Modification of Natural Clays with Magnetite to Provide Boosted Antimicrobial Properties and Chemopreventive Activity Against Melanoma
Abstract
1. Introduction
2. Materials and Methods
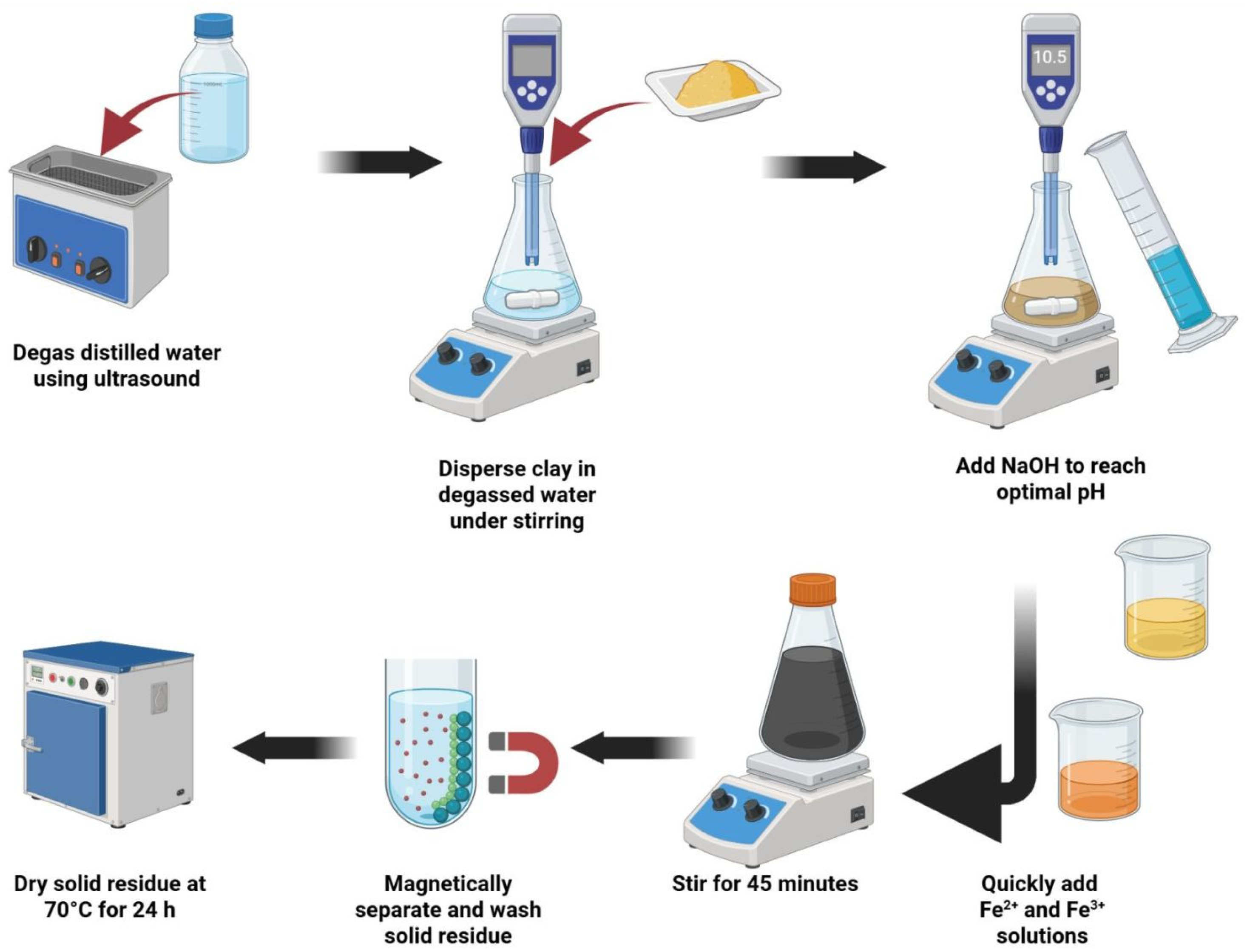
2.1. Synthesis of Magnetite–Clay Complexes
2.2. Characterization of Magnetic Complexes
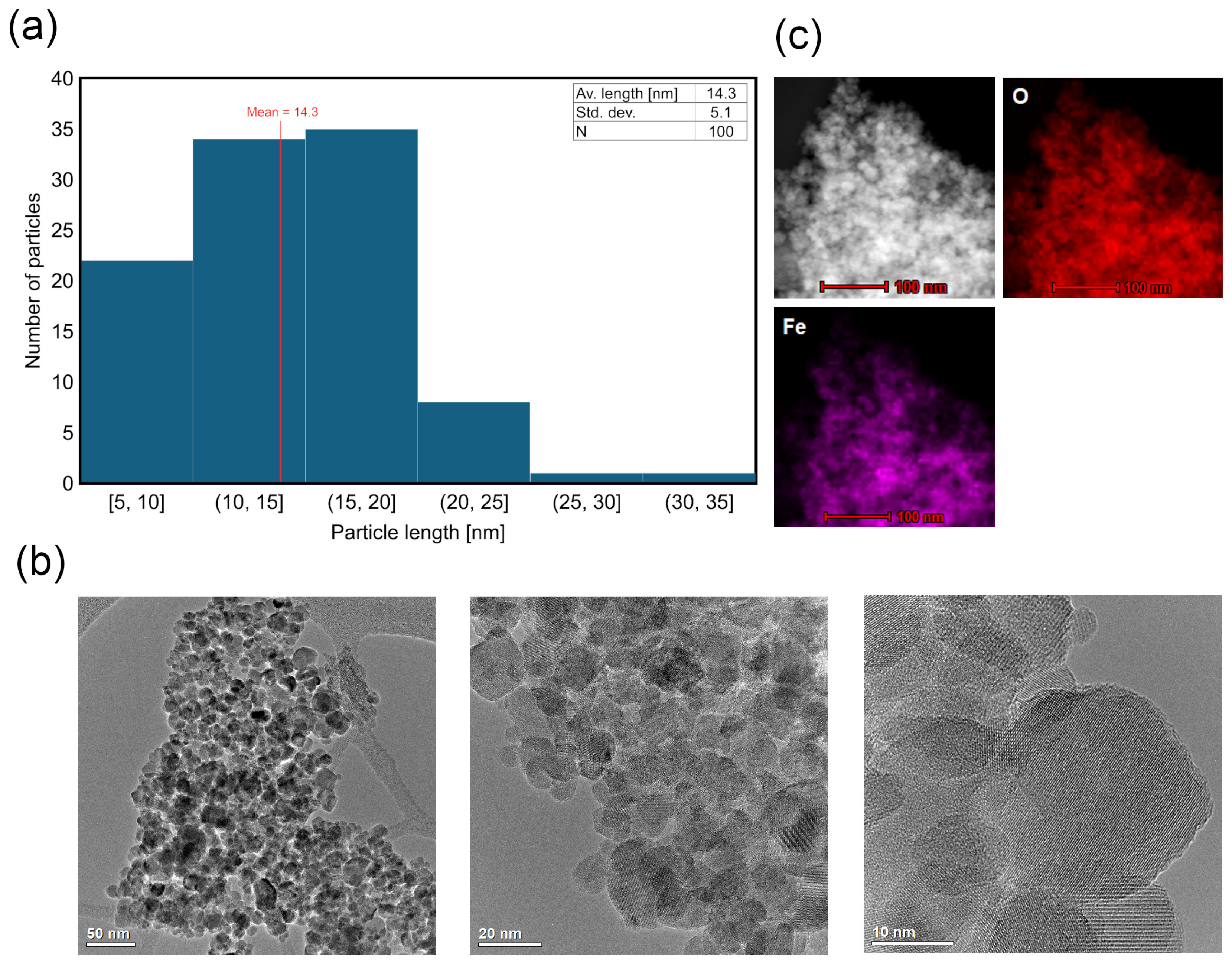
2.2.1. TEM and STEM-EDS Analysis
2.2.2. SEM Imaging
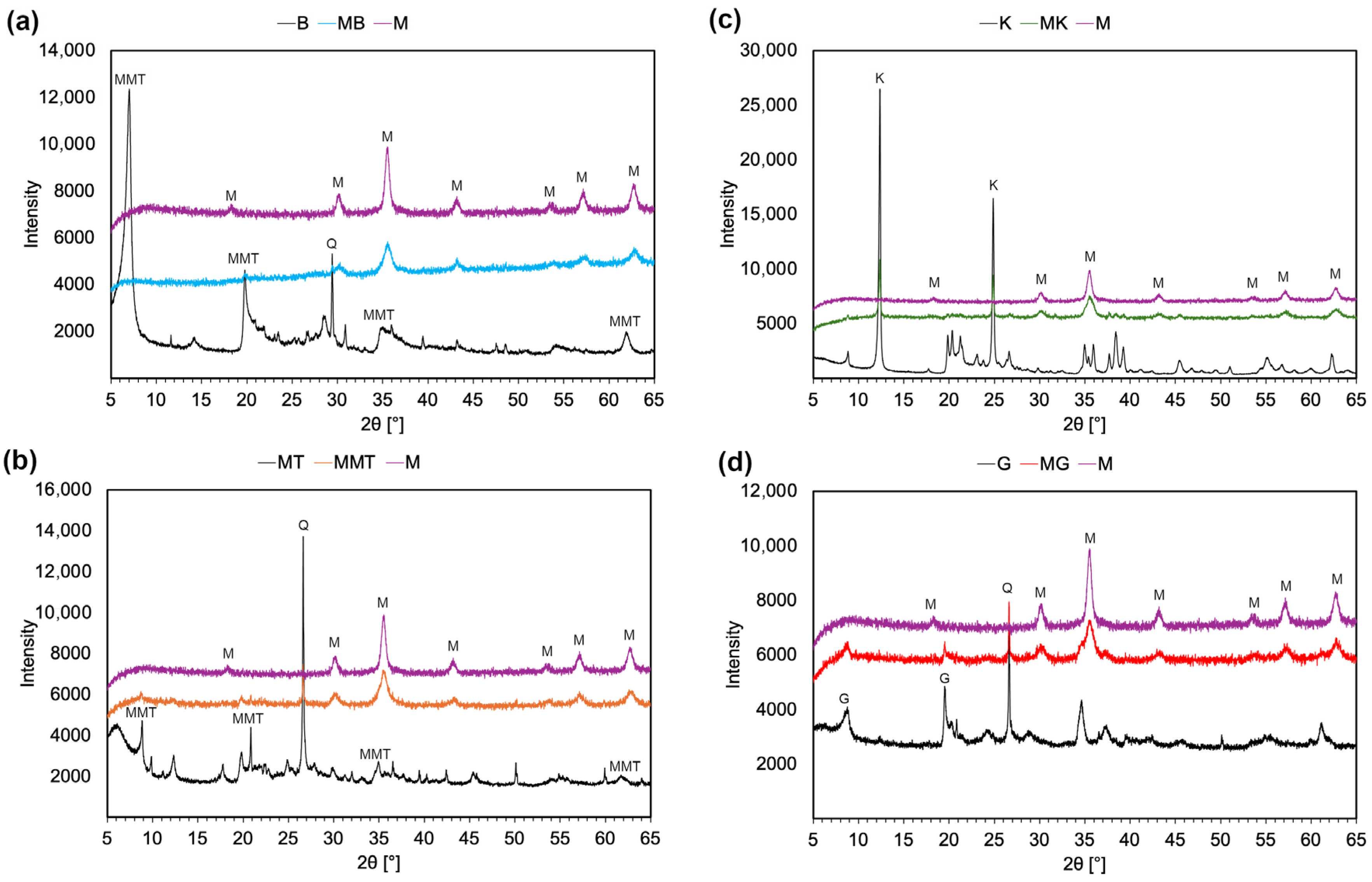
2.2.3. X-Ray Diffraction Analysis
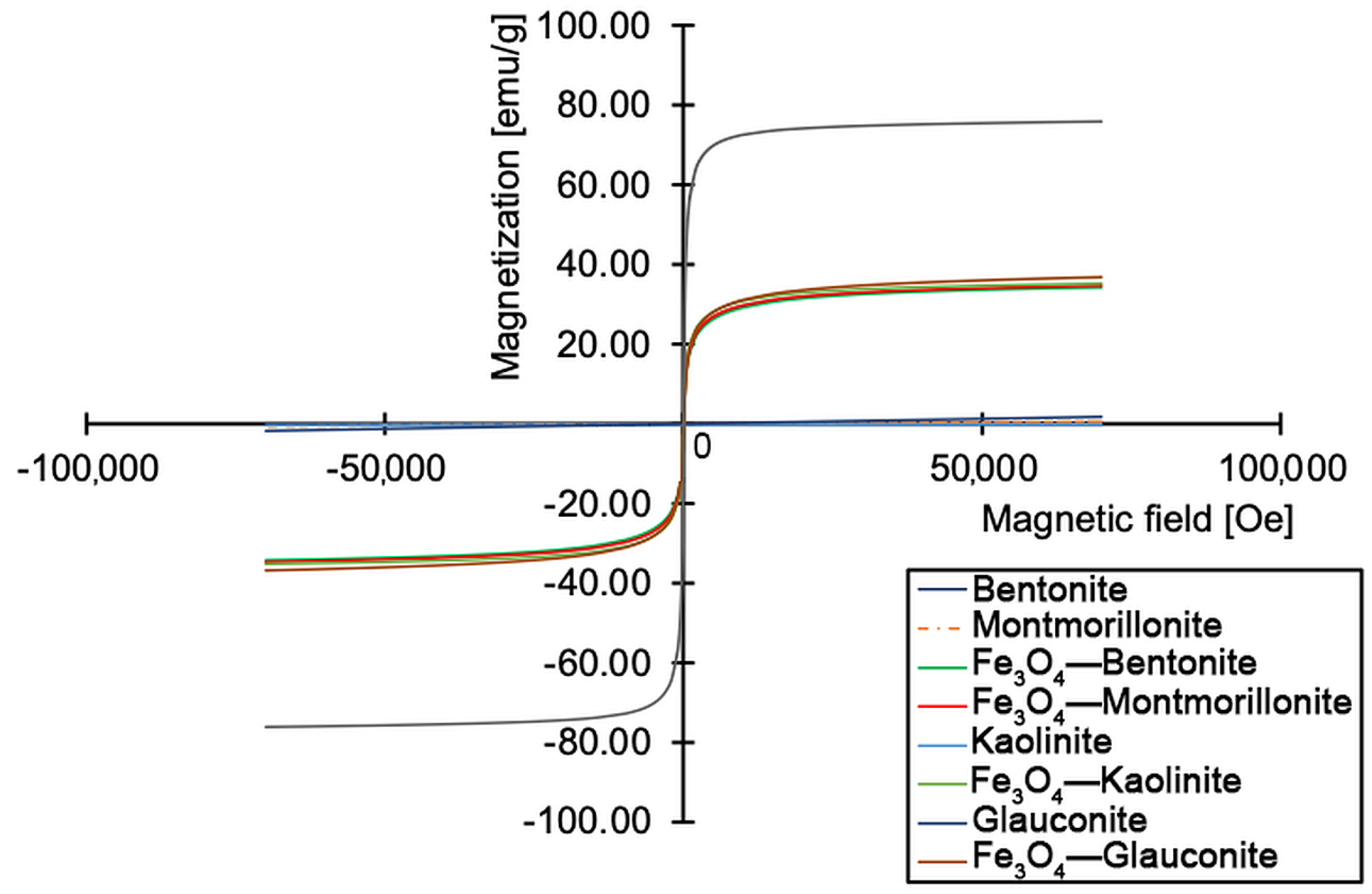
2.2.4. Magnetization Measurements
2.3. Antimicrobial Activity Tests
2.3.1. MIC Value Determination
2.3.2. Direct Contact Antibacterial Test
2.4. Cell Culture Experiments
2.4.1. Cells
2.4.2. Cytotoxicity Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Magnetite–Clay Complexes
3.2. Antimicrobial Activity Assessment
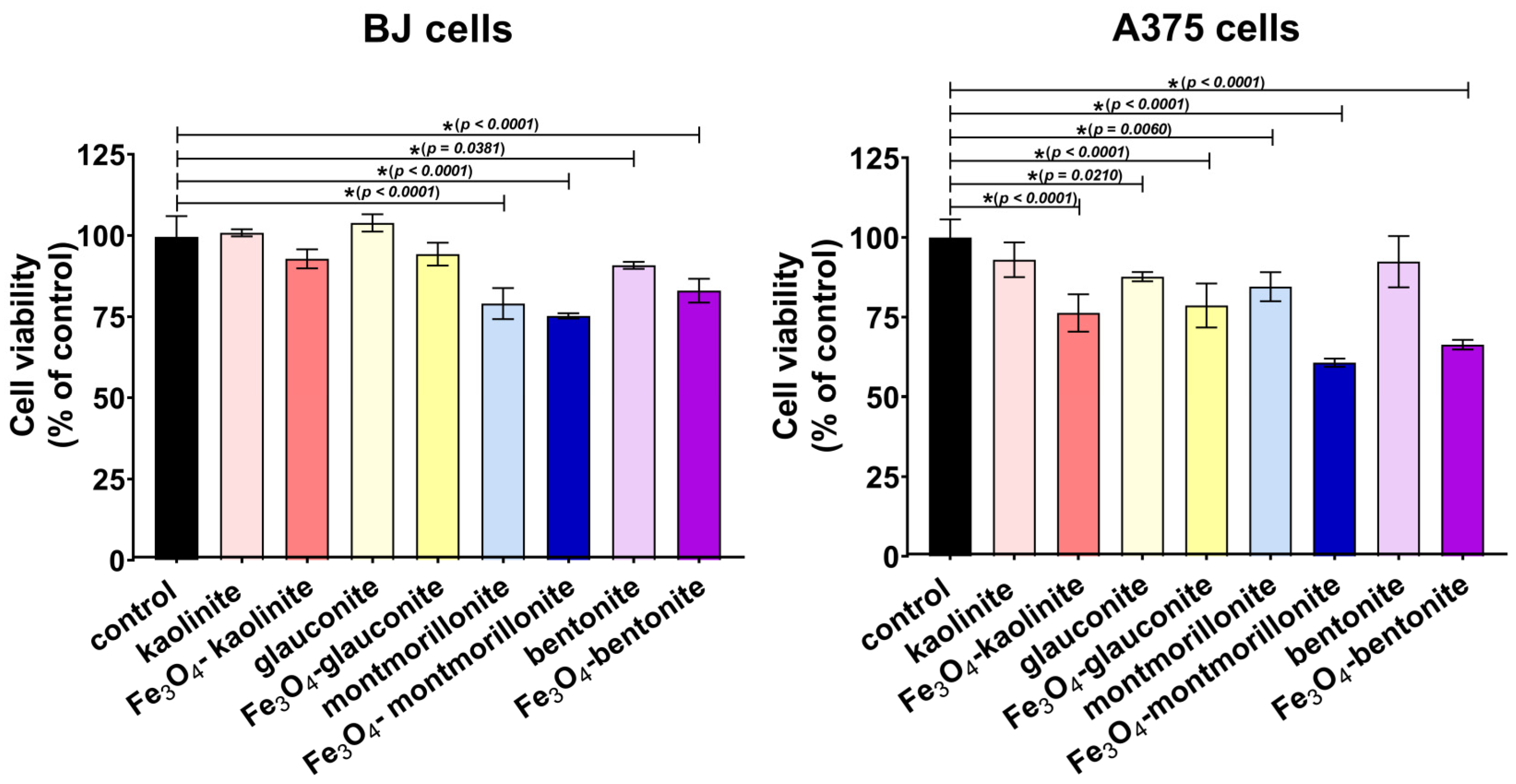
3.3. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| CAP | Cold Atmospheric Plasma |
| DNA | Deoxriboynucleic acid |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| MGO | Methyloglyoxal |
| MIC | Minimum inhibitory concentration |
| MMPs | matrix metalloproteinases |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ND | Not determined |
