Influence of Heat Treatment on Precipitate and Microstructure of 38CrMoAl Steel
Abstract
1. Introduction
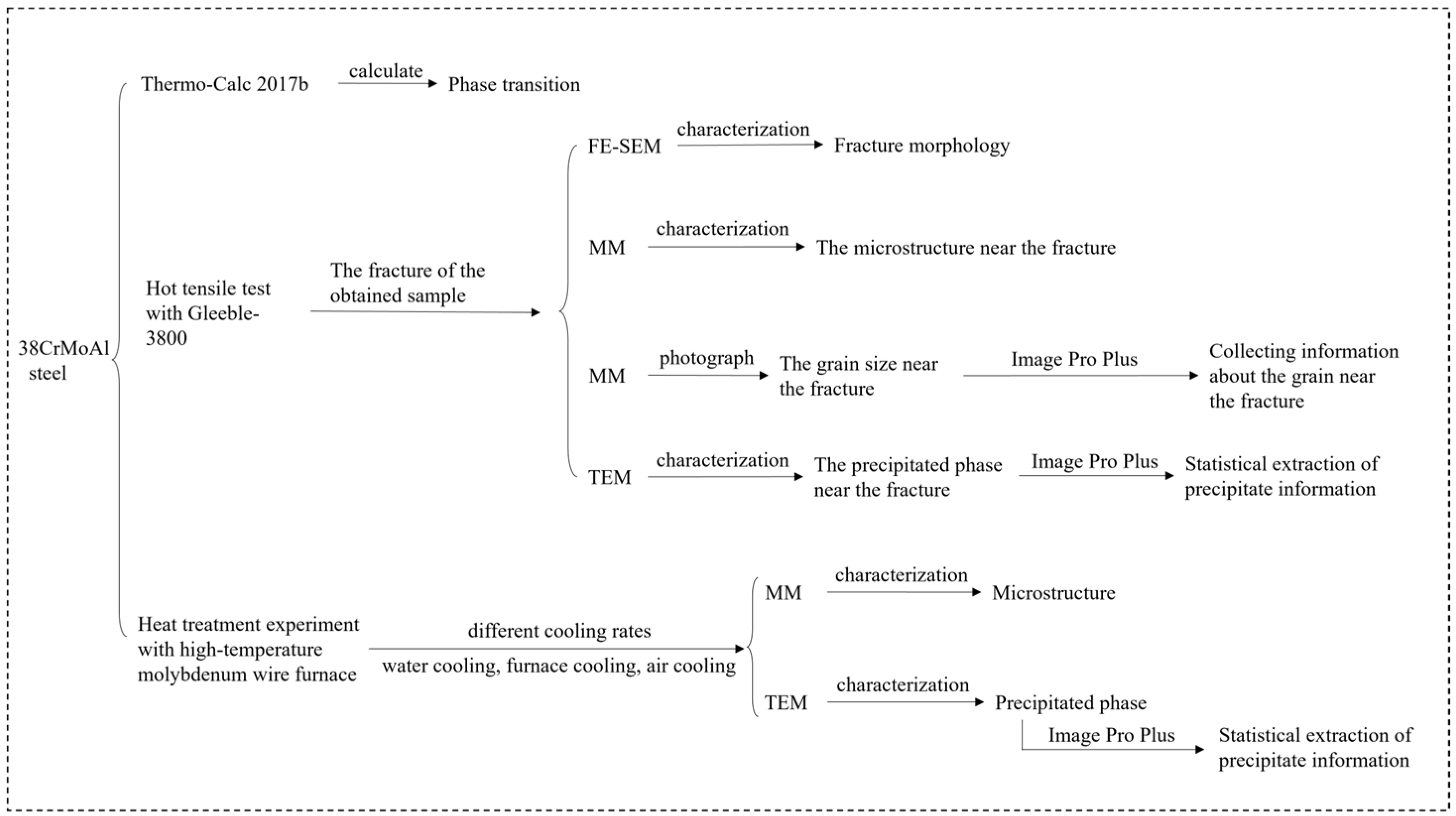
2. Experimental Materials and Methods
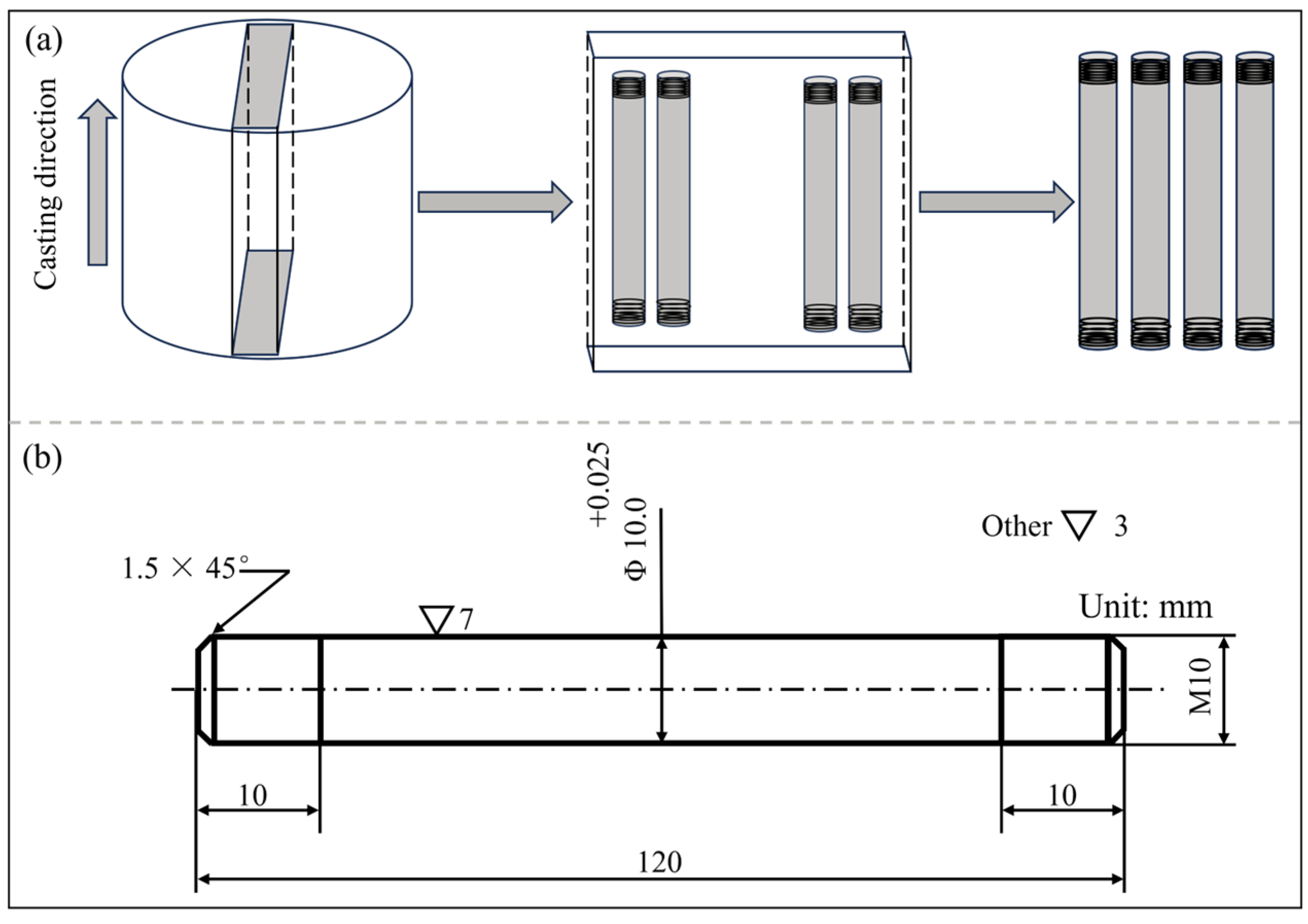
2.1. Experimental Materials
2.2. Experimental Methods
- (1)
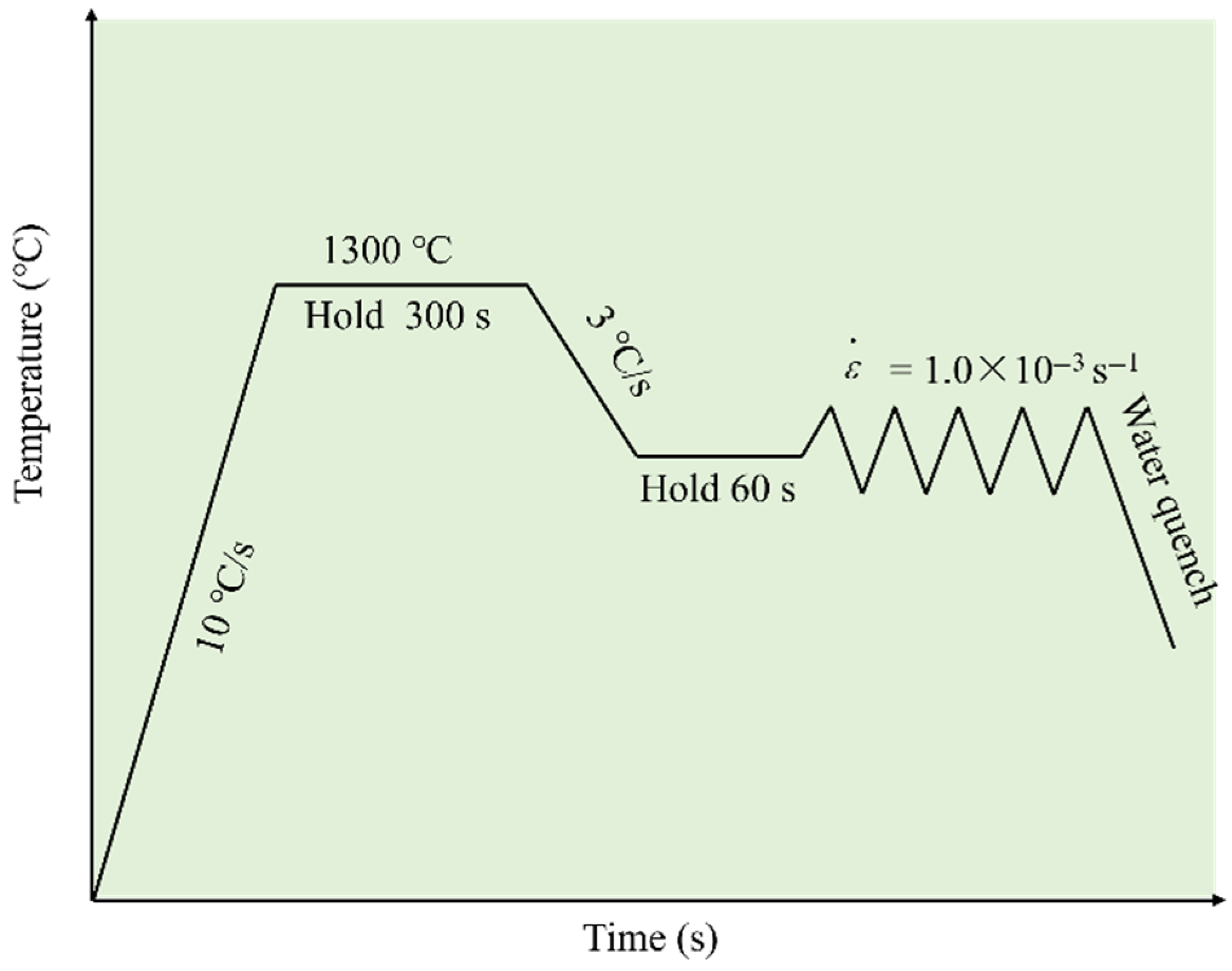
