Production of Food-Grade Monocalcium Phosphate from Meat-Bone Meal
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Methods
2.2. Utilization of Meat Waste to Produce MBM
2.2.1. MBM Properties
- A commercial process for converting the by-product into a usable commodity;
- An actual or potential market for the commodity;
- Sufficient volumes of economically priced material in one location for processing;
- Appropriate facilities for storing the perishable by-products before processing and the finished products after processing;
- A critical mass of trained technical operators.
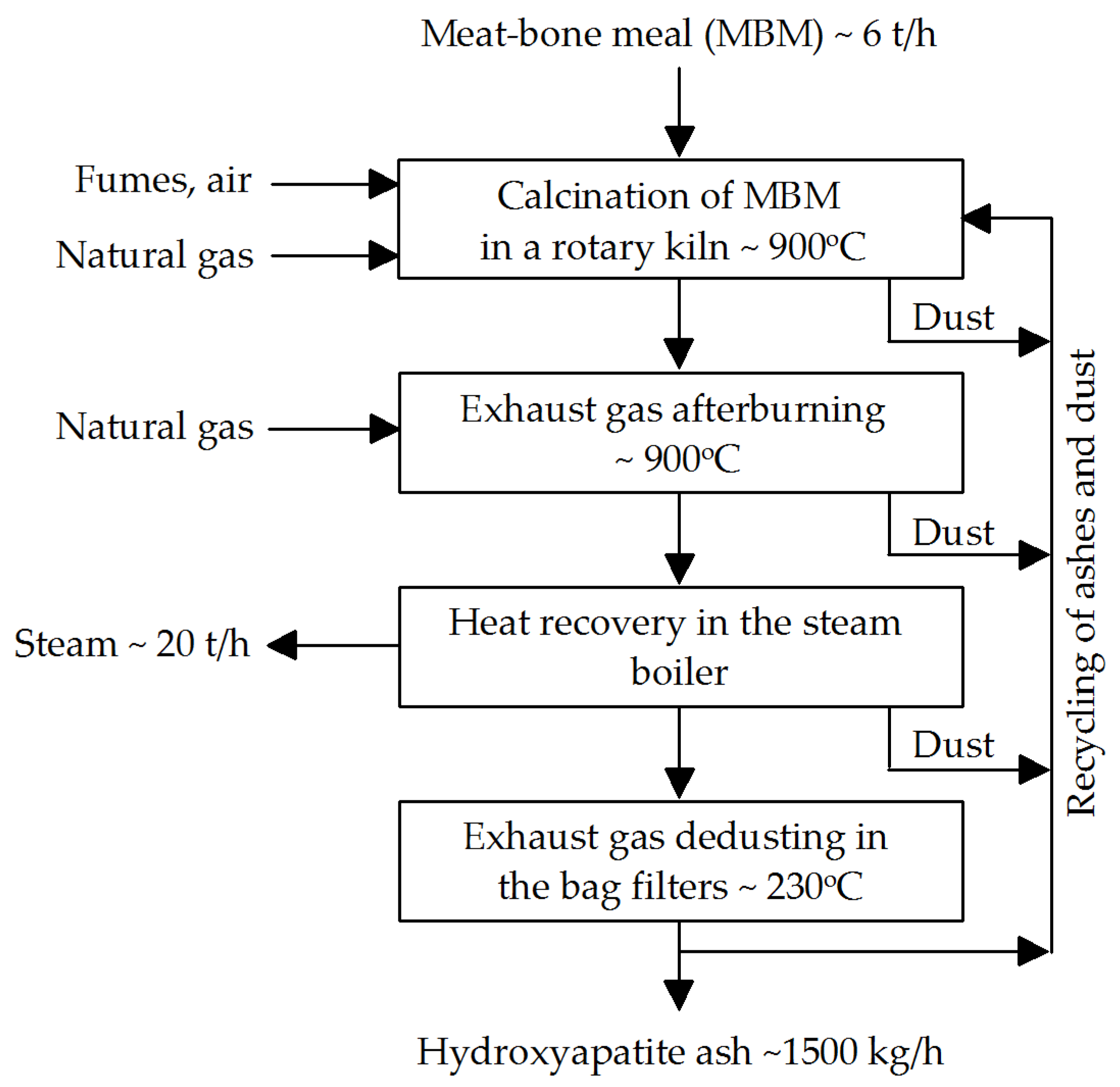
2.2.2. MBM Incineration Process Description
- Calcination time: 30–60 min for a feed rate of approximately 50 kg/m2/h.
- Maximum feedstock temperature: 950 °C.
- Co-current rotary kiln operation with adjustable rotation speed of 1–2 rpm.
- Gas flow velocity in the rotary kiln: up to 4 m/s.
- Oxygen concentration in exhaust gases after the rotary kiln: ~11%.
- Mass ratio of recycled HA to MBM in the feed charge: 1:1; recycled HA can be introduced via the kiln dosing screw.
- In the afterburner chamber, exhaust gases are combusted within 3 s at 850–900 °C.
- Steam produced in the steam boiler: pressure 6 bar.
- Exhaust gas flow rate during dedusting in bag filters: ~2 m/s at 200–250 °C.
- Specific consumption per 1000 kg of HA produced: 4000 kg MBM, 80 kWh electricity, 0.1 m3 process water, and 90 m3 natural gas.
3. Results
3.1. Properties of MBM and Ashes Obtained from Thermal Processing of MBM: Laboratory Test Results
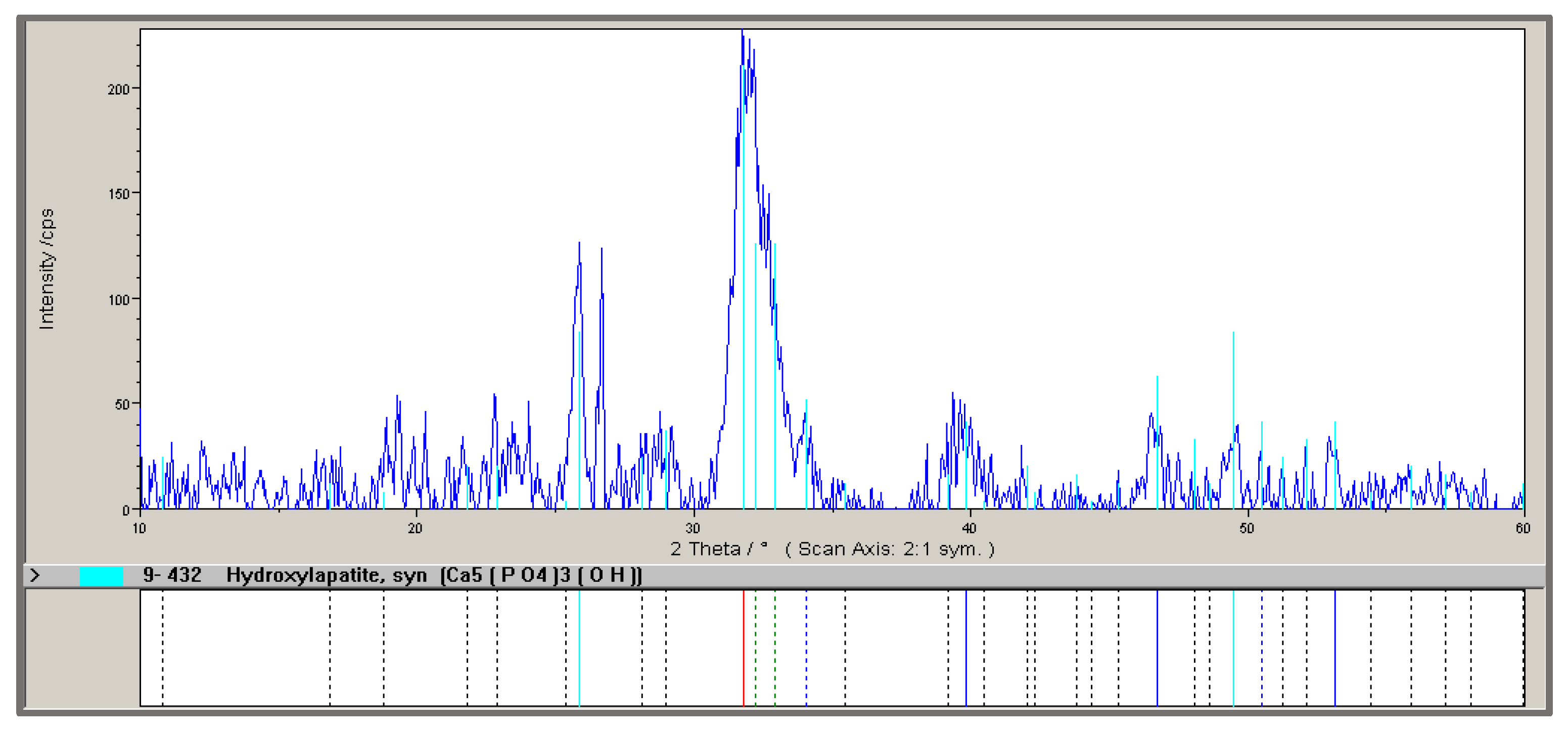
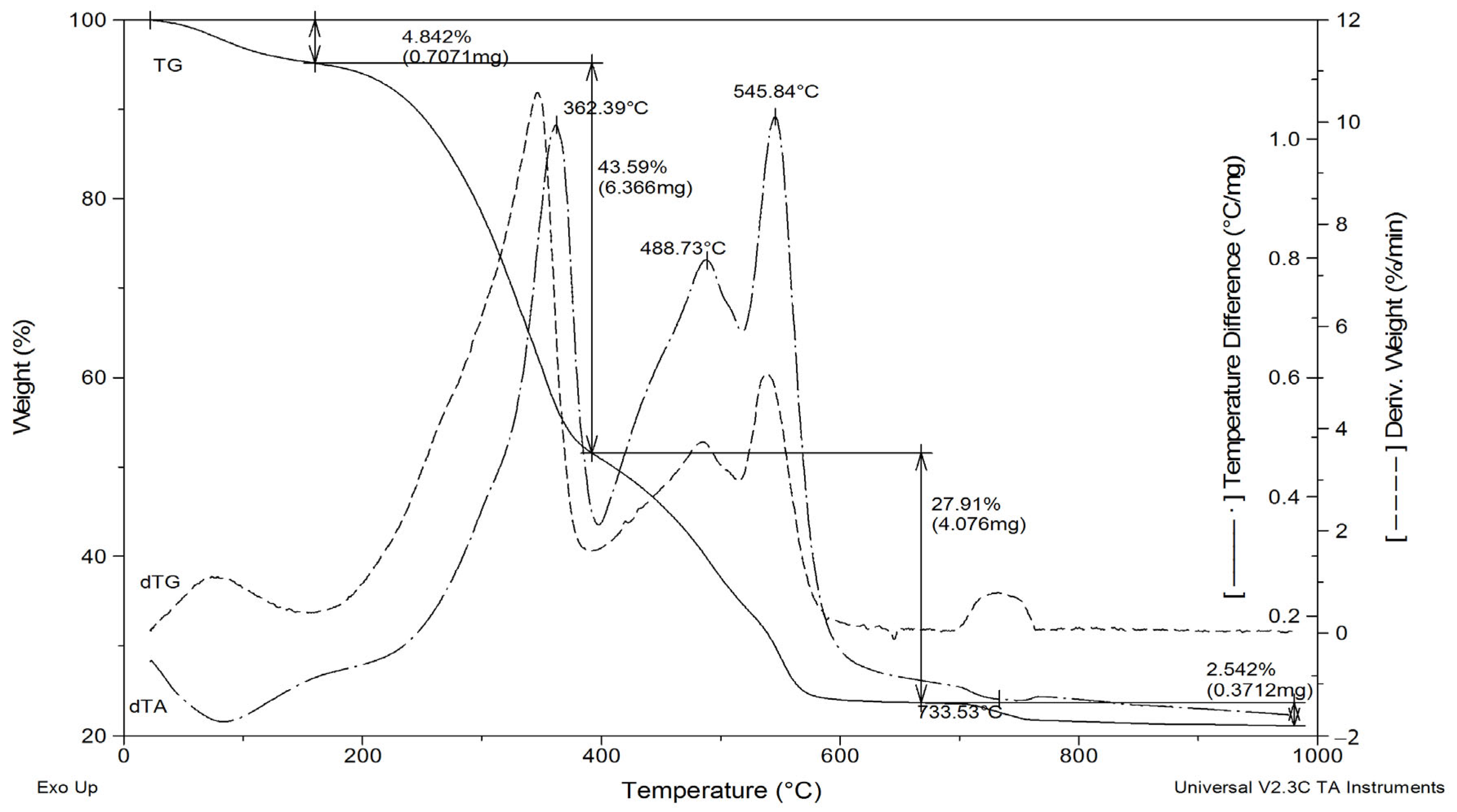