| PEMF | Pulsed Electromagnetic Fields |
| PET | Polyethylene terephthalate |
| PVP | Polivinyl pyrrolidone |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TEM-EDS | Transmission electron microscopy with energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
References
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurter, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.S.; El-Baky, R.M.A.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial activity of silver-treated bacteria against other multi-drug resistant pathogens in their environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef] [PubMed]
- Schabikowski, M.; Kowalczyk, P.; Karczmarska, A.; Gawdzik, B.; Wypych, A.; Kramkowski, K.; Wrzosek, K.; Laskowski, Ł. Aluminium(III) Oxide—The Silent Killer of Bacteria. Molecules 2023, 28, 401. [Google Scholar] [CrossRef]
- Moradi, M.; Montazeri, E.A.; Rafiei Asl, S.; Pormohammad, A.; Farshadzadeh, Z.; Dayer, D.; Turner, R.J. In Vitro and In Vivo Antibacterial and Antibiofilm Activity of Zinc Sulfate (ZnSO4) and Carvacrol (CV) Alone and in Combination with Antibiotics Against Pseudomonas aeruginosa. Antibiotics 2025, 14, 367. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, A.E.A.; Al-Shammari, G.M.; Al-Harbi, L.N.; Subash-Babu, P.; Elsayim, R.; Mohammed, M.A.; Yahya, M.A.; Fattiny, S.Z.A. Antimicrobial Properties of Zinc Oxide Nanoparticles Synthesized from Lavandula pubescens Shoot Methanol Extract. Appl. Sci. 2022, 12, 11613. [Google Scholar] [CrossRef]
- Saqib, S.; Munis, M.F.H.; Zaman, W.; Ullah, F.; Shah, S.N.; Ayaz, A.; Faroop, M.; Bahadur, S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc. Res. Tech. 2019, 82, 415–420. [Google Scholar] [CrossRef]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Hovhannisyan, A.; Gevorgyan, V.; Ananyan, M.; Trchounian, A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019, 103, 2773–2782. [Google Scholar] [CrossRef]
- Ovincy, C.; Babel, S.; Baral, S.; Poudel, S.; Jain, S. Clay Therapy in Wound Healing: A Brief Review of the Literature. J. Wound Manag. Res. 2024, 20, 1–8. [Google Scholar] [CrossRef]
- Williams, L.B.; Haydel, S.E. Evaluation of the medicinal use of clay minerals as antibacterial agents. Int. Geol. Rev. 2010, 52, 745–770. [Google Scholar] [CrossRef]
