Non-Invasive Rayleigh, Raman, and Chromium-Fluorescence Study of Phase Transitions: β-Alumina into γ-Alumina ‘Single’ Crystal and Then to α-Alumina
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Raman Spectroscopy
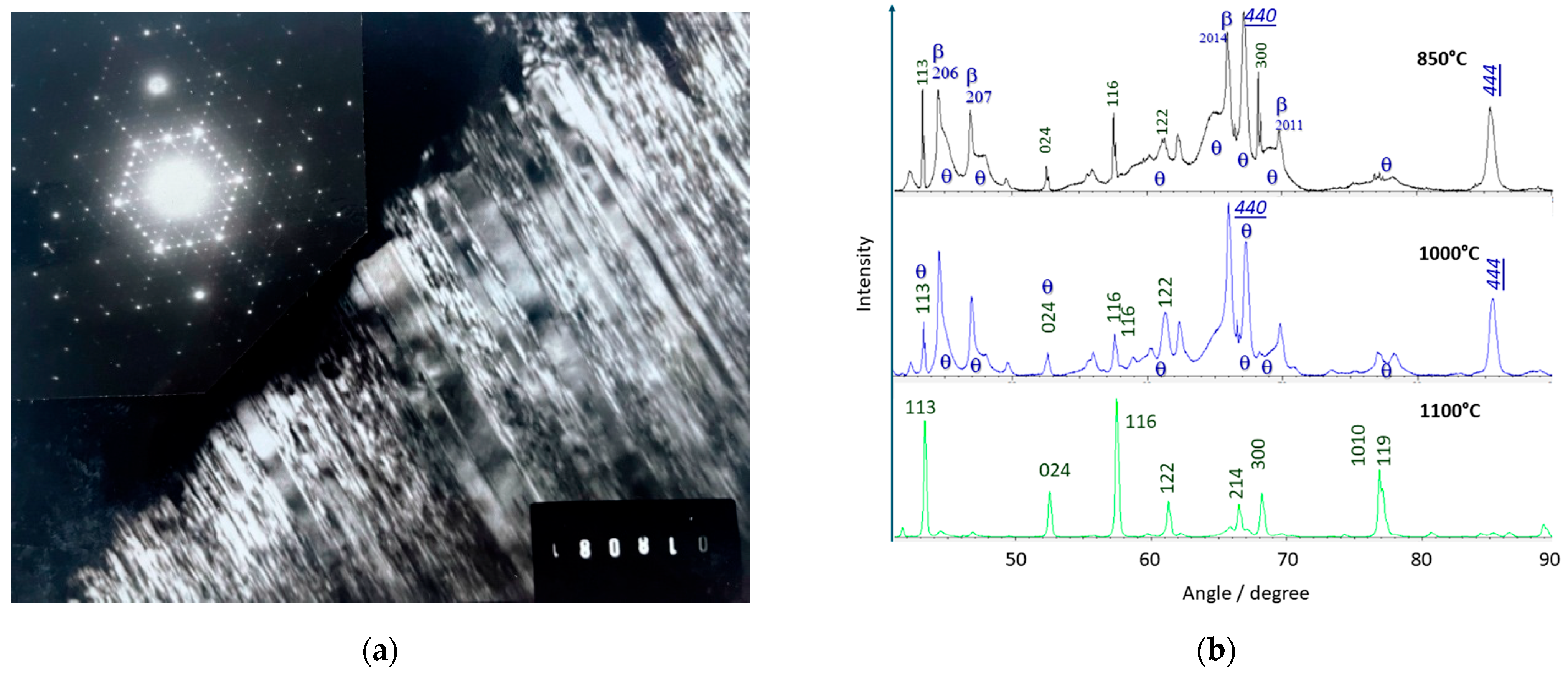
2.3. X-Ray Diffraction
3. Results
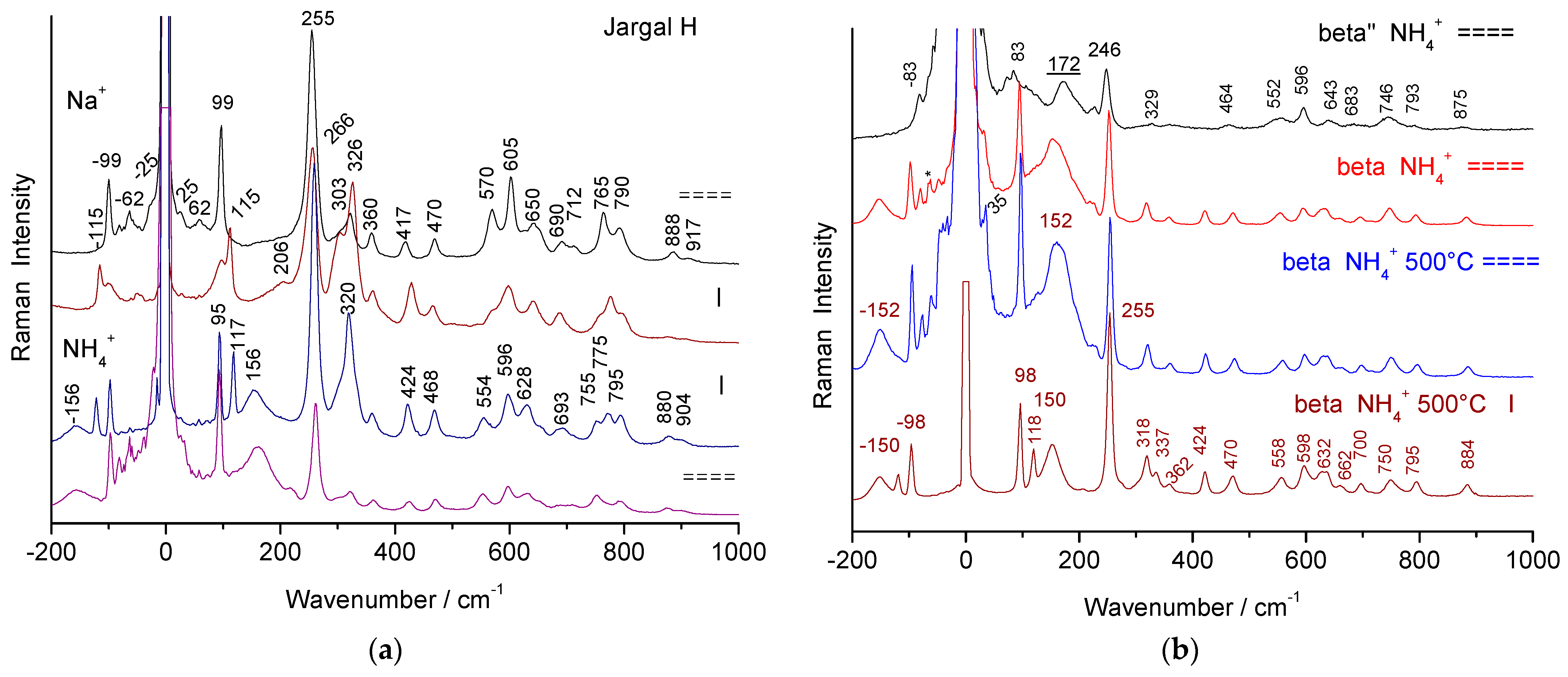
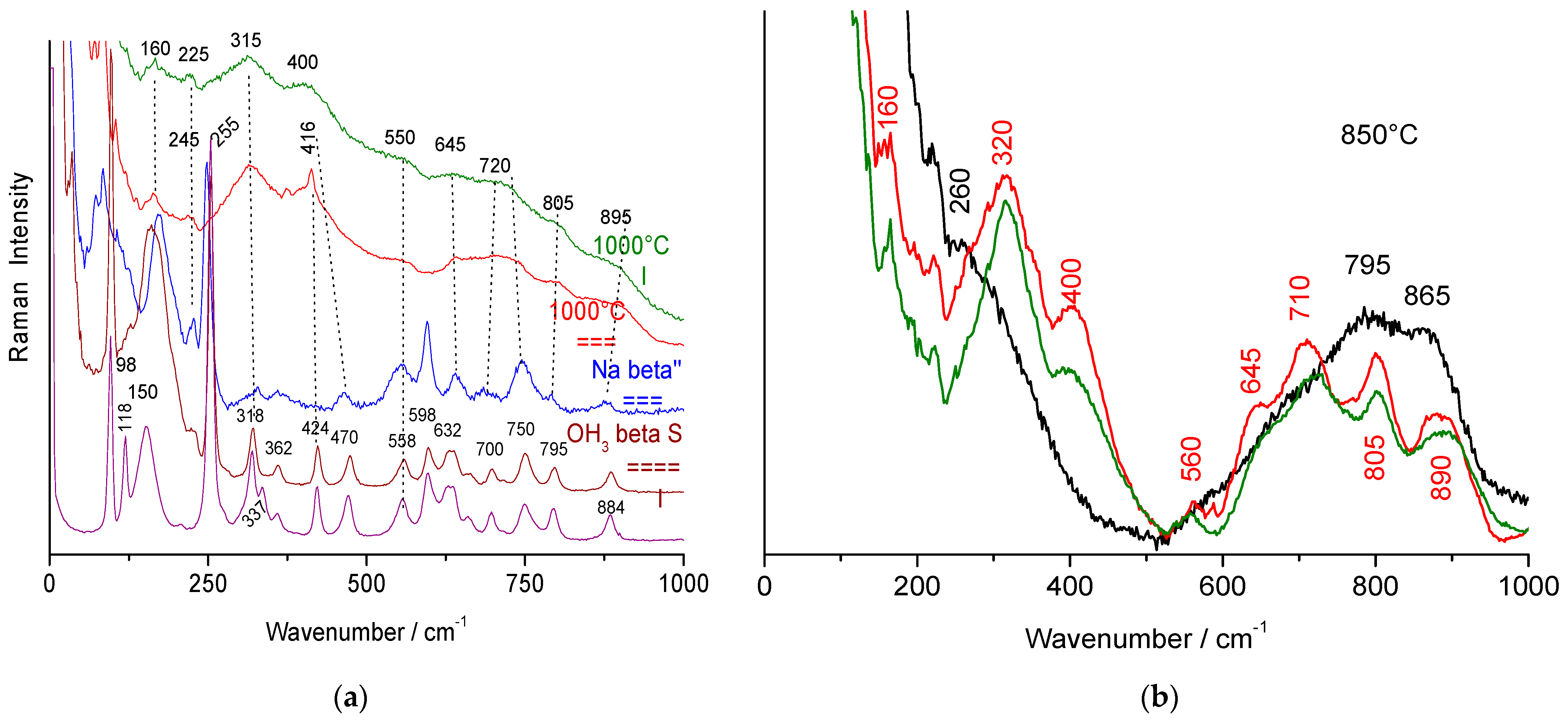
3.1. Raman Signatures of Thermally Treated Beta Alumina
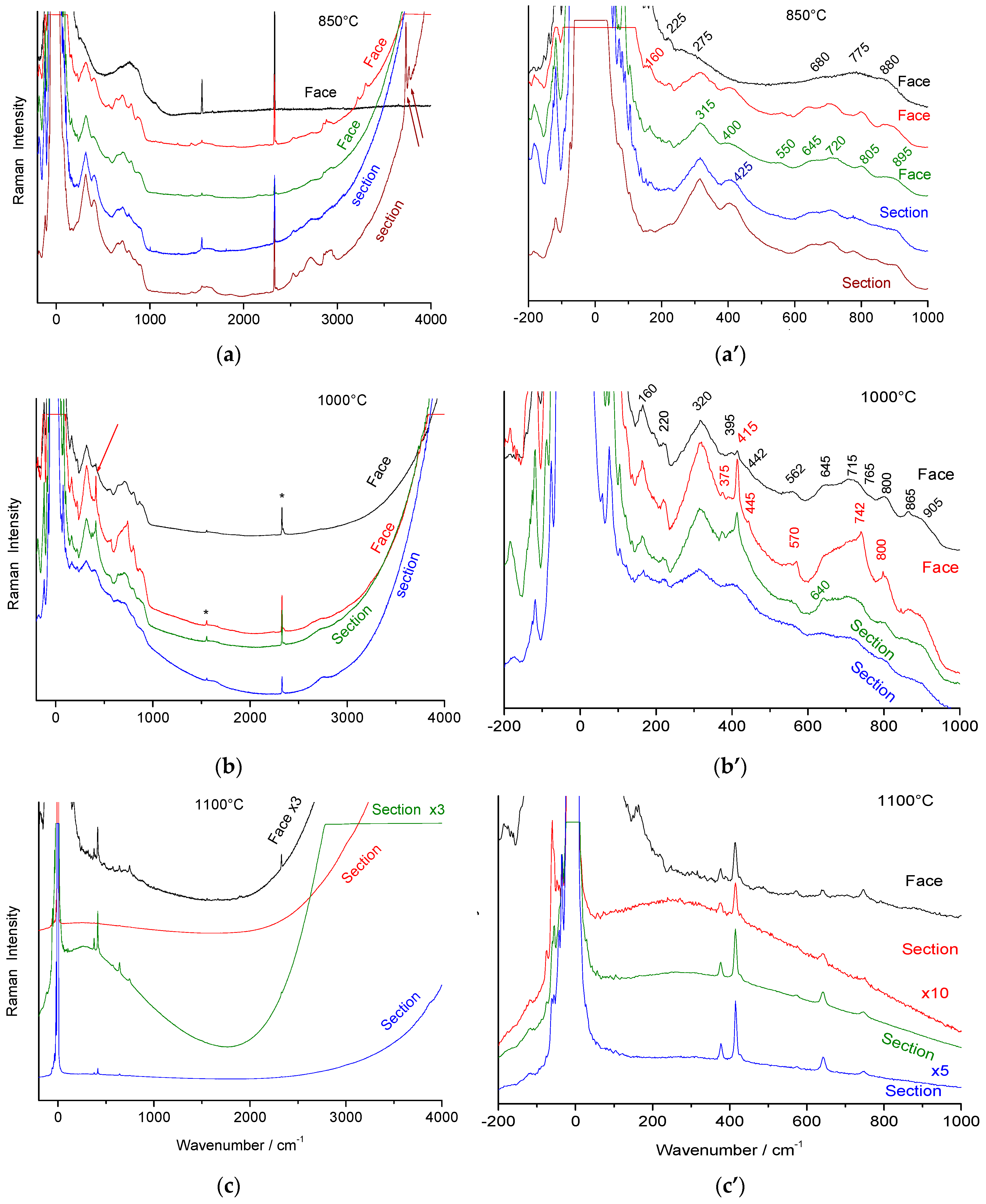
3.2. Raman Spectra Evolution with the Transition from Stoichiometric Beta Alumina to Transition Alumina
3.3. Comparison with Raman Spectra of Alumina Xerogel and Glass
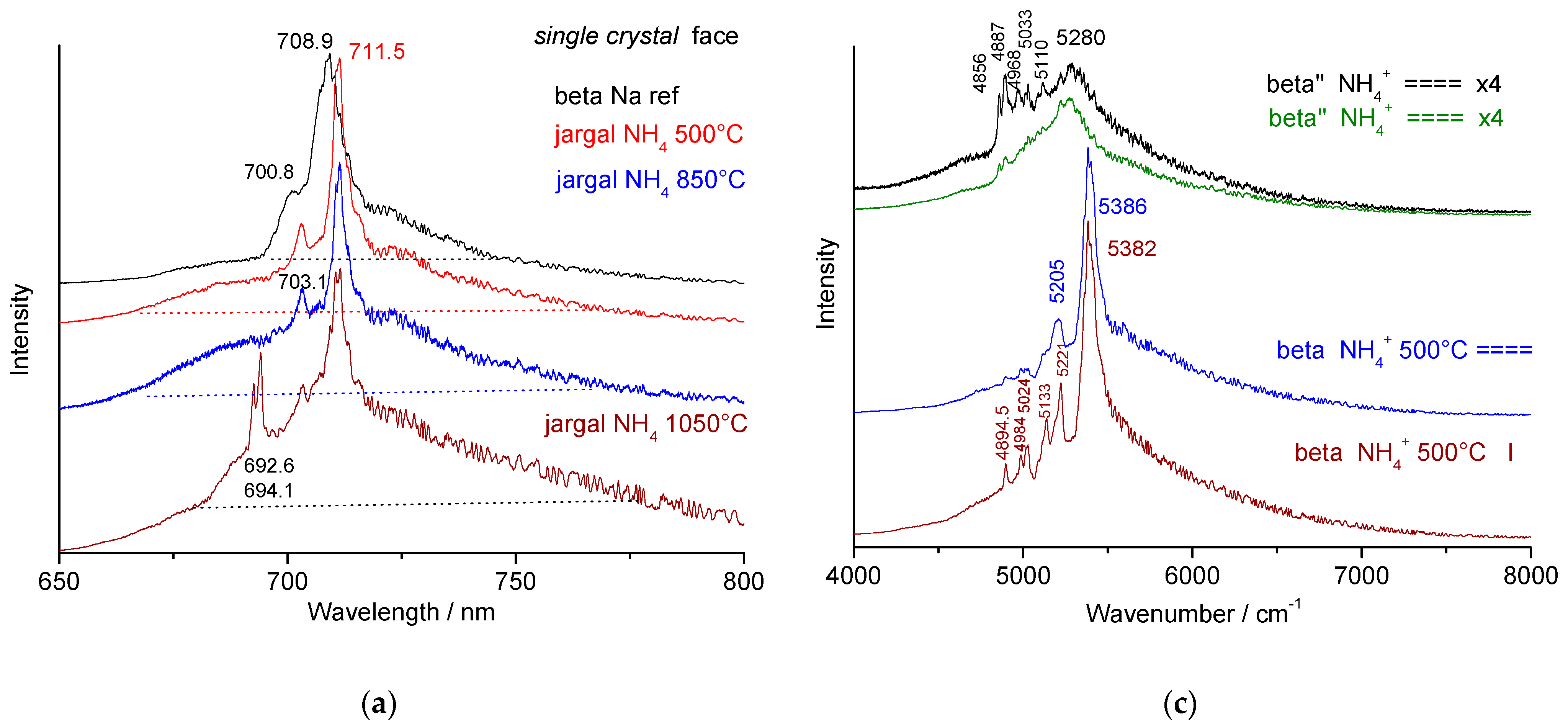
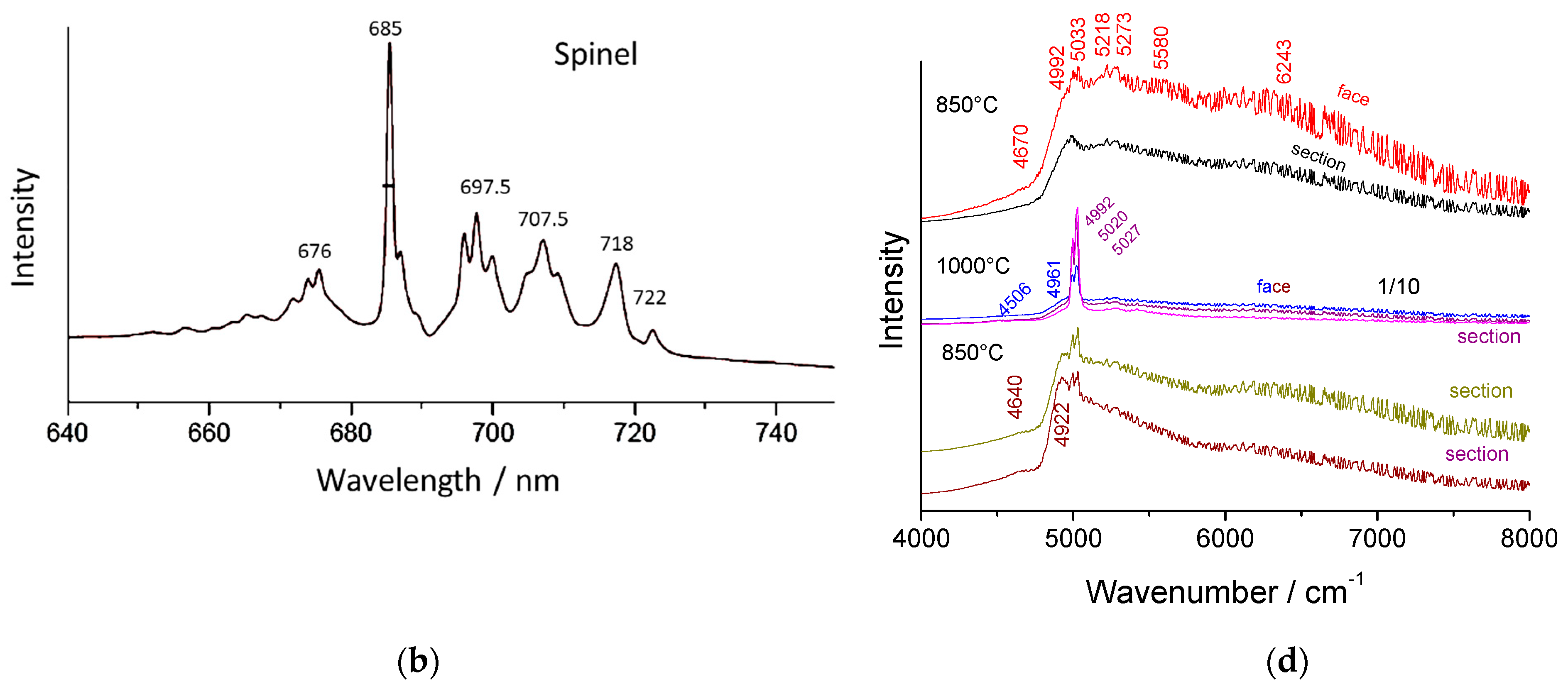
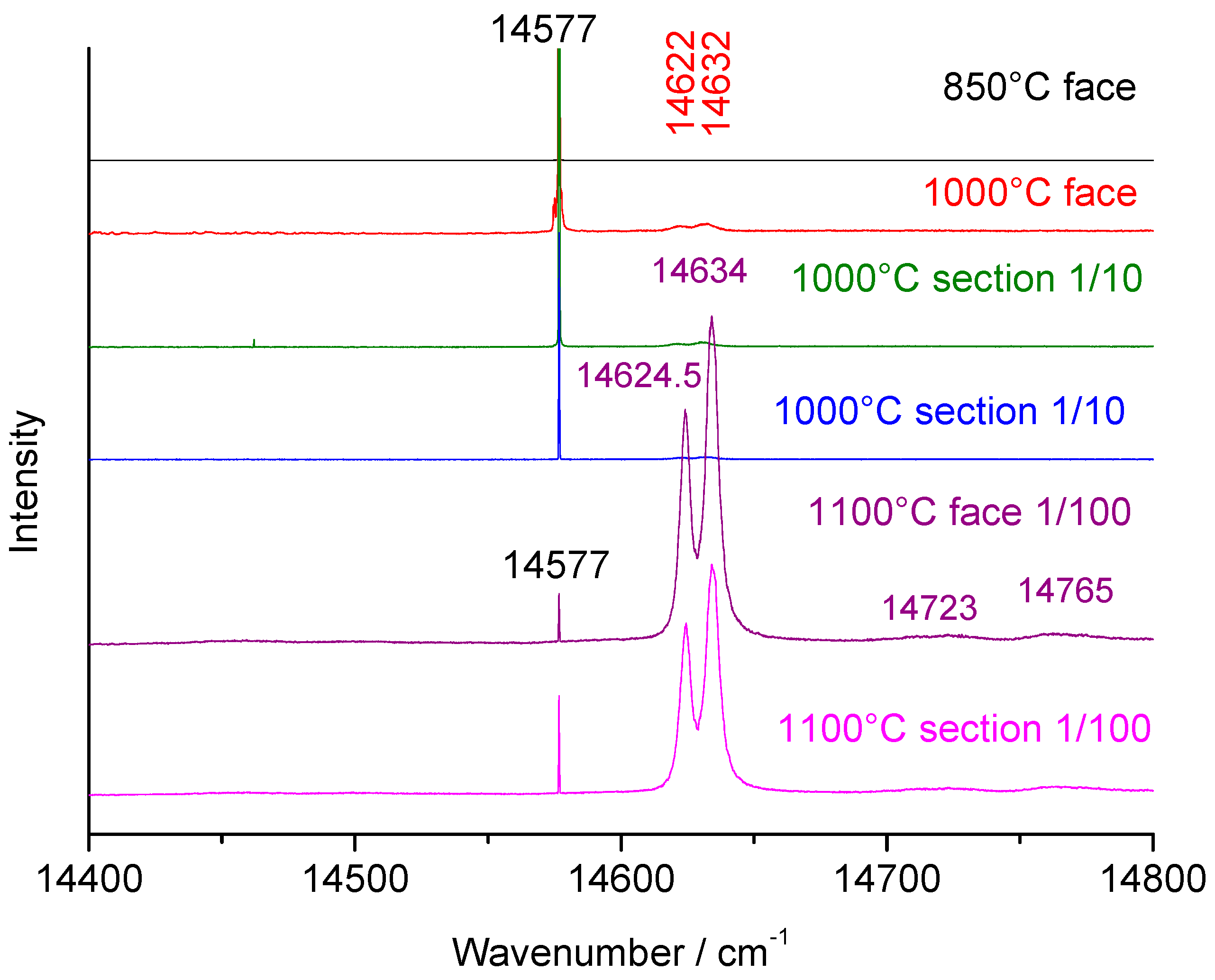
3.4. Fluorescence Signatures
- (i)
- (ii)
- A broader peak around 709 nm (5380 cm−1), characteristic of non-stoichiometric (NS) beta alumina, which shifts to 711.5 nm (5386 cm−1) for stoichiometric (S) beta alumina;
- (iii)
- Very broad bands with maxima at 706.3 nm (5280 cm−1) for ammonium beta″ alumina, 712.5 nm (5400 cm−1) for thermally treated ammonium beta alumina, and 692.7–694 nm (5000–5027 cm−1) for alumina thermally treated at 1150 °C and above.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porto, S.P.S.; Krishnan, R.S. Raman effect of corundum. J. Chem. Phys. 1967, 47, 1009–1012. [Google Scholar] [CrossRef]
- Colomban, P. Structure of oxide gels and glasses by infrared and Raman scattering: Part 1 Alumina. J. Mater. Sci. 1989, 24, 3002–3010. [Google Scholar] [CrossRef]
