Universal Bulk-Fill Composites: An Investigation into the Efficiency of Rapid Curing with Reversible Addition–Fragmentation-Chain Transfer (RAFT)-Mediated Polymerisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Spectroscopy: LCU Characteristics
2.2.2. ATR-FTIR Spectroscopy: Degree of Conversion (DC)
2.3. Statistical Analyses
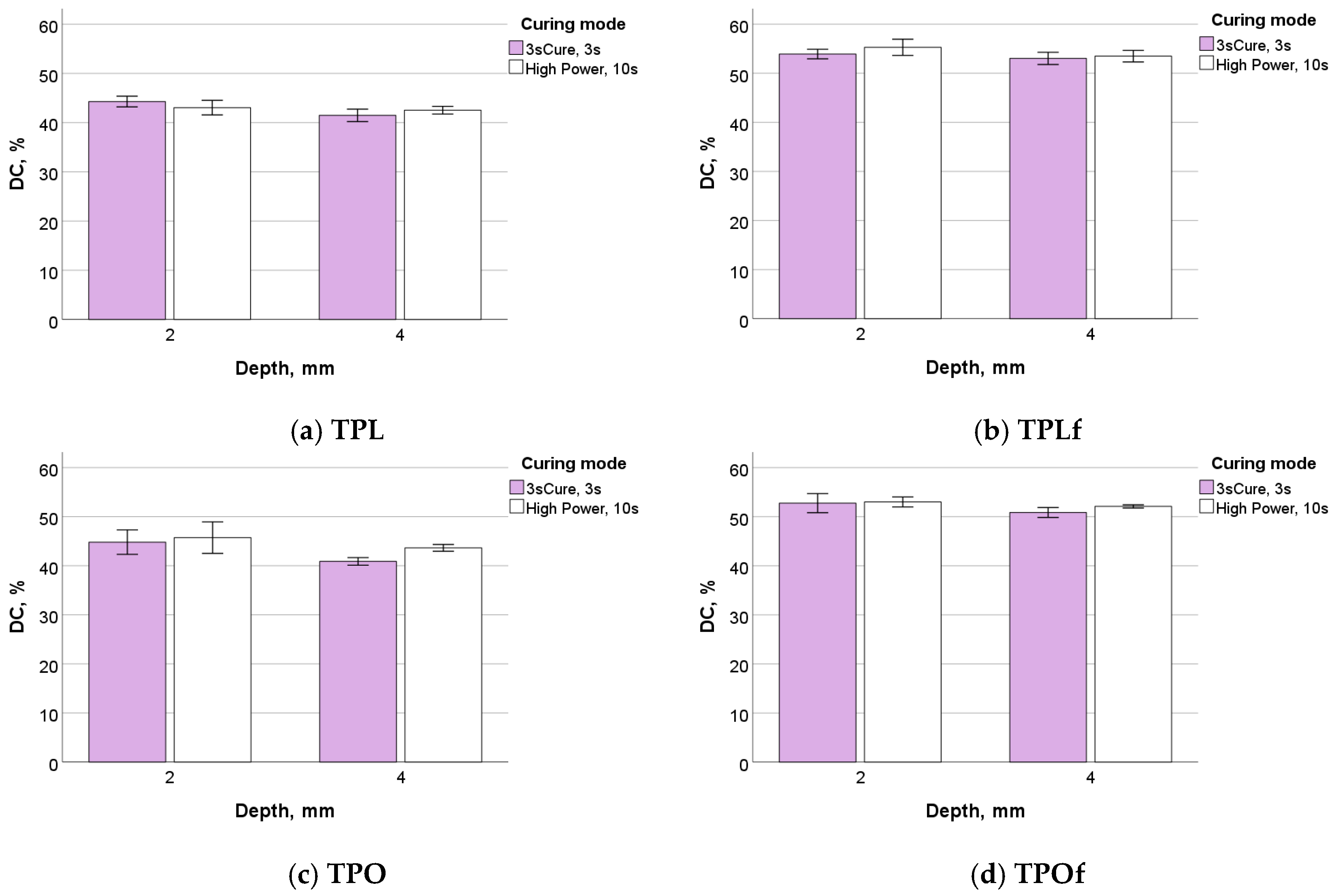
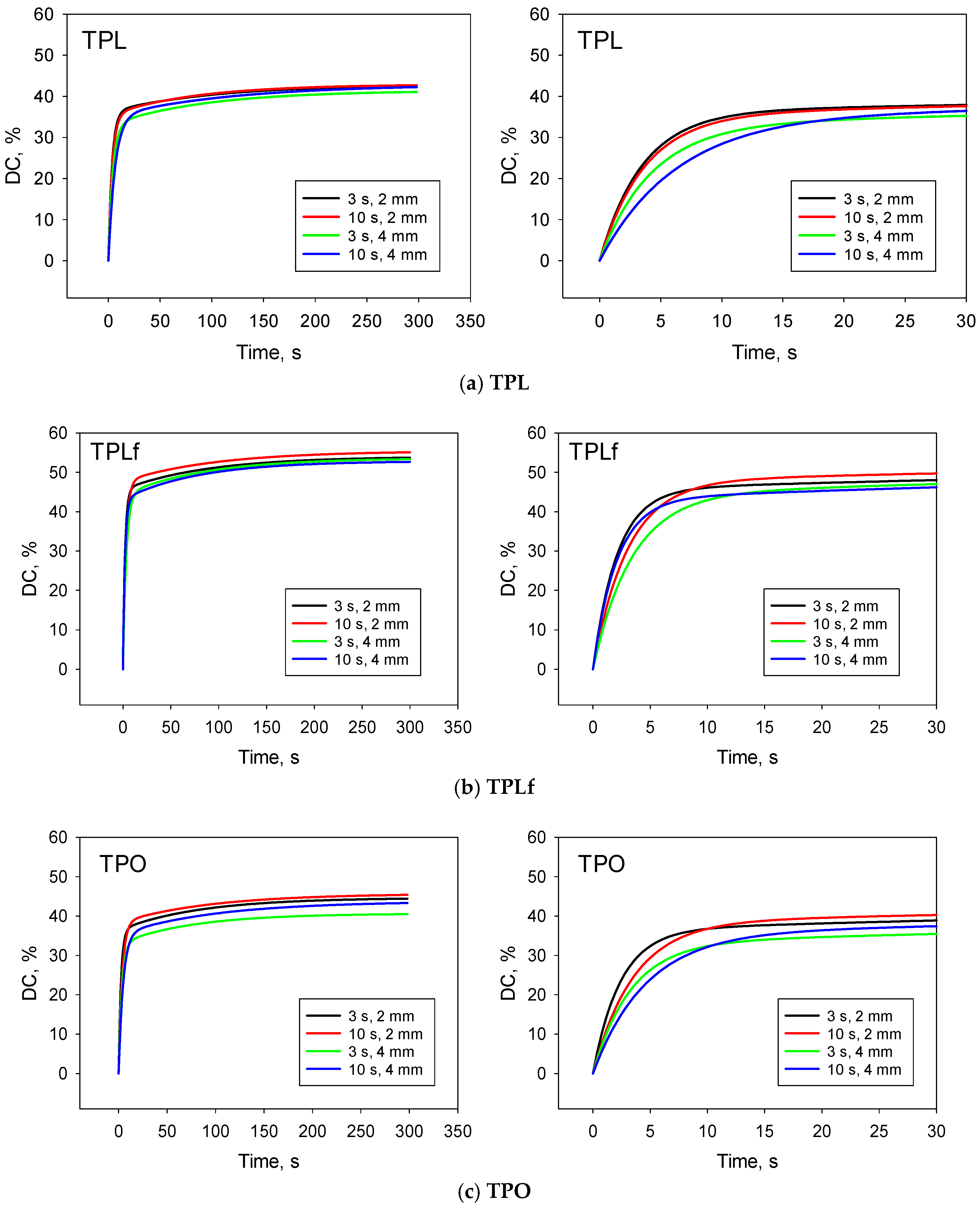
3. Results
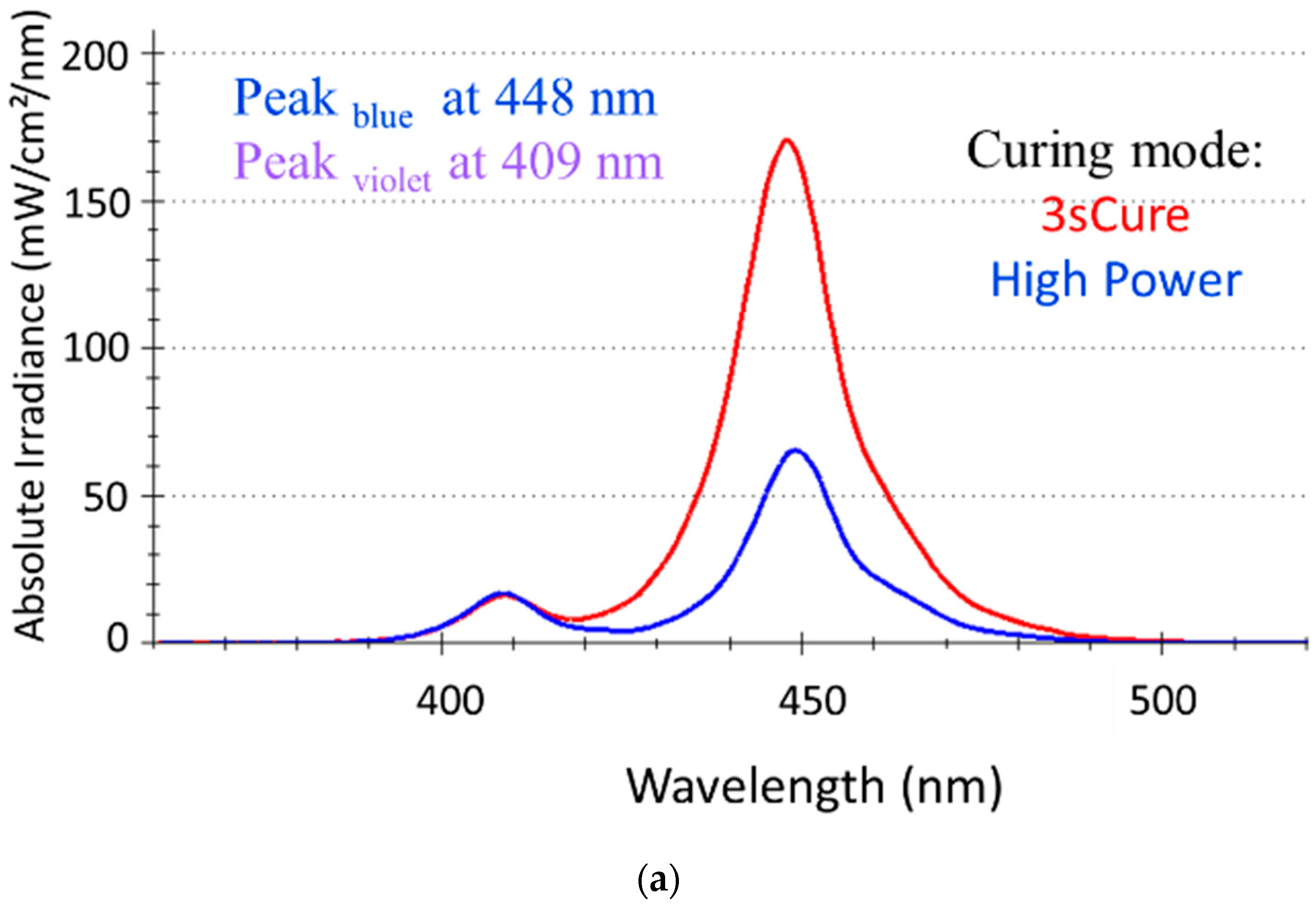
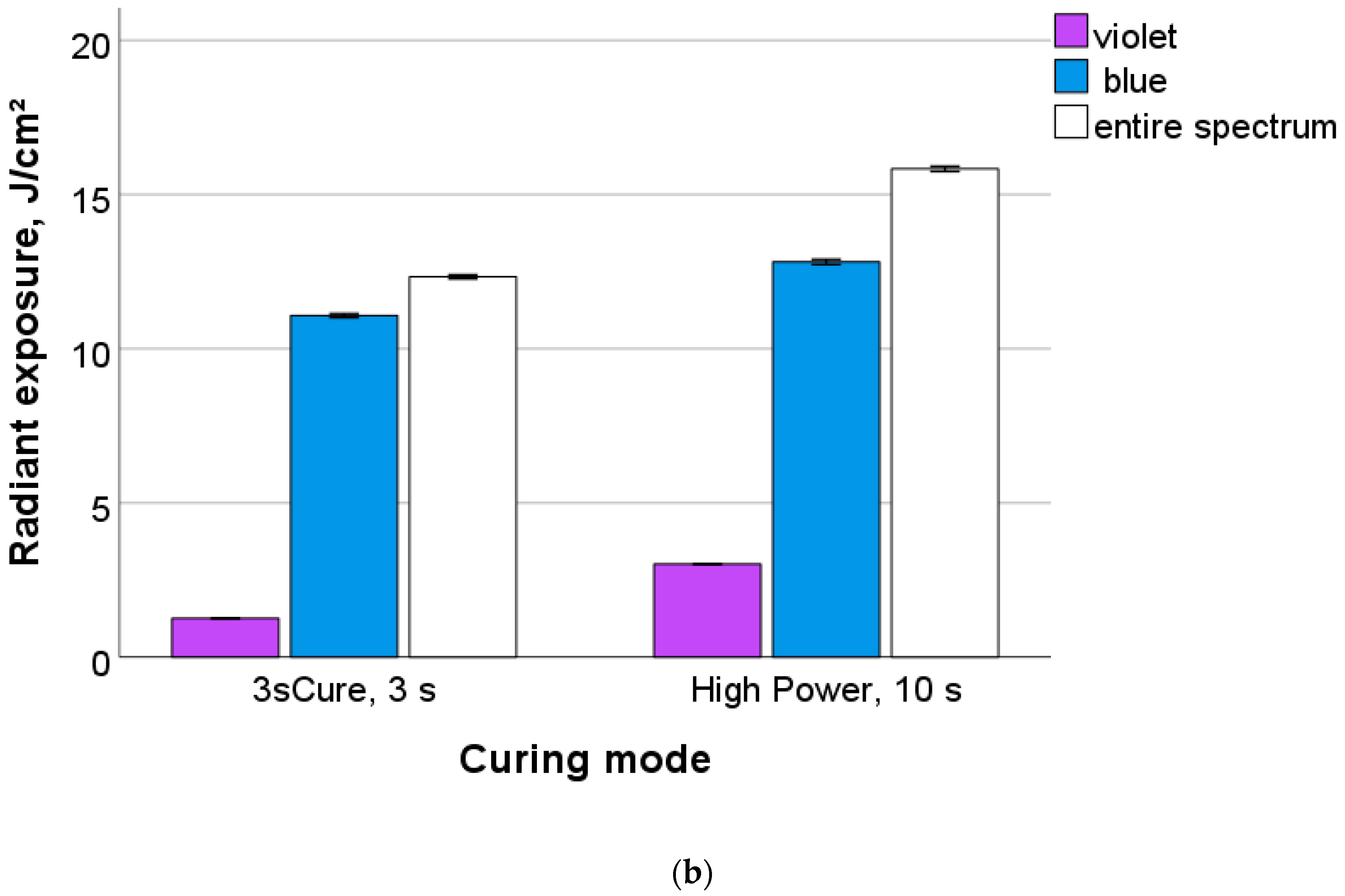
