the highest SBS values. The SBS values in the laser groups B and C were comparable. D group had lower SBS values than B and C. | The highest bond strength is achieved through classical phosphoric acid etching. Treating dentin with an Er,Cr:YSGG laser does not increase the holding strength of the material. |
| Felemban [58] | Comparison of the bond interface quality between restorations placed after bur preparation and Er:YAG laser preparation. | 80 carious primary molars (class I caries) from 40 patients (9–12 years old) treated in one quadrant by bur preparation and in the other by Er:Yag (2940 nm) laser preparation (treatment performed using rubber dam), after 1 year all were assessed clinically and radiographically, 20 teeth were extracted and after preparation assessed using SEM (integration of the adhesive with enamel wall, dentin wall and dentin floor) | No statistically significant difference in bond interface quality of the restorations placed in cavities prepared by bur caries removal or laser method. | The bond interface quality of class I restorations placed in primary molars after Er:YAG laser preparation and bur preparation performed similarly. |
| Oznurhan [67] | Analysis of the resin-dentin interface in primary teeth after laser or bur cavity preparation. | The V class cavities were prepared in 20 extracted primary molars (10 by bur and 10 by Er,Cr:YSGG laser). Later palatal/lingual cavities were restored following acid-etching and buccal without acid-etching, creating four groups: G1: Er,Cr:YSGG laser G2: Er,Cr:YSGG laser and acid etching G3: Bur G4: Bur and acid etching Then teeth were sectioned and immersed in ammoniacal silver nitrate solution for 24 h in a dark chamber. The resin–dentin interface was assessed by SEM and ion analysis was performed with SEM-energy-dispersive X-ray spectroscopy (EDX). | Surface structure: G1, G2 wavy; G3, G4 smooth. Microcracks: G1, G2. Dentin tubules exposure: G1, G2. Gaps and smear layer: G1, G2 no smear layer, gaps; G3 smear layer and gaps; G4 none. Resin tags: broken in G1, G2 cavities, increased in G2. | For better adhesion after laser preparation including acid-etching is recommended. |
| Scatena [59] | In vitro assessment of the influence of Er:YAG laser irradiation distance on the shear strength of the adhesive bond. | 60 exfoliated/extracted primary molars (embedded in acrylic resin and mechanically treated to expose a flat dentin surface) were divided into 6 groups (n = 10): control (etched with 37% phosphoric acid) and rest irradiated (80 mJ, 2 Hz) at different irradiation distances (11, 12, 16, 17, 20 mm), then acid-etched. Later Single bond (adhesive agent) was applied, then resin cylinders (Filtek Z250) were prepared. The shear bond strength were tested by a universal testing machine (0.5 mm/min). | The mean shear bond strengths (MPa) were as follows: Control 7.32 ± 3.83 11 mm 5.07 ± 2.62 12 mm 6.49 ± 1.64 16 mm 7.71 ± 0.66 17 mm 7.33 ± 0.02 20 mm 9.65 ± 2.41 Statistically significant differences were found between 11 mm and 16 mm groups; 11 mm and 20 mm groups | Increasing the laser irradiation distance enhanced the shear bond strength to primary dentin. Er:YAG laser treatment combined with acid etching at a 20 mm distance shows superior adhesive resistance compared to acid etching alone |
| Nisar [71] | Evaluation of disinfection of caries-effected dentin using KTP laser, Er,YAG laser and other methods on the shear bond strength of adhesive resin. | 50 primary molar teeth were qualified by radiological and physical (dental explorer, staining with Caries Detector and visually examined) and divided into 5 groups. Laser conditioning was employed: For KTP laser- 4 times in the row 532 nm applied for 10 s with 5 s waiting period for 1 min at 1 W energy output, 10 t pulsed mode and focal distance 1 mm. Er YAG laser was executed with output 1.2 W, pulse repetition 10 Hz, 2940 nm wavelength, 18.9 J/cm2 | Caries affected dentin when disinfected with Er YAG have high SBS value. Eradicating carious dentine with Er YAG provided eradication of smear layer and formed resin tags allowing adhesive to reach surface with high roughness leading to high SBS values. KTP laser as cavity disinfectant provoked surface melting and demolished dentinal tubules making them incompetent to form hybrid layers, decreasing SBS values. | Er YAG laser can be implemented as cavity disinfectant in primary teeth reliably as it demonstrated better shear bond strength without negating adhesive binding capacity of used resins. |