- Colomban, P.; Lucazeau, G. Vibrational study of and conduction mechanism in β alumina. I. Stoichiometric β alumina. J. Chem. Phys. 1980, 72, 1213–1224. [Google Scholar] [CrossRef]
- Colomban, P.H. Raman study of the formation of transition alumina single crystal from protonic β/β″ aluminas. J. Mater. Sci. Lett. 1988, 7, 1324–1326. [Google Scholar] [CrossRef]
- Remush, D.; Grimsditch, M.; Jorgensen, J.D.; Hodges, J.P. Pressure Dependence of Cr3+ Fluorescence in θ-Alumina. Oxid. Met. 2001, 56, 299–311. [Google Scholar] [CrossRef]
- Wen, Q.; Lipkin, D.M.; Clarke, D.R. Luminescence Characterization of Chromium-Containing theta-Alumina. J. Am. Ceram. Soc. 1998, 81, 3345–3348. [Google Scholar] [CrossRef]
- Clarke, D.R.; Christensen, R.J.; Tolpygo, V. The evolution of oxidation stresses in zirconia thermal barrier coated superalloy leading to spalling failure. Surf. Coat. Techn. 1997, 94–95, 89–93. [Google Scholar] [CrossRef]
- Singh, J.P.; Nair, B.G.; Renusch, D.P.; Sutaria, M.P.; Grimsditch, M.H. Damage evolution and stress analysis in zirconia thermal barrier coatings during cyclic and isothermal oxidation. J. Am. Ceram. Soc. 2001, 84, 2385–2393. [Google Scholar] [CrossRef]
- Bunsell, A.R.; Berger, M.H. Fine diameter ceramic fibres. J. Eur. Ceram. Soc. 2000, 20, 2249–2260. [Google Scholar] [CrossRef]
- Bunsell, A.R. Fibre Reinforcements for Composite Materials; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Schneibel, J.H.; George, E.P.; McKamey, C.G.; Ohriner, E.K.; Santella, M.L.; Carmichael, C.A. Fabrication and tensile properties of continuous-fiber reinforced Ni3Al–Al2O3 composites. J. Mater. Res. 1991, 6, 1673–1679. [Google Scholar] [CrossRef]
