The Effect of Various Types of Polymeric Packaging Materials on the Quality of Copioba Cassava Flour
Abstract
1. Introduction
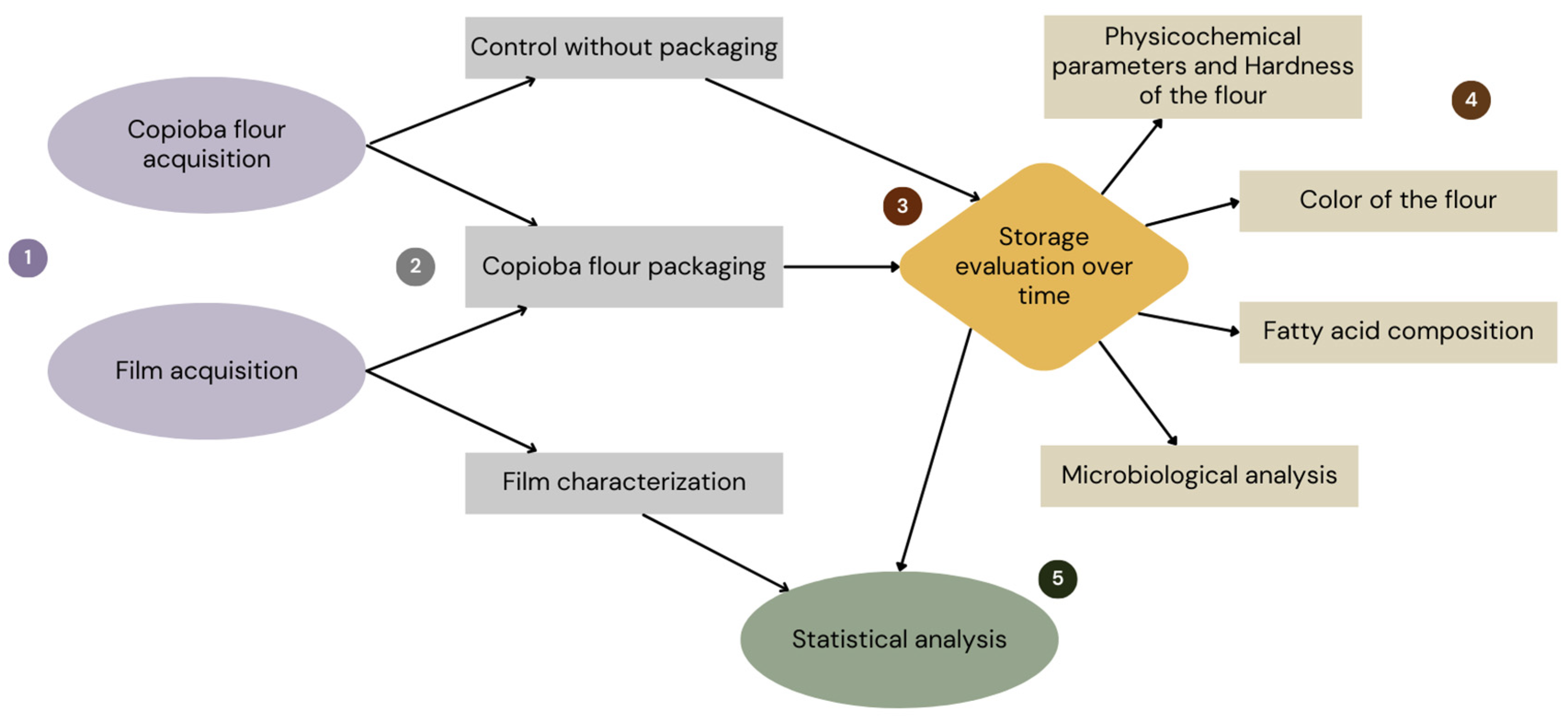
2. Materials and Methods
2.1. Characterization of Packaging Material
2.1.1. Thickness
2.1.2. Mechanical Properties
2.1.3. Water Vapor Transmission Rate (WVTR)
2.2. Assessment of Storage of Copioba Cassava Flour in Various Packaging Materials
2.3. Measurement of Physicochemical Parameters and Hardness of Copioba Cassava Flour
2.4. Color of Copioba Cassava Flour
2.5. Determination of Fatty Acid Composition and Content
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results
3.1. Packaging Characterization
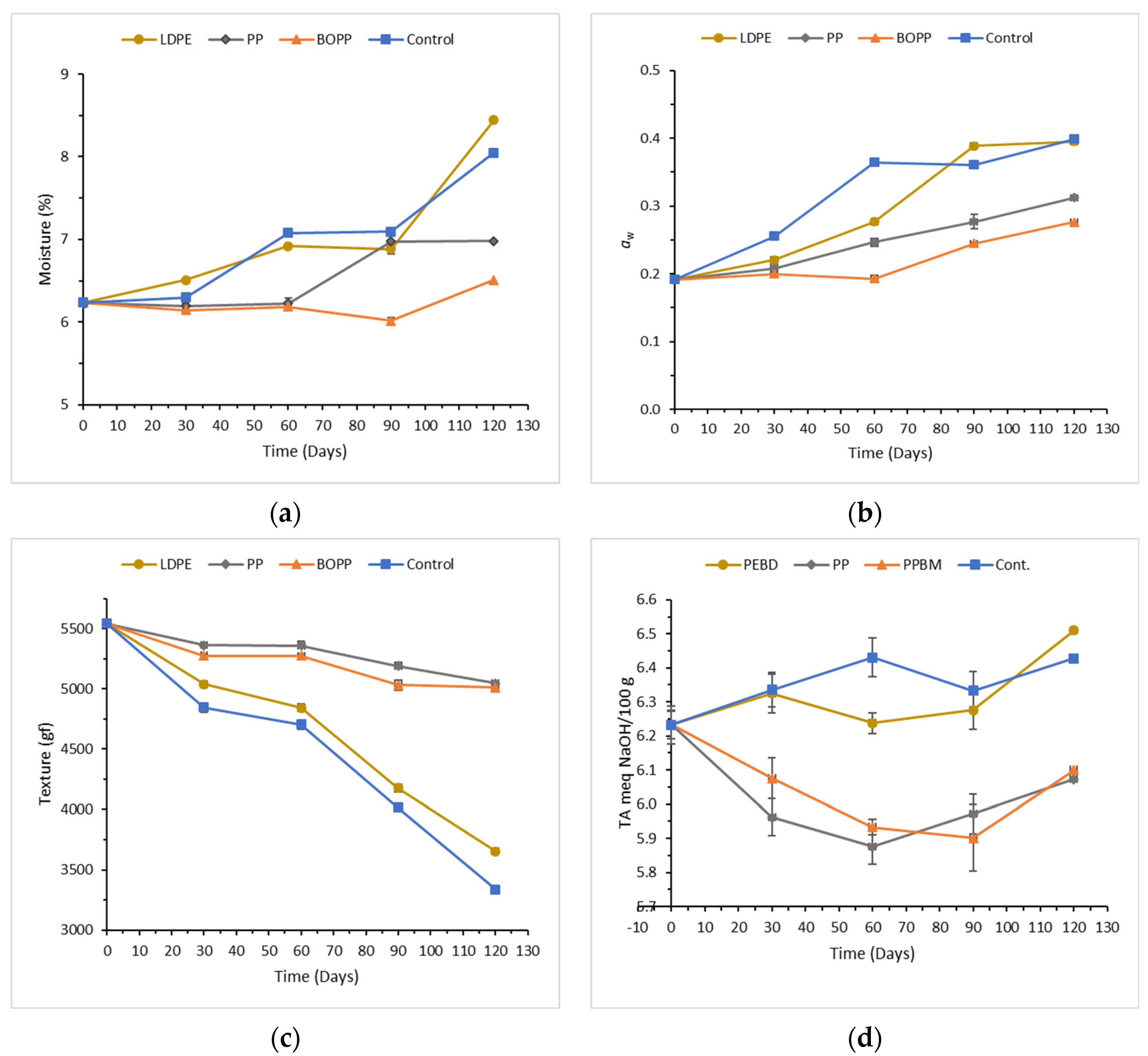
3.2. Effect of Type of Packaging Material on the Physicochemical Parameters and Texture of Copioba Cassava Flour
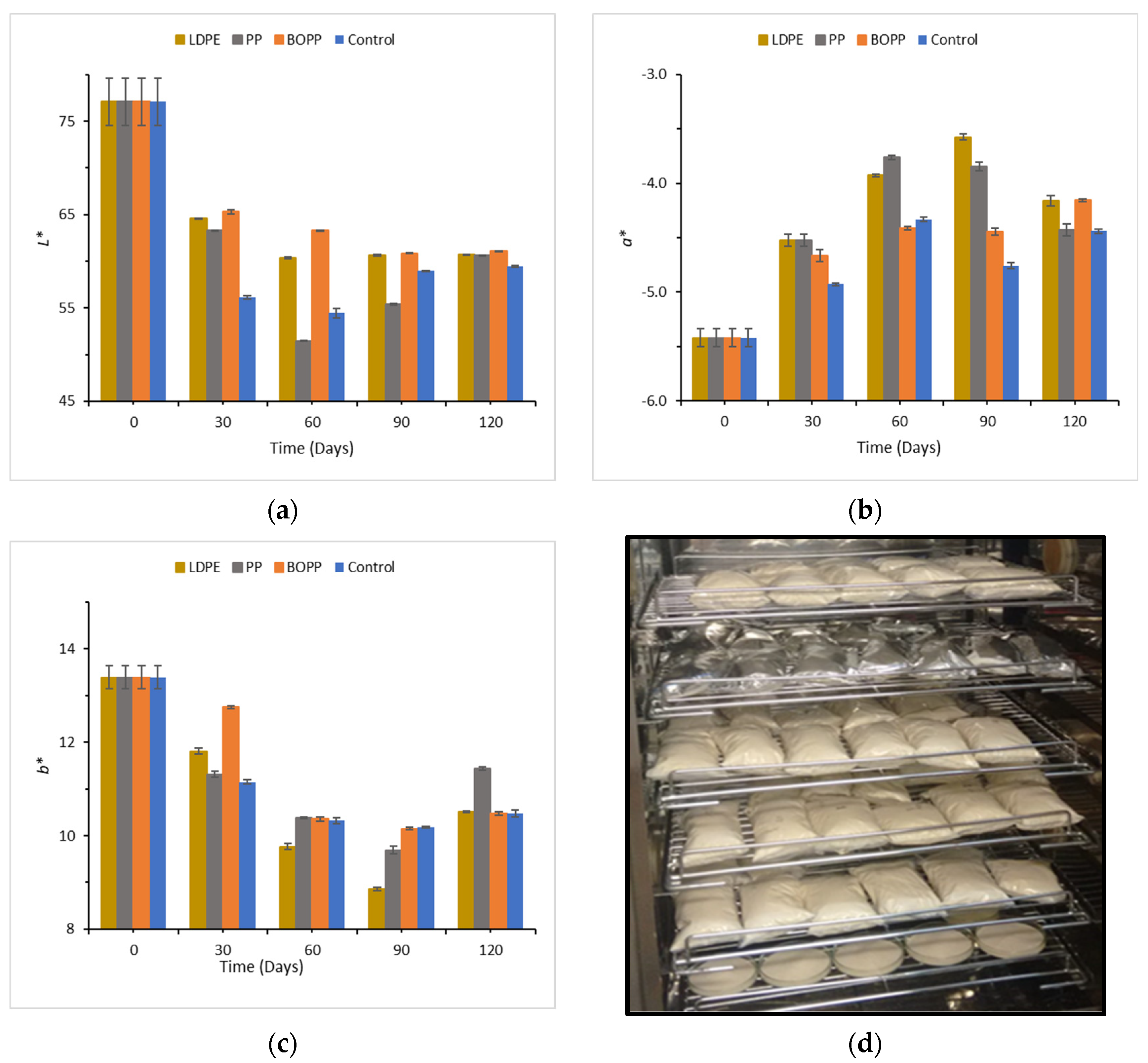
3.3. Effect of Storage Time on the Color of Copioba Cassava Flour
3.4. Effect on Fatty Acid Composition
3.5. Microbiological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDPE | Low-density polyethylene |
| PP | Polypropylene |
| BOPP | Metallized biaxially oriented polypropylene |
| aw | Water activity |
| TA | Titratable acidity |
| CFU | Colony forming unit |
References
- FAOSTAT. Food and Agriculture Organization Corporate Statistical Database; FAO: Rome, Italy, 2023. [Google Scholar]
- IBGE. Instituto Brasileiro de Geografia e Estatística—Produção Agropecuária; IBGE: Rio de Janeiro, Brazil, 2023.
