Barium Titanate-Based Glass–Ceramics Crystallized from Multicomponent Oxide Glasses: Phase Composition and Microstructure
Highlights
- Glass preparation possible for ≤3 mol% ZrO2 in the Na2O∙BaO∙ZrO2∙TiO2∙B2O3∙SiO2∙Al2O3 system.
- Controlled crystallization of the glasses leads to precipitation of BaTiO3 and fresnoite.
- Phase separation occurring during quenching restricts the size of the growing BaTiO3.
- The crystalline structures sizes increase for longer annealing time due to fresnoite growth.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
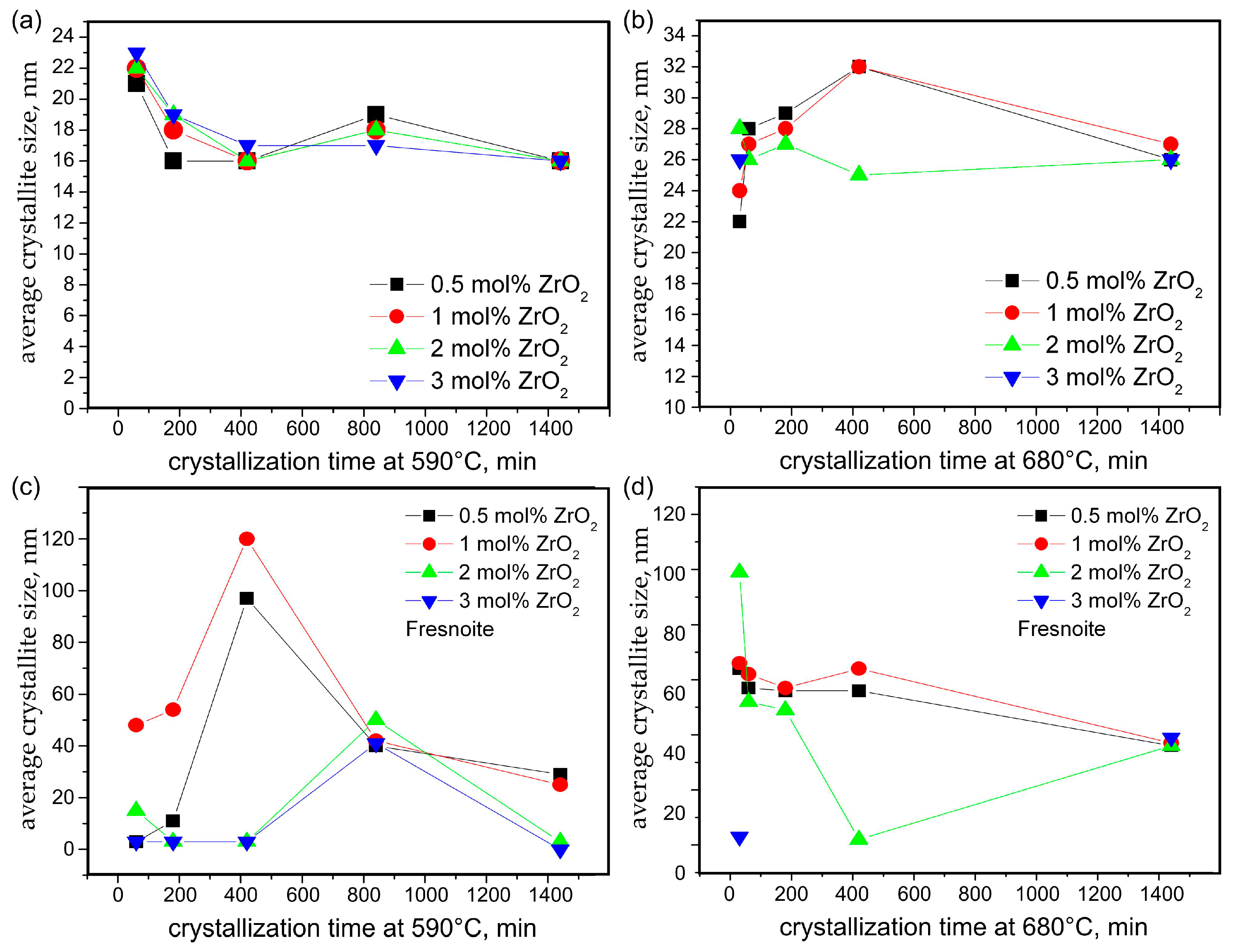
3.1. XRD
3.2. SEM
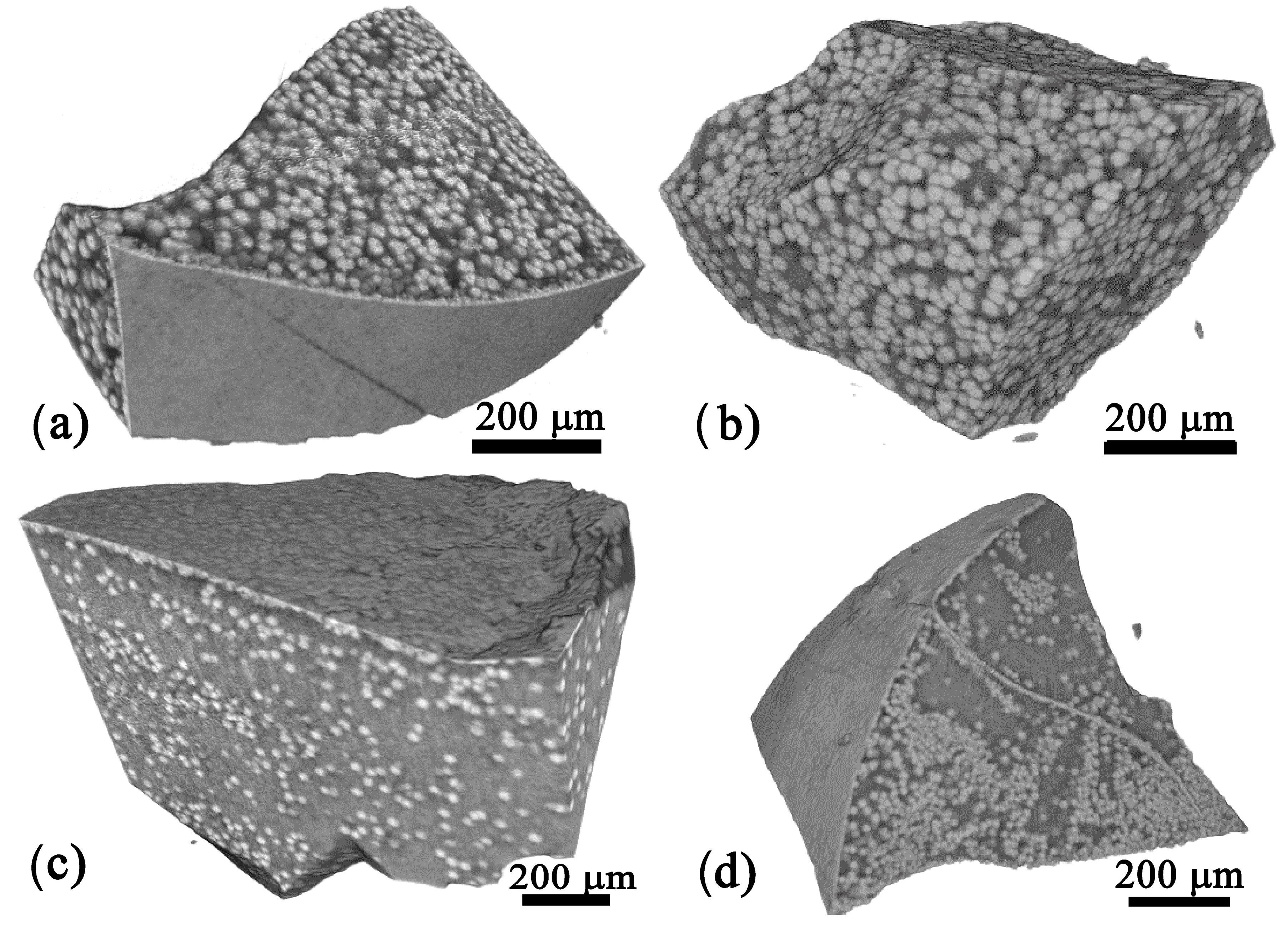
3.3. X-Ray Computed Tomography
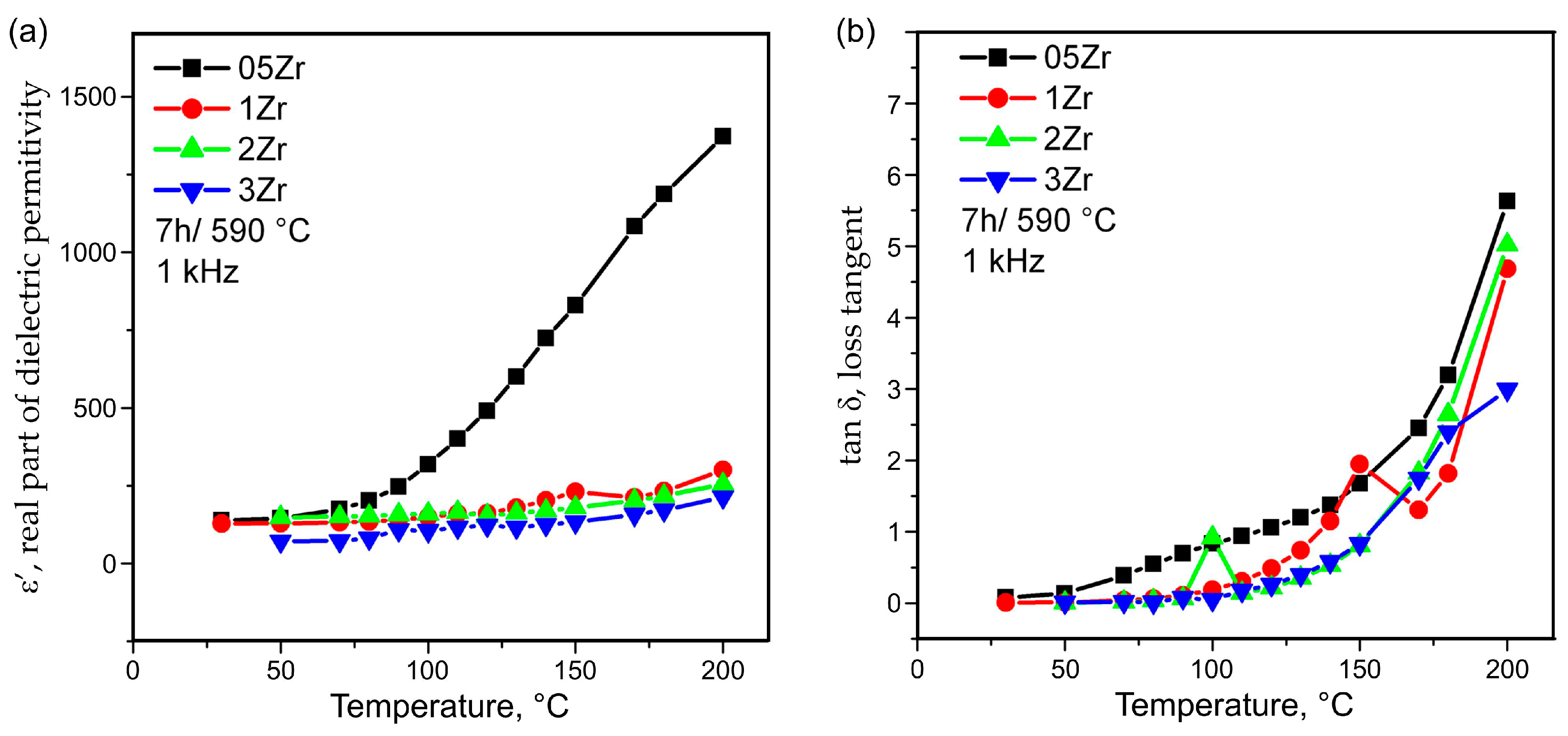
3.4. Electrical Impedance Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray diffraction in the Bragg–Brentano configuration |
| SEM | Scanning electron microscopy |
| EDXS | Energy-dispersive X-ray spectroscopy |
| XTM | Microcomputed X-ray tomography |
| EBSD | Electron back-scatter diffraction |
References
- Vogel, W. Glass Chemistry, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1994. [Google Scholar] [CrossRef]
- Bhargava, A.; Snyder, R.L.; Condrate, R.A. Preparation of BaTiO3 glass-ceramic in the system Ba-Ti-B-O II. Mater. Lett. 1988, 7, 190–196. [Google Scholar] [CrossRef]
- Rüssel, C.; Wisniewski, W. Glass-ceramic engineering: Tailoring the microstructure and properties. Prog. Mater. Sci. 2025, 152, 101437. [Google Scholar] [CrossRef]
