Research Progress on the Hydrogen Embrittlement Resistance Performance of High-Entropy Alloys
Abstract
1. Introduction
2. Occurrence and Failure Modes of Hydrogen Embrittlement
2.1. Occurrence of HE
2.2. Overview of Hydrogen Traps
- (1)
- The weakest traps (5–10 kJ/mol), represented by lattice interstices, primarily interact with diffusible hydrogen species that maintain high mobility within the crystalline structure.
- (2)
- Weak traps (15–25 kJ/mol), encompassing low-angle grain boundaries, martensite lath interfaces, and dislocation networks, demonstrate intermediate hydrogen retention capacity for mobile hydrogen populations.
- (3)
- Moderate-strength traps (30–50 kJ/mol), exemplified by microvoids and incipient cracks, effectively immobilize hydrogen through stronger interactions while permitting limited short-range diffusion.
- (4)
2.3. Failure Mechanism of Hydrogen Embrittlement
- (1)
- Hydrogen Internal Pressure Theory
- (2)
- Surface Energy Reduction HE Model
- (3)
- Hydrogen-Induced Lattice Weakening Mechanism
- (4)
- Hydrogen-Enhanced Local Plasticity Mechanism
- (5)
- Hydrogen-Enhanced Strain-Induced Vacancy Formation Mechanism
- (6)
- Hydrogen-Induced Phase Transformation Theory
3. Research on HE Resistance of HEAs
3.1. Effects of Hydrogen on Mechanical Properties of High-Entropy Alloys
3.2. Microstructural Factors of HE in HEAs
3.2.1. Effects of Alloying Elements
3.2.2. Diffusible Hydrogen Content
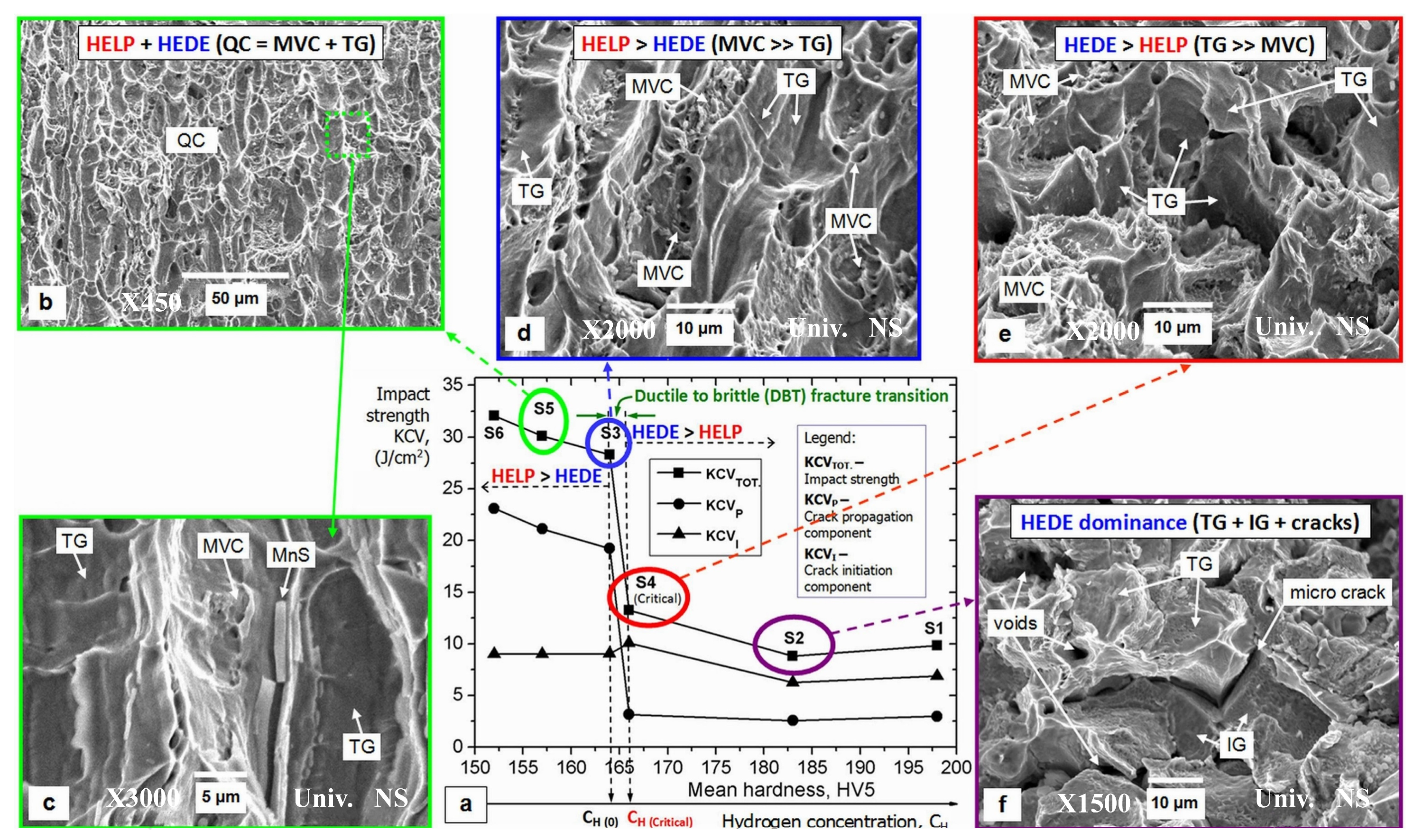
3.2.3. Hydrogen–Alloy Microstructure Interaction
3.2.4. Hydrogen Diffusion Behavior
4. Design Strategies and Prospects for HE Resistance in High-Strength and High-Toughness HEAs
4.1. Design Ideas for Hydrogen Embrittlement-Resistant HEAs
4.1.1. Oxide Film Protection and Phase Structure Regulation
4.1.2. Nano-Precipitate Trap Design
4.1.3. Grain Boundary Strengthening
4.1.4. Optimization of HEAs Fabrication Methods
4.2. Research Directions and Prospects for HE Resistance in HEAs
4.2.1. Conclusions
- (1)
- The concealment and delayed nature of hydrogen embrittlement (HE) pose significant challenges for material development. High-entropy alloys (HEAs), due to their multi-principal-element design, exhibit excellent hydrogen embrittlement resistance and comprehensive properties.
- (2)
- Microstructurally, single-phase FCC (face-centered cubic) structures show superior HE resistance, while grain refinement may promote the formation of secondary phases (e.g., σ phase), increasing HE susceptibility.
- (3)
- EHEAs can achieve high-strength and high-toughness HE-resistant alloys through microstructural regulation of phase composition.
- (4)
- Additive manufacturing, alloying element addition (e.g., Cr, Mo, and Al), and heat treatment are effective strategies to optimize the HE resistance of HEAs.
- (5)
- Future research should leverage machine learning modeling, cross-scale experiments, and environmentally friendly processing to advance HEA applications in the hydrogen energy field.
- (6)
- In the mechanism analysis, we proposed the influence of key mechanisms, indicating that HEAs may face a situation where the HELP mechanism mediates HEDE; that is to say, the key lies in the role of hydrogen concentration, which determines the form of fracture.
4.2.2. Future Research Directions
- (1)
- Synergistic Mechanisms of HE and Corrosion
- (2)
- Multi-Mechanism Coupled Modeling
- (3)
- Optimized HEA Composition and Microstructure Design
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HE | Hydrogen embrittlement |
| HIC | Hydrogen-induced cracking |
| HAC | Hydrogen-assisted cracking |
| MEA | Medium-entropy alloy |
| φHU | Reduction of area before hydrogen charging |
| φHC | Reduction of area after hydrogen charging |
| CL(δ) | Rate of change of the subsurface concentration |
| rad | Adsorption and Desorption Rates (Surface Processes) |
| CL(δ) | Subsurface Concentration Change Rate |
| Rads | Surface-to-Subsurface Adsorption Rate |
| kads | Subsurface Adsorption Rate Constant |
| kdes | Subsurface Desorption Rate Constant |
| kr | Desorption Rate Constant |
| kad | Adsorption Rate Constant |
| JL | Bulk Diffusion Flux |
| rr | Surface Desorption Rate |
| rdes | Subsurface-to-Surface Desorption Rate |
