The Oxidation and Corrosion Resistance of AlCrNbSiTiN Multi-Principal Element Nitride Coatings
Abstract
1. Introduction
2. Experimental Details
2.1. Coatings Preparation
2.2. Coatings Characterization
3. Results and Discussion
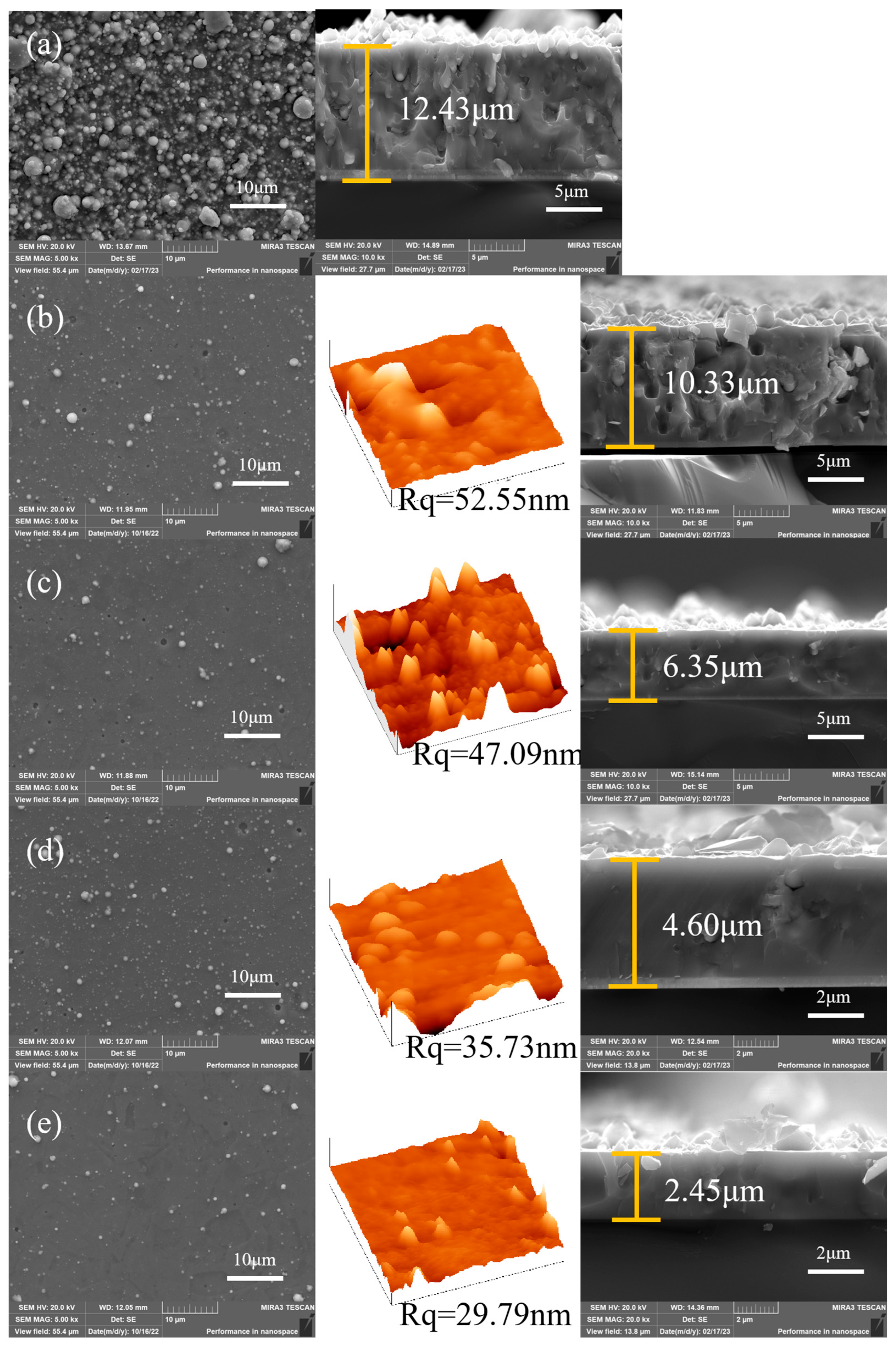
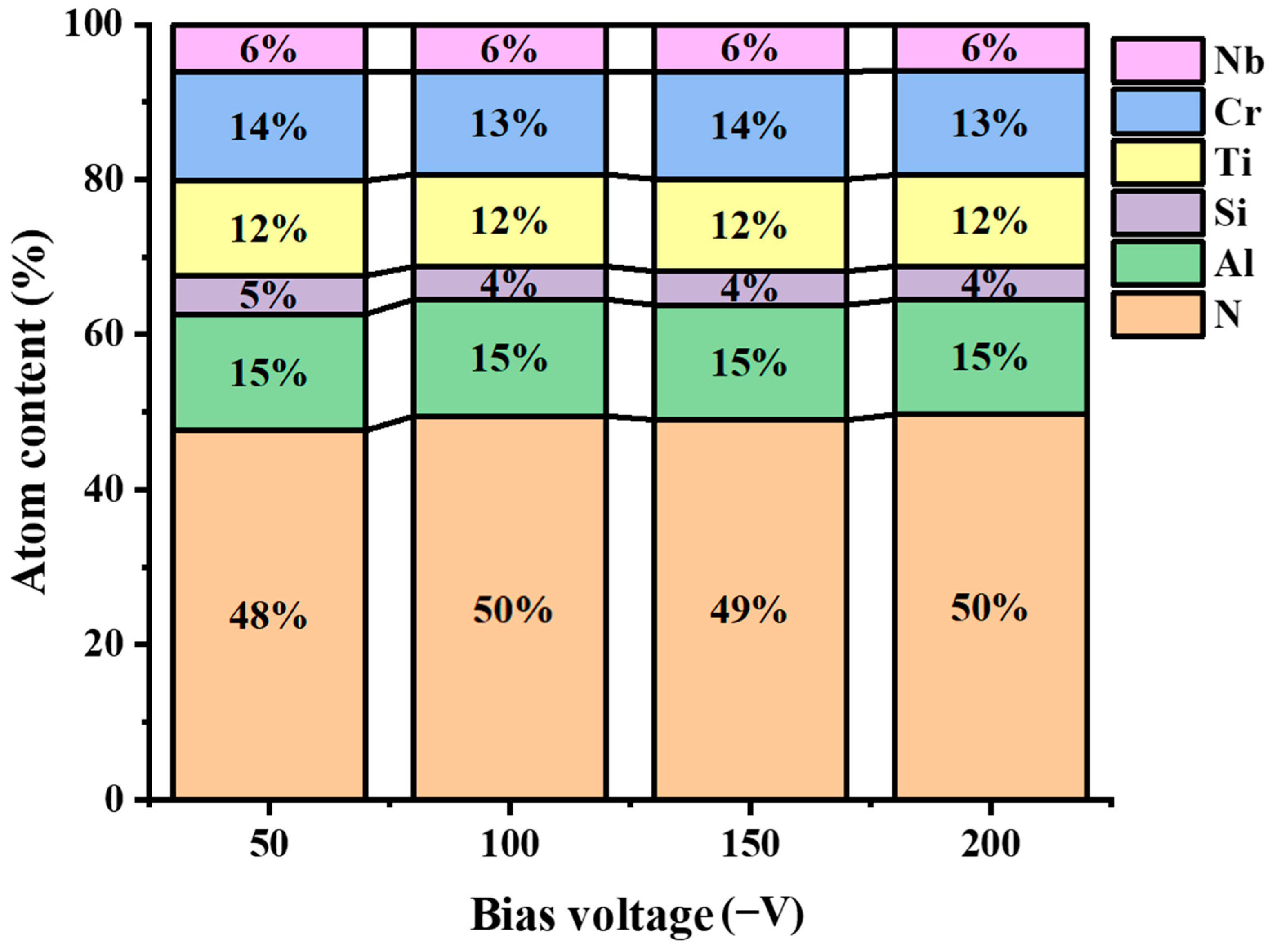
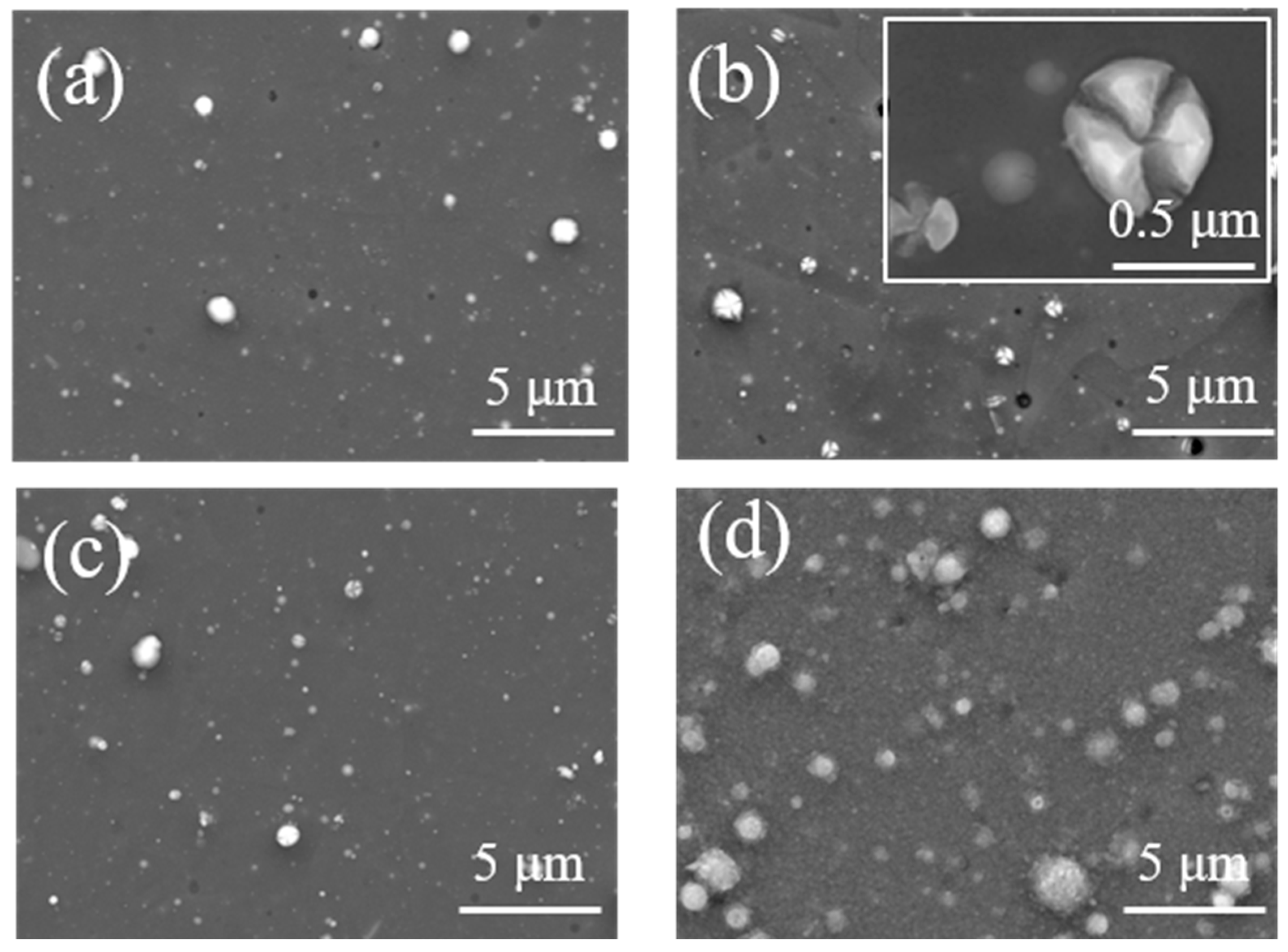
3.1. Morphology and Composition
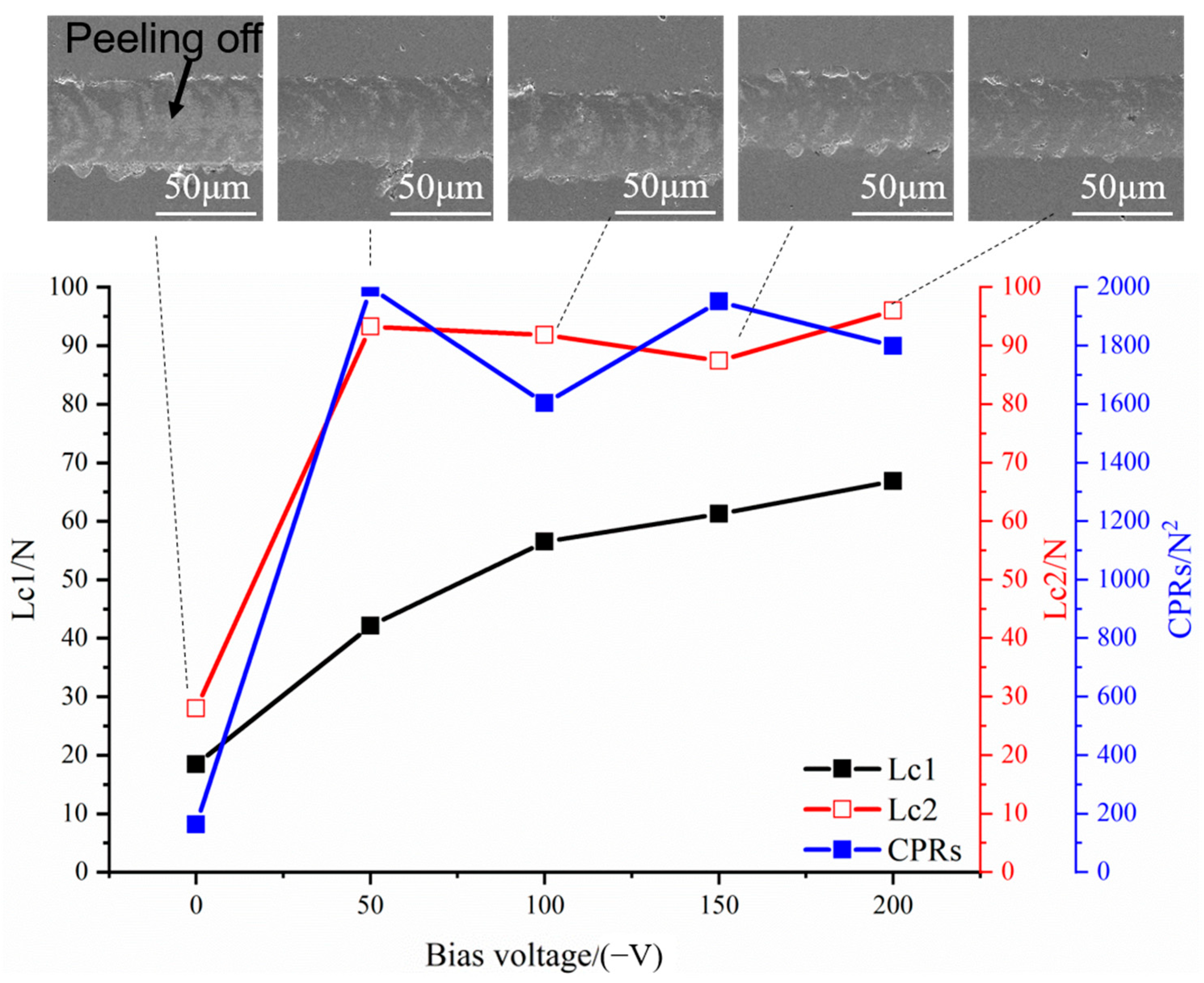
3.2. Mechanical and Tribological Properties
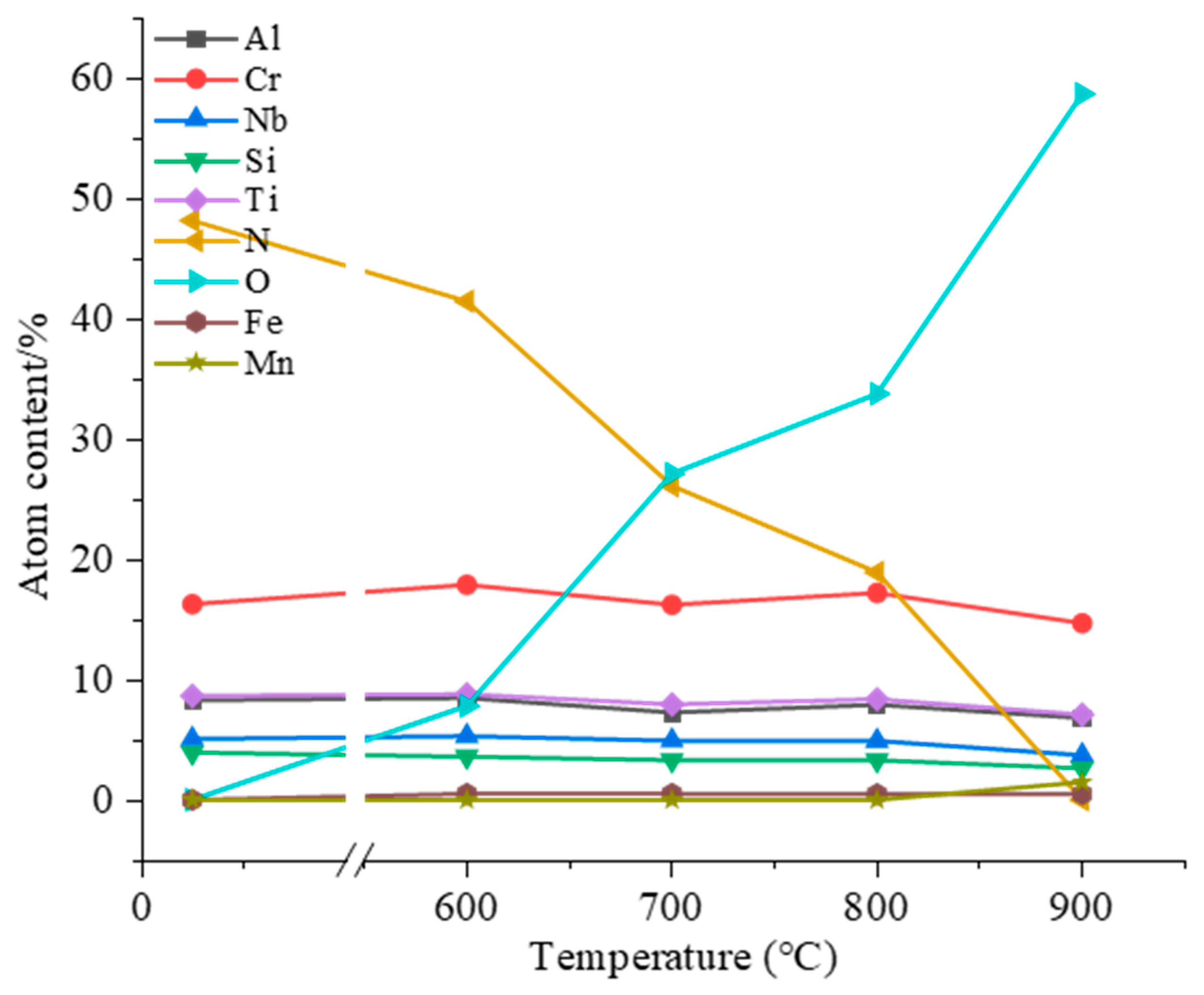
3.3. High-Temperature Oxidation Behavior
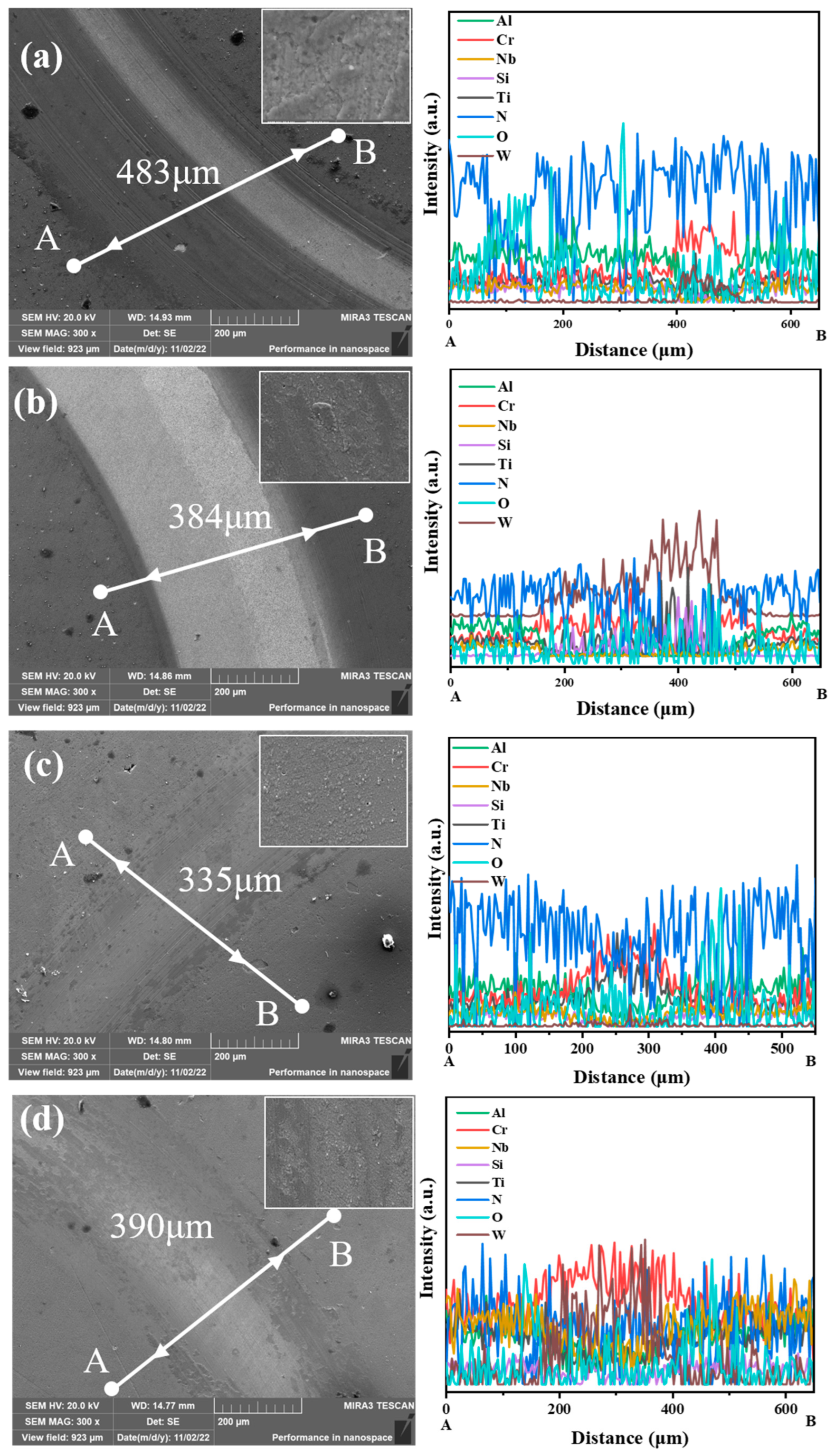
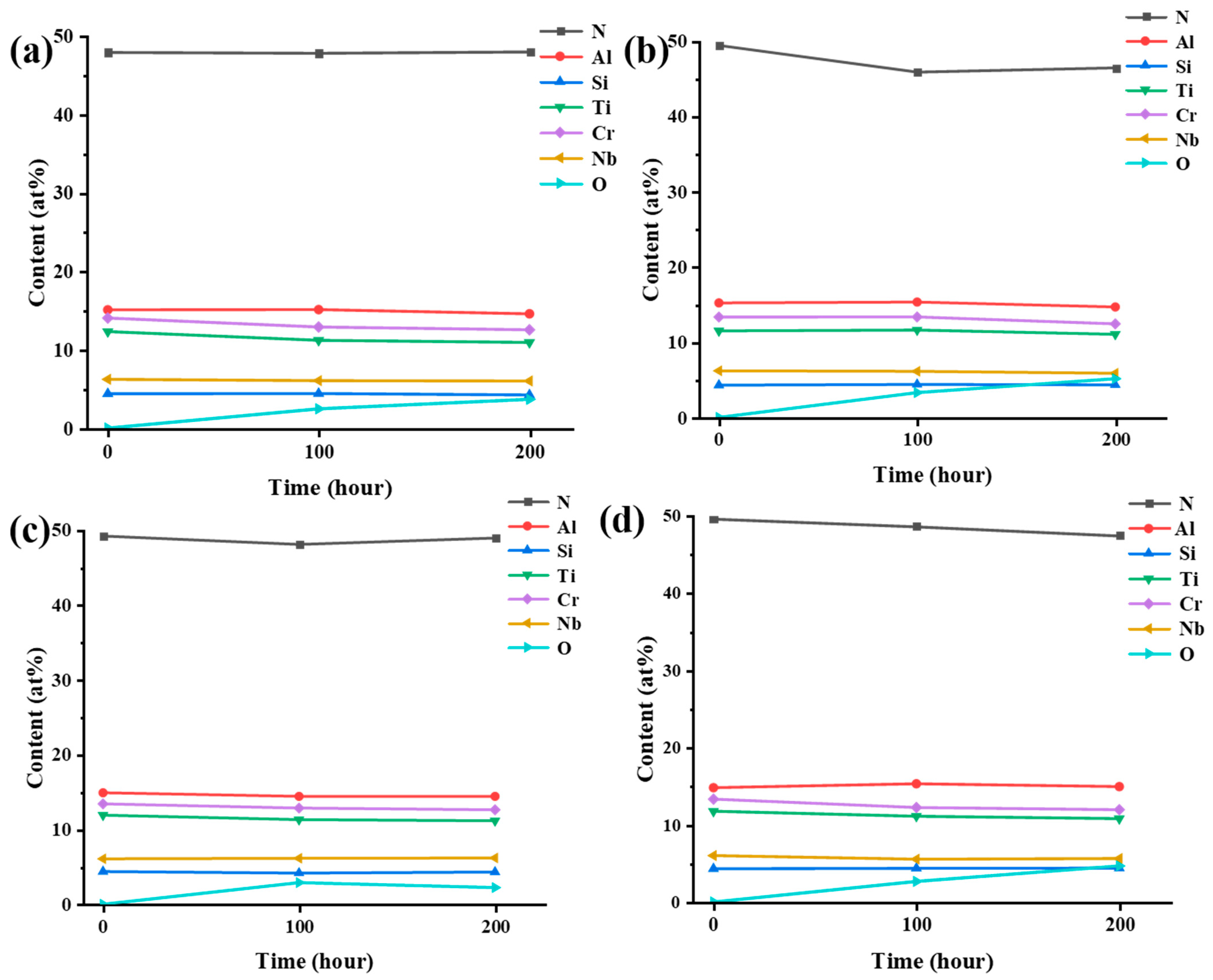
3.4. Water Vapor Corrosion Resistance
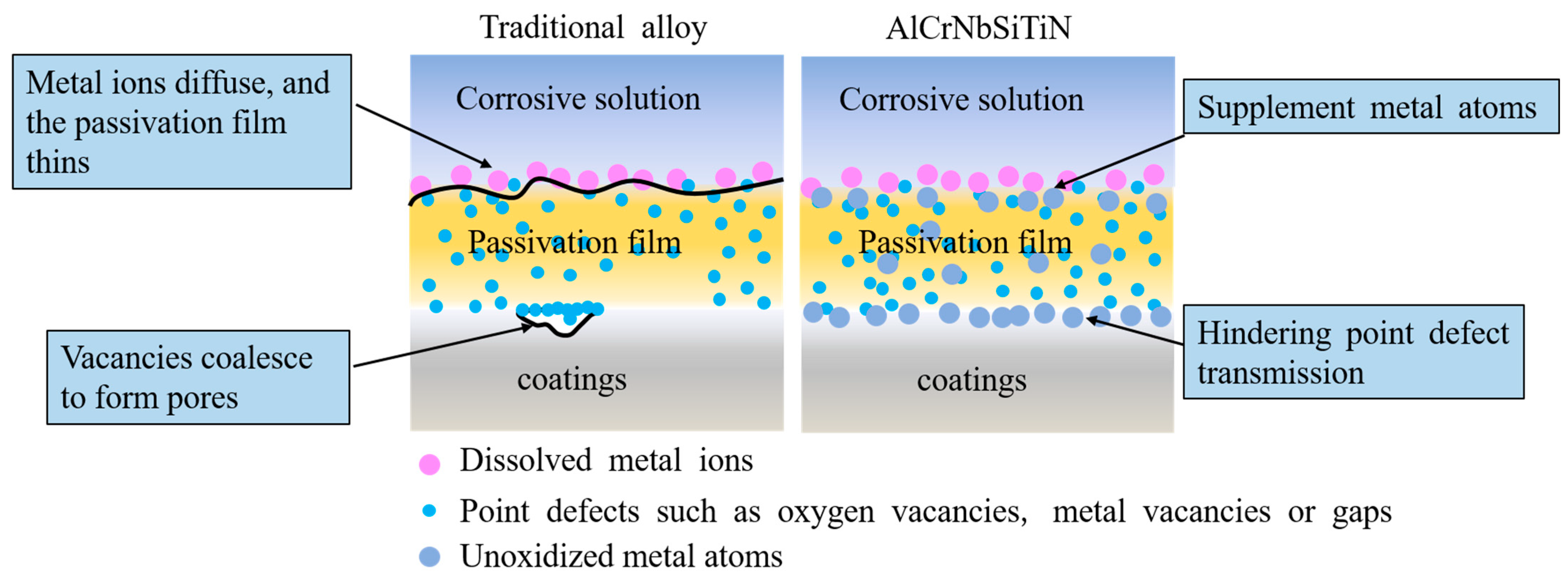
3.5. Electrochemical Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novikov, V.; Stepanov, N.; Zherebtsov, S.; Salishchev, G. Structure and Properties of High-Entropy Nitride Coatings. Metals 2022, 12, 847. [Google Scholar] [CrossRef]
- Derakhshandeh, M.; Eshraghi, M.; Razavi, M. Recent developments in the new generation of hard coatings applied on cemented carbide cutting tools. Int. J. Refract. Met. Hard Mater. 2023, 111, 106077. [Google Scholar] [CrossRef]
- Panjan, P.; Miletic, A.; Drnovsek, A.; Terek, P.; Cekada, M.; Kovacevic, L.; Panjan, M. Cracking Resistance of Selected PVD Hard Coatings. Coatings 2024, 14, 1452. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Liu, X.; Zheng, J.; Zhang, S. Tribocorrosion of CrN coatings on different steel substrates. Surf. Coat. Technol. 2024, 484, 130829. [Google Scholar] [CrossRef]
