Bioactivity of Glass Carbomer Versus Conventional GICs in Sound Enamel and Dentine: A 12-Month SEM-EDS Study
Abstract
1. Introduction
- Characterise microstructural changes in GICs over 12 months;
- Monitor elemental composition changes in GICs during this period;
- Assess compositional modifications in non-demineralised enamel adjacent to GICs;
- Evaluate chemical changes in non-demineralised dentine near the materials.
2. Materials and Methods
2.1. Study Design
- Ketac Universal Aplicap (3M ESPE, Seefeld, Germany), a conventional glass ionomer cement composed primarily of fluoroaluminosilicate glass powder and a liquid phase containing polyalkenoic acid copolymers and tartaric acid [21];
- Fuji IX GP Fast (GC Corporation, Tokyo, Japan), a high-viscosity conventional GIC containing strontium fluoroaluminosilicate glass and polyacrylic acid [22];
- Equia Forte Fil (GC Corporation, Tokyo, Japan), a resin-coated high-viscosity conventional GIC combining strontium fluoroaluminosilicate glass with a resin coating for improved wear resistance [23];
- Glass Carbomer GlassFill (GCP Dental, Vianen, The Netherlands), an enhanced formulation incorporating fluoroaluminosilicate glass, hydroxyapatite nanoparticles and bioactive glass [13].
2.2. Sample Preparation
2.3. Sample Size and Statistical Power
2.4. FE-SEM/EDS Methodology
- E (enamel distant from the material);