| v | Rate of change of surface coverage |
| DL | Bulk Diffusion Coefficient |
| δ | Subsurface Thickness |
| CL | Bulk Hydrogen Concentration |
| σb | Ultimate tensile strength |
| δ | Percentage elongation after fracture |
References
- Gupta, A.K.; Choudhari, A.; Rane, A.; Tiwari, A.; Sharma, P.; Gupta, A.; Sapale, P.; Tirumala, R.T.A.; Muthaiah, R.; Kumar, A. Advances in Nickel-Containing High-Entropy Alloys: From Fundamentals to Additive Manufacturing. Materials 2024, 17, 3826. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.C.; Dong, C.F.; Ni, X.Q.; Zhang, L.; Luo, H.; Li, R.X.; Wang, L.; Man, C.; Li, X.G. Superior resistance to hydrogen damage for selective laser melted 316L stainless steel in a proton exchange membrane fuel cell environment. Corros. Sci. 2020, 166, 108425. [Google Scholar] [CrossRef]
- Lee, D.H.; Sun, B.; Lee, S.; Ponge, D.; Jägle, E.A.; Raabe, D. Comparative study of hydrogen embrittlement resistance between additively and conventionally manufactured 304L austenitic stainless steels. Mater. Sci. Eng. A 2021, 803, 140499. [Google Scholar] [CrossRef]
- Lee, D.H.; Jung, J.Y.; Lee, K.H.; Lee, S.Y.; Zhao, Y.; Lau, K.B.; Wang, P.; Ramamurty, U. Distinct effects of in-situ and ex-situ hydrogen charging methods on the mechanical behavior of CoCrFeNi high-entropy alloy fabricated by laser-powder bed fusion. J. Alloys Compd. 2023, 940, 168858. [Google Scholar] [CrossRef]
- Shiratori, H.; Kimura, T.; Kuwabara, K. Hydrogen embrittlement susceptibility of CoCrFeNiTi-based multiprincpal element alloys formed by laser powder bed fusion. Mater. Today Commun. 2024, 40, 109755. [Google Scholar] [CrossRef]
- Lu, Y.P.; Gao, X.Z.; Jiang, L.; Chen, Z.N.; Wang, T.M.; Jie, J.C.; Kang, H.J.; Zhang, Y.B.; Guo, S.; Ruan, H.H.; et al. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef]
- Wang, Z.J.; Bai, X.Y.; Wang, J.B.; Jiang, H.; Jiao, W.N.; Li, T.X.; Lu, Y.P. A Decade Review of Eutectic High-Entropy Alloys (2014—2024): Design, Preparation and Applications. Acta Metall. Sin. 2024, 1, 17. [Google Scholar]
- Fan, X.K.; Wu, B.; Zhao, C.J.; Dai, W.J. New Progress in the Research on Preparation Technologies of High-Entropy Alloys. Henan Sci. 2024, 42, 1561–1569. [Google Scholar]
- Wang, Z.F.; Zhang, S. Research and Application Progress of High-Entropy Alloys. Coatings 2023, 13, 1916. [Google Scholar] [CrossRef]
- Li, Z.J.; Song, J.; Xu, G.Z.; Hao, W.K.; Luo, H. Research Progress on Brittle Behavior of High-Entropy Metal Materials in Hydrogen Environment. Surf. Technol. 2024, 53, 321–348. [Google Scholar]
- Guo, Z.H.; Liu, M.; Ma, Y.; Yu, H.; Jing, S.R.; Yan, Y. Hydrogen embrittlement and corrosion resistance of NiCoCr-based equimolar face-centered cubic medium-/high-entropy alloys. Corros. Sci. 2025, 245, 112700. [Google Scholar] [CrossRef]
- Koyama, M.; Wang, H.Y.; Verma, V.K.; Tsuzaki, K.; Akiyama, E. Effects of Mn Content and Grain Size on Hydrogen Embrittlement Susceptibility of Face-Centered Cubic High-Entropy Alloys. Metall. Mater. Trans. A 2020, 51, 5612–5616. [Google Scholar] [CrossRef]
- Zhang, S.D. Study on Hydrogen Embrittlement Resistance of Al-Co-Cr-Fe-Ni High-Entropy Alloys. Master’s Thesis, University of Shanghai for Science and Technology, Shanghai, China, 2022; pp. 21–23. [Google Scholar] [CrossRef]
- Zhao, Y.K.; Lee, D.H.; Seok, M.Y.; Lee, J.A.; Phaniraj, M.P.; Suh, J.Y.; Ha, H.Y.; Kim, J.Y.; Ramamurty, U.; Jang, J.I. Resistance of CoCrFeMnNi high-entropy alloy to gaseous hydrogen embrittlement. Scr. Mater. 2017, 135, 54–58. [Google Scholar] [CrossRef]
- Zhou, X.; Tehranchi, A.; Curtin, W.A. Mechanism and Prediction of Hydrogen Embrittlement in fcc Stainless Steels and High Entropy Alloys. Phys. Rev. Lett. 2021, 127, 175501. [Google Scholar] [CrossRef]
- Luo, H.; Sohn, S.S.; Lu, W.J.; Li, L.L.; Li, X.G.; Soundararajan, C.K.; Krieger, W.; Li, Z.M.; Raabe, D. A strong and ductile medium-entropy alloy resists hydrogen embrittlement and corrosion. Nat. Commun. 2020, 11, 3081. [Google Scholar] [CrossRef]
- Pu, Z.; Chen, Y.; Dai, L.H. Strong resistance to hydrogen embrittlement of high-entropy alloy. Mater. Sci. Eng. A 2018, 736, 156–166. [Google Scholar] [CrossRef]