- High-temperature Tensile Test
- (2)
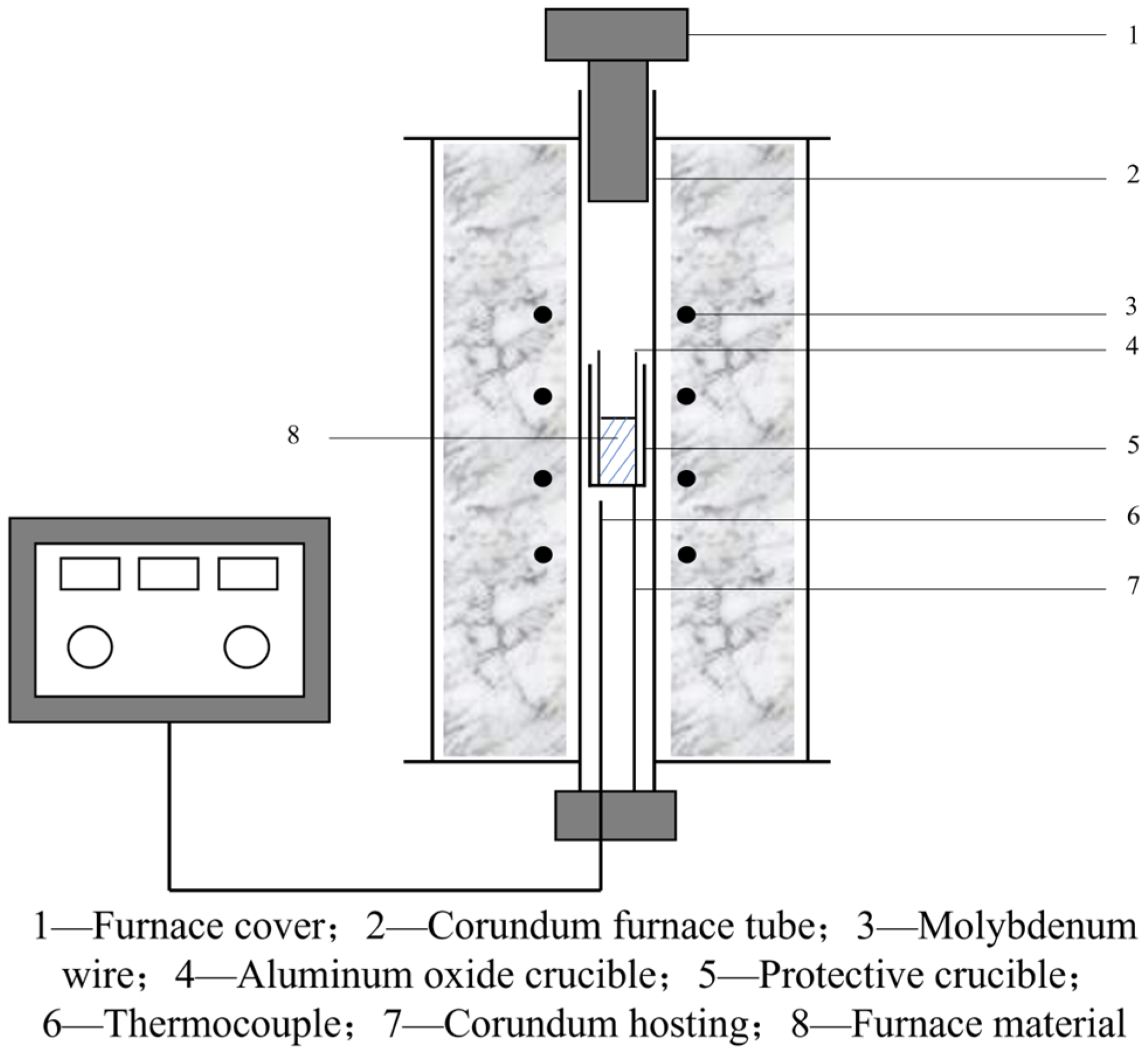
- Heat Treatment Experiment
- (3)
- Characterization of Fracture Morphology, Microstructure, Grain Size, and Precipitated Phases
3. Results and Discussion
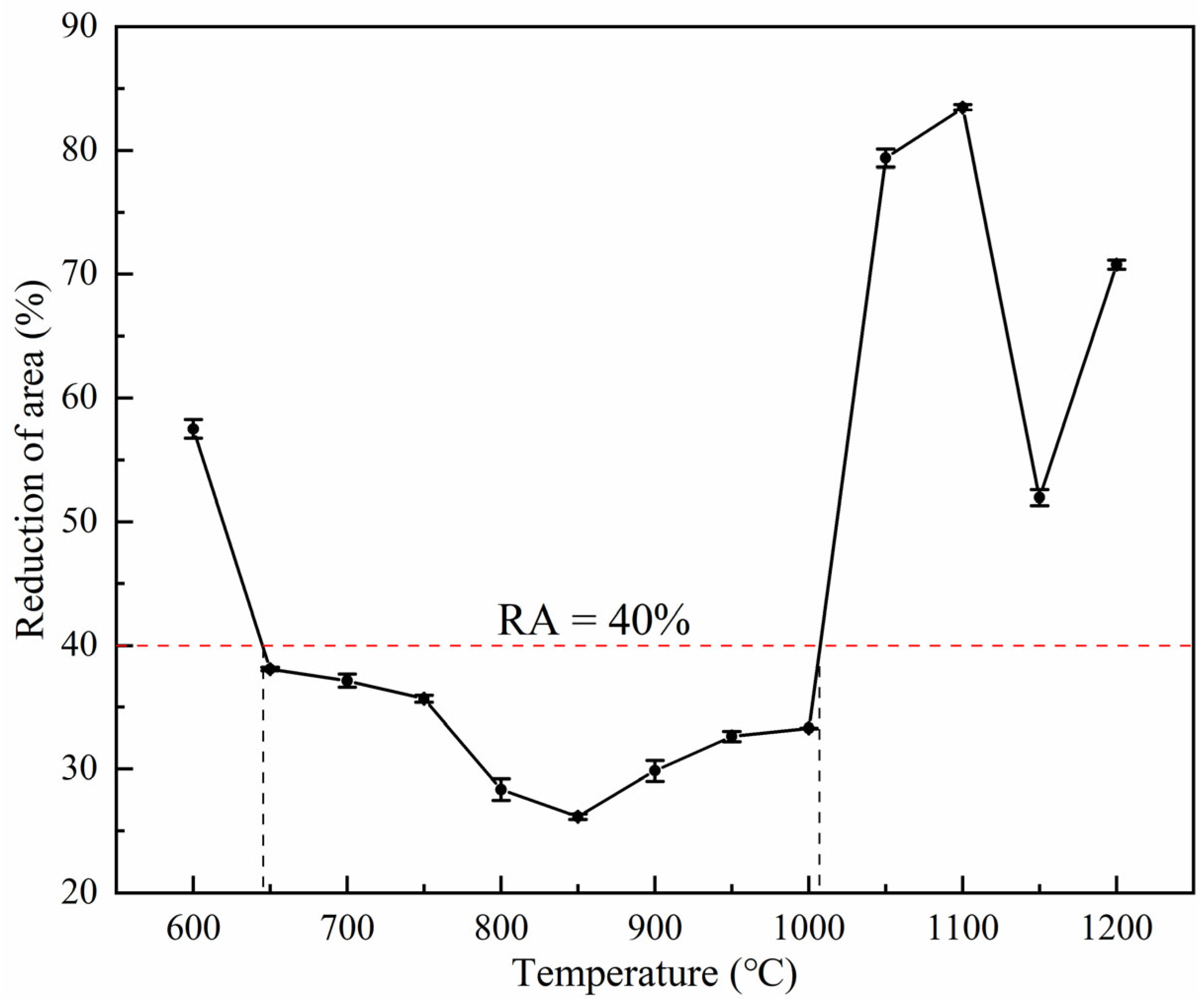
3.1. Hot Ductility