3.1. Light Curing Unit (LCU) Characteristics
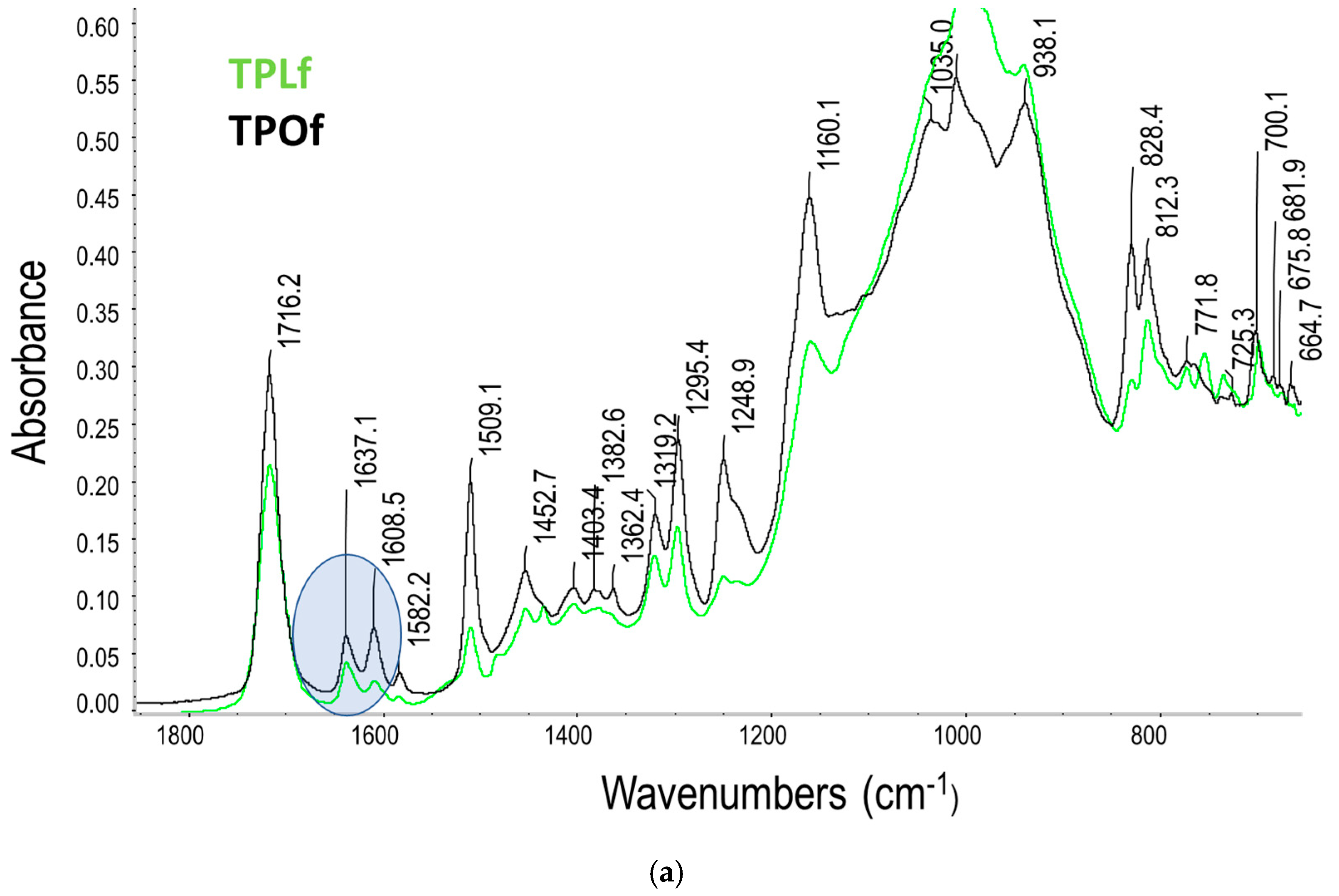
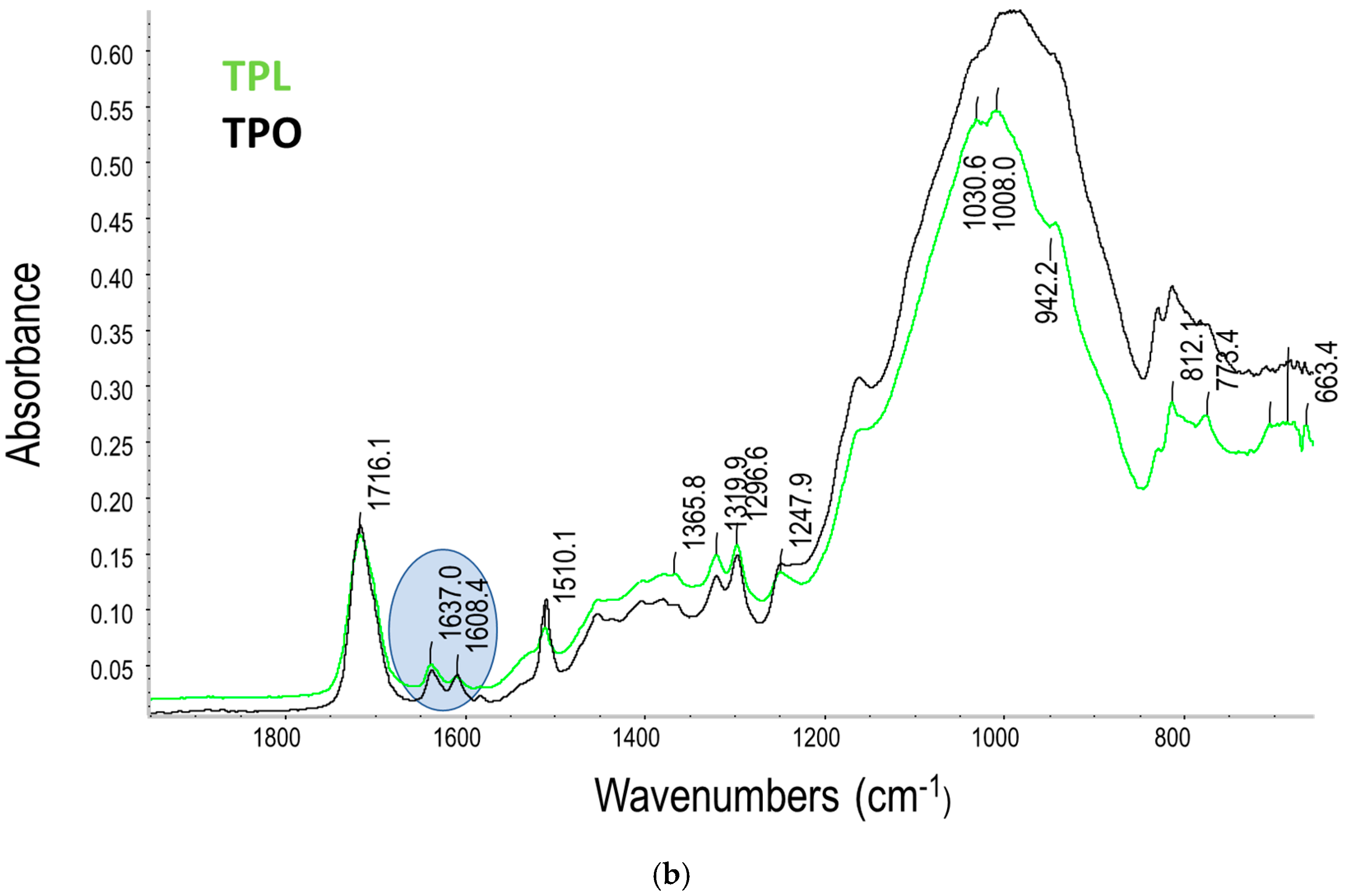
3.2. Degree of Conversion (DC) and Kinetic of Polymerisation
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarapultseva, M.; Hu, D.; Sarapultsev, A. Clinical Performance of Bulk-Fill Versus Incremental Composite Restorations in Primary Teeth: A Systematic Review of In Vivo Evidence. Dent. J. 2025, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Wolf, E.; Lücker, S.; Frankenberger, R.; Wöstmann, B.; Krämer, N. Marginal