- Silva, S.B.; Pena, L.C.C.; Cardoso, R.C.V. Copioba ad common cassava flour know-how: Establishing similarities and distinctions in São Felipe, Brazil. Int. J. Gastron. Food Sci. 2023, 32, 100713. [Google Scholar] [CrossRef]
- Silva, I.R.C.; Cardoso, R.C.V.; Góes, J.A.W.; Druzian, J.I.; Vidal Júnior, P.O.; Andrade, A.C.B. Food safety in cassava “flour houses” of Copioba valley, Bahia, Brazil: Diagnosis and contribution to geographical indication. Food Control 2017, 72, 97–104. [Google Scholar] [CrossRef]
- de Oliveira, O.S.; Brito, V.H.S.; Cereda, M.P. Establishing a standard for handmade Brazilian cassava flour from Baixada Cuiabana (Mato Grosso, Brazil) to support its processing and sale. Food Sci. Technol. 2018, 39, 559–566. [Google Scholar] [CrossRef]
- Freitas-Sá, D.G.C.; Teixeira, K.T.R.; Mattos, C.T.G.B.; Monteiro, R.P. Atributos de aparência da farinha de copioba da Bahia como contribuição à indicação geográfica. In Proceedings of the XXV Congresso Brasileiro de Ciência e Tecnologia de Alimentos, Gramado, Brazil, 24–27 October 2016. [Google Scholar]
- Pascoal, D.R.C.; Moura, L.E.; da Silva, J.R.; Assis, D.J.; Costa, S.S.; Druzian, J.I. Characteristics volatiles of cassava flours and their relationship to parameters other, process and geographical origin: A preliminary study. Food Sci. Technol. 2022, 42, E80221. [Google Scholar] [CrossRef]