- Song, H.; Jo, M.; Kim, D.W. Vanadium or copper alloyed duplex lightweight steel with enhanced hydrogen embrittlement resistance at room temperature. Mater. Sci. Eng. A. 2021, 817, 141347. [Google Scholar] [CrossRef]
- Qu, S.S.; Tan, Y.; Li, W.; Xia, L.; Xu, Y.; Zhang, G.; Yang, G. Review on the Mechanical Properties of Austenitic Stainless Steel in Hydrogen Environment. Shandong Chem. Ind. 2022, 51, 100–103. [Google Scholar]
- Kobayashi, N.; Koyama, M.; Kobayashi, K.; Hojo, T.; Akiyama, E. Hydrogen Embrittlement Behavior of Pure Ni and Ni20Cr Alloy with Different Grain Sizes. Mater. Trans. 2022, 63, 247–256. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.Y.; Yao, C.L.; Shen, J.; Zhang, Y.P.; Ge, Y.H.; Zhang, G.J. Microstructural stability of as-cast and directionally solidified AlCoCrFeNi2.1 eutectic high - entropy alloys at elevated temperatures. Met. Mater. Trans. A 2020, 51, 5781–5789. [Google Scholar] [CrossRef]
- Xiong, T.; Zheng, S.J.; Pang, J.Y.; Ma, X.L. High-strength and high-ductility AlCoCrFeNi2.1 eutectic high-entropy alloy achieved via precipitation strengthening in a heterogeneous structure. Scr. Mater. 2020, 186, 336–340. [Google Scholar] [CrossRef]
- Chu, W.Y. Hydrogen Embrittlement and Stress Corrosion: Basic Part; Science Press: Beijing, China, 2013; p. 11. [Google Scholar]
- Li, X.G. Hydrogen Embrittlement of Metals and Its Generation Mechanism. Shanghai Met. 2023, 45, 1–16. [Google Scholar]
- Pundt, A.; Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Johnson, W.H. On some remarkable changes produced in iron and steel by the action of hydrogen and acids. Proc. R. Soc. Lond. 1874, 11, 168–179. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Song, W.; Li, Y.X. Research Progress on Hydrogen Embrittlement Prevention of Hydrogen Transmission Pipeline Steel. Surf. Technol. 2022, 51, 126–137. [Google Scholar]
- Wang, J.; Liu, X.Y.; Gao, L.Q.; Zha, X.Q. Research Status of Hydrogen-Induced Damage Mechanism of Titanium Alloys. Mater. Prot. 2020, 53, 98–105. [Google Scholar]
- Jiang, B.; Chen, G.; Cui Y., H.; Ren, X.C.; Chu, W.Y. Flakes in Wheel Steel (Part II). Iron. Steel. 2007, 42, 74–78. [Google Scholar]
- Petch, N.J.; Stables, P.D. elayed fracture of metals under static load. Nature. 1952, 169, 842–843. [Google Scholar] [CrossRef]
- Wu, S.D.; Chen, L.; Liu, M.Z. Study on the Hydrogen Concentration Distribution at the Front of Notch under Mode I Loading. Acta Met. Sin. 1990, 25, 86–91. [Google Scholar]
- Turnage, S.A.; Rajagopalan, M.; Darling, K.A.; Garg, P.; Kale, C.; Bazehhour, B.G.; Adlakha, I.; Hornbuckle, B.C.; Williams, C.L.; Peralta, P.; et al. Anomalous mechanical behavior of nanocrystalline binary alloys under extreme conditions. Nat. Commun. 2018, 9, 2699. [Google Scholar] [CrossRef]
- Troiano, A.R. The role of hydrogen and other interstitials in the mechanical behavior of metals. Met. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Oriani, R.A.; Josephic, P.H. Equilibrium aspects of hydrogen-induced cracking of steels. Acta Metall. Sin. 1974, 22, 1065–1074. [Google Scholar] [CrossRef]
- Knott, J.F. Fracture toughness and hydrogen-assisted crack growth in engineering alloys. In Proceedings of the 5th International Conference on Hydrogen Effects in Materials, Moran, WY, USA, 11–14 September 1994; TMS: Warrendale, PA, USA, 1994; pp. 387–408. [Google Scholar]
- Lynch, S.P. Environmentally assisted cracking: Overview of evidence for an adsorption-induced localised-slip process. Acta Metall. 1988, 36, 2639–2661. [Google Scholar] [CrossRef]
- Bernstein, I.M.; Thompson, A.W. Hydrogen in metals. In Proceedings of the International Conference on the Effects of Hydrogen on Materials Properties and Selection and Structural Design, Champion, PA, USA, 23–27 September 1973; ASM: Metals Park, OH, USA, 1974. [Google Scholar]
- Lynch, S.P. Environmentally assisted cracking at high velocities. Scr. Met. 1987, 21, 157–162. [Google Scholar] [CrossRef]
- Lynch, S.P. Progress towards understanding mechanisms of hydrogen embrittlement and stress corrosion cracking. In Proceedings of the NACE International Corrosion 2007 Conference Proceedings, Nashville, TN, USA, 11–15 March 2007; p. 74931. [Google Scholar]