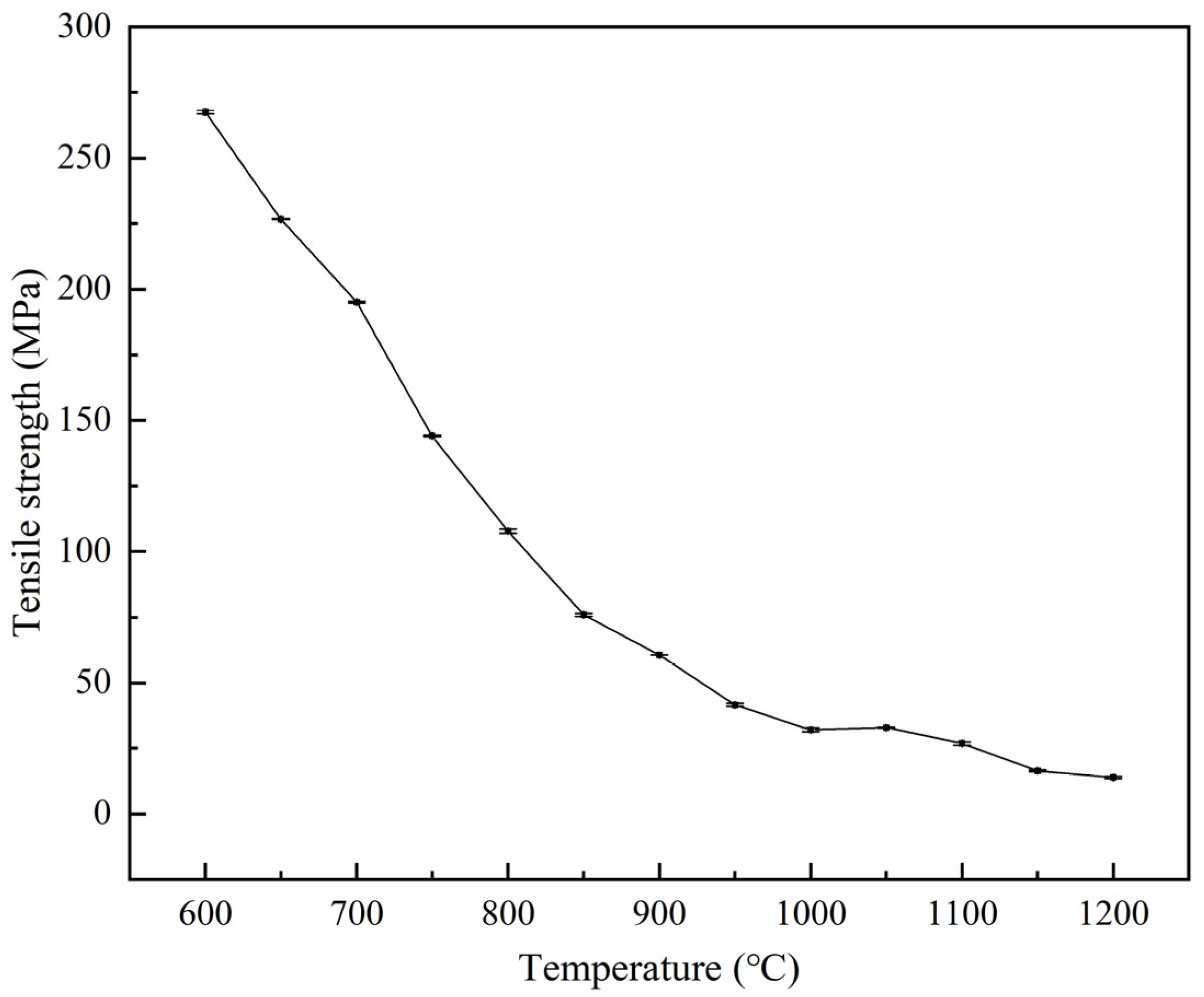
3.2. Tensile Strength
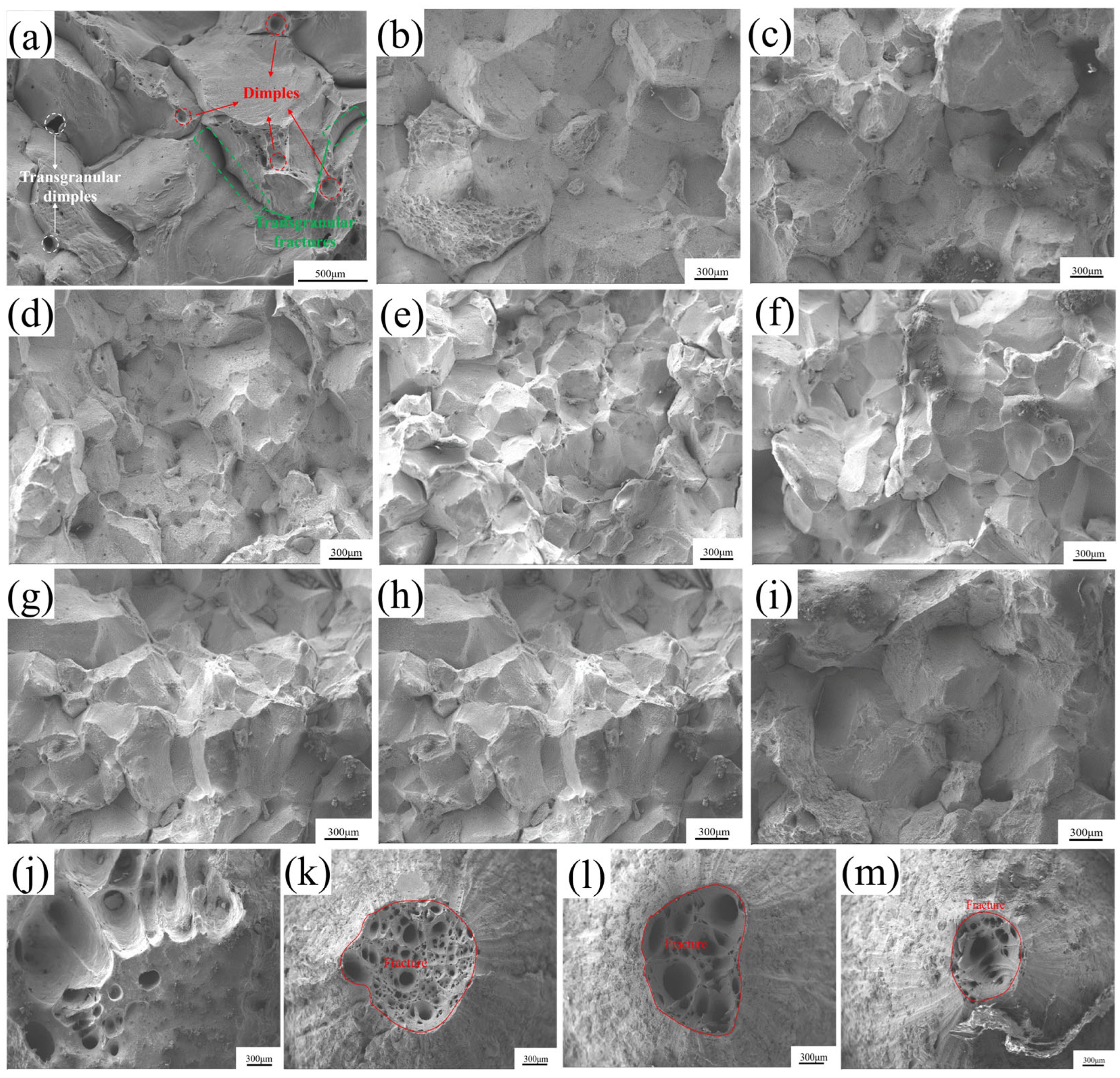
3.3. Fracture Morphology
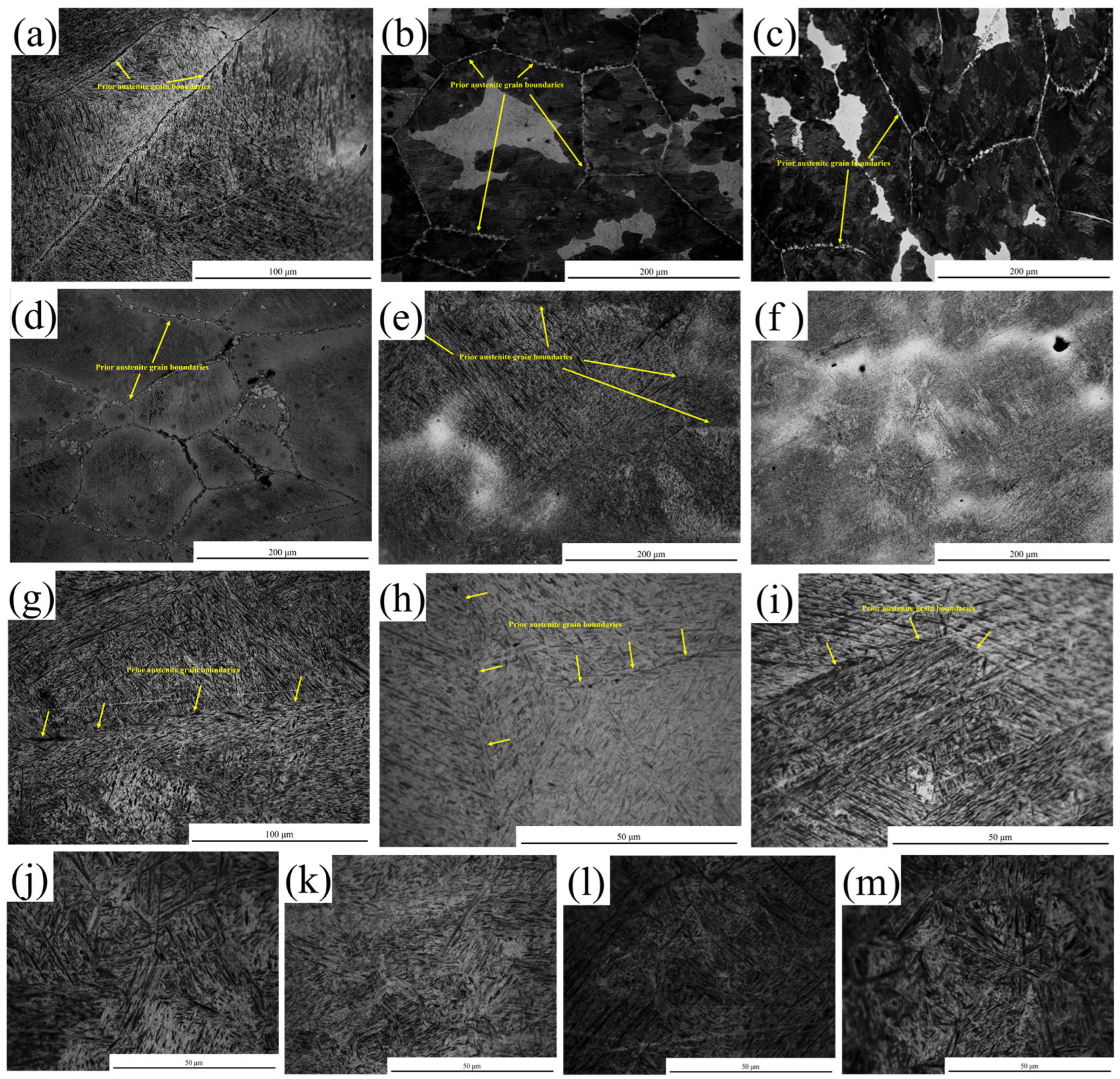
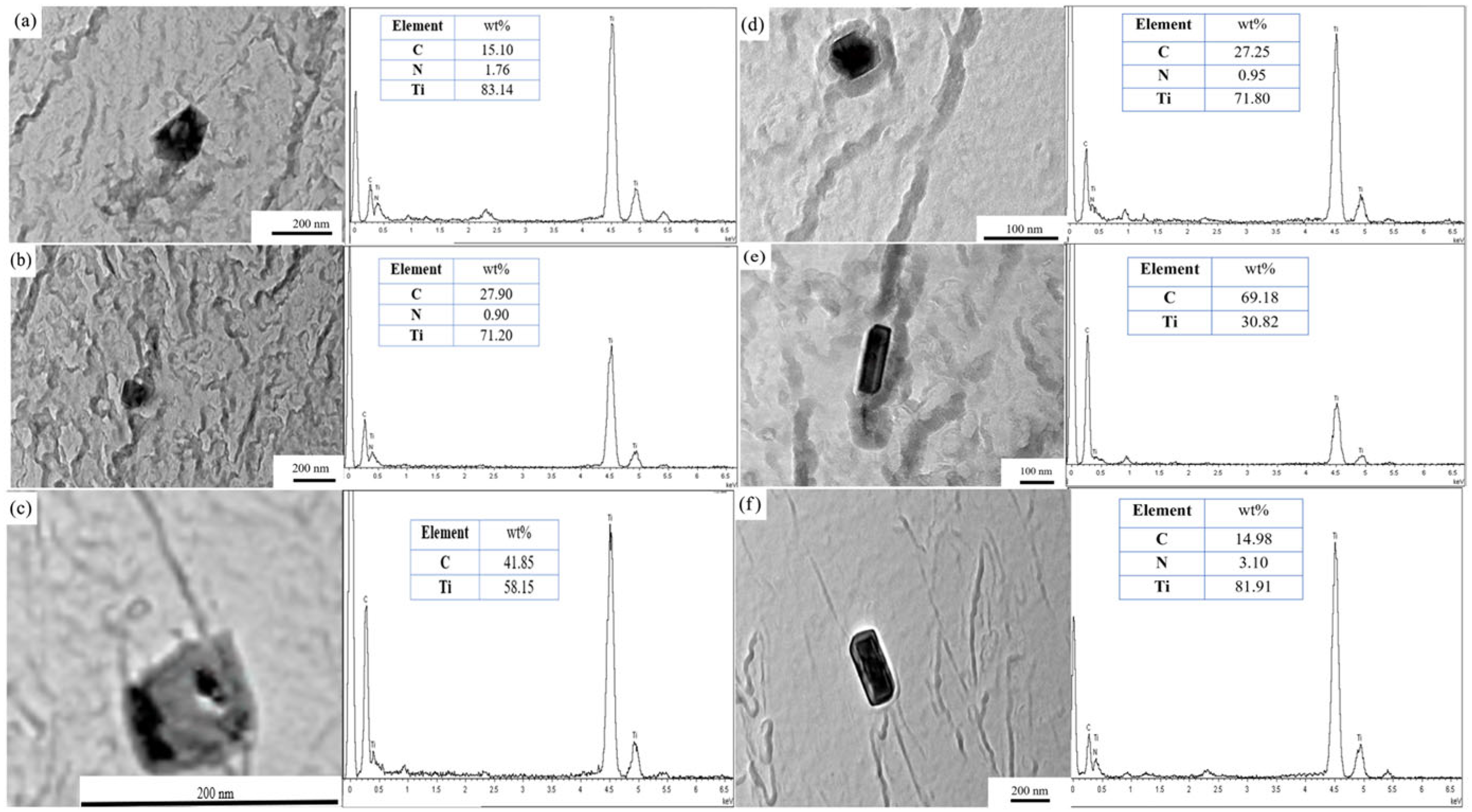
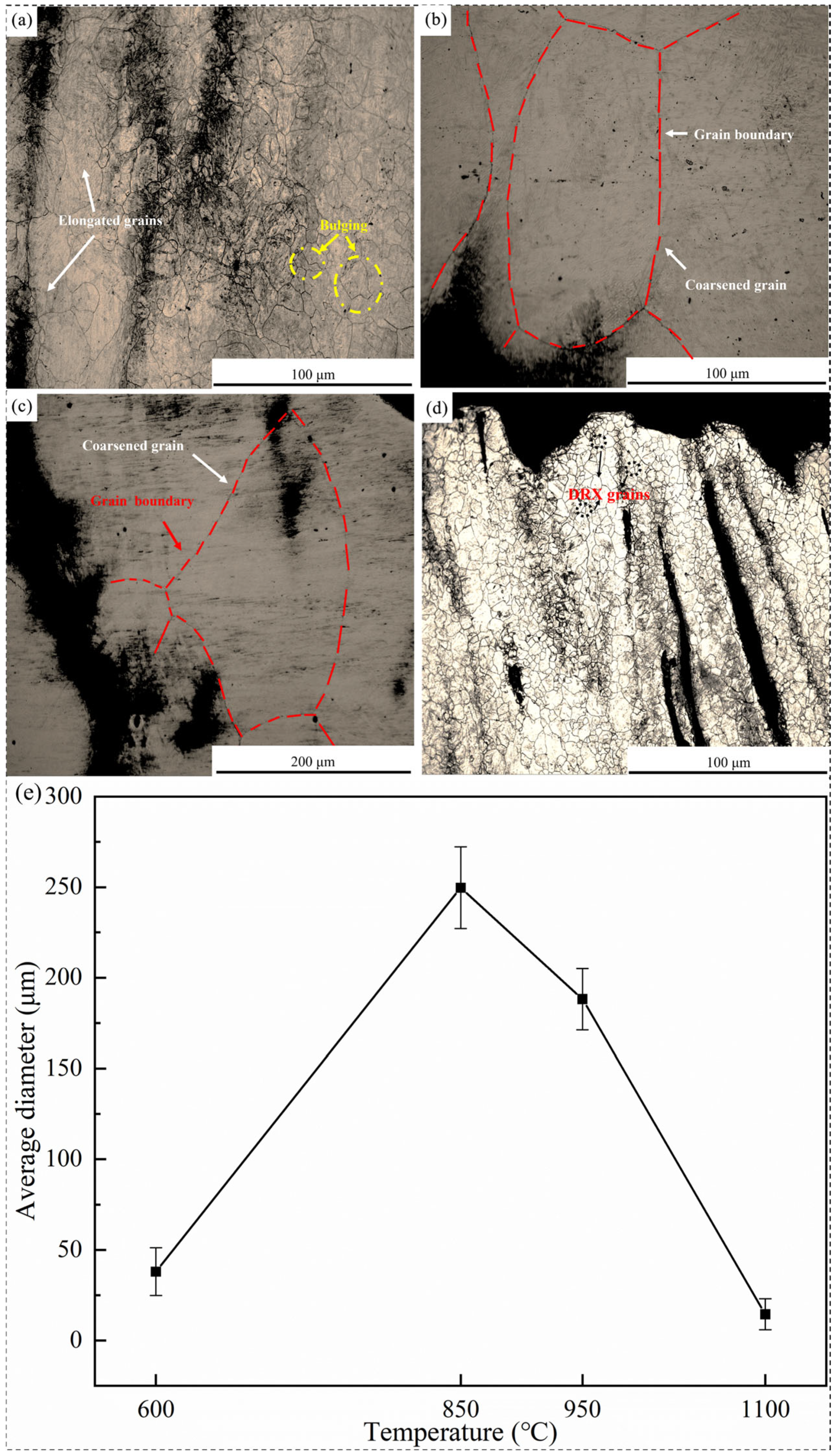
3.4. Microstructural Characterization
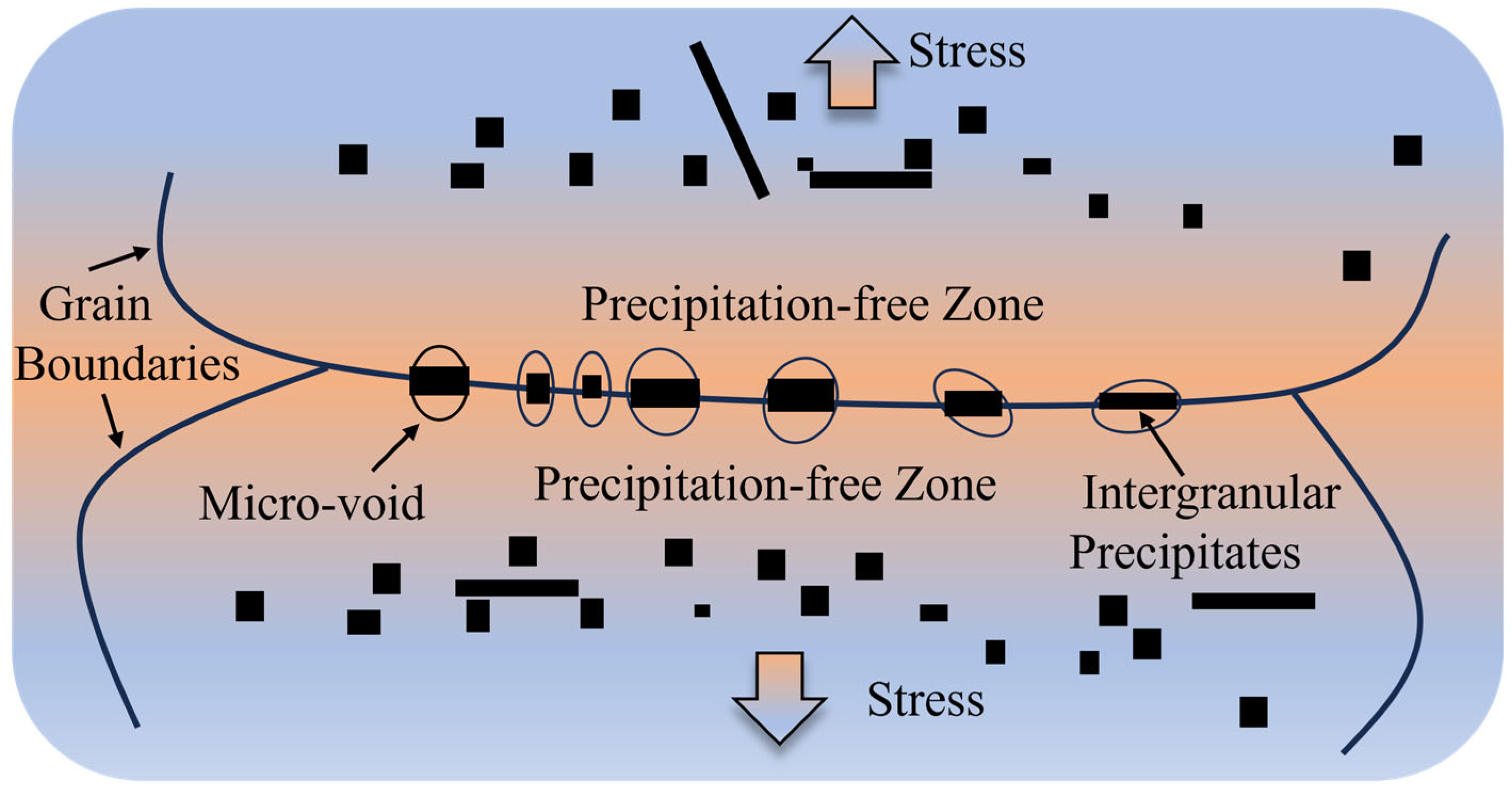
