High-Temperature Oxidation Resistance of Fe-Free AlCoCrNiNb0.2 and AlCoCr0.5NiNb0.2 High-Entropy Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructure and Phase Composition of the As-Cast HEAs
3.2. High-Temperature Oxidation
3.3. Morphology and Phase Composition of the Oxide Film
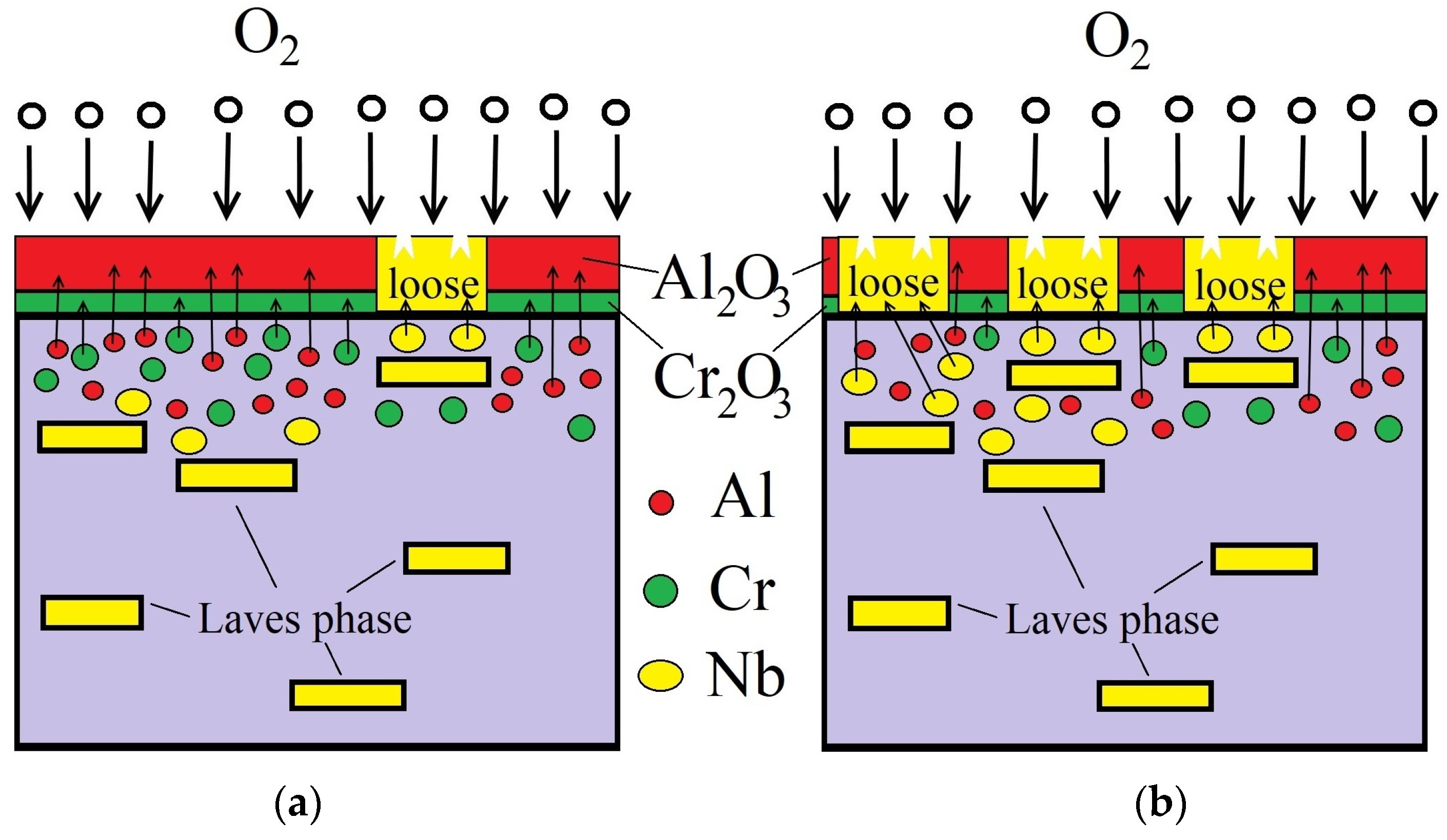
3.4. Oxidation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEA | High-entropy alloy |
| SEM | Scanning electron microscope |
| EDS | Energy-dispersive X-ray spectroscopy detector |
| XRD | X-ray diffraction |
References
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological properties of high-entropy alloys under dry conditions for a wide temperature range—A review. Materials 2021, 14, 5814. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, O.; Shaburova, N.; Krymsky, V.; Myasoedov, V.; Ostovari Moghaddam, A.; Trofimov, E. Effect of electromagnetic pulses on the microstructure and abrasive gas wear resistance of Al0.25CoCrFeNiV high entropy alloy. Coatings 2022, 12, 688. [Google Scholar] [CrossRef]
- Samoilova, O.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. Al0.25CoCrFeNiSi0.6 high entropy alloy with high hardness and improved wear resistance. Mater. Lett. 2022, 328, 133190. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. Npj Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corr. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Kumar, P.; Lam, T.-N.; Tripathi, P.K.; Singh, S.S.; Liaw, P.K.; Huang, E.-W. Recent progress in oxidation behavior of high-entropy alloys: A review. APL Mater. 2022, 10, 120701. [Google Scholar] [CrossRef]