| Kiomarsi [68] | To assess microshear bond strength of a self-adhering flowable composite compared with conventional composite, requiring adhesive and etching procedures to primary enamel treated with graphite disc and laser irradiation. | 72 primary teeth was used in the study, being tested no longer than 3 months after exfoliation/extraction. Smooth enamel surface was achieved after treating tooth with grit disc with silicone carbide particles. Then half of the teeth (36) were treated with Er,Cr YSSG laser with wavelength of 2780 nm, 20 Hz frequency, 1.5 W power, 60% water and 60% air cooling with 1 mm distance and sweeping motion, 60 μs pulse duration. Then 2 different composites were applied to laser and non-laser (control) group (self-adhering-VF and one requiring etching and adhesive procedure-PF) | µSBS of self-adhering composites is increased after laser surface treatment of enamel surface of primary teeth. (13.60 ± 5.47) vs. (5.89 ± 2.42) No significant difference was observed when comparing conventional composite µSBS values applied on laser treated enamel vs. non-treated samples (13.18 ± 3.45) vs. (16.06 ± 3.52) | Conventional flowable composites presented higher µSBS values than self-adhering composites for primary teeth. Laser treatment of enamel surface increases µSBS value of self-adhering composites when compared to non-laser treated samples |
| Bahrololoomi [60] | To evaluate effect of different concentrations of NaOCl on shear bond strength of composite resin to dentin prepared with bur and laser | 48 extracted primary human molars with sound buccal and lingual surfaces were used in the study. Specimens were prepared by root sectioning and crowns were divided into buccal and lingual parts, overall 96 specimens were assessed. 12 of them were assessed for SEM scan. Remaining specimens were divided into 2 groups n = 42 to be prepared with laser or bur. Then each of those groups were divided into 3: without sodium hypochlorite, with 2,5% and with 5.25% sodium hypochlorite. Laser used for preparation was Er:YAG laser (Fotona, Fidelis Plus III, Slovenia), with 2.94 μm wavelength, 200 mJ energy, pulse repetition rate of 10 Hz and micro-short pulse (MSP) along with air-water cooling of 7 mL/min, in non-contact mode from 17 mm distance. Then all surfaces were etched and NaOCl was applied to specimens and after that bond and composite were applied and after 1000 thermocycles bond strength was calculated in MPa using instron testing machine | Shear bond strength to dentin in laser treated group was a little, but not significantly higher compared to bur group. NaOCl application increased SBS in both groups but also without any significant differences. | Difference between laser and bur group was not statistically significant, although laser treated surface of primary teeth presented increase in SBS. This is probably connected to morphological properties of laser treated surface compared to bur prepared surface. The structural difference in primary teeth dentin with permanent ones is probably the reason for this insignificant difference, despite the complete removal of collagen. Primary teeth are less calcified than permanent teeth, and contain more water and organic substances. The dentinal tubules are less aligned and intertubular dentin contains more water than peritubular dentin. Therefore, primary teeth may need different application time and concentration of sodium hypochlorite in order to improve the bond strength compared to permanent teeth; thus the need for more research in this field. |
| Study | Laser Type and Parameters | Sample Type | Adhesive/Restorative Material | Shear/Tensile Bond Strength | Morphological Analysis |
|---|---|---|---|---|---|
| Ersan [61] | Er, Cr:YSGG Device: Waterlase iPlus (Biolase, San Clemente, CA, USA) Wavelength: 2780 nm Pulse time: 140 μs Tip: sapphire, diameter 600 μm, length 6 mm Power: 0.75 W Cooling: 15% water, 15% air Frequency: 20 Hz Distance: 1–2 mm Operating mode: 5 applications of 10 s each, with 5 s breaks | Enamel | pit and fissure sealant ClinPro Sealant (3M ESPE, St. Paul, MN, USA) | Laser + CPP–ACP: 18.23 ± 5.9 MPa Laser: 18.29 ± 7.6 MPa NaOCl: 6.42 ± 2.5 MPa NaOCl + CPP–ACP: 6.23 ± 3.2 MPa CPP–ACP: 15.61 ± 7.9 Mpa Control: 16.09 ± 6.8 MPa | In the Laser, Laser + CPP–ACP, CPP–ACP, and control groups, cohesive and mixed fractures predominated. In the NaOCl and NaOCl + CPP–ACP groups, adhesive fractures predominated. |