3.5. Mechanism of Hot Ductility Evolution
- (i)
- Precipitate
- (ii)
- Microstructure
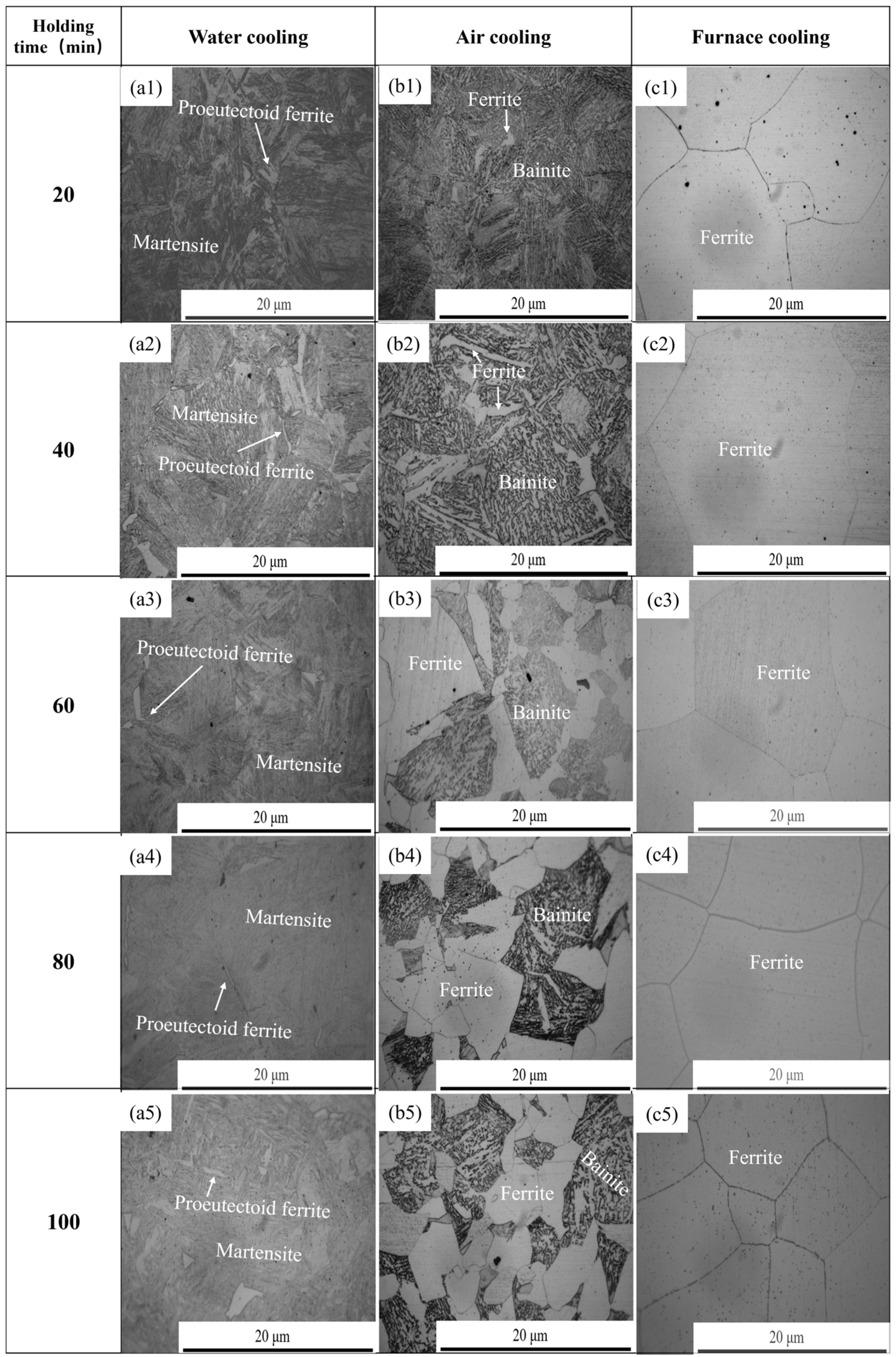
4. Effect of Heat Treatment on Precipitated Phase and Microstructure
5. Conclusions
- (1)
- The high-temperature tensile test of 38CrMoAl steel was carried out using the Gleeble-3800. Based on the criterion of RA = 40%, the steel did not exhibit the first and second brittle regions, and the temperature region of the third brittle was 645–1009 °C. Straightening or processing within the brittle temperature region of the casting slabs should be avoided as much as possible.