- Veselkov, S.; Samoilova, O.; Shaburova, N.; Trofimov, E. High-temperature oxidation of high-entropic alloys: A review. Materials 2021, 14, 2595. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloys Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Murakami, H.; Araki, H.; Watanabe, M.; Kuroda, S.; Yeh, A.-C.; Yeh, J.-W. A study of NiCo0.6Fe0.2CrxSiAlTiy high-entropy alloys for applications as a high-temperature protective coating and a bond coat in thermal barrier coating systems. J. Electrochem. Soc. 2018, 165, C524–C531. [Google Scholar] [CrossRef]
- Jadhav, M.; Singh, S.; Srivastava, M.; Vinod Kumar, G.S. An investigation on high entropy alloy for bond coat application in thermal barrier coating system. J. Alloys Compd. 2019, 783, 662–673. [Google Scholar] [CrossRef]
- Ossiansson, M.; Gupta, M.; Löbel, M.; Lindner, T.; Lampke, T.; Joshi, S. Assessment of CrFeCoNi and AlCrFeCoNi high-entropy alloys as bond coats for thermal barrier coatings. J. Therm. Spray Technol. 2022, 31, 1404–1422. [Google Scholar] [CrossRef]
- Shahbazi, H.; Vakilifard, H.; Nair, R.B.; Liberati, A.C.; Lima, R.S.; Stoyanov, P.; Moreau, C. High entropy alloy bond coats for thermal barrier coatings: A review. J. Therm. Spray Technol. 2024, 33, 430–446. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.; Mu, R.; Zhao, X.; Guo, F. Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corr. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]