| Chikkanarasaiah and Hrishida [62] | Er, Cr:YSGG Wavelength: 2780 nm Energy: 0.5 W, 25 mJ/pulse Energy density: 9 J/cm2 Tip: sapphire, distance: 2 mm Scanning mode | Dentin | Conventional glass ionomer cement—GC Fuji IX (GC Corporation, Tokyo, Japan) | Control: 3.033 ± 0.065 MPa Laser: 3.381 ± 0.088 MPa | The laser removes the smear layer and opens the dentinal tubules, creating an irregular, rough surface that promotes adhesion. |
| Borsatto [46] | Er:YAG Device: KaVo Key Laser 2 (KaVo, Biberach, Germany) Wavelength: 2.94 μm Pulse energy: 80 mJ Frequency: 2 Hz Operating mode: defocused mode Exposure time: 30 s Focal distance: 17 mm Water jet: 1.5 mL/min Beam spot diameter: 0.63 mm Beam area: ~0.312 mm2 | Enamel | Pit and fissure sealant FluroShield (Caulk-Dentsply, Milford, DE, USA) | 37% phosphoric acid: 10.80 ± 3.28 MPa Er:YAG + 37% phosphoric acid: 12.85 ± 2.14 MPa Er:YAG: 4.17 ± 2.31 MPa | Irregular, non-uniform etch pattern, excessive subsurface cracking, micropores partially submerged by fused/resolidified enamel; this resulted in a higher rate of adhesive fractures. |
| Kotb [69] | Diode Laser Wavelength: 980 nm Power: 1 W Operation mode: continuous Fiber diameter: 300 μm Distance: ~1 mm Traverse speed: 1 mm/s Time: 10 s Irradiation field diameter: 1 mm (area ~0.785 mm2) | Enamel Dentin | Activa Bioactive Restorative material (Pulpdent, Watertown, MA, USA) Filtek Z350XT (3M ESPE, St. Paul, MN, USA)—nanohybrid composite All-Bond Universal Adhesive (Bisco, Schaumburg, IL, USA)—universal bonding system | Enamel/Activa Laser = 19.68 ± 3.65 Mpa control = 24.70 ± 2.92 Mpa Enamel/Composite Laser = 18.44 ± 7.49 MPa control = 20.25 ± 3.68 Mpa Dentin/Activa Laser = 13.61 ± 3.07 MPa control = 17.88 ± 3.46 Mpa Dentin/Composite Laser = 19.74 ± 3.64 Mpa control = 17.63 ± 2.63 MPa | The highest number of adhesive fractures occurred in the Dentin/Activa/No Laser group (76.9%) and the Dentin/Composite/Laser group (75%). The lowest number of adhesive fractures occurred in the Enamel/Activa/Laser group (18%). |
| Babu [47] | Er:YAG Wavelength: 2940 nm Max output power: 10 W Pulse energy: 200 mJ Pulse repetition rate: 10 Hz Pulse duration: 450 µs Spot size: 0.785 mm2 Distance: 8–12 mm, non-contact mode Preparation parameters: enamel—4 W, 10 Hz, 60% air-water; dentin—3 W, 60% air, 30% water Er,Cr:YSGG Wavelength: 2780 nm Max output power: 10 W Max pulse energy: 600 mJ Pulse repetition rate: 5–100 Hz Pulse duration: 600–700 µs Spot size: 0.442 mm2 Distance: 8–10 mm, non-contact mode Preparation parameters: enamel—4 W, 15 Hz, 60% air-water; dentin—3 W, 60% air, 30% water | Enamel dentin | Composite Filtek™ 350 XT (3M ESPE, St. Paul, MN, USA) Adper™ Single Bond 2 Adhesive (3M ESPE, St. Paul, MN, USA) | Bur + acid: 17.562 ± 0.810 MPa Er:YAG+acid: 15.928 ± 0.415 MPa Er,Cr:YSGG + acid: 14.964 ± 0.566 MPa Er:YAG without acid: 11.833 ± 0.533 MPa Er,Cr:YSGG without acid: 11.187 ± 0.517 MPa | The laser creates an irregular, scaly surface with open dentinal tubules and no smear layer. This may include a remelted layer, microcracks, adhered collagen fibers and porous layers of recrystallized minerals after microblasting. |
| AlHumaid [63] | Er,Cr:YSGG Wavelength: 2780 nm Fiber diameter: 600 μm Power: 2.5 W or 3.5 W Pulse time: 140 μs Frequency: 20 Hz Pulse energy: 75 mJ/pulse Operating mode: non-contact, 1 mm distance Irradiation time: 15 s Air/water cooling: 70% air/60% water (2.5 W) and 80% air/70% water (3.5 W) | enamel | Pit and fissure sealant ClinPro™ Sealant (3M ESPE, St. Paul, MN, USA) Composite Filtek Z350 (3M ESPE, St. Paul, MN, USA) | 3.5 W laser + acid = 15.57 ± 3.71 MPa 2.5 W laser + acid = 14.18 ± 2.83 MPa 3.5 W laser = 14.78 ± 1.71 MPa 2.5 W laser = 9.66 ± 2.20 MPa Acid = 14.63 ± 3.73 MPa | Er,Cr:YSGG causes micro-explosions in the enamel, leading to macro- and microscopic irregularities, removal of water and mineral components. |