3.1.1. Properties of Hydroxyapatite Ashes Obtained from MBM in a Laboratory Chamber Kiln
3.1.2. MBM Incineration with In-Process Recycling of HA: Laboratory Chamber Kiln Tests
3.2. Quarter-Scale MBM Incineration Test Results
3.3. Characteristics of Inorganic Feed Phosphates (IFP)
- Angle of repose: measured after the powder is poured into a heap. Smaller angles indicate better flowability.
- Tapping tests: used to evaluate the bulk density of the powder. Powders that compact more easily generally exhibit better flowability.
- Rheological tests: assess the resistance of the powder to flow under various conditions of stress and deformation.
- Shear cell measurements: apply shear stress to the powder, measuring the force required to induce movement, providing a precise quantitative assessment of flowability.
Quality Classification of Inorganic Feed Phosphates (IFP)
4. Preparation of MCP from Hydroxyapatite Ashes Obtained from the Thermal Processing of MBM
- Variant A: HA was ground with H3PO4, without the addition of recycled MCP.
- Variant B: HA was ground with H3PO4, followed by the addition of recycled MCP, and the mixture was ground again.
- Variant C: HA was mixed with recycled MCP and then ground with H3PO4.
Preparation of MCP with Recycling of the Final Product
- Variant A: HA was mixed with recycled MCP, followed by the addition of H3PO4.
- Variant B: HA was mixed with H3PO4, followed by the addition of recycled MCP.
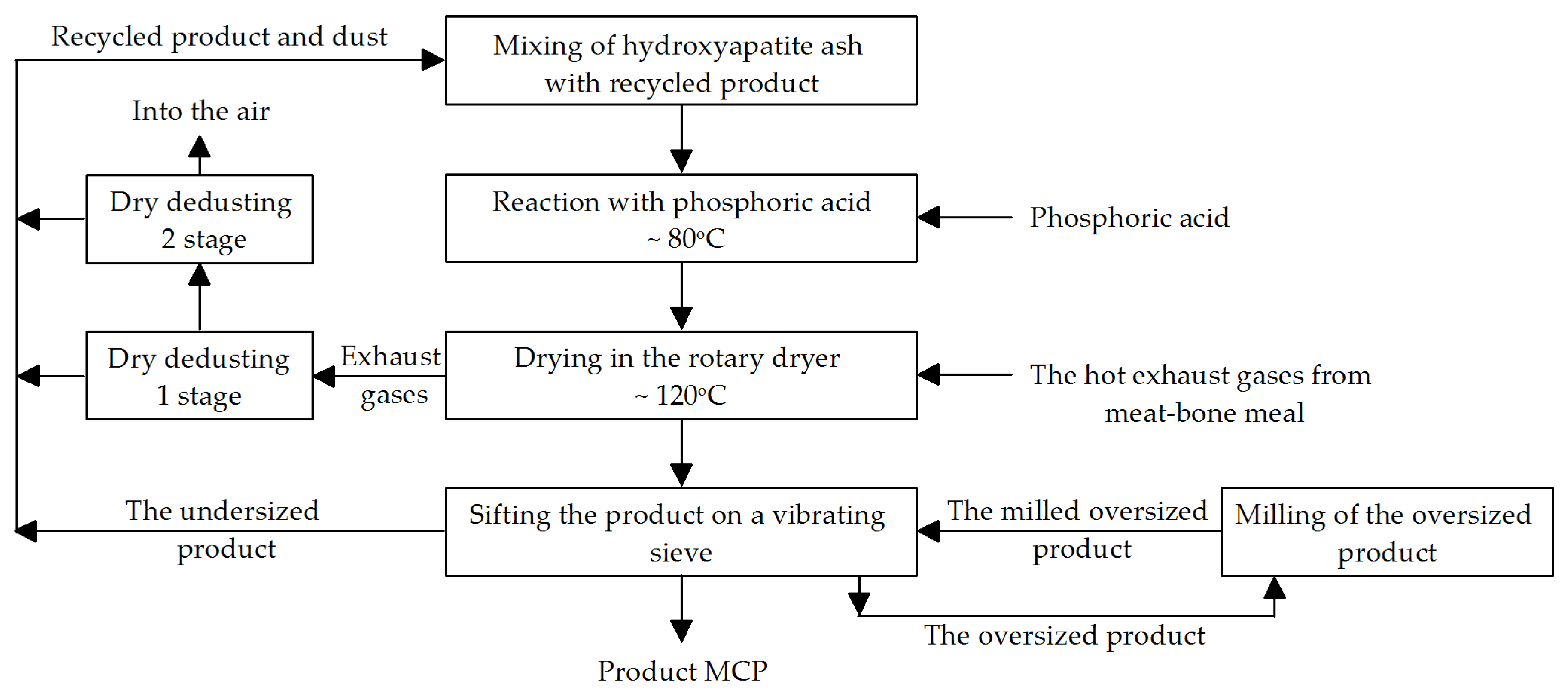
5. Flow Sheet of the MCP Industrial Production Process
Process Parameters
- Mixing of HA ash with recycled MCP for 20 min at a temperature of up to 50 °C.