- Hu, J.; Gu, C.; Li, J.; Li, C.; Feng, J.; Jiang, Y. Microstructure and oxidation behavior of the Y/Ta/Hf co-doped AlCoCrFeNi high-entropy alloys in air at 1100 °C. Corr. Sci. 2023, 212, 110930. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Chen, Y.; Ling, L.; Liu, X.; Xiao, W.; Ni, N.; Zhao, X.; Guo, F.; Xiao, P. Y-doped AlCoCrFeNi2.1 eutectic high-entropy alloy with excellent oxidation resistance and structure stability at 1000 °C and 1100 °C. Corr. Sci. 2021, 180, 109191. [Google Scholar] [CrossRef]
- Samoilova, O.; Pratskova, S.; Suleymanova, I.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. Effect of Pt addition on the oxidation and corrosion resistance of Al0.25CoCrFeNi high-entropy alloy. Metals 2023, 13, 1709. [Google Scholar] [CrossRef]
- Yang, J.-J.; Kuo, C.-M.; Lin, P.-T.; Liu, H.-C.; Huang, C.-Y.; Yen, H.-W.; Tsai, C.-W. Improvement in oxidation behavior of Al0.2Co1.5CrFeNi1.5Ti0.3 high-entropy superalloys by minor Nb addition. J. Alloys Compd. 2020, 825, 153983. [Google Scholar] [CrossRef]
- Wu, T.; Yu, L.; Chen, G.; Wang, R.; Xue, Y.; Lu, Y.; Luan, B. Effects of Mo and Nb on the microstructure and high temperature oxidation behaviors of CoCrFeNi-based high entropy alloys. J. Mater. Res. Technol. 2023, 27, 1537–1549. [Google Scholar] [CrossRef]
- Feng, M.; Lin, T.; Lian, G.; Chen, C.; Huang, X. Effects of Nb content on the microstructure and properties of CoCrFeMnNiNbx high-entropy alloy coatings by laser cladding. J. Mater. Res. Technol. 2024, 28, 3835–3848. [Google Scholar] [CrossRef]
- Malatji, N.; Popoola, A.P.I.; Lengopeng, T.; Pityana, S. Effect of Nb addition on the microstructural, mechanical and electrochemical characteristics of AlCrFeNiCu high-entropy alloy. Int. J. Miner. Metall. Mater. 2020, 27, 1332–1340. [Google Scholar] [CrossRef]