- Maire, E.; Grabon, S.; Adrien, J.; Lorenzino, P.; Asanuma, Y.; Takakuwa, O.; Matsunaga, H. Role of hydrogen charging on nucleation and growth of ductile damage in austenitic stainless steels. Materials 2019, 12, 1426. [Google Scholar] [CrossRef]
- Wang, Z. Hydrogen Diffusion Behavior in High-Strength DP Steel and Its Influence on Hydrogen Embrittlement Sensitivity; Wuhan University of Science and Technology: Wuhan, China, 2021. [Google Scholar]
- Lynch, S.P. Mechanisms of Hydrogen Assisted Cracking—A review. In Proceedings of the International Conference on Hydrogen Effects on Material Behaviour and Corrosion Deformation Interactions, Moran, WY, USA, 22–26 September 2002; TMS: Warrendale, PA, USA, 2003; pp. 189–200. [Google Scholar]
- Westlake, D.G. Generalized model for hydrogen embrittlement. ASM-Trans. 1969, 3–7. [Google Scholar] [CrossRef]
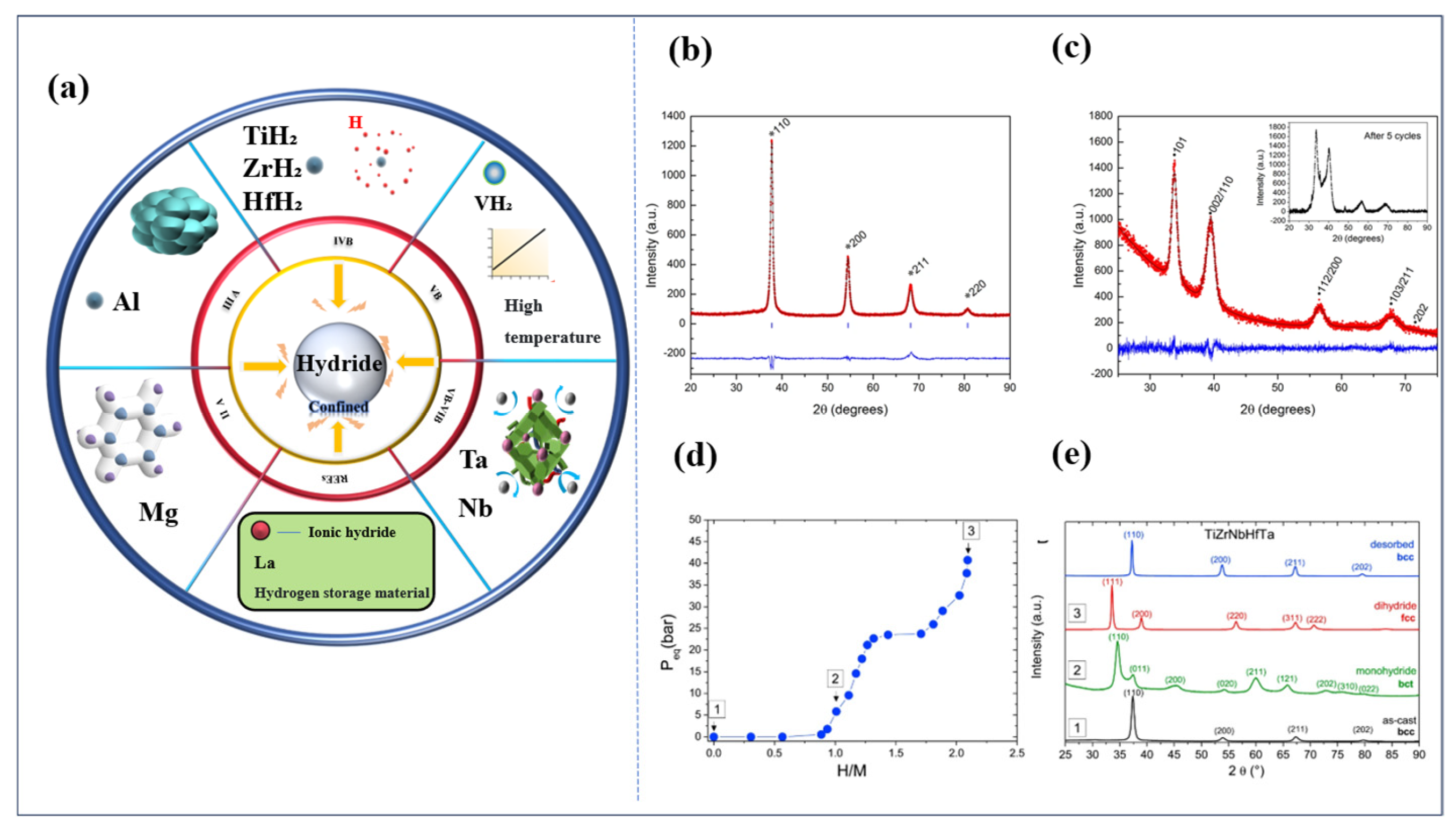
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef]
- Zlotea, M.A.; Sow, G.; Ek, T.R. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.L.; Yang, C.; Hu, H. Fracture strain model for hydrogen embrittlement based on hydrogen enhanced localized plasticity mechanism. Int. J. Hydrogen Energy. 2020, 45, 25541–25554. [Google Scholar] [CrossRef]
- Li, X.F.; Yin, J.; Zhang, J.; Wang, Y.F.; Song, X.L.; Zhang, Y.; Ren, X.C. Hydrogen embrittlement and failure mechanisms of multi-principal element alloys: A review. J. Mater. Sci. Technol. 2022, 122, 20–32. [Google Scholar] [CrossRef]
- Claeys, L.; Verbeken, K.; Depover, T.; Rizi, M.B. Hydrogen interaction with FCC CoCrFeMnNi high entropy alloy. Procedia Struct. Integr. 2024, 54, 250–255. [Google Scholar] [CrossRef]
- Li, X.F.; Feng, Z.; Song, X.L.; Wang, Y.F.; Zhang, Y. Effect of hydrogen charging time on hydrogen embrittlement of CoCrFeMnNi high-entropy alloy. Corros. Sci. 2022, 198, 110073. [Google Scholar] [CrossRef]
- Cheng, H.X.; Luo, H.; Pan, Z.M.; Wang, X.F.; Zhao, Q.C.; Li, X.G. Hydrogen embrittlement of a precipitation-strengthened high-entropy alloy. Corros. Sci. 2024, 227, 111708. [Google Scholar] [CrossRef]
- Nygren, K.E.; Wang, S.; Bertsch, K.M.; Bei, H.B.; Nagao, A.; Robertson, I.M. Hydrogen embrittlement of the equi-molar FeNiCoCr alloy. Acta Mater. 2018, 157, 218–227. [Google Scholar] [CrossRef]
- An, X.D.; Zhang, H.; Zhang, D.H.; Zhu, J.L.; Wang, Q.Q.; Zhu, T.; Shi, Y.M.; Cao, X.Z.; Deng, H.Q.; Hu, W.Y.; et al. Mechanisms of hydrogen embrittlement resistances in FCC concentrated solid solution alloys. Corros. Sci. 2024, 229, 111894. [Google Scholar] [CrossRef]
- Marques, S.C.; Castilho, A.V.; Santos, D.S.D. Effect of Alloying Elements on the Hydrogen Diffusion and Trapping in High Entropy Alloys. Scr. Mater. 2021, 201, 113957. [Google Scholar] [CrossRef]
- Martin, M.; Weber, S.; Theisen, W.; Michler, T.; Naumann, J. Effect of Alloying Elements on Hydrogen Environment Embrittlement of AISI Type 304 Austenitic Stainless Steel. Int. J. Hydrogen Energy 2011, 36, 15888–15898. [Google Scholar] [CrossRef]
- Yi, J.; Zhuang, X.Q.; He, J.; He, M.L.; Liu, W.H.; Wang, S. Effect of Mo Doping on the Gaseous Hydrogen Embrittlement of a CoCrNi Medium-Entropy Alloy. Corros. Sci. 2021, 189, 109628. [Google Scholar] [CrossRef]
- Li, X.F.; Cui, Y.; Zhang, J. Enhancement of hydrogen embrittlement resistance in CoCrFeNi high-entropy alloy through the addition of MoB elements. Int. J. Hydrogen Energy 2024, 92, 1306–1319. [Google Scholar] [CrossRef]
- An, X.D.; Zhang, D.H.; Zhang, H.; Wang, Q.Q.; Zhu, T.; Wu, Z.G.; Zhang, W.D.; Deng, H.Q.; Hu, W.Y.; Cao, X.Z.; et al. Deuterium induced defects and embrittlement behavior of a Co-free high entropy alloy. J. Alloy. Compd. 2023, 940, 168800. [Google Scholar] [CrossRef]
- Wang, H.Y.; Koyama, M.; Hojo, T.; Akiyama, E. Hydrogen embrittlement and associated surface crack growth in fine-grained equiatomic CoCrFeMnNi high-entropy alloys with different annealing temperatures evaluated by tensile testing under in situ hydrogen charging. Int. J. Hydrogen Energy. 2021, 46, 33028–33038. [Google Scholar] [CrossRef]
- Allam, T.; Guo, X.; Lipińska-Chwałek, M.; Hamada, A.; Ahmed, E.; Bleck, W. Impact of precipitates on the hydrogen embrittlement behavior of a V-alloyed medium-manganese austenitic stainless steel. J. Mater. Res. Technol. 2020, 9, 13524–13538. [Google Scholar] [CrossRef]
