Hot-Pressed Reinforced Photocatalyzed TiO2/Chitosan/SiO2 Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
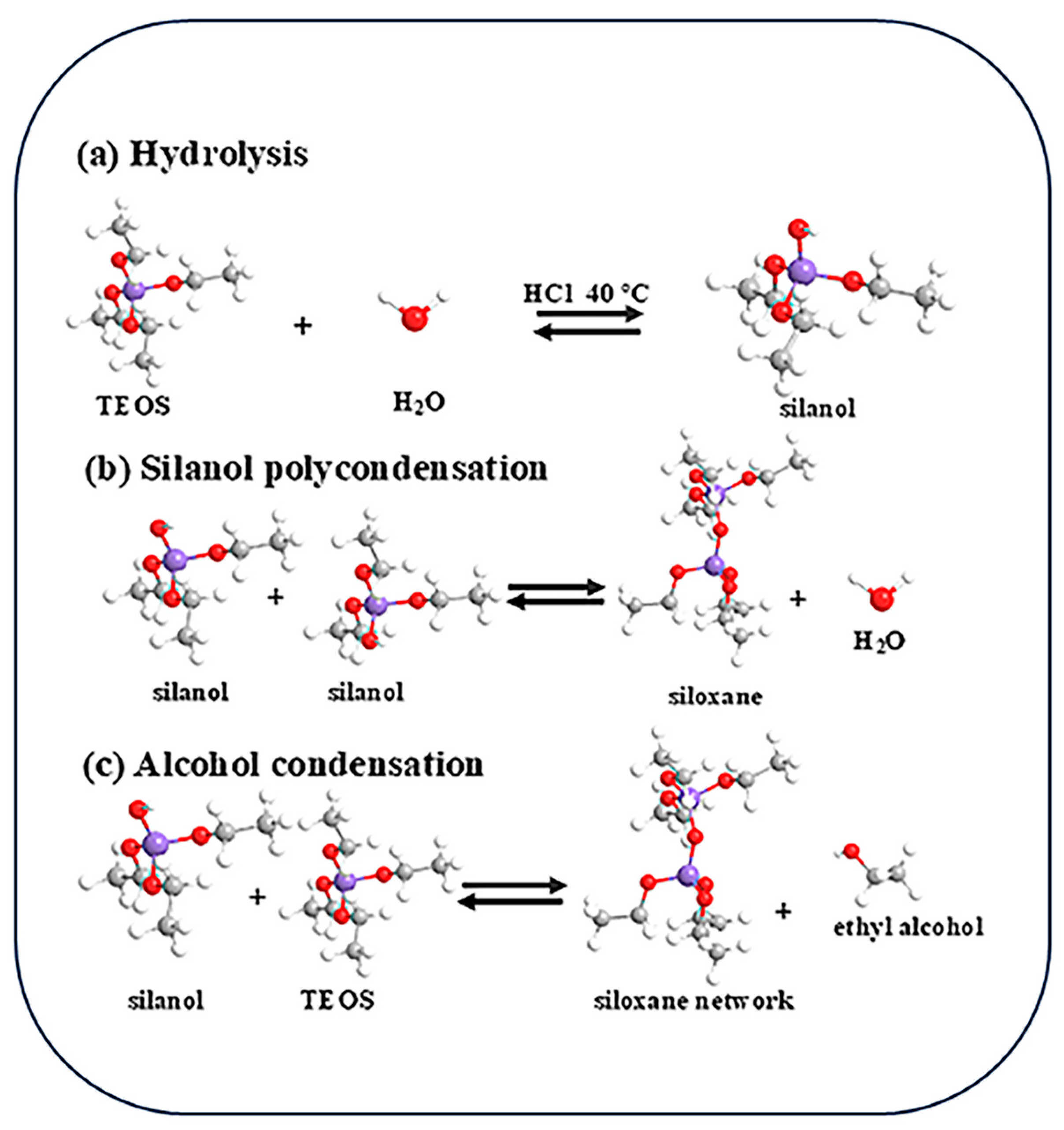
2.2. Preparation of Silicon Dioxide Nanofiber Membrane
2.3. Coating of SiO2 Nanofiber Membrane with TiO2/Chitosan Solution
2.4. Characterization of TiO2/Chitosan/SiO2 Nanofibers
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Nanoindentation
2.4.3. Fourier Transform Infrared Spectroscopy Characterization (FT-IR)
2.4.4. Photocatalytic Degradation Test
2.4.5. Tensile Property
2.4.6. Atomic Force Microscopy (AFM)
3. Results
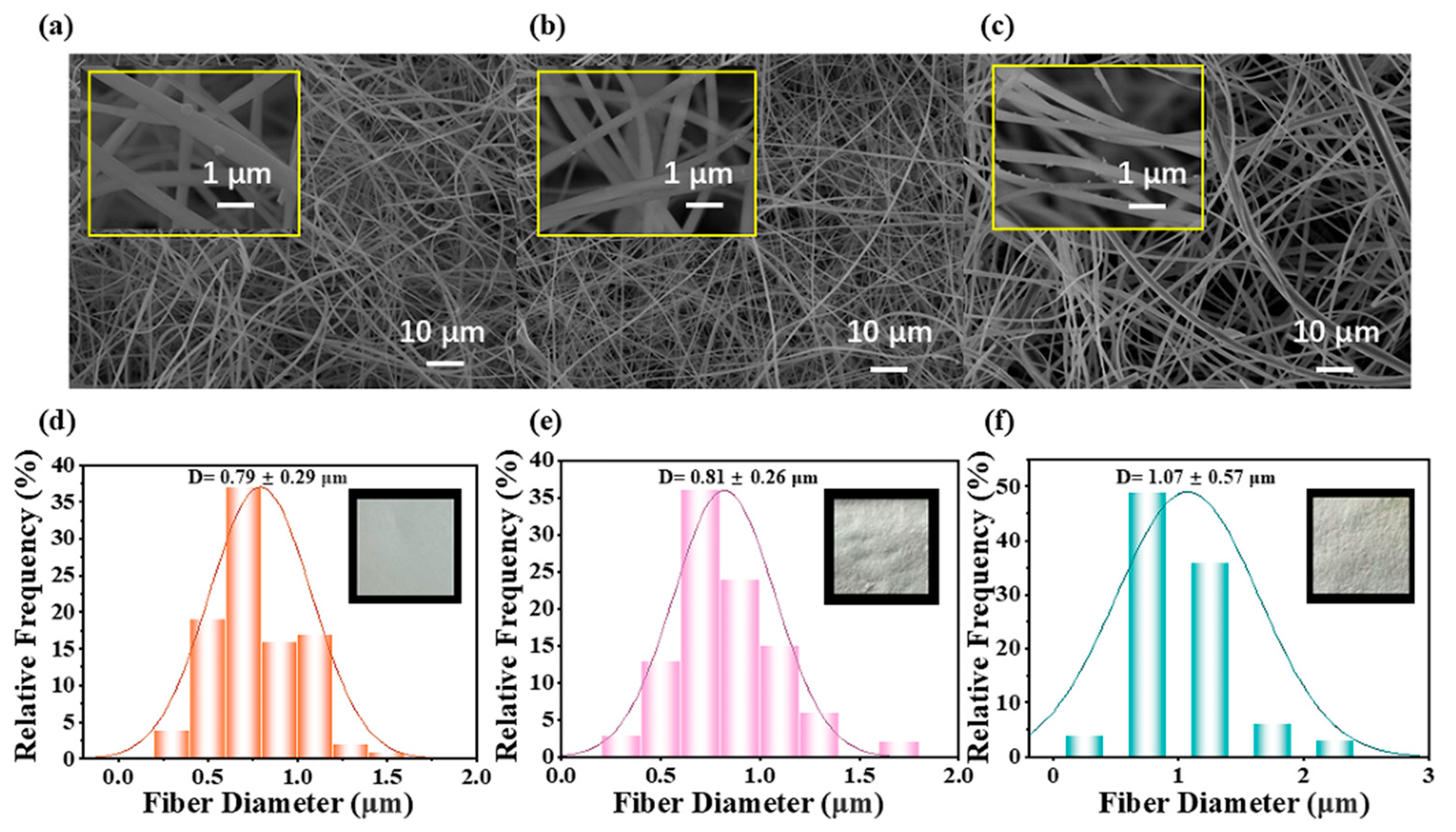
3.1. Morphology of TiO2/CTS/SiO2 Nanofibers
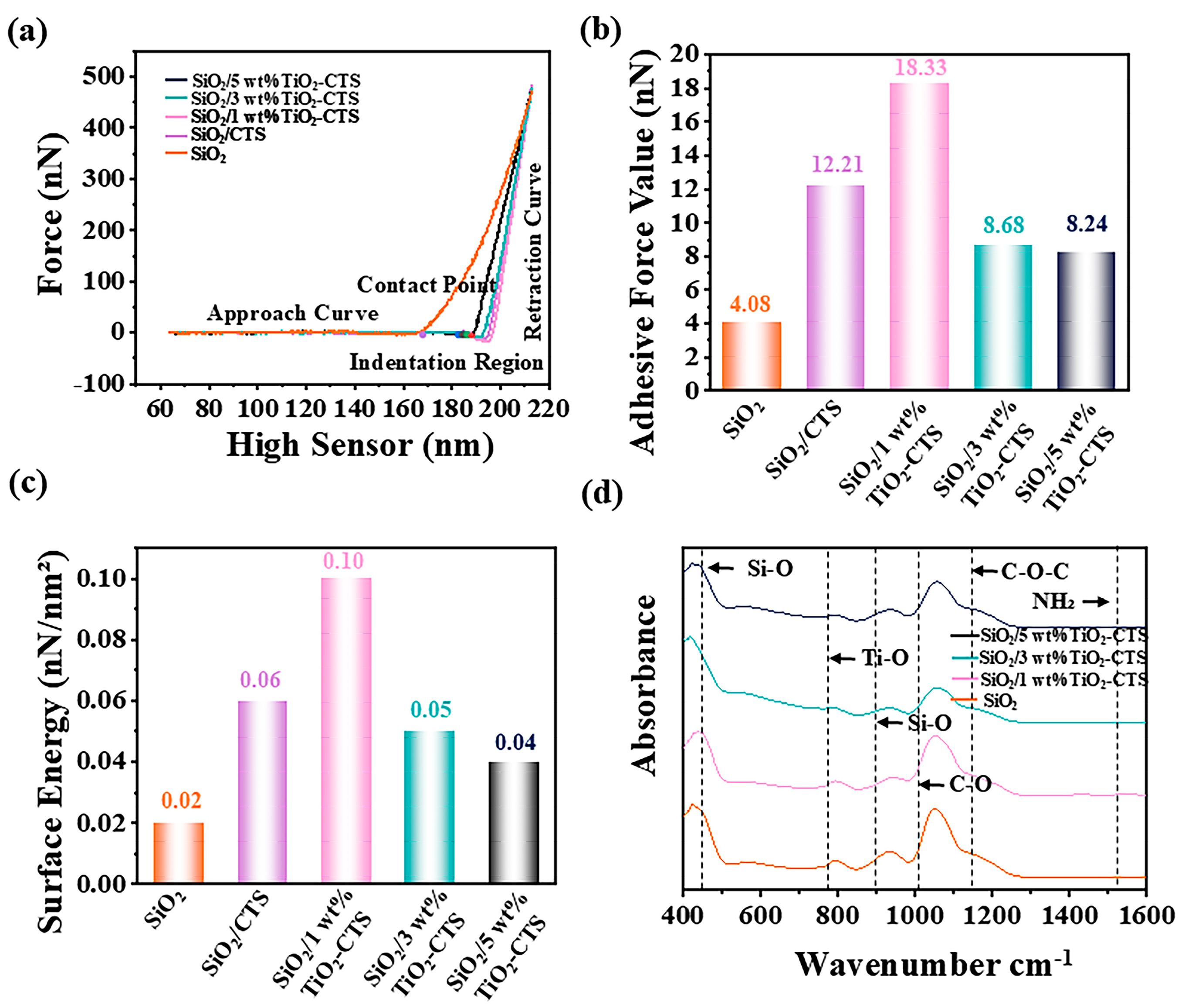
3.2. AFM Force Curves
3.3. FT-IR Characterization
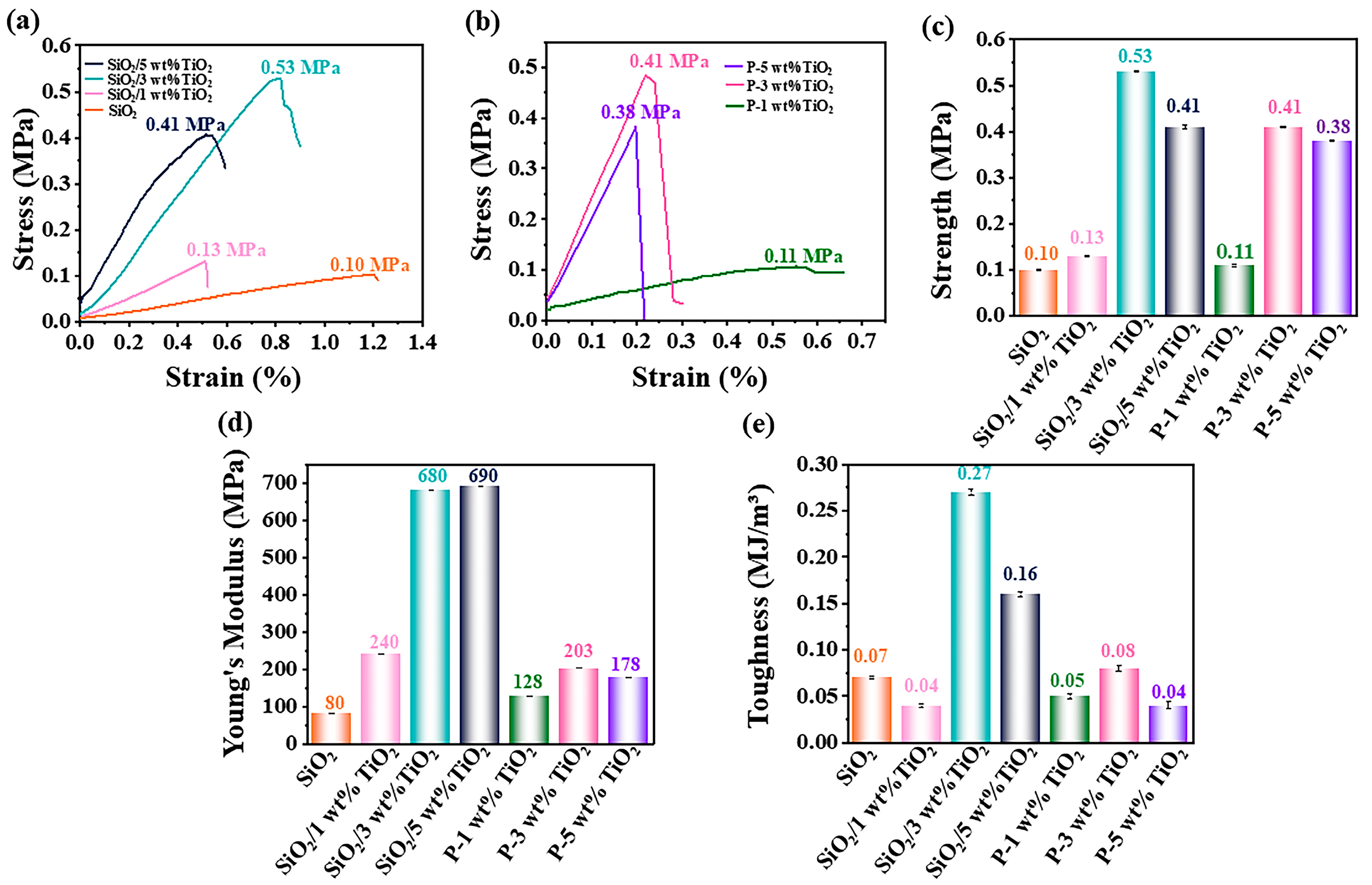
3.4. Mechanical Characterization
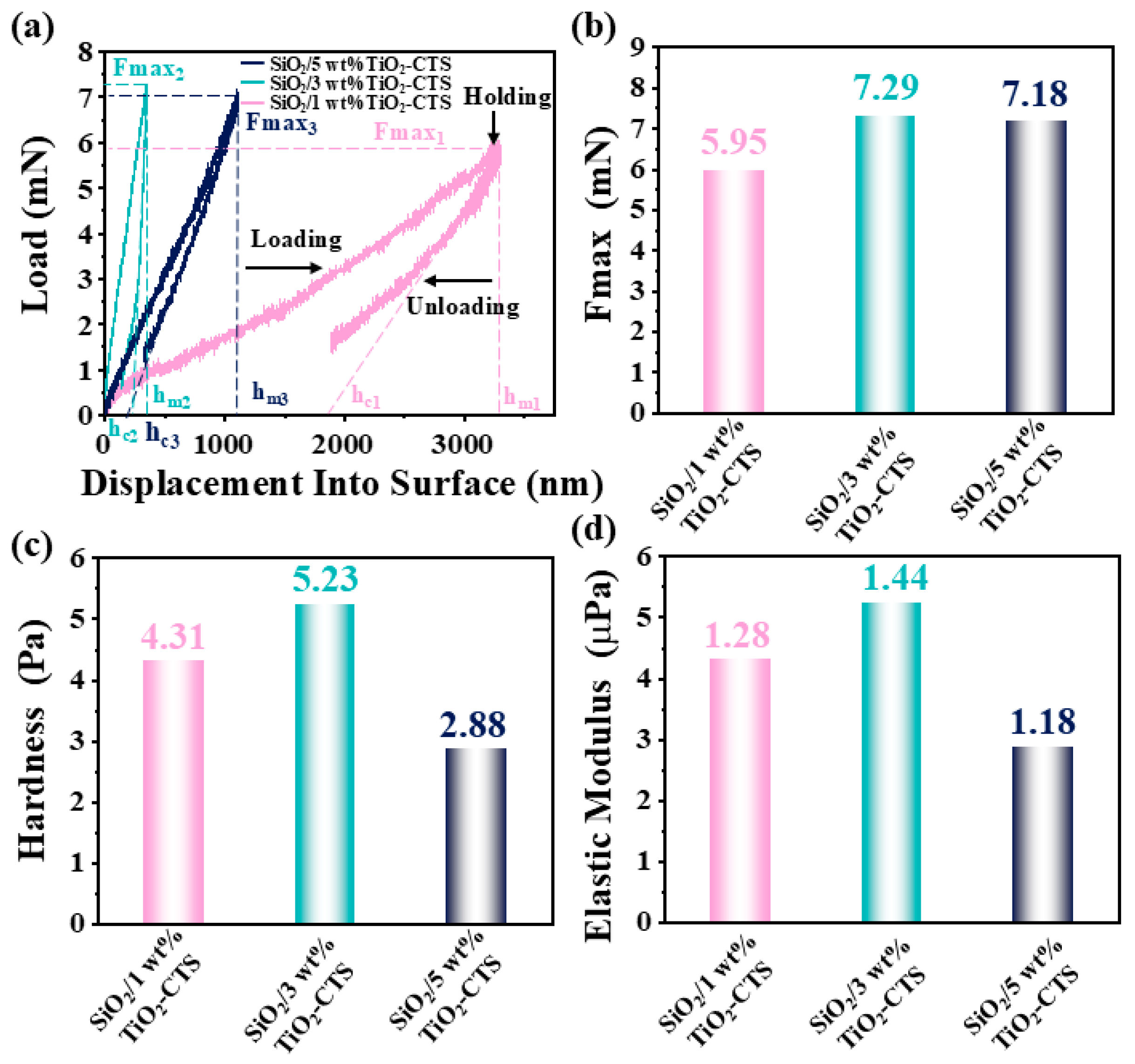
3.5. Nanoindentation
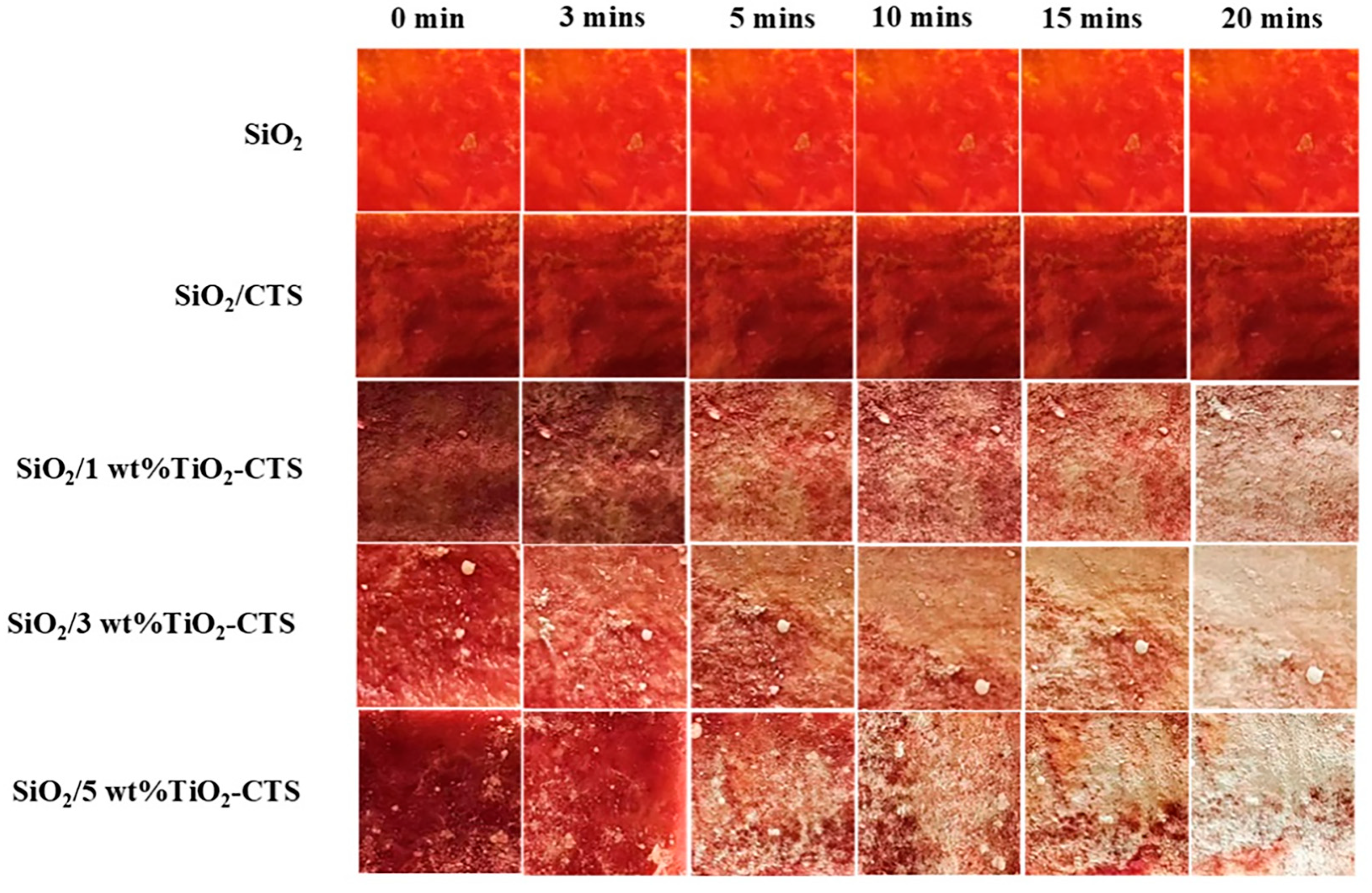
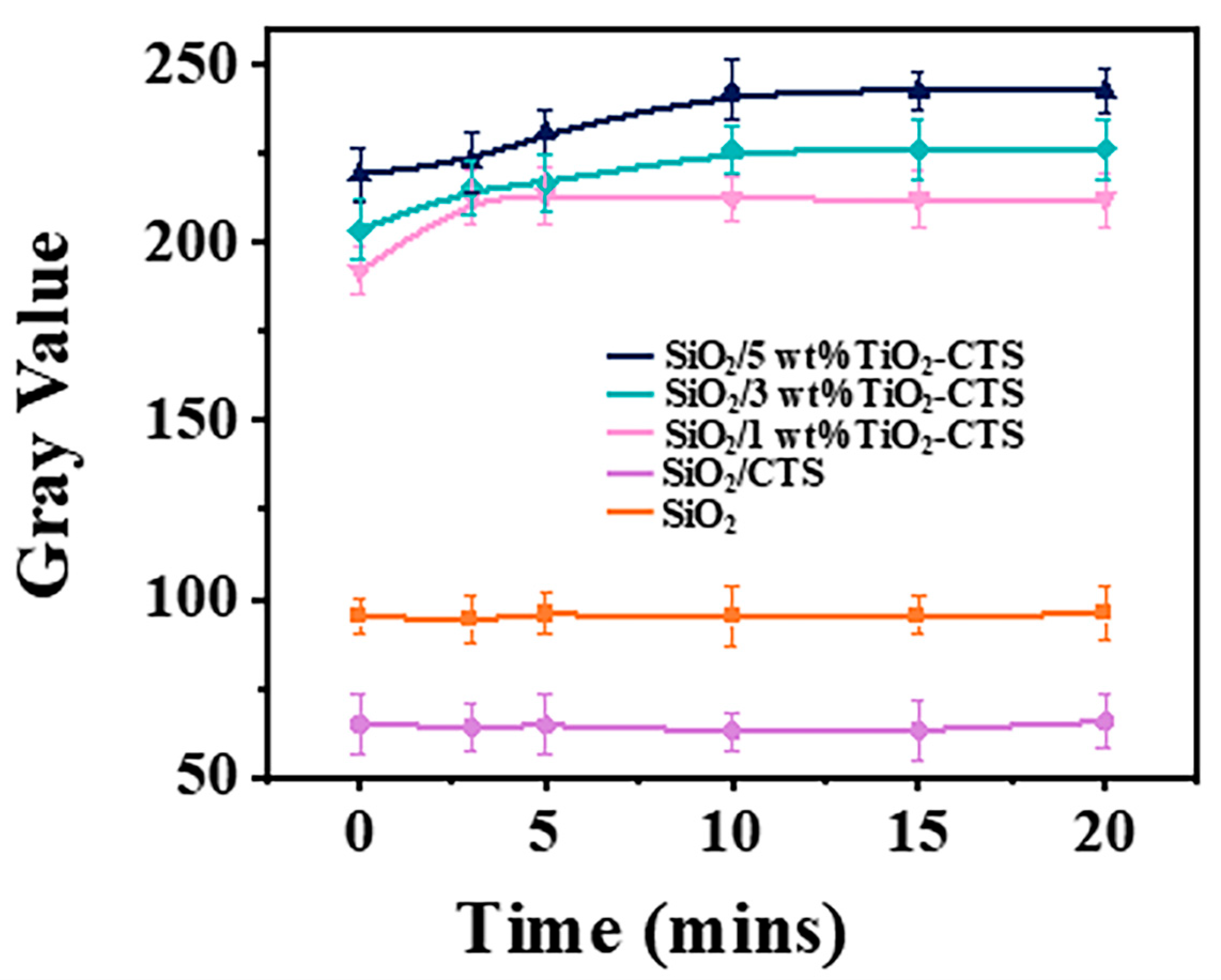
3.6. Photocatalytic Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Z.; Wang, Y.; Wen, D.; Peng, J.; Zhao, L.; Zhai, M. Recent progress in environmental applications of functional adsorbent prepared by radiation techniques: A review. J. Hazard. Mater. 2022, 424, 126887. [Google Scholar] [CrossRef]
- Huang, Z.; An, X.; Cai, X.; Chen, Y.; Liang, Y.; Hu, S.; Wang, H. The impact of new urbanization on PM2.5 concentration based on spatial spillover effects: Evidence from 283 cities in China. Sustain. Cities. Soc. 2023, 90, 104386. [Google Scholar] [CrossRef]
- Chen, H.-S.; Lin, Y.-C.; Chiueh, P.-T. Nexus of ecosystem service-human health-natural resources: The nature-based solutions for urban PM2.5 pollution. Sustain. Cities. Soc. 2023, 91, 104441. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Liao, L.; Li, Z.; Pan, S.; Puyang, C. Review on remediation of organic-contaminated soil by discharge plasma: Plasma types, impact factors, plasma-assisted catalysis, and indexes for remediation. Chem. Eng. J. 2022, 436, 135239. [Google Scholar] [CrossRef]
