Structural and Morphological Characterization of Gd-Doped Ceria (Ce1−xGdxO2−x/2) Synthesized by an Optimized Hydrothermal Method
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z.; Bello, I.T.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Aziz, A.J.A.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications. Ceram. Int. 2020, 46, 23314–23325. [Google Scholar] [CrossRef]
- Ramadhani, M.A.F.; Hussain, M.; Mokhlis, H.; Hajimolana, S. Optimization strategies for Solid Oxide Fuel Cell (SOFC) application: A literature survey. Renew. Sustain. Energy Rev. 2017, 76, 460–484. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Jiang, M.; Gao, X.; Huang, M.; Li, Y.; Ren, L.; Yang, Y.; Yang, Z. Controlled synthesis of CeO2 nanorods and their promotional effect on catalytic activity and aging resistibility for diesel soot oxidation. Appl. Surf. Sci. 2020, 510, 145401. [Google Scholar] [CrossRef]
- Mishra, S.R. Ahmaruzzaman Cerium oxide and its nanocomposites: Structure, synthesis, and wastewater treatment applications. Mater. Today Commun. 2021, 28, 102562. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Matussin, S.N.; Harunsani, M.H.; Khan, M.M. CeO2 and CeO2-based nanomaterials for photocatalytic, antioxidant and antimicrobial activities. J. Rare Earths 2023, 41, 167–181. [Google Scholar] [CrossRef]