- Lavaste, V.; Berger, M.H.; Bunsell, A.R.; Besson, J. Microstructure and mechanical characteristics of alpha-alumina-based fibres. J. Mater. Sci. 1995, 30, 4215–4225. [Google Scholar] [CrossRef]
- Gouadec, G.; Karin, S.; Wu, J.; Parlier, M.; Colomban, P. Physical Chemistry and mechanical imaging of ceramic-fibre-reinforced ceramic-or metal-matrix composites. Compos. Sci. Technol. 2001, 61, 383–388. [Google Scholar] [CrossRef]
- Redonnet, J. Relation Entre Microstructure et Propriétés Mécaniques D’une Fibre D’alumine Continue en Développement. Ph.D. Thesis, Ecole des Mines—PSL Université, Versailles, France, 2025. [Google Scholar]
- Pezzotti, G.; Sbaizero, O.; Sergo, V.; Muraki, N.; Maruyama, K.; Nishida, T. In situ measurements of frictional bridging stresses in alumina using fluorescence spectroscopy. J. Am. Ceram. Soc. 1998, 81, 187–192. [Google Scholar] [CrossRef]
- Pezzotti, G. In situ study of fracture mechanisms in advanced ceramics using fluorescence and Raman microprobe spectroscopy. J. Raman Spectrosc. 1999, 30, 867–875. [Google Scholar] [CrossRef]
- Sinclair, R.; Young, R.J.; Martin, R.D.S. Determination of the axial and radial fibre stress distributions for the Broutman test. Compos. Sci. Technol. 2004, 64, 181–189. [Google Scholar] [CrossRef]
- Gouadec, G.; Colomban, P.; Piquet, N.; Trichet, M.F.; Mazerolles, L. Raman/Cr3+ fluorescence mapping of a melt-grown Al2O3/GdAlO3 eutectic. J. Eur. Ceram. Soc. 2005, 25, 1447–1453. [Google Scholar] [CrossRef]
- Jayaraman, A. Diamond anvil cell and high-pressure physical investigations. Rev. Mod. Phys. 1983, 55, 65–108. [Google Scholar] [CrossRef]
