Enhanced Wear and Corrosion Resistance of AlCoCrFeNiMoTi High-Entropy Alloy via B Addition by Laser Cladding
Abstract
1. Introduction
2. Materials and Experimental Works
3. Results and Discussion
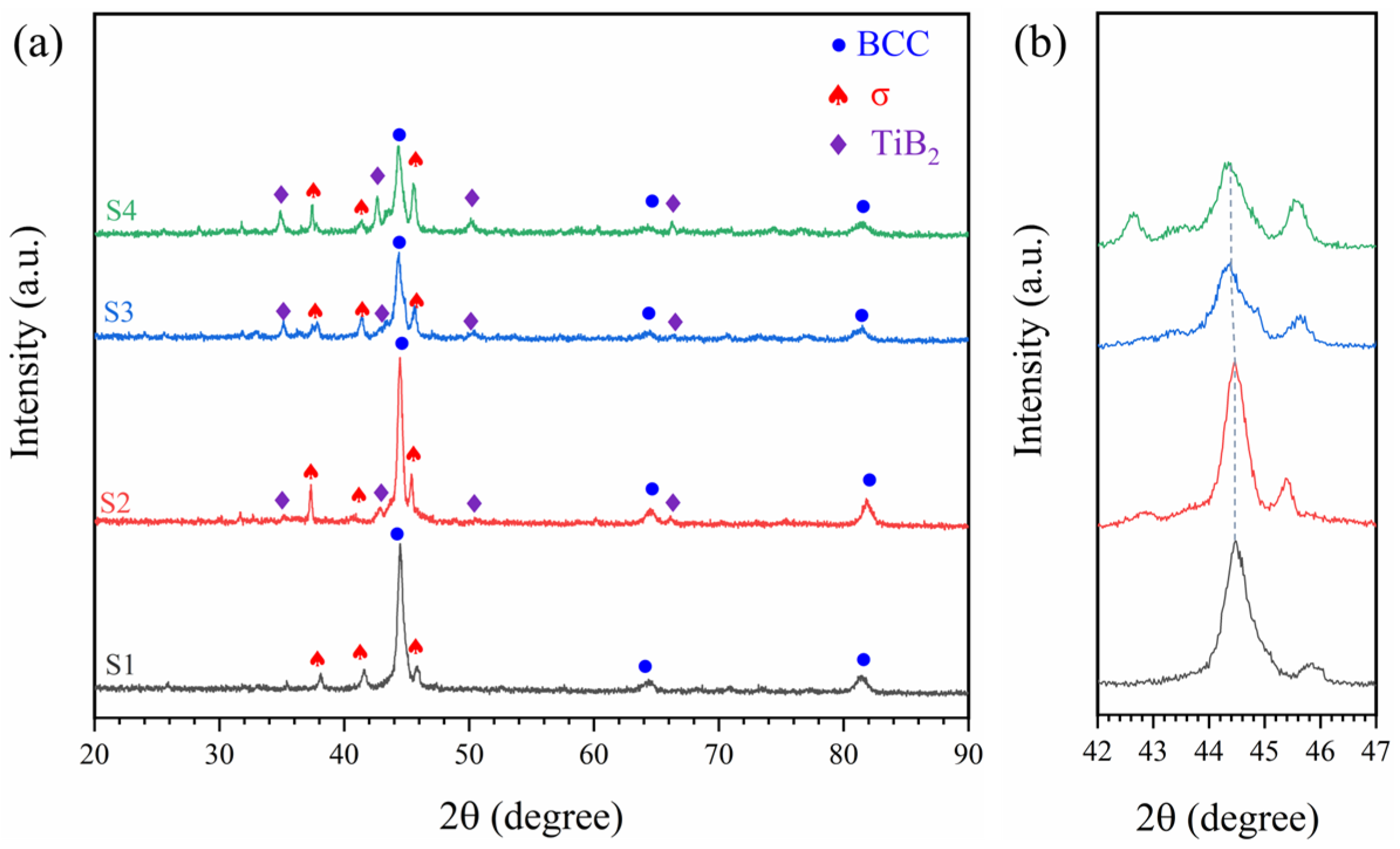
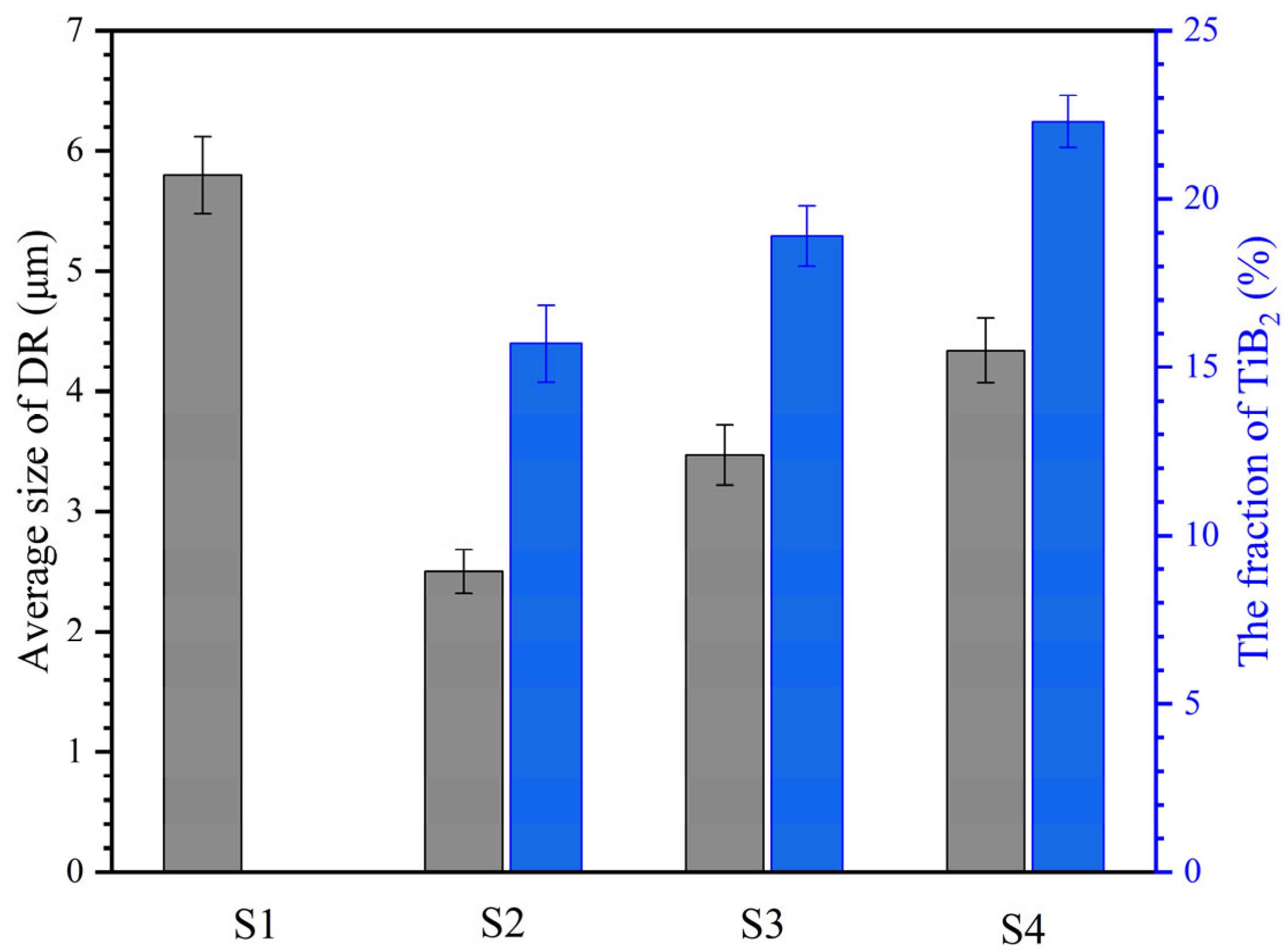
3.1. Microstructure Characterization of HEA Coatings
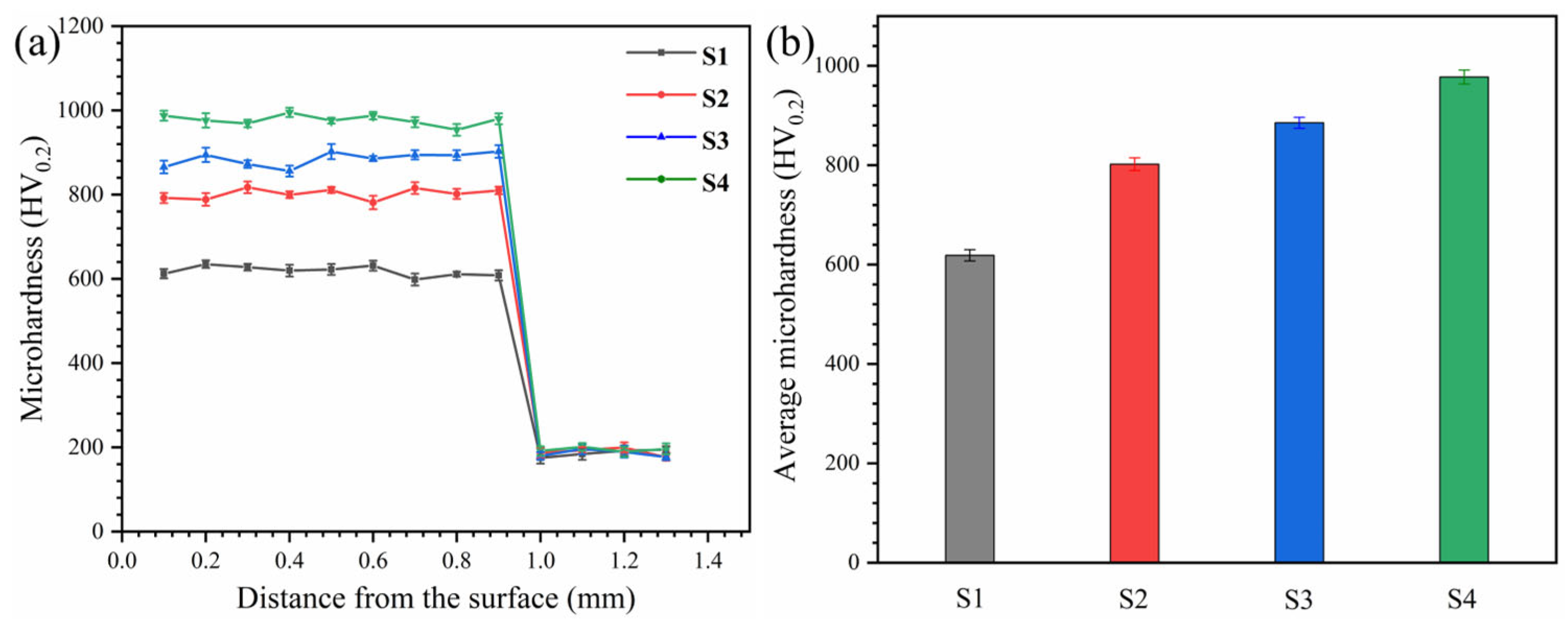
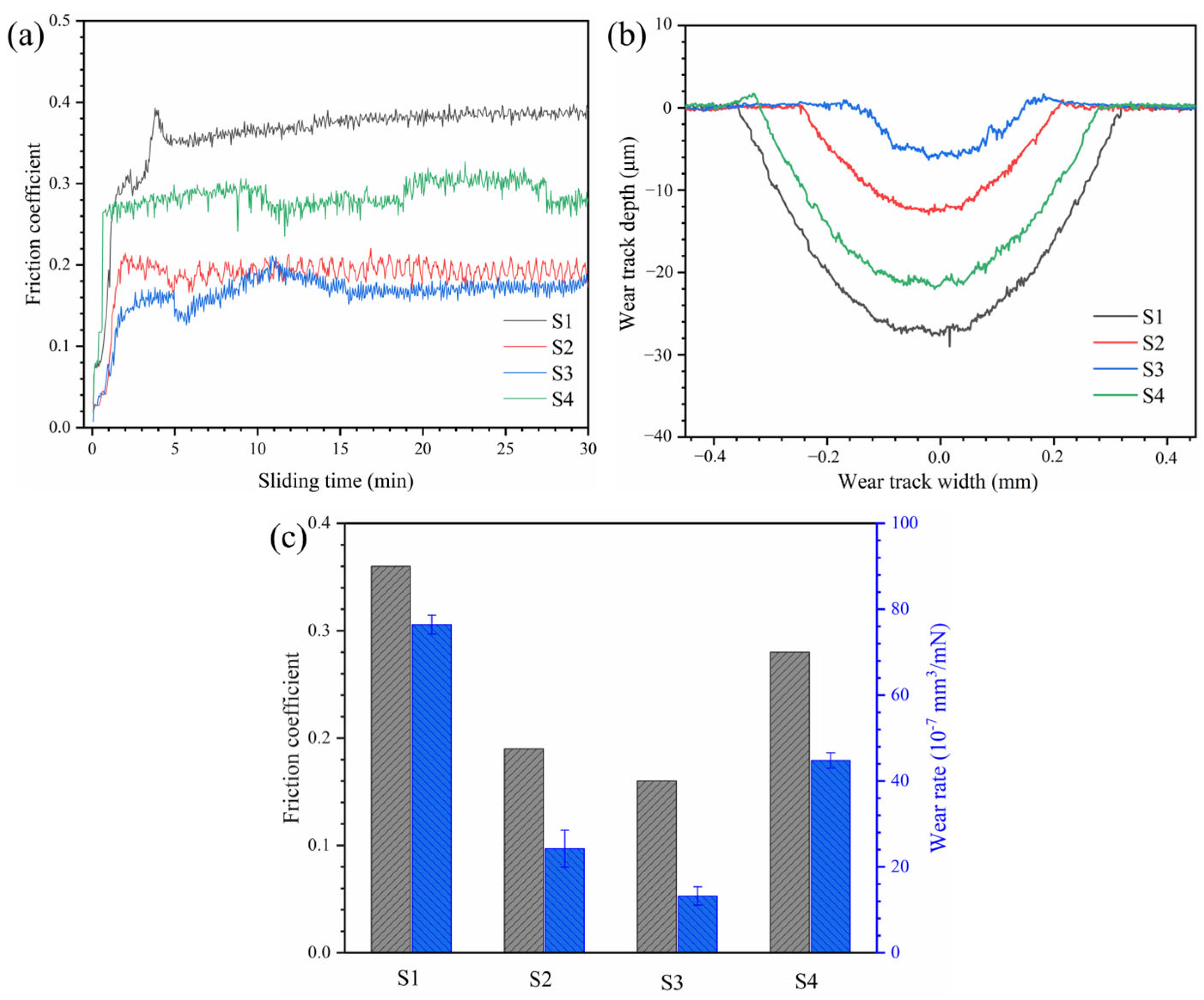
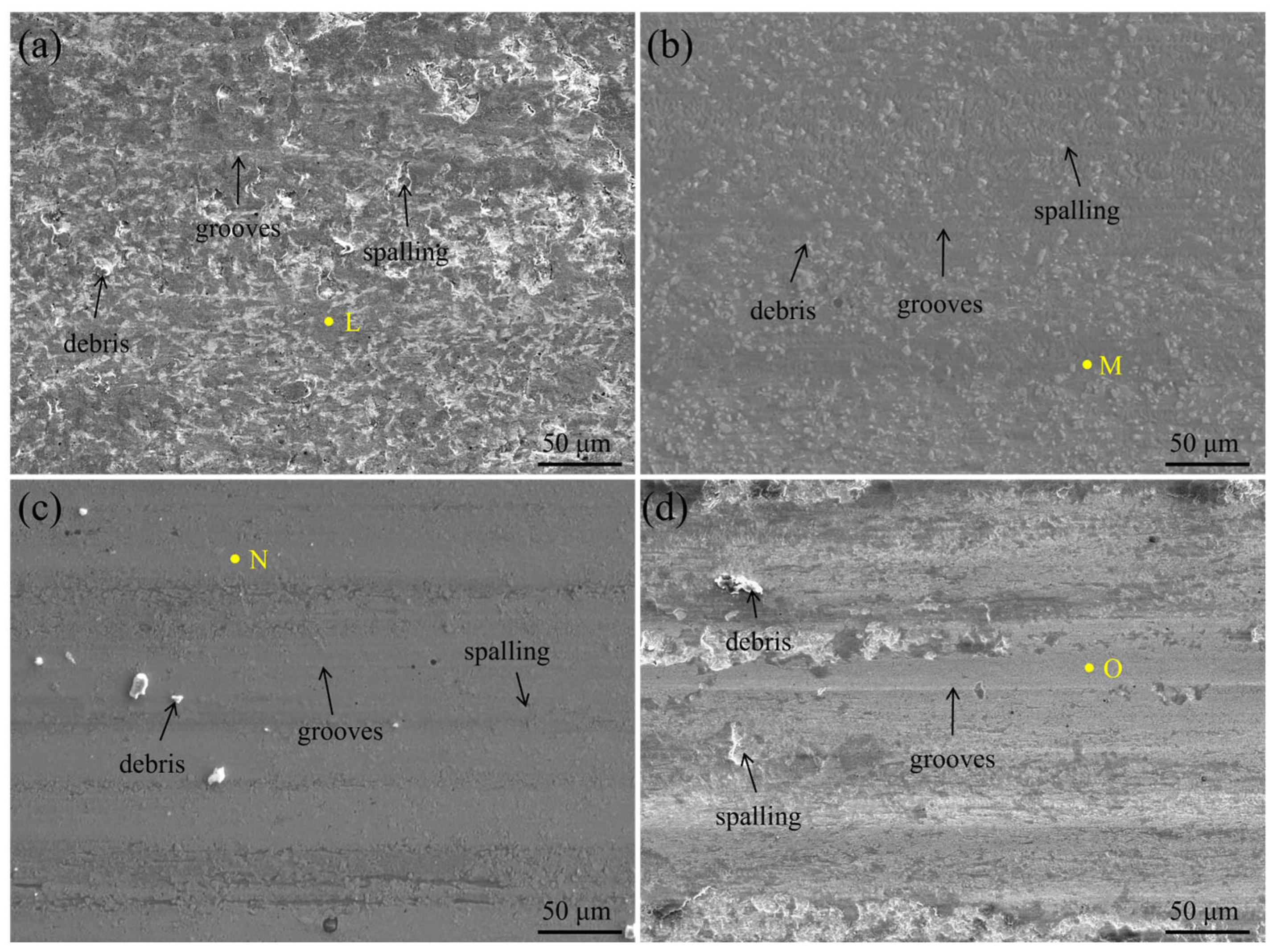
3.2. Microhardness and Room Temperature Wear Resistance Analysis of HEA Coatings
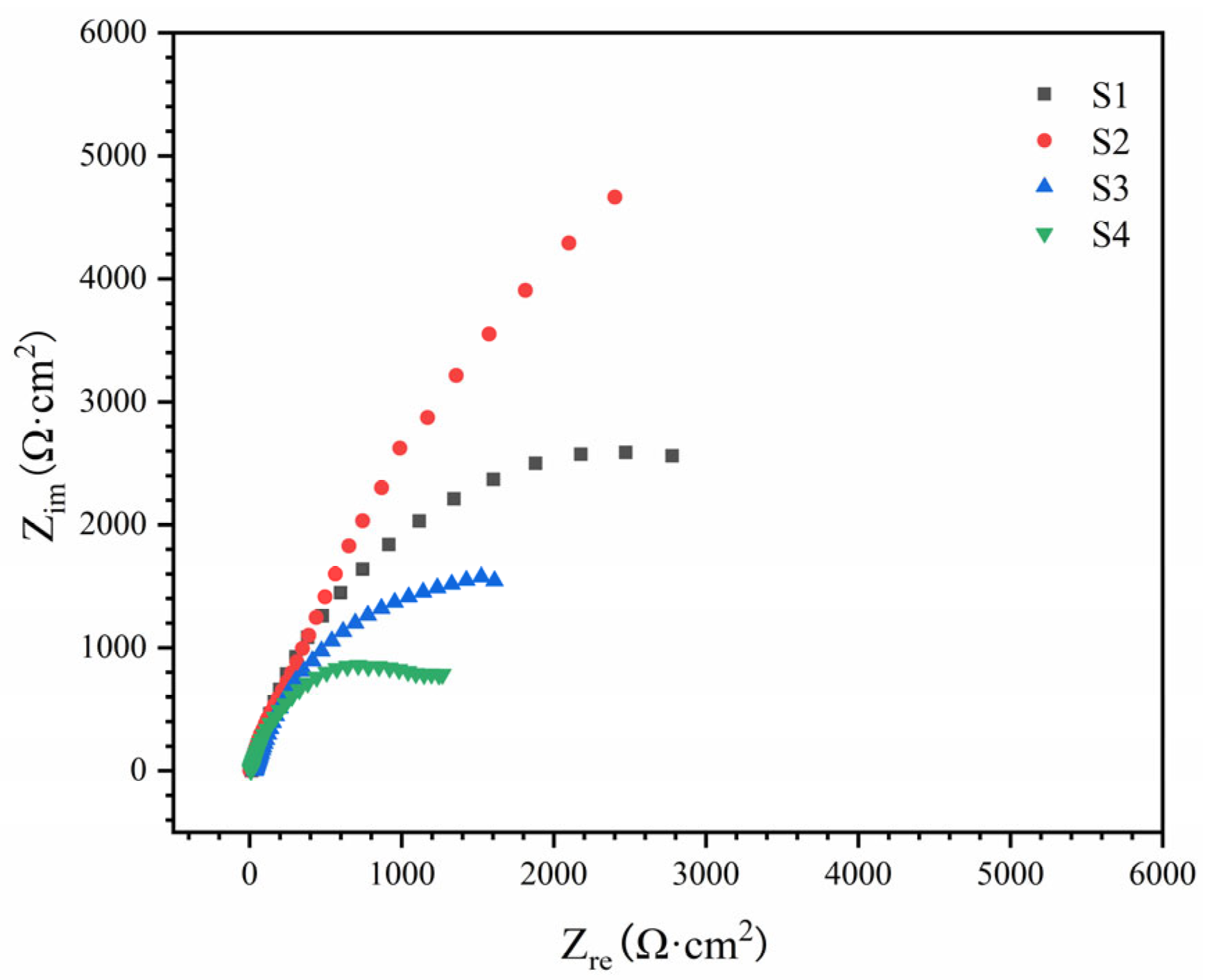
3.3. Corrosion Behavior of HEA Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Cai, Z.; Lin, G.; Chen, F.; Zhang, P.; Gu, L. Microstructure and tribological performance of ultra-high speed laser-cladded AlCrCoFeNi2.1-xTiB2 (x = 0, 0.5, 1.0, 1.5 and 2.0 wt%) high-entropy alloy coatings. Mater. Today Commun. 2023, 36, 106834. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Yu, K.; Guo, N.; Xiao, G.; Wang, Z.; Zhang, H. Effect of Y2O3 on microstructure and properties of CoCrFeNiTiNb high entropy alloy coating on Ti–6Al–4V surface by laser cladding. J. Rare Earths 2024, 42, 586–599. [Google Scholar] [CrossRef]
- Chen, S.; Oh, H.S.; Gludovatz, B.; Kim, S.J.; Park, E.S.; Zhang, Z.; Ritchie, R.O.; Yu, Q. Real-time observations of TRIP-induced ultrahigh strain hardening in a dual-phase CrMnFeCoNi high-entropy alloy. Nat. Commun. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Cantor, B.; Chang, I.T.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2024, 375, 213–218. [Google Scholar] [CrossRef]
- Xu, Z.; Li, D.Y.; Chen, D.L. Effect of Ti on the wear behavior of AlCoCrFeNi high-entropy alloy during unidirectional and bi-directional sliding wear processes. Wear 2021, 476, 203650. [Google Scholar] [CrossRef]