| Wang [48] | Er:YAG Wavelength: 2940 nm Distance: 1 mm Beam diameter: 0.6 mm Time: 10 s (grid scanning mode) Cooling: 60% water, 40% air/steam Energy: 50, 100, 150, 200, 250, 300 mJ (at 10 Hz) Frequency: 5, 10, 15, 20, 25, 30 Hz (at 100 mJ) | Dentin | Composite Filtek Z350 (3M ESPE, St. Paul, MN, USA) | Control 11.06 ± 2.10 MPa Acid etching 13.74 ± 1.73 MPa 50 mJ 17.74 ± 2.63 MPa 100 mJ 18.07 ± 2.03 MPa 150 mJ 18.11 ± 2.15 MPa 200 mJ 17.56 ± 2.54 MPa 250 mJ 13.39 ± 2.41 MPa 300 mJ 11.27 ± 2.30 MPa | Er:YAG 50–200 mJ/5–20 Hz: open dentinal tubules, absent smear layer, irregular surface with fish-scale-like (lamellar) pattern, protruding areas of peritubular dentin; at 150–200 mJ, fine cracks are visible. Er:YAG > 200 mJ > 20 Hz: loss of lamellar pattern, blurred tubule boundaries, enlarged cracks, collapse of dentin structure, localized charring. |
| Bahrololoomi [49] | Er:YAG Wavelength: 2940 nm Energy: Enamel: 300 mJ, 10 Hz Dentin: 200 mJ, 10 Hz Operating mode: non-contact Distance: 17 mm Cooling: water 7 mL/min | Enamel Dentin | Adhesive Adper™ Single Bond 2 (3M ESPE, St. Paul, MN, USA) Composite Filtek™ Z250 (3M ESPE, St. Paul, MN, USA) | Drill+acid 14.53 ± 3.53 MPa Laser + acid 10.69 ± 2.08 MPa Laser without acid 3.53 ± 0.89 MPa | Possible closure of the dentinal tubule orifices and fusion of collagen fibers |
| Malekafzali [64] | Er,Cr:YSGG Wavelength: 2780 nm Tip diameter: 600 μm Power: 1.5 W Energy: 75 mJ/pulse Pulse duration: 140 μs Frequency: 20 Hz Total energy delivered: 15 J Scanning speed: 1 mm/s Distance from surface: 1–2 mm Irradiation pattern: vertical and horizontal Cooling: 55% water, 65% air, water flow 12 mL/min | enamel | adhesive: Single Bond (3M ESPE, St. Paul, MN, USA) Composite: Z100 (3M ESPE, St. Paul, MN, USA) | Control = 24.62 ± 5.56 MPalaser = 18.55 ± 6.41 MPa | No data |
| Sungurtekin-Ekci and Oztas [65] | Er,Cr:YSGG Wavelength: 2780 nm Pulse duration: 140–200 μs Frequency: 20 Hz Power: 2.5 W or 3.5 W Operating mode: non-contact, distance 1 mm from the surface Tip: sapphire, diameter 600 μm, length 6 mm Spot area: 0.442 mm2 Cooling: 90% air, 80% water Exposure time: 10–15 s | Enamel | ClinPro™ (3M ESPE, St. Paul, MN, USA)—resin fissure sealant Composite Filtek™ Z250 (Shade A3.5, 3M ESPE, St. Paul, MN, USA) | Control = 12.18 ± 3.95 MPa Laser 2.5 W = 8.30 ± 1.84 MPa Laser 3.5 W = 11.57 ± 3.27 MPa Laser 2.5 W + acid = 12.67 ± 4.51 MPa Laser 3.5 W + acid = 13.04 ± 3.62 MPa | SEM revealed that in the laser groups the surface was irregular, with microcracks and depressions. |
| Koyuturk [50] | Er:YAG Wavelength: 2940 nm Pulse energy: 200 mJ Frequency: 20 Hz Power: 4.0 W Pulse duration: 100 μs Tip: sapphire, diameter 1.3 mm, length 8 mm | dentine | Adhesives: Clearfil S3 Bond (Kuraray, Osaka, Japan), Xeno V Bond (Dentsply Detrey, Konstanz, Germany) Dyract Extra (Dentsply Detrey, Konstanz, Germany)—compomer | Sound enamel: Bur + Clearfil S3 = 20.32 ± 7.54 MPa Laser + Clearfil S3 = 24.57 ± 7.27 MPa Bur + Xeno V = 12.10 ± 4.80 MPa Laser + Xeno V = 11.02 ± 3.90 MPa Caries: Bur + Clearfil S3 = 15.51 ± 5.49 MPa Laser + Clearfil S3 = 19.30 ± 6.12 MPa Bur + Xeno V = 12.40 ± 5.04 MPa Laser + Xeno V = 13.12 ± 4.33 MPa | For Clearfil S3—in sound and caries-affected dentine after laser and drilling, the hybrid layer was continuous, well-adhered and had numerous, long tags. For Xeno V—also a continuous layer, but shorter and less distinct tags compared to Clearfil S3. |
| Paryab [51] | Er:YAG Wavelength: 2940 nm Pulse duration: 450 μs Focal spot size: 0.785 mm2 Cooling: air/water Distance: 0.8–1.2 cm, non-contact, defocused mode Power: 3 or 4 W Energy: 300 or 400 mJ Frequency: 10 Hz | Dentine | Adhesives: Single Bond (3M ESPE, St. Paul, MN, USA) or Clearfil SE Bond (Kuraray, Osaka, Japan) Composite Filtek Z250 (3M ESPE, St. Paul, MN, USA) | Drill + Single Bond = 6.94 ± 2.12 MPa Drill + Clearfil SE = 9.00 ± 2.47 MPa Laser 300 mJ + Single Bond = 6.28 ± 2.58 MPa Laser 300 mJ + Clearfil SE = 6.42 ± 3.90 MPa Laser 400 mJ + Single Bond = 6.00 ± 3.16 MPa Laser 400 mJ + Clearfil SE = 5.62 ± 2.99 | The laser prepares a dentin surface with open tubules, no smear layer and no signs of charring within the parameters used. |
| Memarpour [52] | Laser type: Er:YAG Wavelength: not reported Time: not reported Distance: 1 mm Power: 1.20 W Power density: not reported Beam diameter: 0.8 mm Surface area: not reported Fluence: not reported Frequency: 10 Hz Pulse duration: 100 μs Cooling: Water 8, Air 4 Energy: 120 mJ | Enamel and dentin | One-step self-etch adhesive: OptiBond All-In-One (Kerr, Orange, CA, USA) Conventional flowable composite: Premise Flowable (Kerr, Orange, CA, USA) Self-adhering flowable composite: Vertise Flow (Kerr, Orange, CA, USA) Group combinations:
| Enamel—SiC pretreatment
| Enamel:
on SiC: partial smear layer removal incomplete, partial exposure of the tubules.
