Electrospun Parallel, Crossed Fibers for Promoting Cell Adhesion and Migration
Highlights
- Parallel and crossed fibers were fabricated utilizing electrospinning technology;
- To eliminate confounding adhesion effects, a precisely engineered non-adhesive platform was developed with controlled surface properties;
- The fiber spacing range of 30–60 μm served as the critical threshold for distinguishing single-cell migration behaviors on parallel fibers;
- The 100 μm inter-fiber spacing emerged as a critical parameter defining cellular behavioral transitions (turning, anchoring, bridging) within crossed fiber matrices.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Micro-Scale Fiber
2.3. Surface Morphology
2.4. Chemical Structure
2.5. Cell Compatibility
2.6. Cell Migration
3. Results and Discussion
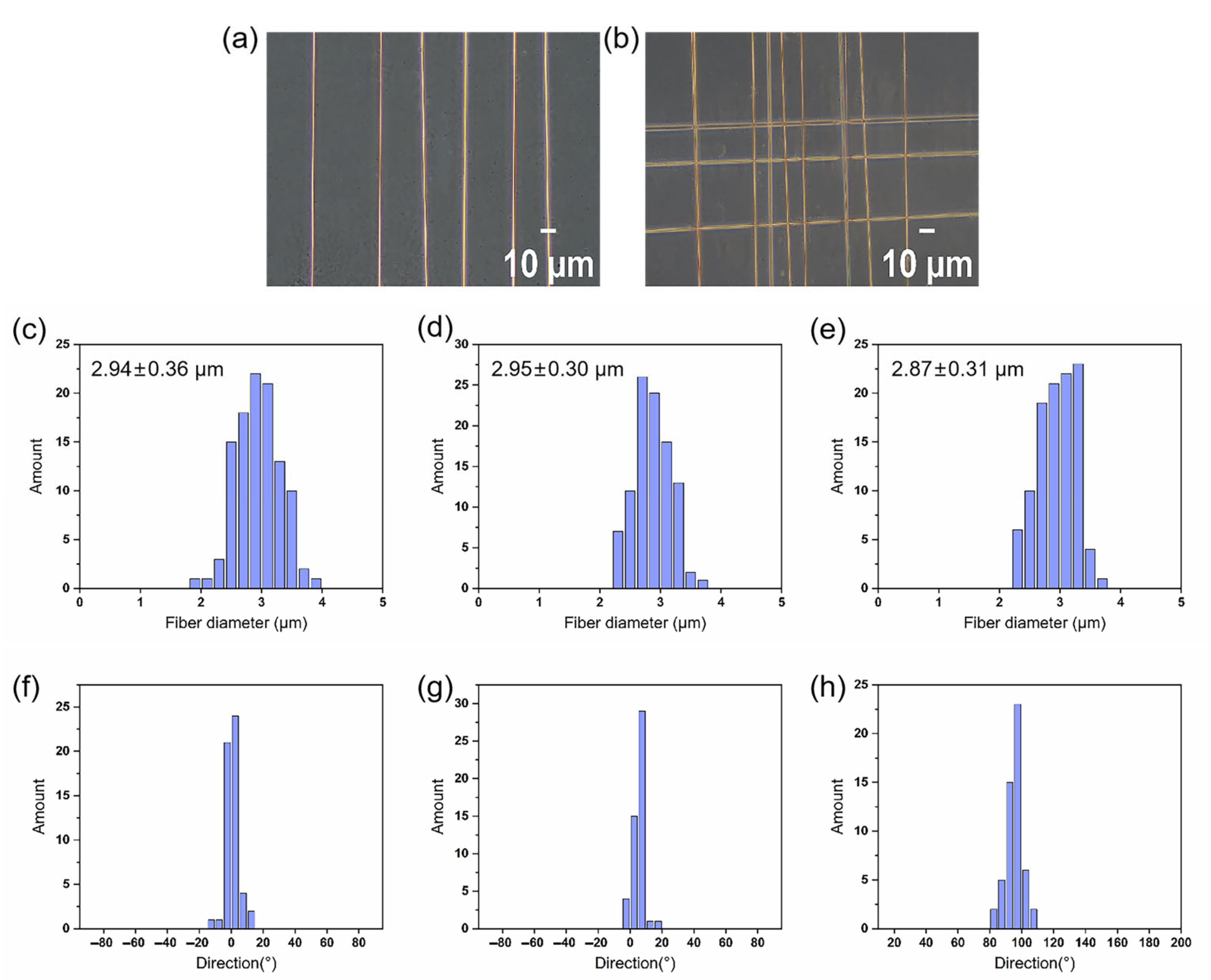
3.1. Morphology and Structure of Electrospun Fibers
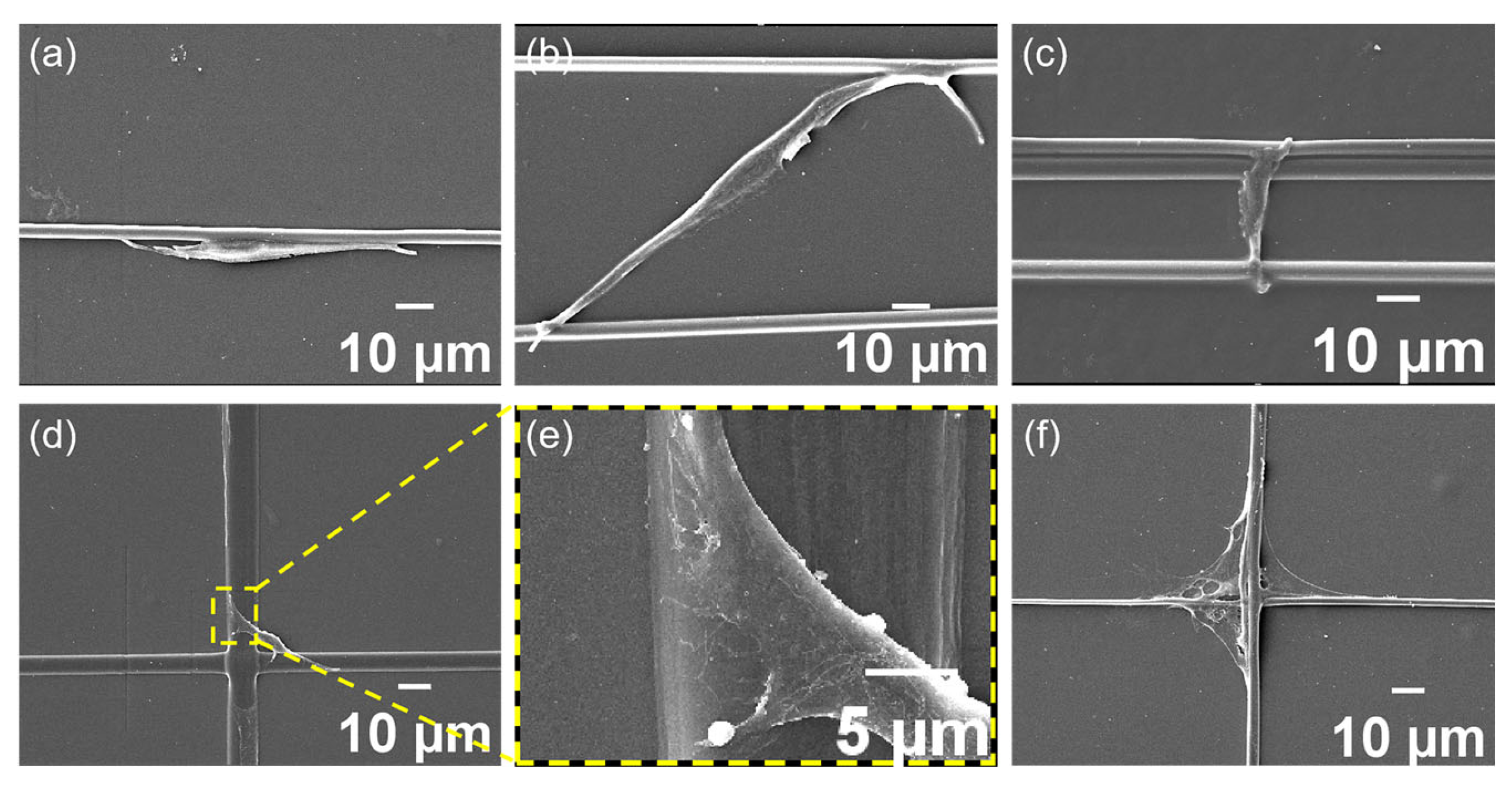
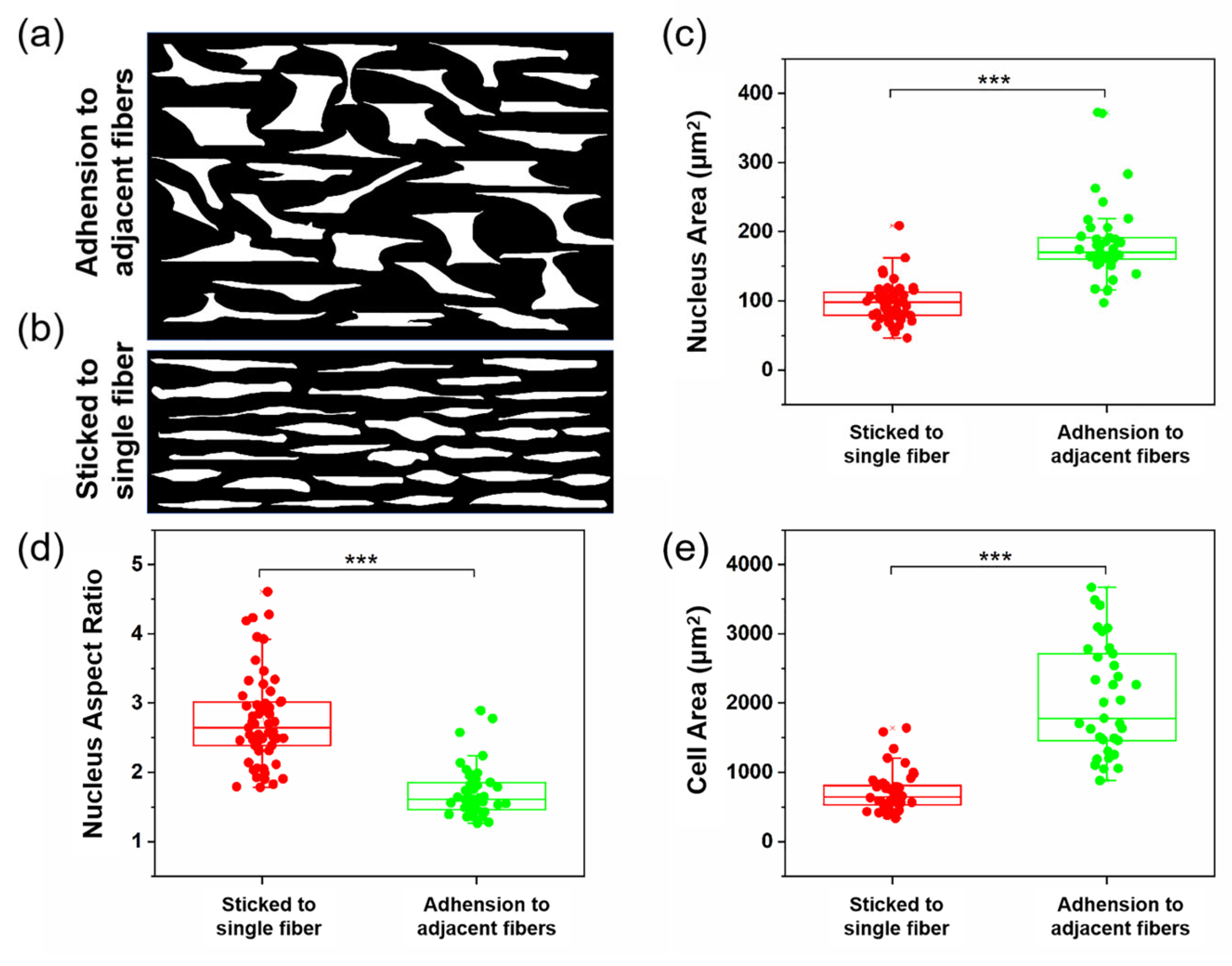
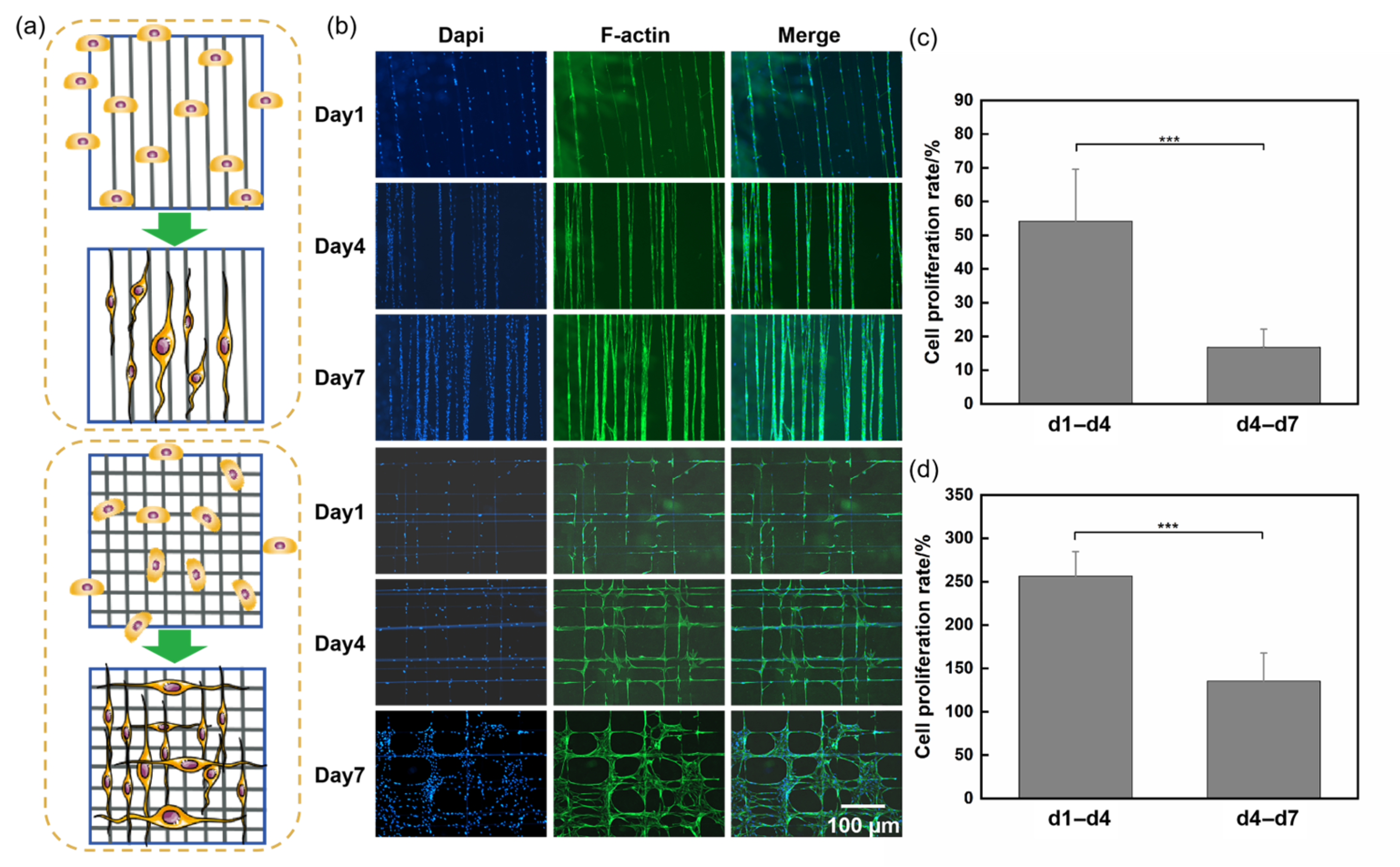
3.2. Cell Spreading and Proliferation
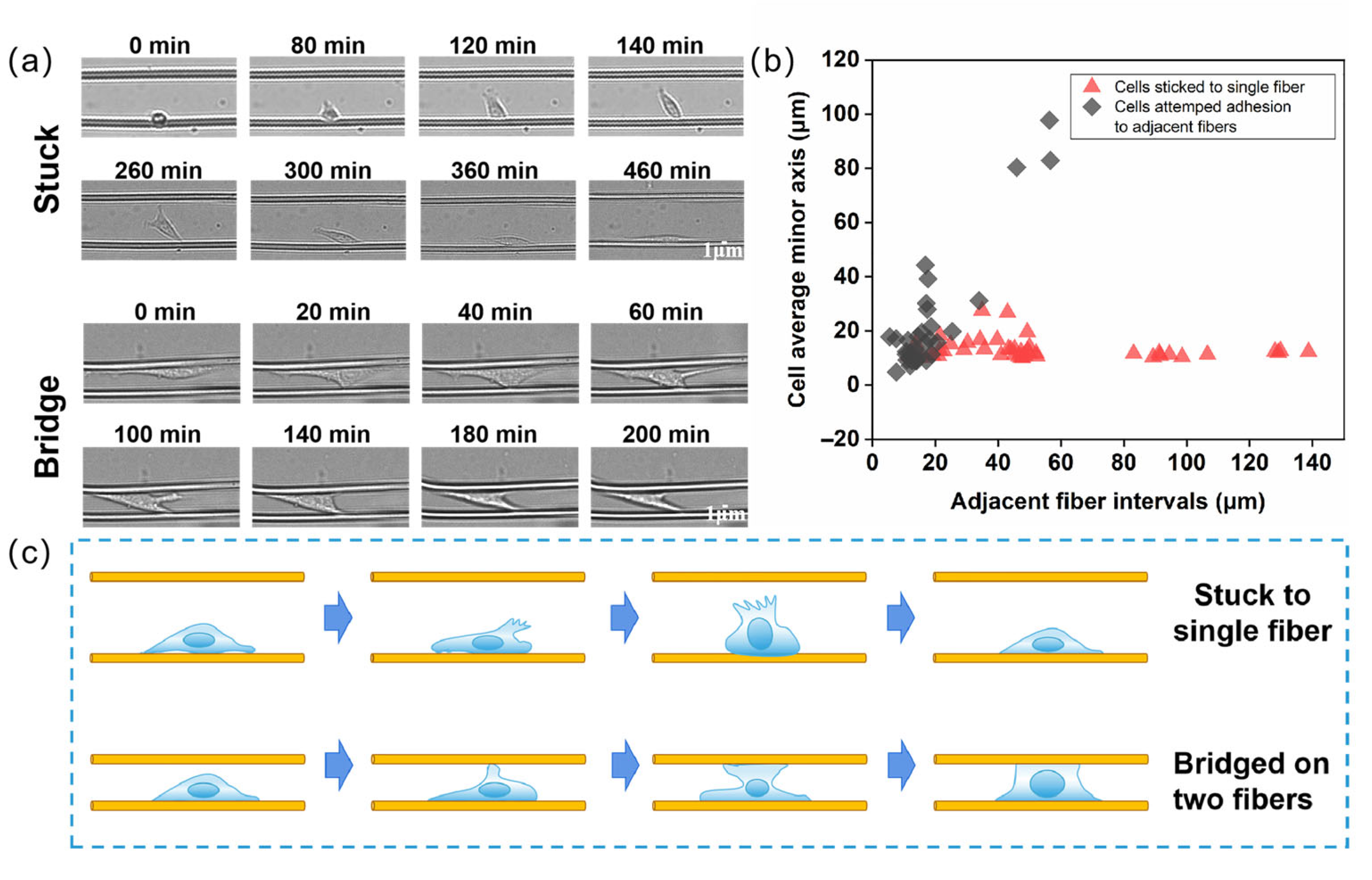
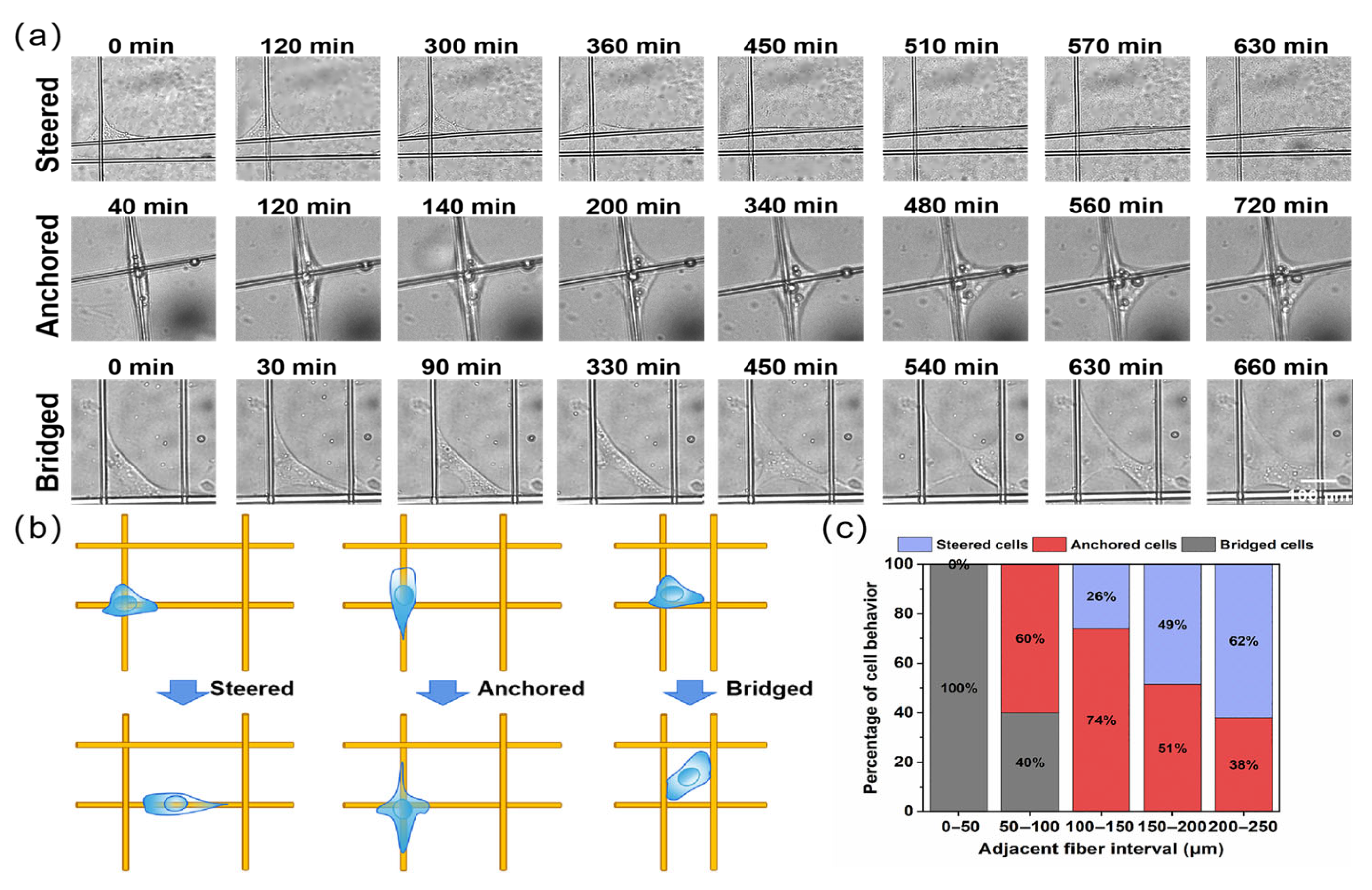
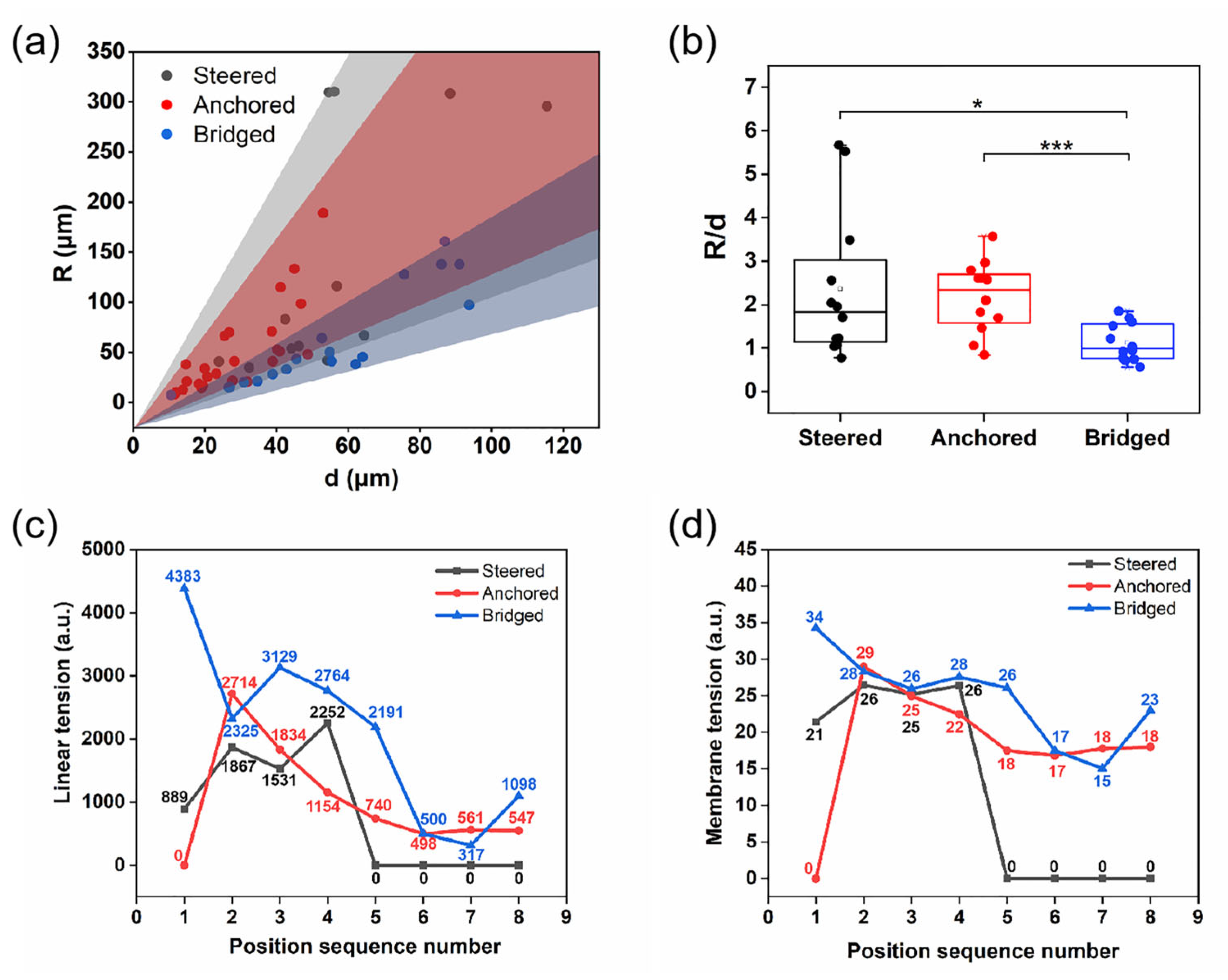