- Yadav, A.K.; Gautam, C.R.; Mishra, A. Mechanical and dielectric behaviors of perovskite (Ba,Sr)TiO3 borosilicate glass ceramics. J. Adv. Cer. 2014, 3, 137–146. [Google Scholar] [CrossRef]
- McCauley, D.; Newnham, R.E.; Randall, C.A. Intrinsic Size Effects in a Barium Titanate Glass-Ceramic. J. Am. Ceram. Soc. 1998, 81, 979–987. [Google Scholar] [CrossRef]
- Kageyama, K.; Takahashi, J. Tunable Microwave Properties of Barium Titanate-Based Ferroelectric Glass-Ceramics. J. Am. Ceram. Soc. 2004, 87, 1602–1605. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Deng, C.; Dai, X.; Li, L. Effect of the Ba/Ti Ratio on the Microstructures and Dielectric Properties of Barium Titanate-Based Glass–Ceramics. J. Am. Ceram. Soc. 2009, 92, 1350–1353. [Google Scholar] [CrossRef]
- Libor, Z.; Wilson, S.A.; Zhang, Q. Rheological properties of magnetic and electro-active nano-particles in non-polar liquids. J. Mater. Sci. 2011, 46, 5385–5393. [Google Scholar] [CrossRef]
- Capsal, J.F.; Dantras, E.; Laffont, L.; Dandurand, L.; Lacabanne, C. Nanotexture influence of BaTiO3 particles on piezoelectric behaviour of PA 11/BaTiO3 nanocomposites. J. Non-Cryst. Solids 2010, 356, 629–634. [Google Scholar] [CrossRef]
- Langhammer, H.T.; Müller, T.; Walther, T.; Böttcher, R.; Hesse, D.; Pippel, E.; Ebbinghaus, S.G. Ferromagnetic properties of barium titanate ceramics doped with cobalt, iron, and nickel. J. Mater. Sci. 2016, 51, 10429–10441. [Google Scholar] [CrossRef]
- Du, G.P.; Hu, Z.J.; Han, Q.F.; Qin, X.M.; Shi, W.J. Effects of niobium donor doping on the phase structures and magnetic properties of Fe doped BaTiO3 ceramics. J. Alloys Comp. 2010, 492, L79–L81. [Google Scholar] [CrossRef]