- Vos, W.L.; Schouten, J.A. On the temperature correction to the ruby pressure scale. J. Appl. Phys. 1991, 69, 6744–6746. [Google Scholar] [CrossRef]
- Colomban, P.; Boilot, J.-P.; Kahn, A.; Lucazeau, G. Structural Investigation of Protonic Conductor NH4+ Beta Alumina and Stoichiometric H3O+ beta Alumina. Nouv. J. Chim. 1978, 2, 21–32. [Google Scholar]
- Colomban, P. Structure of oxide gels and glasses by infrared and Raman scattering: Part 2 Mullites. J. Mater. Sci. 1989, 24, 3011–3020. [Google Scholar] [CrossRef]
- Colomban, P. Contribution à L’étude des Mécanismes de Conductivité dans les Composes de Type β et β’’ Al2O3. Ph.D. Thesis, Université Pierre-et-Marie Curie, Paris, France, 1979. [Google Scholar]
- Collin, G.; Boilot, J.P.; Colomban, P.; Comes, R. Host lattices and superionic properties in β-and β’’-alumina. I. Structures and local correlations. Phys. Rev. B 1986, 34, 5838–5849. [Google Scholar] [CrossRef]
- Bragg, W.L.; Gottfried, C.; West, J. The structure of β alumina. Zeitschr. Krist.-Cryst. Mater. 1931, 77, 255–274. [Google Scholar] [CrossRef]
- Hao, C.H.; Chase, L.L.; Mahan, G.D. Raman scattering in β−alumina. Phys. Rev. B 1976, 13, 4306–4313. [Google Scholar] [CrossRef]
- Frech, R.; Bates, J.B. Raman, ir reflection, and emission spectra of sodium β-alumina. Spectrochim. Acta Part A Mol. Spectrosc. 1979, 35, 685–694. [Google Scholar] [CrossRef]
- Bates, J.B. Raman scattering from NH4+ and ND4+ in beta-alumina. J. Chem. Phys. 1980, 73, 1503–1513. [Google Scholar] [CrossRef]
- Hayes, W.; Holden, L.; Tofield, B.C. Infrared studies of hydrogen beta alumina. J. Phys. C Solid State Phys. 1980, 13, 4217–4228. [Google Scholar] [CrossRef]