3.3. Cell Migration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Chapter 1-Anatomy and function of the skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 1–14. [Google Scholar]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ní Annaidh, A.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. 2012, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Gao, W.D.; Fu, X.L.; Shi, M.; Xie, W.H.; Zhang, W.; Zhao, F.J.; Chen, X.F. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater. Sci. 2018, 6, 340–349. [Google Scholar] [CrossRef]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef]
- Hinz, B.; Pittet, P.; Smith-Clerc, J.; Chaponnier, C.; Meister, J.J. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol. Biol. Cell 2004, 15, 4310–4320. [Google Scholar] [CrossRef]
- Chen, S.X.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J.W. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2014, 5, 119–136. [Google Scholar] [CrossRef]
- Doyle, A.D.; Yamada, K.M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 2016, 343, 60–66. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Sunyer, R.; Conte, V.; Escribano, J.; Elosegui-Artola, A.; Labernadie, A.; Valon, L.; Navajas, D.; García-Aznar, J.M.; Muñoz, J.J.; Roca-Cusachs, P.; et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 2016, 353, 1157–1161. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.J.; Wu, T.; Dai, Y.Q.; Xia, Y.N. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Wang, K.; Liu, L.P.; Xie, J.; Shen, L.; Tao, J.; Zhu, J. Facile strategy to generate aligned polymer nanofibers: Effects on cell adhesion. ACS Appl. Mater. Interfaces 2018, 10, 1566–1574. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Chen, N.; Ramakrishna, S.; Mo, X. The cellular response of nerve cells on poly-l-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 715–726. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite-tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 342–351. [Google Scholar] [CrossRef]
- Yao, C.; Qiu, Z.; Li, X.; Zhu, H.; Li, D.; He, J. Electrohydrodynamic printing of microfibrous architectures with cell-scale spacing for improved cellular migration and neurite outgrowth. Small 2023, 19, 2207331. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Gao, X.; Wen, M.; Liu, Y.; Hou, T.; Niu, B.; An, M.W. Synthesis and characterization of PU/PLCL/CMCS electrospun scaffolds for skin tissue engineering. Polymers 2022, 14, 5029. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hou, T.; Wang, L.; Liu, Y.; Guo, J.Q.; Zhang, L.; Yang, T.T.; Tang, W.J.; An, M.W.; Wen, M.L. Aligned electrospun fibers of different diameters for improving cell migration capacity. Colloids Surf. B Biointerfaces 2024, 234, 113674. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Shen, Q.C.; Lin, Y.; Xu, S.Y.; Meng, Q. Evaluation of the potential of chimeric spidroins/poly(L-lactic-co-ε-caprolactone) (PLCL) nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 111, 110752. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.Y.; Mi, X.Y.; Midgley, A.C.; Du, X.C.; Huang, Z.Q.; Wei, T.T.; Liu, R.H.; Ma, T.Z.; Zhi, D.K.; Zhu, D.; et al. Targeted repair of vascular injury by adipose-derived stem cells modified with p-selectin binding peptide. Adv. Sci. 2020, 7, 1903516. [Google Scholar] [CrossRef]
- Gu, Z.H.; Fan, S.N.; Kundu, S.C.; Yao, X.; Zhang, Y.P. Fiber diameters and parallel patterns: Proliferation and osteogenesis of stem cells. Regen. Biomater. 2023, 10, rbad001. [Google Scholar] [CrossRef]
- Lei, Q.; He, J.K.; Li, D.C. Electrohydrodynamic 3D printing of layer-specifically oriented, multiscale conductive scaffolds for cardiac tissue engineering. Nanoscale 2019, 11, 15195–15205. [Google Scholar] [CrossRef]