- Maiti, R.P.; Basu, S.; Bhattacharya, S.; Chakravorty, D. Multiferroic behavior in silicate glass nano composite having a core-shell microstructure. J. Non-Cryst. Solids 2009, 355, 2254–2259. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Abo-Mosallam, H.A.; Darwish, H. Electrical and mechanical properties of alkali barium titanium aluminoborosilicate glass-ceramics containing strontium or magnesium. J. Mater. Sci. Mater. Electron. 2009, 20, 507–516. [Google Scholar] [CrossRef]
- Jackson, T.J.; Jones, I.P. Nanoscale defects and microwave properties of (Ba,Sr)TiO3 ferroelectric thin films. J. Mater. Sci. 2009, 44, 5288–5296. [Google Scholar] [CrossRef]
- Sulekar, S.; Kim, J.H.; Han, H.; Dufour, P.; Tenailleau, C.; Nino, J.C.; Cordoncillo, E.; Beltran-Mir, H.; Dupuis, S.; Guillemet-Fritsch, S. Internal barrier layer capacitor, nearest neighbor hopping, and variable range hopping conduction in Ba1-xSrxTiO3-δ nanoceramics. J. Mater. Sci. 2016, 51, 7440–7450. [Google Scholar] [CrossRef][Green Version]
- Kreuer, K.D.; Adams, S.; Munch, W.; Fuchs, A.; Klock, U.; Maier, J. Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid State Ion. 2001, 145, 295–306. [Google Scholar] [CrossRef]
- Tachafine, A.; Aoujgal, A.; Rguiti, M.; Graça, M.P.F.; Costa, L.C.; Outzourhit, A.; Carru, J.C. Classical and Relaxor Ferroelectric Behavior of Titanate of Barium and Zirconium Ceramics. Spectros. Lett. Intern. J. Rapid Commun. 2014, 47, 404–410. [Google Scholar] [CrossRef]