- Zhang, H.; Mane, A.U.; Yang, X.; Xia, Z.; Barry, E.F.; Luo, J. Visible-Light-Activated Photocatalytic Films toward Self-Cleaning Membranes. Adv. Funct. Mater. 2020, 30, 2002847. [Google Scholar] [CrossRef]
- Tang, X.; Rosseler, O.; Chen, S.; Houzé de l’Aulnoit, S.; Lussier, M.J.; Zhang, J. Self-cleaning and de-pollution efficacies of photocatalytic architectural membranes. Appl. Catal. B 2021, 281, 119260. [Google Scholar] [CrossRef]
- Xu, J.-F.; Wu, J.-C.; Ruan, L.-J.; Tian, W.; Yan, X.; Chen, Y. Ag@SnS/PVDF membranes with self-cleaning ability driven by photocatalysis process. J. Membr. Sci. 2024, 707, 123015. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Shao, J.; Chen, Y.; Wang, Z.L.; Li, H. Self-cleaning triboelectric nanogenerator based on TiO2 photocatalysis. Nano Energy 2020, 70, 104499. [Google Scholar] [CrossRef]
- Liu, G.; Xia, H.; Niu, Y.; Zhao, X.; Zhang, G.; Song, L.; Chen, H. Fabrication of self-cleaning photocatalytic durable building coating based on WO3-TNs/PDMS and NO degradation performance. Chem. Eng. J. 2021, 409, 128187. [Google Scholar] [CrossRef]
- Parasuraman, V.; Sekar, P.P.; Lee, H.; Sheraz, M.; Ly, H.N.; Bin Azizar, G.A. Photocatalytic self-cleaning eco-friendly paint: A unique approach for efficient indoor air pollutant removal and surface disinfection. Constr. Build. Mater. 2024, 412, 134671. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.-J.; Wu, G.-P.; Darling, S.B.; Xu, Z.-K. Photocatalytic Nanofiltration Membranes with Self-Cleaning Property for Wastewater Treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.F. Development of nanostructured photocatalytic coatings for anti-bioadhesion and self-cleaning of residual bacterial cells. Chem. Eng. J. 2018, 338, 513–525. [Google Scholar] [CrossRef]