- Viseras, C.; Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C. Clay minerals in skin drug delivery. Clays Clay Miner. 2019, 67, 59–71. [Google Scholar] [CrossRef]
- Londono, S.C.; Hartnett, H.E.; Williams, L.B. Antibacterial Activity of Aluminum in Clay from the Colombian Amazon. Environ. Sci. Technol. 2017, 51, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Wang, Z.; Huang, Z.; Xie, Z.; Xia, L.; Zhang, Y. Clays and Wound Healing. Materials 2024, 17, 1691. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Mulla, N.R.; Khot, V.M.; Patil, R.S. Anticancer activity of surface functionalized magnetite (Fe3O4) nanoparticles—Effect of polymer coating. Emergent Mater. 2024, 7, 1071–1080. [Google Scholar] [CrossRef]
- Genc, S.; Taghizadehghalehjoughi, A.; Yeni, Y.; Jafarizad, A.; Hacimuftuoglu, A.; Nikitovic, D.; Docea, A.O.; Mezhuev, Y.; Tsatsakis, A. Fe3O4 Nanoparticles in Combination with 5-FU Exert Antitumor Effects Superior to Those of the Active Drug in a Colon Cancer Cell Model. Pharmaceutics 2023, 15, 245. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Akhtar, M.J. Selective killing of cancer cells by iron oxide nanoparticles mediated through reactive oxygen species via p53 pathway. J. Nanoparticle Res. 2013, 15, 1225. [Google Scholar] [CrossRef]
- Lv, H.; Liu, J.; Zhen, C.; Wang, Y.; Wei, Y.; Ren, W.; Shang, P. Magnetic fields as a potential therapy for diabetic wounds based on animal experiments and clinical trials. Cell Prolif. 2021, 54, e12982. [Google Scholar] [CrossRef] [PubMed]
- Cheing, G.L.Y.; Li, X.; Huang, L.; Kwan, R.L.C.; Cheung, K.K. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics 2014, 35, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Derkowski, A.; Środoń, J.; Franus, W.; Uhlík, P.; Banaś, M.; Zieliński, G.; Caplovicova, M.; Franus, M. Partial dissolution of glauconitic samples: Implications for the methodology of K-Ar and Rb-Sr dating. Clays Clay Miner. 2009, 57, 531–554. [Google Scholar] [CrossRef]
- Franus, W.; Dudek, K. Clay minerals and clinoptilolite from variegated shales formation in the skole unit, polish flysch carpathians. Geol. Carpathica 1999, 50, 23–24. [Google Scholar]
- Series on Testing and Assessment, No. 202; Guidance Document for Quantitative Method for Evaluating Antibacterial Activity of Porous and Non-porous Antibacterial Treated Materials. OECD Publishing: Paris, France, 2014.