- Su, S.; Wang, L.; Feng, C.; Liu, Y.; Li, C.; Du, H.; Tang, Z.; Xu, Y.; Wang, L. Fingerprints of anthocyanins and flavonols of Vaccinium uliginosum berries from different geographical origins in the Greater Khingan Mountains and their antioxidant capacities. Food Control 2016, 64, 218–225. [Google Scholar] [CrossRef]
- Crescenzi, R.; Filippis, F.; Giua, M.; Vaquero-Piñero, C. Geographical indications and local development: The strength of territorial embeddedness. Reg. Stud. 2022, 56, 381–393. [Google Scholar] [CrossRef]
- INPI. Instituto Nacional de Propriedade Industrial. In Indicações Geográficas: Indicações de Procedências Reconhecidas; INPI: Rio de Janeiro, Brazil, 2025. [Google Scholar]
- Chea, V.; Angellier-Coussy, H.; Peyron, S.; Kemmer, D.; Gontard, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging: Physical–chemical and structural stability under food contact conditions. J. Appl. Polym. Sci. 2016, 133, 41850. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Yang, X.; Deshmukh, R.K.; Gaikwad, K.K.; Bahmid, N.A.; Castro-Muñoz, R. Recent advances in reinforced bioplastics for food packaging—A critical review. Int. J. Biol. Macromol. 2024, 263, 130399. [Google Scholar] [CrossRef]
- Dey, A.; Dhumal, C.V.; Sengupta, P.; Kumar, A.; Pramanik, N.K.; Amal, T. Challenges and possible solutions to mitigate the problems of single-use plastics used for packaging food items: A review. J. Food Sci. Technol. 2021, 58, 3251–3269. [Google Scholar] [CrossRef]