- Seifikar, F.; Habibi-Yangjeh, A. Floating photocatalysts as promising materials for environmental detoxification and energy production: A review. Chemosphere 2024, 355, 141686. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Wang, M.; Cai, L.; Wang, Y.; Zhou, F.; Xu, K.; Tao, X.; Chai, Y. Graphene-Draped Semiconductors for Enhanced Photocorrosion Resistance and Photocatalytic Properties. J. Am. Chem. Soc. 2017, 139, 4144–4151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, Y.; Huang, H.; Zhang, M.; Sun, X.; Wang, Z. 2D lamellar membrane with nanochannels synthesized by bottom-up assembly approach for the superior photocatalytic hydrogen evolution. Renew. Sust. Energ. Rev. 2022, 168, 112767. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, D.; Guo, Y.; Chen, S.; Lin, W.; Long, Y. Superhydrophilic and polyporous nanofibrous membrane with excellent photocatalytic activity and recyclability for wastewater remediation under visible light irradiation. Chem. Eng. J. 2022, 427, 131685. [Google Scholar] [CrossRef]
- Kumar, D.P.; Park, H.; Kim, E.H.; Hong, S.; Gopannagari, M.; Reddy, D.A.; Kim, T.K. Noble metal-free metal-organic framework-derived onion slice-type hollow cobalt sulfide nanostructures: Enhanced activity of CdS for improving photocatalytic hydrogen production. Appl. Catal. B 2018, 224, 230–238. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Stathatos, E.; Papoulis, D.; Aggelopoulos, C.A.; Panagiotaras, D.; Nikolopoulou, A. TiO2/palygorskite composite nanocrystalline films prepared by surfactant templating route: Synergistic effect to the photocatalytic degradation of an azo-dye in water. J. Hazard. Mater. 2012, 211–212, 68–76. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Kim, H.-E.; Son, A.; Lee, C.; Kim, E.-J.; Hong, S.W. Binder-free immobilization of TiO2 photocatalyst on steel mesh via electrospraying and hot-pressing and its application for organic micropollutant removal and disinfection. J. Hazard. Mater. 2018, 360, 62–70. [Google Scholar] [CrossRef]
