A Comparison of High-Impulse and Direct-Current Magnetron Sputtering Processes for the Formation of Effective Bactericidal Oxide Coatings on Polymer Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. The Coating Deposition Process
2.2. Material Investigation
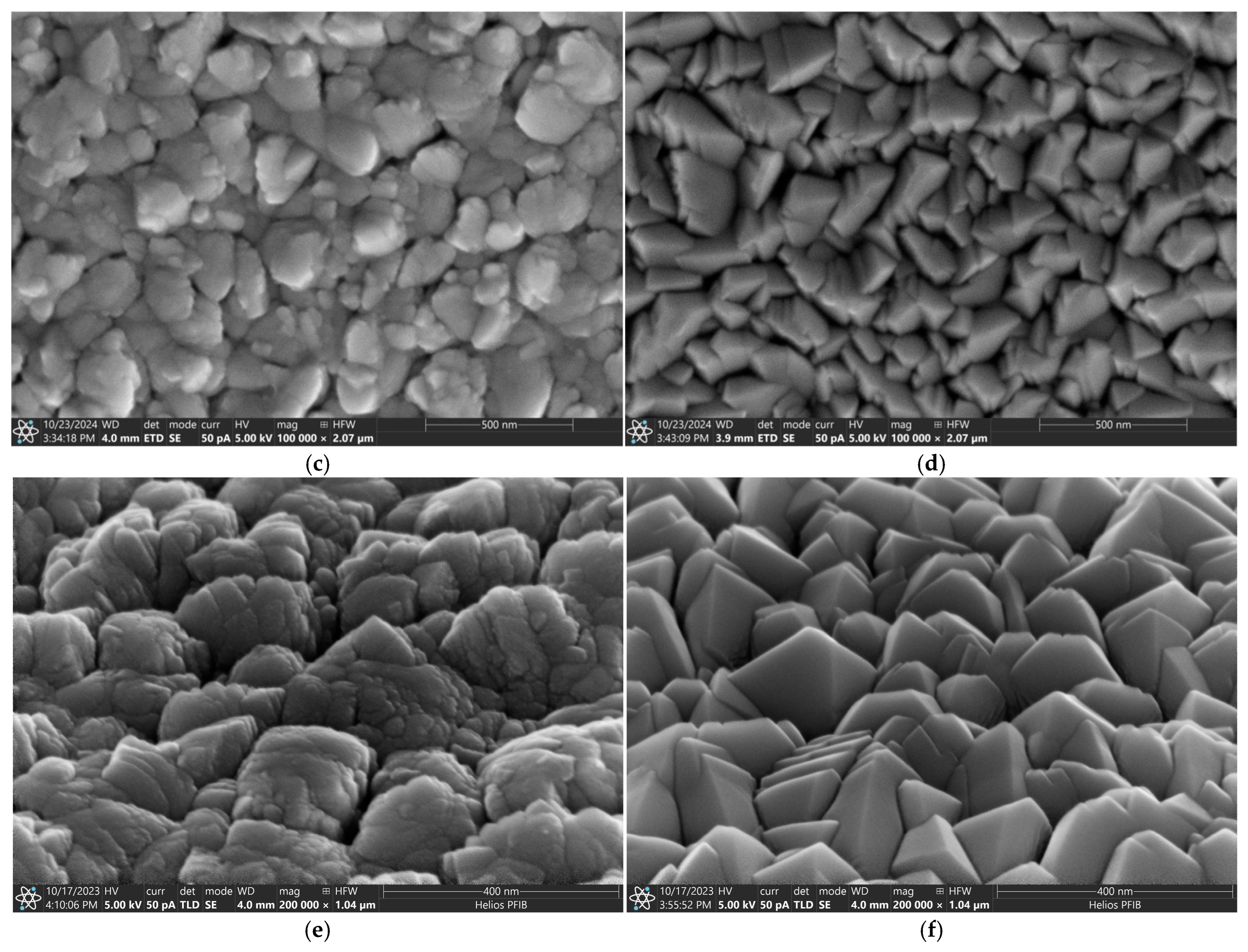
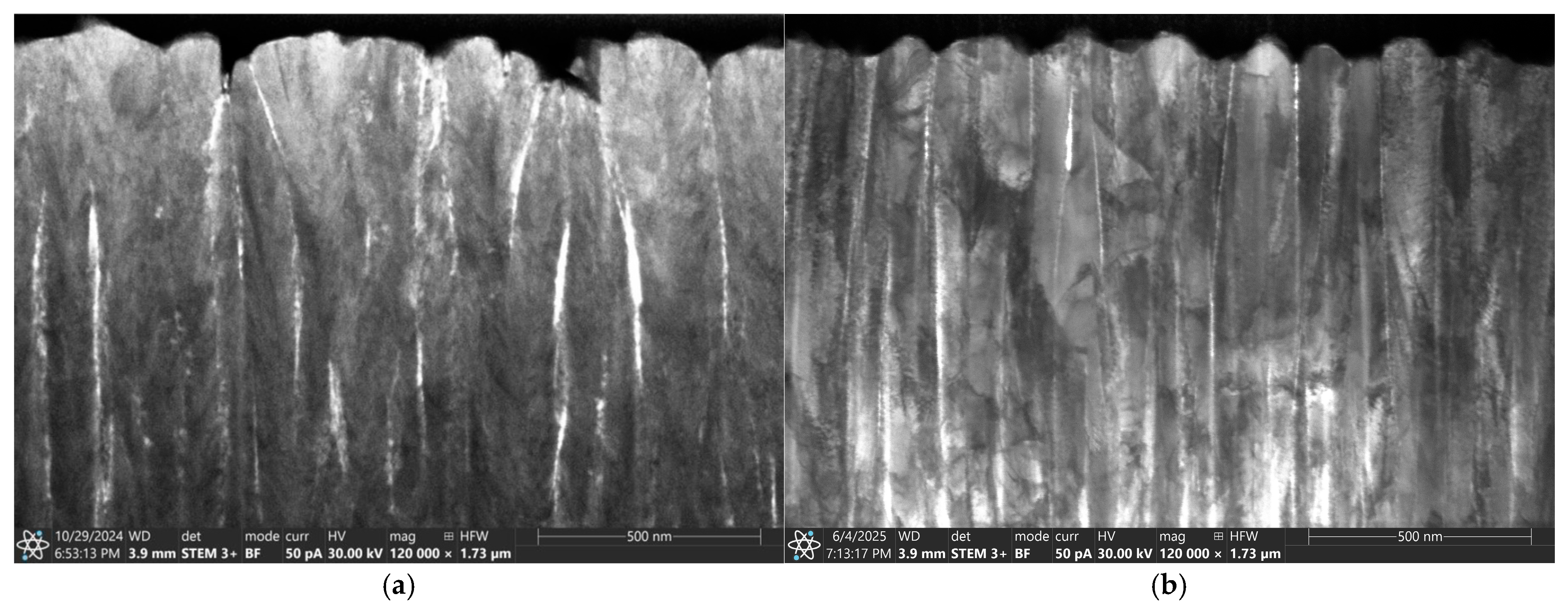
2.2.1. Microstructure
2.2.2. Roughness
2.2.3. Chemical Composition
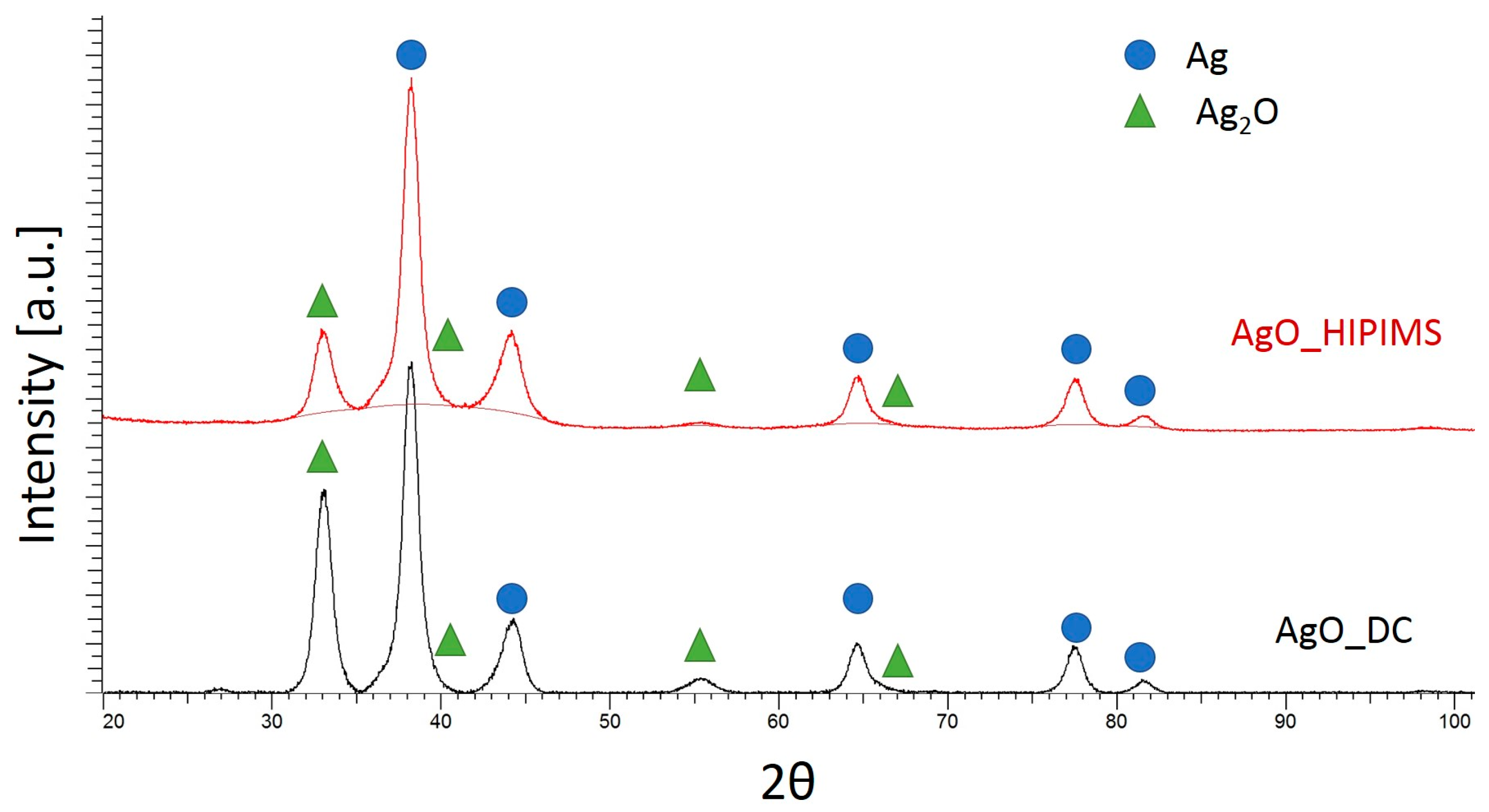
2.2.4. Phase Composition
2.3. Mechanical Properties