- Pan, B.; Xu, X.; Yang, J.; Zhan, H.; Feng, L.; Long, Q.; Yao, Q.; Deng, J.; Cheng, L.; Lu, Z.; et al. Effect of Nb, Ti, and V on wear resistance and electrochemical corrosion resistance of AlCoCrNiMx (M=Nb, Ti, V) high-entropy alloys. Mater. Today Commun. 2024, 39, 109314. [Google Scholar] [CrossRef]
- Gawel, R.; Rogal, Ł.; Grzesik, Z. Behaviour of Al, Co, Cr, Ni-based high entropy alloys under high-temperature thermal shock oxidising conditions. Corr. Sci. 2022, 198, 110116. [Google Scholar] [CrossRef]
- Samoilova, O.; Suleymanova, I.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. The behavior of Al0.5CoCrFeNiCuPt0.3 high-entropy alloy during high-temperature oxidation. High Temp. Corr. Mater. 2024, 101, 811–825. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of niobium on microstructure and properties of the CoCrFeNbxNi high entropy alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Ji, X.; Guan, K.; Bao, Y.; Mao, Z.; Wang, F.; Dai, H. Effect of Nb addition on the corrosion and wear resistance of laser clad AlCr2FeCoNi high-entropy alloy coatings. Lubricants 2024, 12, 5. [Google Scholar] [CrossRef]
- Ogura, M.; Fukushima, T.; Zeller, R.; Dederichs, P.H. Structure of the high-entropy alloy AlxCrFeCoNi: Fcc versus bcc. J. Alloys Compd. 2017, 715, 454–459. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Pakseresht, A.; Omidvar, H.; Shafiei, A. Investigation of the high-temperature oxidation behavior of the Al0.5CoCrFeNi high entropy alloy. Surf. Interfaces 2020, 21, 100724. [Google Scholar] [CrossRef]