- Sala, N.; de Figueiredo, M.; Franz, R.; Kainz, C.; Sánchez-López, J.; Rojas, T.; de los Reyes, D.; Colominas, C.; Abad, M. Microstructural and mechanical properties of TiN/CrN and TiSiN/CrN multilayer coatings deposited in an industrial-scale HiPIMS system: Effect of the Si incorporation. Surf. Coat. Technol. 2024, 494, 131461. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Kenzhegulov, A.; Mamaeva, A.; Panichkin, A.; Kshibekova, B.; Alibekov, Z.; Fischer, D. Tribological Characteristics of Multilayer TiN/TiCN Coatings Compared to TiN Coatings. J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
- Tillmann, W.; Kokalj, D.; Stangier, D.; Fu, Q.; Kruis, F. Bias-voltage effect on the TiN nanoparticle injection into magnetron sputtered CrN thin films towards nc-TiN/nc-CrN composites. Appl. Surf. Sci. Adv. 2021, 6, 100149. [Google Scholar] [CrossRef]
- Baseri, N.; Mohammadi, M.; Ghatee, M.; Yousefieh, M.; Abassi-Firouzjah, M. The effect of substrate bias voltage on the mechanical and electrochemical corrosion properties of multilayered CrN/CrAlN coatings produced by cathodic arc evaporation. Int. J. Appl. Ceram. Technol. 2024, 21, 395–407. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Li, S.; Ai, S.; Liu, Z.; Wang, X.; Zhou, L.; Wu, T.; Bai, Y. Advancements in particle-reinforced high-entropy alloy coatings: Microstructure, mechanical properties and mechanisms. J. Alloys Compd. 2025, 1029, 180787. [Google Scholar] [CrossRef]
- Kumar, S. Comprehensive review on high entropy alloy-based coating. Surf. Coat. Technol. 2024, 477, 130327. [Google Scholar] [CrossRef]
- Lian, G.; Gao, W.; Chen, C.; Huang, X.; Feng, M. Review on hard particle reinforced laser cladding high-entropy alloy coatings. J. Mater. Res. Technol.-JMRT 2024, 33, 1366–1405. [Google Scholar] [CrossRef]
- Huang, Z.; Lang, W.; Chen, Y.; Yang, B.; Wan, Q. A Novel (AlCrNbTaTi)N Multilayer Hard High-Entropy Alloy Nitride Coating with Variable Aluminum Content Deposited by Cathodic Arc Ion Plating. Coatings 2025, 15, 76. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Y. High-Entropy Alloy Films. Coatings 2023, 13, 635. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Lyu, P.; Hua, Y.; Lu, J.; Cai, J. Effect of vacuum heat treatment on microstructure evolution and oxidation behavior of arc ion plated AlCoCrFeNiYHf high entropy alloy coatings. Mater. Charact. 2025, 227, 115211. [Google Scholar] [CrossRef]
- Guo, J.; Su, Y.; Zhang, C.; Cai, W.; Lin, S. Influence of nitrogen flow rate on the structure and properties of (AlTiVCrMoSi)Nx high-entropy alloy nitride coatings via arc ion plating. Surf. Coat. Technol. 2025, 496, 131732. [Google Scholar] [CrossRef]