|
| Flury [53] | Laser type: Er:YAG Wavelength: 2.94 μm Group 2a Energy: Preparation: 200 mJ Finishing: 100 mJ Frequency: Preparation: 25 Hz Finishing: 35 Hz Power: Preparation: 5.0 W Finishing: 3.5 W Pulse duration: ~450 μs Distance: 2 mm Beam diameter: 1 mm Cooling: water-cooled Time: >300 s (preparation + finishing) Power density: no data Fluence: no data Surface area: no data Group 2b: Energy: 400 mJ Frequency: 20 Hz Power: 8.0 W Pulse duration: ~450 μs Distance: 2 mm Beam diameter: 1 mm Cooling: water-cooled Time: 30–40 s Power density: no data Fluence: no data Surface area: no data Group 2c: Energy: 100 mJ Frequency: 35 Hz Power: 3.5 W Pulse duration: ~450 μs Distance: 2 mm Beam diameter: 1 mm Cooling: water-cooled Time: 60–90 s Power density: no data Fluence: no data Surface area: no data Group 2d: Energy: 50 mJ Frequency: 35 Hz Power: 1.75 W Pulse duration: ~450 μs Distance: 2 mm Beam diameter: 1 mm Cooling: water-cooled Time: 100–130 s Power density: no data Fluence: no data Surface area: no data | Dentin | Adhesive: Clearfil SE (self-etch system)—Kuraray, Osaka, Japan Restorative composite: Clearfil Majesty Esthetic—(Kuraray, Osaka, Japan) | μTBS: Group 1a: not measured Group 1b (Grinding + 40 μm diamond bur): 24.8 ± 6.6 MPa Group 2a: not measured Group 2b (Grinding + Er:YAG laser): 26.1 ± 4.3 MPa Group 2c (Grinding + Er:YAG laser): 22.2 ± 8.6 MPa Group 2d (Grinding + Er:YAG laser): 23.9 ± 6.1 MPa No significant differences between groups (p = 0.394) | SEM observations: Bur-treated dentin: smooth surface with a uniform smear layer, no open tubules. Laser-treated dentin: no smear layer, open dentinal tubules and scaly, irregular surface. Group 2d: Incomplete ablation; parts of the SiC-ground surface remained visible. No signs of thermal damage. |
| Yildiz [54] | Laser type: Er:YAG Wavelength: 2.94 μm Time: no data Distance: 1 mm Power: 3.5 W Power density: no data Beam diameter: 1 mm Surface area: no data Fluence: 44 J/cm2 Frequency: 10 Hz Pulse duration: 300 μs Cooling: continuous air and water spray Energy: no data | Dentin | Adhesive systems:
| Microtensile bond strength (μTBS): Rotary bur + Etch-and-rinse: 20.77 ± 5.64 MPa Rotary bur + Self-etch: 21.05 ± 5.19 MPa Er:YAG laser + Etch-and-rinse: 15.70 ± 5.87 MPa Er:YAG laser + Self-etch: 16.51 ± 4.91 MPa Carisolv + Etch-and-rinse: 19.29 ± 4.30 MPa Carisolv + Self-etch: 19.37 ± 3.56 MPa | SEM observations: Bur-treated dentin:
|
| Bolukbasi [70] | Laser type: Nd:YAG Wavelength: 1064 nm Time: 10 s Distance: no data Power: 2 W Power density: no data Beam diameter: 300 μm Surface area: no data Fluence: no data Frequency: 20 Hz Pulse duration: no data Cooling: no data Energy: 100 mJ | Dentin | Composite resin: Filtek Z550 Posterior Restorative (3M ESPE, St. Paul, MN, USA) Glass hybrid cement: GC Equia Forte (GC Corporation, Tokyo, Japan) Adhesive system for composite: Single Bond Universal (3M ESPE, St. Paul, MN, USA) | SBS results Nd:YAG laser—Composite resin: 13.79 ± 1.24 MPa Nd:YAG laser—Glass hybrid: 7.58 ± 0.60 MPa APCP—Composite resin: 13.35 ± 0.65 MPa APCP—Glass hybrid: 7.54 ± 0.99 MPa Control—Composite resin: 12.85 ± 1.34 MPa Control—Glass hybrid: 7.41 ± 0.81 MPa Biodentine—Composite resin: 12.60 ± 1.17 MPa Biodentine®—Glass hybrid: 6.70 ± 0.91 MPa Photobiomodulation—Composite resin: 12.14 ± 1.47 MPa Photobiomodulation—Glass hybrid: 6.92 ± 0.77 MPa Ferric sulfate—Composite resin: 10.17 ± 1.45 MPa Ferric sulfate—Glass hybrid: 7.21 ± 0.76 MPa | SEM observations: Nd:YAG laser: Smear layer removed, partially opened dentinal tubules Biodentine: Tubules occluded by material Ferric sulfate: Some tubules occluded and some open Photobiomodulation: similar to control group, smear layer remained, tubules not clearly opened APCP: Similarly to control group |
