Research on Shrinkage in Lithium Slag Geopolymer Mortar: Effects of Mix Proportions and a Shrinkage Prediction Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Methods
2.2.1. Mix Proportion of Geopolymer
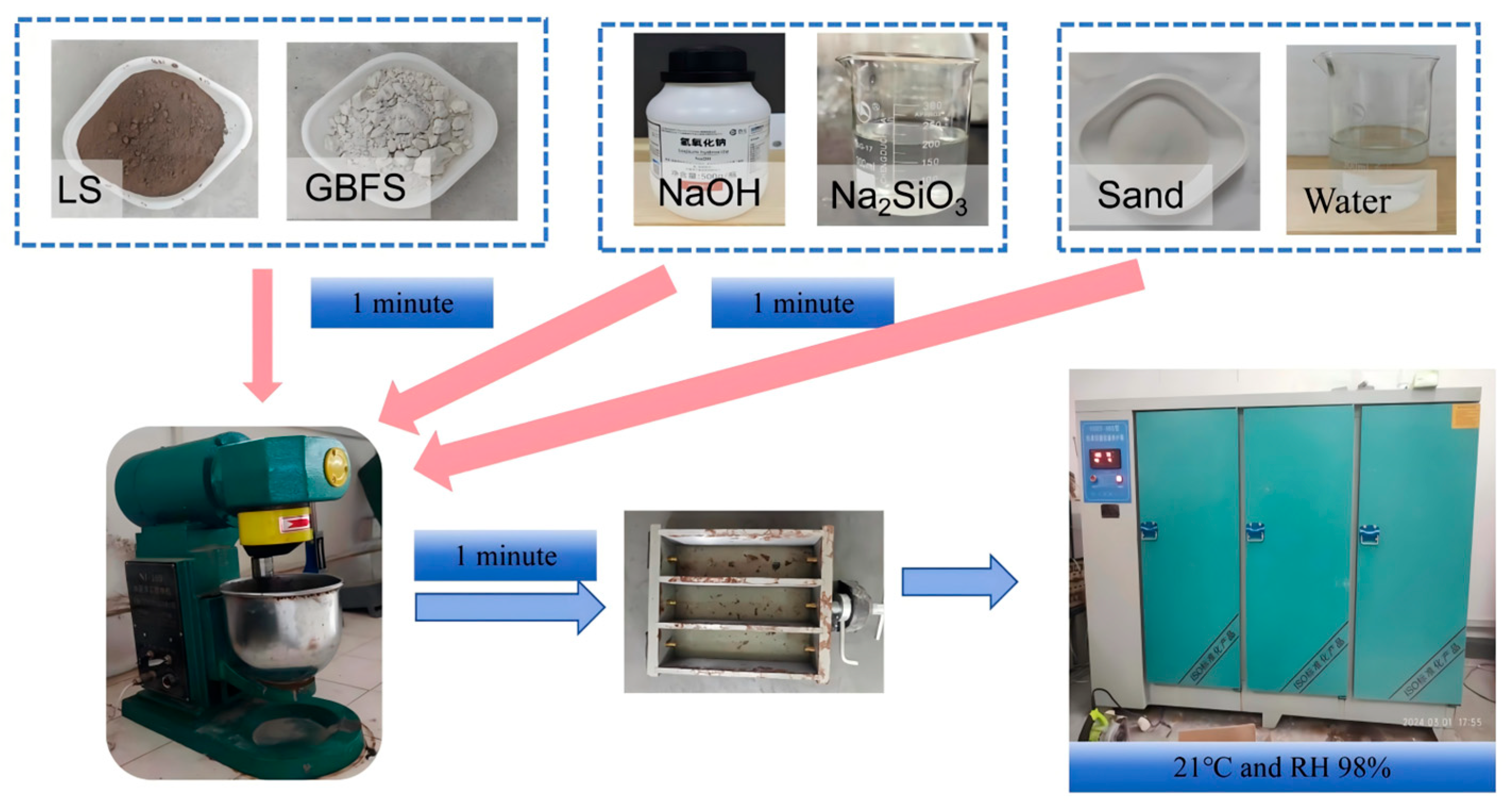
2.2.2. Preparation Sample
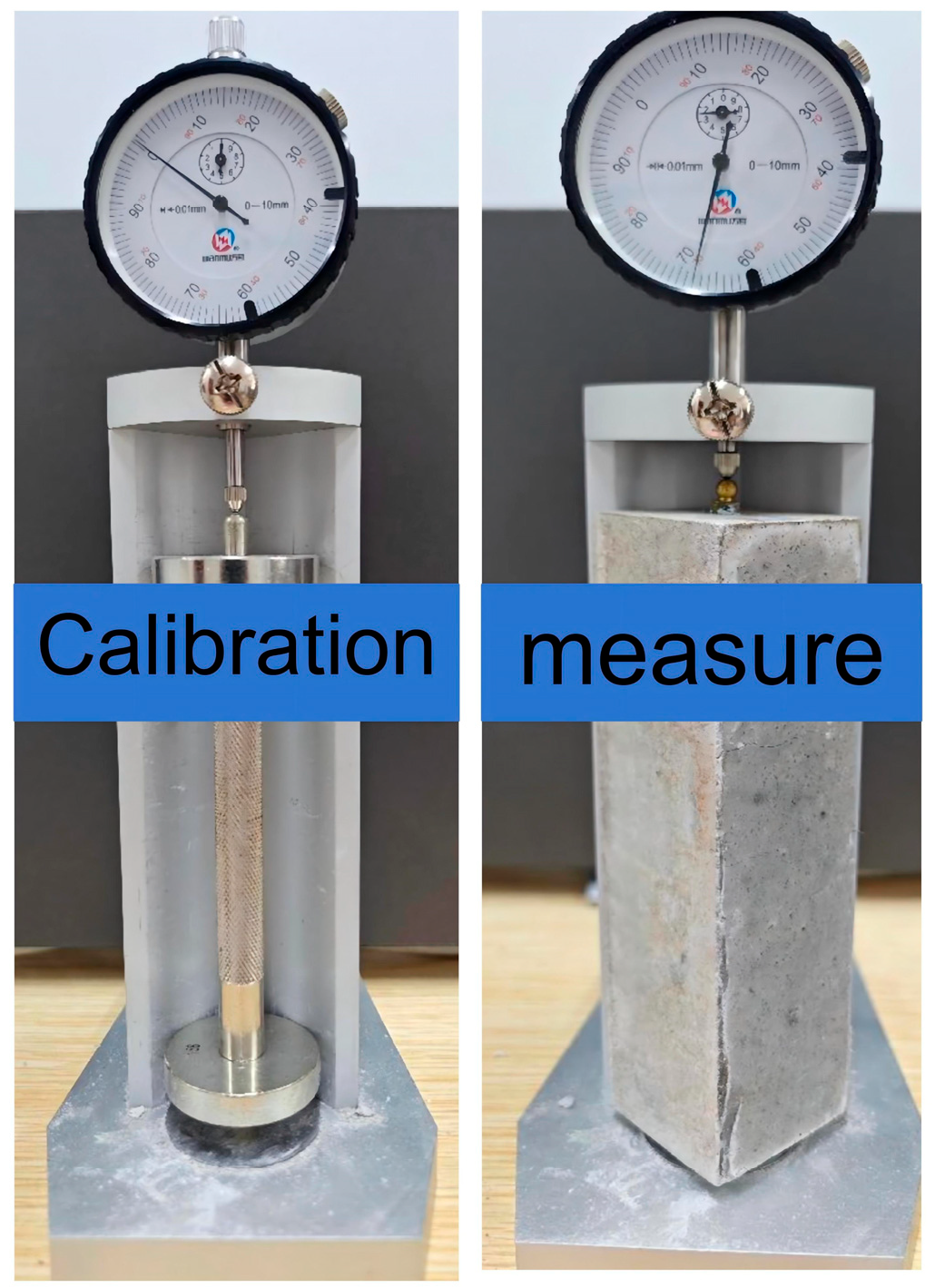
2.2.3. Shrinkage Test
2.2.4. Microstructure Analysis of Geopolymer
3. Results and Analysis
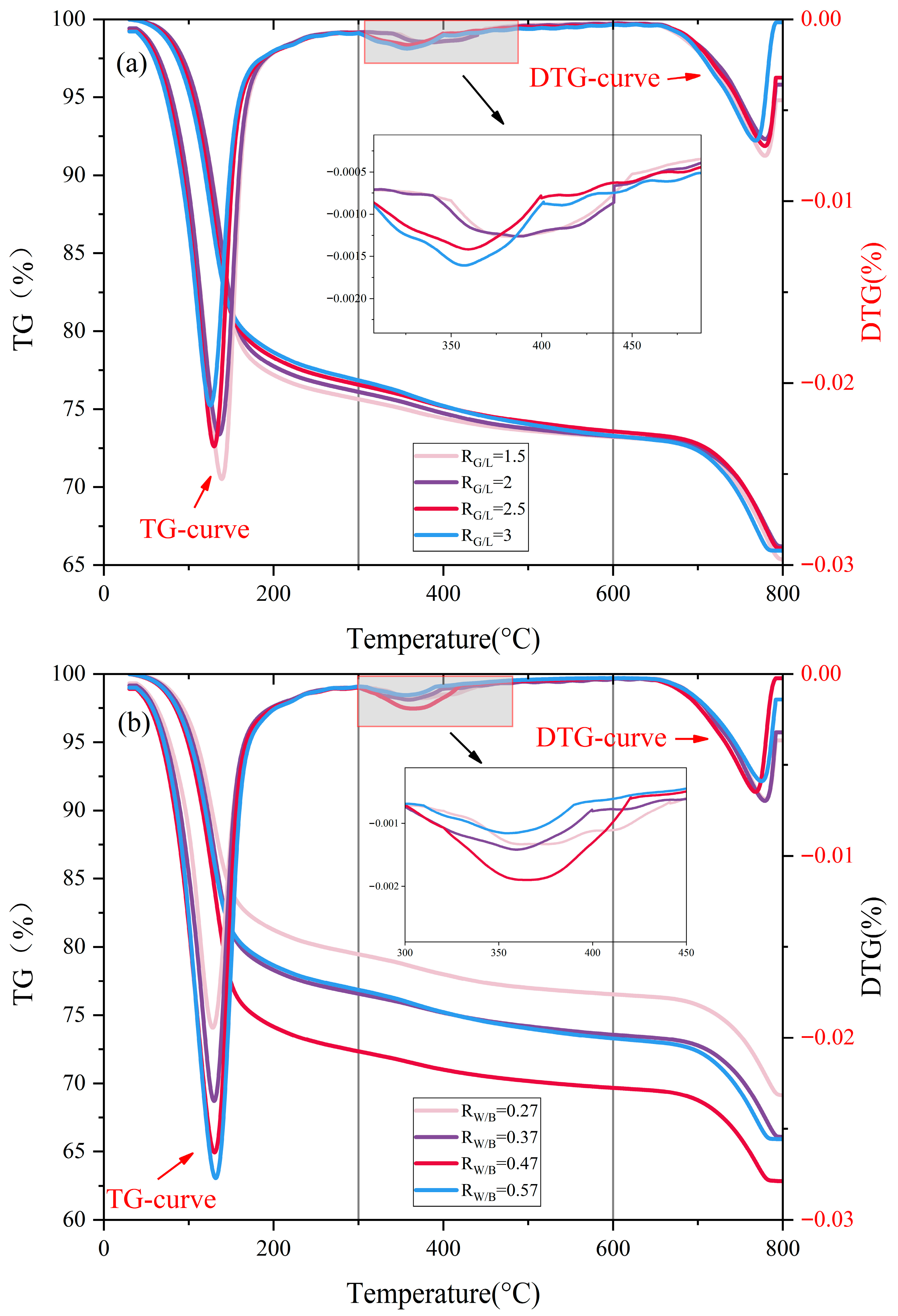
3.1. Simultaneous Thermal Analysis