- Cui, M.; Ding, X.; Lian, Y.; Wu, Y.; Jiao, J.; Cheng, Y.; Yang, J.; Zhang, J.; Sun, Y. Effect of nitrogen flow rates on the structural and properties of TiVCrNiSi (N) high entropy coating deposited by arc ion plating. Surf. Coat. Technol. 2025, 499, 131868. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Pelenovich, V.; Xu, Y.; Tan, K.; Hu, L.; Zeng, X.; Zeng, Z.; Lei, Y.; Chen, Y.; et al. Effects of duty cycle on microstructure and mechanical properties of (AlCrNbSiTi)N high-entropy nitride hard coatings deposited by pulsed arc ion plating. Vacuum 2024, 225, 113219. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Liu, Y.; Li, W.; Chen, Y.; Yang, B. Structure, Mechanical Properties and Water Vapor Corrosion Resistance of AlCrNbSiTiN High-Entropy Nitride Coatings Deposited by RF Magnetron Sputtering. Coatings 2024, 14, 1006. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Tsai, M.H.; Shen, W.J.; Yeh, J.W. Structure and properties of two Al-Cr-Nb-Si-Ti high-entropy nitride coatings. Surf. Coat. Technol. 2013, 221, 118–123. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Tu, M.; Hu, Y.; Wang, H.; Zhang, J.; Li, Z.; Zeng, X.; Wan, Q.; Vasiliy, P.; et al. Structure and mechanical properties of multi-principal-element (AlCrNbSiTi)N hard coating. Surf. Coat. Technol. 2022, 433, 128113. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic-modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Keidar, M.; Beilis, I.; Boxman, R.; Goldsmith, S. Nonstationary macroparticle charging in arc plasma-jet. In Proceedings of the XVI International Symposium on Discharges and Electrical Insulation in Vacuum, Moscow-St. Petersburg, Russian, 1 January 1994; pp. 140–145. [Google Scholar]
- Keidar, M.; Aharonov, R.; Beilis, I. Influence of an electrical field on the macroparticle size distribution in a vacuum arc. J. Vac. Sci. Technol. A-Vac. Surf. Film. 1999, 17, 3067–3073. [Google Scholar] [CrossRef]
- Aouadi, K.; Tlili, B.; Nouveau, C.; Besnard, A.; Chafra, M.; Souli, R. Influence of Substrate Bias Voltage on Corrosion and Wear Behavior of Physical Vapor Deposition CrN Coatings. J. Mater. Eng. Perform. 2019, 28, 2881–2891. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lin, S.-J.; Yeh, J.-W. (AlCrNbSiTi)N/TiN multilayer films designed by a hybrid coating system combining high-power impulse magnetron sputtering and cathode arc deposition. Surf. Coat. Technol. 2023, 468, 129757. [Google Scholar] [CrossRef]
- Wan, Q.; Jia, B.Y.; Liu, P.; Luo, Y.; Chen, J.; Zhang, X.Y.; Xiao, Y.Y.; Abdelkader, T.K.; Refai, M.; Zhang, J.; et al. Microstructure and mechanical properties of FeCoCrNiAl0.1N high entropy alloy nitride coatings synthesized by cathodic arc ion plating using alloy target. Surf. Coat. Technol. 2023, 457, 129305. [Google Scholar] [CrossRef]