- (2)
- Based on the fracture morphology, the specimens exhibited ductile fracture in the temperature ranges of 600 °C and 1050–1200 °C. In the temperature region of 650–750 °C, the specimens showed a combination of ductile fracture and intergranular brittle fracture. Within the temperature region of 800–1000 °C, the specimens underwent intergranular brittle fracture.
- (3)
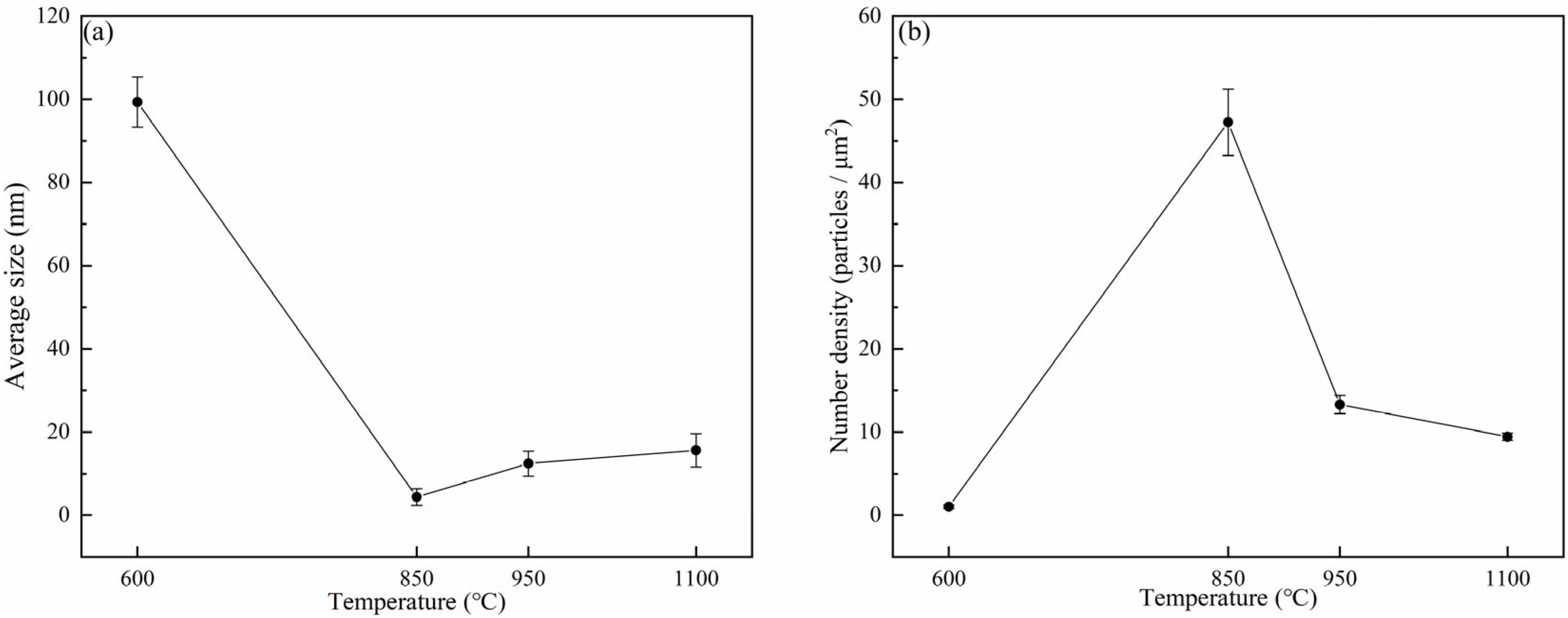
- The fracture mechanisms of 38CrMoAl steel are classified into three types: (I) in the α single-phase region, the thickness of intergranular proeutectoid ferrite increases with rising temperature, reducing hot ductility; (II) in the γ single-phase region, the average size of precipitates increases and the number density decreases with increasing temperature, improving hot ductility; and (III) in the α + γ two-phase region, the precipitation of proeutectoid ferrite facilitates crack propagation, and the dense distribution of precipitates at grain boundaries causes stress concentration, deteriorating hot ductility.
- (4)
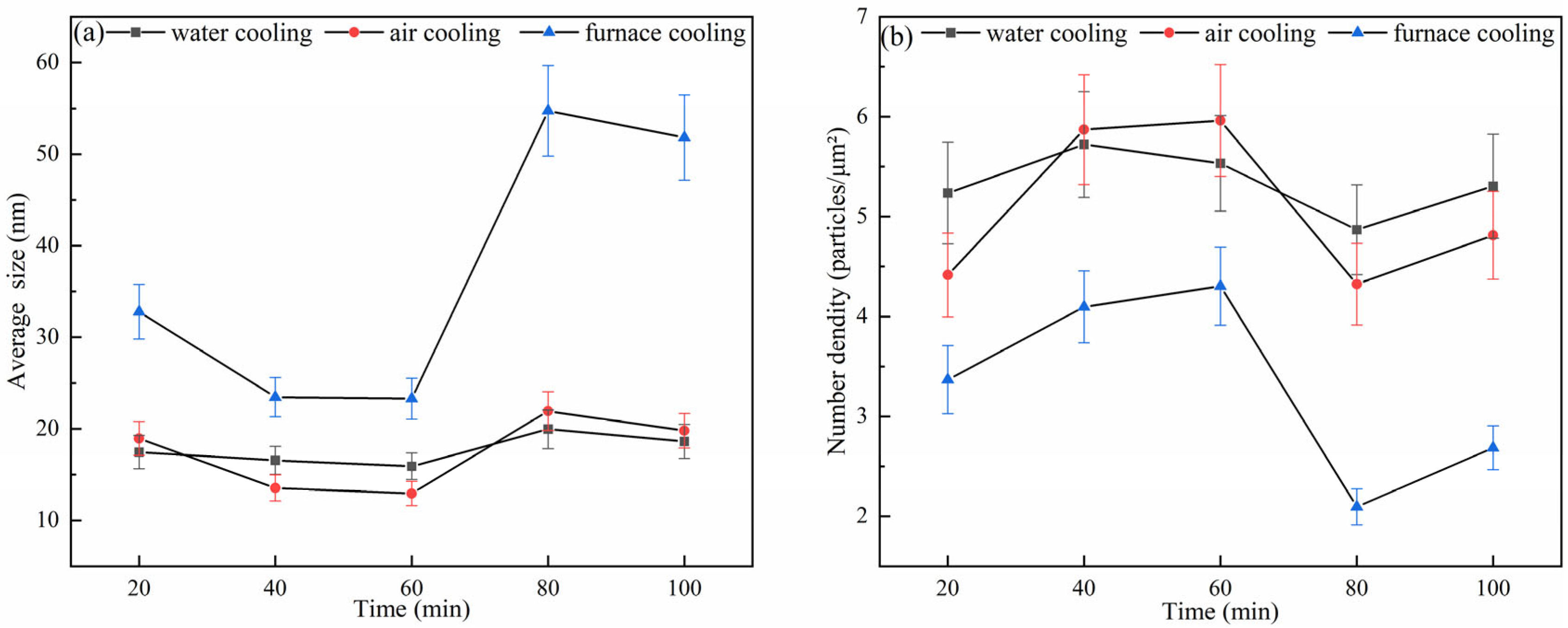
- The analysis of heat treatment for 38CrMoAl steel showed that with the same holding time, the microstructure of the specimen transformed as the cooling rate increased as follows: ferrite → bainite + ferrite → martensite + proeutectoid ferrite. Under three cooling conditions (water cooling, air cooling, and furnace cooling), the average size of precipitates exhibited a trend of decreasing first, then increasing, and decreasing again with the increase in holding time. (I) At holding times of 20 min, 80 min, and 100 min, the order of the average sizes of precipitates was furnace cooling > air cooling > water cooling. (II) At holding times of 40 min and 60 min, the order changed to furnace cooling > water cooling > air cooling. The variation trend of the precipitate number density was opposite to that of the average size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RA | Reductions in area |
| PFZ | Precipitate-free Zone |
| DRX | Dynamic recrystallization |
References
- GB/T 3077-2015; Steels. Standard Press of China: Beijing, China, 2016.