- Guetta-Terrier, C.; Monzo, P.; Zhu, J.; Long, H.; Venkatraman, L.; Zhou, Y.; Wang, P.; Chew, S.Y.; Mogilner, A.; Ladoux, B.; et al. Protrusive waves guide 3D cell migration along nanofibers. J. Cell Biol. 2015, 211, 683–701. [Google Scholar] [CrossRef]
- Sun, Q.; Pei, F.; Zhang, M.; Zhang, B.; Jin, Y.; Zhao, Z.H.; Wei, Q. Curved nanofiber network induces cellular bridge formation to promote stem cell mechanotransduction. Adv. Sci. 2023, 10, e2204479. [Google Scholar] [CrossRef]
- Tabdanov, E.D.; Puram, V.V.; Win, Z.; Alamgir, A.; Alford, P.W.; Provenzano, P.P. Bimodal sensing of guidance cues in mechanically distinct microenvironments. Nat. Commun. 2018, 9, 4891. [Google Scholar] [CrossRef]
- Suraneni, P.; Fogelson, B.; Rubinstein, B.; Noguera, P.; Volkmann, N.; Hanein, D.; Mogilner, A.; Li, R. A mechanism of leading-edge protrusion in the absence of Arp2/3 complex. Mol. Biol. Cell 2015, 26, 901–912. [Google Scholar] [CrossRef]
- Gao, X.; Wen, M.; Liu, Y.; Hou, T.; An, M. Mechanical Performance and Cyocompatibility of Pu/Plcl Nanofibrous Electrospun Scaffolds for Skin Regeneration. Eng. Regen. 2022, 3, 53–58. [Google Scholar] [CrossRef]