| Borsatto [55] | Laser type: Er:YAG Wavelength: 2.94 μm Time: 20 s Distance: 12 mm Power: no data Power density: Beam diameter: 0.63 mm Surface area: no data Fluence: no data Frequency: 2 Hz Pulse duration: no data Cooling: fine water mist during irradiation Energy: 250 mJ | Enamel | Adhesive system: Single Bond 2 Adper (3M ESPE, St. Paul, MN, USA)—etch-and-rinse type adhesive Restorative material: Filtek Z250 light-cured composite resin (3M ESPE, St. Paul, MN, USA) | SBS: AI (Bur, 24 h Water Storage/0 Thermal Cycles): 17.45 ± 2.03 MPa AII (Bur, 7 d WS/500 TCs): 16.38 ± 1.49 MPa AIII (Bur, 1 month WS/2000 TCs): 6.88 ± 0.66 MPa AIV (Bur, 6 months WS/12,000 TCs): 7.77 ± 1.53 MPa BI (Laser, 24 hWS/0 TCs): 12.32 ± 0.99 MPa BII (Laser, 7 d WS/500 TCs): 15.37 ± 2.24 MPa BIII (Laser, 1 month WS/2000 TCs): 15.05 ± 2.01 MPa BIV (Laser, 6 months WS/12,000 TCs): 5.51 ± 1.01 MPa | The fractured specimens were examined under a stereomicroscope at 40× magnification to classify the type of failure. SEM analysis was not used in this study. In the groups where enamel was prepared with the Er:YAG laser, failures were predominantly adhesive failures. As the duration of water storage and the number of thermal cycles increased, the proportion of mixed failures became higher. Importantly, no cohesive fractures were detected in any of the groups. |
| Wanderley [56] | Laser type: Er:YAG Wave length: 2.94 μm Energy: 60, 80, 100 mJ Distance: 17 mm Time: 1 min and 30 sec Frequency: 2 Hz | Enamel | Hybrid light-curing composite resin (Filtek Z250, 3M ESPE, St. Paul, MN, USA) | Control—35% phosphoric acid= 14.28 (±3.24) MPa Er:YAG laser 60 mJ/2 Hz + acid= 18.48 (±4.58) MPa Er:YAG laser 80 mJ/2 Hz + acid= 17.82 (±4.38) MPa Er:YAG laser 100 mJ/2 Hz + acid= 16.59 (±5.40) MPa | Control- even surface, type II etching pattern, demineralized interprismatic part, surface microporosity Er:YAG 60 mJ/2 HZ- irradiation: uneven surface, no etching pattern, after acid etching: slightly micro-rough surface + few crevices and melting points, type I etching pattern + demineralization of the prism centers Er:YAG 80 mJ/2 HZ- Laser irradiation: irregular surface, gaps, small areas of re-melting, unclear etching pattern, acid etching: flaking surface, gaps, small areas of re-melting Er:YAG 100 mJ/2 HZ- Laser irradiation: fractal image, multiple fissures, no etching pattern. Acid etching: microrough surface, uneven topography, multiple adhesions. |
| Moghini [57] | Laser type: Er:YAG Wave length: 2.94 μm Energy: 60, 80, 100 mJ Distance: 17 mm Time: 1 min and 30 sec Frequency: 2 Hz | Dentin | Hybrid light-curing composite resin (Filtek Z250, 3M ESPE, St. Paul, MN, USA) | Control—35% phosphoric acid= 17.89 (4.75) MPa Er:YAG laser 60 mJ/2 Hz + acid= 12.34 (4.85) MPa Er:YAG laser 80 mJ/2 Hz + acid= 10.30 (3.67) MPa Er:YAG laser 100 mJ/2 Hz + acid= 10.41 (4.20) MPa | Control- regular topography, demineralized peritubular dentin, open and widened tubule entrances Er:YAG 60 mJ/2 HZ- Laser irradiation: uneven tissue surfaces, few open tubules, after acid etching, uneven tissue, more open tubules, absent smear layer, cratered intertubular dentin Er:YAG 80 mJ/2 HZ- Irradiation: irregular surface—craters, fissures, cracks, few open channels, some with a smear layer, after etching: more regular, flaky surface, wider channel openings Er:YAG 100 mJ/2 HZ- irradiation: uneven and flaking surface, noticeable fissures, cracks, open tubules. After etching: irregular, flaking surface, many cracks over the entire surface, numerous open tubules, noticeable peritubular dentin, irregular intertubular dentin. |
| Kattan [66] | Laser type: Er,Cr:YSGG Wave length: 2780 nm Pulse duration: 140μs Frequency: 20 Hz | Dentin | -Rely X ARC (3M ESPE, St. Paul, MN, USA) -Rely X Unicem (AplicapTM/MaxicapTM,3M ESPE, St. Paul, MN, USA) -GIC: Ketac Cem Aplicap (3M, Seefeld, Germany) ESPE, St. Paul, MN, USA) | Group (Rely-X ARC) A - 25.38 Mpa Group B (ECL-RelyX-Uni) - 20.47 Mpa Group C (ECL-RelyX-ARC) - 21.65 Mpa Group D (ECL-GIC) - 15.91 Mpa | SEM was not performed. |