- Colomban, P.; Fillaux, F.; Tomkinson, J.; Kearley, G.J. Inelastic neutron-scattering study of proton dynamics in β-alumina. Solid State Ion. 1995, 77, 45–50. [Google Scholar] [CrossRef]
- McWhan, D.B.; Shapiro, S.M.; Remeika, J.P.; Shirane, G. Neutron-scattering studies on beta-alumina. J. Phys. C Solid State Phys. 1975, 8, L487–L491. [Google Scholar] [CrossRef]
- Colomban, P. Vibrational study of hydrogen beta alumina. J. Phys. C Solid State Phys. 1981, 14, 4325–4333. [Google Scholar] [CrossRef]
- Lassègues, J.C.; Fouassier, M.; Baffier, N.; Colomban, P.; Dianoux, A.J. Neutron scattering study of the proton dynamics in NH+ 4 and OH+ 3 β alumina. J. Phys. 1980, 41, 273–280. [Google Scholar] [CrossRef]
- Lucazeau, G. Infrared, Raman and neutron scattering studies of β-and β″-alumina: A static and dynamical structure analysis. Solid State Ion. 1983, 8, 1–25. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G.; Bougeard, D. Vibrational and normal-mode analysis of stoichiometric β-Al2O3. Solid State Ion. 1983, 11, 1–18. [Google Scholar] [CrossRef]
- Łodziana, Z.; Parliński, K. Dynamical stability of the α and θ phases of alumina. Phys. Rev. B 2003, 67, 174106. [Google Scholar] [CrossRef]
- Colomban, P.; Vivien, D. EPR study of ordering in Stoichiometric β-aluminate. Phys. Stat. Sol. (a) 1983, 76, 565–574. [Google Scholar] [CrossRef]
- Smrčok, Ľ.; Vratislav, L.; Křesťan, J. γ-Alumina: A single-crystal X-ray diffraction study. Cryst. Struct. Commun. 2006, 62, i83–i84. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data. Available online: https://www.icdd.com (accessed on 15 March 2025).