- Choo, W.; Lee, J.Y. Thermal analysis of trapped hydrogen in pure iron. Metall. Trans. A 1982, 13, 135–140. [Google Scholar] [CrossRef]
- Gao, Z.; Lee, D.H.; Zhao, Y.K.; Wang, P.; Murakami, K.; Komazaki, S.I.; Suh, J.Y.; Kim, H.S.; Ramamurty, U.; Jang, J.I. Hydrogen trapping and micromechanical behavior in additively manufactured CoCrFeNi high-entropy alloy in as-built and pre-strained conditions. Acta Mater. 2024, 271, 119886. [Google Scholar] [CrossRef]
- Rudomilova, D.; Prosek, T.; Luckeneder, G. Techniques for investigation of hydrogen embrittlement of advanced high strength steels. Corros. Rev. 2018, 36, 413–434. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Effect of hydrogen in advanced high strength steel materials. Int. J. Hydrogen Energy 2019, 44, 28007–28030. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, E.; Wan, J.; Liu, J.; Huang, Y.; Li, X. Effect of Nb on the hydrogen-induced cracking of high-strength low-alloy steel. Corros. Sci. 2018, 139, 83–96. [Google Scholar] [CrossRef]
- Shoda, H.; Suzuki, H.; Takai, K.; Hagihara, Y. Hydrogen desorption behavior of pure iron and Inconel 625 during elastic and plastic deformation. ISIJ Int. 2010, 50, 115–123. [Google Scholar] [CrossRef]
- Metalnikov, P.; Eliezer, D.; Ben-Hamu, G. Hydrogen trapping in additive manufactured Ti-6Al-4V alloy. Mater. Sci. Eng. A 2021, 811, 141050. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.M. Hydrogen trapping phenomena in metals with Bcc and Fcc crystal-structures by the desorption thermal-analysis technique. Surf. Coat. Technol. 1986, 28, 301–314. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Conforto, E.; Berziou, C.; Savall, C.; Feaugas, X. Grain size and grain-boundary effects on diffusion and trapping of hydrogen in pure nickel. Acta Mater. 2012, 60, 6814–6828. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, J.; Cui, Y.; Djukic, M.B.; Feng, H.; Wang, Y.F. Review of the hydrogen embrittlement and interactions between hydrogen and microstructural interfaces in metallic alloys: Grain boundary, twin boundary, and nano-precipitate. Int. J. Hydrogen Energy 2024, 72, 74–109. [Google Scholar] [CrossRef]
- Luo, H.; Lu, W.J.; Fang, X.F.; Ponge, D.; Li, Z.M.; Raabe, D. Beating hydrogen with its own weapon: Nano-twin gradients enhance embrittlement resistance of a high-entropy alloy. Mater. Today 2018, 21, 1003–1009. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.F.; Song, X.L.; Gu, T.; Zhang, Y. Hydrogen Embrittlement of CoCrFeMnNi High-Entropy Alloy Compared with 304 and IN718 Alloys. Metals 2022, 6, 998. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Han, J.H.; Li, J.X.; Cao, F.H.; Wang, S. Tailoring hydrogen embrittlement resistance of pure Ni by grain boundary engineering. Corros. Commun. 2022, 6, 48–51. [Google Scholar] [CrossRef]
- Zhou, C.S.; Fang, B.; Wang, J.; Tang, D.; Tao, H.M.; He, Y.M.; Zhou, Z.R.; Chen, C.F.; Zhang, L. Hydrogen embrittlement resistance of TWIP (twinning-induced plasticity) steel in high pressure hydrogen environment. Int. J. Fatigue 2021, 151, 106362. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M.; Kuramoto, S.; Hojo, T. Unraveling the effect of dislocations and deformation-induced boundaries on environmental hydrogen embrittlement behavior of a cold-rolled Al-Zn-Mg-Cu alloy. Int. J. Hydrogen Energy 2021, 46, 8285–8299. [Google Scholar] [CrossRef]
- Zhao, Q.C.; Luo, H.; Yang, Z.S.; Pan Z., M.; Wang, Z.J.; Islamgaliev, R.K.; Li, X.G. Hydrogen induced cracking behavior of the dual-phaseCo30Cr10Fe10Al18Ni30Mo2 eutectic high entropy alloy. Int. J. Hydrogen Energy 2024, 50, 134–147. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D. Mechanisms of hydrogen trapping in austenitic, duplex, and super martensitic stainless steels. J. Alloys Compd. 2017, 720, 451–459. [Google Scholar] [CrossRef]
- Cheng, H.X.; Luo, H.; Pan, Z.M.; Wang, X.F.; Zhao, Q.C.; Fu, Y.; Li, X.G. Effects of laser powder bed fusion process parameters on microstructure and hydrogen embrittlement of high-entropy alloy. J. Mater. Sci. Technol. 2023, 155, 211–226. [Google Scholar] [CrossRef]
- He, J.; Liu, Q.; He, M.L.; Li, J.X.; Wang, S. The hydrogen embrittlement of pure Ni fabricated by additive manufacturing. Int. J. Hydrogen Energy 2023, 48, 16910–16922. [Google Scholar] [CrossRef]