| Felemban [58] | Er:YAG laser Wavelength: 2940 nm, used by the instructions of manufacturer (not included in the paper) | Enamel and dentin | Clearfil Universal Bond Quick system (Kuraray Noritake Dental, Tokyo, Japan) 3MTM FiltekTM Z350 XT Universal Restorative, 3M ESPE, St. Paul, MN, USA | Data not included in the study | Final SEM analysis on 20 teeth-one tooth prepared by bur, other by laser (10 subjects: one tooth prepared by bur and one by laser at each subject) -all 20 restorations were integrated with enamel and dentin walls (prepared conventionally or with laser) -adhesive integration with the dentin floor: 6 subjects had both their restorations fully integrated, 4 restorations were not integrated (2 subjects had restoration prepared conventionally not integrated, while prepared by laser integrated; other 2 subjects had restoration prepared by bur integrated to dentin floor, while prepared by laser were not) |
| Oznurhan [67] | Erbium, chromium:yttrium–scandium–gallium–garnet (Er,Cr:YSGG) laser Power output: 6.0 W (85–90% air and 80–85% water) for Enamel; 3.5 W (70% air and 65% water) for dentine working distance of 1.5–2 mm. (defocused mode) | Dentin | Single Bond AdperTM 3M ESPE FiltekTM Z250, 3M ESPE, St. Paul, MN, USA | Data not included in the study | SEM analysis: -G1 and G2, the surfaces were wavy -G3 and G4, the surfaces were smooth -Microcracks were observed in some of the lased cavities -G1 and G2 the dentin tubules were exposed, and there were the lack of smear layer and gaps -G3 the smear layer and gaps were observed -G4 no gaps and smear layer -G2 increased resin tags were observed (-G1, a thinner hybrid layer or in some parts, the absence of the hybrid layer was observed). -the hybrid layer was thicker in G2 compared to G4 -some resin tags were broken in cavities that were prepared with laser. SEM-energy-dispersive X-ray spectroscopy (EDX) analysis: in the acid-etched groups (G2, G4) the silver ions were observed in hybrid layer and dentin tubules |
| Scatena [59] | Er:YAG laser -wavelength 2.94 μm at pulse energy 80 mJ -repetition rate: 2 Hz -laser beam: 0.63 mm -varying irradiation Distance: 11, 12, 16, 17, 20 [mm] | Dentin | Single Bond (3M ESPE, St. Paul, MN, USA) Filtek Z250 (3M ESPE, St. Paul, MN, USA) | SBS MPa: G1 7.32 ± 3.83 (control) G2 5.07 ± 2.62 (11 mm) G3 6.49 ± 1.64 (12 mm) G4 7.71 ± 0.66 (16 mm) G5 7.33 ± 0.02 (17 mm) G6 9.65 ± 2.41 (20 mm) | Data not included in the study |
| Nisar [71] | KTP Laser: Device: (Smartlite D, Deka, Calenzano Firenze, Italy) Wavelength: 532 nm Energy output: 1 W 10 t pulsed mode Focal distance 1 mm Er:YAG Laser: Device (Nubway, Beijing, China) Wavelength: 2940 nm Energy density: 18.9 J/cm2 Pulse duration 150 s Repetition rate: 10 Hz Irrigation 5 mL/min | Caries affected dentin | Adper prime NT Bond (Dentsply Detrey, Konstanz, Germany) Resin composite: Tetric N-Ceram (Ivoclar Vivadent, Schaan, Liechtenstein) | SBS MPa KTP Laser group 8.25 ± 0.41 Er:YAG laser group: 10.23 ± 0.33 Control group (Dentin disinfected with CHX) 8.19 ± 0.73 | Samples were characterized under 40× magnification with stereomicroscope to assess fracture mode. |
| Kiomarsi [68] | Er,Cr:YSGG Device: iPlus Waterlase; Biolase, USA Gold MZ6 tip Frequency: 20 Hz Output power 1.5 W Coolant: 60% water, 60% air Distance: 1 mm to the enamel surface Sweeping motion 60 μs pulse duration (10 s for each surface) | Enamel | Vertise Flow (Kerr, Orange, CA, USA) Premise Flow (Kerr Italia S.r.l., Scafati, Italy) Adper Single Bond 2 (3M ESPE St Paul, MN, USA) | µSBS MPa For Vertise Flow Laser treated samples (13.60 ± 5.47) Non treated samples (5.89 ± 2.42) For Premise Flow (13.18 ± 3.45) Non treated samples (16.06 ± 3.52) | Data not included with the study |
| Bahrololoomi [60] | Er:Yag laser Device: Fotona, Fidelis Plus III, Slovenia Handpiece: RO2-C-919 Wavelength: 2940 nm Energy: 200 mJ Pulse repetition 10 Hz Water cooling 7 mL/min Non-contact mode within 17 mm distance | Enamel (removed with bur or laser, respectively), dentin | One-Step Plus (Bisco, Schamburg, IL, USA) Bonding Agent AELITE Composite (Bisco, Schamburg, IL, USA) | SBS MPa Bur treated samples 13.56 ± 3.36 (No hypochlorite used) 13.53 ± 3.64 (2,5% hypochlorite used) 14.36 ± 3.64 (5,25% hypochlorite used) Laser treated samples: 13.39 ± 3.1 (No hypochlorite used) 14.63 ± 3.93 (No hypochlorite used) 14.51 ± 4 (No hypochlorite used) | SEM Microscopy with 1000× and 5000× magnification. Surface roughness, presence of smear layer, appearance of dentinal tubules and presence of melting or crack were analyzed. |