- de Mimérand, Y.d.R.; Li, K.; Zhou, C.; Jin, X.; Hu, X.; Chen, Y.; Guo, J. Functional Supported ZnO/Bi2MoO6 Heterojunction Photocatalysts with 3D-Printed Fractal Polymer Substrates and Produced by Innovative Plasma-Based Immobilization Methods. ACS Appl. Mater. Interfaces 2020, 12, 43138–43151. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Gao, F.; Jiang, L.; Wang, D.; Pan, H. From Single Atom Photocatalysts to Synergistic Photocatalysts: Design Principles and Applications. Adv. Funct. Mater. 2025, 35, 2418427. [Google Scholar] [CrossRef]
- Li, H.; Su, Z.; Hu, S.; Yan, Y. Free-standing and flexible Cu/Cu2O/CuO heterojunction net: A novel material as cost-effective and easily recycled visible-light photocatalyst. Appl. Catal. B 2017, 207, 134–142. [Google Scholar] [CrossRef]
- Mudhoo, A.; Paliya, S.; Goswami, P.; Singh, M.; Lofrano, G.; Carotenuto, M. Fabrication, functionalization and performance of doped photocatalysts for dye degradation and mineralization: A review. Environ. Chem. Lett. 2020, 18, 1825–1903. [Google Scholar] [CrossRef]
- Villegas-Fuentes, A.; Rosillo-de la Torre, A.; Vilchis-Nestor, A.R.; Luque, P.A. Improvement of the optical, photocatalytic and antibacterial properties of ZnO semiconductor nanoparticles using different pepper aqueous extracts. Chemosphere 2023, 339, 139577. [Google Scholar] [CrossRef] [PubMed]
- Tseng, I.H.; Liu, Z.-C.; Chang, P.-Y. Bio-friendly titania-grafted chitosan film with biomimetic surface structure for photocatalytic application. Carbohydr. Polym. 2020, 230, 115584. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, S.; Xing, B.; Liu, Y.; Zhang, C.; Xia, H. Preparation of magnetic adsorbent-photocatalyst composites for dye removal by synergistic effect of adsorption and photocatalysis. J. Clean. Prod. 2022, 348, 131301. [Google Scholar] [CrossRef]