- Reaction of the HA/MCP mixture in an Eirich mixer with phosphoric acid: reaction temperature: ~80 °C, continuous dosing of phosphoric acid into the HA/MCP mixture, with continuous stirring, reaction time: ~1 h.
- Drying in a rotary dryer: time: ~2 h (adjustable), consistency of the dried material: coarse paste; operating temperature: up to 150 °C, material temperature <105 °C; co-current operation, 0.2–2.0 rpm; dryer load: average 7 t/h, or ~50 kg/m2h.
- Screen operating parameters: temperature around 50 °C.
- Disintegrator operating parameters: temperature 30–40 °C.
- Dry cyclone operating parameters after the dryer: temperature around 100 °C, pressure according to the supplier’s specifications.
- Bag filter operating parameters after the dryer: temperature around 100 °C, pressure according to the supplier’s specifications.
6. Preliminary Calculation of MCP Production Costs
- Raw material costs (e.g., phosphate rock, phosphoric acid, energy);
- Global demand and production capacities;
- Geopolitical issues and supply chain disruptions;
- Environmental regulations and sustainability requirements;
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MBM | Meat-bone meal |
| MM | Meat meal |
| MCP | Monocalcium phosphate |
| DCP | Dicalcium phosphate |
| TCP | Tricalcium phosphate |
| DFP | Defluorinated calcium phosphate |
| HA | Hydroxyapatite |
| IS | Industrial symbiosis |
| CE | Circular economy |
| CP | Cleaner production |
| SD | Sustainable development |
| IFP | Inorganic feed phosphates |
| BM | Blood meal |
| MB | Bone meal |
| SM | Skin Meal |
| LM | Liquex meal |
References
- Nweze, J.A.; Gupta, S.; Akor, J.; Nwuche, C.O.; Nweze, J.E.; Unah, V.U. Animal Waste: An Environmentally Sustainable Management Approach. In Climate Changes Mitigation and Sustainable Bioenergy Harvest Through Animal Waste; Arshad, M., Ed.; Springer: Cham, Switzerland, 2023; pp. 1–33. [Google Scholar] [CrossRef]
- Sakadevan, K.; Nguyen, M.L. Chapter four—Livestock production and its impact on nutrient pollution and greenhouse gas emissions. Adv. Agron. 2017, 141, 147–184. [Google Scholar] [CrossRef]
- European Food Safety Authority. Available online: www.efsa.europa.eu (accessed on 24 August 2024).
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of food waste per product group along the food supply chain in the European Union: A mass flow analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef]
- Pinto, J.; Boavida-Dias, R.; Matos, H.A.; Azevedo, J. Analysis of the food loss and waste valorisation of animal by-products from the retail sector. Sustainability 2022, 14, 2830. [Google Scholar] [CrossRef]
- Gwyther, C.L.; Williams, A.P.; Golyshin, P.N.; Edward-Jones, G.; Jones, D.L. The environmental and biosecurity characteristics of livestock carcass disposal methods: A review. Waste Manag. 2011, 31, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Kantorek, M.; Jesionek, K.; Polesek-Karczewska, S.; Ziółkowski, P.; Badur, J. Thermal utilization of meat and bone meals. Performance analysis in terms of drying process, pyrolysis and kinetics of volatiles combustion. Fuel 2019, 254, 115548. [Google Scholar] [CrossRef]
- Kowalski, Z.; Makara, A. Sustainable systems for the production of district heating using meat-bone meal as biofuel: A Polish case study. Energies 2022, 15, 3615. [Google Scholar] [CrossRef]
- Silvasy, T.; Ahmad, A.A.; Wang, K.H.; Radovich, T.J.K. Rate and timing of meat and bone meal applications influence growth, yield, and soil water nitrate concentrations in sweet corn production. Agronomy 2021, 11, 1945. [Google Scholar] [CrossRef]
- Nogalska, A.; Załuszniewska, A. The effect of meat and bone meal applied without or with mineral nitrogen on macronutrient content and uptake by winter oilseed rape. J. Elem. 2020, 25, 905–915. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Basu, L. By-Products|Inedible. In Encyclopedia of Meat Sciences, 2nd ed.; Reference Module in Food Science; Dikeman, M., Devine, C., Eds.; Academic Press: Oxford, UK, 2014; pp. 125–136. [Google Scholar] [CrossRef]
- Cascarosa, E.; Boldrin, A.; Astrup, T. Pyrolysis and gasification of meat-and-bone-meal: Energy balance and GHG accounting. Waste Manag. 2013, 33, 2501–2508. [Google Scholar] [CrossRef]
- Jeng, A.S.; Haraldsen, T.K.; Grønlund, A.; Pedersen, P.A. Meat and bone meal as nitrogen and phosphorus fertilizer to cereals and rye grass. Nutr. Cycl. Agroecosyst. 2006, 76, 183–191. [Google Scholar] [CrossRef]
- Central Statistical Office of Poland. 2021. Available online: https://www.statista.com/statistics/1036706/poland-deliveries-of-meat-product/ (accessed on 5 February 2021).
- Kowalski, Z.; Makara, A. Innovative System for Animal Waste Utilization Using Closed-Loop Material and Energy Cycles and Bioenergy: A Case Study. Energies 2025, 18, 2579. [Google Scholar] [CrossRef]
- Kowalski, Z.; Makara, A.; Generowicz, A.; Ciuła, J. Improving the Quality of Hydroxyapatite Ashes from the Combustion of Meat-Bone Meal in an Industrial Rotary Kiln. Energies 2023, 16, 5911. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal By-Products Regulation). Off. J. Eur. Union 2009, 300, 1–33. [Google Scholar]
- European Union. Commission Regulation (EU) No 142/2011 of 25 February 2011 Implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council Laying Down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Implementing Council Directive 97/78/EC as Regards Certain Samples and Items Exempt from Veterinary Checks at the Border Under That Directive. Off. J. Eur. Union 2011, 54, 1–254. [Google Scholar]
- Ellen MacArthur Foundation. Towards the Circular Economy, Vol. 3: Accelerating the Scale-Up Across Global Supply Chains; Ellen Macarthur Foundation: Cowes, UK, 2014; Available online: https://www.ellenmacarthurfoundation.org/towards-the-circular-economy-vol-3-accelerating-the-scale-up-across-global (accessed on 12 November 2023).