- Parvathy, S.; Subramanian, P.; Karthick, S.A.; Subbaiya, R. The structural, optical, antimicrobial and anticancer properties of biocompatible astaxanthin coated ZnO and CeO2 nanoparticles. Mater. Lett. 2022, 312, 131669. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Lv, C.; Chen, X.; Zhao, Z.; Qu, Y.; Zhu, Y. Effects of CeO2 geometry on corrosion resistance of epoxy coatings. Surf. Eng. 2020, 36, 175–183. [Google Scholar] [CrossRef]
- Pîslaru-Dănescu, L.; Telipan, G.; Ion, I.; Marinescu, V. Prototyping a gas sensors using CeO2 as a matrix or dopant in oxide semiconductor systems. In Cerium Oxide—Applications and Attributes; Sher, B.K., Kalsoom, A., Eds.; IntechOpen Limited: London, UK, 2018; pp. 61–94. [Google Scholar]
- Anirban, S.; Dutta, A. Revisiting ionic conductivity of rare earth doped ceria: Dependency on different factors. Int. J. Hydrogen Energy 2020, 45, 25139–25166. [Google Scholar] [CrossRef]
- Chaubey, N.; Wani, B.N.; Bharadwaj, S.R.; Chattopadhyaya, M.C. Chattopadhyaya Physicochemical properties of rare earth doped ceria Ce0.9Ln0.1O1.95 (Ln = Nd, Sm, Gd) as an electrolyte material for IT-SOFC/SOEC. Solid State Sci. 2013, 20, 135–141. [Google Scholar] [CrossRef]
- Artini, C. Rare-Earth-Doped Ceria Systems and Their Performance as Solid Electrolytes: A Puzzling Tangle of Structural Issues at the Average and Local Scale. Inorg. Chem. 2018, 57, 13047–13062. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.S.; Katta, K.K.; Chanakya, N.; Prasad, G.; Upender, G. Gd3+ and Sm3+ doped CeO2 for IT-SOFC and room temperature formaldehyde gas sensing applications. Inorg. Chem. Commun. 2024, 160, 111843. [Google Scholar] [CrossRef]