Quality and Wear of Bulk-Fill Composites: Differences Between Dentitions. J. Adhes. Dent. 2025, 27, 9–19. [Google Scholar] [CrossRef]
- Lucchi, P.; Mazzoleni, S.; Parcianello, R.G.; Gatto, R.; Gracco, A.; Stellini, E.; Ludovichetti, F.S. Bulk-flow composites in paediatric dentistry: Long term survival of posterior restorations. A retrospective study. J. Clin. Pediatr. Dent. 2024, 48, 108–114. [Google Scholar] [CrossRef]
- de Menezes, A.J.O.; do Nascimento Barbosa, L.; Leite, J.V.C.; Barbosa, L.M.M.; Montenegro, R.V.; Dantas, R.V.F.; de Souza, G.M.; de Andrade, A.K.M.; Lima, R.B.W. Clinical Outcomes of Bulk-Fill Resin Composite Restorations: A 10-Year Mapping Review and Evidence Gap Map. J. Esthet. Restor. Dent. 2025, 37, 920–933. [Google Scholar] [CrossRef]
- Schoilew, K.; Fazeli, S.; Felten, A.; Sekundo, C.; Wolff, D.; Frese, C. Clinical evaluation of bulk-fill and universal nanocomposites in class II cavities: Five-year results of a randomized clinical split-mouth trial. J. Dent. 2023, 128, 104362. [Google Scholar] [CrossRef]
- Rueggeberg, F.A. State-of-the-art: Dental photocuring—A review. Dent. Mater. 2011, 27, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Sehmi, H. A comparative study of the properties of dental resin composites polymerized with plasma and halogen light. Dent. Mater. 2003, 19, 517–522. [Google Scholar] [CrossRef]
- Randolph, L.D.; Palin, W.M.; Watts, D.C.; Genet, M.; Devaux, J.; Leloup, G.; Leprince, J.G. The effect of ultra-fast photopolymerisation of experimental composites on shrinkage stress, network formation and pulpal temperature rise. Dent. Mater. 2014, 30, 1280–1289. [Google Scholar] [CrossRef]
- Joly, G.D.; Abuelyaman, A.S.; Fornof, A.R.; Craig, B.D.; Krepski, L.R.; Moser, W.H.; Yurt, S.; Oxman, J.D.; Falsafi, A. Dental Compositions Comprising Addition-Fragmentation Agents (Patent). 2015. Available online: https://patents.google.com/patent/US9907733B2/en (accessed on 23 September 2025).