- Neves, A.; Godina, R.; Azevedo, S.G.; Matias, J.C.O. A comprehensive review of industrial symbiosis. J. Clean. Prod. 2020, 247, 119113. [Google Scholar] [CrossRef]
- Shi, L. Industrial symbiosis: Context and relevance to the sustainable development goals (SDGs). In Responsible Consumption and Production; Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–12. [Google Scholar]
- Asif, F.M.A.; Lieder, M.; Rashid, A. Multi-method simulation based tool to evaluate economic and environmental performance of circular product systems. J. Clean. Prod. 2016, 139, 1261–1281. [Google Scholar] [CrossRef]
- Liu, C.; Côté, R.P.; Zhang, K. Implementing a three-level approach in industrial symbiosis. J. Clean. Prod. 2015, 87, 318–327. [Google Scholar] [CrossRef]
- Cecchin, A.; Salomone, R.; Deutz, P.; Raggi, A.; Cutaia, L. Relating industrial symbiosis and circular economy to the sustainable development debate. In Industrial Symbiosis for the Circular Economy; Strategies for Sustainability; Salomone, R., Cecchin, A., Deutz, P., Raggi, A., Cutaia, L., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–25. [Google Scholar]
- Bocken, N.M.P.; de Pauw, I.; Bakker, C.; van der Grinten, B. Product design and business model strategies for a circular economy. J. Ind. Prod. Eng. 2016, 33, 308–320. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, J.; Xu, S.; Xiong, Q.; Xu, X.; Li, J.; Huang, H. Meat & bone meal (MBM) incineration ash for phosphate removal from wastewater and afterward phosphorus recovery. J. Clean. Prod. 2019, 238, 117960. [Google Scholar] [CrossRef]
- Tan, Z.; Lagerkvist, A. Phosphorus recovery from the biomass ash: A review. Renew. Sust. Energ. Rev. 2011, 15, 3588–3602. [Google Scholar] [CrossRef]
- Kwon, W.B.; Kim, B.G. Standardized total tract digestibility of phosphorus in various inorganic phosphates fed to growing pigs. Anim. Sci. J. 2017, 88, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Smol, M.; Kowalski, Z.; Makara, A.; Henclik, A. Comparative LCA study of different methods of the feed phosphates (FPs) production. J. Clean. Prod. 2019, 239, 117963. [Google Scholar] [CrossRef]
- Lamp, A.E.; Mereu, A.; Ruiz-Ascacibar, I.; Moritz, J.S. Inorganic feed phosphate type determines mineral digestibility, broiler performance, and bone mineralization. J. Appl. Poult. Res. 2020, 29, 559–572. [Google Scholar] [CrossRef]
- Kleyn, R. Chicken Nutrition: A Guide for Nutritionists and Poultry Professionals; Context Publications: Leicestershire, UK, 2013. [Google Scholar]
- Shastak, Y.; Witzig, M.; Hartung, K.; Rodehutscord, M. Comparison of retention and prececal digestibility measurements in evaluating mineral phosphorus sources in broilers. Poult. Sci. 2012, 91, 2201–2209. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, C.; Yang, S.; Yin, H.; Yang, Z.; Chi, R. Life cycle assessment of feed grade mono-dicalcium phosphate production in China, a case study. J. Clean. Prod. 2021, 290, 125182. [Google Scholar] [CrossRef]
- Woyengo, A.T.; Nørgaard, J.V.; van der Heide, M.E.; Nielsen, T.S. Calcium and phosphorus digestibility in rock- and bone-derived calcium phosphates for pigs and poultry: A review. Anim. Feed Sci. Technol. 2022, 294, 115509. [Google Scholar] [CrossRef]
- Wilkosz-Język, A. Production of Calcium Phosphate from Meat and Bone Meal. Ph.D. Thesis, Cracow University of Technology, Cracow, Poland, 2007. (In Polish). [Google Scholar]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- EUR-Lex, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Closing the Loop—An EU Action Plan for the Circular Economy, COM/2015/0614 final, Document 52015DC0614, Brussels 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015DC0614 (accessed on 25 May 2025).
- Lieder, M.; Rashid, A. Towards circular economy implementation: A comprehensive review in the context of manufacturing industry. J. Clean. Prod. 2016, 115, 36–51. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kalantar, M. The analysis of the effects of clean technologies from economic point of view. J. Clean. Prod. 2015, 102, 394–407. [Google Scholar] [CrossRef]
- Kowalski, Z. (Mineral and Energy Economy Research Institute of the Polish Academy of Sciences, Kraków, Poland); Makara, A. (Cracow University of Technology, Faculty of Chemical Engineering and Technology, Kraków, Poland). Technological Assumptions for the Meat Bone Meal MBM Combustion Unit for Farmutil HS SA. Unpublished Report. 2017. (In Polish)
- Kowalski, A.; Banach, M.; Makara, A. Optimization of the co-combustion of meat–bone meal and sewage sludge in terms of the quality produced ashes used as substitute of phosphorites. Environ. Sci. Pollut. Res. 2021, 28, 8205–8214. [Google Scholar] [CrossRef] [PubMed]
- International Fertilizer Industry Association (IFA). Evaluation of Commonly Used Methods for the Analysis of Acid-Soluble Phosphate in Internationally Traded Inorganic Fertilizers, A/14/92 June 2014, France. Available online: https://fertilizer.org/wp-content/uploads/2023/01/2014_ifa_phosphate_method.pdf (accessed on 25 May 2025).
- ISO 15959:2016; Fertilizers—Determination of Extracted Phosphorus. ISO: Geneva, Switzerland, 2016.
- StatSoft Electronic Statistics Handbook. Available online: http://www.statsoft.pl/textbook/stathome.html (accessed on 25 June 2025). (In Polish).
- Ockerman, H.W.; Hansen, C.L. Animal by-product processing. In Ellis Horwood Series in Food Science and Technology; VCH Verlagsgesellschaft: Weinheim, Germany, 1988. [Google Scholar]
- Kowalski, Z.; Makara, A. The circular economy model used in the polish agro-food consortium: A case study. J. Clean. Prod. 2021, 284, 124751. [Google Scholar] [CrossRef]
- PN-R-64801:1999; Animal Feeding Stuffs. Feed Meals of Animal Origin. Polish Standard: Warsaw, Poland, 2013. (In Polish)
- Hicks, T.M.; Verbeek, C.J.R. Chapter 3-Meat Industry Protein By-Products: Sources and Characteristics. In Protein Byproducts: Transformation from Environmental Burden into Value-Added Products; Dhillon, G.S., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 37–61. [Google Scholar] [CrossRef]
- Buckley, M.; Penkman, K.E.H.; Wess, T.J.; Reaney, S.; Collins, M.J. Protein and mineral characterization of rendered meat and bone meal. Food Chem. 2012, 134, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- BREF. European Commission, Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques in the Slaughterhouses and Animal By-Products Industries. 2005. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2020-01/sa_bref_0505.pdf (accessed on 15 May 2025).
- Tang, W.; Xu, W.; Zhong, M.; Zhang, Z. Slightly doped hydroxyapatite pigments of subtractive color with high near-infrared reflectance. J. Solid State Chem. 2023, 322, 123947. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials (Text with EEA relevance). Off. J. Eur. Union 2013, 29, 1–29. [Google Scholar]
- Phosphea. Quality Classification of Inorganic Feed Phosphates. 2025. Available online: https://www.phosphea.com/expert-files/quality-classification-of-inorganic-feed-phosphates/ (accessed on 12 August 2025).
- Campos, I.; Valente, L.M.P.; Matos, E.; Marques, P.; Freire, F. Life-cycle assessment of animal feed ingredients: Poultry fat, poultry by-product meal and hydrolyzed feather meal. J. Clean. Prod. 2020, 252, 119845. [Google Scholar] [CrossRef]
- Jamroz, D. Animal Nutrition and Feed Science; PWN: Warsaw, Poland, 2001; Volumes 1–3. (In Polish) [Google Scholar]
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed—Council Statement. OJ L 140. 30 May 2002, pp. 10–22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM:l12069 (accessed on 2 August 2025).
- European Chemicals Agency (ECHA). Guidance for Identification and Naming of Substances Under REACH and CLP. Version 3.0. 2023. Available online: https://echa.europa.eu/documents/10162/2324906/substance_id_en.pdf (accessed on 24 July 2025).
- Cauduro, J. In Vitro Testing of Inorganic Phosphorus Sources for Phosphorus Availability in Swine. Master’s Thesis, RMIT University, School of Applied Sciences, Science, Engineering and Technology Portfolio, Melbourne, Australia, 2009. Available online: https://core.ac.uk/download/pdf/15614781.pdf (accessed on 15 July 2025).
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquacult. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Petersen, G.I.; Pedersen, C.; Lindemann, M.D.; Stein, H.H. Relative bioavailability of phosphorus in inorganic phosphorus sources fed to growing pigs. J. Anim. Sci. 2011, 89, 460–466. [Google Scholar] [CrossRef]
- Santos, A.A.d.L.; Leal, G.F.; Marques, M.R.; Reis, L.C.C.; Junqueira, J.R.d.J.; Macedo, L.L.; Corrêa, J.L.G. Emerging Drying Technologies and Their Impact on Bioactive Compounds: A Systematic and Bibliometric Review. Appl. Sci. 2025, 15, 6653. [Google Scholar] [CrossRef]
- Monocalcium Phosphate Price Trend and Forecast. Available online: https://www.chemanalyst.com/Pricing-data/monocalcium-phosphate-1572 (accessed on 25 June 2025).