- Cvejic, Z.; Rakic, S.; Kremenovic, A.; Antic, B.; Jovalekic, C.; Colomban, P. Nanosize ferrites obtained by ball milling: Crystal structure, cation distribution, size-strain analysis and Raman investigations. Solid State Sci. 2006, 8, 908–915. [Google Scholar] [CrossRef]
- Koenig, J.L. Raman scattering of synthetic polymers—A review. Appl. Spectrosc. Rev. 1971, 4, 233–305. [Google Scholar] [CrossRef]
- Colomban, P. (Ed.) Proton Conductors: Solids, Membranes and Gels-Materials and Devices; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar] [CrossRef]
- Colomban, P. Proton and protonic species: The hidden face of solid-state chemistry. How to measure H-content in materials? Fuel Cells 2013, 13, 6–18. [Google Scholar] [CrossRef]
- Tournié, A.; Ricciardi, P.; Colomban, P. Glass corrosion mechanisms: A multiscale analysis. Solid State Ion. 2008, 179, 2142–2154. [Google Scholar] [CrossRef]
- Colomban, P. Vibrational characterization of the various forms of (solvated or unsolvated) mobile proton in the solid state. Advantages, limitations and open questions. Solid State Ion. 2023, 393, 116187. [Google Scholar] [CrossRef]
- Vendange, V.; Colomban, P. Determination of the hydroxyl content in gels and porous “glasses” from alkoxide hydrolysis by combined TGA and BET analysis. J. Porous Mater. 1996, 3, 193–200. [Google Scholar] [CrossRef]
- Colomban, P.; Vendange, V. Sintering of alumina and mullites prepared by slow hydrolysis of alkoxides: The role of the protonic species and pore topology. J. Non-Crystall. Solids 1992, 147–148, 245–250. [Google Scholar] [CrossRef]
- Doss, C.J.; Zallen, R. Raman studies of sol-gel alumina: Finite-size effects in nanocrystalline AlO(OH). Phys. Rev. B 1993, 48, 15626–15637. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Thyagarajan, G. Intensity studies in Raman effect: Part II. On the wing accompanying the Rayleigh line in liquids and liquid mixtures. Z. Phys. 1959, 154, 81–89. [Google Scholar] [CrossRef]
- Perrot, M.; Brooker, M.H.; Lascombe, J. Raman light scattering studies of the depolarized Rayleigh wing of liquids and solutions. J. Chem. Phys. 1981, 74, 2787–2794. [Google Scholar] [CrossRef]
- Gochiyaev, V.Z.; Malinovsky, V.K.; Novikov, V.N.; Sokolov, A.P. Structure of the Rayleigh line wing in highly viscous Iiquids. Philos. Mag. B 1991, 63, 777–787. [Google Scholar] [CrossRef]
- Cortie, D.L.; Cyster, M.J.; Ablott, T.A.; Richardson, C.; Smith, J.S.; Iles, G.N.; Wang, X.L.; Mitchell, D.R.G.; Mole, R.A.; de Souza, N.R.; et al. Boson peak in ultrathin alumina layers investigated with neutron spectroscopy. Phys. Rev. Res. 2020, 2, 023320. [Google Scholar] [CrossRef]
- Roy, A.; Sood, A.K. Phonons and fractons in sol-gel alumina: Raman study. Pramana 1995, 44, 201–209. [Google Scholar] [CrossRef]
- Malinovsky, V.K.; Sokolov, A.P. The nature of boson peak in Raman scattering in glasses. Solid State Commun. 1986, 57, 757–761. [Google Scholar] [CrossRef]
- Schroeder, J.; Wu, W.; Apkarian, J.L.; Lee, M.; Hwa, L.G.; Moynihan, C.T. Raman scattering and Boson peaks in glasses: Temperature and pressure effects. J. Non-Crystall. Solids 2004, 349, 88–97. [Google Scholar] [CrossRef]
- Macêdo, M.I.F.; Bertran, C.A.; Osawa, C.C. Kinetics of the γ → α-alumina phase transformation by quantitative X-ray diffraction. J. Mater. Sci. 2007, 42, 2830–2836. [Google Scholar] [CrossRef]
- Santos, P.S.; Santos, H.S.; Toledo, S.P.D. Standard transition aluminas. Electron microscopy studies. Mater. Res. 2000, 3, 104–114. [Google Scholar] [CrossRef]
- Krishnan, R.S. Raman spectrum of alumina and the luminescence of ruby. Proc. Indian Acad. Sci.—Sect. A 1947, 26, 450. [Google Scholar] [CrossRef]
- Zvonarev, S.V.; Smirnov, N.O. Luminescence quenching in magnesium-doped alumina ceramics. Phys. Solid State 2019, 61, 835–839. [Google Scholar] [CrossRef]
- Choudhari, K.S.; Hebbar, D.; Kulkarni, S.D.; Santhosh, C.; George, S.D. Cr3+ doped nanoporous anodic alumina: Facile microwave assisted doping to realize nanoporous ruby and phase dependent photoluminescence. Ceram. Int. 2019, 45, 12130–12137. [Google Scholar] [CrossRef]
- Jankowiak, R.; Roberts, K.; Tomasik, P.; Sikora, M.; Small, G.J.; Schilling, C.H. Probing the crystalline environment of α-alumina via luminescence of metal ion impurities: An optical method of ceramic flaw detection. Mater. Sci. Engn. A 2000, 281, 45–55. [Google Scholar] [CrossRef]
- Redonnet, J.; Colomban, P.; Berger, M.H.; Joannès, S.; Francy, O. (to be submitted in J. Eur. Cer. Soc., 2026).