- Zhang, X.; Pelenovich, V.; Liu, Y.; Ke, X.; Zhang, J.; Yang, B.; Ma, G.; Li, M.; Wang, X. Effect of bias voltages on microstructure and properties of (TiVCrNbSiTaBY)N high entropy alloy nitride coatings deposited by RF magnetron sputtering. Vacuum 2022, 195, 110710. [Google Scholar] [CrossRef]
- Zhang, X.; Pelenovich, V.; Zeng, X.; Wan, Q.; Liu, J.; Pogrebnjak, A.; Guo, Y.; Liu, Y.; Lei, Y.; Yang, B. Unravel hardening mechanism of AlCrNbSiTi high-entropy alloy coatings. J. Alloys Compd. 2023, 965, 171222. [Google Scholar] [CrossRef]
- Bharti, R.; Butt, M.; Dey, A. Nanoindentation and Nanoscratch Testing for the Mechanical Characterization of Stealth Thin Film Coatings. Russ. J. Non-Ferr. Met. 2024, 65, 151–161. [Google Scholar] [CrossRef]
- Lackner, J.; Major, L.; Kot, M. Microscale interpretation of tribological phenomena in Ti/TiN soft-hard multilayer coatings on soft austenite steel substrates. Bull. Pol. Acad. Sci.-Tech. Sci. 2011, 59, 343–355. [Google Scholar] [CrossRef]
- Masanta, M.; Shariff, S.; Choudhury, A. Evaluation of modulus of elasticity, nano-hardness and fracture toughness of TiB2-TiC-Al2O3 composite coating developed by SHS and laser cladding. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2011, 528, 5327–5335. [Google Scholar] [CrossRef]