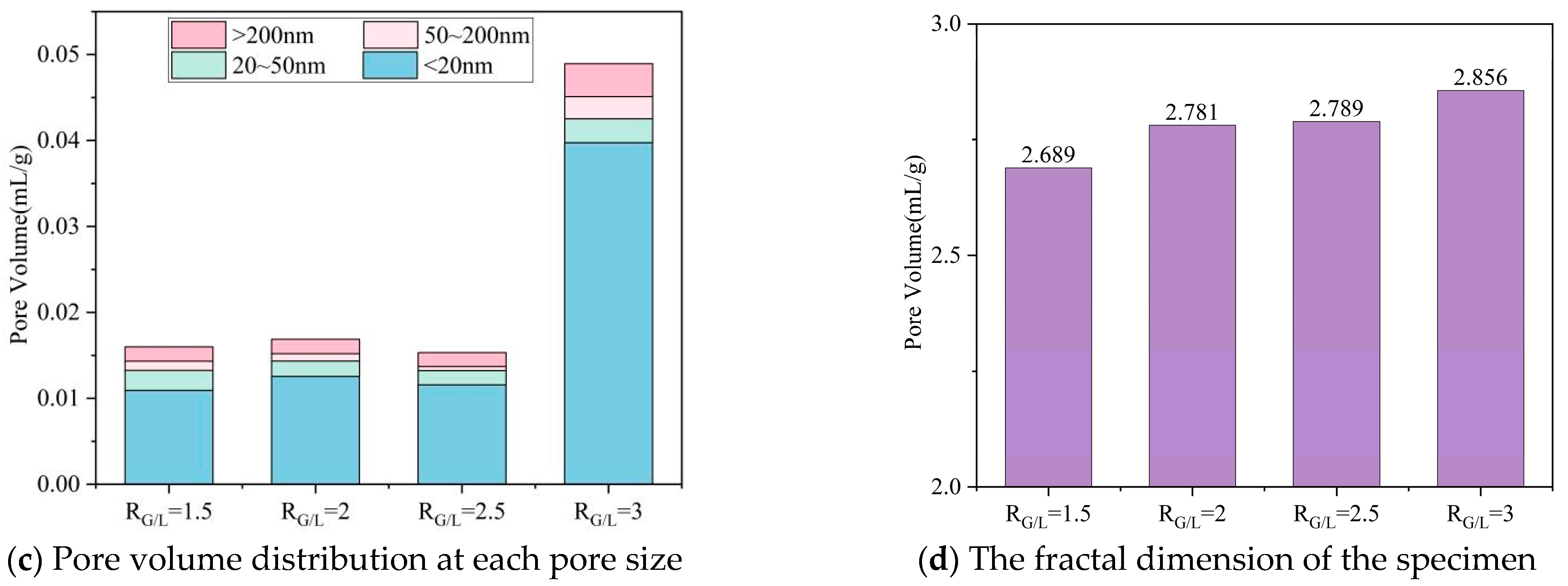
3.2. Pore Structure
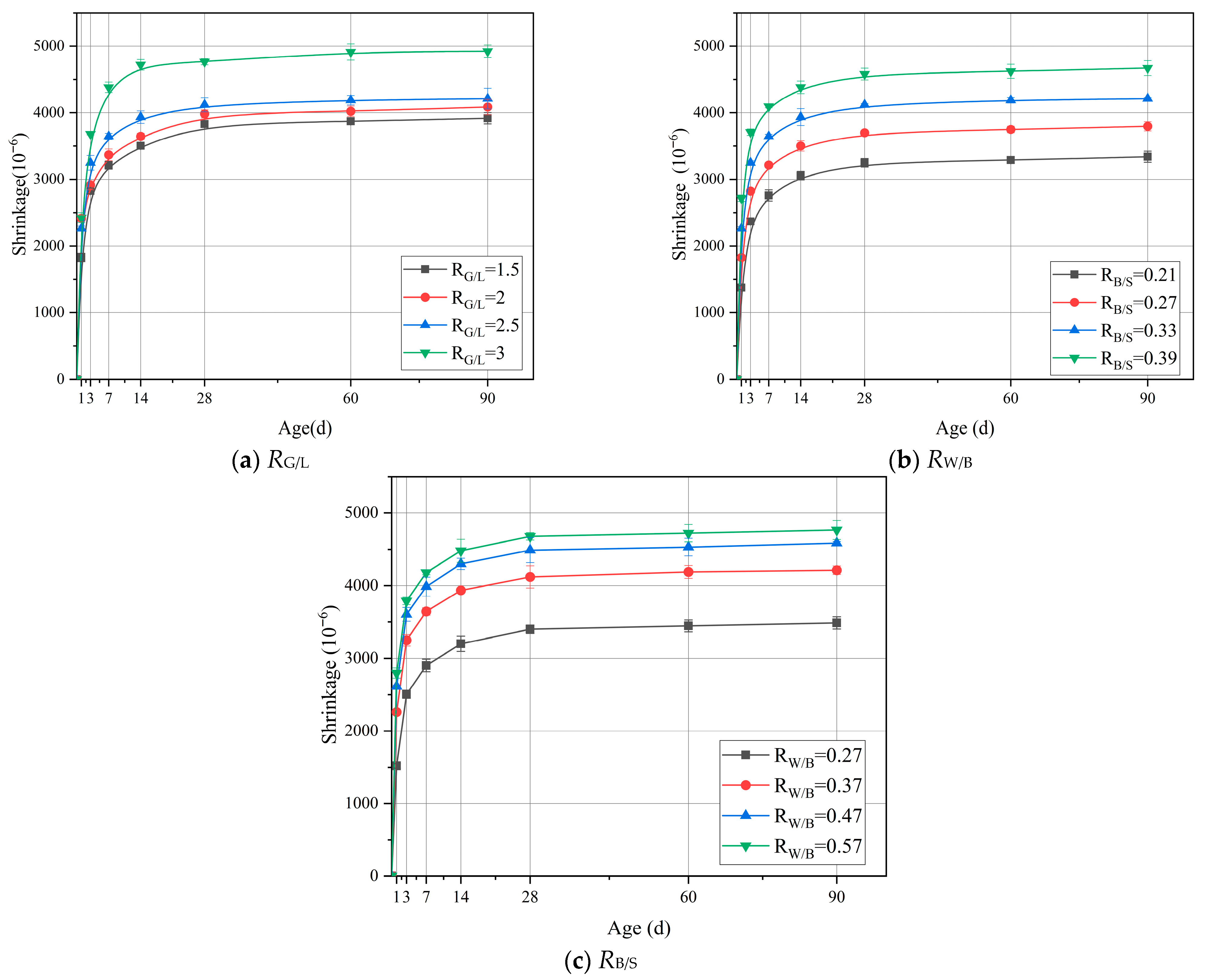
3.3. Shrinkage Strain
4. Mathematical Prediction Model
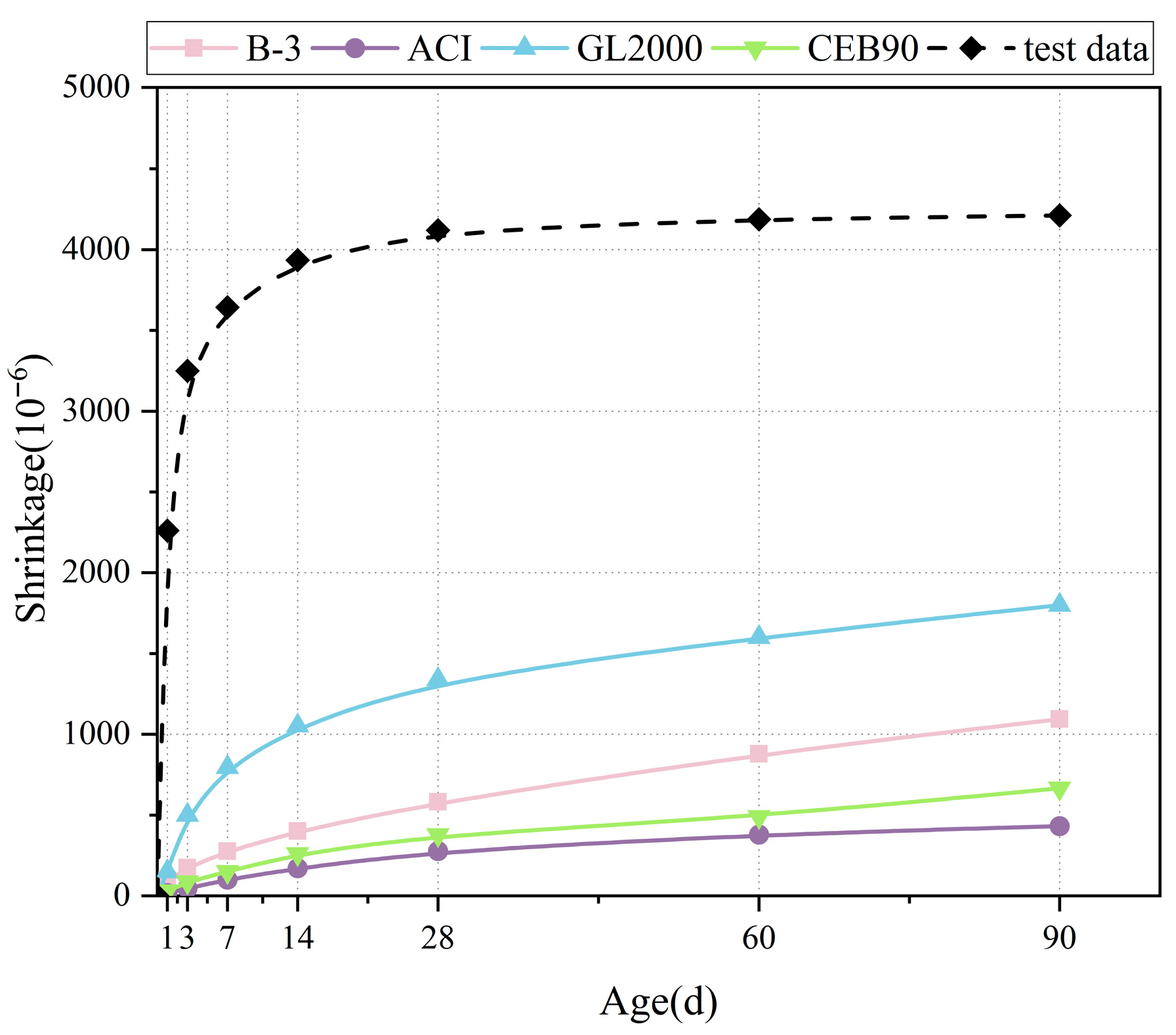
4.1. Existing Mathematical Model
4.2. Modified Mathematical Model
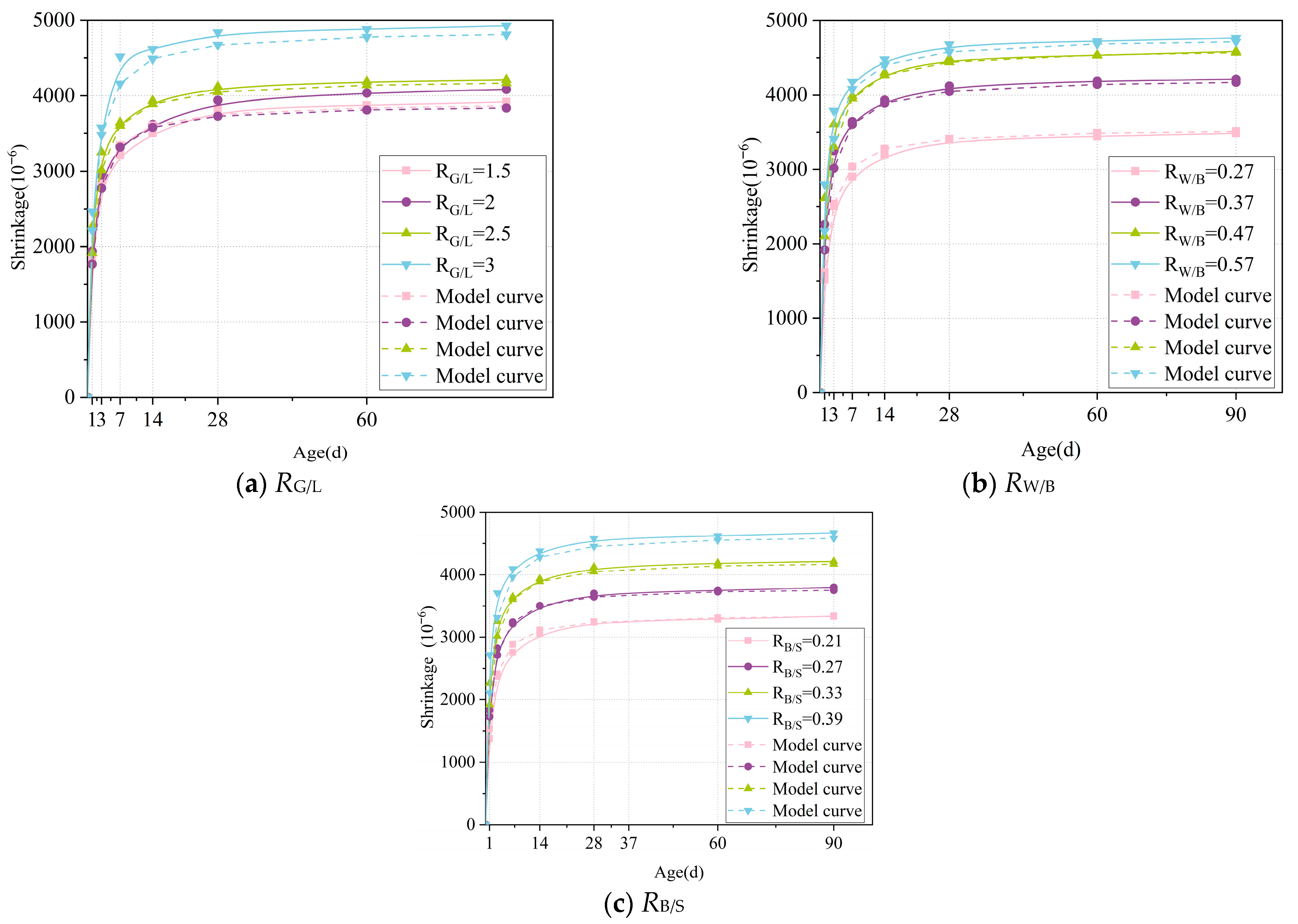
4.3. Model Verification
5. Conclusions
- (1)
- Compared with the sample (RG/L), the low calcium property of LS alters the polymerization path of silicate