- Li, G.; Ye, J.; Shen, Y.; Fang, Q.; Liu, F. Covalent triazine frameworks composite membrane (CdS/CTF-1) with enhanced photocatalytic in-situ cleaning and disinfection properties for sustainable separation. Chem. Eng. J. 2021, 421, 127784. [Google Scholar] [CrossRef]
- Liang, D.; Tang, J.; Sun, Q.; Zhao, W.; Li, J.; Liu, Y. Achieving ultra-low latent heat of water evaporation in capillary water by regulating hydrophilic groups and pore structure of cellulose/chitosan gel. Carbohydr. Polym. 2025, 353, 123302. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Saito, Y.; Iwamoto, S.; Tanaka, Y.; Hontama, N.; Endo, T. Suppressing aggregation of quinacridone pigment and improving its color strength by using chitosan nanofibers. Carbohydr. Polym. 2021, 255, 117365. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Fu, Z.; Lu, L.; Cheng, J.; Fei, Y. A ‘top modification’ strategy for enhancing the ability of a chitosan aerogel to efficiently capture heavy metal ions. J. Colloid Interface Sci. 2021, 594, 141–149. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Han, T.; Cheng, M.; Zhang, W.; Long, J.; Fu, X. A biomimetic SiO2@chitosan composite as highly-efficient adsorbent for removing heavy metal ions in drinking water. Chemosphere 2019, 214, 738–742. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Tang, H.; Wan, S.; Qin, W.; Zeng, Q. Depletion stabilization of emulsions based on bacterial cellulose/carboxymethyl chitosan complexes. Carbohydr. Polym. 2022, 297, 119904. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, T.; He, R.; Ding, Y.; Duan, T.; Xiao, B. Understanding the interfacial interactions of bioinspired chitosan-calcite nanocomposites by first principles molecular dynamics simulations and experimental FT-IR spectroscopy. Carbohydr. Polym. 2019, 223, 115054. [Google Scholar] [CrossRef]