- Albedwawi, S.H.; AlJaberi, A.; Haidemenopoulos, G.N.; Polychronopoulou, K. High entropy oxides-exploring a paradigm of promising catalysts: A review. Mater. Des. 2021, 202, 109534. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Phase constitution, surface chemistry and corrosion behavior of electrodeposited MnFeCoNiCu high entropy alloy-graphene oxide composite coatings. Surf. Coat. Technol. 2022, 429, 127943. [Google Scholar] [CrossRef]
- Gu, Z.; Xi, S.; Mao, P.; Wang, C. Microstructure and wear behavior of mechanically alloyed powder AlxMo0.5NbFeTiMn2 high entropy alloy coating formed by laser cladding. Surf. Coat. Technol. 2020, 401, 126244. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; ur Rehman, E.; Ullah, S.; Atif, M.; Tariq, A. A review on laser cladding of high-entropy alloys, their recent trends and potential applications. J. Manuf. Process. 2021, 68, 225–273. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Asabre, A.; Gemagami, P.; Parsa, A.B.; Wagner, C.; Kostka, A.; Laplanche, G. Influence of Mo/Cr ratio on the lamellar microstructure and mechanical properties of as-cast Al0.75CoCrFeNi compositionally complex alloys. J. Alloys Compd. 2022, 899, 163183. [Google Scholar] [CrossRef]
- Hao, X.; Liu, H.; Zhang, X.; Tao, J.; Wang, Y.; Yang, C.; Liu, Y. Microstructure and wear resistance of in-situ TiN/(Nb, Ti)5Si3 reinforced MoNbTaWTi-based refractory high entropy alloy composite coatings by laser cladding. Appl. Surf. Sci. 2023, 626, 157240. [Google Scholar] [CrossRef]
- Geng, J.; Yang, X.; Wang, G.; Yin, M.; Li, J.; Li, Y. Corrigendum to “Effect of TiB2 content on the microstructure, corrosion behavior, and wear resistance of (Fe50Mn30Co10Cr10)0.8−x (TiB2)x Mo0.2 high-entropy alloy coatings by laser cladding”. Surf. Coat. Technol. 2025, 501, 131915. [Google Scholar] [CrossRef]