ions: it reduces the concentration of Ca2+ in the system, promoting the polymerization of Na+ with silicate to form a high-density N-A-S-H gel. At the same time, the unreacted LS particles can fill the 50–100 nm micropores of the C-A-S-H gel, reducing the average pore diameter from 53.5 nm to 28.75 nm, further blocking the capillary water migration channels.
- (2)
- When the water–binder ratio decreases from 0.57 to 0.27, the 90-day shrinkage reduces by 36.7%. The MIP test shows that at this time, the proportion of mesopores increases by 27.6%, the capillary stress caused by the evaporation of free water significantly increases, and the shrinkage strain is 36.7% higher than that when RW/B = 0.37; when RW/B < 0.27, insufficient fluidity of the slurry occurs, resulting in decreased vibration compactness, and causes a slight increase in the proportion of harmful pores.
- (3)
- The ratio of RB/S has a significant impact on the performance of LSGM, with the core mechanism being that the integrity of cementitious material coating sand particles determines interfacial porosity. The binder–sand ratio increased from 0.39 to 0.21; the 90-day shrinkage rate decreased by 39.8%. This is because at a low RB/S ratio, there is sufficient cementitious material to fully encapsulate the sand particles, optimize the transition zone between sand and cement, and reduce loose contact surfaces and voids.