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- Li, Y.; Wang, Z.; Liu, R. Superparamagnetic α-Fe2O3/Fe3O4 heterogeneous nanoparticles with enhanced biocompatibility. Nanomaterials 2021, 11, 834. [Google Scholar] [CrossRef]
- Sant’Angelo, D.; Descamps, G.; Lecomte, V.; Stanicki, D.; Penninckx, S.; Dragan, T.; Van Gestel, D.; Laurent, S.; Journe, F. Therapeutic Approaches with Iron Oxide Nanoparticles to Induce Ferroptosis and Overcome Radioresistance in Cancers. Pharmaceuticals 2025, 18, 325. [Google Scholar] [CrossRef]
- Kgabi, D.P.; Ambushe, A.A. Characterization of South African Bentonite and Kaolin Clays. Sustainability 2023, 15, 12679. [Google Scholar] [CrossRef]
- Cuevas, J.; Cabrera, M.Á.; Fernández, C.; Mota-Heredia, C.; Fernández, R.; Torres, E.; Turrero, M.J.; Ruiz, A.I. Bentonite Powder XRD Quantitative Analysis Using Rietveld Refinement: Revisiting and Updating Bulk Semiquantitative Mineralogical Compositions. Minerals 2022, 12, 772. [Google Scholar] [CrossRef]
- Rouibah, K.; Ferkous, H.; Abdessalam-Hassan, M.; Mossab, B.L.; Boublia, A.; Pierlot, C.; Abdennouri, A.; Avramova, I.; Alam, M.; Benguerba, Y.; et al. Exploring the Efficiency of Algerian Kaolinite Clay in the Adsorption of Cr(III) from Aqueous Solutions: Experimental and Computational Insights. Molecules 2024, 29, 2135. [Google Scholar] [CrossRef]
- Bansal, U.; Banerjee, S.; Ruidas, D.K.; Pande, K. Origin and geochemical characterization of the glauconites in the Upper Cretaceous Lameta Formation, Narmada Basin, central India. J. Palaeogeogr. 2018, 7, 99–116. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic characterization of iron oxide nanoparticles for biomedical applications. In Biomedical Nanotechnology: Methods and Protocols; Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2017; pp. 47–71. [Google Scholar] [CrossRef]
- Hadadian, Y.; Masoomi, H.; Dinari, A.; Ryu, C.; Hwang, S.; Kim, S.; Cho, B.K.; Lee, J.Y.; Yoon, J. From Low to High Saturation Magnetization in Magnetite Nanoparticles: The Crucial Role of the Molar Ratios between the Chemicals. ACS Omega 2022, 7, 15996–16012. [Google Scholar] [CrossRef]
- Svensson, S.L.; Behroozian, S.; Xu, W.; Surette, M.G.; Li, L.; Davies, J. Kisameet Glacial Clay: An Unexpected Source of Bacterial Diversity. mBio 2017, 8, e00590-17. [Google Scholar] [CrossRef]
- Herrera, P.; Burghardt, R.C.; Phillips, T.D. Adsorption of Salmonella enteritidis by cetylpyridinium-exchanged montmorillonite clays. Vet. Microbiol. 2000, 74, 259–272. [Google Scholar] [CrossRef]
- Haydel, S.E.; Remenih, C.M.; Williams, L.B. Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens. J. Antimicrob. Chemother. 2008, 61, 353–361. [Google Scholar] [CrossRef]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Morrison, K.D.; Misra, R.; Williams, L.B. Unearthing the Antibacterial Mechanism of Medicinal Clay: A Geochemical Approach to Combating Antibiotic Resistance. Sci. Rep. 2016, 6, 19043. [Google Scholar] [CrossRef]
- Azmi, N.N.; Mahyudin, N.A.; Wan Omar, W.H.; Mahmud Ab Rashid, N.K.; Ishak, C.F.; Abdullah, A.H.; Sharples, G.J. Antibacterial activity of clay soils against food-borne salmonella typhimurium and staphylococcus aureus. Molecules 2022, 27, 170. [Google Scholar] [CrossRef]
- Jee, S.C.; Han, D.H.; Kim, M.; Bu, K.B.; Sung, J.S.; Kadam, A.A. Montmorillonite modified with Fe3O4 and tannic acid for inhibition of S. aureus and MRSA biofilm formation. Appl. Clay Sci. 2025, 276, 107913. [Google Scholar] [CrossRef]
- Abd Qasim, M.; Yaaqoob, L.A. Evaluation of Antibacterial Activity of Iron Oxide Nanoparticles Synthesis by Extracellular Lactobacillus against Pseudomonas Aeruginosa. J. Med. Chem. Sci. 2023, 6, 1100–1111. [Google Scholar] [CrossRef]
- Abdelghany, S.; Elsayed, A.; Kabary, H.; Salaheldin, H. Iron oxide/silver-doped iron oxide nanoparticles: Facile synthesis, characterization, antibacterial activity, genotoxicity and anticancer evaluation. Sci. Rep. 2025, 15, 29593. [Google Scholar] [CrossRef]
- Afzal, R.K.; Khalid, F.; Hannan, A.; Ahmed, S.A. Methylglyoxal: Antimicrobial activity against blood culture isolates of Salmonella Typhi and other Gram negative rods. Pak. J. Med. Sci. 2019, 35, 1110–1114. [Google Scholar] [CrossRef]
- Long, M.; Liu, Q.; Wang, D.; Wang, J.; Zhang, Y.; Tang, A.; Liu, N.; Bui, B.; Chen, W.; Yang, H. A new nanoclay-based bifunctional hybrid fiber membrane with hemorrhage control and wound healing for emergency self-rescue. Mater. Today Adv. 2021, 12, 100190. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Liu, X.-W.; Hu, M.; Hu, Y.-H. Chemical composition and surface charge properties of montmorillonite. J. Cent. South Univ. Technol. 2008, 15, 193–197. [Google Scholar] [CrossRef]
- Maryan, A.S.; Montazer, M.; Rashidi, A.; Rahimi, M.K. Antibacterial properties of clay layers silicate: A special study of montmorillonite on cotton fiber. Asian J. Chem. 2013, 25, 2889–2892. [Google Scholar] [CrossRef]
- AATCC 100-2004; Test Method for Antibacterial Finishes on Textile Materials. AATCC: Durham, NC, USA, 2004.