- Kowalski, Z.; Kulczycka, J.; Skowron, G.; Sobczak, A. Comparative evaluation of calcium feed phosphate production methods using Life Cycle Assessment. Arch. Environ. Prot. 2007, 33, 83–94. [Google Scholar]
- Leiva, H.; Julian, I.; Ventura, L.; Wallin, E.; Vendt, M.; Fornell, R.; Paniagua, F.G.; Ascaso, S.; Gomez-Perez, M. Advancing Sustainability Through Industrial Symbiosis: A Technoeconomic Approach Using Material Flow Cost Accounting and Cost–Benefit Analysis. Sustainability 2025, 17, 2730. [Google Scholar] [CrossRef]
- Alrawashdeh, S.J.M.; Kumar, K.; Deshpande, M.R.; Raathi, R.; Teltumbade, G.R. Circular Economy—Innovative Strategies and Sustainable Solutions. In Management of Waste to Control Environmental Pollutions: Sustainability and Economic Feasibility; Sustainable Landscape Planning and Natural Resources Management; Parray, J.A., Shameem, N., Haghi, A.K., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
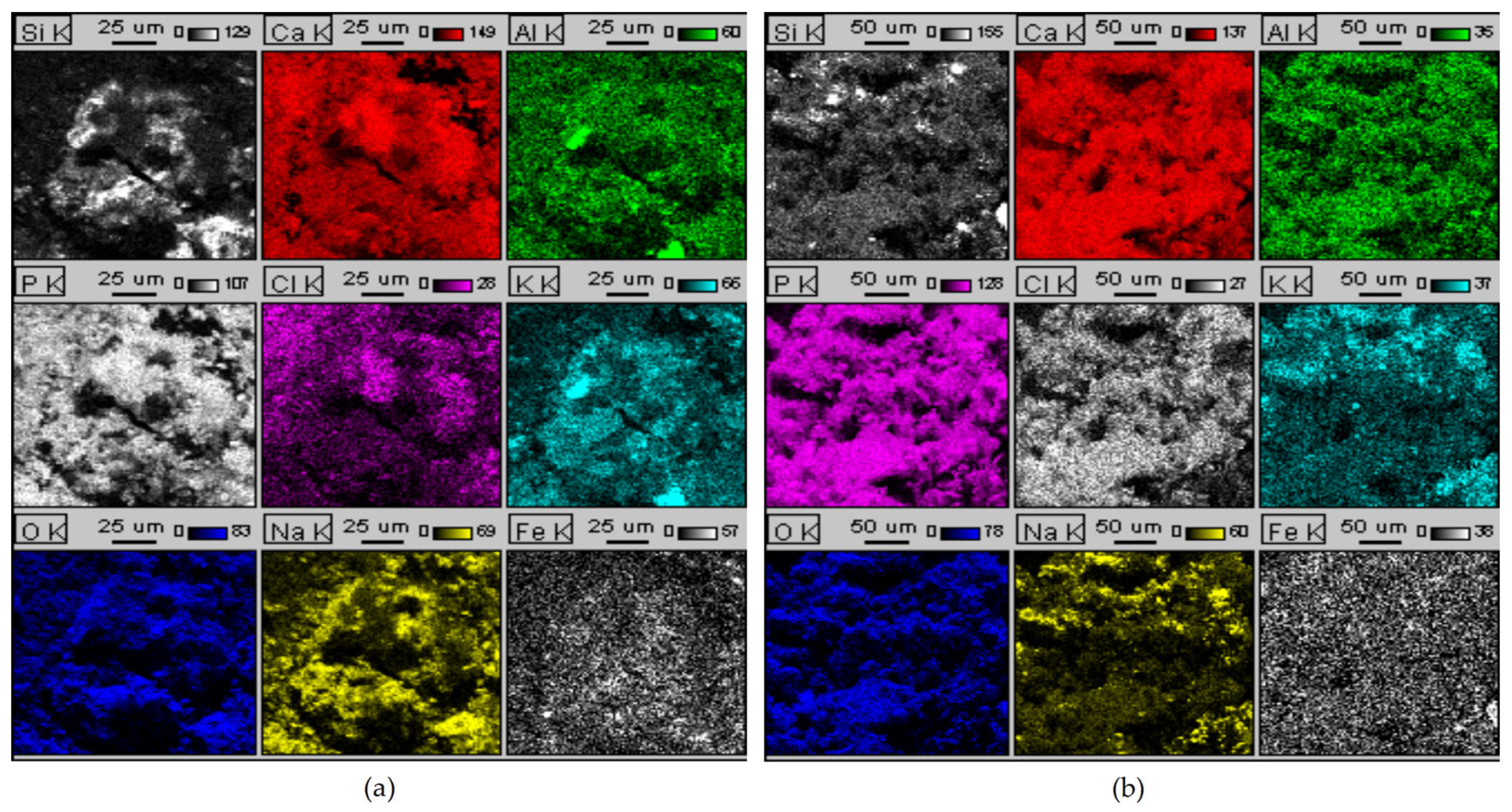
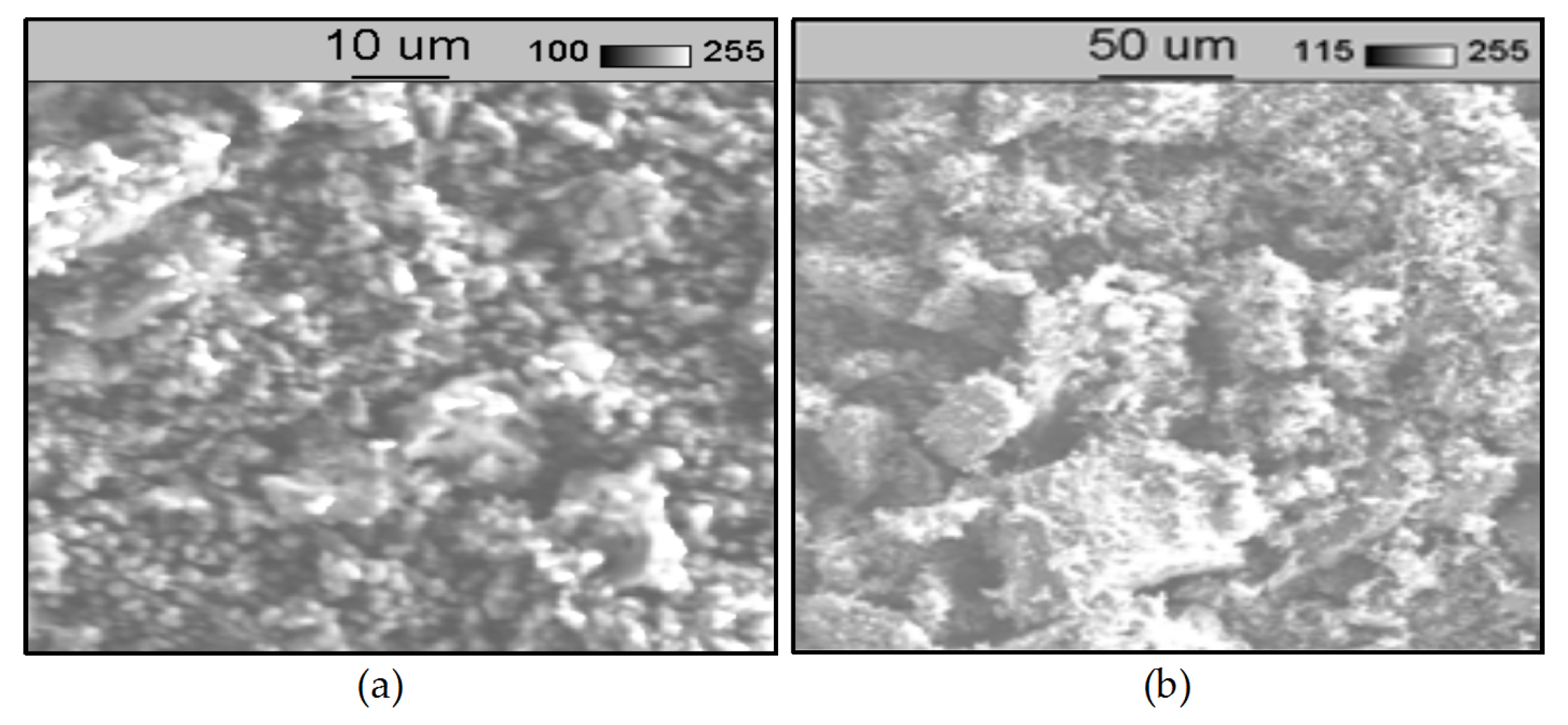




| Features | Physicochemical Requirements of Different Types of Meal | |||||
|---|---|---|---|---|---|---|
| MM | MBM | MB | BM | SM | LM | |
| Appearance | Loose, homogeneous, not charred | |||||
| Odor | Characteristic, without moldy or musty odors | |||||
| Fineness, sieving through a 4 mm square mesh sieve (%) | 100 | |||||
| Moisture, ≤ (%) | 10 | |||||
| Crude fiber content, ≤ (%) | 1 | |||||
| Crude ash content, ≤ (%) | As the producer declares | 5.5 | As the producer declares | |||
| Total phosphorus content, ≥ (%) | 5.5 | 9 | 9 | Not standardized | ||
| Total Protein Content, ≥ (%) | 55.0 | 40.0 | 26.5 | 89.0, 80.0 1 | 45.0 | 70.0 |
| Content of Digestible Proteins in total protein, ≥ (%) | 87.0 | 87.0 | 80.0 | 90.0 | 80.0 | 80.0 |
| Calcination Temperature of MBM | Weight Loss (%) | P Content (%) | Ca Content (%) | X-Ray Phase Composition |
|---|---|---|---|---|
| 600 °C | 70 | 14.5 ± 0.5 * | 33.8 ± 0.4 * | Ca10(PO4)6(OH)2, SiO2, Ca3(PO4)2, CaCO3 |
| 950 °C | 77 | 15.0 ± 0.4 * | 36.6 ± 0.6 * | Ca10(PO4)6(OH)2, SiO2, Ca3(PO4)2 |
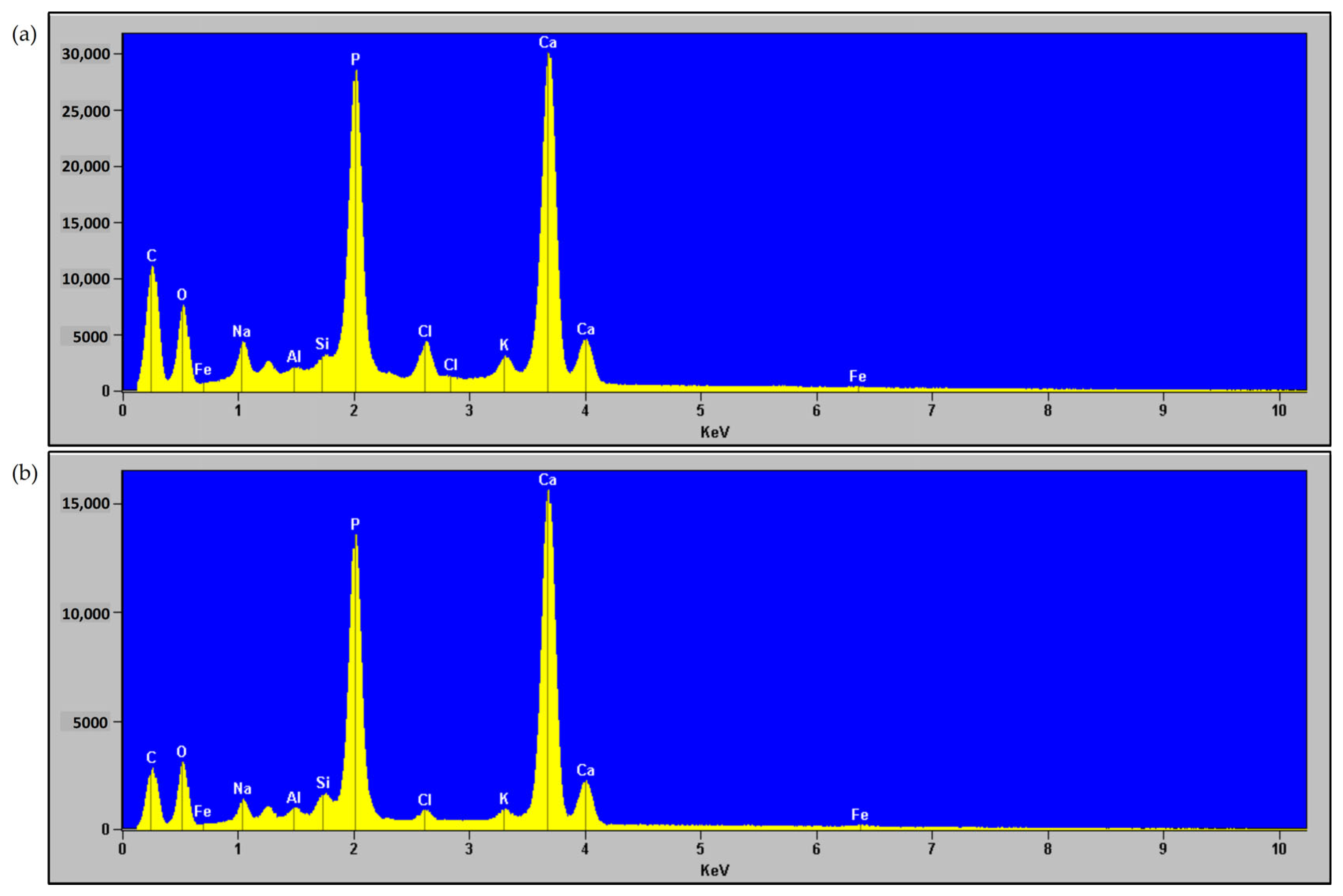
| Element | MBM | HA After MBM Incineration at 750 °C | ||
|---|---|---|---|---|
| Content (%) | Uncertainty ± % | Content (%) | Uncertainty ± % | |
| Ca | 33.3 | 6.6 | 7.95 | 1.59 |
| K | 0.68 | 0.136 | 0.452 | 0.090 |
| Na | 1.58 | 0.32 | 0.440 | 0.088 |
| P | 4.18 | 0.84 | 17.9 | 3.6 |
| N | 8.10 | 1.60 | 0.16 | 0.03 |
| Mg | 0.784 | 0.157 | 0.199 | 0.040 |
| Content (mg/kg) | Uncertainty ± mg/kg | Content (mg/kg) | Uncertainty ± mg/kg | |
| As | <0.010 | - | 0.84 | 0.13 |
| Cd | <0.002 | - | 0.014 | 0.004 |
| Cu | 7.5 | 1.1 | 43 | 6 |
| Fe | 3410 | 680 | 1010 | 200 |
| Hg | 0.013 | 0.002 | <0.10 | - |
| Pb | 1.0 | 0.1 | 1.3 | 0.2 |
| Zn | 129 | 19 | 189 | 28 |
| Si | 76 | 11 | 3410 | 680 |
| Cl− | 385 | 58 | 146 | 22 |