- Reddy, K.P.; Swetha, C.; Murugadoss, A. Pd/Chitosan Nanoparticle Catalysts Prepared by Solid Mortar Grinding for Hydrogenation of Nitroarenes. ACS Sustain. Chem. Eng 2023, 11, 1643–1654. [Google Scholar] [CrossRef]
- Imai, K.; Fukushima, T.; Kobayashi, H.; Higashimoto, S. Visible-light responsive TiO2 for the complete photocatalytic decomposition of volatile organic compounds (VOCs) and its efficient acceleration by thermal energy. Appl. Catal. B-Environ. 2024, 346, 123745. [Google Scholar] [CrossRef]
- Dong, Z.; Meng, C.; Li, Z.; Zeng, D.; Wang, Y.; Cheng, Z. Novel Co3O4@TiO2@CdS@Au double-shelled nanocage for high-efficient photocatalysis removal of U(VI): Roles of spatial charges separation and photothermal effect. J. Hazard. Mater. 2023, 452, 131248. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Devarayapalli, K.C.; Kim, B.; Lim, Y.; Lee, D.S. Fabrication of MXene-derived TiO2/Ti3C2 integrated with a ZnS heterostructure and their synergistic effect on the enhanced photocatalytic degradation of tetracycline. J. Mater. Sci. Technol. 2024, 198, 186–199. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Zheng, X.; Zhao, W.; Wu, M.; Wang, Z. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat. Toxicol. 2015, 158, 1–13. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Liu, Y.; Deng, S.; Wu, H.; Wang, G. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2012, 84, 155–162. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Dalai, S.; Chandrasekaran, N.; Mukherjee, A. Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicol. Environ. Saf. 2011, 74, 1180–1187. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Lao, Y.; Lv, X.; Tao, Y.; Huang, B. TiO2 nanoparticles in the marine environment: Physical effects responsible for the toxicity on algae Phaeodactylum tricornutum. Sci. Total Environ. 2016, 565, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef]