- Zhao, Q.C.; Luo, H.; Djukic, M.B.; Pan, Z.M.; Cheng, H.X.; Islamgaliev, R.K. Hydrogen-induced crack behavior of a precipitation-strengthened Ni50Cr20Co15Al10V5 high entropy alloy. Corros. Sci. 2024, 241, 112–562. [Google Scholar] [CrossRef]
- Feng, D.C.; Wang, W.J.; Liu, Y.F.; Zheng, W.J.; Yan, D.J.; Li, C.A.; Huang, M.; He, Y.M.; Lai, S.B.; Yang, J.G. Hydrogen-induced evolution associated with nano-scale precipitated phases in AlCoCrFeNi2.1 high-entropy alloy. J. Alloys Compd. 2023, 944, 169116. [Google Scholar] [CrossRef]
- Young, G.A.; Scully, J.R. The diffusion and trapping of hydrogen in high purity aluminum. Acta Mater. 1998, 46, 6337–6349. [Google Scholar] [CrossRef]
- Wan, D.; Guan, S.; Wang, D.; Lu, X.; Ma, J. Hydrogen embrittlement of additively manufactured AlCoCrFeNi2.1 eutectic high-entropy alloy. Corros. Sci. 2022, 195, 110007. [Google Scholar] [CrossRef]
- Dong, C.F.; Li, X.G.; Liu, Z.Y.; Zhang, Y.R. Hydrogen-induced cracking and healing behaviour of X70 steel. J. Alloys Compd. 2009, 484, 966–972. [Google Scholar] [CrossRef]
- You, Z.Y.; Tang, Z.Y.; Li, J.P.; Chu, F.B.; Ding, H.; Misra, R.D.K. Effect of grain boundary engineering on grain boundary character distribution and deformation behavior of a TRIP-assisted high-entropy alloy. Mater. Charact. 2023, 205, 113294. [Google Scholar] [CrossRef]
- Li, X.Y.; Gao, X.J.; Shi, C.C.; Zhu, G.M.; Guo, N.N. Research Progress of Eutectic High-Entropy Alloys. Spec. Cast. Nonferrous Alloys 2021, 41, 32–74. [Google Scholar]
- Hussein, A.; Kim, B.; Verbeken, K.; Depover, T. Effect of Grain Boundary Misorientation on Hydrogen Flux Using a Phase-Field-Based Diffusion and Trapping Model. Adv. Eng. Mater. 2024, 26, 22. [Google Scholar] [CrossRef]
- Page, D.E.; Varela, K.F.; Johnson, O.K.; Fullwood, D.T.; Homer, E.R. Measuring simulated hydrogen diffusion in symmetric tilt nickel grain boundaries and examining the relevance of the Borisov- relationship for individual boundary diffusion. Acta Mater. 2021, 212, 116882. [Google Scholar] [CrossRef]
- Mohammadi, A.; Edalati, P.; Arita, M.; Bae, J.W.; Kim, H.S.; Edalati, K. High strength and high ductility of a severely deformed high-entropy alloy in the presence of hydrogen. Corros. Sci. 2023, 216, 111097. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.H.; Park, H.J.; Kang, N.H. Effective hydrogen diffusion coefficient for CoCrFeMnNi high-entropy alloy and microstructural behaviors after hydrogen permeation. Int. J. Hydrogen Energy 2020, 45, 10227–10232. [Google Scholar] [CrossRef]
- Xie, Z.C.; Wang, Y.J.; Lu, C.S.; Dai, L.H. Sluggish hydrogen diffusion and hydrogen decreasing stacking fault energy in a high-entropy alloy. Mater. Today Commun. 2021, 26, 101902. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhu, J.H.; Wu, Y.; Yang, X.S.; Lookman, T.; Wu, H.H. Machine learning assisted design of FeCoNiCrMn high-entropy alloys with ultra-low hydrogen diffusion coefficients. Acta Mater. 2022, 224, 117535. [Google Scholar] [CrossRef]
- Himanen, L.; Jäger, M.O.J.; Morooka, E.V.; Canova, F.F.; Ranawat, Y.S.; Gao, D.Z.; Rinke, P.; Foster, A.S. Library of descriptors for machine learning in materials science. Comput. Phys. Commun. 2020, 247, 106949. [Google Scholar] [CrossRef]
- Wang, S.; Hashimoto, N.; Wang, Y.M.; Ohnuki, S. Activation volume and density of mobile dislocations in hydrogen-charged iron. Acta Mater. 2013, 61, 4734–4742. [Google Scholar] [CrossRef]
- Wilcox, B.A.; Smith, G.C. The Portevin-Le Chatelier effect in hydrogen charged nickel. Acta Met. 1964, 12, 371–376. [Google Scholar] [CrossRef]
- Wang, S.; Nagao, A.; Edalati, K.; Horita, Z.; Robertson, I.M. Influence of hydrogen on dislocation self-organization in Ni. Acta Mater. 2017, 135, 96–162. [Google Scholar] [CrossRef]
- Ogawa, Y.H.; Birenis, D.; Matsunaga, H.; Thøgersen, A.; Prytz, Ø.; Takakuwa, O.; Yamabe, J. Multi-scale observation of hydrogen-induced, localized plastic deformation in fatigue-crack propagation in a pure iron. Scr. Mater. 2017, 140, 13–147. [Google Scholar] [CrossRef]