- Ilie, N.; Watts, D.C. Outcomes of ultra-fast (3 s) photo-cure in a RAFT-modified resin-composite. Dent. Mater. 2020, 36, 570–579. [Google Scholar] [CrossRef]
- Watts, D.C.; Algamaiah, H. Characterizing surface viscoelastic integrity of ultra-fast photo-polymerized composites: Methods development. Dent. Mater. 2020, 36, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Marovic, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Effect of rapid high-intensity light-curing on polymerization shrinkage properties of conventional and bulk-fill composites. J. Dent. 2020, 101, 103448. [Google Scholar] [CrossRef]
- Marovic, D.; Par, M.; Daničić, P.; Marošević, A.; Bojo, G.; Alerić, M.; Antić, S.; Puljić, K.; Badovinac, A.; Shortall, A.C.; et al. The Role of Rapid Curing on the Interrelationship Between Temperature Rise, Light Transmission, and Polymerisation Kinetics of Bulk-Fill Composites. Int. J. Mol. Sci. 2025, 26, 2803. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Diegelmann, J. Impact of ultra-fast (3 s) light-cure on cell toxicity and viscoelastic behavior in a dental resin-based composite with RAFT-mediated polymerization. J. Mech. Behav. Biomed. Mater. 2021, 124, 104810. [Google Scholar] [CrossRef] [PubMed]
- Baabdullah, F.; Bakitian, F.; Alshammari, H.; Aljanakh, M.; Holdar, A.; Ozcan, I.; Antonson, S.A. Comparative analysis of pulpal temperature changes in bulk fill resin composites with different photoinitiators activated using various light curing units: An in vitro study. Dent. Mater. 2025, 41, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Klarić, E.; Bosnić, J.V.; Par, M.; Tarle, Z.; Marovic, D. One-Year Evaluation of High-Power Rapid Curing on Dentin Bond Strength. Materials 2024, 17, 2297. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Marovic, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Rapid high-intensity light-curing of bulk-fill composites: A quantitative analysis of marginal integrity. J. Dent. 2021, 111, 103708. [Google Scholar] [CrossRef]
- Beres, M.A.; Boyer, C.; Hartlieb, M.; Konkolewicz, D.; Qiao, G.G.; Sumerlin, B.S.; Perrier, S. RAFT with Light: A User Guide to Using Thiocarbonylthio Compounds in Photopolymerizations. ACS Polym. Au 2025, 5, 184–213. [Google Scholar] [CrossRef]
- Barner-Kowollik, C. Handbook of RAFT Polymerization; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Gorsche, C.; Griesser, M.; Gescheidt, G.; Moszner, N.; Liska, R. β-Allyl Sulfones as Addition–Fragmentation Chain Transfer Reagents: A Tool for Adjusting Thermal and Mechanical Properties of Dimethacrylate Networks. Macromolecules 2014, 47, 7327–7336. [Google Scholar] [CrossRef]
- Ilie, N. An In Vitro Comparison of Elastoplastic and Viscoelastic Behavior of Dental Composites with Reversible Addition–Fragmentation Chain Transfer-Mediated Polymerization. J. Compos. Sci. 2023, 7, 247. [Google Scholar] [CrossRef]
- Ilie, N. Relationship Between Fracture Toughness and Fracture Mirror in Modern Polymer-Based Dental Composites. J. Funct. Biomater. 2025, 16, 290. [Google Scholar] [CrossRef]