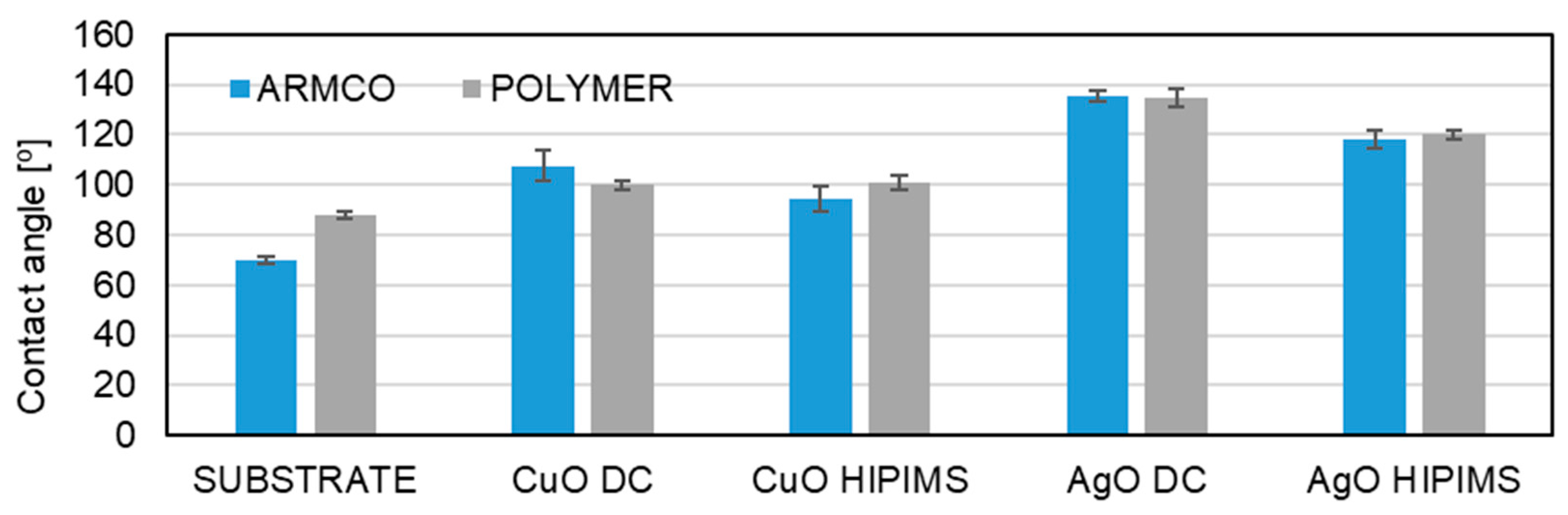
2.4. Wettability
2.5. Bactericidal Properties
3. Results
3.1. The Surface Morphology and Microstructure of AgO and CuO Coatings
3.2. The Mechanical Properties of AgO and CuO Coatings
3.3. Wettability
3.4. The Bactericidal Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral potential of nanoparticles for the treatment of Coronavirus infections. J. Trace Elem. Med. Biol. 2022, 72, 126977. [Google Scholar] [CrossRef]
- Ozono, S.; Zhang, Y.; Ode, H.; Sano, K.; Tan, T.S.; Imai, K.; Miyoshi, K.; Kishigami, S.; Ueno, T.; Iwatani, Y.; et al. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat. Commun. 2021, 12, 848. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and metal oxide-based antiviral nanoparticles: Properties, mechanisms of action, and applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/director-general/speeches/detail/antimicrobial-resistance-no-action-today-no-cure-tomorrow (accessed on 7 April 2011).