- Swalin, R.A. Thermodynamics of Solids, 2nd ed.; Wiley: New York, NY, USA, 1972; 400p. [Google Scholar]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Mroczka, K.; Berent, K.; Kulik, T.; Danielewski, M. Influence of Cu content on high temperature oxidation behavior of AlCoCrCuxFeNi high entropy alloys (x = 0; 0.5; 1). Intermetallics 2017, 84, 52–61. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Tan, Z.; He, D.; Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. High temperature oxidation behavior of Al0.6CrFeCoNi and Al0.6CrFeCoNiSi0.3 high entropy alloys. J. Alloys Compd. 2018, 764, 845–852. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, S.; Jin, Y.; Xu, L.; Xu, X.; Yin, C.; Jia, Y. High–temperature oxidation behaviours of AlCoCrFeNi high–entropy alloy at 1073–1273 K. Oxid. Met. 2020, 94, 265–281. [Google Scholar] [CrossRef]
- Lussana, D.; Baldissin, D.; Massazza, M.; Baricco, M. Thermodynamic and kinetics aspects of high temperature oxidation on a 304L stainless steel. Oxid. Met. 2014, 81, 515–528. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Yang, R.; Yang, S.; Jia, Y.; Chen, M.; Qiao, Y.; Guo, P.; Zhu, S.; Wang, F. Oxidation behavior of a nanocrystalline coating with low Ta content at high temperature. Corr. Sci. 2021, 180, 109182. [Google Scholar] [CrossRef]
- Gomes, G.H.d.M.; de Andrade, R.R.; Mohallem, N.D.S. Investigation of phase transition employing strain mapping in TT- and T-Nb2O5 obtained by HRTEM micrographs. Micron 2021, 148, 103112. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, J.; Zhao, Y.; Shao, J. Diffusion of metal ions from a substrate into oxide coatings. Opt. Mater. Express 2016, 6, 3119–3126. [Google Scholar] [CrossRef]