- Ridha, N.; Mahmood Mat Yunus, W.; Halim, S.A.; Talib, Z.A. Effect of Sr Substitution on Structure and Thermal Diffusivity of Ba1-xSrxTiO3 Ceramic. Am. J. Engin. Appl. Sci. 2009, 2, 661–664. [Google Scholar]
- Harizanova, R.; Vladislavova, L.; Bocker, C.; Avdeev, G.; Rüssel, C. Sr-Substituted Barium Titanate Glass Ceramics from Oxide Glasses As Potential Material for Sensor Preparation. In Advanced Nanotechnologies for Detection and Defence against CBRN Agents; NATO Science for Peace and Security Series—B: Physics and Biophysics; Petkov, P., Tsiulyanu, D., Popov, C., Kulisch, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 349–360. [Google Scholar] [CrossRef]
- Kyong, S.; Han, Y.H. Effect of Sr and Ca on addition on the microstructure and the PTCR properties of Ho-doped (Ba,Sr)TiO3. J. Korean Phys. Soc. 2009, 55, 765–769. [Google Scholar] [CrossRef]
- Harizanova, R.; Pernikov, M.; Wisniewski, W.; Avdeev, G.; Mihailova, I.; Rüssel, C. Phase formation and dielectric properties of strontium-substituted barium titanate glass-ceramics. Bulg. Chem. Commun. 2022, 54, 45–51. [Google Scholar] [CrossRef]
- Laulhé, C.; Hippert, F.; Bellissent, R.; Simon, A.; Cuello, G.J. Local structure in BaTi1−xZrxO3 relaxors from neutron pair distribution function analysis. Phys. Rev. B 2009, 79, 064104. [Google Scholar] [CrossRef]
- Laulhé, C.; Pasturel, A.; Hippert, F.; Kreisel, J. Random local strain effects in homovalentsubstituted relaxor ferroelectrics: A first-principles study of BaTi0.74Zr0.26O3. Phys. Rev. B 2010, 82, 132102. [Google Scholar] [CrossRef]