- Li, W.; Xie, D.; Li, D.; Zhang, Y.; Gao, Y.; Liaw, P.K. Mechanical behavior of high-entropy alloys. Prog. Mater. Sci. 2021, 118, 100777. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, T.-J.; Chen, S.-K.; Yeh, J.-W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0≤x≤2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Li, Y.; Fu, H.; Wang, K.; Yang, X.; Zong, B.; Lin, J. Effect of Mo addition on microstructure and wear resistance of laser clad AlCoCrFeNi-TiC composite coatings. Appl. Surf. Sci. 2023, 623, 157071. [Google Scholar] [CrossRef]
- Wang, E.; Li, J.; Kang, F.; Jiang, F.; Lv, L.; Dai, B.; Cao, Y.; Jiang, W. Balancing the mechanical properties of Al0.45CoCrFeNiTix high-entropy alloys by tailoring titanium content. J. Mater. Res. Technol. 2024, 28, 967–979. [Google Scholar] [CrossRef]
- Li, Z.; Jing, C.; Feng, Y.; Wu, Z.; Lin, T.; Zhao, J. Microstructure evolution and properties of laser cladding Nb containing eutectic high entropy alloys. Int. J. Refract. Met. Hard Mater. 2023, 110, 105992. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Song, C.; Wang, M.; Xiao, G.; Zhang, H.; Li, X.; Yu, K.; Xu, L. Al and Mo synergistic enhancement of CoCrFeNi high-entropy alloy laser cladding layer. J. Mater. Res. Technol. 2024, 28, 2572–2581. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Wang, Z.Y.; Zhang, C.H.; Chen, H.T.; Chen, J. New studies on wear and corrosion behavior of laser cladding FeNiCoCrMox high entropy alloy coating: The role of Mo. Int. J. Refract. Met. Hard Mater. 2022, 102, 105721. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, T.; Guan, C.; Sun, J.; Tan, X. Microstructure and friction coefficient of ceramic (TiC, TiN and B4C) reinforced Ni-based coating by laser cladding. Ceram. Int. 2019, 45, 20824–20836. [Google Scholar] [CrossRef]