| F− | 117 | 23 | 432 | 86 |
| Mass Ratio of MBM:HA | P Content (%) | Ca Content (%) |
|---|---|---|
| 1:4 | 16.8 ± 0.5 * | 36.7 ± 0.8 * |
| 1:5 | 16.4 ± 0.6 * | 36.3 ± 0.7 * |
| 1:6 | 16.6 ± 0.5 * | 35.5 ± 1.0 * |
| 1:7 | 16.8 ± 0.7 * | 36.1 ± 0.7 * |
| 1:8 | 16.7 ± 0.4 * | 36.0 ± 0.6 * |
| 1:9 | 16.6 ± 0.5 * | 37.5 ± 1.0 * |
| 1:10 | 16.8 ± 0.5 * | 35.7 ± 0.5 * |
| No | MBM:HA Mass Ratio | Temperature (°C) | Time (min) | P Content (%) | Ca Content (%) |
|---|---|---|---|---|---|
| 1 | 1:8.2 | 879 | 150 | 16.67 ± 0.4 * | 36.16 ± 0.7 * |
| 2 | 1:8.2 | 671 | 150 | 16.85 ± 0.5 * | 36.05 ± 0.5 * |
| 3 | 1:2.8 | 879 | 150 | 16.27 ± 0.4 * | 36.29 ± 0.8 * |
| 4 | 1:2.8 | 671 | 150 | 16.30 ± 0.5 * | 35.80 ± 0.8 * |
| 5 | 1:8.2 | 879 | 60 | 16.57 ± 0.5 * | 36.59 ± 0.7 * |
| 6 | 1:8.2 | 671 | 60 | 16.74 ± 0.5 * | 35.80 ± 0.6 * |
| 7 | 1:2.8 | 879 | 60 | 16.72 ± 0.7 * | 36.18 ± 0.8 * |
| 8 | 1:2.8 | 671 | 60 | 16.90 ± 0.4 * | 37.05 ± 1.0 * |
| 9 | 1:1 | 775 | 105 | 16.36 ± 0.5 * | 34.99 ± 0.8 * |
| 10 | 1:10 | 775 | 105 | 16.80 ± 0.5 * | 35.24 ± 1.0 * |
| 11 | 1:5.5 | 600 | 105 | 16.69 ± 0.6 * | 35.70 ± 1.1 * |
| 12 | 1:5.5 | 950 | 105 | 17.27 ± 0.4 * | 35.93 ± 0.9 * |
| 13 | 1:5.5 | 775 | 30 | 17.02 ± 0.5 * | 35.69 ± 0.8 * |
| 14 | 1:5.5 | 775 | 180 | 16.93 ± 0.6 * | 35.49 ± 0.7 * |
| 15 | 1:5.5 | 775 | 105 | 16.58 ± 0.7 * | 36.11 ± 1.1 * |
| 16 | 1:5.5 | 775 | 105 | 16.66 ± 0.5 * | 35.82 ± 0.9 * |
| 17 | 1:5.5 | 775 | 105 | 16.86 ± 0.5 * | 35.30 ± 1.0 * |
| 18 | 1:5.5 | 775 | 105 | 16.74 ± 0.4 * | 36.43 ± 1.0 * |
| 19 | 1:5.5 | 775 | 105 | 17.07 ± 0.5 * | 35.67 ± 0.6 * |
| 20 | 1:5.5 | 775 | 105 | 17.03 ± 0.6 * | 35.77 ± 0.8 * |
| Test | Temperature (°C) | Mass Ratio of MBM:HA | P Content (%) | Ca Content (%) | X-Ray Phase Composition |
|---|---|---|---|---|---|
| 1 | 600 | 1:1 | 16.11 ± 0.4 * | 36.49 ± 0.9 * | Ca10(PO4)6(OH)2 |
| 2 | 600 | 1:2 | 14.98 ± 0.5 * | 37.29 ± 0.7 * | Ca10(PO4)6(OH)2 |
| 3 | 600 | 1:3 | 16.70 ± 0.6 * | 36.95 ± 0.8 * | Ca10(PO4)6(OH)2 |
| 4 | 800 | 1:1 | 17.42 ± 0.7 * | 36.97 ± 1.0 * | Ca10(PO4)6(OH)2 |
| 5 | 800 | 1:2 | 16.95 ± 0.5 * | 36.71 ± 0.7 * | Ca10(PO4)6(OH)2 |
| 6 | 800 | 1:3 | 16.49 ± 0.6 * | 37.11 ± 0.6 * | Ca10(PO4)6(OH)2 |
| Features | Feed Phosphate Type | |||||
|---|---|---|---|---|---|---|
| Monocalcium | Dicalcium | Tricalcium | Calcium-Sodium | Sodium Calcium Magnesium | Ammonium | |
| Appearance | Loose or granulated | |||||
| Odor and color | specific | |||||
| Fineness: loose phosphates—residue on a sieve with a mesh of 0.3 mm, ≤ (%) | 10 | |||||
| Granulated and loose phosphates, sifting throughout a sieve mesh of 3 mm, (%) | 100 | |||||
| Phosphorus content, ≤ (%) | 22 | 16 | 18 | 16 | 17 | 25 |
| Phosphorus content, soluble in 0.4% HCl solution, ≤ (%) | 20.0 | 14.5 | 16.0 | 14.5 | 15.3 | 22.5 |
| Calcium content, (%) | 15–20 | 21–30 | 31–35 | 12–26 | 5–10 | |