- Harizanova, R.; Tasheva, T.; Gaydarov, V.; Avramova, I.; Lilova, V.; Nedev, S.; Zamfirova, G.; Nedkova-Shtipska, M.; Rüssel, C. Structure and physicochemical characteristics of the glasses in the system Na2O/BaO/ZrO2/TiO2/SiO2/B2O3/Al2O3—Influence of the ZrO2 addition on the physico-chemical and mechanical properties. Solid State Sci. 2024, 151, 107515. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Harizanova, R.; Wisniewski, W.; Avdeev, G.; Rüssel, C. Crystallization and growth morphology of barium titanate and fresnoite from a glass with the composition 20.1Na2O·23.1BaO·23TiO2·9.8B2O3·21SiO2·3Al2O3. CrystEngComm 2017, 19, 6208–6214. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen; Nachrichten von der Gesellschaft der Wissenschaften: Göttingen, Germany, 1918; pp. 98–100. [Google Scholar]
- Harizanova, R.; Abrashev, M.; Avramova, I.; Vladislavova, L.; Bocker, C.; Tsutsumanova, G.; Avdeev, G.; Rüssel, C. Phase composition identification and microstructure of BaTiO3-containing sodium-aluminoborosilicate glass-ceramics. Solid State Sci. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Niepce, J.-C.; Pizzagalli, L. Chapter 2: Structure and Phase Transitions in Nanocrystals. In Nanomaterials and Nanochemistry, 1st ed.; Brechignac, C., Houdy, P., Lahmani, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 35–54. [Google Scholar]
- Wisniewski, W.; Thieme, K.; Rüssel, C. Fresnoite Glass-Ceramics—A Review. Prog. Mater. Sci. 2018, 98, 68–107. [Google Scholar] [CrossRef]