- (4)
- Based on a thorough consideration of the characteristics of LSGM and combined with experimental data, the influence coefficients of the GBFS-LS ratio (RG/L), water–binder ratio (RW/B), and cement–sand ratio (RB/S) were introduced into the ACI model framework to construct the LSGM shrinkage prediction model. The fitting goodness R2 of this model is greater than 0.98, and its adaptability to different mix ratios is significantly better than the traditional model. This model provides a quantitative tool for LSGM engineering design, which can significantly reduce the trial-making cost and cycle.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Hao, H.; Zhao, F.; Liu, Z. Global Lithium Flow 1994–2015: Implications for Improving Resource Efficiency and Security. Environ. Sci. Technol. 2018, 52, 2827–2834. [Google Scholar] [CrossRef]
- Sverdrup, H.U. Modelling global extraction, supply, price and depletion of the extractable geological resources with the LITHIUM model, Resources. Resour. Conserv. Recycl. 2016, 114, 112–129. [Google Scholar] [CrossRef]
- Swart, P.; Dewulf, J.; Biernaux, A. Resource demand for the production of different cathode materials for lithium ion batteries. J. Clean. Prod. 2014, 84, 391–399. [Google Scholar] [CrossRef]
- Liu, D.; Gao, X.; An, H.; Qi, Y.; Sun, X.; Wang, Z.; Chen, Z.; An, F.; Jia, N. Supply and demand response trends of lithium resources driven by the demand of emerging renewable energy technologies in China. Resour. Conserv. Recycl. 2019, 145, 311–321. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Q.; Li, H.; Wang, W.; Hua, J. Heavy metal pollution characteristics and systemic risk assessment of the environment around the tailings site. J. Soils Sediments 2024, 24, 217–229. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, Z.; Zhu, Y.; Ren, Q.; Provis, J.L.; Wang, Q.; Jiang, Z. Leaching behavior of lithium slag at various pH conditions. Cem. Concr. Compos. 2025, 159, 105985. [Google Scholar] [CrossRef]
- Xue, W.; Heng, W.; Qiang, W. Research progress on resource utilization of lithium slag in China. Mater. Rev. 2022, 36, 63–73. (In Chinese) [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction-recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A comparative study on geopolymers synthesized by different classes of fly ash after exposure to elevated temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Ahn, N.; Le, T.A.; Lee, K. Theoretical and experimental study on mechanical properties and flexural strength of fly ash-geopolymer concrete. Constr. Build. Mater. 2016, 106, 65–77. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, C.; Long, G.; Xie, Y. A novel non-Portland cementitious material: Mechanical properties, durability and characterization. Constr. Build. Mater. 2020, 238, 117671. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Li, Q.; Wang, J.; Ling, Y. Fabrication and engineering properties of concretes based on geopolymers/alkali-activated binders—A review. J. Clean. Prod. 2020, 258, 120896. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.; Mishra, J.; Lazorenko, G.; Kasprzhitskii, A. Mine tailings-based geopolymers: Properties, applications and industrial prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, X.; Liu, S.; Luo, L.; Hao, Y.; Lv, Y.; Xiao, Y.; Yang, J.; Tian, H. Heterogeneous Variations on Historical and Future Trends of CO2 and Multiple Air Pollutants from the Cement Production Process in China: Emission Inventory, Spatial-Temporal Characteristics, and Scenario Projections. Environ. Sci. Technol. 2022, 56, 14306–14314. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Negahban, E.; Bagheri, A.; Sanjayan, J. One-Year study of restrained shrinkage and creep behaviours of geopolymer concrete. Constr. Build. Mater. 2023, 376, 131057. [Google Scholar] [CrossRef]
- Archez, J.; Farges, R.; Gharzouni, A.; Rossignol, S. Influence of the geopolymer formulation on the endogeneous shrinkage. Constr. Build. Mater. 2021, 298, 123813. [Google Scholar] [CrossRef]
- Wan, C.; Jiang, T. Early shrinkage characteristics of high performance geopolymer concrete. Acta Mater. Compos. Sin. 2024, 41, 952–964. [Google Scholar] [CrossRef]
- Zhang, T.; Fu, H.; Han, J. Deformation Mechanisms of Magnesium Silicate Hydrate Cement with a Shrinkage-Reducing Admixture under Different Curing Conditions. Minerals 2023, 13, 563. [Google Scholar] [CrossRef]
- Seyrek, Y.; Rudić, O.; Juhart, J.; Grengg, C.; Charry, E.M.; Freytag, B.; Mittermayr, F. On drying shrinkage of geopolymer and how to mitigate it with vegetable oil. Constr. Build. Mater. 2024, 436, 137013. [Google Scholar] [CrossRef]
- Hanumananaik, M.; Subramaniam, K.V.L. Shrinkage in low-calcium fly ash geopolymers for precast applications: Reaction product content and pore structure under drying conditions. J. Build. Eng. 2023, 78, 107583. [Google Scholar] [CrossRef]
- Wu, H.; He, M.; Wu, S.; Cheng, J.; Wang, T.; Che, Y.; Du, Y.; Deng, Q. Effects of binder component and curing regime on compressive strength, capillary water absorption, shrinkage and pore structure of geopolymer mortars. Constr. Build. Mater. 2024, 442, 137707. [Google Scholar] [CrossRef]
- Samuel, D.; Kriven, W.M. Depth dependence of hardness and reaction in metakaolin-based geopolymers cured at low humidity. J. Am. Ceram. Soc. 2024, 107, 543–560. [Google Scholar] [CrossRef]