- Ito, M.; Horiguchi, G.; Hariu, T.; Ito, A.; Kamiya, H.; Okada, Y. Controlling fly ash adhesion at high temperatures via porosity effect. Powder Technol. 2020, 374, 492–495. [Google Scholar] [CrossRef]
- Luan, X.; Chang, S.; Yu, R.; Zou, Y.; Riedel, R.; Cheng, L. Effect of PSO and TiB2 content on the high temperature adhesion strength of SiBCNO ceramic. Ceram. Int. 2019, 45, 9515–9521. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Yuan, Z.; Ye, L. Construction of sacrificial bonds and hybrid networks in EPDM rubber towards mechanical performance enhancement. Appl. Surf. Sci. 2019, 484, 616–627. [Google Scholar] [CrossRef]
- Zhang, G.; Ge, F.; Wang, M.; Liu, Z.; Ren, Y.; Fu, K. Self-Healable, Recyclable, and Reprocessable Poly(urethane–urea) Elastomers with Tunable Mechanical Properties Constructed by Incorporating Triple Dynamic Bonds. Ind. Eng. Chem. Res. 2023, 62, 1425–1437. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Wang, Y.; Zhang, Y.; Dou, H.; Zhong, H.; Guo, M. Laminated composites with an ultra-high cellulose content exhibit high strength and toughness. Cellulose 2024, 31, 7521–7530. [Google Scholar] [CrossRef]
- Ren, Y.; Zhong, Y.; Yang, Y.; Huo, H.; Zhang, L.; Zhang, J. Green recyclable biocomposite prepared from lignin and bamboo. J. Clean. Prod. 2024, 449, 141710. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Luo, Y.; Shi, Y.; Ma, X.; Zhang, M. A self-healing elastomer based on an intrinsic non-covalent cross-linking mechanism. J. Mater. Chem. A 2019, 7, 15207–15214. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, H. Self-introduction of carbon nitride quantum dots into carbon nitride planar structure for enhanced photocatalytic hydrogen production. Appl. Catal. B 2023, 339, 123101. [Google Scholar] [CrossRef]