- Hu, C.H.; Xu, Z.R.; Xia, M.S. Antibacterial effect of Cu2+-exchanged montmorillonite on Aeromonas hydrophila and discussion on its mechanism. Vet. Microbiol. 2005, 109, 83–88. [Google Scholar] [CrossRef]
- Magaña, S.M.; Quintana, P.; Aguilar, D.H.; Toledo, J.A.; Ángeles-Chávez, C.; Cortés, M.A.; León, L.; Freile-Pelegrín, Y.; López, T.; Torres Sánchez, R.M. Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. A Chem. 2008, 281, 192–199. [Google Scholar] [CrossRef]
- Abdullayev, E.; Paterson, J.R.; Kuszynski, E.P.; Hamidi, M.D.; Nahar, P.; Greenwell, H.C.; Neumann, A.; Sharples, G.J. Evaluation of the antibacterial properties of commonly used clays from deposits in central and southern Asia. Clays Clay Miner. 2024, 72, e9. [Google Scholar] [CrossRef]
- Cunningham, T.M.; Koehl, J.L.; Summers, J.S.; Haydel, S.E. pH-dependent metal ion toxicity influences the antibacterial activity of two natural mineral mixtures. PLoS ONE 2010, 5, e9456. [Google Scholar] [CrossRef] [PubMed]
- Caflisch, K.M.; Schmidt-Malan, S.M.; Mandrekar, J.N.; Karau, M.J.; Nicklas, J.P.; Williams, L.B.; Patel, R. Antibacterial activity of reduced iron clay against pathogenic bacteria associated with wound infections. Int. J. Antimicrob. Agents 2018, 52, 692–696. [Google Scholar] [CrossRef]
- Otto, C.C.; Cunningham, T.M.; Hansen, M.R.; Haydel, S.E. Effects of antibacterial mineral leachates on the cellular ultrastructure, morphology, and membrane integrity of Escherichia coli and methicillin-resistant Staphylococcus aureus. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 26. [Google Scholar] [CrossRef]
- Karaubayeva, A.A.; Bekezhanova, T.; Zhaparkulova, K.; Susniak, K.; Sobczynski, J.; Kazimierczak, P.; Przekora, A.; Skalicka-Wozniak, K.; Kulinowski, Ł.; Glowniak-Lipa, A.; et al. Antimicrobial Mixture Based on Micronized Kaolinite and Ziziphora Essential Oil as a Promising Formulation for the Management of Infected Wounds. Int. J. Mol. Sci. 2024, 25, 13192. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, X.; Cai, P.; Huang, Q.; Rong, X.; Liang, W. Preferential adsorption of extracellular polymeric substances from bacteria on clay minerals and iron oxide. Colloids Surf. B Biointerfaces 2011, 83, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xia, Q.; Zeng, Q.; Wang, X.; Dong, H. Antibacterial Mechanisms of Reduced Iron-Containing Smectite-Illite Clay Minerals. Environ. Sci. Technol. 2021, 55, 15256–15265. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Behroozian, S.; Svensson, S.L.; Li, L.Y.; Davies, J.E. Broad-Spectrum Antimicrobial and Antibiofilm Activity of a Natural Clay Mineral from British Columbia, Canada. mBio 2020, 11, e02350-20. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- NIH. National Cancer Institute [Internet]. 2025. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/chemoprevention (accessed on 24 September 2025).