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Slezakova, K.; Morais, S.; Pereira, M.D.C. Atmospheric Nanoparticles and Their Impacts on Public Health. In Current Topics in Public Health; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Cornu, R.; Béduneau, A.; Martin, H. Influence of nanoparticles on liver tissue and hepatic functions: A review. Toxicology 2020, 430, 152344. [Google Scholar] [CrossRef]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J.; et al. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef]
- Valentini, X.; Rugira, P.; Frau, A.; Tagliatti, V.; Conotte, R.; Laurent, S.; Colet, J.; Nonclercq, D. Hepatic and Renal Toxicity Induced by TiO2 Nanoparticles in Rats: A Morphological and Metabonomic Study. J. Toxicol. 2019, 1, 5767012. [Google Scholar] [CrossRef] [PubMed]
- Bahamonde, J.; Brenseke, B.; Chan, M.Y.; Kent, R.D.; Vikesland, P.J.; Prater, M.R. Gold Nanoparticle Toxicity in Mice and Rats: Species Differences. Toxicol. Pathol. 2018, 46, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Ding, W.; Zhang, F. Acute toxicity of ferric oxide and zinc oxide nanoparticles in rats. J. Nanosci. Nanotechnol. 2010, 10, 8617–8624. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Behrangi, S.; Sedláček, I.; Štěrba, J.; Suková, G.; Czigány, Z.; Buršíková, V.; Souček, P.; Sochora, V.; Balázsi, K.; Vašina, P. An Assessment of the Bactericidal and Virucidal Properties of ZrN-Cu Nanostructured Coatings Deposited by an Industrial PVD System. Coatings 2022, 12, 1330. [Google Scholar] [CrossRef]
- Franklin, T.; Yang, R. Vapor-Deposited Biointerfaces and Bacteria: An Evolving Conversation. ACS Biomater. Sci. Eng. 2019, 6, 182–197. [Google Scholar] [CrossRef]
- Rebelo, R.; Calderon, S.V.; Fangueiro, R.; Henriques, M.; Carvalho, S. Influence of oxygen content on the antibacterial effect of Ag-O coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2016, 305, 1–10. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Banfi, F.; Gavioli, L. Antimicrobial Nanostructured Coatings: A Gas Phase Deposition and Magnetron Sputtering Perspective. Materials 2020, 13, 784. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P. Microfiltration Membranes Modified with Zinc by Plasma Treatment. Membranes 2023, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P.; Woskowicz, E.; Życki, M. Influence of deposition parameters of TiO2+CuO coating on the membranes surface used in the filtration process of dairy wastewater on their functional properties. Membranes 2021, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ju, H.; Figueiredo, N.M.; Ferreira, F. Exploring the Potential of High-Power Impulse Magnetron Sputtering for Nitride Coatings: Advances in Properties and Applications. Coatings 2025, 15, 130. [Google Scholar] [CrossRef]