- He, S.; Sun, S.; Xue, H.; Kang, C.; Yu, S. Polypropylene microplastics aging under natural conditions in winter and summer and its effects on the sorption and desorption of nonylphenol. Environ. Res. 2023, 225, 115615. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, Y.; Fan, L.; Yang, S.; Zhang, M.; An, L. Preparation and mechanical properties of SiCw-Al2O3-YAG ceramic composite by hot oscillatory pressing. Ceram. Int. 2021, 47, 21231–21235. [Google Scholar] [CrossRef]
- Balalan, Z.; Gulan, F. Microstructure and mechanical properties of Cu-B4C and CuAl-B4C composites produced by hot pressing. Rare Met. 2019, 38, 1169–1177. [Google Scholar] [CrossRef]
- Wang, A.; Liu, C.; Hu, L.; Tian, T.; He, Q.; Wang, W. Effects of processing on mechanical properties of B4C-graphene composites fabricated by hot pressing. Mater. Sci. Eng. A 2021, 808, 140872. [Google Scholar] [CrossRef]
- ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012.
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, Z.; Ferrell, R.E. Elastic modulus and hardness of muscovite and rectorite determined by nanoindentation. Appl. Clay Sci. 2009, 43, 271–281. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, Z.; Zhang, J.; Liu, Y.; Hou, C.; Cheng, H.; Wang, Y.; Liu, X. Hot-Pressed Reinforced Photocatalyzed TiO2/Chitosan/SiO2 Nanofibers. Materials 2025, 18, 4828. https://doi.org/10.3390/ma18214828
Wang J, Liu Z, Zhang J, Liu Y, Hou C, Cheng H, Wang Y, Liu X. Hot-Pressed Reinforced Photocatalyzed TiO2/Chitosan/SiO2 Nanofibers. Materials. 2025; 18(21):4828. https://doi.org/10.3390/ma18214828
Chicago/Turabian StyleWang, Jingwen, Zunzhi Liu, Jingmei Zhang, Yang Liu, Chunjing Hou, Hui Cheng, Yaru Wang, and Xiang Liu. 2025. "Hot-Pressed Reinforced Photocatalyzed TiO2/Chitosan/SiO2 Nanofibers" Materials 18, no. 21: 4828. https://doi.org/10.3390/ma18214828
APA StyleWang, J., Liu, Z., Zhang, J., Liu, Y., Hou, C., Cheng, H., Wang, Y., & Liu, X. (2025). Hot-Pressed Reinforced Photocatalyzed TiO2/Chitosan/SiO2 Nanofibers. Materials, 18(21), 4828. https://doi.org/10.3390/ma18214828