| Sodium content, (%) | 6–8 | 11–14 | ||||
| Magnesium content, ≤ (%) | 3 | |||||
| Nitrogen content, % | 11–12 | |||||
| Chlorides as NaCl, ≤ (%) | 1 | 1 | ||||
| Fluorine content, ≤ (%) | 0.2 | |||||
| Lead content, ≤ (%) | 0.0030 | |||||
| Cadmium content, ≤ (%) | 0.0010 | |||||
| Mercury content, ≤ (%) | 0.00010 | |||||
| Arsenic content, ≤ (%) | 0.0010 | |||||
| Variant | P (%) | Ca Content (%) | X-Ray Phase Composition | ||
|---|---|---|---|---|---|
| Total Content | Solubility in 0.4% HCl | Solubility in 2% Citric Acid | |||
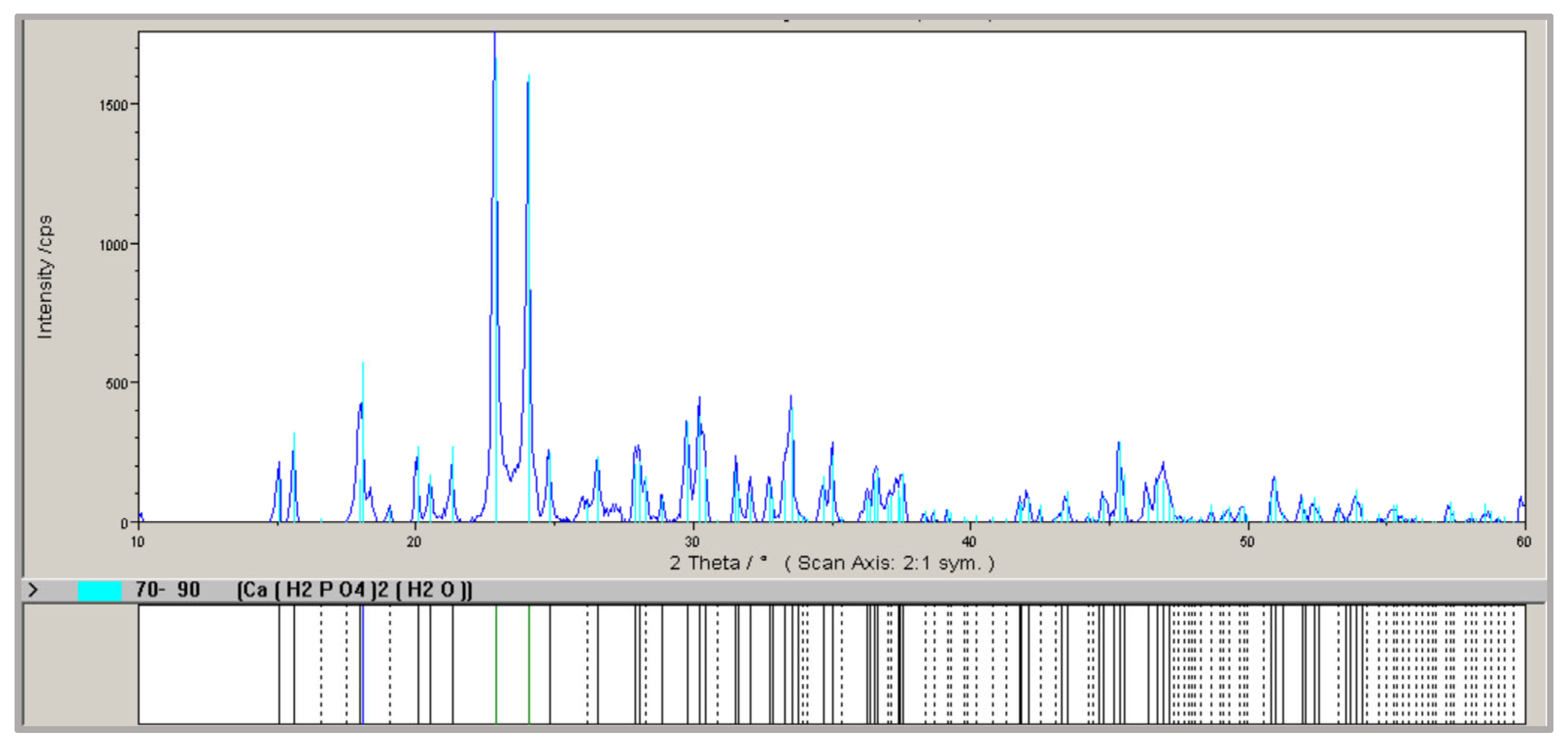
| A | 18.5 ± 0.4 * | 100 ± 0.8 * | 100 ± 0.7 * | 16.0 ± 0.5 * | Ca(H2PO4)2 · H2O, Ca10(PO4)6(OH)2 |
| B | 21.9 ± 0.5 * | 100 ± 0.8 * | 100 ± 0.9 * | 16.5 ± 0.4 * | Ca(H2PO4)2 · H2O, Ca10(PO4)6(OH)2 |
| C | 22.5 ± 0.5 * | 100 ± 0.6 * | 100 ± 0.8 * | 16.5 ± 0.4 * | Ca(H2PO4)2 · H2O, Ca10(PO4)6(OH)2 |
| Recycled MCP:HA Mass Ratio | Test | P Content (%) | Ca Content (%) | X-Ray Phase Composition | ||
|---|---|---|---|---|---|---|
| Total | Soluble in 0.4% HCl | Soluble in 2% Citric Acid | ||||
| 0 | 0(1) | 23.5 ± 0.4 * | 23.3 ± 0.8 * | 23.5 ± 0.6 * | 15.1 ± 0.4 * | Ca(H2PO4)2·H2O |
| 0(2) | 23.5 ± 0.5 * | 23.4 ± 0.7 * | 23.5 ± 0.5 * | 14.9 ± 0.3 * | Ca(H2PO4)2·H2O | |
| 0.5 | 0.5A | 24.0 ± 0.5 * | 22.0 ± 0.4 * | 23.2 ± 0.5 * | 16.4 ± 0.4 * | Ca(H2PO4)2·H2O |
| 0.5B | 24.3 ± 0.7 * | 22.6 ± 0.6 * | 23.6 ± 0.5 * | 15.9 ± 0.7 * | Ca(H2PO4)2·H2O | |
| 1 | 1A(1) | 24.0 ± 0.4 * | 22.8 ± 0.5 * | 23.3 ± 0.7 * | 14.4 ± 0.4 * | Ca(H2PO4)2·H2O |
| 1A(2) | 23.7 ± 0.7 * | 22.8 ± 0.6 * | 24.0 ± 0.5 * | 15.4 ± 0.6 * | Ca(H2PO4)2·H2O | |
| 1B(1) | 23.9 ± 0.7 * | 21.8 ± 0.6 * | 23.9 ± 0.5 * | 15.1 ± 0.6 * | Ca(H2PO4)2·H2O | |
| 1B(2) | 23.2 ± 0.4 * | 22.4 ± 0.4 * | 23.6 ± 0.3 * | 14.8 ± 0.3 * | Ca(H2PO4)2·H2O | |
| 1A(H) | 23.0 ± 0.5 * | 21.9 ± 0.4 * | 22.6 ± 0.3 * | 14.8 ± 0.5 * | Ca(H2PO4)2·H2O | |
| 1B(H) | 22.9 ± 0.6 * | 22.5 ± 0.7 * | 23.1 ± 0.5 * | 16.0 ± 0.6 * | Ca(H2PO4)2·H2O | |
| 2 | 2A | 23.7 ± 0.7 * | 22.3 ± 0.5 * | 23.2 ± 0.4 * | 15.7 ± 0.5 * | Ca(H2PO4)2·H2O |
| 2B | 23.9 ± 0.6 * | 22.0 ± 0.6 * | 23.3 ± 0.5 * | 16.1 ± 0.7 * | Ca(H2PO4)2·H2O | |
| 3 | 3A | 24.4 ± 0.3 * | 23.0 ± 0.5 * | 23.8 ± 0.7 * | 15.5 ± 0.5 * | Ca(H2PO4)2·H2O |
| 3B | 23.6 ± 0.6 * | 22.7 ± 0.5 * | 24.1 ± 0.5 * | 15.0 ± 0.4 * | Ca(H2PO4)2·H2O | |
| 4 | 4A | 24.3 ± 0.5 * | 22.6 ± 0.6 * | 24.0 ± 0.4 * | 15.1 ± 0.6 * | Ca(H2PO4)2·H2O |
| 4B | 24.2± 0.4 * | 22.4 ± 0.8 * | 24.6 ± 0.5 * | 14.9 ± 0.7 * | Ca(H2PO4)2·H2O | |
| 5 | 5A | 24.5 ± 0.7 * | 22.2 ± 0.5 * | 24.5 ± 0.7 * | 14.8 ± 0.5 * | Ca(H2PO4)2·H2O |
| 5B | 24.3 ± 0.5 * | 22.5 ± 0.4 * | 23.7 ± 0.6 * | 15.0 ± 0.7 * | Ca(H2PO4)2·H2O | |
| No | Calculation Position | Unit | Consumption Figure | Price per Unit ($) | Cost per | |
|---|---|---|---|---|---|---|
| 1 t ($) | Year ($) | |||||
| 1 | Direct materials | 825 | 17,902,500 | |||