- Seidel, S.; Patzig, C.; Höche, T.; Krause, M.; Ebert, M.; Hu, Y.; Zuin, L.; Gawronski, A.; Rüssel, C. The crystallization of MgO–Al2O3–SiO2–ZrO2 glass-ceramics with and without the addition of Y2O3—Aa combined STEM/XANES study. RSC Adv. 2016, 6, 62934–62943. [Google Scholar] [CrossRef]
- Kleebusch, E.; Patzig, C.; Krause, M.; Hu, Y.; Höche, T.; Rüssel, C. The Formation of Nanocrystalline ZrO2 Nuclei in a Li2O-Al2O3-SiO2 Glass- a Combined XANES and TEM Study. Sci. Rep. 2017, 7, 10869. [Google Scholar] [CrossRef]
- Montorsi, M.; Leonelli, C.; Menziani, M.C.; Du, J.; Cormack, A.N. Molecular dyn amics study of zirconia containing glasses. Phys. Chem. Glas. 2002, 43, 137–142. [Google Scholar]
- Galoisy, L.; Pélegrin, E.; Arrio, M.-A.; Ildefonse, P.; Calas, G.; Ghaleb, D.; Fillet, C.; Pacaud, F. Evidence for 6-coordinated zirconium in inactive nuclear waste glasses. J. Am. Ceram. Soc. 1999, 82, 2219–2224. [Google Scholar] [CrossRef]
- Osman, R.A.M.; Idris, M.S. Electrical Properties of Fresnoite Ba2TiSi2O8 Using Impedance Spectroscopy. Adv. Mater. Res. 2013, 795, 640–643. [Google Scholar] [CrossRef]
- Abbott, E.E.; Mann, M.; Kolis, J.W. Hydrothermal synthesis of compounds in the fresnoite mineral family (Ba2TiSi2O8). J. Solid State Chem. 2011, 184, 1257–1262. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, H.; Wang, D.; Wang, J.; Boughton, R.I. Optical Properties of the Fresnoite Ba2TiSi2O8 Single Crystal. Crystals 2017, 7, 53. [Google Scholar] [CrossRef]
- Sharma, S.; Shamim, K.; Ranjan, A.; Rai, R.; Kumari, P.; Sinha, S. Impedance and modulus spectroscopy characterization of lead free barium titanate ferroelectric ceramics. Ceram. Int. 2015, 41, 7713–7722. [Google Scholar] [CrossRef]