- D (dentine distant from the material);
- M (restorative material).
- EM (enamel adjacent to the material);
- DM (dentine adjacent to the material).
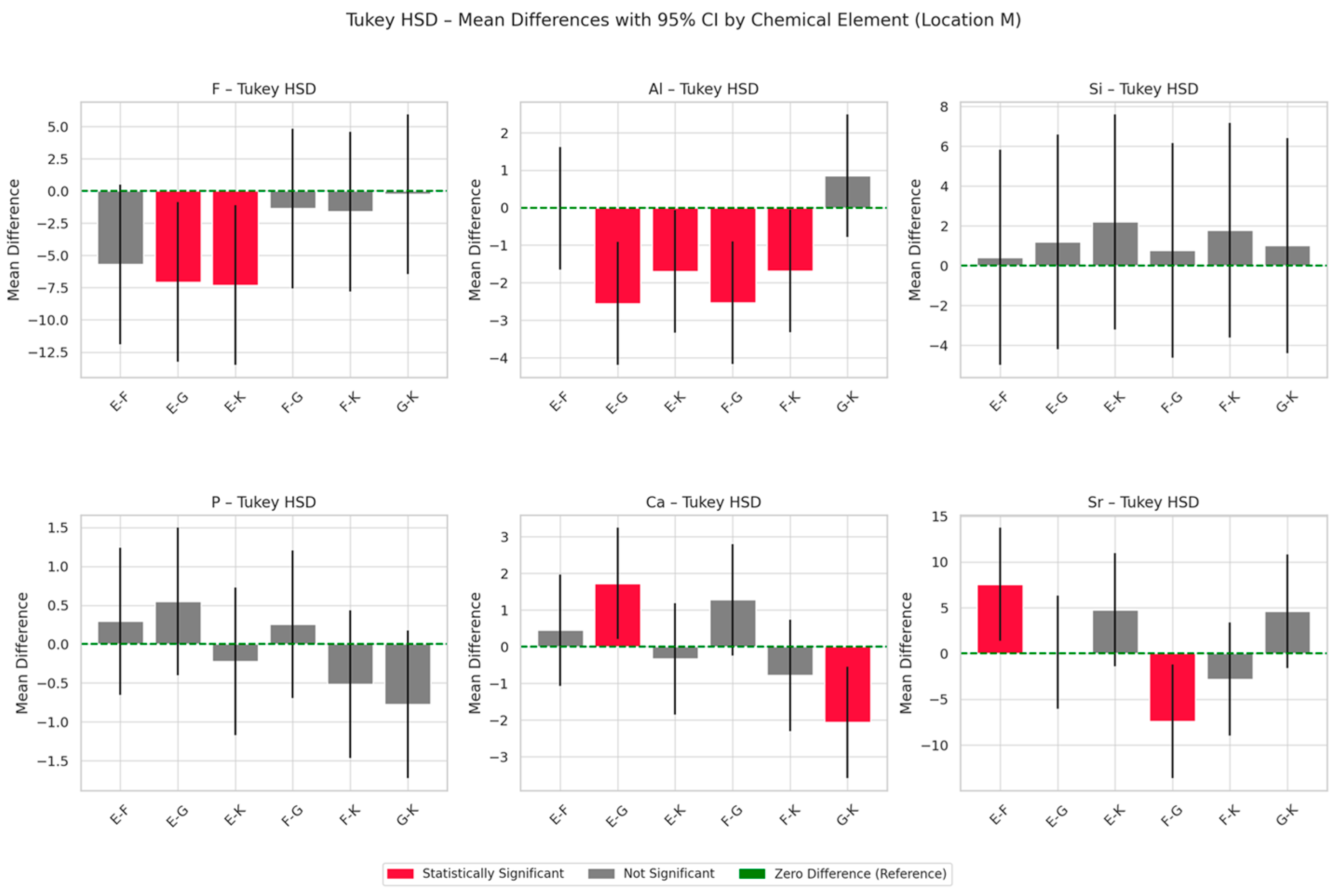
2.5. Statistical Analysis
3. Results
3.1. FE-SEM Analysis of Material Microstructure
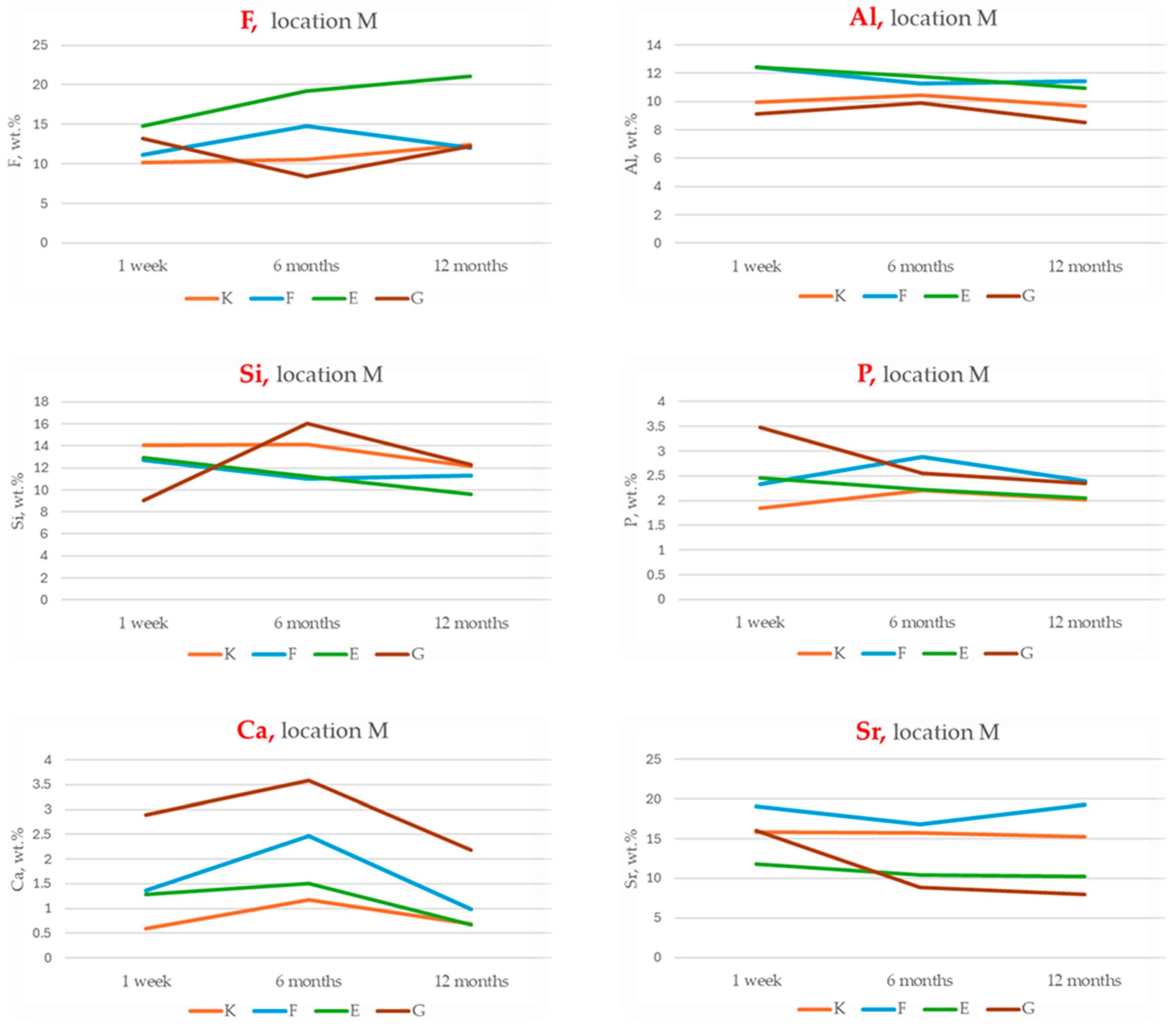
3.2. EDS Analysis of Chemical Composition Changes Within the Materials
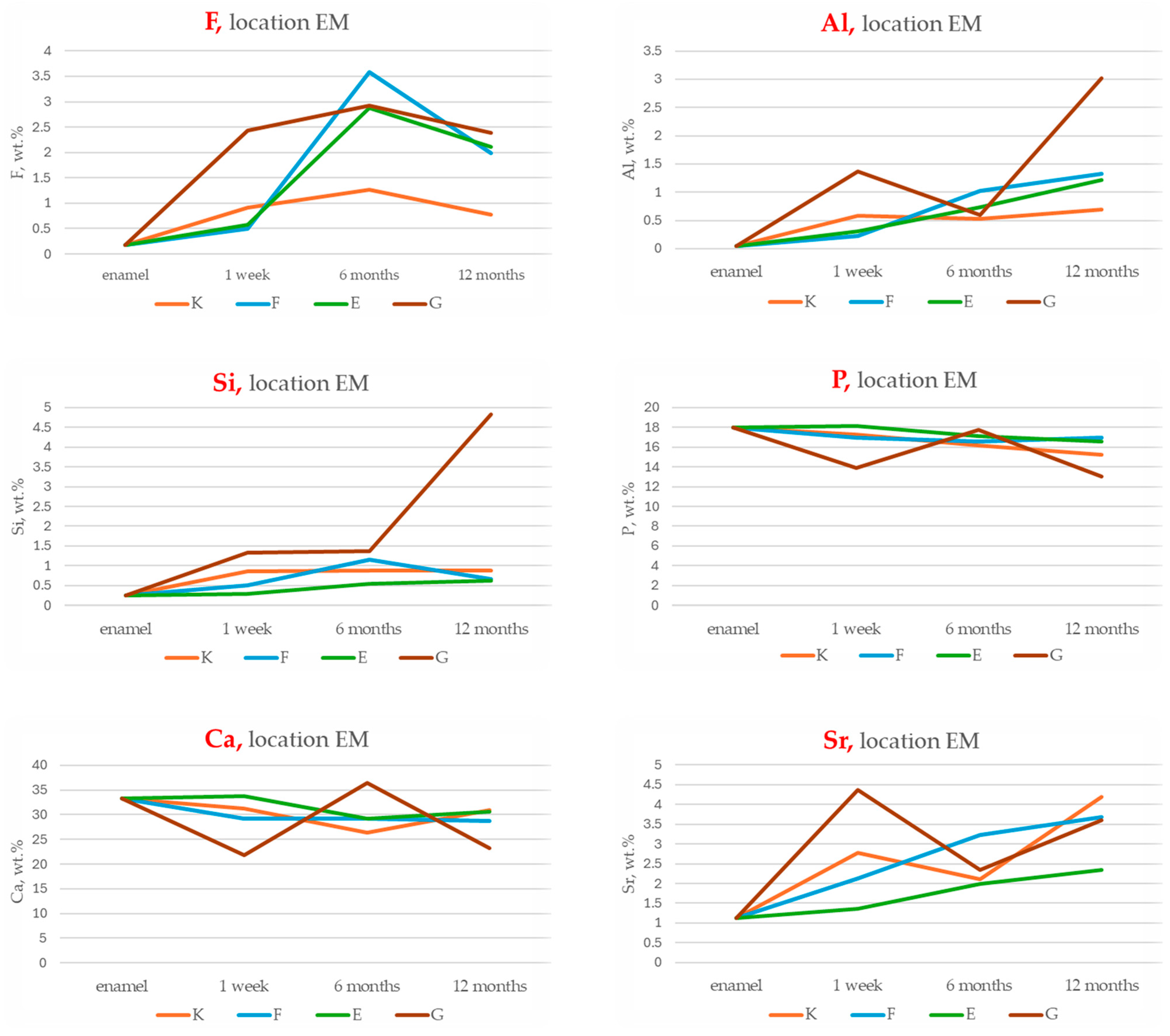