- Persano, F.; Leporatti, S. Nano-Clays for Cancer Therapy: State-of-the Art and Future Perspectives. J. Pers. Med. 2022, 12, 1736. [Google Scholar] [CrossRef]
- Gianni, E.; Avgoustakis, K.; Papoulis, D. Kaolinite group minerals: Applications in cancer diagnosis and treatment. Eur. J. Pharm. Biopharm. 2020, 154, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, J.; Przekora, A.; Franus, W. Zeolites and zeolite imidazolate frameworks on a quest to obtain the ideal biomaterial for biomedical applications: A review. Mater. Today 2023, 67, 495–517. [Google Scholar] [CrossRef]
- Kheirabadi, B.S.; Mace, J.E.; Terrazas, I.B.; Fedyk, C.G.; Estep, J.S.; Dubick, M.A.; Blackbourne, L.H. Safety evaluation of new hemostatic agents, smectite granules, and kaolin-coated gauze in a vascular injury wound model in swine. J. Trauma Inj. Infect. Crit. Care 2010, 68, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, P.A.M.; Ouédraogo, R.; Sombié, B.C.; Yabré, Z.; Semdé, R. Clay minerals used in formulations for cutaneous wound healing: An educational review. Health Sci. Rev. 2025, 17, 100242. [Google Scholar] [CrossRef]
- Dário, G.M.; da Silva, G.G.; Gonçalves, D.L.; Silveira, P.; Junior, A.T.; Angioletto, E.; Bernardin, A.M. Evaluation of the healing activity of therapeutic clay in rat skin wounds. Mater. Sci. Eng. C 2014, 43, 109–116. [Google Scholar] [CrossRef]

| Tested Clay Mineral | E. coli | P. aeruginosa | S. aureus | C. albicans | |
|---|---|---|---|---|---|
| MIC | MIC | MIC | MIC | MFC | |
| kaolinite | ND | ND | 5 | 5 | ND |
| Fe3O4–kaolinite | 10 | 10 | 5 | 5 | 5 |
| glauconite | ND | ND | 2.5 | 20 | ND |
| Fe3O4–glauconite | 20 | 20 | 1.25 | 5 | 20 |
| montmorillonite | ND | ND | 10 | 20 | 20 |
| Fe3O4–montmorillonite | 0.156 | 0.156 | 0.156 | 1.25 | 1.25 |
| bentonite | 10 | 20 | 2.5 | 5 | ND |
| Fe3O4–bentonite | 5 | 5 | 0.313 | 2.5 | ND |
| Bacteria Strain | Mean of the Number of Viable Bacteria (CFU) | Antibacterial Activity | ||||
|---|---|---|---|---|---|---|
| Control Material Ti t = 0 | Control Material Ti t = 24 h | Tested Clay Mineral | Tested Materials | Log CFU Reduction | % CFU Reduction | |
| S. aureus | 1.31 × 104 | 1.17 × 104 | montmorillonite | <1 | 4.07 | 99.99 |
| Fe3O4–montmorillonite | <1 | 4.07 | 99.99 | |||
| bentonite | 3.76 × 104 | NA | NA | |||
| Fe3O4–bentonite | 1.41 × 104 | NA | NA | |||
| E. coli | 4.14 × 104 | 6.35 × 106 | montmorillonite | <1 | 6.8 | 99.99 |
| Fe3O4–montmorillonite | <1 | 6.8 | 99.99 | |||
| bentonite | 5.06 × 106 | 0.1 | 20.31 | |||
| Fe3O4–bentonite | 2.42 × 105 | 1.42 | 96.19 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, A.; Matusiak, J.; Trzaskowska, M.; Maciejczyk, A.; Kazimierczak, P.; Suśniak, K.; Palka, K.; Korona-Glowniak, I.; Franus, W.; Przekora, A. Modification of Natural Clays with Magnetite to Provide Boosted Antimicrobial Properties and Chemopreventive Activity Against Melanoma. Materials 2025, 18, 4759. https://doi.org/10.3390/ma18204759
Wójcik A, Matusiak J, Trzaskowska M, Maciejczyk A, Kazimierczak P, Suśniak K, Palka K, Korona-Glowniak I, Franus W, Przekora A. Modification of Natural Clays with Magnetite to Provide Boosted Antimicrobial Properties and Chemopreventive Activity Against Melanoma. Materials. 2025; 18(20):4759. https://doi.org/10.3390/ma18204759
Chicago/Turabian StyleWójcik, Alicja, Jakub Matusiak, Marta Trzaskowska, Aleksandra Maciejczyk, Paulina Kazimierczak, Katarzyna Suśniak, Krzysztof Palka, Izabela Korona-Glowniak, Wojciech Franus, and Agata Przekora. 2025. "Modification of Natural Clays with Magnetite to Provide Boosted Antimicrobial Properties and Chemopreventive Activity Against Melanoma" Materials 18, no. 20: 4759. https://doi.org/10.3390/ma18204759
APA StyleWójcik, A., Matusiak, J., Trzaskowska, M., Maciejczyk, A., Kazimierczak, P., Suśniak, K., Palka, K., Korona-Glowniak, I., Franus, W., & Przekora, A. (2025). Modification of Natural Clays with Magnetite to Provide Boosted Antimicrobial Properties and Chemopreventive Activity Against Melanoma. Materials, 18(20), 4759. https://doi.org/10.3390/ma18204759