- Miao, X.; Pang, X.; Li, S.; Wei, H.; Yin, J.; Kong, X. Mechanical strength and the degradation mechanism of metakaolin based geopolymer mixed with ordinary Portland cement and cured at high temperature and high relative humidity. Chin. J. Chem. Eng. 2023, 60, 118–130. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, X.; Ding, L.; Zhou, J.; Hao, X.; Xie, J.; Wu, Z. Effects of additives on alkali-activated slag/fly ash based geopolymer mortar: Mechanical properties, shrinkage properties, and shrinkage prediction model. Constr. Build. Mater. 2025, 494, 143556. [Google Scholar] [CrossRef]
- Sherwani, A.F.H.; Younis, K.H.; Arndt, R.W. Fresh, Mechanical, and Durability Behavior of Fly Ash-Based Self Compacted Geopolymer Concrete: Effect of Slag Content and Various Curing Conditions. Polymers 2022, 14, 3209. [Google Scholar] [CrossRef]
- Vickers, L.; Rickard, W.D.A.; van Riessen, A. Strategies to control the high temperature shrinkage of fly ash based geopolymers. Thermochim. Acta 2014, 580, 20–27. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Zhang, W.; Duan, X.; Su, F.; Zhu, J.; Hama, Y. Drying shrinkage inhibition effect and mechanism of polyol shrinkage-reducing admixture on the metakaolin-based geopolymer. J. Mater. Res. Technol. 2024, 28, 2021–2032. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mitigation strategies in alkali-activated slag. Cem. Concr. Res. 2017, 101, 131–143. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Castel, A.; Foster, S.J.; Ng, T.; Sanjayan, J.G.; Gilbert, R.I. Creep and drying shrinkage of a blended slag and low calcium fly ash geopolymer Concrete. Mater. Struct. 2015, 49, 1619–1628. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z. Influence of fly ash on the pore structure and shrinkage characteristics of metakaolin-based geopolymer pastes and mortars. Constr. Build. Mater. 2017, 153, 284–293. [Google Scholar] [CrossRef]
- Hawa, A.; Tonnayopas, D.; Prachasaree, W. Performance Evaluation and Microstructure Characterization of Metakaolin-Based Geopolymer Containing Oil Palm Ash. Sci. World J. 2013, 2013, 857586. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Shrinkage and strength of alkaline activated ground steel slag/ultrafine palm oil fuel ash pastes and mortars. Mater. Des. 2014, 63, 710–718. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- De Silva, P.; Sagoe-Crenstil, K. Medium-term phase stability of Na2O-Al2O3-SiO2-H2O geopolymer systems. Cem. Concr. Res. 2008, 38, 870–876. [Google Scholar] [CrossRef]
- Vidal, L.; Joussein, E.; Colas, M.; Absi, J.; Rossignol, S. Effect of the addition of ammonium molybdate on metakaolin-based geopolymer formation: Shrinkage and crystallization. Powder Technol. 2015, 275, 211–219. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Fu, C. Shrinkage behavior of fly ash based geopolymer pastes with and without shrinkage reducing admixture. Cem. Concr. Compos. 2019, 98, 74–82. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, H.; Dong, H.; Zhao, Y. Effect of reactive magnesia on dry shrinkage of geopolymer concrete and its bond property with BFRP bar. Constr. Build. Mater. 2025, 465, 140208. [Google Scholar] [CrossRef]
- Cui, C.; Tai, W.; Luo, C.; Wang, L.; Peng, H. Mechanisms underlying drying shrinkage in ASM-based geopolymer: Capillary tensile stress and its prediction method. Constr. Build. Mater. 2024, 450, 138698. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Lin, H.; Li, Y. Development of autogenous shrinkage prediction model of alkali-activated slag-fly ash geopolymer based on machine learning. J. Build. Eng. 2023, 71, 106538. [Google Scholar] [CrossRef]
- Khair, S.; Rahman, S.A.; Shaikh, F.U.A.; Sarker, P.K. Evaluating lithium slag for geopolymer concrete: A review of its properties and sustainable construction applications. Case Stud. Constr. Mater. 2024, 20, e02822. [Google Scholar] [CrossRef]
- Hou, Y.; Yin, S.; Yang, S.; Chen, X.; Du, H. Mechanical properties, damage evolution and energy dissipation of cemented tailings backfill under impact loading. J. Build. Eng. 2023, 66, 105912. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Zhu, Y. Effect of lithium slag on hydration behavior of Portland cement paste. Constr. Build. Mater. 2025, 463, 138909, ISSN 0950-0618. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, A.; Wu, C.; Zhu, D. Effect of modified multi-walled carbon nanotubes on mechanical properties and microscopic mechanism of lithium slag geopolymers. Case Stud. Constr. Mater. 2024, 21, e03819. [Google Scholar] [CrossRef]
- Luo, X.; Huang, L.; Chen, Z.; Li, Y.; Zhou, Z.; Qu, Y. Alkali-fused lithium slag as a substitute for slag in one-part geopolymers: Mechanism of reactivity enhancement and microstructure. Constr. Build. Mater. 2024, 442, 137696. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, C.; Ai, L.; Wang, L.; Zhu, D. Effects of modified multi-walled carbon nanotubes and PVA fibers on mechanical properties and microstructure of lithium slag base geopolymer. Constr. Build. Mater. 2024, 438, 137237. [Google Scholar] [CrossRef]
- JGJ/T 70-2009; Standard for Test Method of Basic Properties of Construction Mortar. China Architecture & Building Press: Beijing, China, 2009.