- Yoon, K.H.; Cho, W.S.; Kang, D.H. Molten salt synthesis of lead-based relaxors. J. Mater. Sci. 1993, 33, 2977–2984. [Google Scholar] [CrossRef]
- Qiao, L.; Bi, X. Dielectric phase transition and relaxor behaviour in BaTiO3/LaNiO3 superlattice. CrystEngComm 2011, 13, 1693–1696. [Google Scholar] [CrossRef]
- Salak, A.N.; Shvartsman, V.V.; Seabra, M.P.; Kholkin, A.L.; Ferreira, V.M. Ferroelectric-to-relaxor transition behaviour of BaTiO3 ceramics doped with La(Mg1/2Ti1/2)O3. J. Phys. Condens. Matter 2014, 16, 2785–2794. [Google Scholar] [CrossRef]
- Mahesh Kumar, M.; Srinivas, K.; Suryanarayana, S.V. Relaxor behavior in BaTiO3. Appl. Phys. Lett. 2000, 76, 1330–1332. [Google Scholar] [CrossRef]
- Ravez, J.; Simon, A. Lead-free ferroelectric relaxor ceramics derived from BaTiO3. Eur. Phys. J. Appl. Phys. 2000, 11, 9–13. [Google Scholar] [CrossRef]
- Halder, S.; Schneller, T.; Böttger, U.; Waser, R. Fabrication and electrical characterisation of Zr-substituted BaTiO3 thin films. Appl. Phys. A 2005, 81, 25–29. [Google Scholar] [CrossRef]

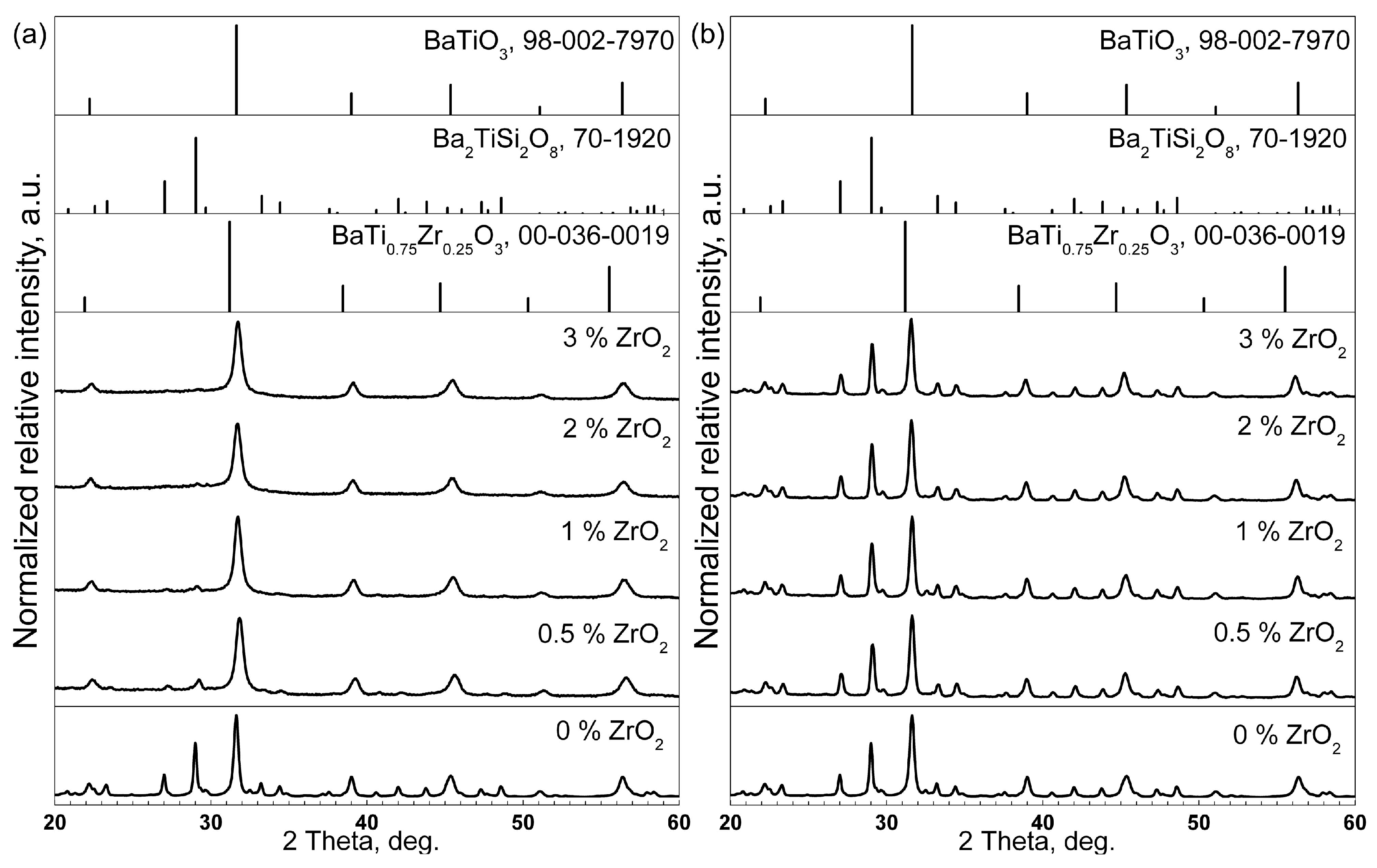
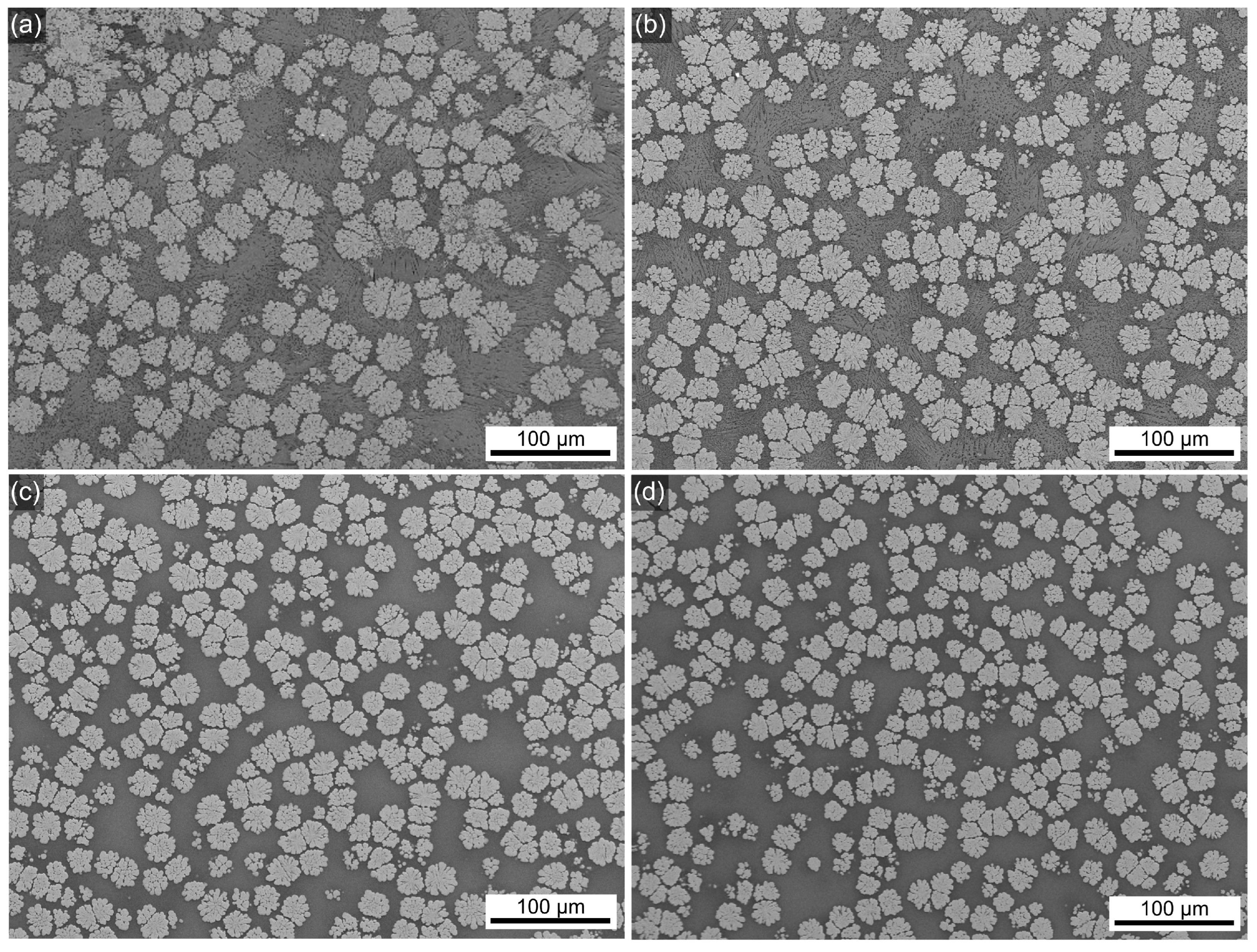

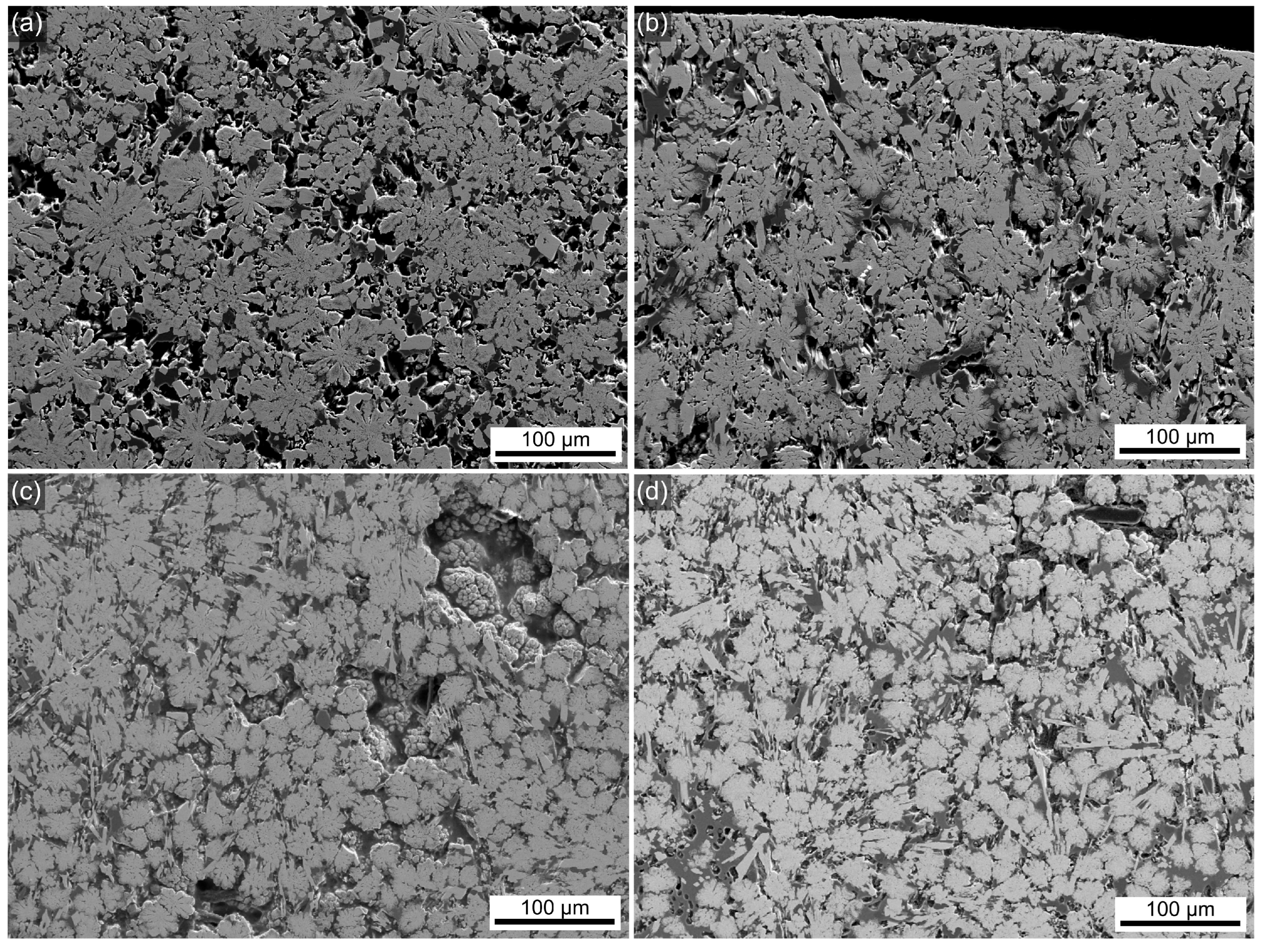
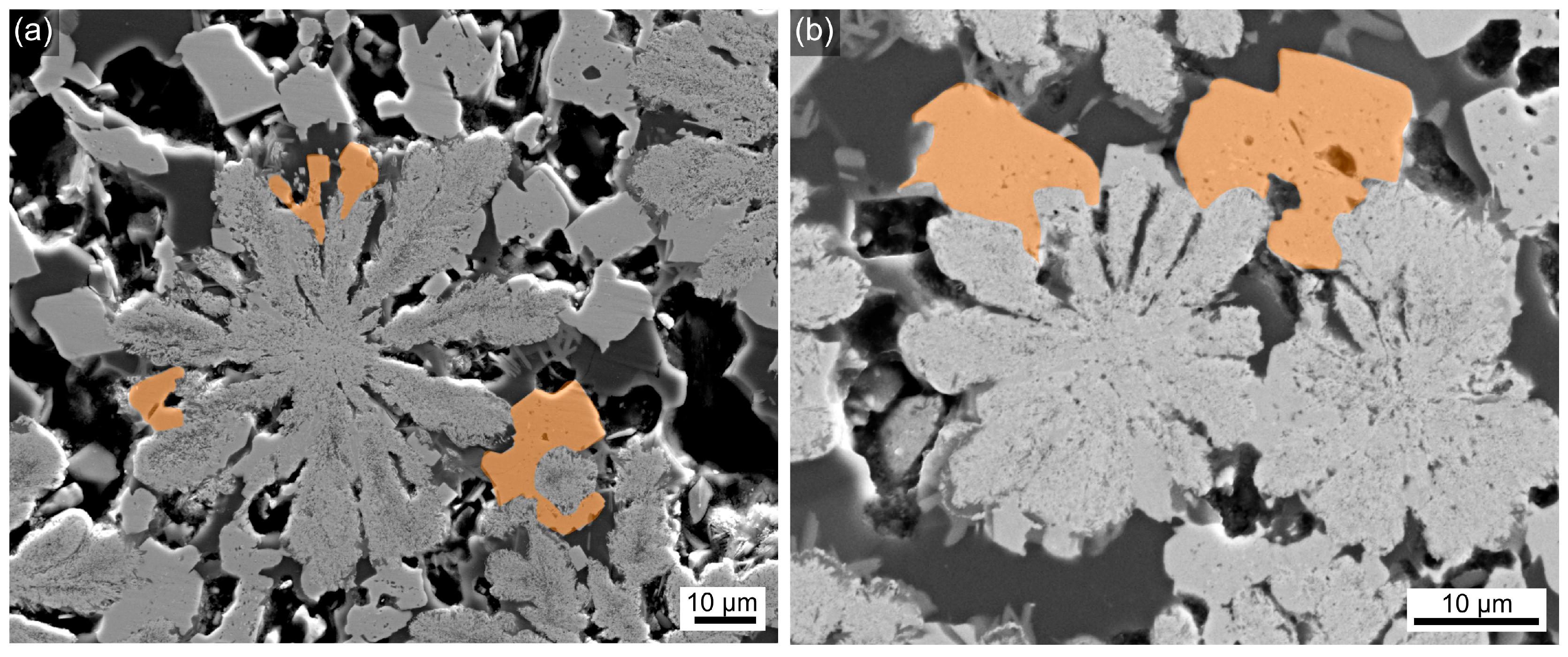




| Sample Name | Tg, °C ±3 °C | Tc1, °C ±3 °C | Tc2, °C ±3 °C |
|---|---|---|---|
| 05Zr | 433 | 585 | 670 |
| 1Zr | 443 | 585 | 683 |
| 2Zr | 447 | 590 | 688 |
| 3Zr | 450 | 590 | 683 |
| Sample Name | Thermal History, Time/Temperature | Average Bright Structure Size, µm | Bright Structure Volume Fraction, vol% (±15%) |