- Awol, S.M.; Kuyu, C.G.; Bereka, T.Y. Physicochemical stability, microbial growth, and sensory quality of teff flour as affected by packaging materials during storage. LWT 2023, 189, 115488. [Google Scholar] [CrossRef]
- Macedo, I.S.M.; Sousa-Gallagher, M.J.; Oliveira, J.C.; Byrne, E.P. Quality by design for packaging of granola breakfast product. Food Control 2013, 29, 438–443. [Google Scholar] [CrossRef]
- Opara, U.L.; Mditshwa, A. A review on the role of packaging in securing food system: Adding value to food products and reducing losses and waste. Afr. J. Agric. Res. 2013, 8, 2621–2630. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Jha, K. Influence of storage and packaging conditions on the quality of soy flour from sprouted soybean. J. Food Sci. Technol. 2011, 48, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Golestan, M.N.; Ghosta, Y.; Pourmirza, A.A.; Valizadegan, O. Study on laser perforated films as gas permeable packaging for confused flour beetle control inside food packaging. J. Stored Prod. Res. 2015, 60, 54–59. [Google Scholar] [CrossRef]
- Opara, U.L.; Caleb, O.J.; Uchechukwu-Agua, A.D. Evaluating the Impacts of Selected Packaging Materials on the Quality Attributes of Cassava Flour (cvs. TME 419 and UMUCASS 36). J. Food Sci. 2016, 81, 324–331. [Google Scholar] [CrossRef]
- Adejumo, B.A.; Raji, A.O. Microbiological safety and sensory attributes of garri in selected packaging materials. Acad. Res. Int. 2012, 3, 2–8. [Google Scholar]