- Wang, C.; Pureza, J.; Yang, Y.; Chung, Y. Investigation of hardness and fracture toughness properties of Fe/VC multilayer coatings with coherent interfaces. Surf. Coat. Technol. 2016, 288, 179–184. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Chung, Y. Commentary on using H/E and H3/E2 as proxies for fracture toughness of hard coatings. Thin Solid Film. 2019, 688, 137265. [Google Scholar] [CrossRef]
- Otani, Y.; Hofmann, S. High temperature oxidation behaviour of (Ti1-xCrx)N coatings. Thin Solid Film. 1996, 287, 188–192. [Google Scholar] [CrossRef]
- Lee, D. TEM study on oxidized TiCrN coatings ion-plated on a steel substrate. Surf. Coat. Technol. 2003, 173, 81–86. [Google Scholar] [CrossRef]
- Brachet, J.; Idarraga-Trujillo, I.; Le Flem, M.; Le Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, C.; Tupin, M.; et al. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Kravchenko, Y.; Kislitsyn, S.; Ruzimov, S.; Noli, F.; Misaelides, P.; Hatzidimitriou, A. TiN/Cr/Al2O3 and TiN/Al2O3 hybrid coatings structure features and properties resulting from combined treatment. Surf. Coat. Technol. 2006, 201, 2621–2632. [Google Scholar] [CrossRef]


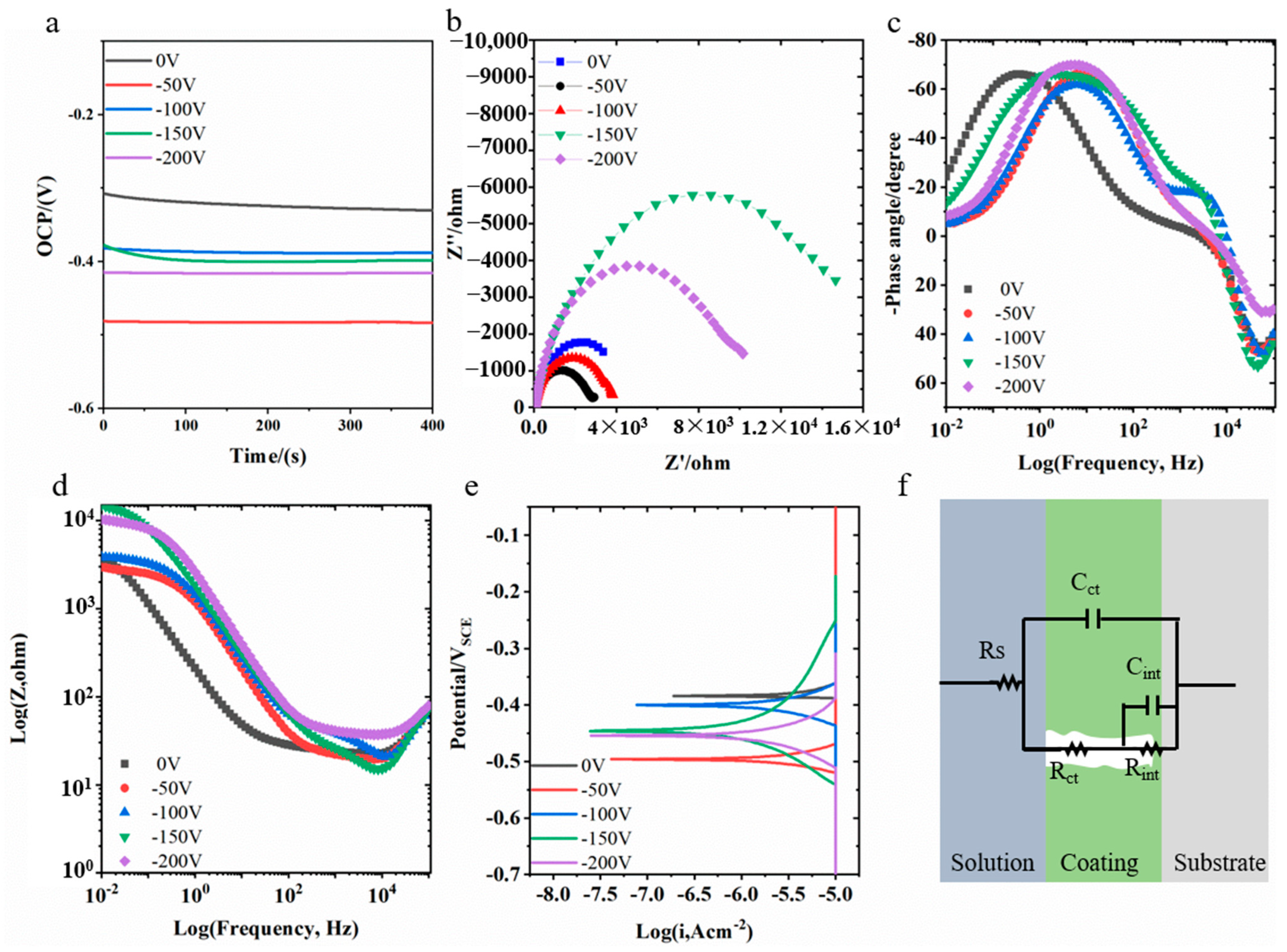
| 0 V | −50 V | −100 V | −150 V | −200 V | |
|---|---|---|---|---|---|
| Rs/Ω·cm2 | 19.47 | 23.20 | 21.59 | 15.14 | 37.50 |
| CPEct/10−5 F·cm−2 | 7.2 | 7.2 | 1.1 | 9.9 | 3.0 |
| nct | 0.92 | 0.89 | 0.87 | 0.91 | 0.98 |