- Abideen, H.Z.U.; Maqsood, A.; Gul, A. Effects of Sm and Gd co-doping on ionic conductivity of ceria-based electrolyte materials. Ceram. Int. 2024, 50, 44165–44174. [Google Scholar] [CrossRef]
- Arabacı, A.; Öksüzömer, M.F. Preparation and characterization of 10 mol% Gd doped CeO2 (GDC) electrolyte for SOFC applications. Ceram. Int. 2012, 38, 6509–6515. [Google Scholar] [CrossRef]
- Conceição, E.J.F.; Aragón, F.F.H.; Urian, Y.A.; Castro, T.J.; Coaquira, J.A.H.; Morais, P.C.; da Silva, S.W. Effects of Gd doping on the structural, optical and magnetic properties of Ce1−xGdxO2−δ nanoparticles and the impact of the oxygen vacancy. J. Alloys Compd. 2023, 960, 170628. [Google Scholar] [CrossRef]
- Li, X.; Feng, Z.; Lu, J.; Wang, F.; Xue, M.; Shao, G. Synthesis and electrical properties of (Ce)1−x(Gd)x(O)2−x/2 (x = 0.05–0.3) solid solutions prepared by a citrate–nitrate combustion method. Ceram. Int. 2012, 38, 3203–3207. [Google Scholar] [CrossRef]
- Ilcheva, V.; Boev, V.; Lefterova, E.; Avdeev, G.; Dimitrov, O.; Bojanova, N.; Kolev, H.; Petkova, T. Effect of gadolinium doping on the structure of (Ce)1–x(Gd)x(O)2−x/2 solid solutions prepared by ionic gelation approach. Emerg. Sci. J. 2024, 8, 1686–1696. [Google Scholar] [CrossRef]
- Acharya, S.A.; Gaikwad, V.M.; Sathe, V.; Kulkarni, S.K. Influence of gadolinium doping on the structure and defects of ceria under fuel cell operating temperature. Appl. Phys. Lett. 2014, 104, 113508. [Google Scholar] [CrossRef]