- Padhi, A.; Thomson, A.H.; Perry, J.B.; Davis, G.N.; McMillan, R.P.; Loesgen, S.; Kaweesa, E.N.; Kapania, R.; Nain, A.S.; Brown, D.A. Bioenergetics underlying single-cell migration on aligned nanofiber scaffolds. Am. J. Physiol. Cell Physiol. 2020, 318, C476–C485. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Kim, D.S.; Ju, J.H.; Shin, H.S. Assessment of Spirulina-PCL nanofiber for the regeneration of dermal fibroblast layers. Vitr. Cell Dev. Biol. Anim. 2013, 49, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S. Cadherin-actin interactions at adherens junctions. Curr. Opin. Cell Biol. 2011, 23, 515–522. [Google Scholar] [CrossRef]
- Armingol, E.; Baghdassarian, H.M.; Lewis, N.E. The diversification of methods for studying cell-cell interactions and communication. Nat. Rev. Gene. 2024, 25, 381–400. [Google Scholar] [CrossRef]
- Sun, Q.; Hou, Y.; Chu, Z.Q.; Wei, Q. Soft overcomes the hard: Flexible materials adapt to cell adhesion to promote cell mechanotransduction. Bioact. Mater. 2022, 10, 397–404. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, T.C.; Liu, X.J.; Wei, Q. Cellular spatial sensing determines cell mechanotransduction activity on the aligned nanofibers. Small 2025, 21, e2410351. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, H.Y.; Xu, X.; Dou, S.X.; Li, W.; Guan, D.; Ye, F.; Chen, X.; Guo, M.; Wang, P.Y.; et al. Switch of cell migration modes orchestrated by changes of three-dimensional lamellipodium structure and intracellular diffusion. Nat. Commun. 2023, 14, 5166. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Rahman-Zaman, A.; VanderBurgh, J.A.; Taufalele, P.V.; Jain, A.; Erickson, D.; Bordeleau, F.; Reinhart-King, C.A. Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nat. Commun. 2019, 10, 4185. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef]
- Nishimura, R.; Kanchanawong, P. Nanoscale mechano-adaption of integrin-based cell adhesions: New tools and techniques lead the way. Curr. Opin. Cell Biol. 2025, 94, 102509. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Das, J.; Subramanyam, D. Traffic flow and signals: Regulating the movement within cells. Curr. Opin. Cell Biol. 2025, 94, 102518. [Google Scholar] [CrossRef] [PubMed]
- Isomursu, A.; Park, K.Y.; Hou, J.; Cheng, B.; Mathieu, M.; Shamsan, G.A.; Fuller, B.; Kasim, J.; Mahmoodi, M.M.; Lu, T.J.; et al. Directed cell migration towards softer environments. Nat. Mater. 2022, 21, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