- Ogiehor, I.S.; Ikenebomeh, M.J. The effects of different packaging materials on the shelf stability of garri. Afr. J. Biotechnol. 2006, 5, 2412–2416. [Google Scholar]
- Uchechukwu-Agua, A.D.; Caleb, O.J.; Manley, M.; Opara, U.L. Effects of storage conditions and duration on physicochemical and microbial quality of the flour of two cassava cultivars (TME 419 and UMUCASS 36). CyTA J. Food 2015, 13, 635–645. [Google Scholar] [CrossRef]
- Ekeledo, E.; Abass, A.; Müller, J. Effect of packaging and storage conditions on the pasting and functional properties of pretreated yellow-fleshed cassava flour. Appl. Food Res. 2024, 4, 100467. [Google Scholar] [CrossRef]
- de Matos, M.F.R.; da Silva, I.R.C.; Mendonça, T.A.; Santos, L.F.P.; Nunes, I.L.; Druzian, J.I. Conformity of cassava flours type copioba commercialized in the fairs in salvador (BA) with the parameters of the legislation: A contribution to geographical indication (GI) of the product. Rev. Geintec 2012, 2, 307–326. [Google Scholar] [CrossRef]
- Silva, A.C.M.S.; Pinho, L.S.; Sousa, L.S.; Moura, L.E.; Souza, C.O.; Druzian, J.I. Classificação, identidade e matérias estranhas de farinha de mandioca copioba: Conformidade com a legislação brasileira e contribuição a indicação geográfica. Cad. Prospec. 2015, 8, 192–202. [Google Scholar] [CrossRef]
- IBGE. Instituto Brasileiro de Geografia e Estatística—Portal de Mapas; IBGE: Rio de Janeiro, Brazil, 2025.