- Fernández-García, S.; Tinoco, M.; Hungría, A.B.; Chen, X.; Calvino, J.J.; Martínez-Munuera, J.C.; Giménez-Mañogil, J.; García-García, A. NO Oxidation on Lanthanum-Doped Ceria Nanoparticles with Controlled Morphology. Catalysts 2023, 13, 894. [Google Scholar] [CrossRef]
- Khadar, Y.A.S.; Balamurugan, A.; Devarajan, V.; Subramanian, R. Hydrothermal Synthesis of Gadolinium (Gd) Doped Cerium Oxide (CeO2) Nanoparticles: Characterization and Antibacterial Activity. Orient. J. Chem. 2017, 33, 2405–2411. [Google Scholar] [CrossRef]
- Kang, J.-G.; Min, B.-K.; Sohn, Y. Synthesis and characterization of Gd(OH)3 and Gd2O3 nanorods. Ceram. Int. 2015, 41, 1243–1248. [Google Scholar] [CrossRef]
- Acharya, S.; Gaikwad, V.; D’Souza, S.; Barman, S. Gd/Sm dopant-modified oxidation state and defect generation in nano-ceria. Solid State Ion. 2014, 260, 21–29. [Google Scholar] [CrossRef]
- Mc Bride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and X-ray studies of Ce1−xRExO2−y, where RE = La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Nakajima, A.; Yoshihara, A.; Ishigame, M. Defect-Induced Raman Spectra in Doped CeO2. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 13297–13307. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Ankita; Chahal, S.; Singh, S.; Kumar, L.; Gupta, V.; Kumar, S.; Kumar, S.; Kumar, P. Gd doped Cerium Oxide for organic dye degradation and tuning of magnetic properties. Mater. Sci. Eng. B 2024, 300, 117049. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M., III; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Mi, R.; Li, D.; Hu, Z.; Yang, R.T. Morphology Effects of CeO2 Nanomaterials on the Catalytic Combustion of Toluene: A Combined Kinetics and Diffuse Reflectance Infrared Fourier Transform Spectroscopy Study. ACS Catal. 2021, 11, 7876–7889. [Google Scholar] [CrossRef]
- Nolan, M.; Parker, S.C.; Watson, G.W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 2005, 595, 223–232. [Google Scholar] [CrossRef]
- Anjaneya, K.; Nayaka, G.; Manjannaa, J.; Govindaraj, G.; Ganesha, K. Preparation and characterization of Ce1−xGdxO2−δ (x = 0.1–0.3) as solid electrolyte for intermediate temperature SOFC. J. Alloys Compd. 2013, 578, 53–59. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Sun, W.; Chen, C.; Luo, S.; Shen, J.; Jiang, C.; Jing, F. Porous Silica Coated Ceria as a Switch in Tandem Oxidative Dehydrogenation and Dry Reforming of Ethane with CO2. ChemCatChem 2021, 13, 3501–3509. [Google Scholar] [CrossRef]
- Ji, N.; Chen, Y.; Li, Z.; Wang, J.; Duan, X.; Jiang, H. Characterization of LuGdVO3 mixed crystal prepared by one new method. Results Phys. 2019, 12, 335–338. [Google Scholar] [CrossRef]
- Yu, X.; Li, G. XPS study of cerium conversion coating on the anodized 2024 aluminum alloy. J. Alloys Compd. 2004, 364, 193–198. [Google Scholar] [CrossRef]
- Arabaci, A. Effect of Sm and Gd dopants on structural characteristics and ionic conductivity of ceria. Ceram. Int. 2015, 41, 5836–5842. [Google Scholar] [CrossRef]