| 75% H3PO4 | kg/t | 654 | ||||
| 100% H3PO4 | kg/t | 490 | 1642 | 805 | 17,468,500 | |
| 2 | Purchase costs | |||||
| 75% H3PO4 | kg/t | 654 | 31 | 20 | 434,000 | |
| 3 | Total material costs (items 1–2) | 825 | ||||
| 4 | Own semi-finished products | - | ||||
| Hydroxyapatite ash | kg/t | 346 | - | - | ||
| 5 | Process energy | 4.2 | ||||
| - electricity | kWh/t | 26 | 0.16 | 4.2 | ||
| 6 | Direct salaries | 30.14 | ||||
| 7 | Total direct costs (items 3–6) | 829 | ||||
| 8 | Chemical analyses costs | 3 | 65,100 | |||
| 9 | Environmental Use Fees | 0.5 | 10,850 | |||
| 10 | Variable line costs (items 7–9) | 833 | 18,069,590 | |||
| 11 | Maintaining Machinery and Equipment | 8.65 | 189,000 | |||
| - repairs and maintenance | 0.91 | 21,000 | ||||
| - amortization 8% | 7.74 | 168,000 | ||||
| - auxiliary materials | - | |||||
| 12 | Total Production Cost at Plant (items 10–11) | 841 | 18,258,590 | |||
| 13 | Labor resources maintenance, including: | 0.5 | 10,850 | |||
| - occupational health & safety costs | 0.5 | 10,850 | ||||
| 14 | Plant’s fixed costs | 4.35 | 100,000 | |||
| 15 | General production process management | 4.7 | 102,000 | |||
| - technical supervisors’ salaries (1700 $/person × 4 employees/month + 25% overhead) | 4.7 | 102,000 | ||||
| 16 | Total Net Production Cost at the Plant (items 12–15) | 851 | 18,471,440 | |||
| 17 | Collected for further processing (item 16) | 851 | 18,471,440 | |||
| 18 | Cost of product without packaging (item 17) | 851 | 18,471,440 | |||
| 19 | Packaging | - | - | |||
| - paper bags (25 kg) | pieces/t | 40 | 0.50 | 20 | 434,000 | |
| 20 | Main product manufacturing cost (items 18–19) | 871 | 18,905,440 | |||
| 21 | Total administrative costs | % | 3 | 26 | 567,021 | |
| 22 | Factory manufacturing cost (items 20–21) | 897 | 19,472,461 | |||
| 23 | Cost of sales | 27 | 586,486 | |||
| 22 | Total production costs (items 22–23) | 924 | 20,058,947 | |||
| 25 | MCP sales revenue | 1400 | 1400 | 30,380,000 | ||
| 26 | Profit | $ | 10,321,053 | |||
| Profit margin | % | 34 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, Z.; Wilkosz-Język, A.; Makara, A. Production of Food-Grade Monocalcium Phosphate from Meat-Bone Meal. Materials 2025, 18, 4653. https://doi.org/10.3390/ma18204653
Kowalski Z, Wilkosz-Język A, Makara A. Production of Food-Grade Monocalcium Phosphate from Meat-Bone Meal. Materials. 2025; 18(20):4653. https://doi.org/10.3390/ma18204653
Chicago/Turabian StyleKowalski, Zygmunt, Agnieszka Wilkosz-Język, and Agnieszka Makara. 2025. "Production of Food-Grade Monocalcium Phosphate from Meat-Bone Meal" Materials 18, no. 20: 4653. https://doi.org/10.3390/ma18204653
APA StyleKowalski, Z., Wilkosz-Język, A., & Makara, A. (2025). Production of Food-Grade Monocalcium Phosphate from Meat-Bone Meal. Materials, 18(20), 4653. https://doi.org/10.3390/ma18204653