- Colomban, P.; Hsieh, J.; Shi, C.-F. (to be submitted in Ceramics, 2026).
- Frankberg, E.J.; Lambai, A.; Zhang, J.; Kalikka, J.; Khakalo, S.; Paladino, B.; Cabrioli, M.; Mathews, N.G.; Salminen, T.; Hokka, M.; et al. Exceptional microscale plasticity in amorphous aluminum oxide at room temperature. Adv. Mater. 2023, 35, e2303142. [Google Scholar] [CrossRef]
- Cocke, D.L.; Johnson, E.D.; Merrill, R.P. Planar models for alumina-based catalysts. Catal. Rev. Sci. Engn. 1984, 26, 163–231. [Google Scholar] [CrossRef]
- Rashkeev, S.N.; Sohlberg, K.; Glazoff, M.V.; Novak, J.; Pennycook, S.J.; Pantelides, S.T. Transition metal atoms on different alumina phases: The role of subsurface sites on catalytic activity. Phys. Rev. B 2003, 67, 115414. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 17, 3393–3403. [Google Scholar] [CrossRef]
- Wachs, I.E.; Roberts, C.A. Monitoring surface metal oxide catalytic active sites with Raman spectroscopy. Chem. Soc. Rev. 2010, 39, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, L.; Bowden, M.; Szanyi, J. High temperature transition aluminas in δ-Al2O3/θ-Al2O3 stability range. J. Catal. 2021, 393, 357–368. [Google Scholar] [CrossRef]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Miao, C.; Wang, R.; Zhang, R.; Li, X.; Wang, J.; Wang, X.; Yao, J. Advances in morphology-controlled alumina and its supported Pd catalysts: Synthesis and applications. Chem. Soc. Rev. 2024, 53, 5014–5053. [Google Scholar] [CrossRef] [PubMed]
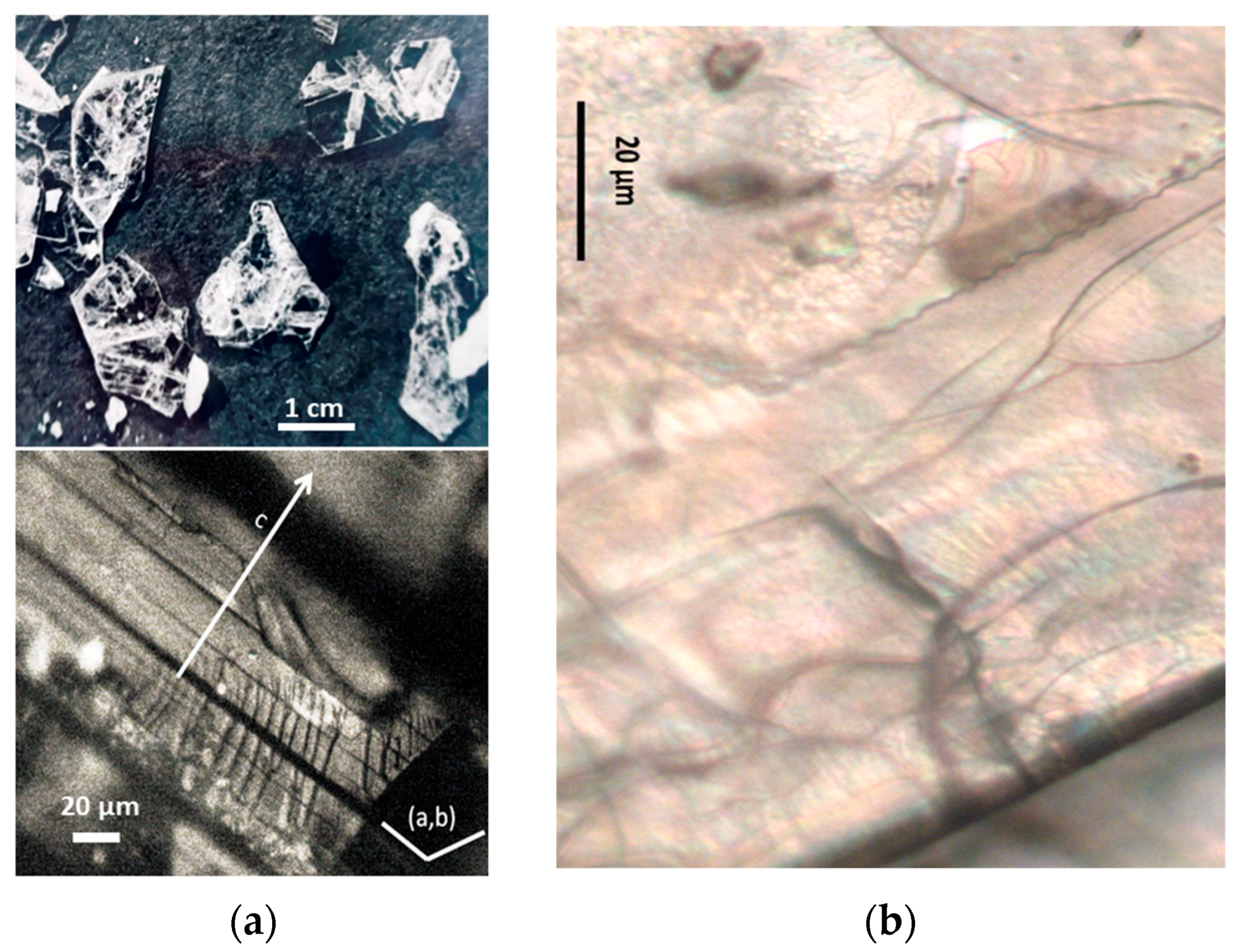


| Samples | View | Synthesis | Thermal Treatments | Remarks (Composition) | Refs. |
|---|---|---|---|---|---|
| Na beta alumina single crystal |  | Cooling from the melt | no | Non-stoichiometric (11 Al2O3 1.3 Na2O) | [22,23] |
| Na beta″ alumina single crystal |  | Cooling from the melt | no | Non-stoichiometric (11 Al2O3 1.6 Na2O) | [22,23] |
| NH4 beta alumina single crystal |  | Ion exchange | ~180 °C | Non-stoichiometric (11 Al2O3 1.3 (NH4)2O) | [22,23] |
| NH4 beta″ alumina single crystal |  | Ion exchange | ~180 °C | Non-stoichiometric (11 Al2O3 1.3 (NH4)2O) | [22,23] |
| NH4 beta alumina single crystal |  | Ion exchange + thermal treatment | 500 °C | Stoichiometric (11 Al2O3 (OH3)2O) | [22,23] |
| OH3 beta alumina crystal |  | Ion exchange + thermal treatment | 750 °C | Phase transformation (Al2O3 (Hn)) | [3,21,23] |
| H+ transition alumina |  | Ion exchange + thermal treatment | 850 °C | Transition Alumina (Al2O3 (Hn)) | |
| Alpha/transition alumina |  | Ion exchange + thermal treatment | 1000 °C | (Al2O3) | |
| Alumina xerogel |  | Very slow hydrolysis-polycondensation + thermal treatment | 700 °C | Optically clear monolith (Al2O2.5(OH)0.9, 0.4H2O) | [2,23] |
| Alumina glass |  | Very slow hydrolysis-polycondensation + thermal treatment | 1000 °C | Phase transformation (A12O2.8(OH)0.35, 0.3H2O) | [2,23] |
| Alumina (nanocrystalline) |  | Very slow hydrolysis-polycondensation + thermal treatment | 1100 °C | A12O3 | [2,23] |
| Phase (Characteristics) | T | Phase (Characteristics) | T | Phase (Characteristics) | T | Phase (Characteristic) | T | Phase (Characteristic) |
|---|---|---|---|---|---|---|---|---|