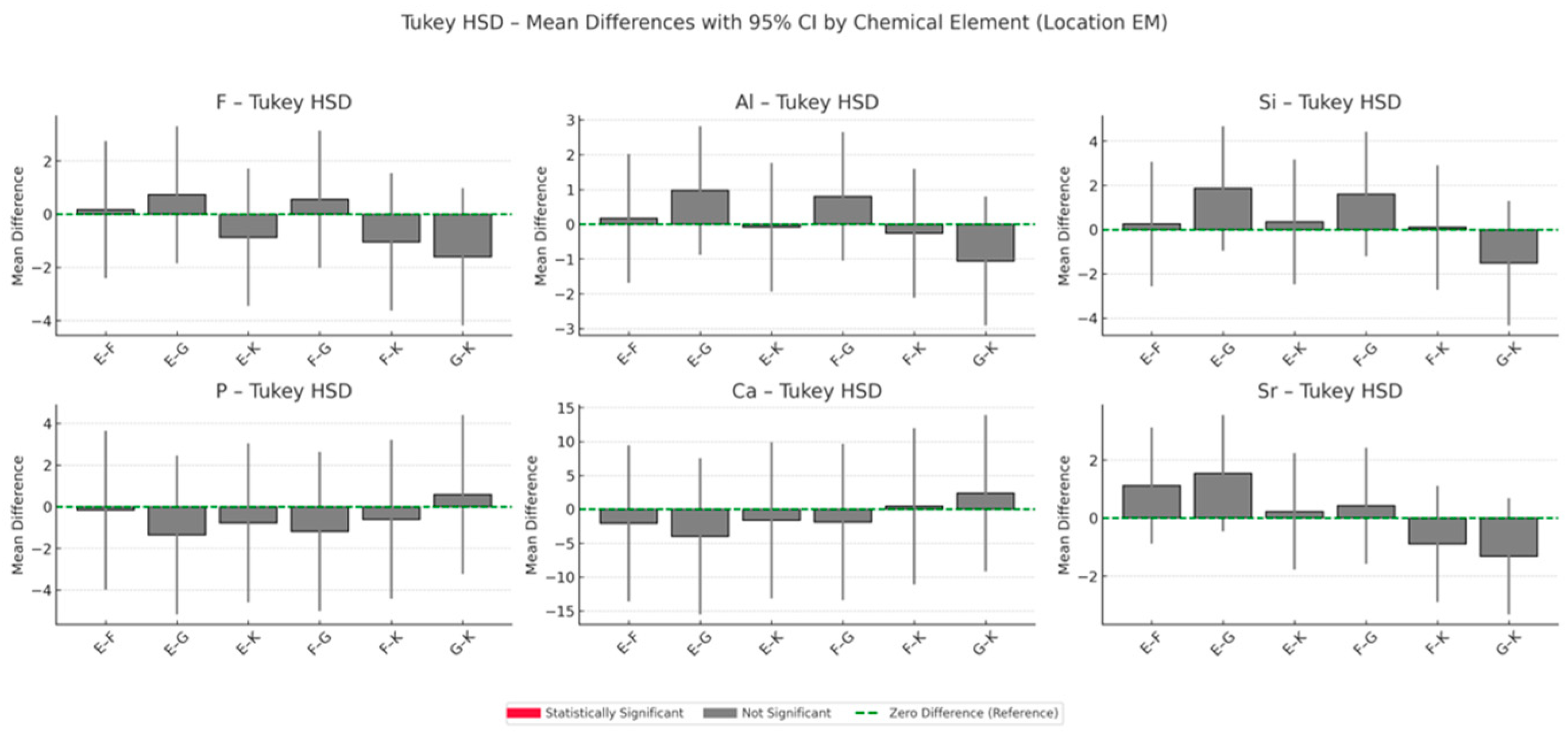
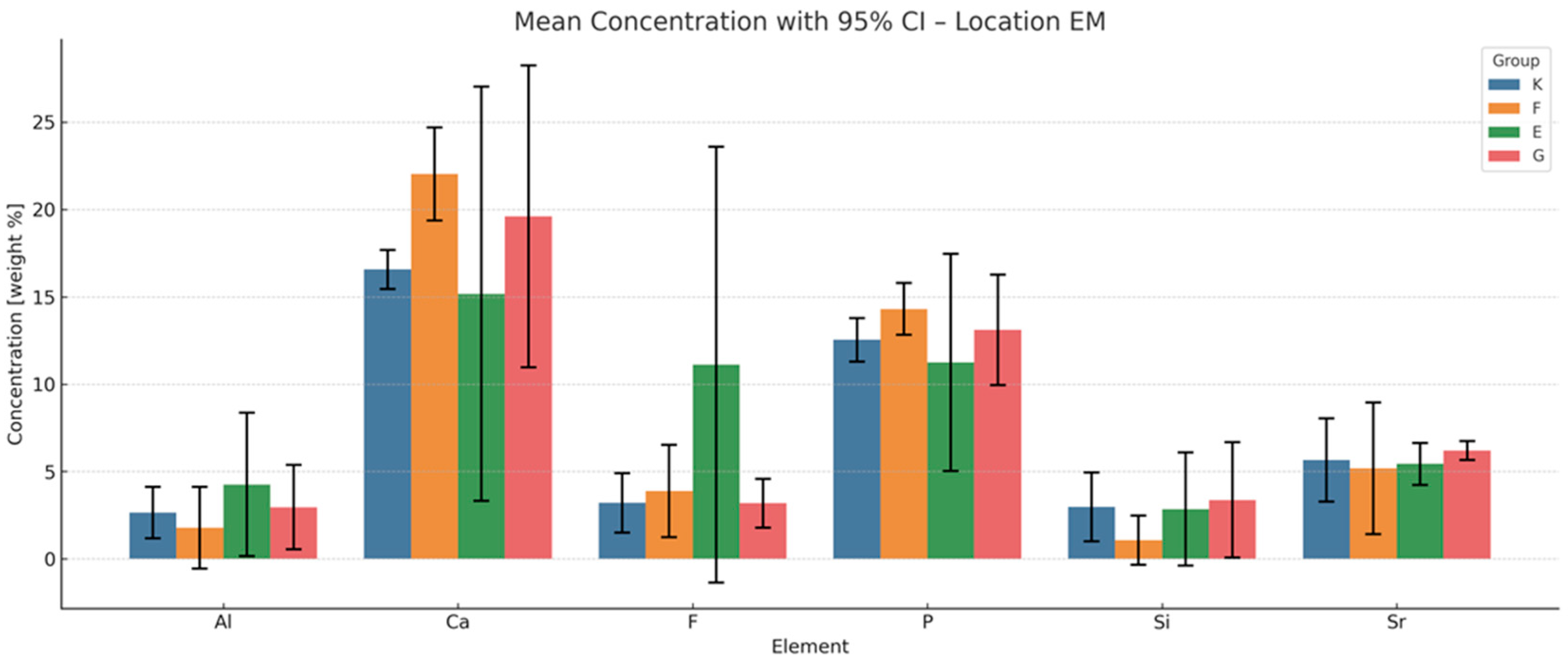
3.3. EDS Analysis of Elemental Composition Changes in the Enamel Adjacent to the Material
Cumulative Indices of Material–Enamel Interaction
- Total Ion Exchange with Enamel (peak concentrations of F, Al, Si, Sr):
- This index reflects the maximum ion exchange between the material and enamel, incorporating peak values for fluoride (F), aluminium (Al), silicon (Si) and strontium (Sr).