- Tiron, V.; Velicu, I.-L.; Matei, T.; Cristea, D.; Cunha, L.; Stoian, G. Ultra-Short Pulse HiPIMS: A Strategy to Suppress Arcing during Reactive Deposition of SiO2 Thin Films with Enhanced Mechanical and Optical Properties. Coatings 2020, 10, 663. [Google Scholar] [CrossRef]
- Hála, M.; Vernhes, R.; Zabeida, O.; Klemberg-Sapieha, J.-E.; Martinu, L. Reactive HiPIMS deposition of SiO2/Ta2O5 optical interference filters. J. Appl. Phys. 2014, 116, 213302. [Google Scholar] [CrossRef]
- Strzelecki, G.W.; Nowakowska-Langier, K.; Chodun, R.; Okrasa, S.; Wicher, B.; Zdunek, K. Influence of modulation frequency on the synthesis of thin films in pulsed magnetron sputtering processes. Mater. Sci. Pol. 2018, 36, 697–703. [Google Scholar] [CrossRef]
- Velicu, I.-L.; Tiron, V.; Rusu, B.G.; Popa, G. Copper thin films deposited under different power delivery modes and magnetron configurations: A comparative study. Surf. Coat. Technol. 2017, 327, 192–199. [Google Scholar] [CrossRef]
- Tiron, V.; Velicu, I.-L.; Cristea, D.; Lupu, N.; Stoian, G.; Munteanu, D. Influence of ion-to-neutral flux ratio on the mechanical and tribological properties of TiN coatings deposited by HiPIMS. Surf. Coat. Technol. 2018, 352, 690–698. [Google Scholar] [CrossRef]
- Tiron, V.; Velicu, I.-L.; Pana, I.; Cristea, D.; Rusu, B.G.; Dinca, P.; Porosnicu, C.; Grigore, E.; Munteanu, D.; Tascu, S. HiPIMS deposition of silicon nitride for solar cell application. Surf. Coat. Technol. 2018, 344, 197–203. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, T.; Mei, H.; Yun, J.M.; Jeong, S.H.; Wang, Q.; Kim, K.H. Effects of Negative Bias Voltage and Ratio of Nitrogen and Argon on the Structure and Properties of NbN Coatings Deposited by HiPIMS Deposition System. Coatings 2017, 8, 10. [Google Scholar] [CrossRef]
- Audronis, M.; Hinder, S.J.; Mack, P.; Bellido-Gonzalez, V.; Bussey, D.; Matthews, A.; Baker, M.A. A comparison of reactive plasma pre-treatments on PET substrates by Cu and Ti pulsed-DC and HIPIMS discharges. Thin Solid Films 2011, 520, 1564–1570. [Google Scholar] [CrossRef]
- Baghriche, O.; Ehiasarian, A.P.; Kusiak-Nejman, E.; Pulgarin, C.; Sanjines, R.; Morawski, A.W.; Kiwi, J. High power impulse magnetron sputtering (HIPIMS) and traditional pulsed sputtering (DCMSP) Ag-surfaces leading to E. coli inactivation. J. Photochem. Photobiol. A Chem. 2012, 277, 11–17. [Google Scholar] [CrossRef]