| Chemically prepared ‘gel’ (Rayleigh wing; CHn modes, O-H modes) | <800 °C | Microporous protonated glassy alumina (Boson peak; strong O-H modes) | 800 °C –900 °C | Deprotonated transition alumina (Rayleigh wing; weak O-H modes) | 900–1000 °C | Transition alumina Nucleation of alpha alumina (alpha alumina peaks; disappearance of Rayleigh wing) | 1050 °C –1100 °C | Growth of alpha alumina at the expand of transition alumina (alpha alumina peaks, fluorescence background) |
| S beta alumina single crystal | 850 °C | Syntaxic formation of gamma and theta alumina single crystals | 1000 °C | Development of gamma alumina and nucleation of alpha alumina | 1100 °C | |||
| Beta Na | Beta’’ NH4 500 °C | Beta-1 NH4 500 °C | Beta-2 NH4 500 °C | J NH4 500 °C | Beta NH4 850 °C | J NH4 1050° | Beta NH4 1000 | A 1050 °C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm−1 | nm | cm−1 | nm | cm−1 | nm | cm−1 | nm | cm−1 | nm | cm−1 | nm | cm−1 | nm | cm−1 | nm | cm−1 | nm | cm−1 | nm | |
| 4294 | 660.4 | |||||||||||||||||||
| 4506 | 4499.8 | 669.5 | ||||||||||||||||||
| 4570.9 | 672.7 | |||||||||||||||||||
| 4643.5 | 676 | |||||||||||||||||||
| 4670 | 4665 | 677 | ||||||||||||||||||
| 4695 | 678.4 | |||||||||||||||||||
| 4856 | 685.8 | Θ | ||||||||||||||||||
| 4887 | 687.3 | 4894.5 | 687.7 | 4922 | ||||||||||||||||
| 4961 | ||||||||||||||||||||
| 4968 | 691.2 | |||||||||||||||||||
| 4984 | 691.9 | 4992 | 692.6 | 4992 | 5002 | 692.8 | 5002 | 692.8 | β | |||||||||||
| 5020 | ||||||||||||||||||||
| 5033 | 694.3 | 5024 | 693.8 | 5033 | 694.1 | 5027 | 5037 | 694.5 | 5035.5 | 694.4 | β | |||||||||
| 5110 | 698 | |||||||||||||||||||
| 5133 | 699.1 | |||||||||||||||||||
| 5205 | 702.7 | |||||||||||||||||||
| 5221 | 703.5 | 52,135 | 703.1 | 5218 | 5213.5 | 703.1 | 5211.6 | 703 | ||||||||||||
| 5166.9 | 700.8 | 5273 | β NS | |||||||||||||||||
| 5330 | 708.9 | β NS | ||||||||||||||||||
| 5280 | 706.4 | β″ NS | ||||||||||||||||||
| 5382 | 711.5 | 5386 | 711.7 | 5381.5 | 711.5 | 53,815 | 711.5 | 5381.5 | 711.5 | β S | ||||||||||
| 5282.1 | 706.5 | |||||||||||||||||||
| 5411.1 | 713 | |||||||||||||||||||
| 5460.1 | 715.5 | |||||||||||||||||||
| 5545.5 | 720 | 5606 | 5545.5 | 5605 | 5545.5 | 5605 | 5545.5 | 720 | 5545.5 | 720 | ||||||||||
| 5580 | ||||||||||||||||||||
| 6243 | ||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redonnet, J.; Simsek-Franci, G.; Colomban, P. Non-Invasive Rayleigh, Raman, and Chromium-Fluorescence Study of Phase Transitions: β-Alumina into γ-Alumina ‘Single’ Crystal and Then to α-Alumina. Materials 2025, 18, 4682. https://doi.org/10.3390/ma18204682
Redonnet J, Simsek-Franci G, Colomban P. Non-Invasive Rayleigh, Raman, and Chromium-Fluorescence Study of Phase Transitions: β-Alumina into γ-Alumina ‘Single’ Crystal and Then to α-Alumina. Materials. 2025; 18(20):4682. https://doi.org/10.3390/ma18204682
Chicago/Turabian StyleRedonnet, Juliette, Gulsu Simsek-Franci, and Philippe Colomban. 2025. "Non-Invasive Rayleigh, Raman, and Chromium-Fluorescence Study of Phase Transitions: β-Alumina into γ-Alumina ‘Single’ Crystal and Then to α-Alumina" Materials 18, no. 20: 4682. https://doi.org/10.3390/ma18204682
APA StyleRedonnet, J., Simsek-Franci, G., & Colomban, P. (2025). Non-Invasive Rayleigh, Raman, and Chromium-Fluorescence Study of Phase Transitions: β-Alumina into γ-Alumina ‘Single’ Crystal and Then to α-Alumina. Materials, 18(20), 4682. https://doi.org/10.3390/ma18204682