- Ren, X.; Wang, R.; Wei, D.; Huang, Y.; Zhang, H. Study on surface alloying of 38CrMoAl steel by electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B 2021, 505, 44–49. [Google Scholar] [CrossRef]
- Chen, Y.; Song, L.; Zhang, C.; Ye, X.; Song, R.; Wang, Z.; Zhao, X.; Hu, J. Plasma nitriding without formation of compound layer for 38CrMoAl hydraulic plunger. Vacuum 2017, 143, 98–101. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, H.; Chen, Y.; Wu, X.; Zhou, S.; Hu, W. Dynamic fracture toughness and damage mechanism of 38CrMoAl steel under salt spray corrosion. Theor. Appl. Fract. Mech. 2022, 119, 103382. [Google Scholar] [CrossRef]
- Tan, R.; Liu, W.; Song, B.; Yang, S.; Chen, Y.; Zuo, X.; Huang, Y. Numerical simulation on solidification behavior and structure of 38CrMoAl large round bloom using CAFE model. J. Iron Steel Res. Int. 2023, 30, 1222–1233. [Google Scholar] [CrossRef]
- Steenken, B.; Rezende, J.L.L.; Senk, D. Hot ductility behaviour of high manganese steels with varying aluminium contents. Mater. Sci. Technol. 2017, 33, 567–573. [Google Scholar] [CrossRef]
- Ba, L.; Di, X.; Li, C.; Pan, J.; Ma, C.; Qu, Y.; Yang, X.; Hu, W. Enhancing hot ductility of a cryogenic high manganese steel at a high strain rate by matrix homogenization. Mater. Sci. Eng. A 2023, 872, 145002. [Google Scholar] [CrossRef]
- Kang, S.E.; Kang, M.H.; Mintz, B. Influence of vanadium, boron and titanium on hot ductility of high Al, TWIP steels. Mater. Sci. Technol. 2021, 37, 42–58. [Google Scholar] [CrossRef]
- Kang, S.E.; Banerjee, J.R.; Mintz, B. Influence of S and AlN on hot ductility of high Al, TWIP steels. Mater. Sci. Technol. 2012, 28, 589–596. [Google Scholar] [CrossRef]
- Kang, S.E.; Tuling, A.; Lau, I.; Banerjee, J.R.; Mintz, B. The hot ductility of Nb/V containing high Al, TWIP steels. Mater. Sci. Technol. 2011, 27, 909–915. [Google Scholar] [CrossRef]
- Trang, T.T.T.; Lee, S.; Heo, Y.; Kang, M.; Lee, D.; Lee, J.S.; Yim, C.H. Improved hot ductility of an as-cast high Mn TWIP steel by direct implementation of an MnS-containing master alloy. Scr. Mater. 2022, 215, 114685. [Google Scholar] [CrossRef]
- Calvo, J.; Cabrera, J.M.; Rezaeian, A.; Yue, S. Evaluation of the hot ductility of a C–Mn steel produced from scrap recycling. ISIJ Int. 2007, 47, 1518–1526. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhao, S.; Song, R.; Hu, B. Hot ductility behavior of a Fe-0.3C-9Mn-2Al medium Mn steel. Int. J. Miner. Metall. Mater. 2021, 28, 422–429. [Google Scholar] [CrossRef]
- Hassan, M.M.; Shafiq, M.A.; Mourad, S.A. Experimental study on cracked steel plates with different damage levels strengthened by CFRP laminates. Int. J. Fatigue 2021, 142, 105914. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Roy, T.K.; Mahashabde, V.V. A study to establish correlation between intercolumnar cracks in slabs and off-center defects in hot-rolled products. J. Fail. Anal. Prev. 2016, 16, 95–103. [Google Scholar] [CrossRef]
- Zhang, M.; Bao, Y.; Zhao, L.; Chen, J.; Zheng, H. Formation and control of central cracks in alloy steel ZKG223. Steel Res. Int. 2022, 93, 2200289. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, R.; Xin, X.; Xu, L. Effects of non-metallic inclusions on hot ductility of high manganese TWIP steels containing different aluminum contents. Metall. Mater. Trans. B-Proc. Metall. Mater. Proc. Sci. 2016, 47, 1697–1712. [Google Scholar] [CrossRef]
- Wang, Y. Solidification structure, non-metallic inclusions and hot ductility of continuously cast high manganese TWIP steel slab. ISIJ Int. 2019, 59, 872–879. [Google Scholar] [CrossRef]
- Qaban, A.; Mintz, B.; Kang, S.E.; Naher, S. Hot ductility of high Al TWIP steels containing Nb and Nb-V. Mater. Sci. Technol. 2017, 33, 1645–1656. [Google Scholar] [CrossRef]
- Kang, S.E.; Banerjee, J.R.; Tuling, A.; Mintz, B. Influence of P and N on hot ductility of high Al, boron containing TWIP steels. Mater. Sci. Technol. 2014, 30, 1328–1335. [Google Scholar] [CrossRef]
- Vedani, M.; Dellasega, D.; Mannuccii, A. Characterization of grain-boundary precipitates after hot-ductility tests of microalloyed steels. ISIJ Int. 2009, 49, 446–452. [Google Scholar] [CrossRef][Green Version]
- Qian, G.; Cheng, G.; Hou, Z. Effect of the induced ferrite and precipitates of Nb–Ti bearing steel on the ductility of continuous casting slab. ISIJ Int. 2014, 54, 1611–1620. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, J.; Wu, R.; Liu, G.; Wang, S. Effect of V on the hot ductility behavior of high-strength hot-stamped steels and associated microstructural features. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2023, 54, 3476–3488. [Google Scholar] [CrossRef]
- Chen, X.M.; Song, S.H.; Sun, Z.C.; Liu, S.J.; Weng, L.Q.; Yuan, Z.X. Effect of microstructural features on the hot ductility of 2.25Cr–1Mo steel. Mater. Sci. Eng. A 2010, 527, 2725–2732. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wang, C.; Yang, Z.; Wu, J.; Guan, M.; Liu, Q. Analysis and Control of the Slab Hot Ductility Behaviors Based on Nozzle Arrangement during Continuous Casting. Steel Res. Int. 2024, 95, 2300296. [Google Scholar] [CrossRef]
- Zaitsev, A.; Arutyunyan, N.; Koldaev, A. Hot Ductility, Homogeneity of the Composition, Structure, and Properties of High-Strength Microalloyed Steels: A Critical Review. Metals 2023, 13, 1066. [Google Scholar] [CrossRef]
- Sun, Z.; Shen, D.; Liu, G.; Guo, N.; Guo, F.; Zhang, Z.; Sun, W.; Tang, B. Influence of microstructure evolution on hot ductility behavior of a Cr and Mo alloyed Fe-C-Mn steel during hot deformation. Mater. Today Commun. 2024, 39, 109164. [Google Scholar] [CrossRef]
- Tuling, A.; Banerjee, J.R.; Mintz, B. Influence of peritectic phase transformation on hot ductility of high aluminium TRIP steels containing Nb. Mater. Sci. Technol. 2011, 27, 1724–1731. [Google Scholar] [CrossRef]
- Tacikowski, M.; Osinkolu, G.A.; Kobylanski, A. Segregation-induced intergranular brittleness of ultrahigh-purity Fe–S alloys. Mater. Sci. Technol. 1986, 2, 154–158. [Google Scholar] [CrossRef]
- Mejía, I.; Altamirano, G.; Bedolla-Jacuinde, A.; Cabrera, J.M. Effect of boron on the hot ductility behavior of a low carbon advanced ultra-high strength steel (a-UHSS). Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 5165–5176. [Google Scholar] [CrossRef]
- Kizu, T.; Urabe, T. Hot ductility of sulfur-containing low manganese mild steels at high strain rate. ISIJ Int. 2009, 49, 1424–1431. [Google Scholar] [CrossRef][Green Version]