- Audronis, M.; Bellido-Gonzalez, V.; Daniel, B. Control of reactive high power impulse magnetron sputtering processes. Surf. Coat. Technol. 2010, 204, 2159–2164. [Google Scholar] [CrossRef]
- Nelson, J.B.; Riley, D.P. An experimental investigation of extrapolation methods in the derivation of accurate unit-cell dimensions of crystals. Proc. Phys. Soc. 1945, 57, 160–177. [Google Scholar] [CrossRef]
- Rogowska, R.; Rogowski, A. Measurements of contact angle by the sessile drop and the Wilhelmy plate method. Logistyka-Nauka 2011, 3, 1–10. [Google Scholar]
- Zheng, W.; Chen, Y.; Peng, X.; Zhong, K.; Lin, Y.; Huang, Z. The Phase Evolution and Physical Properties of Binary Copper Oxide Thin Films Prepared by Reactive Magnetron Sputtering. Materials 2018, 11, 1253. [Google Scholar] [CrossRef]
- Vasilev, O.; Hayles, A.; Campbell, D.; Jaarsma, R.; Johnson, L.; Vasilev, K. Nanoscale antibacterial coatings incorporating silver nanoparticles derived by plasma techniques—A state-of-the-art perspective. Mater. Today Chem. 2024, 41, 102341. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Brenning, N.; Huo, C.; Lundin, D.; Raadu, M.A.; Vitelaru, C.; Stancu, G.D.; Minea, T.; Helmersson, U. Understanding deposition rate loss in high power impulse magnetron sputtering: I. Ionization-driven electric fields. Plasma Sources Sci. Technol. 2012, 21, 025005. [Google Scholar] [CrossRef]
- Wasa, K.; Hayakawa, S.; Haber, S. Handbook of Sputtering Deposition Technology: Principle, Technology and Applications; William Andrew Publishing: Norwich, NY, USA, 1992. [Google Scholar]
- Kukushkin, S.A.; Osipov, A.V. Phase transitions and the nucleation of catalytic nanostructures under the action of chemical, physical, and mechanical factors. Kinet. Catal. 2008, 49, 79–91. [Google Scholar] [CrossRef]
- Ossai, C.I.; Raghavan, N. Nanostructure and nanomaterial characterization, growth mechanisms, and applications. Nanotechnol. Rev. 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Le, M.-T.; Sohn, Y.-U.; Lim, J.-W.; Choi, G.-S. Effect of Sputtering Power on the Nucleation and Growth of Cu Films Deposited by Magnetron Sputtering. Mater. Trans. 2010, 51, 116–120. [Google Scholar] [CrossRef]
- Luis, E.E.M.; Carrasco, I.S.; Reis, F.D.A. Layer-by-layer growth, apparent instability, and mound coarsening in thin film deposition controlled by thermally activated surface diffusion. Mater. Today Commun. 2025, 42, 111122. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, B.; Zheng, Y. Ultrathin Ferroelectric Films: Growth, Characterization, Physics and Applications. Materials 2014, 7, 6377–6485. [Google Scholar] [CrossRef]