|---|---|---|---|
| 05Zr | 0.5 h/680 °C | 26 ± 6 | 45 ± 7 |
| 05Zr | 3 h/680 °C | 28 ± 8 | 51 ± 8 |
| 1Zr | 24 h/590 °C | 18 ± 9 | 43 ± 6 |
| 1Zr | 0.5 h/680 °C | 31 ± 7 | 40 ± 6 |
| 1Zr | 3 h/680 °C | 32 ± 9 | 61 ± 9 |
| 2Zr | 0.5 h/680 °C | 17 ± 5 | 17 ± 3 |
| 2Zr | 3 h/680 °C | 35 ± 8 | 61 ± 9 |
| 3Zr | 0.5 h/680 °C | 17 ± 5 | 32 ± 5 |
| 3Zr | 3 h/680 °C | 25 ± 5 | 43 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harizanova, R.; Wisniewski, W.; Tatchev, D.M.; Avdeev, G.; Nedev, S.; Rüssel, C. Barium Titanate-Based Glass–Ceramics Crystallized from Multicomponent Oxide Glasses: Phase Composition and Microstructure. Materials 2025, 18, 3783. https://doi.org/10.3390/ma18163783
Harizanova R, Wisniewski W, Tatchev DM, Avdeev G, Nedev S, Rüssel C. Barium Titanate-Based Glass–Ceramics Crystallized from Multicomponent Oxide Glasses: Phase Composition and Microstructure. Materials. 2025; 18(16):3783. https://doi.org/10.3390/ma18163783
Chicago/Turabian StyleHarizanova, Ruzha, Wolfgang Wisniewski, Dragomir M. Tatchev, Georgi Avdeev, Svetlozar Nedev, and Christian Rüssel. 2025. "Barium Titanate-Based Glass–Ceramics Crystallized from Multicomponent Oxide Glasses: Phase Composition and Microstructure" Materials 18, no. 16: 3783. https://doi.org/10.3390/ma18163783
APA StyleHarizanova, R., Wisniewski, W., Tatchev, D. M., Avdeev, G., Nedev, S., & Rüssel, C. (2025). Barium Titanate-Based Glass–Ceramics Crystallized from Multicomponent Oxide Glasses: Phase Composition and Microstructure. Materials, 18(16), 3783. https://doi.org/10.3390/ma18163783