- Aparicio-Fernández, R.; Springer, H.; Szczepaniak, A.; Zhang, H.; Raabe, D. In-situ metal matrix composite steels: Effect of alloying and annealing on morphology, structure and mechanical properties of TiB2 particle containing high modulus steels. Acta Mater. 2016, 107, 38–48. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, D.; Cui, Y.; Manladan, S.M.; Wang, T.; Shan, M.; Han, J. Effect of CoCrFeMnNi transition cladding layer on crack resistance of CoCrFeMnNi + x(TiC) composite cladding layer. Mater. Lett. 2021, 304, 130700. [Google Scholar] [CrossRef]
- Liu, H.; Liang, H.; Peng, G.; Chen, P.; Liu, X.; He, X.; Yu, G. Microstructure evolution and tribological performance of laser-clad Al1.5Co0.5CrFeNi2(TiB)x high-entropy alloy composite coatings with in-situ synthesized complex precipitates. Surf. Coat. Technol. 2025, 499, 131870. [Google Scholar] [CrossRef]
- Liang, G.; Jin, G.; Cui, X.; Qiu, Z.; Wang, J. The directional array TiN-reinforced AlCoCrFeNiTi high-entropy alloy synthesized in situ via magnetic field-assisted laser cladding. Appl. Surf. Sci. 2022, 572, 151407. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Li, X.; Chen, P.; Yang, H.; Hao, J. Effect of heat treatment on phase stability and wear behavior of laser clad AlCoCrFeNiTi0.8 high-entropy alloy coatings. Surf. Coat. Technol. 2020, 392, 125758. [Google Scholar] [CrossRef]
- Zhang, H.; Chong, K.; Zhao, W.; Sun, Z. Effects of pulse parameters on in-situ Ti-V carbides size and properties of Fe-based laser cladding layers. Surf. Coat. Technol. 2018, 344, 163–169. [Google Scholar] [CrossRef]
- Wang, L.; Jia, C.; Yuan, Y.; Huang, Y.; Yang, L. Microstructure and wear behaviors of (TiB2+TiB+TiC)/Ti coating fabricated by laser wire deposition. Mater. Lett. 2022, 328, 133132. [Google Scholar]
- Wang, H.; Li, Y.; Xu, G.; Li, J.; Zhang, T.; Lu, B.; Yu, W.; Wang, Y.; Du, Y. Effect of nano-TiC/TiB2 particles on the recrystallization and precipitation behavior of AA2055-TiC+TiB2 alloys. Mater. Sci. Eng. A 2023, 871, 144927. [Google Scholar] [CrossRef]
- Wu, H.; Huang, S.; Zhu, H.; Xie, Z. Strengthening FeCrNiCu high entropy alloys via combining V additions with in-situ TiC particles. Scr. Mater. 2021, 195, 113724. [Google Scholar] [CrossRef]