- Kumar Dewangan, S.; Mangish, A.; Kumar, S.; Sharma, A.; Ahn, B.; Kumar, V. A review on high-temperature applicability: A milestone for high entropy alloys. Eng. Sci. Technol. Intern. J. 2022, 35, 101211. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Danielewski, M. State-of-the-art diffusion studies in the high entropy alloys. Metals 2020, 10, 347. [Google Scholar] [CrossRef]
- Sabioni, A.C.S.; Souza, J.N.V.; Ji, V.; Jomard, F.; Trindade, V.B.; Carneiro, J.F. Study of ion diffusion in oxidation films grown on a model Fe–15%Cr alloy. Solid State Ionics 2015, 276, 1–8. [Google Scholar] [CrossRef]
- Wiman, P.; Boonpensin, M.; Saranyachot, P.; Thublaor, T. Approximation in Ellingham diagram and calculation approach using heat capacity of oxides and metals constituent in stainless steels. Mater. Today Proc. 2022, 52, 2539–2542. [Google Scholar] [CrossRef]
- Sabioni, A.C.S.; Malheiros, E.A.; Ji, V.; Jomard, F.; de Almeida Macedo, W.A.; Gastelois, P.L. Ion diffusion study in the oxide layers due to oxidation of AISI 439 ferritic stainless steel. Oxid. Met. 2014, 81, 407–419. [Google Scholar] [CrossRef]
- Shaburova, N.A.; Ostovari Moghaddam, A.; Veselkov, S.N.; Sudarikov, M.V.; Samoilova, O.V.; Trofimov, E.A. High-temperature oxidation behavior of AlxCoCrFeNiM (M = Cu, Ti, V) high-entropy alloys. Phys. Mesomech. 2021, 24, 653–662. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Guo, S.; Zhu, L.; Teng, J.; Jiang, L.; Moverare, J.; Li, X.-H.; Peng, R.L. The impact of Al/Cr ratio on the oxidation kinetics of Y-doped AlCoCrFeNi high-entropy alloys at 1100 °C. Intermetallics 2025, 176, 108582. [Google Scholar] [CrossRef]