- Rocha, R.A.; Muccillo, E.N.S. Physical and chemical properties of nanosized powders of gadolinia-doped ceria prepared by the cation complexation technique. Mater. Res. Bull. 2003, 38, 1979–1986. [Google Scholar] [CrossRef]
- Momin, N.; Manjanna, J.; Aruna, S.; Kumar, S.S.; Anjaneya, K. Effect of 20 mol % gadolinium doping on oxide ion conductivity of ceria as electrolyte for intermediate temperature solid oxide fuel cell. Ceram. Int. 2022, 48, 35867–35873. [Google Scholar] [CrossRef]
- Xin, Y.; Qi, Y.; Ma, X.; Wang, Z.; Zhang, Z.; Zhang, S. Rare-earth (Nd, Sm, Eu, Gd and Y) enhanced CeO2 solid solution nanorods prepared by co-precipitation without surfactants. Mater. Lett. 2010, 64, 2659–2662. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S. Hydrothermal synthesis of lanthanide (hydr)oxide micro/nanorods in presence of tetrabutylammonium hydroxide. J. Rare Earths 2016, 34, 618. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
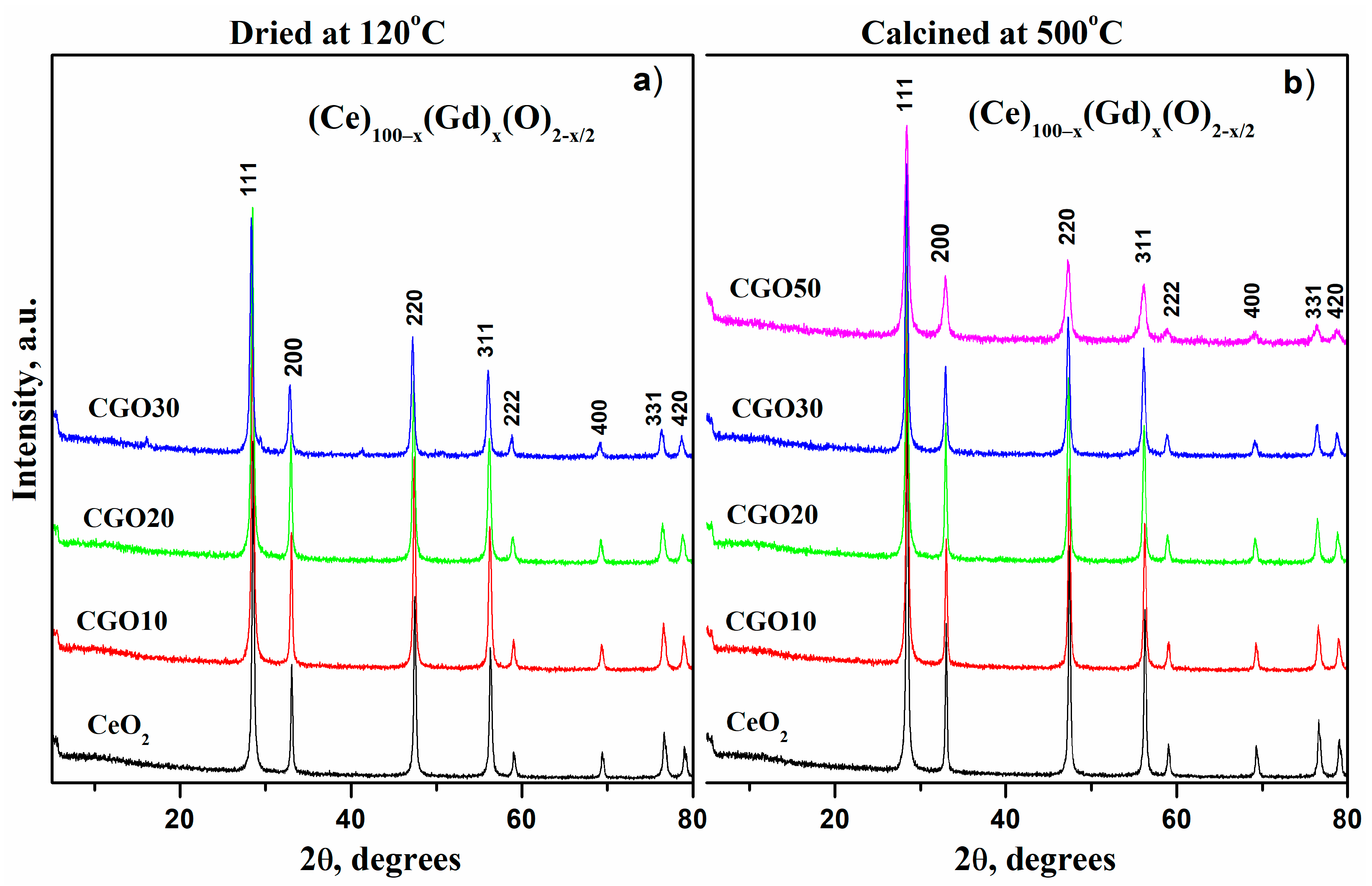
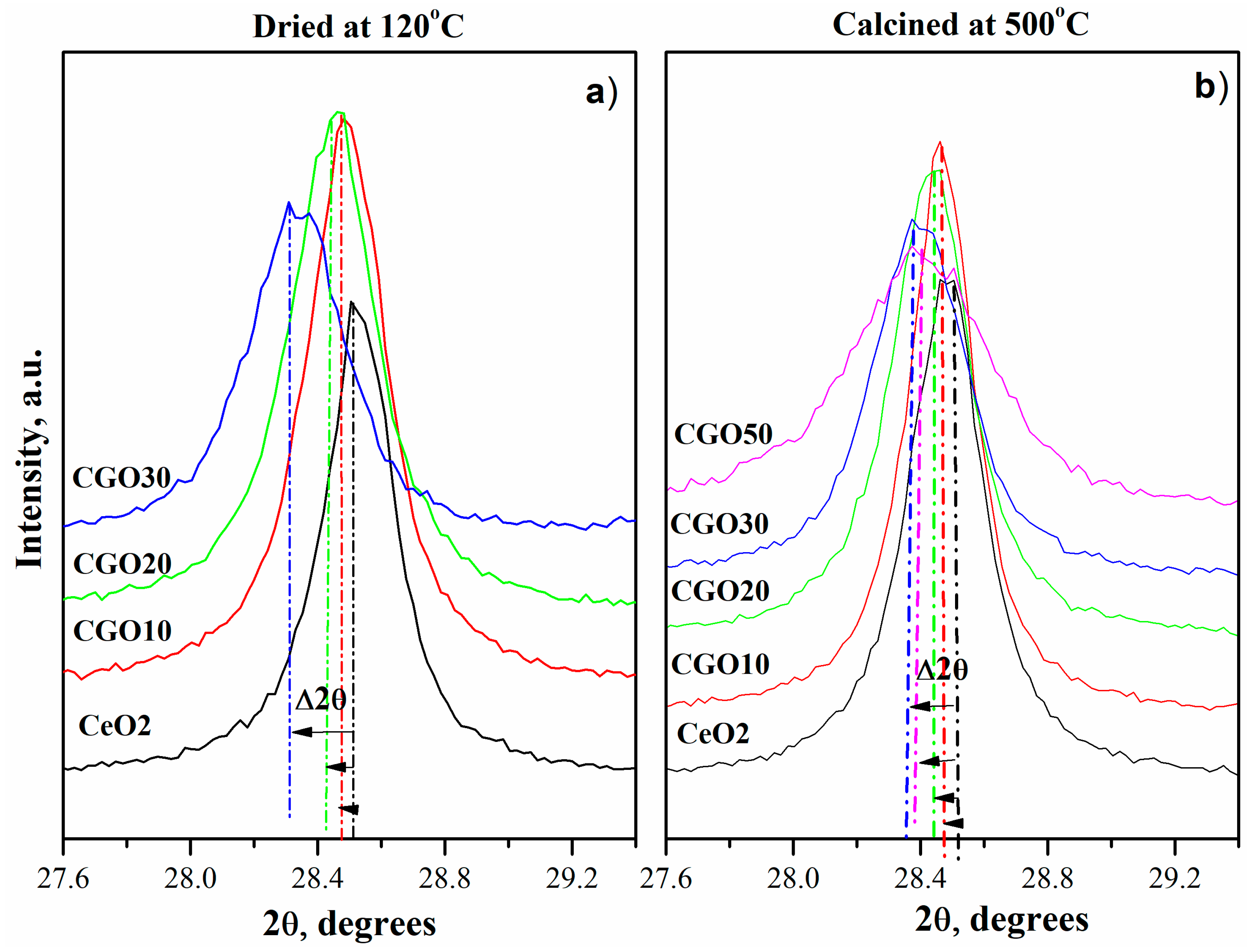


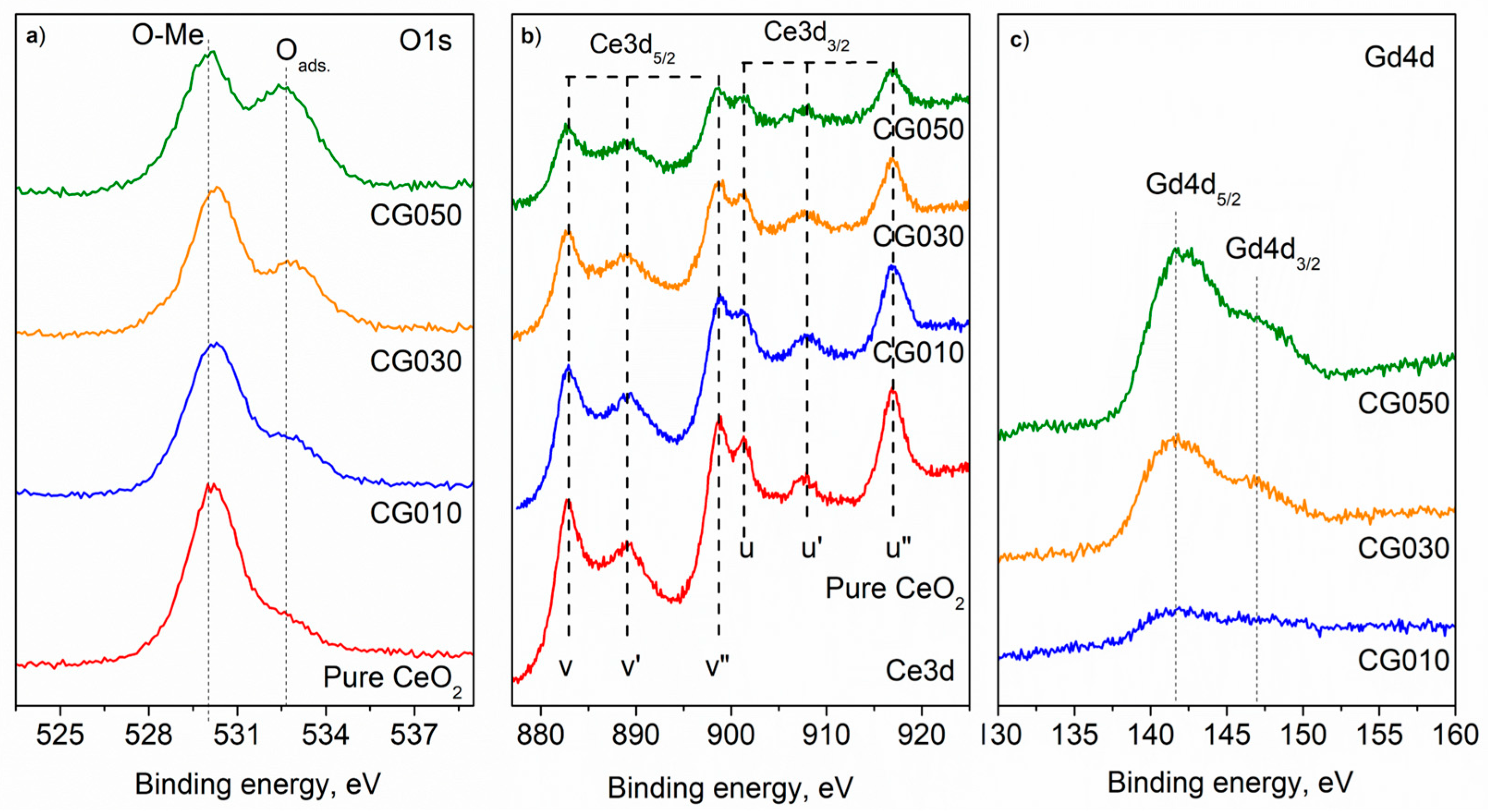
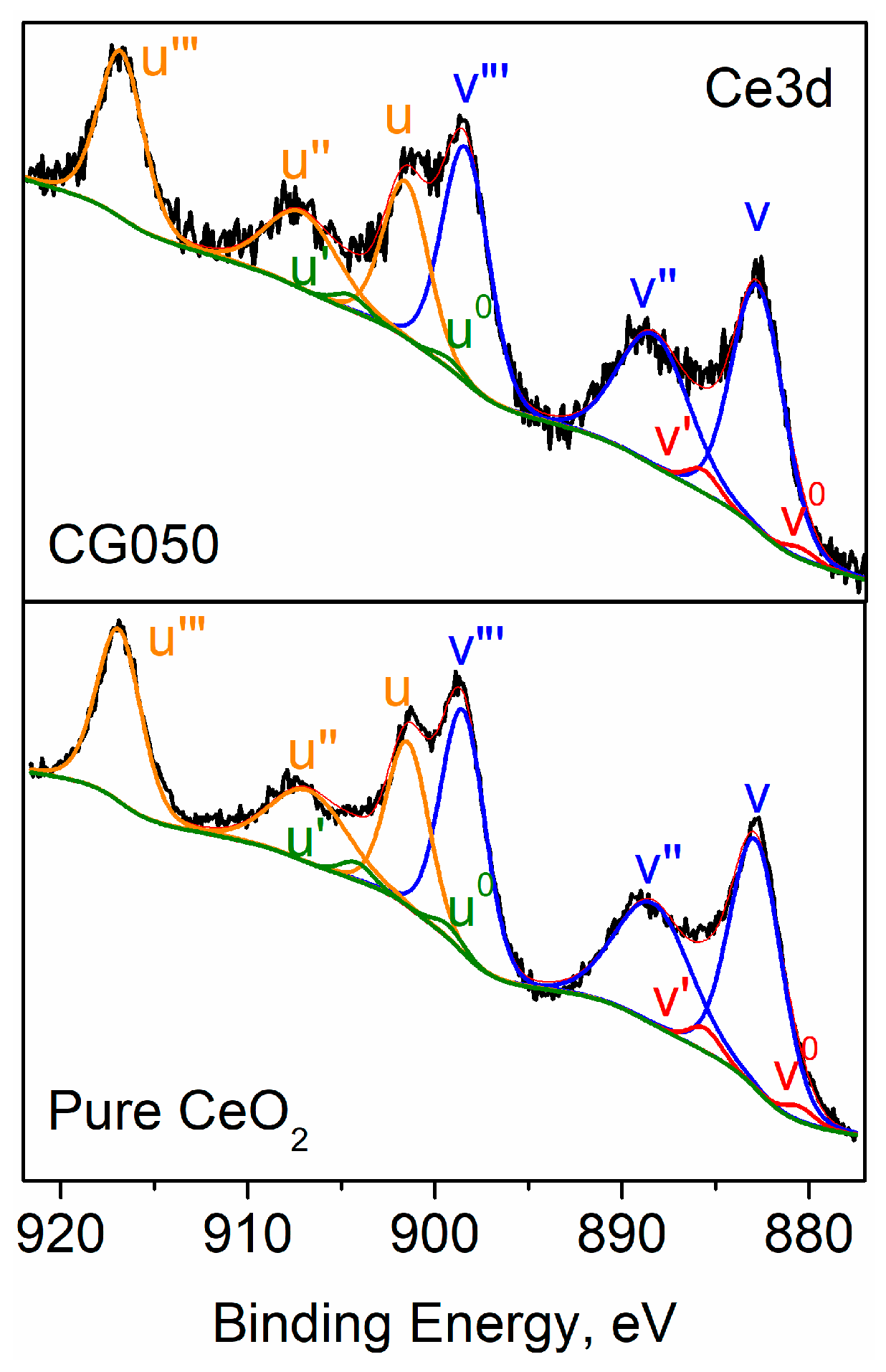


| Composition of (Ce)1−x(Gd)x(O)2−x/2 | Abbreviation |
|---|---|
| CeO2 | CeO2 |
| Ce0.9Gd0.1O1.95 | CGO10 |
| Ce0.8Gd0.2O1.90 | CGO20 |
| Ce0.7Gd0.3O1.85 | CGO30 |
| Ce0.5Gd0.5O1.75 | CGO50 |
| Sample | 120 °C | 500 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| FWHM | Tip Width | Ratio [%] | χ [%] | FWHM | Tip Width | Ratio [%] | χ [%] | |
| CeO2 | 0.2362 | 0.2834 | 19.98 | 45.32 | 0.2187 | 0.2624 | 19.98 | 46.17 |