- Birenis, D.; Ogawa, Y.H.; Matsunaga, H.; Takakuwa, O.; Yamabe, J.; Prytz, Ø.; Thøgersen, A. Interpretation of hydrogen-assisted fatigue crack propagation in BCC iron based on dislocation structure evolution around the crack wake. Acta Mater. 2018, 156, 245–253. [Google Scholar] [CrossRef]
- Robertson, I.M.; Birnbaum, H.K. Effect of hydrogen on the dislocation structure of deformed nickel. Scr. Metall. 1984, 18, 218–269. [Google Scholar] [CrossRef]
- Harris, Z.D.; Lawrence, S.K.; Medlin, D.L.; Guetard, G.; Burns, J.T.; Somerday, B.P. Elucidating the contribution of mobile hydrogen-deformation interactions to hydrogen-induced intergranular cracking in polycrystalline nickel. Acta Mater. 2018, 158, 180–192. [Google Scholar] [CrossRef]
- Cho, L.; Kong, Y.R.; Kathayat, P.; Brown, D.W.; Lawrence, S.K.; Clausen, B.; Vogel, S.C.; Ravkov, L.; Balogh, L.; Ronevich, J.A.; et al. Influence of hydrogen on deformation and embrittlement mechanisms in a high Mn austenitic steel: In-Situ neutron diffraction and diffraction line profile analysis. Acta Mater. 2024, 281, 120420. [Google Scholar] [CrossRef]
- Bertsch, K.M.; Wang, S.; Nagao, A.; Robertson, I.M. Hydrogen-induced compatibility constraints across grain boundaries drive intergranular failure of Ni. Mater. Sci. Eng. A 2019, 760, 58–67. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.M.; Lu, W.J.; Ponge, D.; Raabe, D. Hydrogen embrittlement of an interstitial equimolar high-entropy alloy. Corros. Sci. 2018, 136, 403–408. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, B.R.; Liu, X.J.; Wang, H.; Jiang, S.H.; Naeem, M.; Wang, X.L.; Wu, Y.; Lu, Z.P. Hydrogen embrittlement resistance of precipitation-hardened FeCoNiCr high entropy alloys. Intermetallics 2023, 153, 107800. [Google Scholar] [CrossRef]
- Zhang, S.D.; Liu, M.; Luo, Y.; Wang, L.B.; Wang, Z.M.; Wang, Z.Y.; Li, F.J.; Shen, Q.; Wang, X.W. Immunity of Al0.25CoCrFeNi high-entropy alloy to hydrogen embrittlement. Mater. Sci. Eng. A. 2021, 821, 141590. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, B.; Pan, Z.M.; Fu, Y.; Li, X.G. Hydrogen induced microstructure evolution and cracking mechanism in a metastable dual-phase high-entropy alloy. J. Mater. Res. Technol. 2021, 819, 141490. [Google Scholar] [CrossRef]
- Balaji, V.; Jeyapandiarajan, P.; Joel, J.; Anbalagan, A.; Ashwath, P.; Anouncia, S.M.; Batako, A.; Xavior, M.A. Mitigating hydrogen embrittlement in high-entropy alloys for next-generation hydrogen storage systems. J. Mater. Res. Technol. 2024, 33, 7681–7697. [Google Scholar] [CrossRef]
- Dieudonné, T.; Marchetti, L.; Wery, M.; Chêne, J.; Allely, C.; Cugy, P.; Scott, C.P. Role of copper and aluminum additions on the hydrogen embrittlement susceptibility of austenitic Fe–Mn–C TWIP steels. Corros. Sci. 2014, 82, 218–226. [Google Scholar] [CrossRef]
- Luo, H.; Pan, Z.M.; Yang, T.; Chang, W.W.; Zhang, D.W.; Cheng, H.X.; Li, X.G.; Raabe, D. A High-Entropy Alloy for Superior Resistance to Biogenic Sulfuric Acid Corrosion and Hydrogen Embrittlement. Matter 2025, 8, 101944. [Google Scholar] [CrossRef]
- Yoo, J.S.; Jo, M.C.; Kim, D.W.; Song, H.J.; Koo, M.S.; Sohn, S.S.; Lee, S.H. Influence of Cu Addition on the Hydrogen Embrittlement Resistance of 1GPa-Class Dual-Phase Light Steel. Acta Mater. 2020, 196, 370–383. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Fang, L.M.; Zeng, Z.; Wang, X.L. Ultrahigh hydrogen diffusivities in Al2O3 under high pressure. Phys. Rev. B 2024, 110, 1443102. [Google Scholar] [CrossRef]
- Cai, S.W.; Hua, T.; Zong, Y. Effect of Voltage on Hydrogen Embrittlement Sensitivity Inhibition of Micro-arc Oxidation Coating on 7050 Aluminum Alloy. Heat Treat Met. 2020, 45, 11. [Google Scholar]
- Jiang, Z.Q.; Song, R.G.; Jiang, B. Effect of Micro-arc Oxidation Coating on Hydrogen-Induced Local Plastic Deformation of 7050 Aluminum Alloy. Surf Technol. 2020, 49, 29–33. [Google Scholar]
- Liu, M.; Lu, W.J.; Li, F.J.; Zhang, S.D.; Shen, Q.; Jian, M.L.; Lei, Z.Y.; Wang, Z.Y. Effect of Heat Treatment on Hydrogen Embrittlement Susceptibility of Al0.25CoCrFeNi High Entropy Alloy. Eng. Fail. Anal. 2024, 162, 108432. [Google Scholar] [CrossRef]
- Shi P., J.; Li, Y.; Wen Y., B.; Li Y., Q.; Wang, Y.; Ren, W.L.; Zheng T., X.; Guo Y., F.; Hou, L.; Shen, Z.; et al. Semi-coherent nanoprecipitates induced by Nb microalloying enable high-strength and hydrogen-resistant interstitial high-entropy alloy. Eng. Fract. Mech. 2025, 320, 111044. [Google Scholar]