- Lin, T.; Feng, M.; Lian, G.; Lu, H.; Chen, C.; Huang, X. Effects of C content on the microstructure and properties of CoCrFeNiTi0.5Mo0.5Cx high-entropy alloy coatings by laser cladding. J. Mater. Res. Technol. 2024, 33, 1540–1557. [Google Scholar] [CrossRef]
- Xiang, K.; Chai, L.; Zhang, C.; Guan, H.; Wang, Y.; Ma, Y.; Sun, Q.; Li, Y. Investigation of microstructure and wear resistance of laser-clad CoCrNiTi and CrFeNiTi medium-entropy alloy coatings on Ti sheet. Opt. Laser Technol. 2022, 145, 107518. [Google Scholar] [CrossRef]
- Wu, C.L.; Xu, T.Z.; Wang, Z.Y.; Zhang, C.H.; Zhang, S.; Ni, C.L.; Zhang, D.X. Laser surface alloying of FeCoCrAlNiTi high entropy alloy composite coatings reinforced with TiC on 304 stainless steel to enhance wear behavior. Ceram. Int. 2022, 48, 20690–20698. [Google Scholar] [CrossRef]
- Li, Z.; Mei, K.; Dong, J.; Yang, Y.; Sun, J.; Luo, Z. An investigation on the wear and corrosion resistance of AlCoCrFeNi high-entropy alloy coatings enhanced by Ti and Si. Surf. Coat. Technol. 2024, 487, 130949. [Google Scholar] [CrossRef]
- Deng, G.; Tieu, A.K.; Lan, X.; Su, L.; Wang, L.; Zhu, Q.; Zhu, H. Effects of normal load and velocity on the dry sliding tribological behaviour of CoCrFeNiMo0.2 high entropy alloy. Tribol. Int. 2020, 144, 106116. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Yang, Y.; Ma, Q.; Luo, Z. Structural and property regulation of double ceramic phase–reinforced AlCoCrFeNiTiZr–based high entropy alloy coatings by pulsed–wave lasers. Ceram. Int. 2024, 50, 21193–21202. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Wu, Q.; Li, Y.; Yu, K.; Luo, Z. Substantial enhancement of AlCoCrFeNiTiWC high-entropy alloy coating performance under water cooling and pulsed laser. Tribol. Int. 2025, 201, 110273. [Google Scholar] [CrossRef]