| CGO10 | 0.2752 | 0.3302 | 19.98 | 42.67 | 0.2165 | 0.2598 | 20.00 | 43.90 |
| CGO20 | 0.3033 | 0.3640 | 20.01 | 44.69 | 0.2381 | 0.2858 | 20.03 | 45.22 |
| CGO30 | 0.3482 | 0.4179 | 20.02 | 40.19 | 0.3031 | 0.3637 | 19.99 | 40.64 |
| CGO50 | 0.2949 | 0.3539 | 20.01 | 37.77 | ||||
| Sample | Space Group | Cell Parameter, Å | Crystallite Size, nm | |||||
|---|---|---|---|---|---|---|---|---|
| 120 °C | 500 °C | 120 °C | 500 °C | 120 °C | 500 °C | |||
| a [Å] | V [Å3] | a [Å] | V [Å3] | |||||
| CeO2 | Fm-3m | Fm-3m | 5.4166 | 158.92 | 5.4139 | 158.68 | 37 | 41 |
| CGO10 | Fm-3m | Fm-3m | 5.4214 | 159.34 | 5.4219 | 159.39 | 31 | 34 |
| CGO20 | Fm-3m | Fm-3m | 5.4291 | 160.02 | 5.4286 | 159.97 | 28 | 32 |
| CGO30 | Ia-3 | Fm-3m | 10.8619 | 1281.5 | 5.4308 | 160.17 | 24 | 26 |
| CGO50 | - | Ia-3 | - | 10.8707 | 1284.6 | - | 16 | |
| Sample | ε (120 °C) | ε (500 °C) | Change (%) |
|---|---|---|---|
| CeO2 | 9.41 × 10−6 | 1.40 × 10−6 | −85% ↓ |
| CGO10 | 7.37 × 10−6 | 2.98 × 10−8 | −99.6% ↓ |
| CGO20 | 2.57 × 10−5 | 1.44 × 10−4 | +460% ↑ |
| CGO30 | 5.28 × 10−5 | 3.68 × 10−4 | +597% ↑ |
| CGO50 | — | 1.83 × 10−3 | (very high value) |
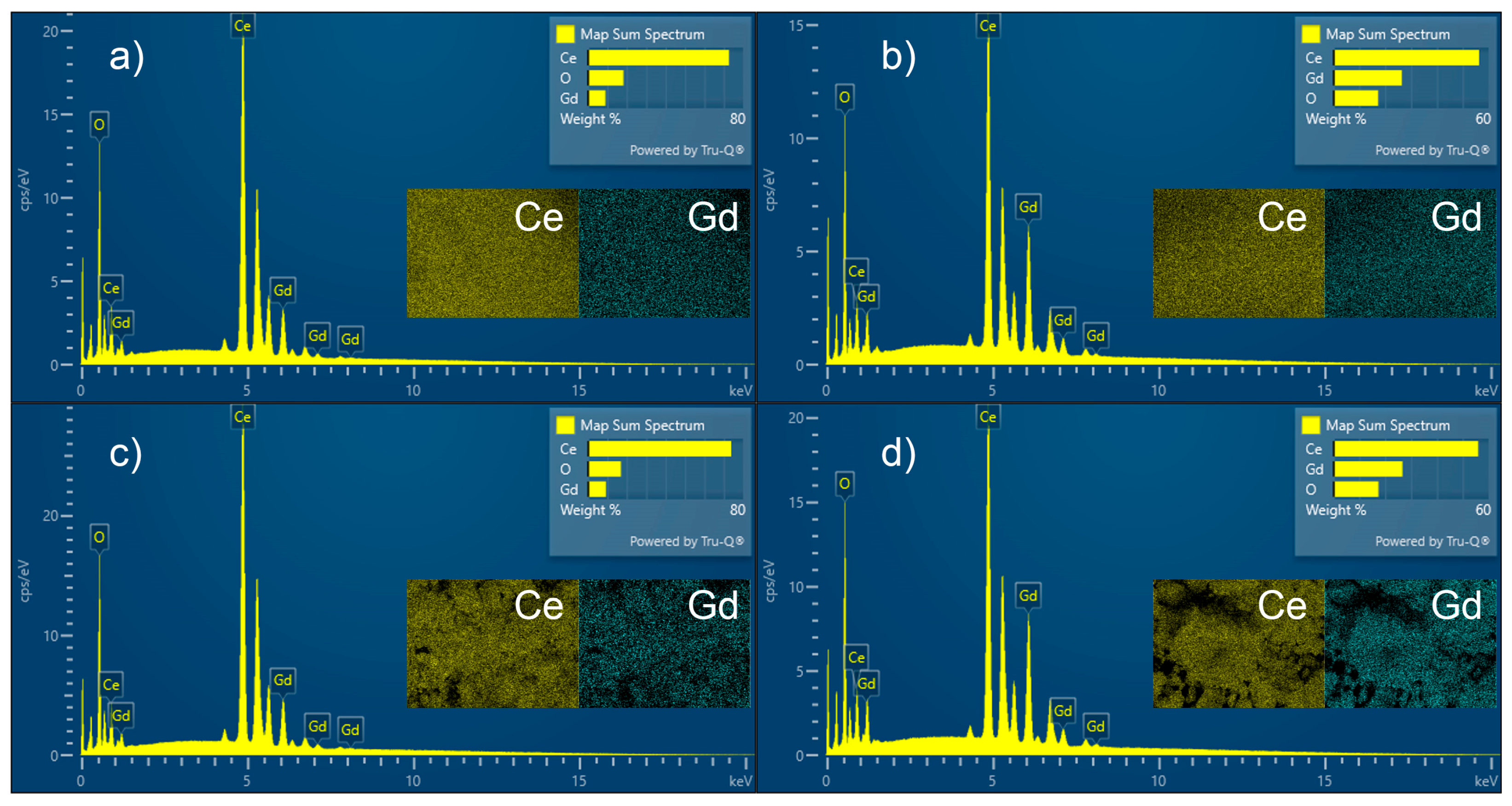
| Samples | O, at. % | Ce, at. % | Gd, at. % | Ce3+, % | Ce4+, % |
|---|---|---|---|---|---|
| CeO2 | 74.1 | 25.9 | - | 2.7 | 97.3 |
| CG010 | 75.3 | 20.8 | 3.9 | 0.2 | 99.8 |
| CG030 | 79.1 | 8.9 | 12.0 | 1.4 | 98.6 |
| CG050 | 76.3 | 9.3 | 14.4 | 2.0 | 98.0 |
| Sample | EDXRF | EDS | ||||
|---|---|---|---|---|---|---|