- Shi, R.J.; Ma, Y.; Wang Z., D.; Gao, L.; Yang X., S.; Qiao, L.J.; Pang X., L. Atomic-scale investigation of deep hydrogen trapping in NbC/α-Fe semi-coherent interfaces. Acta Mater. 2020, 200, 686–698. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Žeravčić, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fail. Anal. 2019, 216, 106528. [Google Scholar]
- Milos, D.; Žeravčić, V.Š.; Gordana, B.; Sedmak, A. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar]
- Koyama, M.; Ichii, K.; Tsuzaki, K. Grain refinement effect on hydrogen embrittlement resistance of an equiatomic CoCrFeMnNi high-entropy alloy. Int. J. Hydrogen Energy 2019, 44, 17163–17167. [Google Scholar] [CrossRef]
- Miao, J.W.; Wang, M.L.; Zhang, A.J.; Lu, Y.P.; Wang, T.M.; Li, T.J. Tribological properties and wear mechanism of AlCr1.3TiNi2 eutectic high-entropy alloy at elevated temperature. Acta Met. Sin. 2023, 59, 267–276. [Google Scholar]
- Turnbull, A.; Zhou, S. Residual stress relaxation in shot peened high strength low alloy steel and its implications for hydrogen assisted cracking. Mater. Sci. Technol. 2010, 26, 824–832. [Google Scholar] [CrossRef]

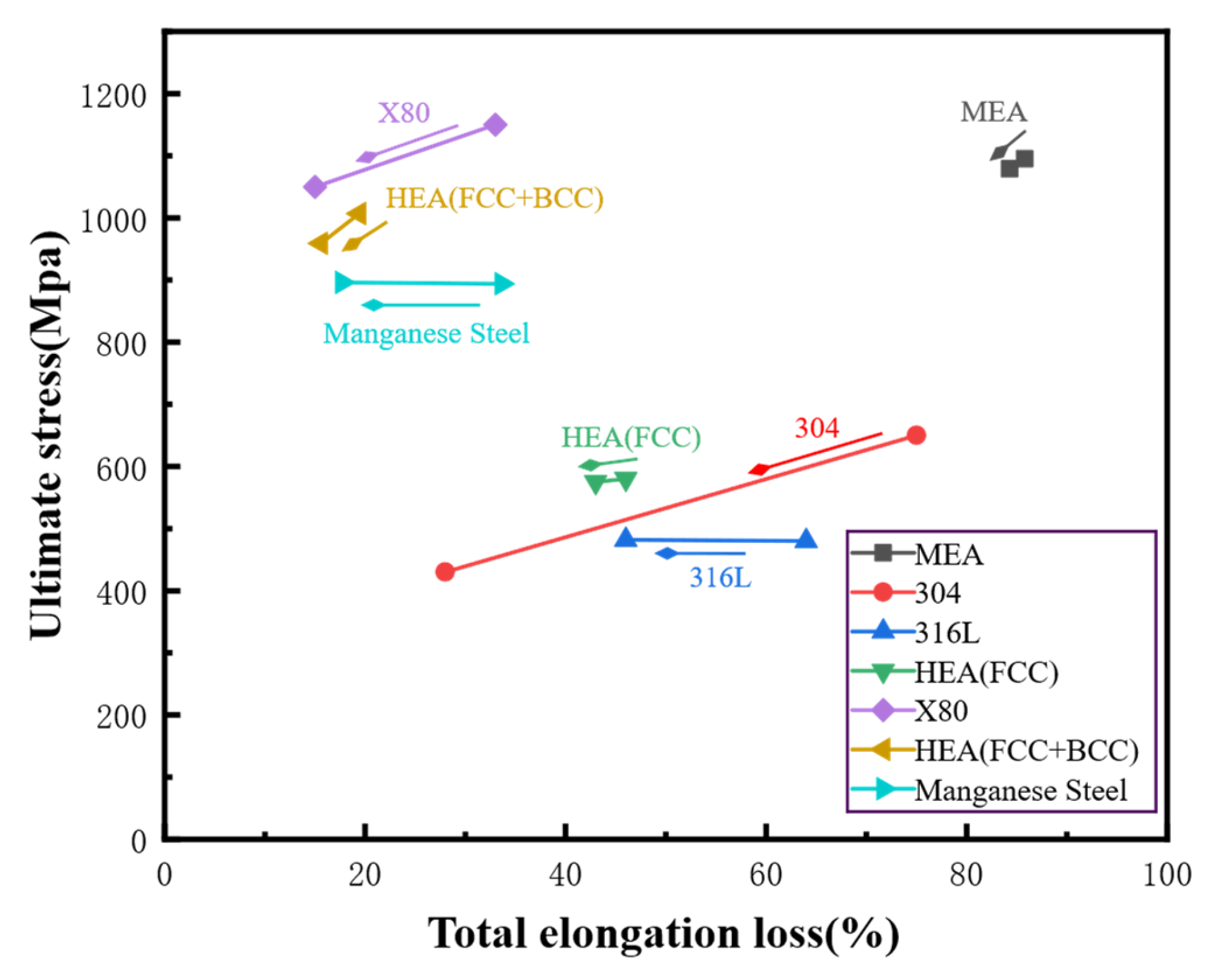
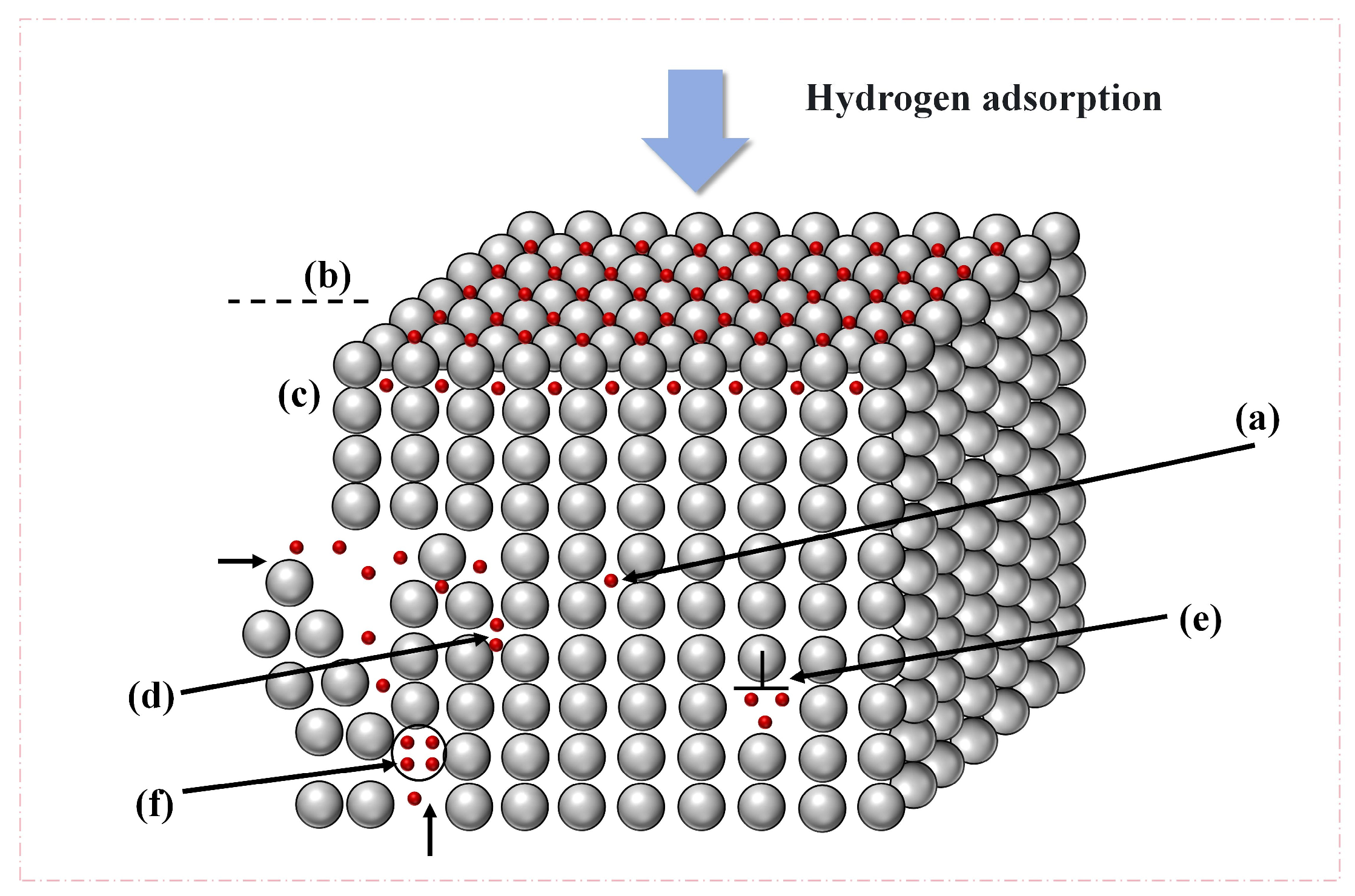
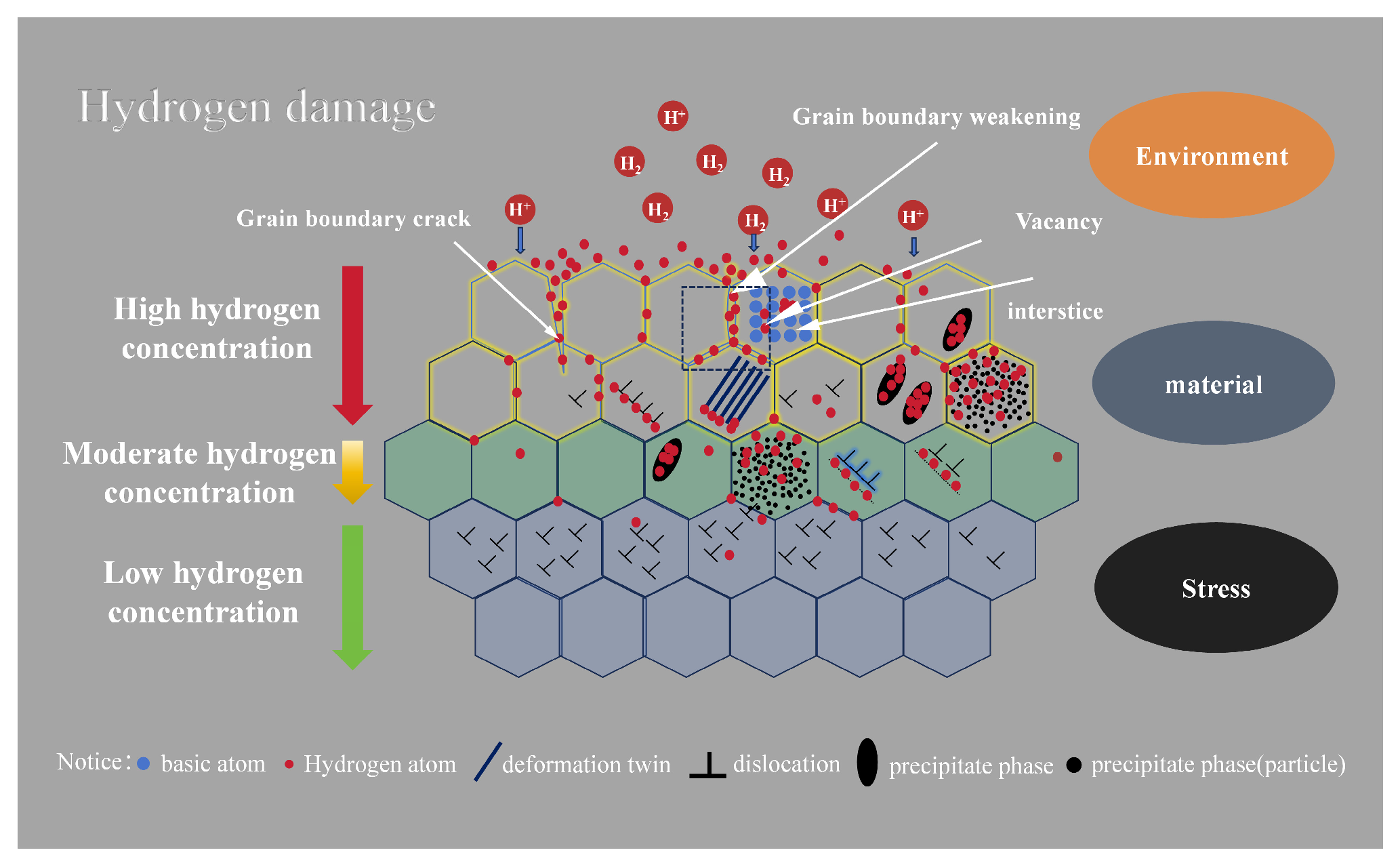
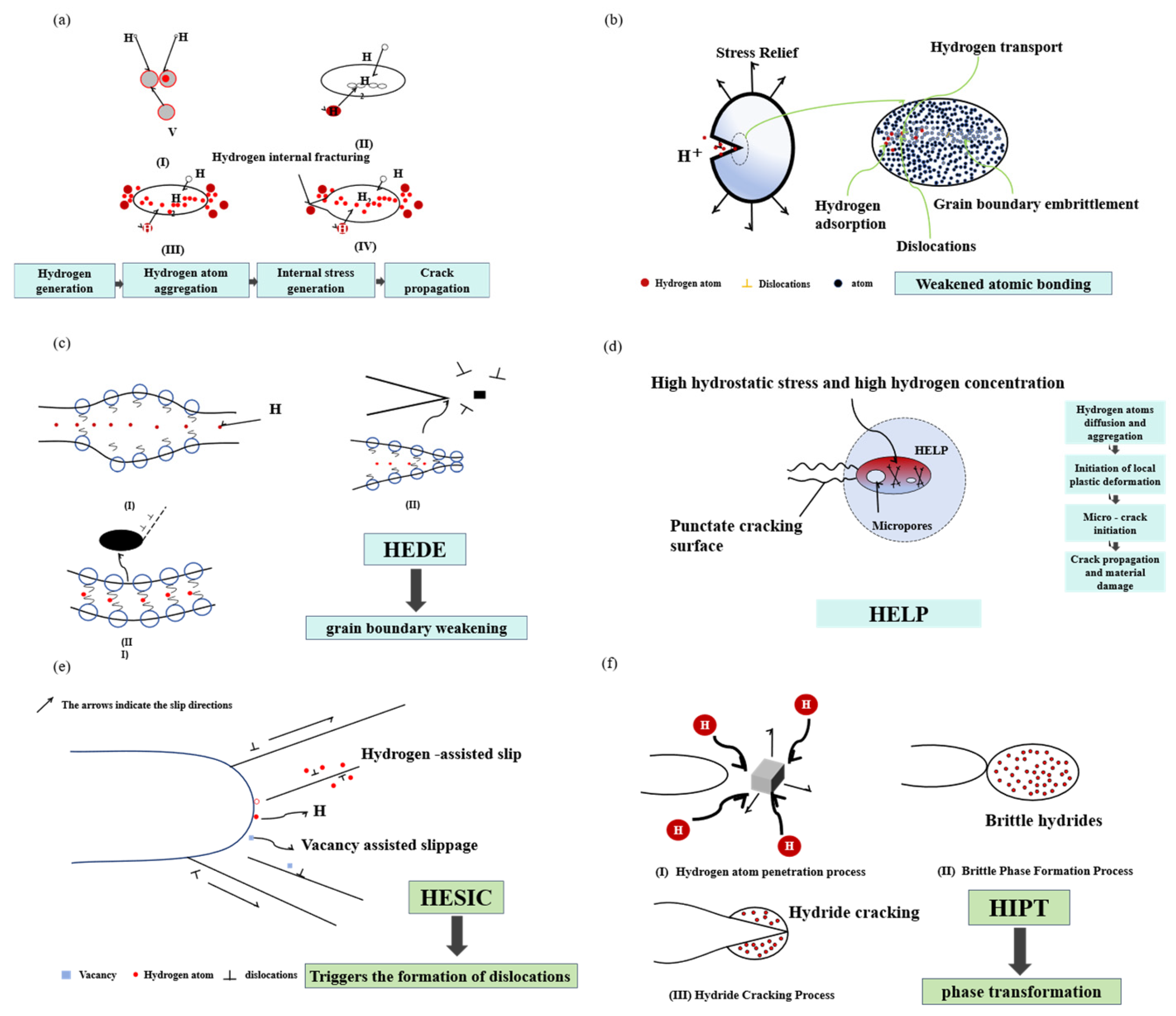
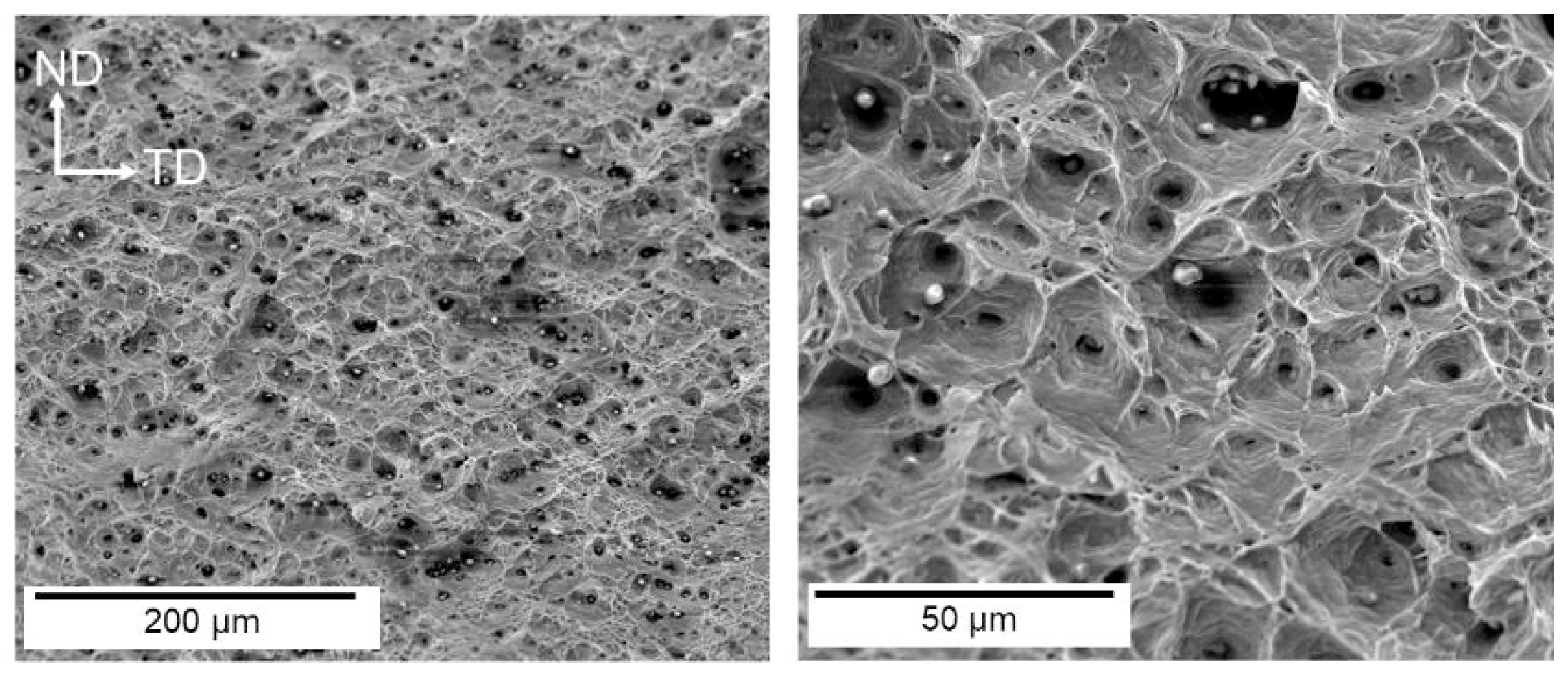
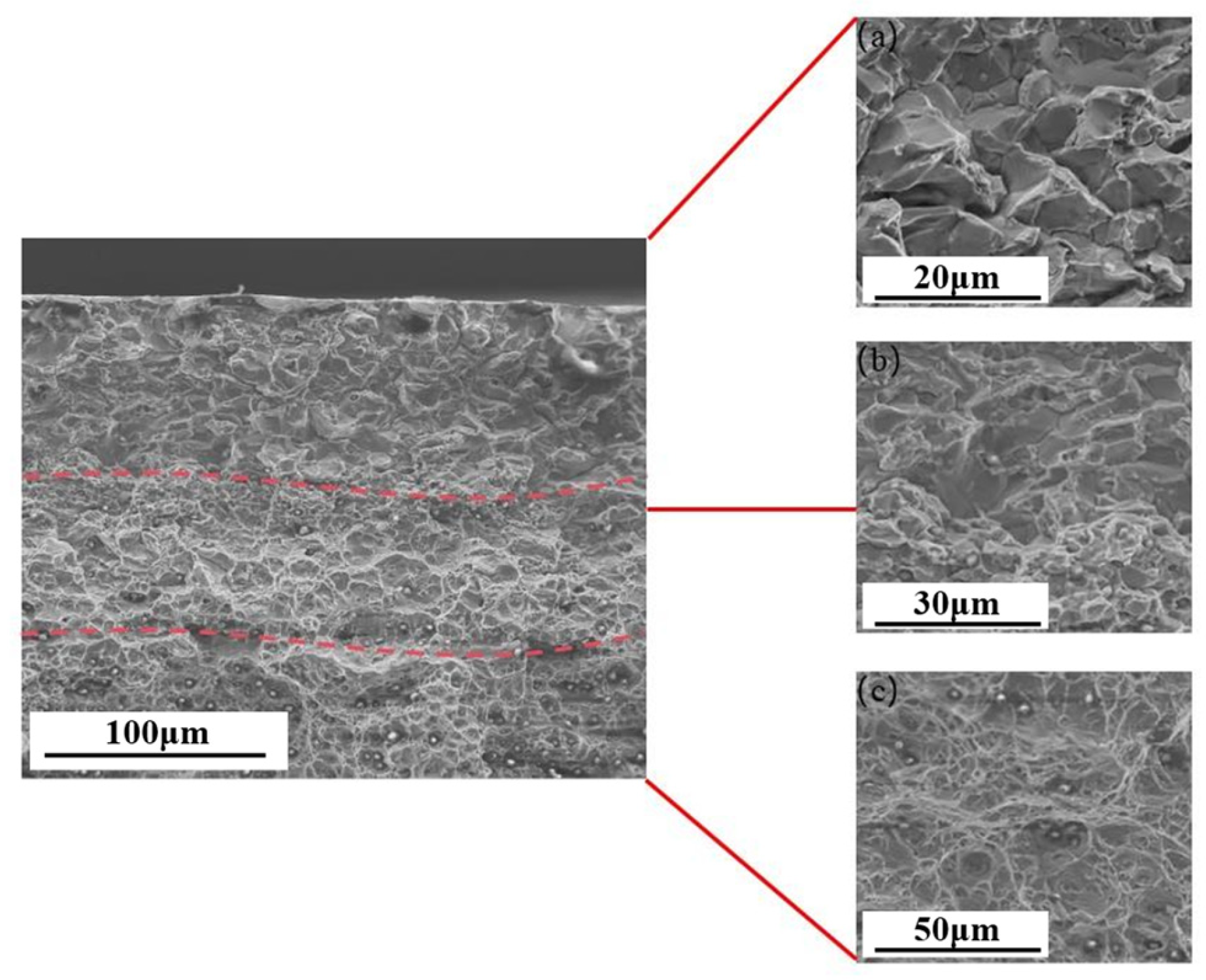
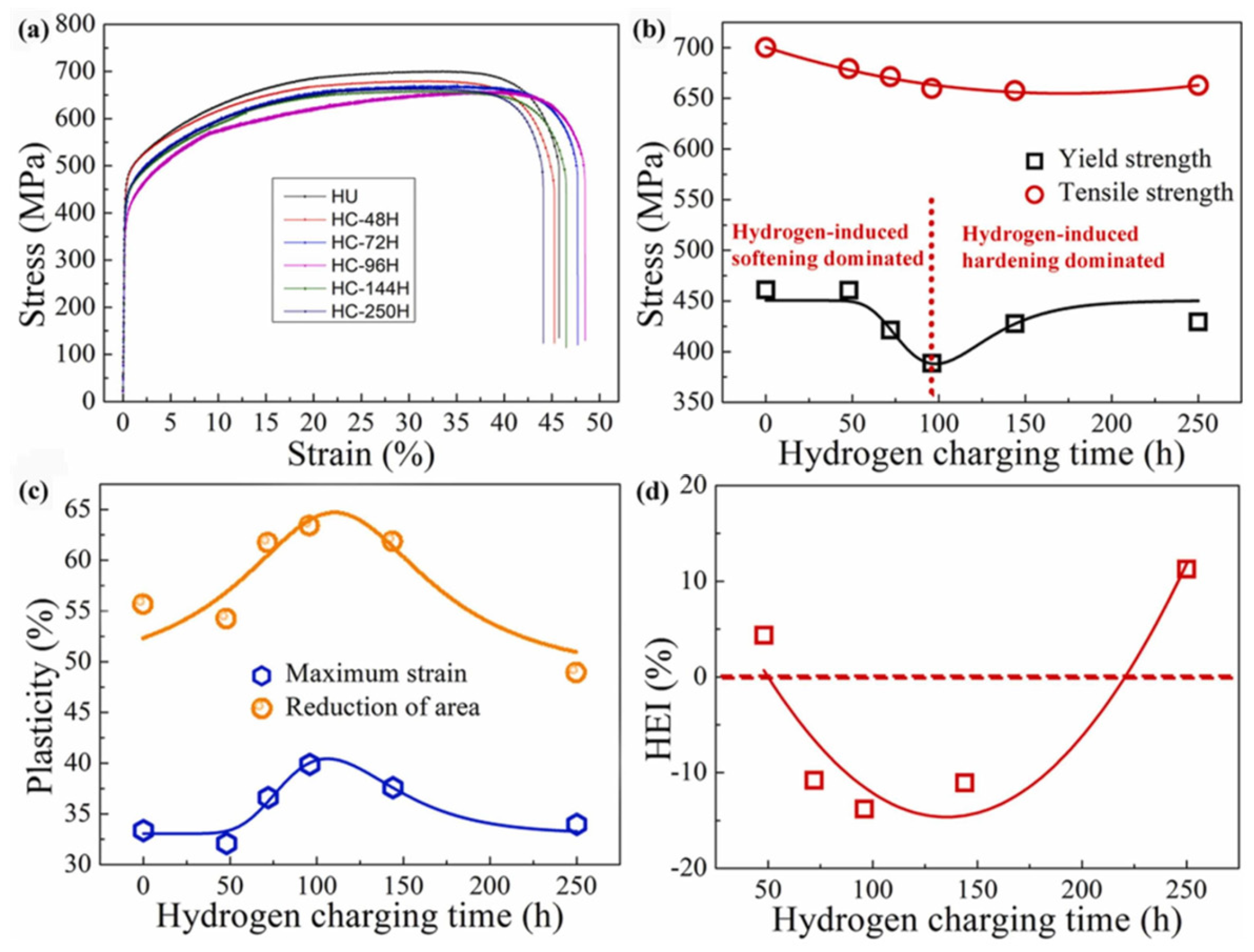
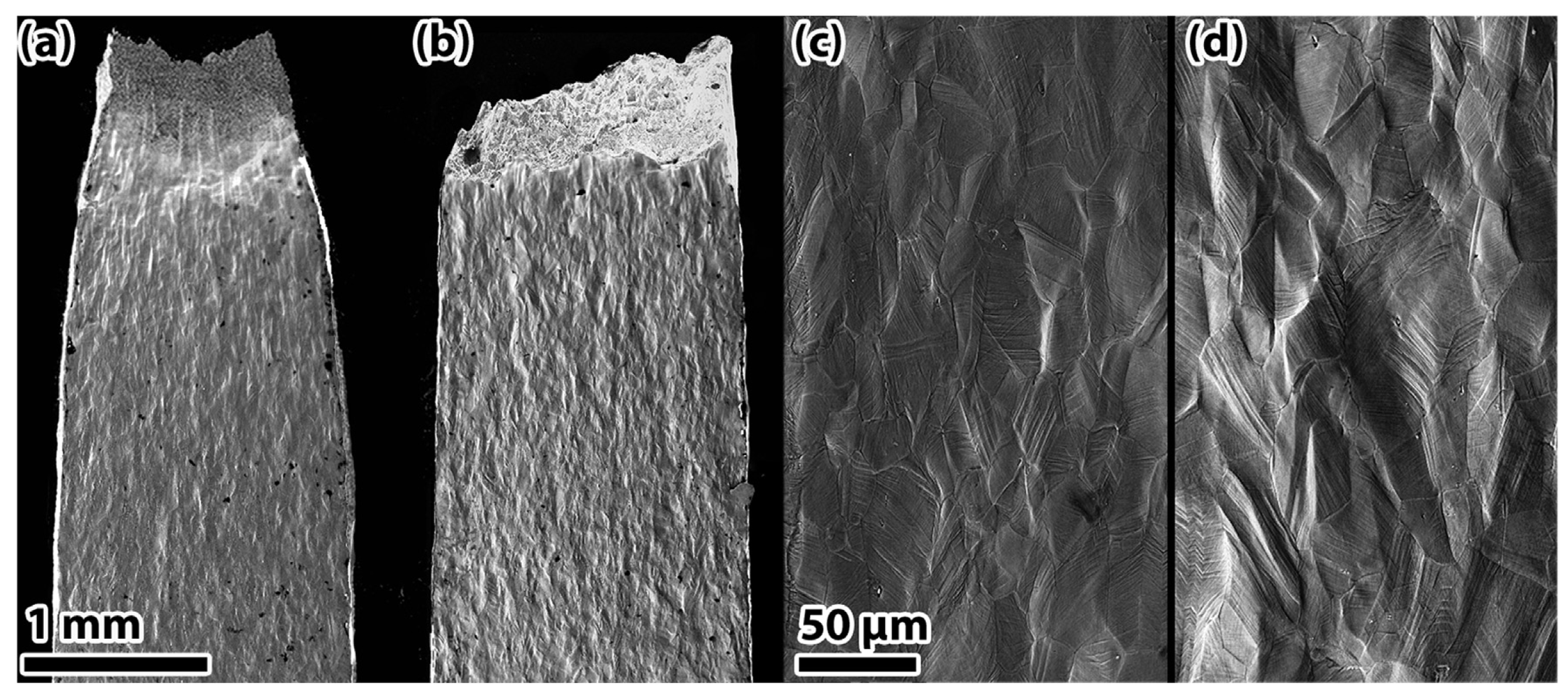



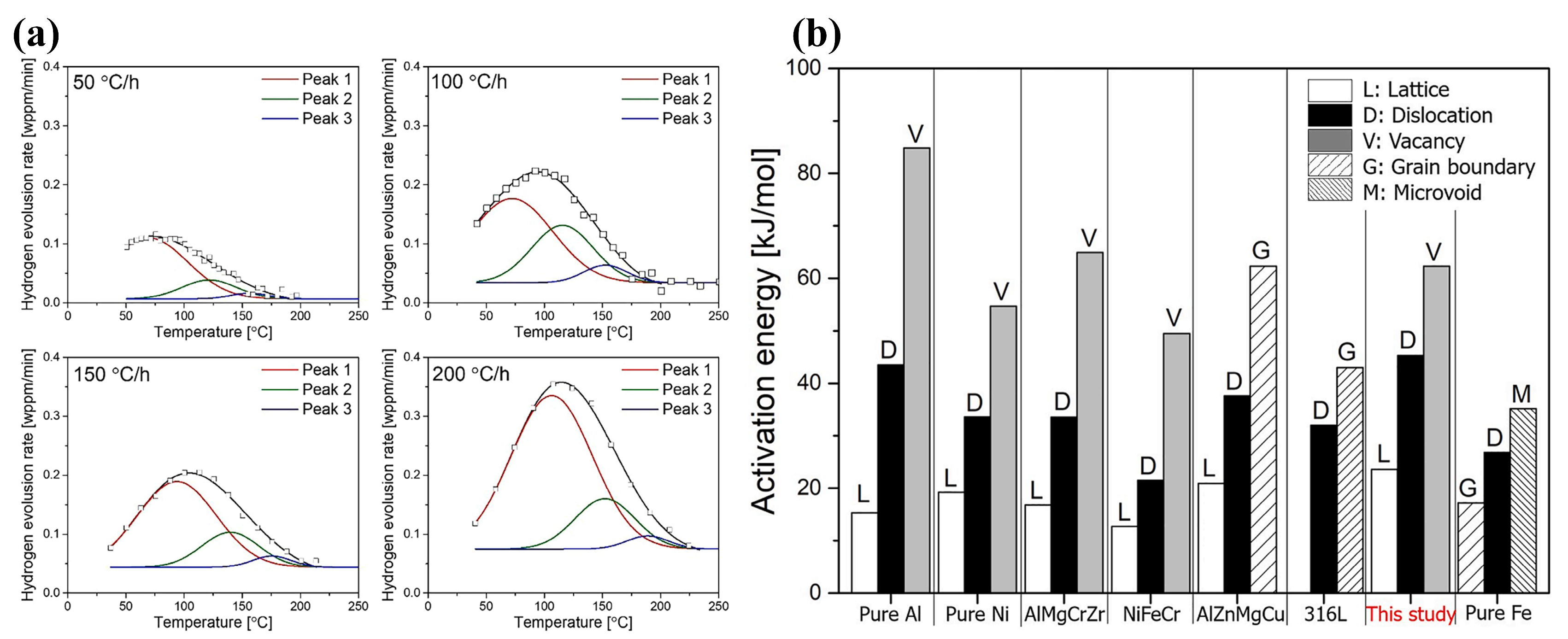
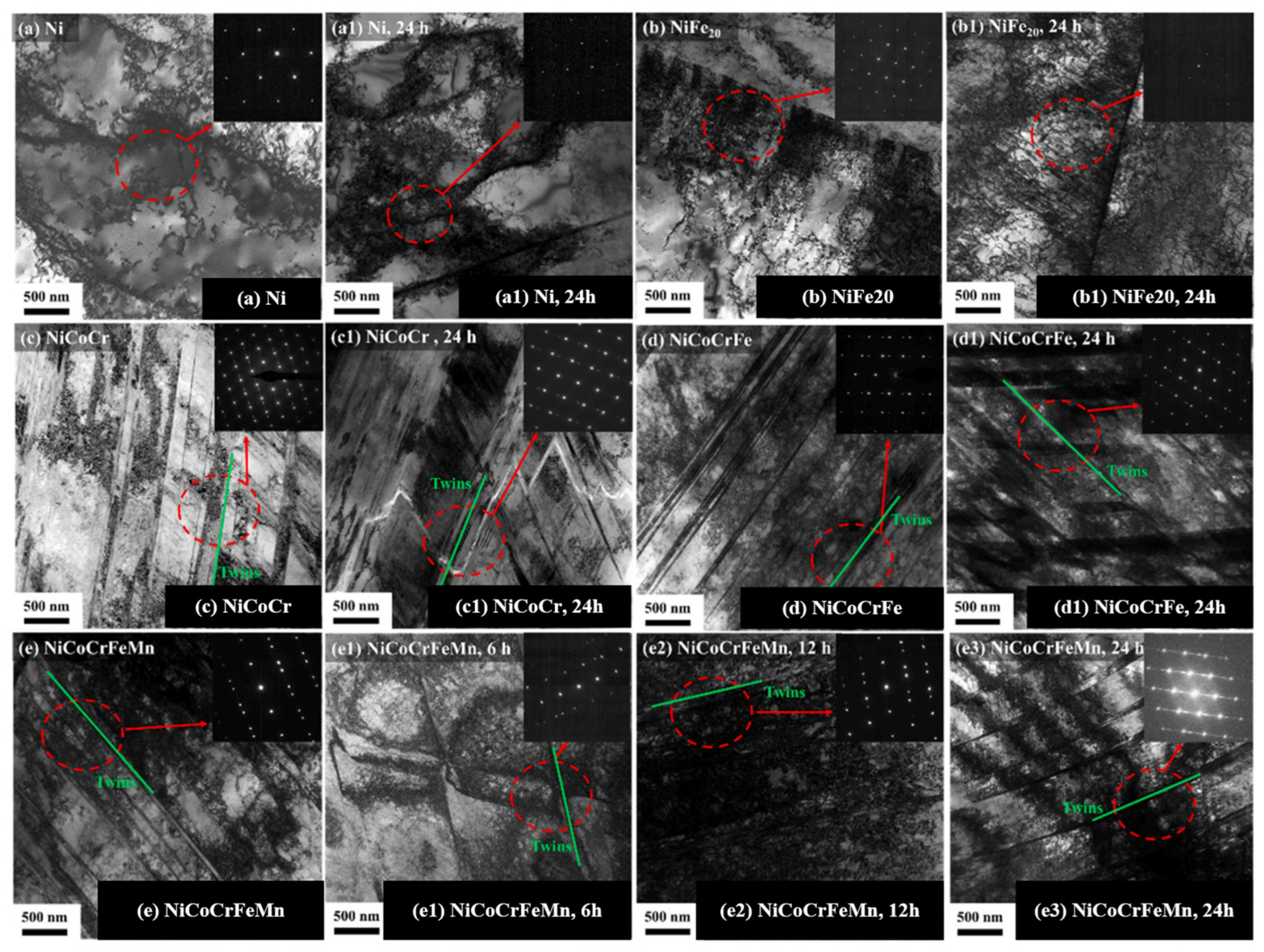
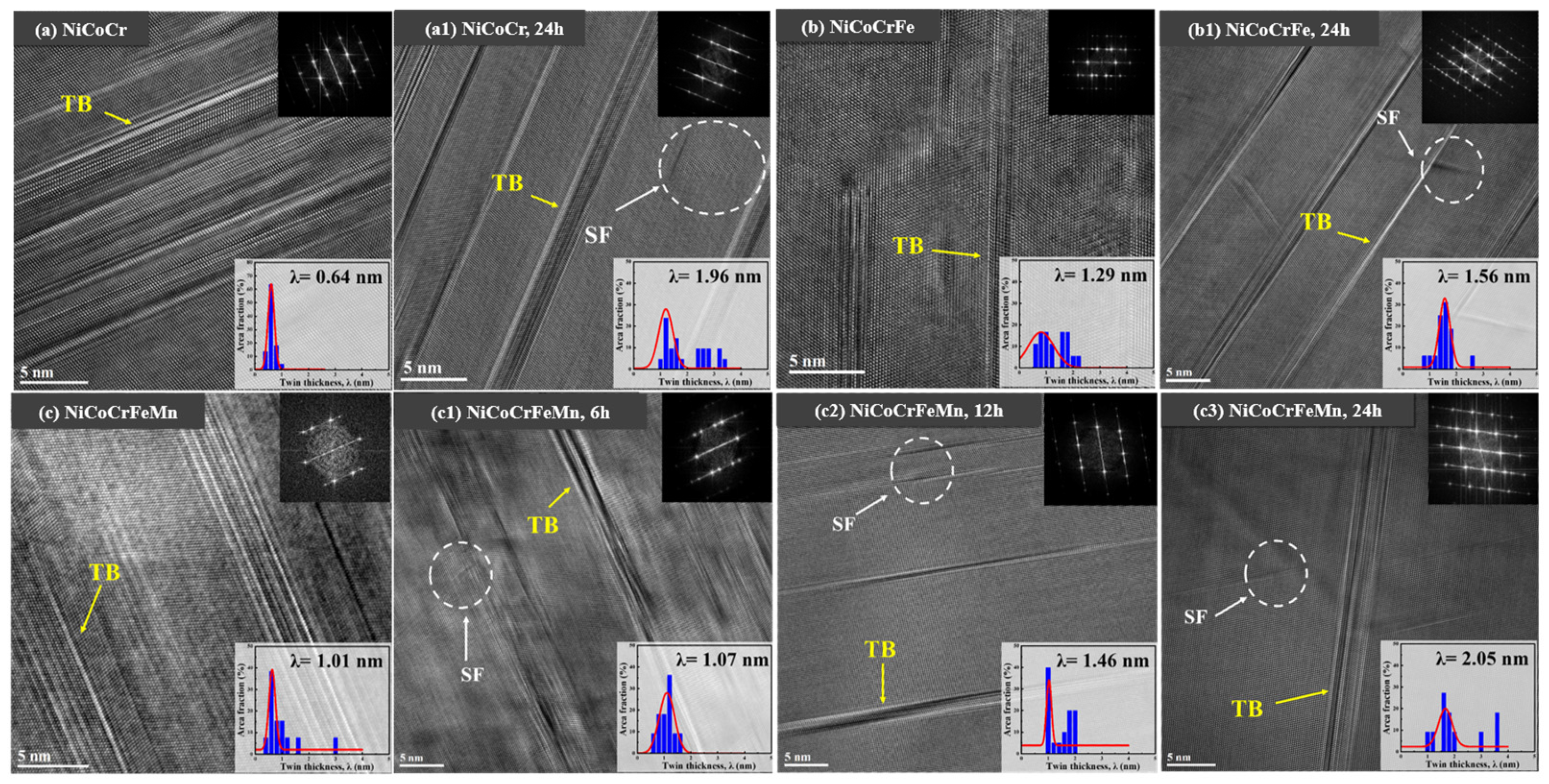

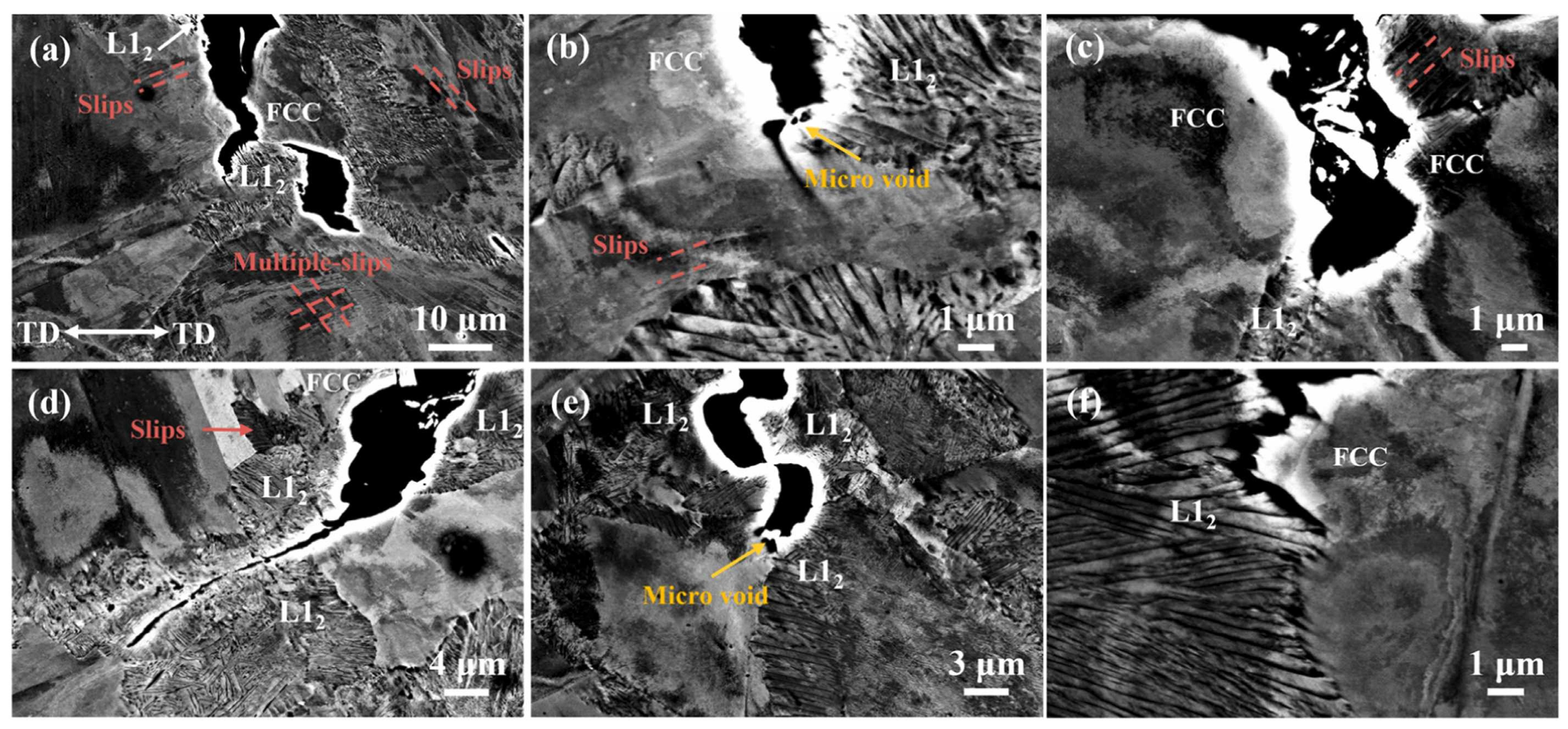
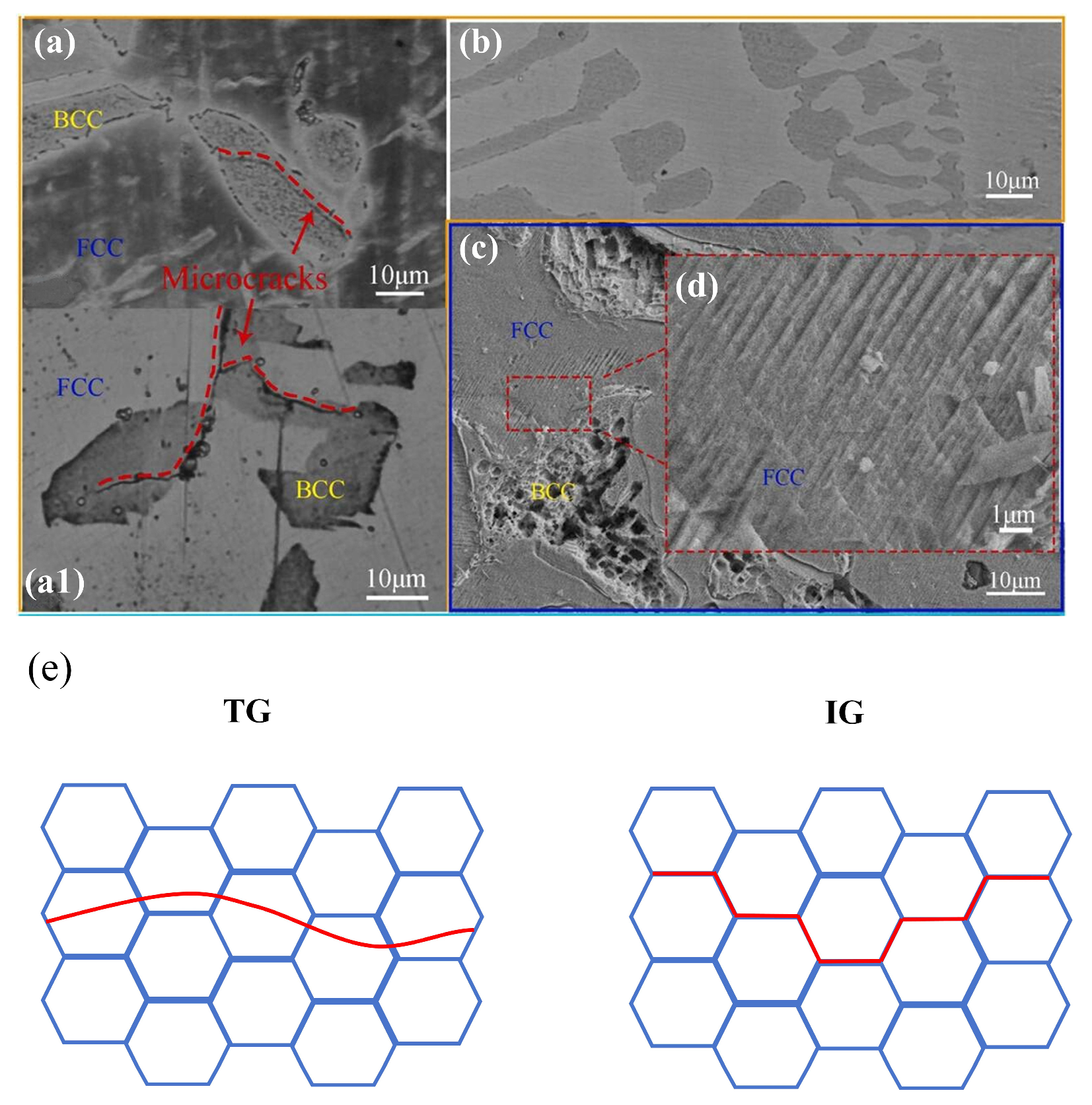

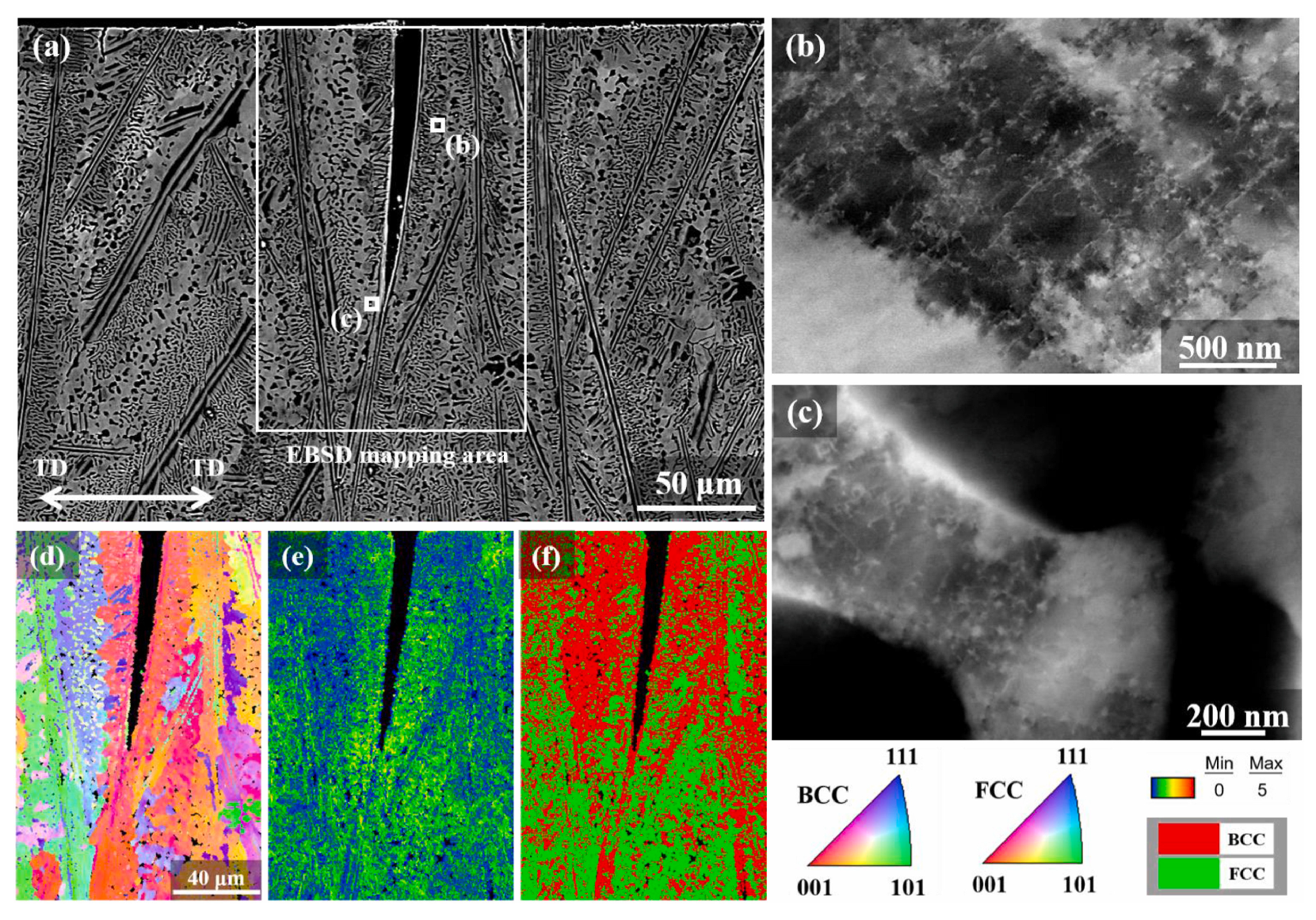
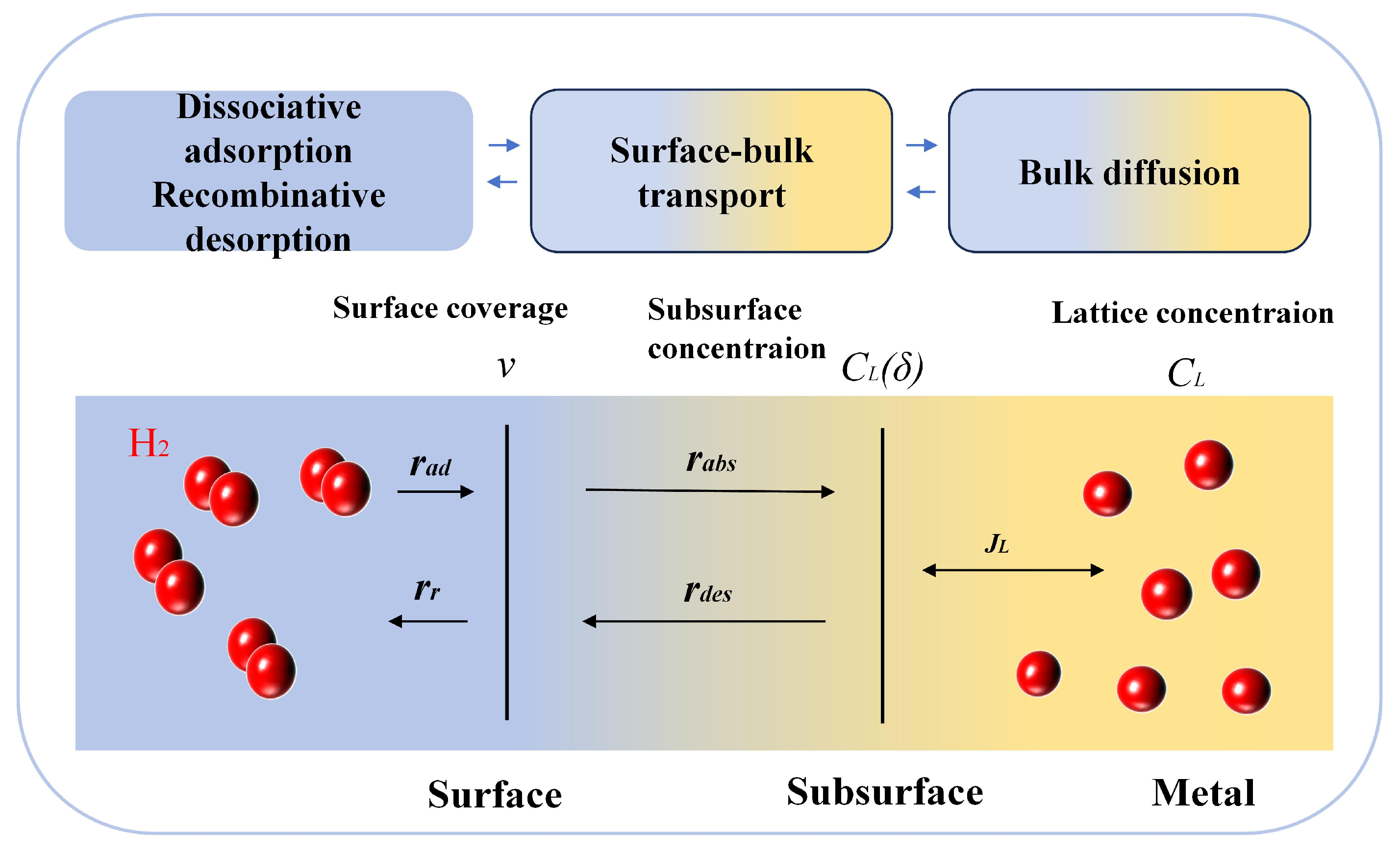

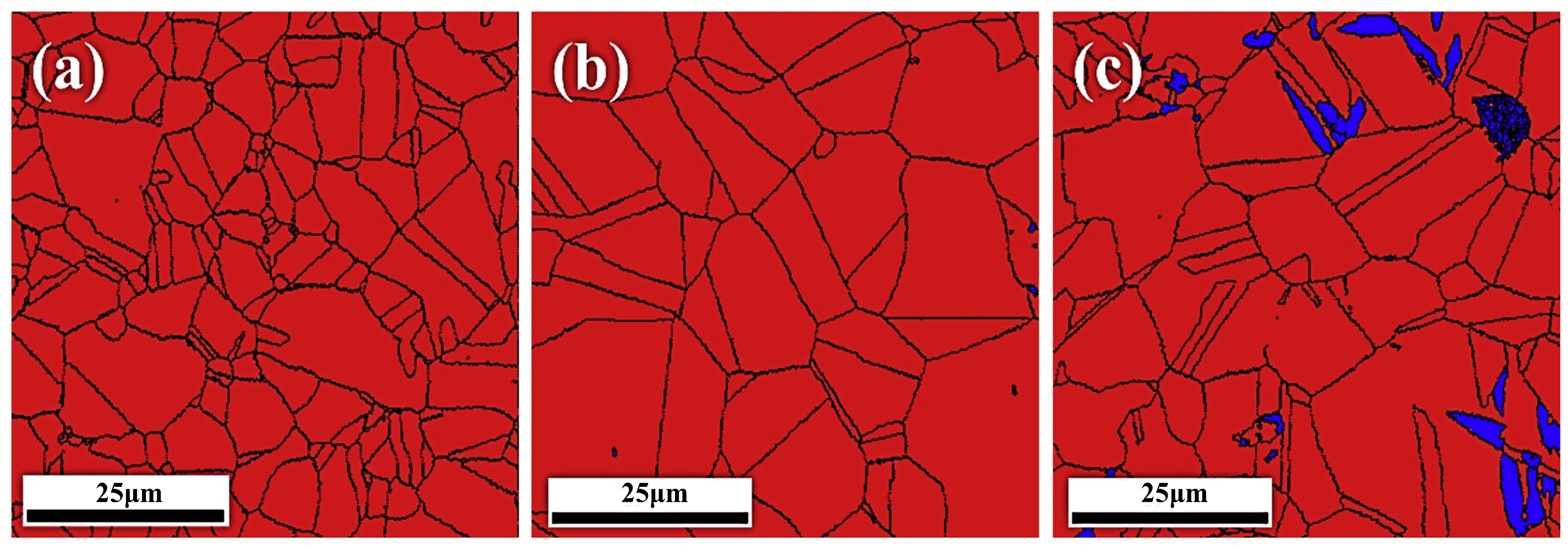
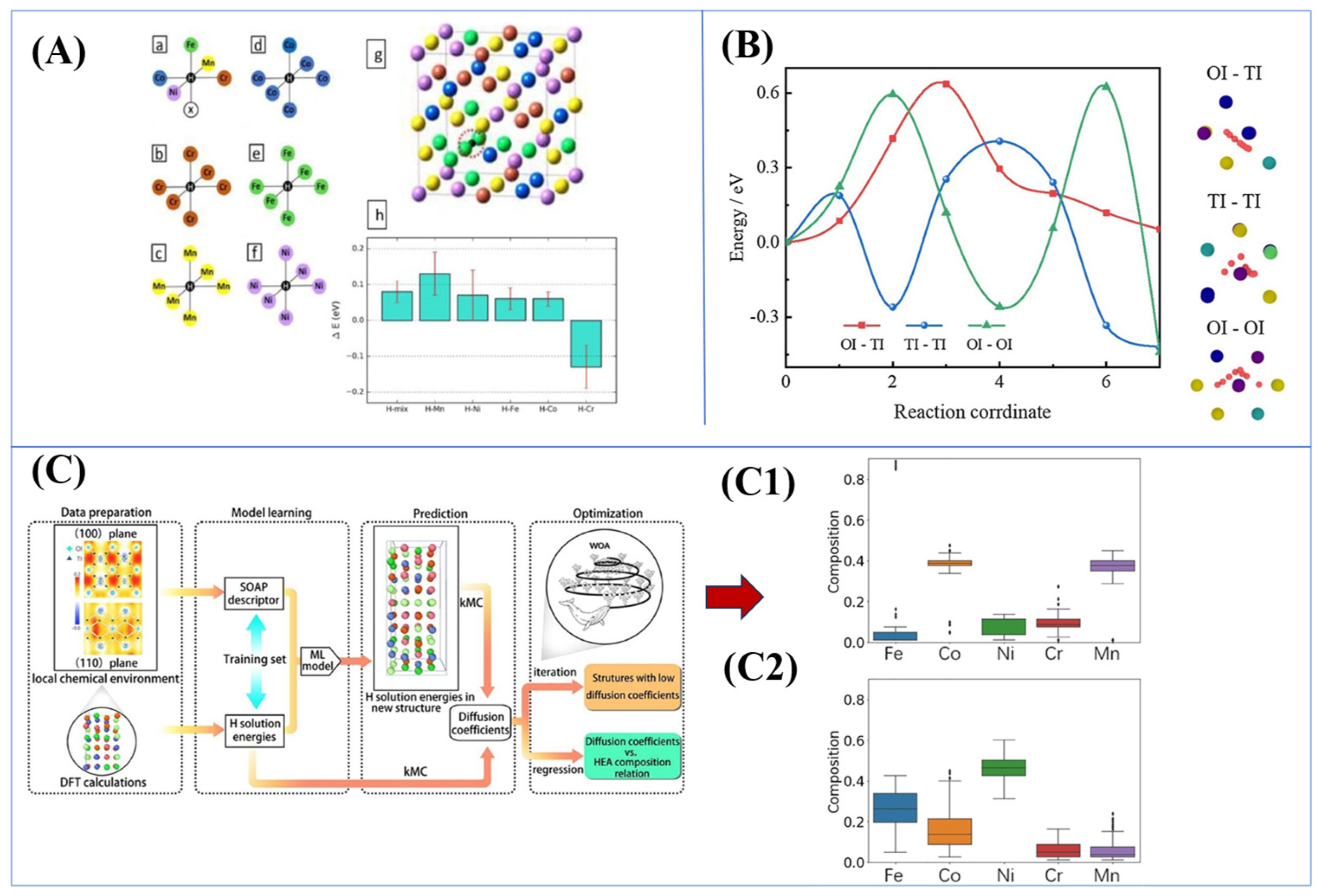
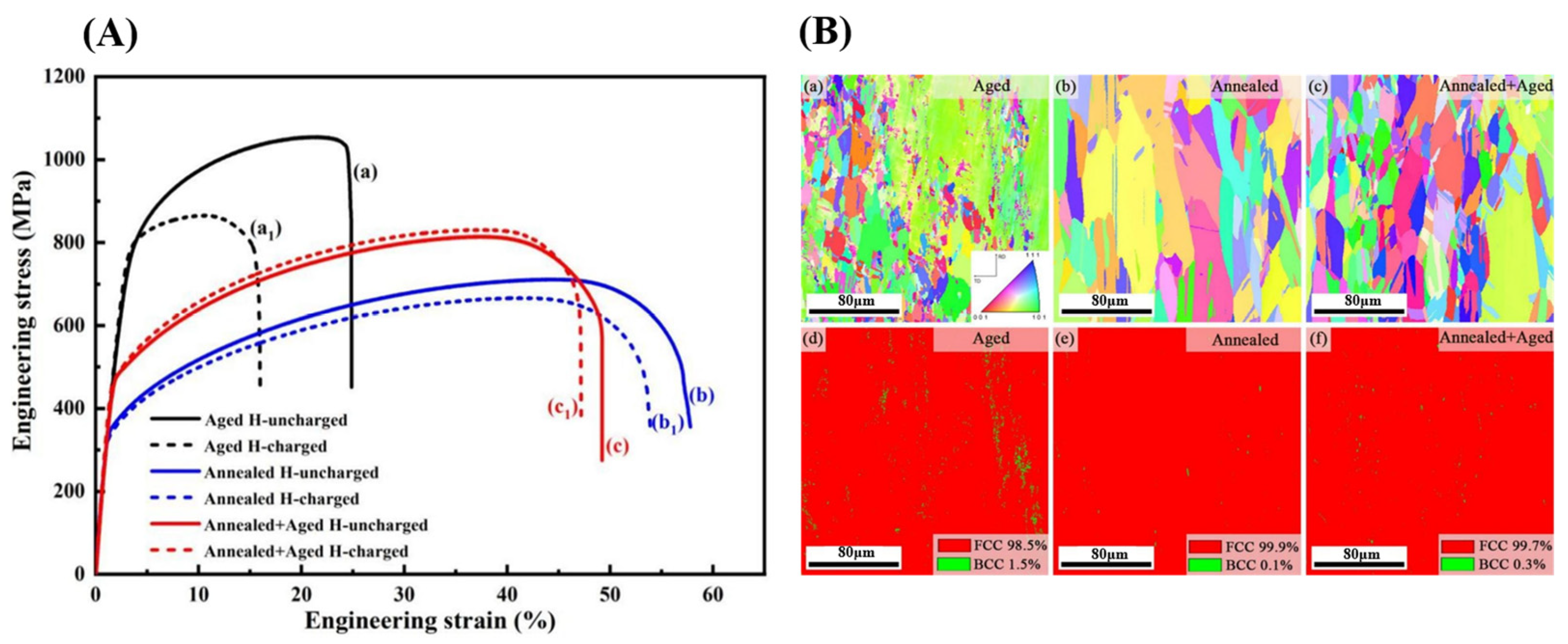
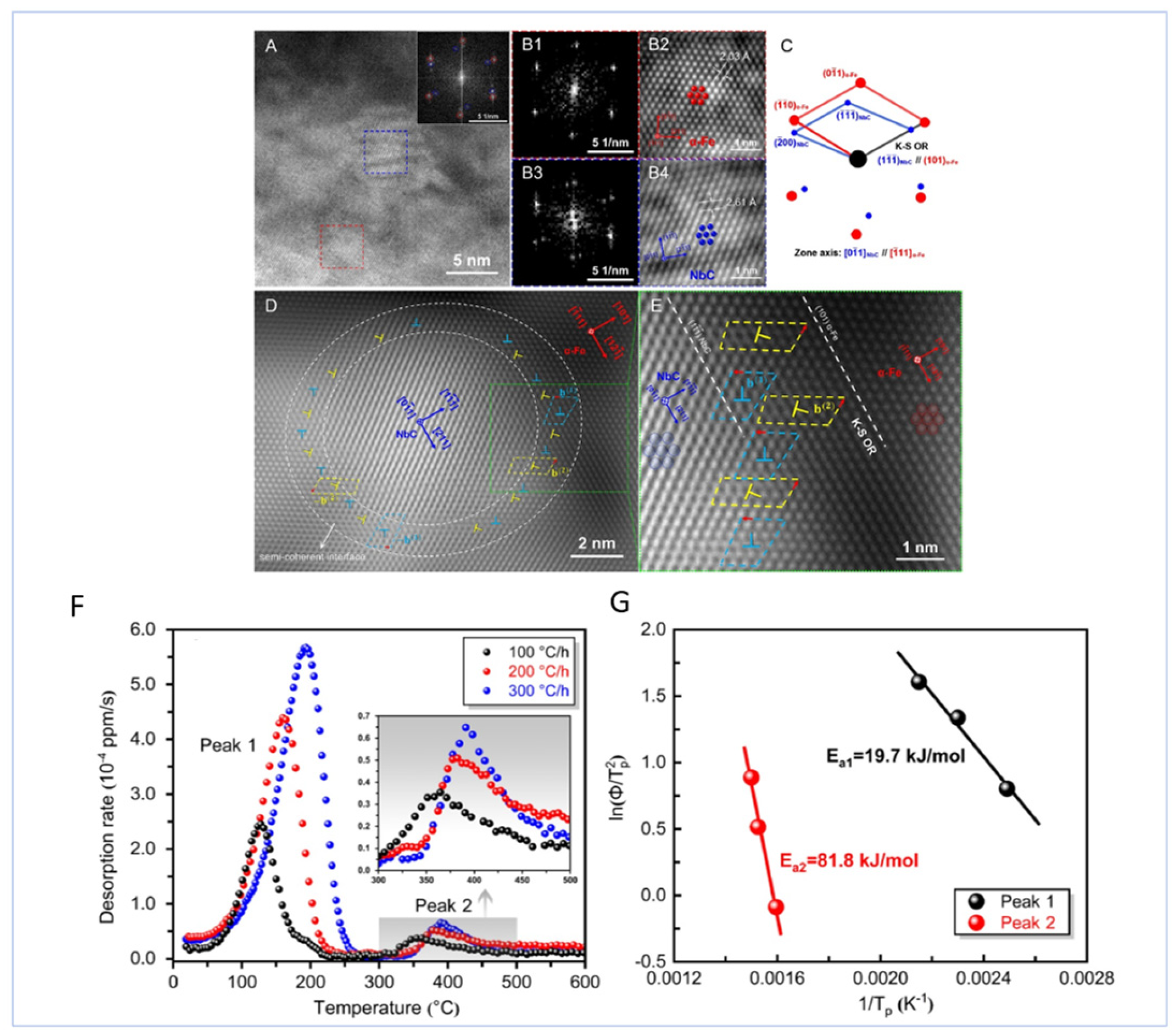
| Material | Deuterium Charged Time (h) | Deuterium Concentration (wt.ppm) | Deuterium Desorption Peak (°C) |
|---|---|---|---|
| Ni | 24 | 115.7 | 353/510 |
| NiFe20 | 24 | 21.21 | 491 |
| NiCoCr | 24 | 82.05 | 485 |
| NiCoCrFe | 24 | 16.29 | 493 |
| NiCoCrFeMn | 6 | 46.51 | 495 |
| NiCoCrFeMn | 12 | 150.88 | 492 |
| NiCoCrFeMn | 24 | 275.35 | 495 |
| Background | 0 | 0.01 | - |
| Specimens | Deff (m2/s) | C0 (mol/m3) |
|---|---|---|
| CoCrFeMnNi | 1.81 × 10−11 | 0.043 |
| SS304 | 0.73 × 10−11 | 0.113 |
| SS316L | 1.31 × 10−11 | 0.048 |
| Material | σb/MPa Un-Charge | σb/MPa (Charge-H) | δ/% (Un-Charge) | δ/% (Charge-H) | Hydrogen Content (wt.ppm) | Crystal Structure | Reference |
|---|---|---|---|---|---|---|---|
| CoCrFeMnNi | 730 | 730 | 58 | 46 | 5.65 | FCC | [102] |
| (NiCoFe)86Al7Ti7 | 1324 | 1351 | 35.5 | 29.6 | - | FCC + L12 | [50] |
| AlCoCrFeNi2.1 | 1007 | 959 | 19.4 | 15.6 | 9.26 | FCC + B2 | [82] |
| Ni50Cr20Co15Al10V5 | 1270 | 1197 | 24.2 | 17 | 26.72 | FCC | [79] |
| FeCoCrNi | 810 | 812 | 38 | 43 | 5.13 | FCC | [103] |
| Co30Cr10Fe10Al18Ni30Mo2 | 1131 | 949.8 | 13.5 | 9 | - | FCC+B2 | [75] |
| Al0.25CoCrFeNi | 578.8 | 622.1 | 63.7 | 70.2 | - | FCC | [104] |
| Fe50Mn30Cr10Co10 | 700 | 739 | 57 | 50 | 2.48 | FCC + HCP | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, X.; Jiang, H.; Lv, Y.; Xie, W.; Lu, S.; Xu, D. Research Progress on the Hydrogen Embrittlement Resistance Performance of High-Entropy Alloys. Materials 2025, 18, 2862. https://doi.org/10.3390/ma18122862
Kong X, Jiang H, Lv Y, Xie W, Lu S, Xu D. Research Progress on the Hydrogen Embrittlement Resistance Performance of High-Entropy Alloys. Materials. 2025; 18(12):2862. https://doi.org/10.3390/ma18122862
Chicago/Turabian StyleKong, Xiao, Hui Jiang, Yuting Lv, Wenlong Xie, Shuoyi Lu, and Dingfeng Xu. 2025. "Research Progress on the Hydrogen Embrittlement Resistance Performance of High-Entropy Alloys" Materials 18, no. 12: 2862. https://doi.org/10.3390/ma18122862
APA StyleKong, X., Jiang, H., Lv, Y., Xie, W., Lu, S., & Xu, D. (2025). Research Progress on the Hydrogen Embrittlement Resistance Performance of High-Entropy Alloys. Materials, 18(12), 2862. https://doi.org/10.3390/ma18122862