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E96/E96M-24a; Standard Test Methods for Gravimetric Determination of Water Vapor Transmission Rate of Materials. ASTM International: West Conshohocken, PA, USA, 2014.
- AOAC. Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Li, Y.; Kloeppel, K.M.; Hsieh, F. Texture of glassy corn cakes as a function of moisture content. J. Food Sci. 1998, 63, 869–872. [Google Scholar] [CrossRef]
- Bible, B.B.; Singha, S. Canopy position influences CIELAB coordinates of peach color. HortScience 1993, 28, 992–993. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.D.; Ackman, R.G. Capillary column gas chromatography method for analysis of encapsulated fish oil and fish oil ethyl esters: Collaborative study. J. AOAC Int. 1992, 75, 488–506. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; Souza, C.O.; Vich, D.V.; Carvalho, G.C.; Nascimento, M.A. Screening microalgae strains for biodiesel production: Lipid productivity and estimation of fuel quality based on fatty acids profiles. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Downes, F.P.; Ito, K. Compendium of Methods for the Microbiological Examination of Foods; APHA: Washington, DC, USA, 2001. [Google Scholar]
- FDA. Bacteriological Analytical Manual, 8th ed.; Revision A; FDA: Washington, DC, USA, 1998; Chapter 14.
- Aryee, F.N.A.; Oduro, I.; Ellis, W.O.; Afuakwa, J.J. The physicochemical properties of flour samples from the roots of 31 varieties of cassava. Food Control 2006, 17, 916–922. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA). Resolução de Diretoria Colegiada Nº 12; Ministério da Saúde: Brasília, Brazil, 2001. (In Portugues)
- MAPA. Ministério da Agricultura, Pecuária e Abastecimento, Instrução Normativa 52/2011; MAPA: Brasília, Brazil, 2011. [Google Scholar]
- ANVISA. Agência Nacional de Vigilância Sanitária, Resolução da Diretoria Colegiada—RDC 711/2022; Ministério da Saúde: São Paulo, Brazil, 2022.
- Chukwu, O.; Abdullahi, H. Effects of moisture content and storage period on proximate composition, microbial counts and total carotenoids of cassava flour. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 753–763. [Google Scholar]
- Famurewa, J.A.V.; Oluwamukomi, M.O.; Alaba, J.O. Storage stability of pupuru flour (a cassava product) at room temperature. Br. J. Appl. Sci. Technol. 2012, 2, 138–145. [Google Scholar] [CrossRef]
- Massey, L.K. Permeability Properties of Plastics and Elastomers, 2nd ed.; Plastics Design Library/William Andrew Publishing: Norwich, NY, USA, 2003. [Google Scholar]
- Lazić, V.; Budinski-Simendić, J.; Gvozdenović, J.; Simendić, B. Barrier properties of coated and laminated polyolefin films for food packaging. Acta Phys. Pol. A 2010, 117, 855–858. [Google Scholar] [CrossRef]
- Kulchan, R.; Boonsupthip, W.; Suppakul, P. Shelf life prediction of packaged cassava-flour-based baked product using empirical models and activation energy for water vapor permeability of polyolefin films. J. Food Eng. 2010, 100, 461–467. [Google Scholar] [CrossRef]