- Ji, X.; Chan, S.Y.N.; Feng, N. Fractal model for simulating the space-filling process of cement hydrates and fractal dimensions of pore structure of cement-based materials. Cem. Concr. Res. 1997, 27, 1691–1699. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Tian, L.; Chen, X.; Liu, X.; Li, H.; Ge, Y. Mechanisms of recycled fine brick aggregate in autogenous shrinkage mitigation, mechanical properties enhancement and microstructure improvement of alkali-activated slag-fly ash mortar. Constr. Build. Mater. 2023, 373, 130755. [Google Scholar] [CrossRef]
- You, N.; Liu, Y.; Gu, D.; Ozbakkaloglu, T.; Pan, J.; Zhang, Y. Rheology, shrinkage and pore structure of alkali-activated slag-fly ash mortar incorporating copper slag as fine aggregate. Constr. Build. Mater. 2020, 242, 118029. [Google Scholar] [CrossRef]
- Ranjbar, N.; Talebian, S.; Mehrali, M.; Kuenzel, C.; Cornelis Metselaar, H.S.; Jumaat, M.Z. Mechanisms of interfacial bond in steel and polypropylene fiber reinforced geopolymer composites. Compos. Sci. Technol. 2016, 122, 73–81. [Google Scholar] [CrossRef]
- ACI (American Concrete Institute). Prediction of Creep, Shrinkage, and Temperature Effects in Concrete Structures, ACI 209R-92; ACI: Farmington Hills, MI, USA, 1992. [Google Scholar]
- CEB-FIB (European Committee for Concrete—International Federation for Structural Concrete). Structural Concrete: Textbook on Behavior, Design and Performance: Updated Knowledge of the CEB/FIP Model Code 1990; CEB-FIB: London, UK, 1999. [Google Scholar]
- Bažant, Z.P.; Baweja, S. Creep and shrinkage prediction model for analysis anddesign of concrete structures—Model B3. Mater. Struct. 1995, 28, 357–365. [Google Scholar] [CrossRef]
- Gardner, N.; Lockman, M. Design provisions for drying shrinkage and creep of normal strength concrete. Acids Mater. J. 2001, 98, 159–167. [Google Scholar]
- Sakata, K. Prediction of concrete creep and shrinkage, creep and shrinkage of concrete. In Proceedings of the Fifth International RILEM Symposium, Barcelona, Spain, 6–9 September 1993; pp. 649–654. [Google Scholar]
- Mokarem, D.W.; Weyers, R.E.; Lane, D.S. Development of a shrinkage performance specifications and prediction model analysis for supplemental cementitious material concrete mixtures. Cem. Concr. Res. 2005, 35, 918–925. [Google Scholar] [CrossRef]





| Raw Material | GBFS | LS | |
|---|---|---|---|
| Chemical composites (%) | SiO2 | 34.2 | 39.8 |
| CaO | 34 | 16.9 | |
| Al2O3 | 17.6 | 15.3 | |
| Fe2O3 | 1.01 | 3.98 | |
| MgO | 6.21 | 1.40 | |
| SO3 | 1.62 | 9.74 | |
| Li2O | / | 0.26 | |
| Other | 5.36 | 12.62 |
| No | Samples | GBFS (g) | LS (g) | Sand (g) | Water (g) |
|---|---|---|---|---|---|
| 1 | RG/L-1.5 | 900 | 600 | 495 | 555 |
| 2 | RG/L-2 | 1000 | 500 | 495 | 555 |
| 3 | RG/L-2.5 | 1072 | 428 | 495 | 555 |
| 4 | RG/L-3 | 1125 | 375 | 495 | 555 |
| 5 | RB/S-0.21 | 1072 | 428 | 315 | 555 |
| 6 | RB/S-0.27 | 1072 | 428 | 405 | 555 |
| 7 | RB/S-0.33 | 1072 | 428 | 495 | 555 |
| 8 | RB/S-0.39 | 1072 | 428 | 585 | 555 |
| 9 | RW/B-0.27 | 1072 | 428 | 495 | 405 |
| 10 | RW/B-0.37 | 1072 | 428 | 495 | 555 |
| 11 | RW/B-0.47 | 1072 | 428 | 495 | 705 |
| 12 | RW/B-0.57 | 1072 | 428 | 495 | 855 |
| Model | Functional Form | |
|---|---|---|
| ACI | ||
| CEB-90 | ||
| B3 | ||
| Gardner | ||
| Sakata | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Pan, G.; Wu, C.; Xu, S.; Zhu, D. Research on Shrinkage in Lithium Slag Geopolymer Mortar: Effects of Mix Proportions and a Shrinkage Prediction Model. Materials 2025, 18, 4766. https://doi.org/10.3390/ma18204766
Wang L, Pan G, Wu C, Xu S, Zhu D. Research on Shrinkage in Lithium Slag Geopolymer Mortar: Effects of Mix Proportions and a Shrinkage Prediction Model. Materials. 2025; 18(20):4766. https://doi.org/10.3390/ma18204766
Chicago/Turabian StyleWang, Lei, Gao Pan, Cai Wu, Sidong Xu, and Daopei Zhu. 2025. "Research on Shrinkage in Lithium Slag Geopolymer Mortar: Effects of Mix Proportions and a Shrinkage Prediction Model" Materials 18, no. 20: 4766. https://doi.org/10.3390/ma18204766
APA StyleWang, L., Pan, G., Wu, C., Xu, S., & Zhu, D. (2025). Research on Shrinkage in Lithium Slag Geopolymer Mortar: Effects of Mix Proportions and a Shrinkage Prediction Model. Materials, 18(20), 4766. https://doi.org/10.3390/ma18204766