- Ranking: G > F > E > K;
- Long-Term Ion Exchange (12-month concentrations of F, Al, Si, Sr):
- This index evaluates ion exchange capacity at the 12-month time point, indicating each material’s ability to sustain ion release over time.
- Ranking: G > F > K > E;
- Total Remineralisation Potential (F + Sr only):
- Based on the highest measured concentrations of fluoride and strontium, this index highlights the theoretical potential for enamel remineralisation.
- Ranking: G > F > K > E;
- Long-Term Remineralisation Activity (12-month concentrations of F + Sr):
- This index focuses on the sustained release of fluoride and strontium after 12 months, reflecting long-term bioactivity.
- Ranking: G > F > K > E.
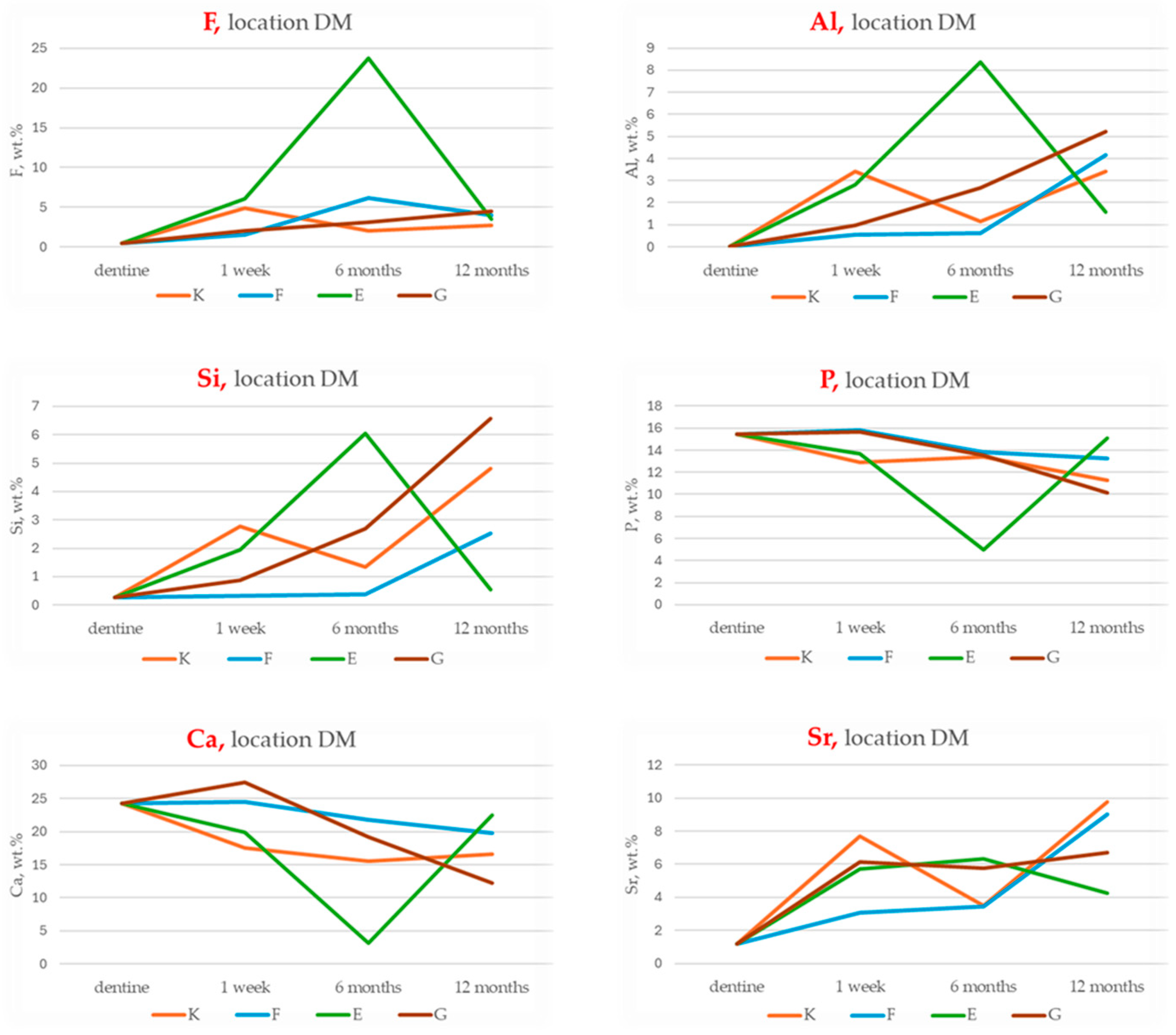
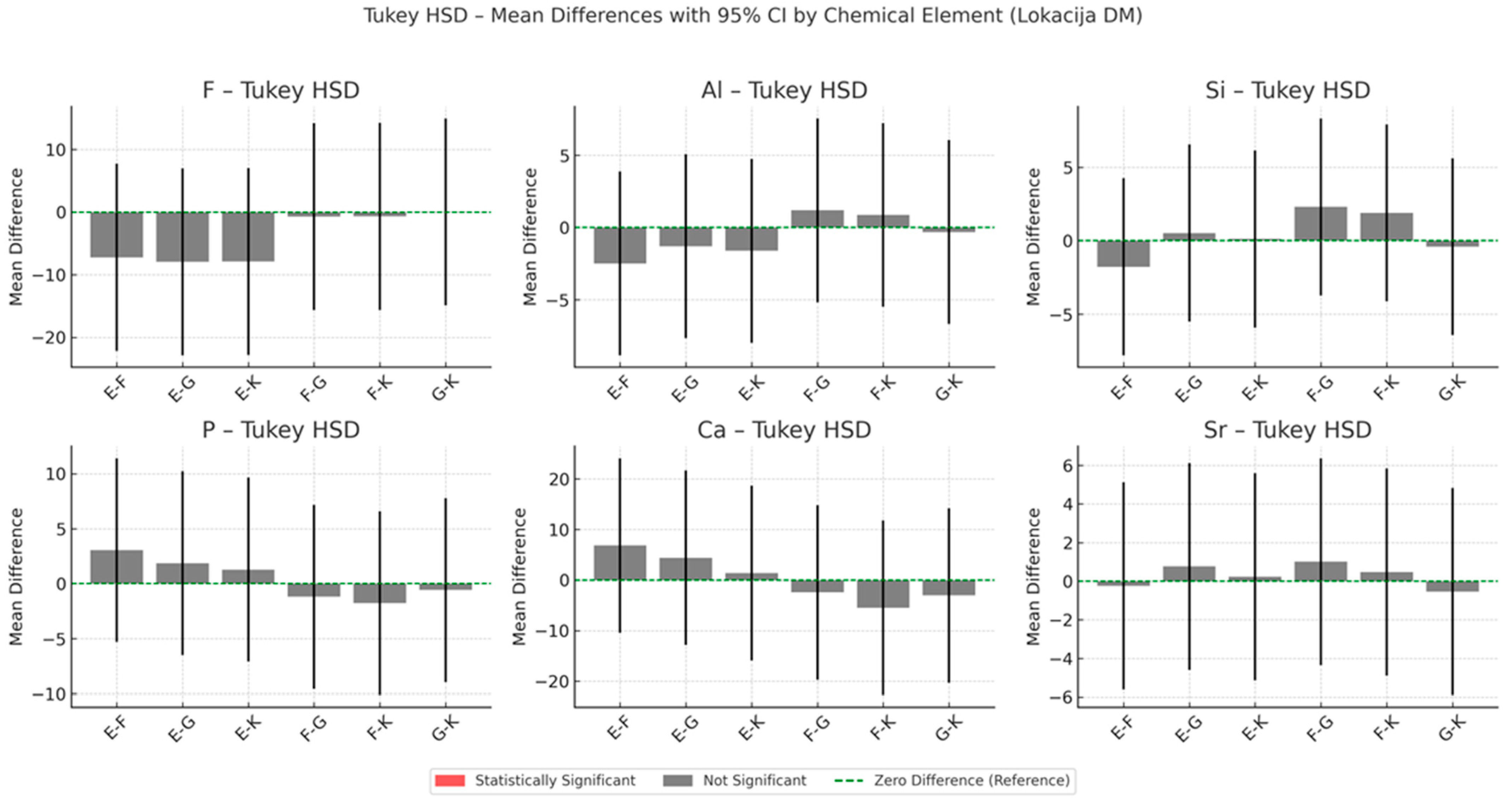
3.4. EDS Analysis of Chemical Composition Changes in the Dentine Adjacent to the Material
Cumulative Indices of Material–Dentine Interaction
- Total Ion Exchange with Dentine (maximum cumulative concentrations of F, Al, Si, Sr):
- Long-Term Ion Exchange with Dentine (12-month cumulative values of F, Al, Si, Sr):