- Spradbery, C.; Mintz, B. Influence of undercooling thermal cycle on hot ductility of C-Mn-Al-Ti and C-Mn-Al-Nb-Ti steels. Ironmak. Steelmak. 2005, 32, 319–324. [Google Scholar] [CrossRef]
- Banks, K.; Koursaris, A.; Verdoorn, F.; Tuling, A. Precipitation and hot ductility of low C-V and low C-V-Nb microalloyed steels during thin slab casting. Mater. Sci. Technol. 2001, 17, 1596–1604. [Google Scholar] [CrossRef]
- Mejía, I.; Salas-Reyes, A.E.; Calvo, J.; Cabrera, J.M. Effect of Ti and B microadditions on the hot ductility behavior of a high-Mn austenitic Fe–23Mn–1.5Al–1.3Si–0.5C TWIP steel. Mater. Sci. Eng. A 2015, 648, 311–329. [Google Scholar] [CrossRef]
- Hornbogen, E.; Graf, M. Fracture toughness of precipitation hardened alloys containing narrow soft zones at grain boundaries. Acta Metall. 1977, 25, 877–881. [Google Scholar] [CrossRef]
- Li, J.; Jiang, B.; Zhang, C.; Zhou, L.; Liu, Y. Hot embrittlement and effect of grain size on hot ductility of martensitic heat-resistant steels. Mater. Sci. Eng. A 2016, 677, 274–280. [Google Scholar] [CrossRef]
- Cai, Z.; An, J.; Cheng, B.; Zhu, M. Effect of austenite grain size on the hot ductility of Nb-bearing peritectic steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2023, 54, 141–152. [Google Scholar] [CrossRef]
- Furumai, K.; Wang, X.; Zurob, H.; Phillion, A. Evaluating the effect of the competition between NbC precipitation and grain size evolution on the hot ductility of Nb containing steels. ISIJ Int. 2019, 59, 1064–1071. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, C. Grain size effect on the hot ductility of high-nitrogen austenitic stainless steel in the presence of precipitates. Materials 2018, 11, 1026. [Google Scholar] [CrossRef]
- Mintz, B.; Kang, S.; Qaban, A. The influence of grain size and precipitation and a boron addition on the hot ductility of a high Al, V containing TWIP steels. Mater. Sci. Technol. 2021, 37, 1035–1046. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Wu, H. Effects of chromium on the microstructure and hot ductility of Nb-microalloyed steel. Int. J. Miner. Metall. Mater. 2021, 28, 1011–1021. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Li, S.; Li, M. Influence of cooling conditions on the hot ductility of Nb-Ti-bearing steels. Metall. Res. Technol. 2015, 112, 604. [Google Scholar] [CrossRef]
- Qayyum, F.; Darabi, A.C.; Guk, S.; Guski, V.; Schmauder, S.; Prahl, U. Analyzing the effects of Cr and Mo on the pearlite formation in hypereutectoid steel using experiments and phase field numerical simulations. Materials 2024, 17, 3538. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, Y.; Meng, F.; Yu, Q.; Sun, F.; Sun, H.; Zhao, Z.; Wang, S.; Huang, L.; Geng, L. Improving the uniform deformation ability and ductility of powder metallurgical Ti2AlNb intermetallic with single-step solution heat treatment. J. Alloys Compd. 2025, 1011, 178377. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, W. Hot ductility loss and recovery in the CGHAZ of T23 steel during post-weld heat treatment at 750 °C. ISIJ Int. 2017, 57, 517–523. [Google Scholar] [CrossRef]
- Qu, H.P.; Lang, Y.P.; Yao, C.F.; Chen, H.T.; Yang, C.Q. The effect of heat treatment on recrystallized microstructure, precipitation and ductility of hot-rolled Fe–Cr–Al–REM ferritic stainless steel sheets. Mater. Sci. Eng. A 2013, 562, 9–16. [Google Scholar] [CrossRef]
- Andersson, J.; Sjöberg, G.P.; Viskari, L.; Chaturvedi, M. Effect of different solution heat treatments on hot ductility of superalloys Part 2–Allvac 718Plus. Mater. Sci. Technol. 2012, 28, 733–741. [Google Scholar] [CrossRef]
- Fu, J.; Du, W.; Jia, L.; Wang, Y.; Zhu, X.; Du, X. Cooling rate controlled basal precipitates and age hardening response of solid-soluted Mg–Gd–Er–Zn–Zr alloy. J. Magnes. Alloys 2021, 9, 1261–1271. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Xia, J.; Peng, Z.; Gao, J.; Sun, H. Recrystallization-precipitation interaction of a Ti microalloyed steel with controlled rolling process. J. Phys. Conf. Ser. 2020, 1676, 12036. [Google Scholar] [CrossRef]
- Jiang, B.; Hu, X.; Zhou, L.; Wang, H.; Liu, Y.; Gou, F. Effect of transformation temperature on the ferrite–bainite microstructures, mechanical properties and the deformation behavior in a hot-rolled dual phase steel. Met. Mater. Int. 2021, 27, 319–327. [Google Scholar] [CrossRef]
- Yin, D.; Qin, Y.; Dai, Z.; Long, X.; Zhang, F.; Yang, Z.; Li, Y. Investigating microstructural properties of ferrite/bainite dual-phase steel through simple process control. Mater. Sci. Technol. 2022, 38, 1348–1357. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Peng, Y.; Zhu, P.; Liu, J.; Feng, Z.; Wu, G.; Huang, X. Unprecedented strength in pure iron via high-pressure induced nanotwinned martensite. Mater. Res. Lett. 2019, 7, 354–360. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Liu, Z.; Yang, Z. Influence of refined hierarchical martensitic microstructures on yield strength and impact toughness of ultra-high strength stainless steel. J. Mater. Sci. Technol. 2020, 51, 130–136. [Google Scholar] [CrossRef]
| C | Si | Mn | P | S | O | N | Ti | Cr | Mo | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.39 | 0.31 | 0.42 | 0.0130 | 0.0010 | 0.0005 | 0.0031 | 0.0127 | 1.53 | 0.10 | 0.85 | Bal. |
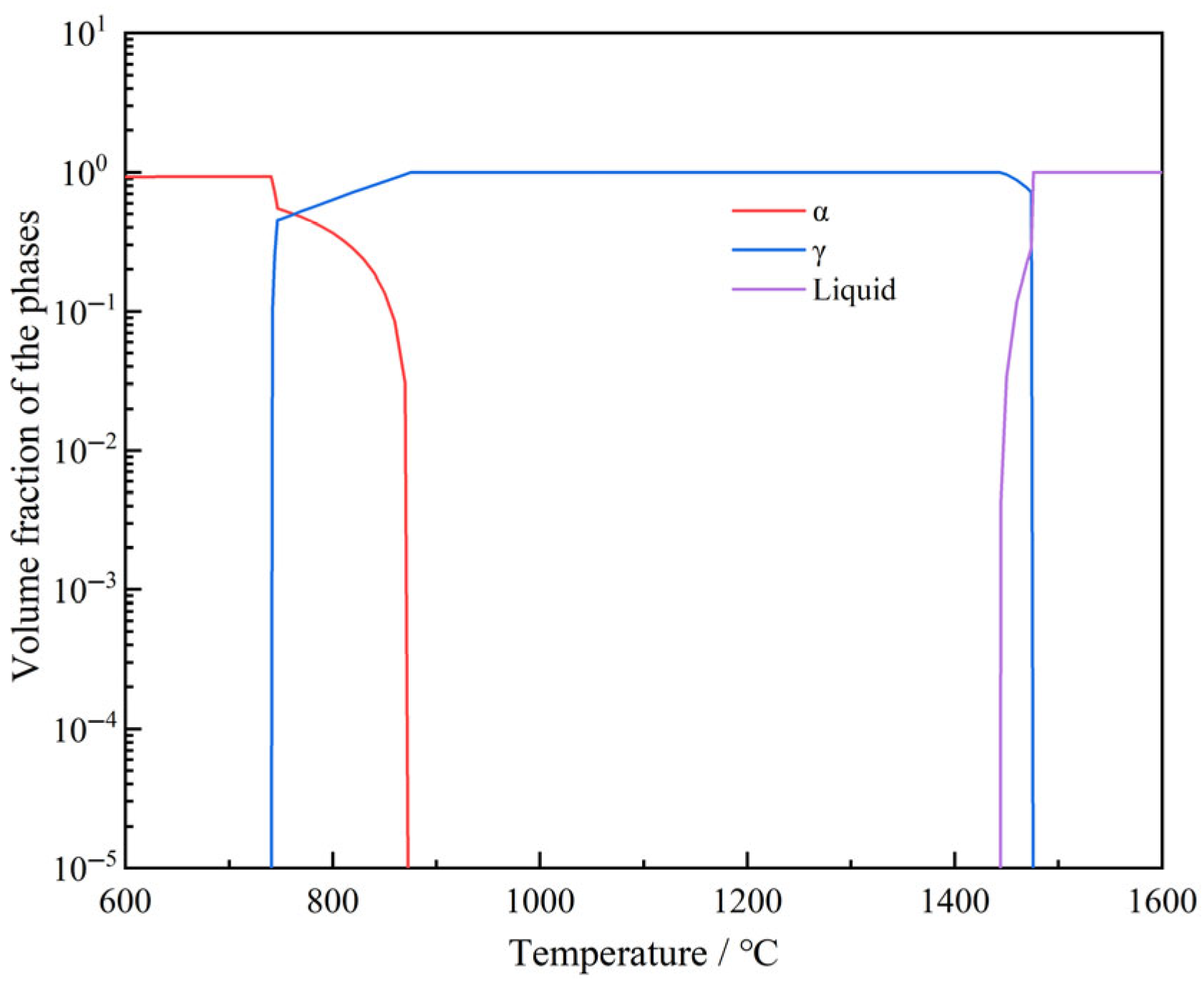
| Precipitation | Starting Precipitation Temperature (°C) | Full Precipitation Temperature (°C) | Maximum Precipitation Amount (Volume Fraction) |
|---|---|---|---|
| α | 870 | - | 0.932 |
| γ | 1474 | 740 | 1 |
| Liquid | - | 1444 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Liang, S.; Chen, B.; Chen, J.; Zhang, Y.; Zuo, X.; Li, Z.; Song, B.; Liu, W. Influence of Heat Treatment on Precipitate and Microstructure of 38CrMoAl Steel. Materials 2025, 18, 3703. https://doi.org/10.3390/ma18153703
Xu G, Liang S, Chen B, Chen J, Zhang Y, Zuo X, Li Z, Song B, Liu W. Influence of Heat Treatment on Precipitate and Microstructure of 38CrMoAl Steel. Materials. 2025; 18(15):3703. https://doi.org/10.3390/ma18153703
Chicago/Turabian StyleXu, Guofang, Shiheng Liang, Bo Chen, Jiangtao Chen, Yabing Zhang, Xiaotan Zuo, Zihan Li, Bo Song, and Wei Liu. 2025. "Influence of Heat Treatment on Precipitate and Microstructure of 38CrMoAl Steel" Materials 18, no. 15: 3703. https://doi.org/10.3390/ma18153703
APA StyleXu, G., Liang, S., Chen, B., Chen, J., Zhang, Y., Zuo, X., Li, Z., Song, B., & Liu, W. (2025). Influence of Heat Treatment on Precipitate and Microstructure of 38CrMoAl Steel. Materials, 18(15), 3703. https://doi.org/10.3390/ma18153703