- Gribanova, E.V.; Kuchek, A.E.; Larionov, M.I. Factors influencing the contact angle value. The contact angle, as a characteristic of the properties of solid surfaces. Russ. Chem. Bull. 2016, 65, 1–13. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A.; Saeed, M.; Al-Zubi, F.; Sedaghat, A.; Bahzad, H. Deposition of stainless steel thin films: Anelectron beam physical vapour deposition approach. Materials 2019, 12, 571. [Google Scholar] [CrossRef]
- Ubuo, E.E.; Udoetok, I.A.; Tyowua, A.T.; Ekwere, I.O.; Al-Shehri, H.S. The Direct Cause of Amplified Wettability: Roughness or Surface Chemistry. J. Compos. Sci. 2021, 5, 213. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Faustini, M.; Garrido, J.J. Effects of the porous texture and surface chemistry of silica xerogels on the sensitivity of fiber-optic sensors toward VOCs. Sens. Actuators B Chem. 2016, 222, 1166–1174. [Google Scholar] [CrossRef]
- Chen, X.M.; Weibel, J.A.; Garimella, S.V. Exploiting microscale roughness on hierarchical superhydrophobic copper surfaces for enhanced dropwise condensation. Adv. Mater. Interfaces 2015, 2, 247. [Google Scholar] [CrossRef]
- Fan, X.; Niu, L.; Wu, Y.; Cheng, J.; Yang, Z. Assembly route toward raspberry-like composite particles and their controlled surface wettability through varied dual-size binary roughness. Appl. Surf. Sci. 2015, 332, 393–402. [Google Scholar] [CrossRef]
- Riahi, S.; Niroumand, B.; Moghadam, A.D.; Rohatgi, P.K. Effect of microstructure and surface features on wetting angle of Fe-3.2. wt% C.E. cast iron with water. Appl. Surf. Sci. 2018, 440, 341–350. [Google Scholar] [CrossRef]
- Wright, A.J.; Kim, Y.; Mack, C.; Sharobem, T.; McGowan, R.; Bravo, L.; Murugan, M.; Dambra, C.; Keyes, B.; Ghosahl, A. Influence of chemistry and surface roughness of various thermal barier coatings on the wettability on molten sand. Surf. Coat. Technol. 2024, 481, 130649. [Google Scholar] [CrossRef]
- Mantel, M.; Wightman, J.P. Influence of the surface chemistry on the wettability of stainless steel. Surf. Interface Anal. 1994, 21, 595–605. [Google Scholar] [CrossRef]
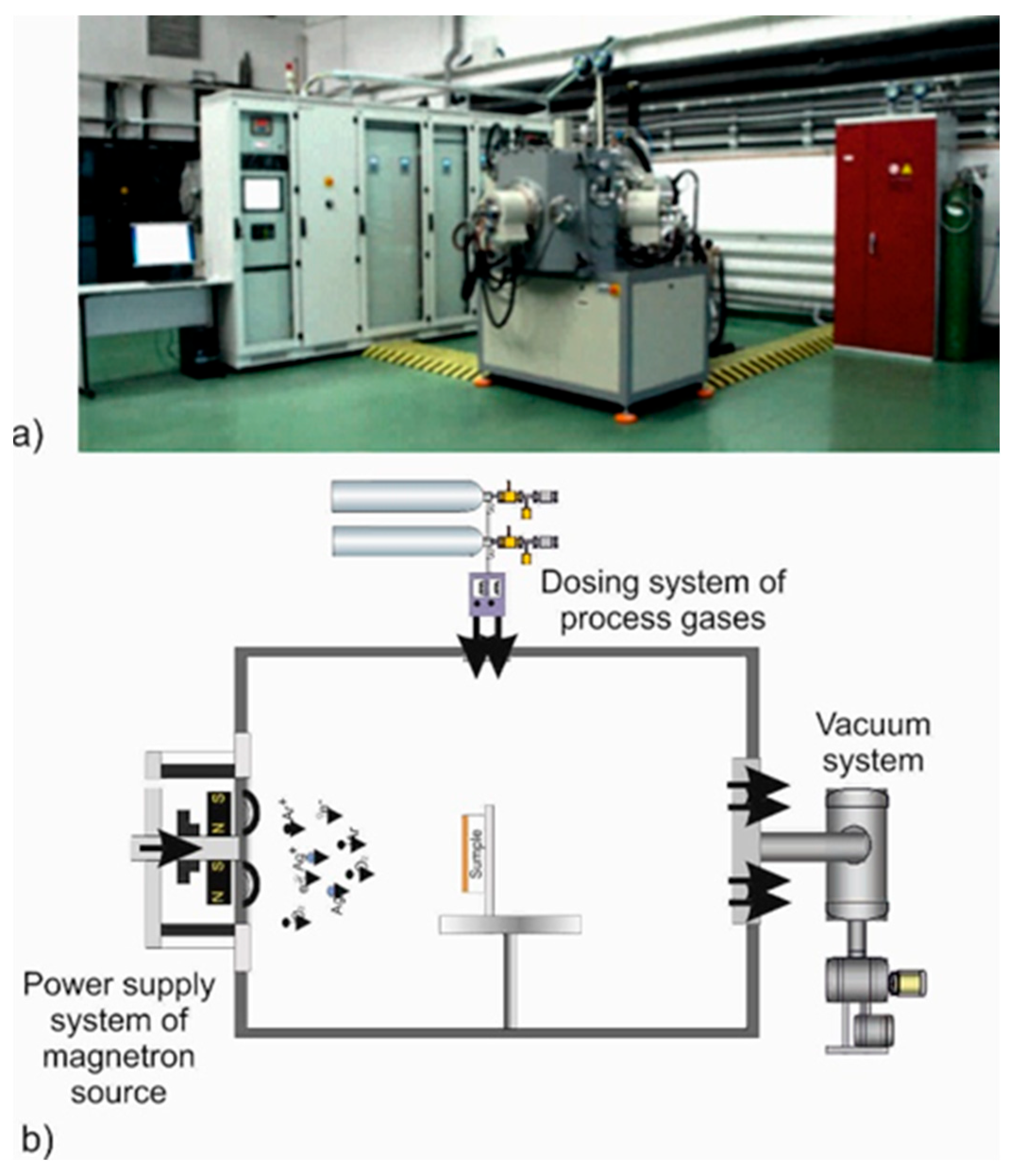
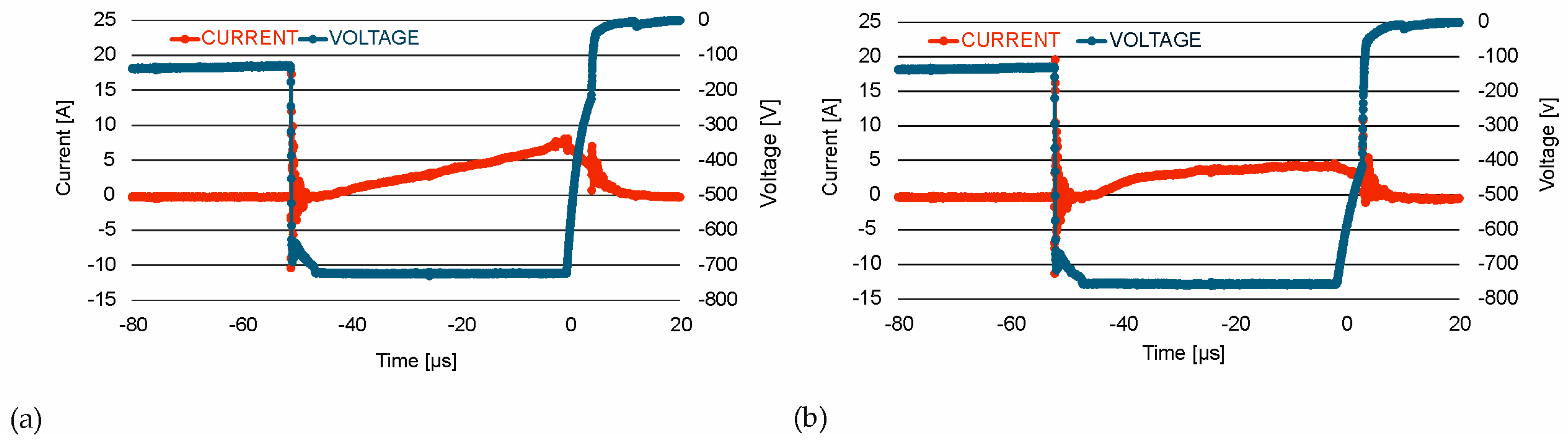
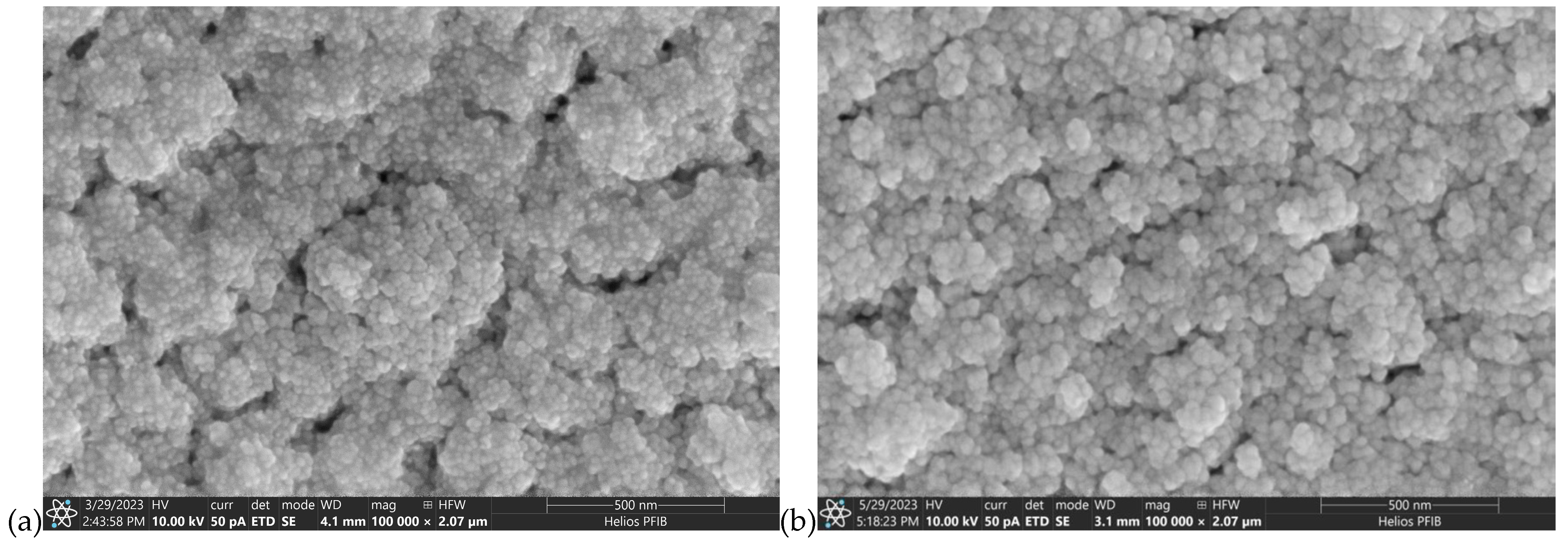
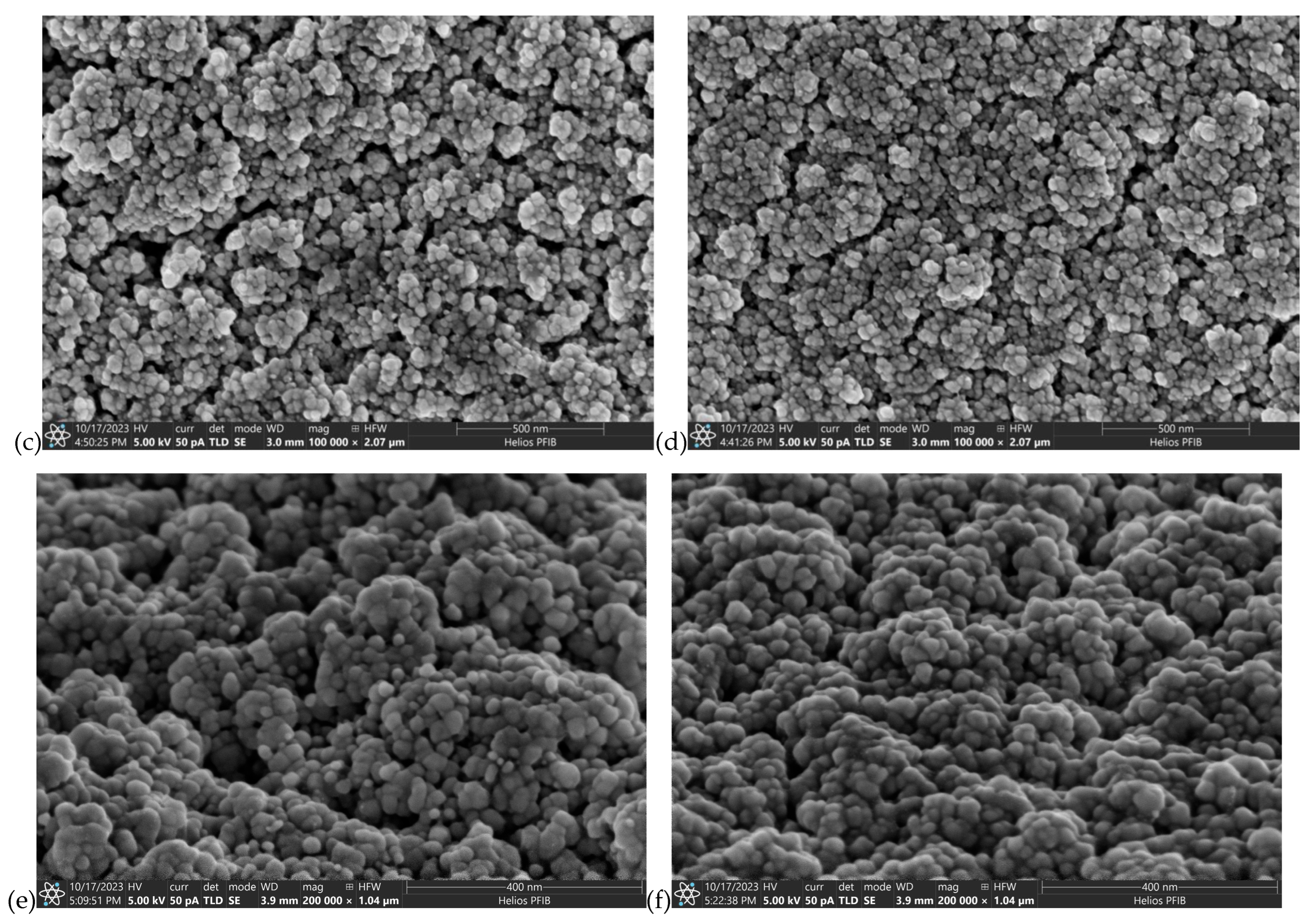




| Material | Atmosphere [sccm] | Pressure [mbar] | Average Magnetron Source Power [W] | Maximum Current Imax [A] | Maximum Voltage Umax [V] | Temperature [°C] | Time [min] |
|---|---|---|---|---|---|---|---|
| DCMS | |||||||