- Guo, W.; Li, J.; Qi, M.; Xu, Y.; Ezatpour, H.R. Effects of heat treatment on the microstructure, wear behavior and corrosion resistance of AlCoCrFeNiSi high-entropy alloy. Intermetallics 2021, 138, 107324. [Google Scholar] [CrossRef]
- Cui, C.; Wu, M.; He, R.; Jie, D.; Gong, Y.; Miao, X. Microstructure, wear and corrosion behavior of high-entropy alloy coatings: The concentration of Mo element and the dual effect of σ-CrMo phase. Surf. Coat. Technol. 2023, 467, 129726. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Zhang, H.; Xiao, G.; Yu, K. Microstructure and corrosion resistance of fusion zone in Ti-6Al-4V alloy welded using pulsed- and continuous-wave lasers. Corros. Sci. 2023, 220, 111269. [Google Scholar] [CrossRef]
- Zhao, K.; Han, G.; Gao, T.; Yang, H.; Qian, Z.; Hu, K.; Liu, G.; Nie, J.; Liu, X. Interface precipitation and corrosion mechanisms in a model Al–Zn–Mg–Cu alloy strengthened by TiC particles. Corros. Sci. 2022, 206, 110533. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Qi, M.; Zhang, Q.; Qi, Y.; Jin, G. Corrosion and passivation behavior of in-situ TiC reinforced Al0.1CrNbSi0.1TaTiV refractory high entropy alloy coatings via doping C. Corros. Sci. 2024, 227, 111736. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Bai, X.; Zhao, W.; Zhang, P.; Rao, W.-F. Laser cladding highly corrosion-resistant nano/submicron ultrafine-grained Fe-based composite layers. Surf. Coat. Technol. 2021, 424, 127636. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Zhang, H.Y.; Wang, R.; Zhang, H.F.; Zhang, C.H.; Wu, C.L.; Chen, H.T. Exploration of wear and slurry erosion mechanisms of laser clad CoCrFeNi + x (NbC) high entropy alloys composite coatings. Tribol. Int. 2024, 193, 109405. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, W.; Wang, D.; Wang, K.S.; Yang, S.F.; Wang, M.Y. High WC content promotes continuous eutectic structure formation and enhances wear resistance in WC/CeO2-Fe laser cladding coatings. Surf. Coat. Technol. 2025, 513, 132491. [Google Scholar]