| Tooth Tissue | Laser Type | Energy (mJ) | Power (W) | Frequency (Hz) | Cooling | Observed Effect on Bond Strength | References |
|---|---|---|---|---|---|---|---|
| Enamel | Er:YAG | 120–200 | 1.2–2.0 | 10–20 | Air–water | Optimal micro-retention; with acid etch → SBS comparable/superior to bur; ≥250 mJ ↓ adhesion | [51,53,60,61,68,70] |
| Enamel | Er,Cr:YSGG | 150–175 | 3.0–3.5 | 20 | Air–water | Clean, micro-rough surfaces; SBS ≈ acid etch; ~3.5 W best; >4 W ↓ adhesion | [68,70] |
| Dentin | Er:YAG | 80–150 | 0.8–1.5 | 5–15 | Air–water | Highest µTBS/SBS; smear removal + open tubules; ≥250–300 mJ → microcracks, thermal change ↓ bonding | [53,55,58,62,65] |
| Dentin | Er,Cr:YSGG | 125–175 | 2.5–3.5 | 20 | Air–water | µTBS ↑ esp. with phosphoric acid; ~3.5 W optimal; >4 W → morphological deterioration | [68,70] |
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 |
|---|---|---|---|---|---|---|---|---|---|
| Ersan [62] | yes | Yes | Yes | no | yes | yes | yes | yes | yes |
| Chikkanarasaiah [62] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Borsatto [46] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Kotb [69] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Babu [47] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Alhumaid [63] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Wang [48] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Bahrololoomi [49] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Malekafzali [64] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Sungurtekin-Ekci [65] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Koyuturk [50] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Paryab [51] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Memarpour [52] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Flury [53] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Yildiz [54] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Bolukbasi [70] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Borsatto [55] | yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Wanderley [56] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Monghini [57] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Kattan [66] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Felemban [58] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | no |
| Oznurhan [67] | Yes | Yes | yes | No | Yes | yes | Yes | Yes | no |
| Scatena [59] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Nisar [68] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Kiomarsi [68] | Yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
| Bahroloomi [60] | yes | Yes | Yes | No | Yes | yes | Yes | Yes | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świenc, W.; Kiryk, J.; Michalak, M.; Majchrzak, Z.; Laszczyńska, M.; Kiryk, S.; Grychowska-Gąsior, N.; Nawrot-Hadzik, I.; Matys, J.; Dobrzyński, M. The Effect of Laser Surface Treatment on the Bond Strength of Adhesive Materials to Primary Teeth: A Systematic Review. Materials 2025, 18, 5212. https://doi.org/10.3390/ma18225212
Świenc W, Kiryk J, Michalak M, Majchrzak Z, Laszczyńska M, Kiryk S, Grychowska-Gąsior N, Nawrot-Hadzik I, Matys J, Dobrzyński M. The Effect of Laser Surface Treatment on the Bond Strength of Adhesive Materials to Primary Teeth: A Systematic Review. Materials. 2025; 18(22):5212. https://doi.org/10.3390/ma18225212
Chicago/Turabian StyleŚwienc, Witold, Jan Kiryk, Mateusz Michalak, Zuzanna Majchrzak, Marzena Laszczyńska, Sylwia Kiryk, Natalia Grychowska-Gąsior, Izabela Nawrot-Hadzik, Jacek Matys, and Maciej Dobrzyński. 2025. "The Effect of Laser Surface Treatment on the Bond Strength of Adhesive Materials to Primary Teeth: A Systematic Review" Materials 18, no. 22: 5212. https://doi.org/10.3390/ma18225212
APA StyleŚwienc, W., Kiryk, J., Michalak, M., Majchrzak, Z., Laszczyńska, M., Kiryk, S., Grychowska-Gąsior, N., Nawrot-Hadzik, I., Matys, J., & Dobrzyński, M. (2025). The Effect of Laser Surface Treatment on the Bond Strength of Adhesive Materials to Primary Teeth: A Systematic Review. Materials, 18(22), 5212. https://doi.org/10.3390/ma18225212