| AgO | Ar:300 O2:30 | 5.0 × 10–3 | 350 | 0.75 | 460 | 20 | 60 |
| CuO | 350 | 0.75 | 465 | ||||
| HIPIMS | |||||||
| AgO | Ar:300 O2:30 | 5.0 × 10–3 | 350 | 5 | 759 | 20 | 60 |
| CuO | 350 | 8 | 752 | ||||
| Coatings | Thickness [nm] | Deposition Rate [nm/min] | Average Particle Size [nm] | Surface Roughness Ra/Sa [nm] | Cu/Ag Content [%wt.] | O Content [%wt.] | |
|---|---|---|---|---|---|---|---|
| AgO | |||||||
| AgODCMS | 5900 | 98.3 | 20.8 | 8.29/8.52 | 93.0 | 7.0 | |
| AgOHIPIMS | 2000 | 33.3 | 20.4 | 7.11/7.12 | 95.5 | 4.5 | |
| CuO | |||||||
| CuODCMS | 3000 | 50 | 75.8 | 3.38/17.6 | 78.7 | 21.3 | |
| CuOHIPIMS | 1700 | 28.3 | 76.9 | 3.21/14.6 | 83.4 | 16.6 | |
| Phase | Crystallite Size [nm] | Microstrain [ε] | Lattice Constant [Å] |
|---|---|---|---|
| AgO DC | |||
| Ag | 5 | −0.0153 | 4.0874 |
| Ag2O | 30 | 0.0184 | 4.6769 |
| AgO HIPIMS | |||
| Ag | 5 | −0.0149 | 4.0884 |
| Ag2O | 10 | 0.0037 | 4.6685 |
| CuO DC | |||
| Cu3O4 | 460 | 0.0320 | 4.2516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacprzyńska-Gołacka, J.; Wieciński, P.; Adamczyk-Cieślak, B.; Sowa, S.; Barszcz, W.; Łożyńska, M.; Kalbarczyk, M.; Krasiński, A.; Garbacz, H.; Smolik, J. A Comparison of High-Impulse and Direct-Current Magnetron Sputtering Processes for the Formation of Effective Bactericidal Oxide Coatings on Polymer Substrates. Materials 2025, 18, 4591. https://doi.org/10.3390/ma18194591
Kacprzyńska-Gołacka J, Wieciński P, Adamczyk-Cieślak B, Sowa S, Barszcz W, Łożyńska M, Kalbarczyk M, Krasiński A, Garbacz H, Smolik J. A Comparison of High-Impulse and Direct-Current Magnetron Sputtering Processes for the Formation of Effective Bactericidal Oxide Coatings on Polymer Substrates. Materials. 2025; 18(19):4591. https://doi.org/10.3390/ma18194591
Chicago/Turabian StyleKacprzyńska-Gołacka, Joanna, Piotr Wieciński, Bogusława Adamczyk-Cieślak, Sylwia Sowa, Wioletta Barszcz, Monika Łożyńska, Marek Kalbarczyk, Andrzej Krasiński, Halina Garbacz, and Jerzy Smolik. 2025. "A Comparison of High-Impulse and Direct-Current Magnetron Sputtering Processes for the Formation of Effective Bactericidal Oxide Coatings on Polymer Substrates" Materials 18, no. 19: 4591. https://doi.org/10.3390/ma18194591
APA StyleKacprzyńska-Gołacka, J., Wieciński, P., Adamczyk-Cieślak, B., Sowa, S., Barszcz, W., Łożyńska, M., Kalbarczyk, M., Krasiński, A., Garbacz, H., & Smolik, J. (2025). A Comparison of High-Impulse and Direct-Current Magnetron Sputtering Processes for the Formation of Effective Bactericidal Oxide Coatings on Polymer Substrates. Materials, 18(19), 4591. https://doi.org/10.3390/ma18194591