| Ce500, % | Gd500, % | Ce120, % | Gd120, % | Ce500, % | Gd500, % | |
| CeO2 | 99.1 | - | 79.6 | - | 84.4 | - |
| CGO10 | 87.6 | 11 | 72.7 | 9.0 | 73.9 | 9.2 |
| CGO20 | 76.4 | 22.5 | 62.3 | 17.9 | 58.3 | 17.7 |
| CGO30 | 64.9 | 33.2 | 56.3 | 26.5 | 56 | 22.6 |
| CGO50 | 44.3 | 54 | - | - | 34.3 | 38.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolev, K.; Dimitrov, O.; Dimitrova, M.; Shipochka, M.; Karashanova, D.; Petkova, T. Structural and Morphological Characterization of Gd-Doped Ceria (Ce1−xGdxO2−x/2) Synthesized by an Optimized Hydrothermal Method. Materials 2025, 18, 4957. https://doi.org/10.3390/ma18214957
Kolev K, Dimitrov O, Dimitrova M, Shipochka M, Karashanova D, Petkova T. Structural and Morphological Characterization of Gd-Doped Ceria (Ce1−xGdxO2−x/2) Synthesized by an Optimized Hydrothermal Method. Materials. 2025; 18(21):4957. https://doi.org/10.3390/ma18214957
Chicago/Turabian StyleKolev, Kolyo, Ognian Dimitrov, Mariela Dimitrova, Maria Shipochka, Daniela Karashanova, and Tamara Petkova. 2025. "Structural and Morphological Characterization of Gd-Doped Ceria (Ce1−xGdxO2−x/2) Synthesized by an Optimized Hydrothermal Method" Materials 18, no. 21: 4957. https://doi.org/10.3390/ma18214957
APA StyleKolev, K., Dimitrov, O., Dimitrova, M., Shipochka, M., Karashanova, D., & Petkova, T. (2025). Structural and Morphological Characterization of Gd-Doped Ceria (Ce1−xGdxO2−x/2) Synthesized by an Optimized Hydrothermal Method. Materials, 18(21), 4957. https://doi.org/10.3390/ma18214957