- Zhang, C.; Li, Q.; Zhang, X.; Zhang, G.; Li, J.; Huang, Y.; Chang, C. Effects of HVOF sprayed, laser cladding and laser remelted preparation process on wear and corrosion properties of Fe-based amorphous alloy coatings. Surf. Coat. Technol. 2025, 511, 132292. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Zhang, M.; Duan, X.; Guo, R.; Niu, B.; Wang, H.F.; Gao, X.H.; Yan, H. Microstructure and Corrosion Resistance of FeCrCoNiAl Coatings on Stainless Steel: Role of Deposition Solution, Parameters, and Aluminum Concentration. J. Alloys Compd. 2025, 1040, 183446. [Google Scholar] [CrossRef]
- Sun, R.Z.; Che, L.; Xie, L.; Zhang, D.D. Wear and corrosion resistant of NiFeSiBPNb amorphous alloy coatings sprayed by HVOF. Intermetallics 2025, 183, 108823. [Google Scholar] [CrossRef]
- Mei, K.T.; Zhang, H.; Gao, H.R.; Guo, N.; Zhao, W. Microstructure and properties of Fe-VC pulsed plasma arc cladding layers under different cooling conditions. Ceram. Int. 2024, 50, 6490–6498. [Google Scholar] [CrossRef]
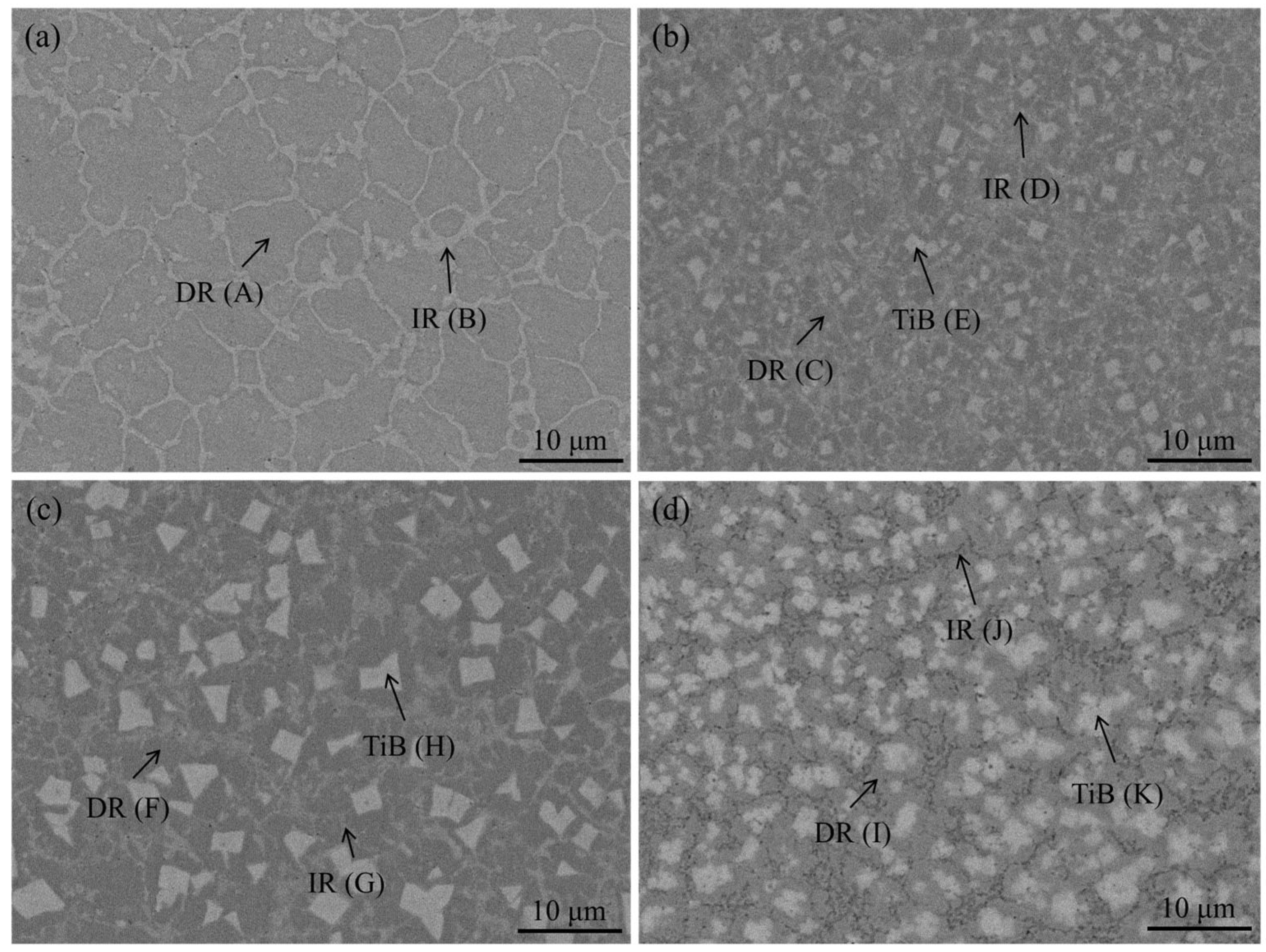

| Point | Al | Co | Cr | Fe | Ni | Ti | Mo | B |
|---|---|---|---|---|---|---|---|---|
| A | 12.4 | 9.3 | 11.6 | 37.8 | 9.7 | 10.5 | 8.7 | – |
| B | 10.1 | 8.5 | 9.4 | 33.4 | 13.1 | 13.2 | 12.3 | – |
| C | 12.5 | 11.7 | 12.5 | 34.9 | 10.5 | 8.9 | 9.0 | – |
| D | 11.3 | 10.3 | 10.1 | 29.7 | 14.3 | 11.4 | 12.9 | – |
| E | 3.4 | 1.8 | 2.1 | 1.3 | 2.5 | 31.0 | 6.4 | 51.5 |
| F | 11.4 | 9.8 | 11.2 | 40.2 | 7.6 | 11.7 | 8.1 | – |
| G | 9.2 | 9.7 | 10.1 | 34.5 | 11.9 | 12.1 | 12.5 | – |
| H | 2.7 | 0.9 | 3.7 | 3.4 | 1.9 | 33.6 | 4.8 | 49.0 |
| I | 9.6 | 9.3 | 11.2 | 38.9 | 10.5 | 9.4 | 11.1 | – |
| J | 8.7 | 8.1 | 10.5 | 35.7 | 12.3 | 10.1 | 14.6 | – |
| K | 1.8 | 1.3 | 3.7 | 2.1 | 2.4 | 38.9 | 3.6 | 46.2 |
| Point | Al | Co | Cr | Fe | Ni | Ti | Mo | B | O |
|---|---|---|---|---|---|---|---|---|---|