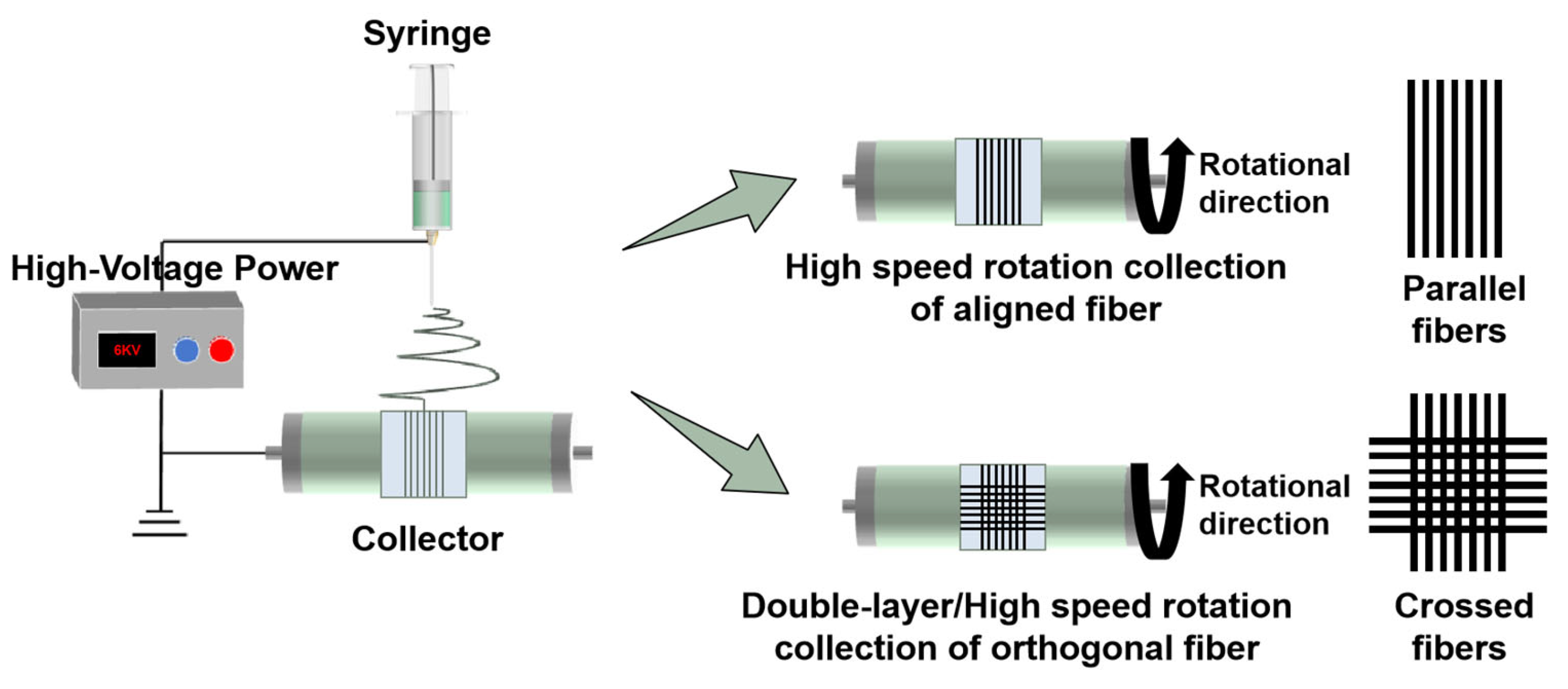
| Voltage (kV) | Flow Rate (mm/s) | Collector Speed (r/min) | Temperature (°C) | Humidity % | Collection Distance (cm) | Oscillation Range |
|---|---|---|---|---|---|---|
| 6 | 0.0013 | 3000 | 25–35 | 20–30 | 17 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Peng, J.; Huang, L.; Peng, X.; Cheng, Y.; Zhang, W.; Jia, W. Electrospun Parallel, Crossed Fibers for Promoting Cell Adhesion and Migration. Materials 2025, 18, 3224. https://doi.org/10.3390/ma18143224
Gao X, Peng J, Huang L, Peng X, Cheng Y, Zhang W, Jia W. Electrospun Parallel, Crossed Fibers for Promoting Cell Adhesion and Migration. Materials. 2025; 18(14):3224. https://doi.org/10.3390/ma18143224
Chicago/Turabian StyleGao, Xiang, Jingjun Peng, Linjie Huang, Xiaoquan Peng, Yanjun Cheng, Wei Zhang, and Wei Jia. 2025. "Electrospun Parallel, Crossed Fibers for Promoting Cell Adhesion and Migration" Materials 18, no. 14: 3224. https://doi.org/10.3390/ma18143224
APA StyleGao, X., Peng, J., Huang, L., Peng, X., Cheng, Y., Zhang, W., & Jia, W. (2025). Electrospun Parallel, Crossed Fibers for Promoting Cell Adhesion and Migration. Materials, 18(14), 3224. https://doi.org/10.3390/ma18143224