| Rct/104 Ω·cm2 | 0.27 | 0.44 | 0.38 | 1.68 | 1.00 |
| Rint/Ω·cm2 | 7.8 | 2.5 | 21.8 | 13.9 | 8.7 |
| CPEint/10−5 F·cm−2 | 14.83 | 115.57 | 14.21 | 12.96 | 7.60 |
| nint | 0.84 | 0.82 | 0.79 | 0.77 | 0.85 |
| Ecorr/V | −0.38 | −0.49 | −0.40 | −0.45 | −0.45 |
| Icorr/μA·cm−2 | 3.01 | 1.62 | 1.66 | 0.79 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Z.; Deng, J.; Xu, H.; Xu, Z.; Wen, Z.; Long, W.; Zhang, L.; Wang, R.; Liu, J.; Chen, Y. The Oxidation and Corrosion Resistance of AlCrNbSiTiN Multi-Principal Element Nitride Coatings. Materials 2025, 18, 4663. https://doi.org/10.3390/ma18204663
Lan Z, Deng J, Xu H, Xu Z, Wen Z, Long W, Zhang L, Wang R, Liu J, Chen Y. The Oxidation and Corrosion Resistance of AlCrNbSiTiN Multi-Principal Element Nitride Coatings. Materials. 2025; 18(20):4663. https://doi.org/10.3390/ma18204663
Chicago/Turabian StyleLan, Zhenbo, Jiangang Deng, Heng Xu, Zhuolin Xu, Zhengqi Wen, Wei Long, Lei Zhang, Ruoxi Wang, Jie Liu, and Yanming Chen. 2025. "The Oxidation and Corrosion Resistance of AlCrNbSiTiN Multi-Principal Element Nitride Coatings" Materials 18, no. 20: 4663. https://doi.org/10.3390/ma18204663
APA StyleLan, Z., Deng, J., Xu, H., Xu, Z., Wen, Z., Long, W., Zhang, L., Wang, R., Liu, J., & Chen, Y. (2025). The Oxidation and Corrosion Resistance of AlCrNbSiTiN Multi-Principal Element Nitride Coatings. Materials, 18(20), 4663. https://doi.org/10.3390/ma18204663