- Chinnici, F.; Guerrero, E.D.; Sonni, F.; Natali, N.; Marín, R.N.; Riponi, C. GC-MS characterization of volatile compounds in quality vinegars with protected European geographical indication. J. Agric. Food Chem. 2009, 57, 4784–4792. [Google Scholar] [CrossRef]
- Nomi, Y.; Masuzaki, R.; Terasawa, N.; Takenaka, M.; Ono, H.; Otsuka, Y.; Murata, M. Formation mechanism and characterization of dilysyldipyrrolones, the Maillard-type yellow pigments. Food Funct. 2013, 4, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L. Food Packaging: Principles and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; 550p. [Google Scholar]
- Stewart-Jones, A.; Stirrup, T.J.; Hodges, R.J.; Farman, D.I.; Hall, D.R. Analysis of free fatty acids in food substrates and in the dust and frass of stored-product pests: Potential for species discrimination? J. Stored Prod. Res. 2009, 45, 119–124. [Google Scholar] [CrossRef]


| Packaging Material | Thickness (mm) | Maximum Tension (MPa) | Maximum Deformation (mm) | Specific Deformation (mm/mm) | WVTR (g/m2∙day) |
|---|---|---|---|---|---|
| LDPE | 0.099 ± 0.009 a | 28.00 ± 3.61 a | 426.33 ± 61.53 a | 8.33 ± 1.53 a | 12.10 ± 2.24 a |
| PP | 0.084 ± 0.009 a | 38.33 ± 1.15 b | 266.33 ± 3.79 b | 5.00 ± 0.00 b | 10.79 ± 2.57 a |
| BOPP | 0.050 ± 0.006 b | 35.00 ± 0.00 b | 33.67 ± 2.52 c | 1.00 ± 0.00 c | 2.93 ± 0.34 b |
| Microorganism | Packaging Material | T = 0 | T = 30 Days | T = 60 Days | T = 90 Days | T = 120 Days |
|---|---|---|---|---|---|---|
| Yeast and molds | LDPE | 2 | 2.30 | 3.47 | 4.69 | 4 |
| PP | 2 | 2.60 | 3 | 3 | ||
| BOPP | 2 | 2 | 3.30 | 3.30 | ||
| CONTROL | 2.90 | 3 | 4 | 4.77 | ||
| B. cereus | LDPE | <1 | 2.30 | 3.30 | 2.60 | 3.30 |
| PP | 2 | 2.30 | 2 | 2 | ||
| BOPP | < 1 | < 1 | 1.60 | 2 | ||
| CONTROL | 2.30 | 3 | 2.77 | 3.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.L.; de Almeida, F.C.; Cardoso, L.G.; Ferreira, E.d.S.; Camilloto, G.P.; Souza, C.O.d. The Effect of Various Types of Polymeric Packaging Materials on the Quality of Copioba Cassava Flour. Materials 2025, 18, 4768. https://doi.org/10.3390/ma18204768
Carvalho AL, de Almeida FC, Cardoso LG, Ferreira EdS, Camilloto GP, Souza COd. The Effect of Various Types of Polymeric Packaging Materials on the Quality of Copioba Cassava Flour. Materials. 2025; 18(20):4768. https://doi.org/10.3390/ma18204768
Chicago/Turabian StyleCarvalho, Andrea Limoeiro, Fabiane Cerqueira de Almeida, Lucas Guimarães Cardoso, Ederlan de Souza Ferreira, Geany Peruch Camilloto, and Carolina Oliveira de Souza. 2025. "The Effect of Various Types of Polymeric Packaging Materials on the Quality of Copioba Cassava Flour" Materials 18, no. 20: 4768. https://doi.org/10.3390/ma18204768
APA StyleCarvalho, A. L., de Almeida, F. C., Cardoso, L. G., Ferreira, E. d. S., Camilloto, G. P., & Souza, C. O. d. (2025). The Effect of Various Types of Polymeric Packaging Materials on the Quality of Copioba Cassava Flour. Materials, 18(20), 4768. https://doi.org/10.3390/ma18204768