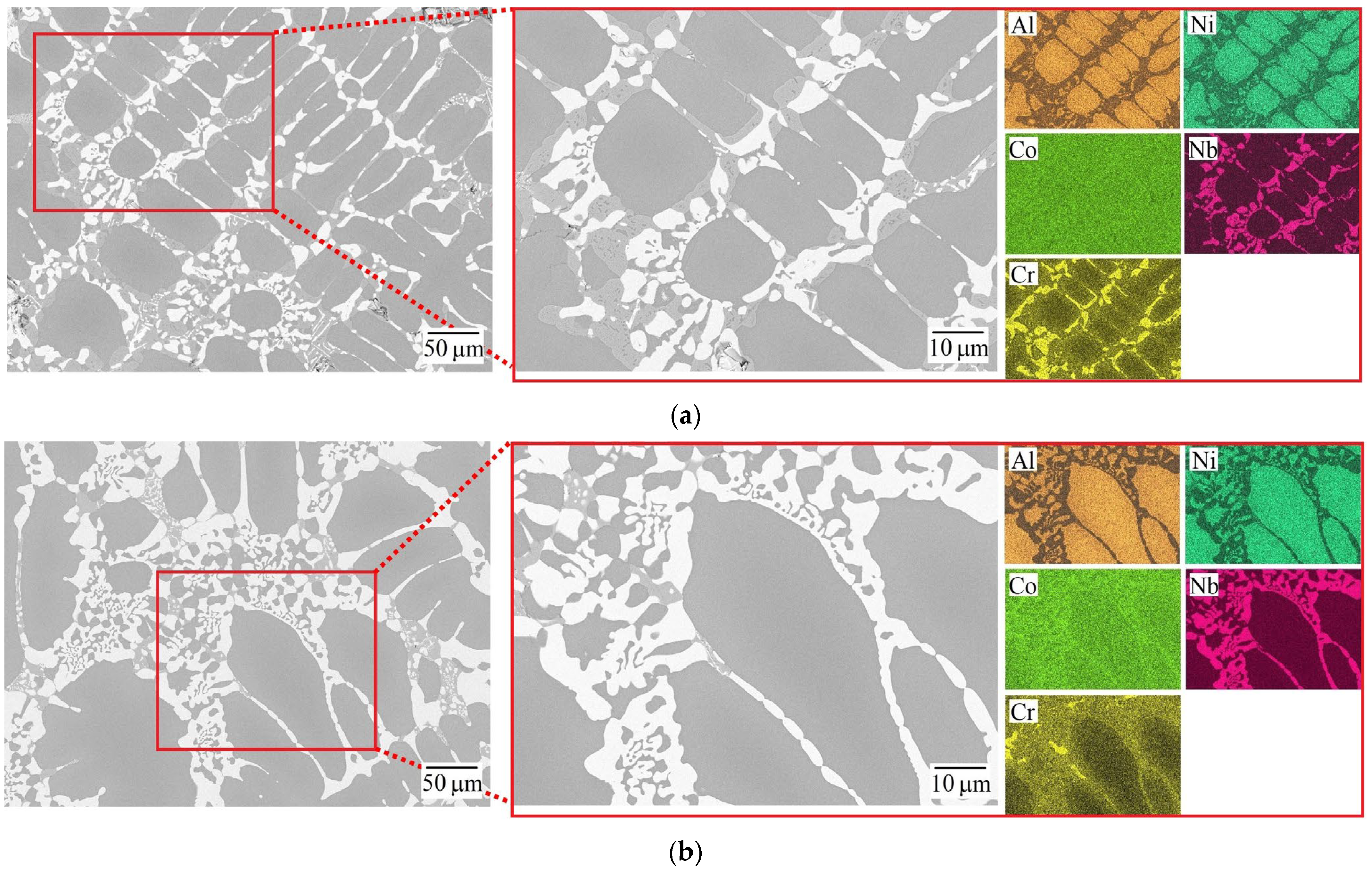
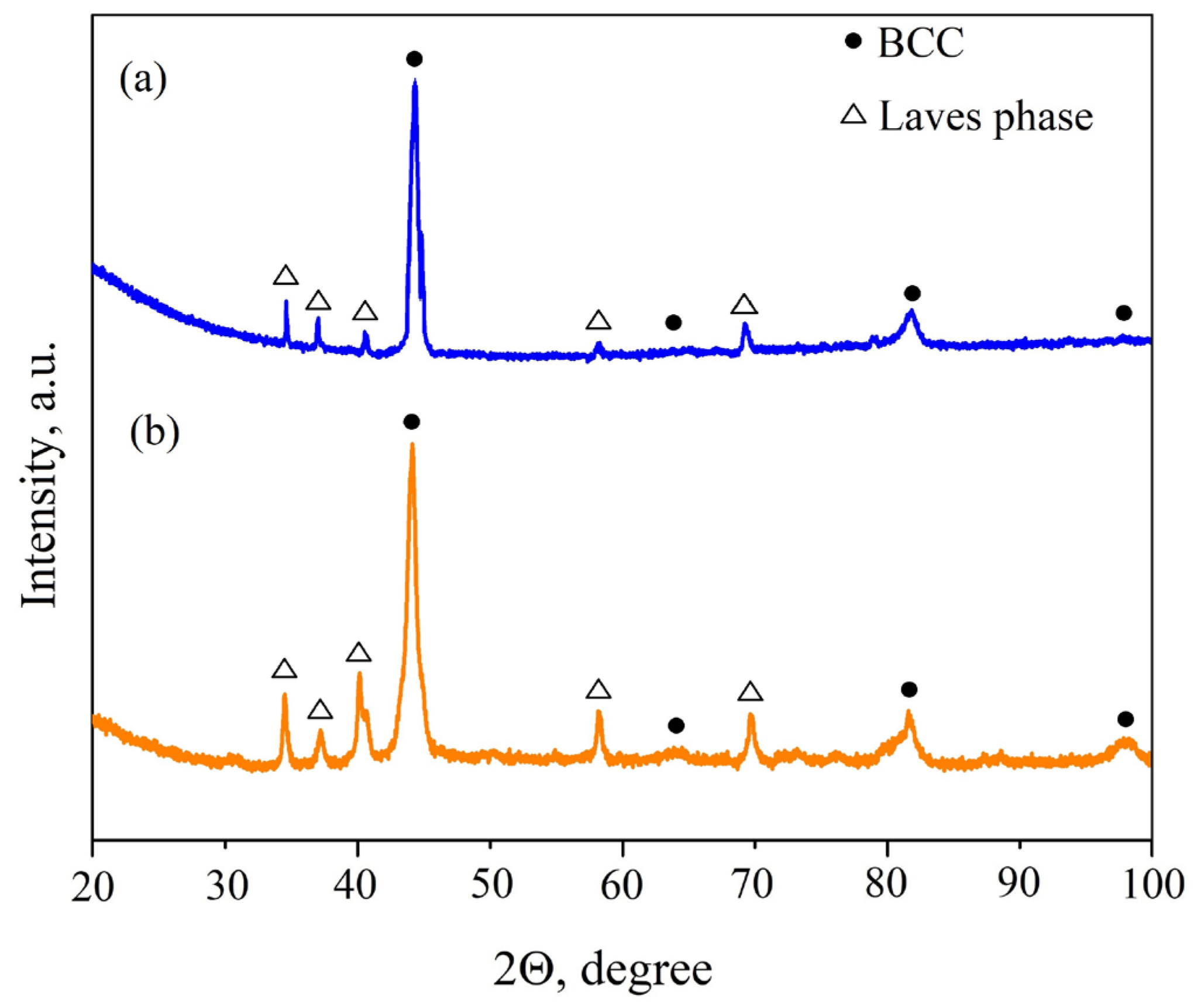
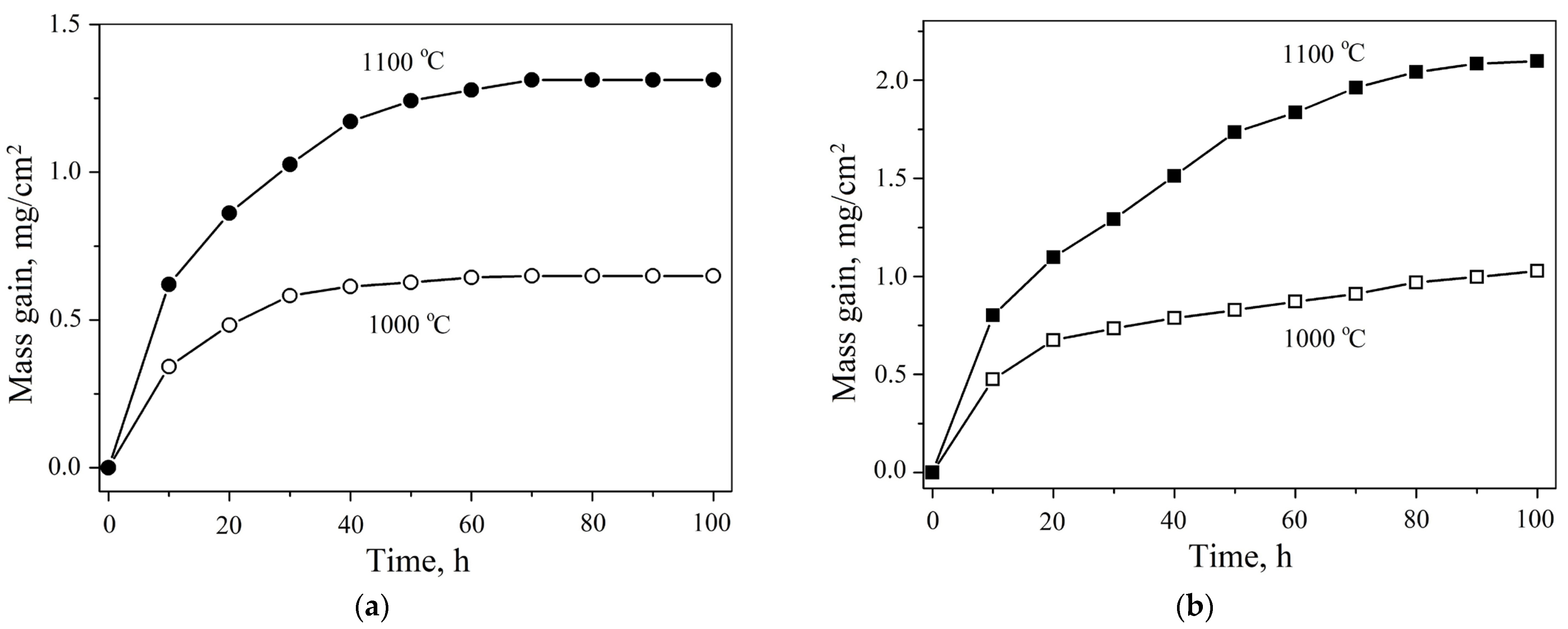
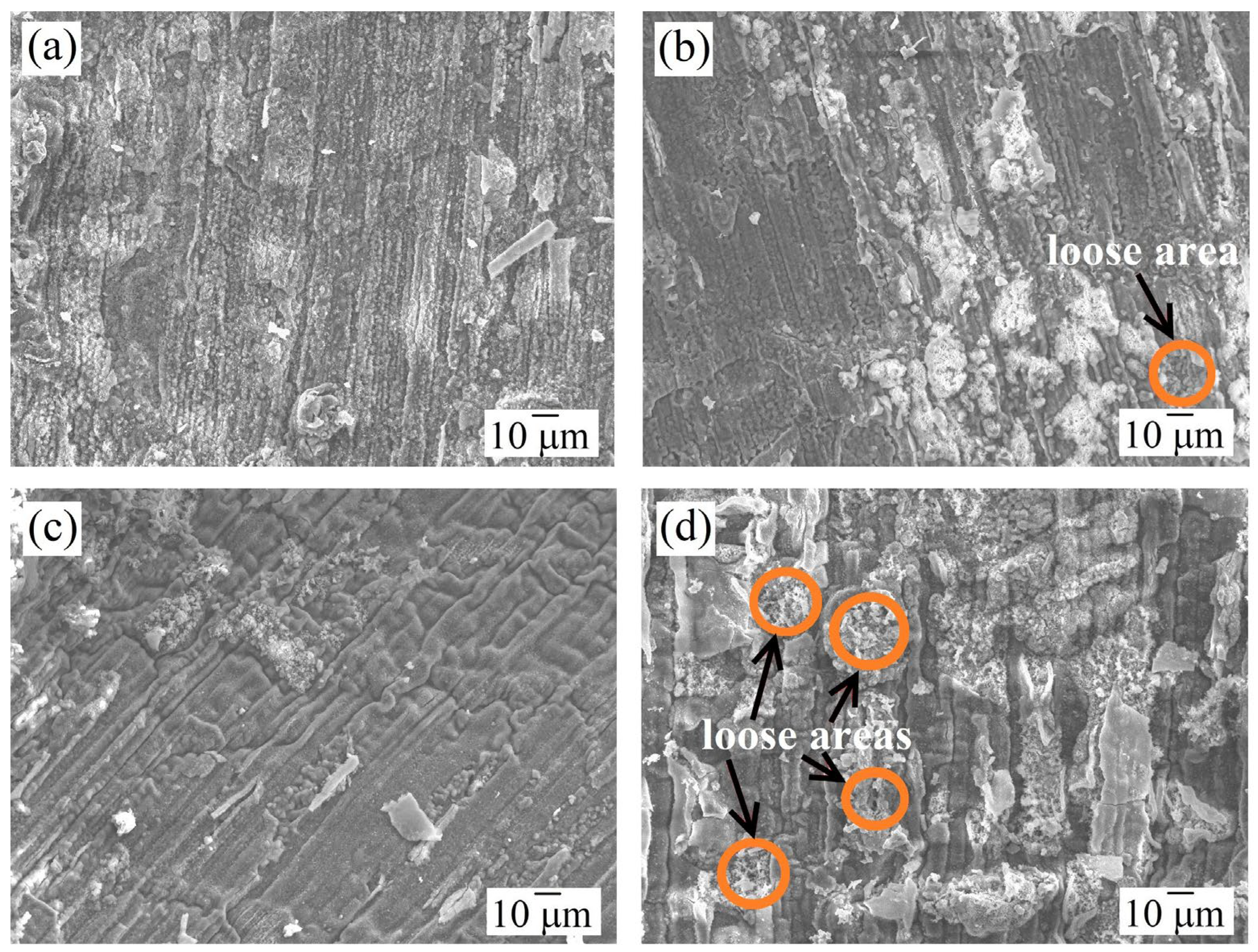
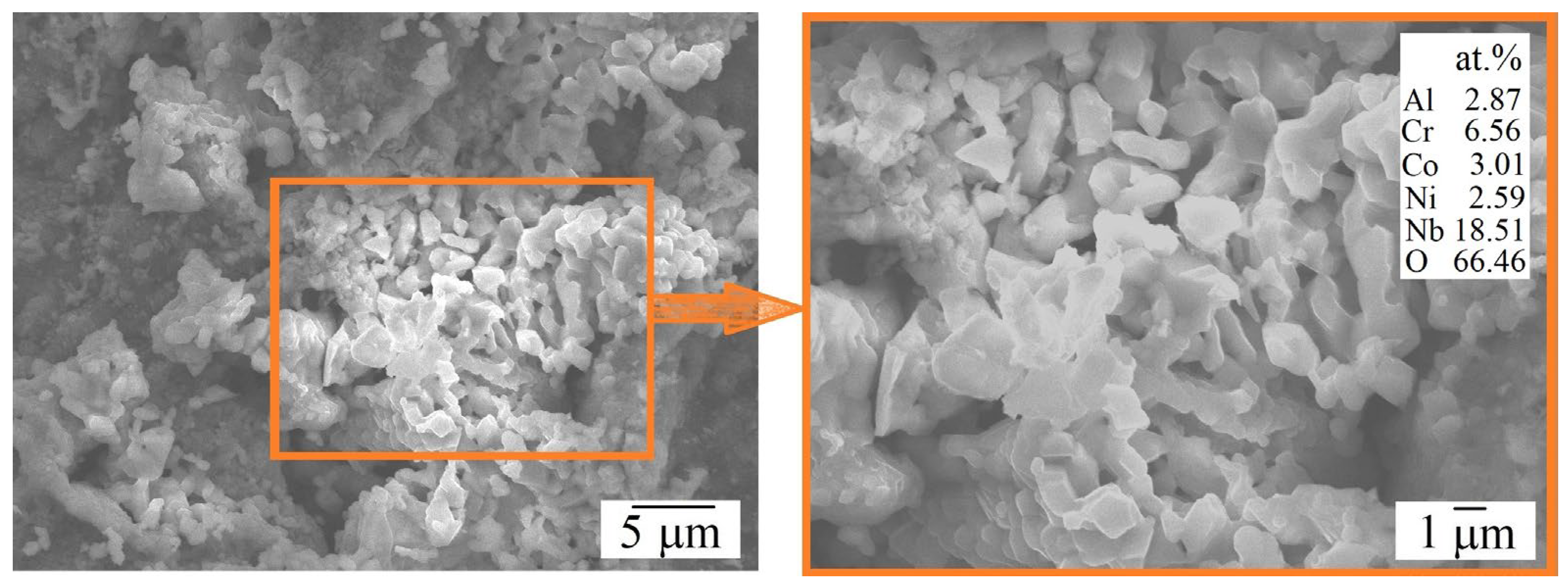
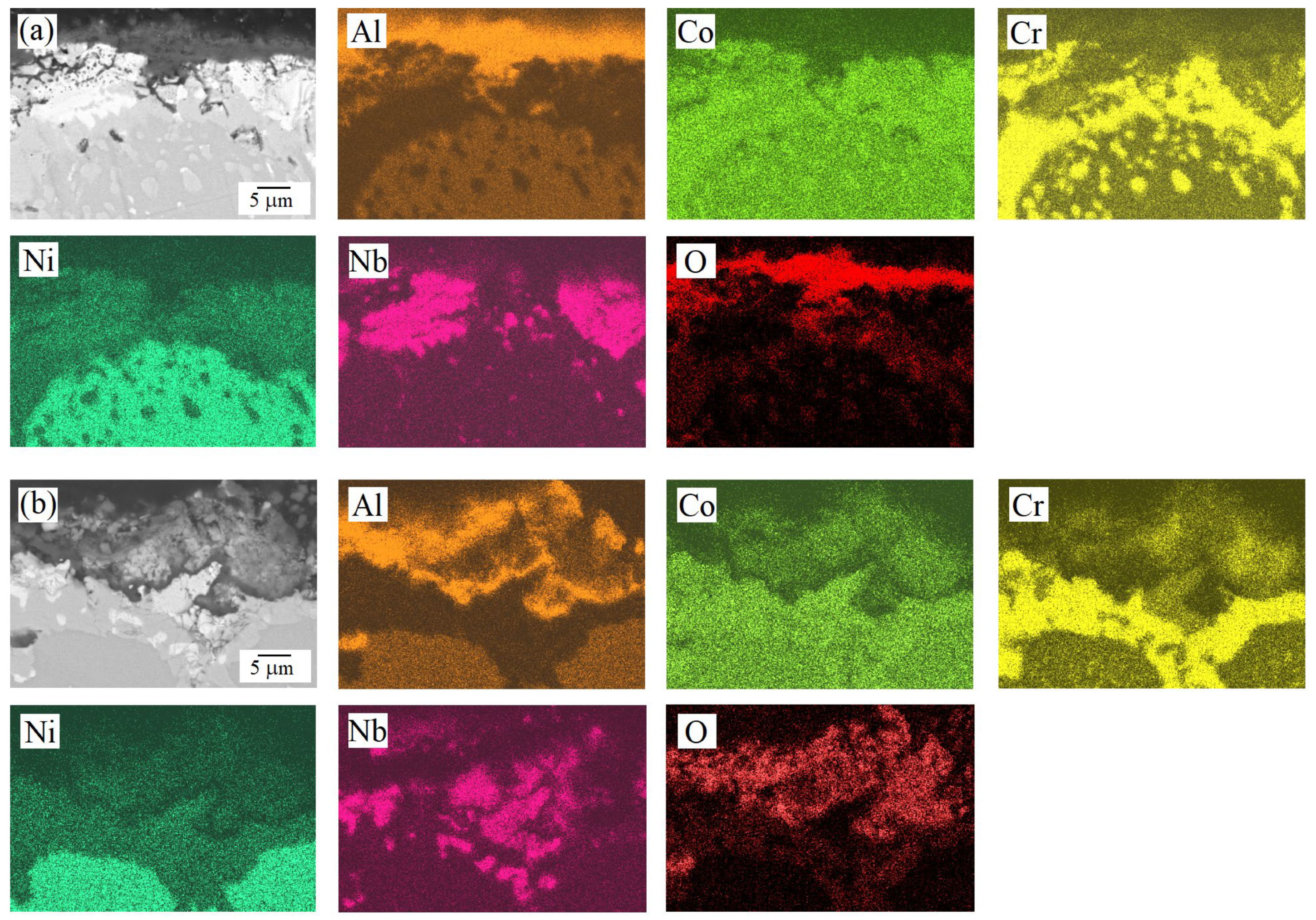
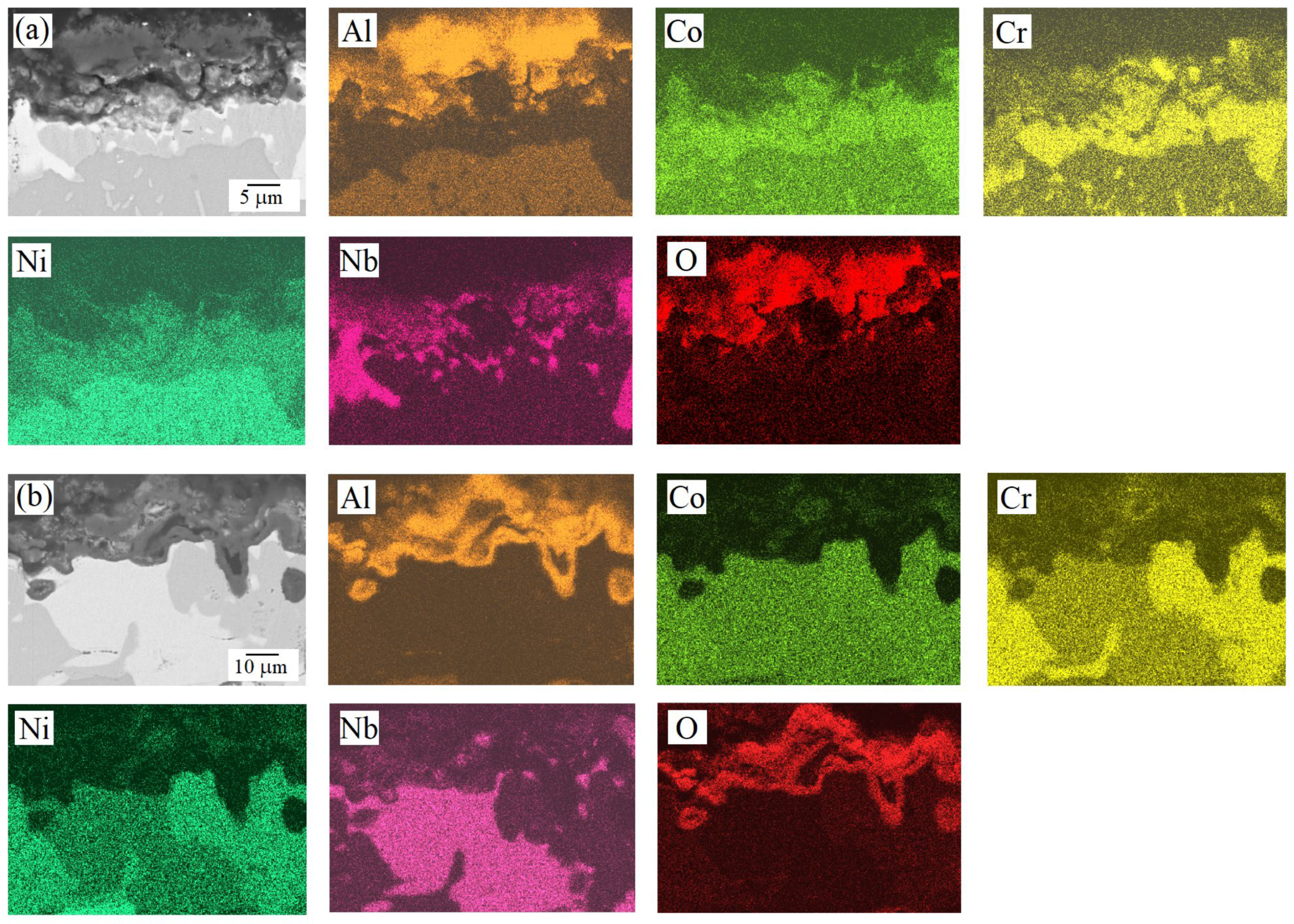
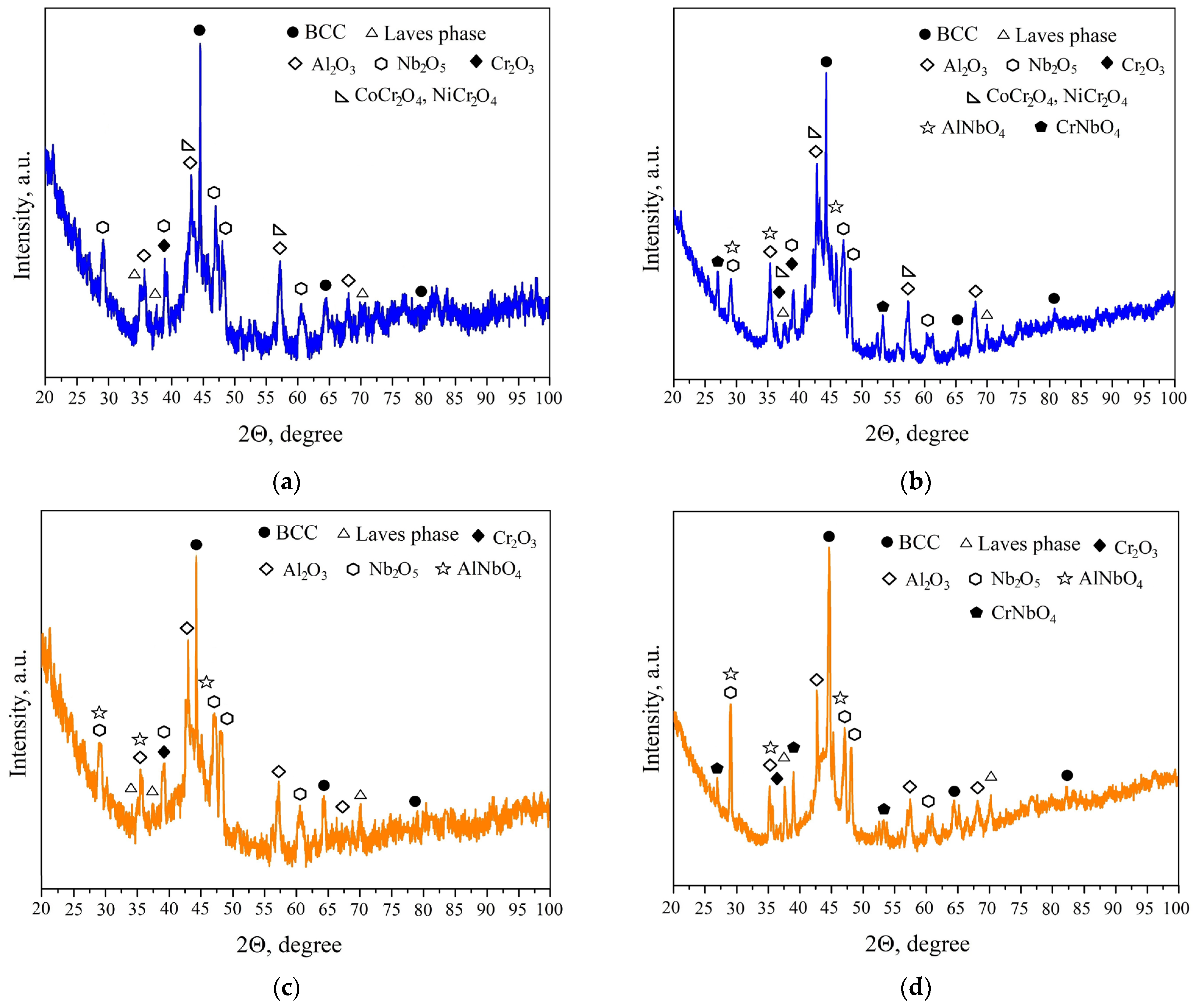
| HEA | MC | Al | Co | Cr | Ni | Nb |
|---|---|---|---|---|---|---|
| AlCoCrNiNb0.2 | Av | 23.76 | 23.87 | 23.66 | 23.85 | 4.86 |
| D | 33.78 | 20.02 | 12.62 | 31.65 | 1.93 | |
| ID1 | 8.41 | 25.26 | 49.78 | 15.26 | 1.29 | |
| ID2 | 4.26 | 30.53 | 26.60 | 14.14 | 24.47 | |
| AlCoCr0.5NiNb0.2 | Av | 27.15 | 26.91 | 13.05 | 26.88 | 6.01 |
| D | 38.52 | 20.38 | 6.21 | 32.85 | 2.04 | |
| ID1 | 9.82 | 33.78 | 30.06 | 23.79 | 2.55 | |
| ID2 | 4.42 | 35.96 | 17.65 | 15.47 | 26.50 |
| Alloy | t, °C | kp | Ea | Alloy | t, °C | kp | Ea |
|---|---|---|---|---|---|---|---|
| AlCoCrNiNb0.2 | 1000 | 19.33 × 10−13 | 192.8 | Al0.5CoCrFeNiCuPt0.3 [26] | 900 | 1.09 × 10−13 | 170 |
| 1100 | 72.87 × 10−13 | 1000 | 4.29 × 10−13 | ||||
| AlCoCr0.5NiNb0.2 | 1000 | 41.79 × 10−13 | 184.6 | Al0.5CoCrFeNi [31] | 900 | 8.91 × 10−13 | 125.7 |
| 1100 | 148.83 × 10−13 | 1000 | 39.3 × 10−13 | ||||
| Al30(CoCrFeNi)70 [11] | 1050 | 19 × 10−13 | – | Al0.6CrFeCoNi [34] | 900 | 11.8 × 10−13 | 196 |
| Al8(CoCrFeNi)92 [11] | 1050 | 25 × 10−13 | – | 1000 | 57.2 × 10−13 | ||
| CoCrFeNi [21] | 1000 | 231 × 10−13 | – | AISI 304L [36] | 900 | 23 × 10−13 | 174 |
| CoCrFeNiMo0.2 [21] | 1000 | 161 × 10−13 | – | 1050 | 93 × 10−13 | ||