- Fernández Godoy, E.; Chaple Gil, A.; Caviedes Thomas, R.; Bersezio Miranda, C.; Martín Casielles, J.; Rodríguez Martínez, G.; Angel Aguirre, P. Efficiency and limitations of polywave light-curing units in restorative dentistry: A systematic review. Clin. Oral. Investig. 2025, 29, 384. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Fischer, U.K.; Ganster, B.; Liska, R.; Rheinberger, V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent. Mater. 2008, 24, 901–907. [Google Scholar] [CrossRef] [PubMed]

| Composite | Code | Shade | LOT | Monomer | Filler wt%/vol% |
|---|---|---|---|---|---|
| Tetric® PowerFill | TPO | IVA | Z04FD5 | Bis-GMA, Bis-PMA, UDMA, Bis-EMA, TDM | 76–77/53–54 |
| Tetric® PowerFlow | TPOf | IVA | Z05FP4 | Bis-EMA, Bis-GMA, TDM | 68.2/46.4 |
| Tetric® plus Fill | TPL | A3 plus | YM0202 | UDMA; AAUDMA; TDM | 70/51–52 |
| Tetric® plus Flow | TPLf | A3 plus | YM0210 | 2-(2-Phenylphenoxy)ethyl 2-methylprop-2-Enoat TDM, UDMA N-(2-Methacryloyloxyethyl)carbaminsäure-(2-methacryloyloxyethyl)ester AAUDMA | 65/50–51 |
| Composite | Depth | Exposure | R2 | amin | amax | bmin | bmax | cmin | cmax | dmin | dmax |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPL | 2 mm | 3 s | 0.95 | 36.02 | 36.41 | 0.28 | 0.29 | 6.63 | 6.94 | 0.01 | 0.01 |
| 10 s | 0.95 | 35.12 | 35.59 | 0.27 | 0.28 | 7.37 | 7.76 | 0.01 | 0.01 | ||
| 4 mm | 3 s | 0.95 | 32.74 | 33.22 | 0.23 | 0.25 | 8.26 | 8.64 | 0.01 | 0.01 | |
| 10 s | 0.95 | 34.70 | 35.29 | 0.15 | 0.16 | 7.70 | 8.13 | 0.01 | 0.01 | ||
| TPLf | 2 mm | 3 s | 0.98 | 45.50 | 45.76 | 0.47 | 0.48 | 8.28 | 8.50 | 0.01 | 0.01 |
| 10 s | 0.98 | 47.49 | 47.77 | 0.33 | 0.34 | 7.70 | 7.93 | 0.01 | 0.01 | ||
| 4 mm | 3 s | 0.99 | 44.07 | 44.35 | 0.29 | 0.30 | 9.30 | 9.54 | 0.01 | 0.01 | |
| 10 s | 0.98 | 42.95 | 43.22 | 0.28 | 0.29 | 9.67 | 9.91 | 0.01 | 0.01 | ||
| TPOf | 2 mm | 3 s | 0.98 | 42.87 | 43.10 | 0.46 | 0.47 | 10.15 | 10.36 | 0.01 | 0.01 |
| 10 s | 0.98 | 45.13 | 45.38 | 0.28 | 0.29 | 7.84 | 8.05 | 0.01 | 0.01 | ||
| 4 mm | 3 s | 0.99 | 41.08 | 41.31 | 0.35 | 0.35 | 9.74 | 9.93 | 0.01 | 0.01 | |
| 10 s | 0.99 | 43.34 | 43.62 | 0.25 | 0.25 | 8.88 | 9.10 | 0.01 | 0.01 | ||
| TPO | 2 mm | 3 s | 0.93 | 36.15 | 36.61 | 0.41 | 0.43 | 8.11 | 8.50 | 0.01 | 0.01 |
| 10 s | 0.95 | 38.05 | 38.48 | 0.28 | 0.29 | 7.29 | 7.64 | 0.01 | 0.01 | ||
| 4 mm | 3 s | 0.93 | 32.72 | 33.24 | 0.30 | 0.31 | 7.47 | 7.91 | 0.01 | 0.01 | |
| 10 s | 0.95 | 35.04 | 35.57 | 0.21 | 0.22 | 8.26 | 8.67 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilie, N. Universal Bulk-Fill Composites: An Investigation into the Efficiency of Rapid Curing with Reversible Addition–Fragmentation-Chain Transfer (RAFT)-Mediated Polymerisation. Materials 2025, 18, 4489. https://doi.org/10.3390/ma18194489
Ilie N. Universal Bulk-Fill Composites: An Investigation into the Efficiency of Rapid Curing with Reversible Addition–Fragmentation-Chain Transfer (RAFT)-Mediated Polymerisation. Materials. 2025; 18(19):4489. https://doi.org/10.3390/ma18194489
Chicago/Turabian StyleIlie, Nicoleta. 2025. "Universal Bulk-Fill Composites: An Investigation into the Efficiency of Rapid Curing with Reversible Addition–Fragmentation-Chain Transfer (RAFT)-Mediated Polymerisation" Materials 18, no. 19: 4489. https://doi.org/10.3390/ma18194489
APA StyleIlie, N. (2025). Universal Bulk-Fill Composites: An Investigation into the Efficiency of Rapid Curing with Reversible Addition–Fragmentation-Chain Transfer (RAFT)-Mediated Polymerisation. Materials, 18(19), 4489. https://doi.org/10.3390/ma18194489