| L | 9.6 | 7.2 | 8.8 | 25.8 | 5.5 | 9.2 | 9.4 | – | 24.3 |
| M | 6.5 | 4.8 | 10.1 | 27.1 | 6.9 | 7.8 | 5.0 | 3.2 | 28.6 |
| N | 8.9 | 8.1 | 6.8 | 22.4 | 7.2 | 11.2 | 6.8 | 7.2 | 21.4 |
| O | 6.7 | 7.6 | 7.6 | 19.7 | 6.3 | 7.5 | 11.2 | 9.7 | 23.7 |
| Sample | Ecorr (mV vs. SCE) | icorr (μA·cm−2) | Ep (mV vs. SCE) | Eb (mV vs. SCE) |
|---|---|---|---|---|
| S1 | –278 | 14.5 | –153 | 542 |
| S2 | –245 | 7.1 | –132 | 537 |
| S3 | –298 | 35.2 | –104 | 345 |
| S4 | –317 | 54.1 | –198 | 185 |
| Sample | Rs (Ω·cm2) | Qf | Rf (kΩ·cm2) | Qdl | Rct (kΩ·cm2) | ||
|---|---|---|---|---|---|---|---|
| Yo (10−4·Ω−1·cm−2·Sn) | nsl | Yo (10−5·Ω−1·cm−2·Sn) | nsl | ||||
| S1 | 12.4 | 5.41 | 0.83 | 2.53 | 14.15 | 0.84 | 5.12 |
| S2 | 14.1 | 4.17 | 0.87 | 3.12 | 8.52 | 0.86 | 7.64 |
| S3 | 13.2 | 7.21 | 0.88 | 1.21 | 17.23 | 0.91 | 3.22 |
| S4 | 12.7 | 8.92 | 0.86 | 0.67 | 25.62 | 0.81 | 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, S.; Sun, J.; Qi, Z.; Wei, Y.; Chen, H.; Li, Y. Enhanced Wear and Corrosion Resistance of AlCoCrFeNiMoTi High-Entropy Alloy via B Addition by Laser Cladding. Materials 2025, 18, 4651. https://doi.org/10.3390/ma18204651
Ao S, Sun J, Qi Z, Wei Y, Chen H, Li Y. Enhanced Wear and Corrosion Resistance of AlCoCrFeNiMoTi High-Entropy Alloy via B Addition by Laser Cladding. Materials. 2025; 18(20):4651. https://doi.org/10.3390/ma18204651
Chicago/Turabian StyleAo, Sansan, Jiaxun Sun, Ziyuan Qi, Youxiang Wei, Hongyu Chen, and Yang Li. 2025. "Enhanced Wear and Corrosion Resistance of AlCoCrFeNiMoTi High-Entropy Alloy via B Addition by Laser Cladding" Materials 18, no. 20: 4651. https://doi.org/10.3390/ma18204651
APA StyleAo, S., Sun, J., Qi, Z., Wei, Y., Chen, H., & Li, Y. (2025). Enhanced Wear and Corrosion Resistance of AlCoCrFeNiMoTi High-Entropy Alloy via B Addition by Laser Cladding. Materials, 18(20), 4651. https://doi.org/10.3390/ma18204651