| CoCrFeNiMo0.2Nb0.1 [21] | 1000 | 125 × 10−13 | – | N5–3Ta nickel-based superalloy [37] | 1050 | 0.61 × 10−13 | 180 |
| CoCrFeNiMo0.2Nb0.2 [21] | 1000 | 63.9 × 10−13 | – | 1150 | 1.93 × 10−13 |
| HEA | t, °C | Al | Co | Cr | Ni | Nb | O |
|---|---|---|---|---|---|---|---|
| AlCoCrNiNb0.2 | 1000 | 32.30 | 2.07 | 4.90 | 1.13 | 1.23 | 58.37 |
| 1100 | 28.81 | 2.30 | 6.91 | 0.94 | 1.63 | 59.41 | |
| AlCoCr0.5NiNb0.2 | 1000 | 31.15 | 3.15 | 3.19 | 2.20 | 2.07 | 58.24 |
| 1100 | 26.97 | 2.80 | 3.02 | 2.15 | 4.87 | 60.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoilova, O.; Pratskova, S.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. High-Temperature Oxidation Resistance of Fe-Free AlCoCrNiNb0.2 and AlCoCr0.5NiNb0.2 High-Entropy Alloys. Materials 2025, 18, 3701. https://doi.org/10.3390/ma18153701
Samoilova O, Pratskova S, Shaburova N, Ostovari Moghaddam A, Trofimov E. High-Temperature Oxidation Resistance of Fe-Free AlCoCrNiNb0.2 and AlCoCr0.5NiNb0.2 High-Entropy Alloys. Materials. 2025; 18(15):3701. https://doi.org/10.3390/ma18153701
Chicago/Turabian StyleSamoilova, Olga, Svetlana Pratskova, Nataliya Shaburova, Ahmad Ostovari Moghaddam, and Evgeny Trofimov. 2025. "High-Temperature Oxidation Resistance of Fe-Free AlCoCrNiNb0.2 and AlCoCr0.5NiNb0.2 High-Entropy Alloys" Materials 18, no. 15: 3701. https://doi.org/10.3390/ma18153701
APA StyleSamoilova, O., Pratskova, S., Shaburova, N., Ostovari Moghaddam, A., & Trofimov, E. (2025). High-Temperature Oxidation Resistance of Fe-Free AlCoCrNiNb0.2 and AlCoCr0.5NiNb0.2 High-Entropy Alloys. Materials, 18(15), 3701. https://doi.org/10.3390/ma18153701