- Total Remineralisation Potential (Sum of peak F and Sr concentrations):
- Long-Term Remineralisation Activity (12-month F and Sr values):
3.5. Comparative Ion Incorporation in Enamel and Dentine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| ART | Atraumatic Restorative Treatment |
| CI | Confidence Interval |
| CT | Computed Tomography |
| DM | Dentine adjacent to Material |
| E | Equia Forte |
| E1, E6, E12 | Equia Forte at 1 week, 6 months and 12 months respectively |
| EDS | Energy Dispersive X-ray Spectroscopy |
| EM | Enamel adjacent to Material |
| F | Fuji IX |
| F1, F6, F12 | Fuji IX at 1 week, 6 months and 12 months, respectively |
| FE-SEM | Field-Emission Scanning Electron Microscopy |
| G | Glass Carbomer |
| G1, G6, G12 | Glass Carbomer at 1 week, 6 months and 12 months, respectively |
| GIC(s) | Glass Ionomer Cement(s) |
| HA | Hydroxyapatite |
| K | Ketac Universal |
| K1, K6, K12 | Ketac Universal at 1 week, 6 months and 12 months, respectively |
| MR | Mineralised Region |
| NaOCl | Sodium Hypochlorite |
| SD | Standard Deviation |
| SEM | Scanning Electron Microscopy |
| wt.% | Weight Percent |
References
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Can glass polyalkenoate (glass-ionomer) dental cements be considered bioactive? A review. Heliyon 2024, 10, e25239. [Google Scholar] [CrossRef]
- Singer, L.; Bourauel, C. Herbalism and glass-based materials in dentistry: Review of the current state of the art. J. Mater. Sci. Mater. Med. 2023, 34, 60. [Google Scholar] [CrossRef]
- Ge, K.X.; Lam, W.Y.-H.; Chu, C.H.; Yu, O.Y. Updates on the clinical application of glass ionomer cement in restorative and preventive dentistry. J. Dent. Sci. 2024, 19 (Suppl. 1), S1–S9. [Google Scholar] [CrossRef]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental luting cements: An updated comprehensive review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive glass applications: A literature review of human clinical trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Zandi Karimi, A.; Rezabeigi, E.; Drew, R.A.L. Aluminium-free glass ionomer cements containing 45S5 Bioglass® and its bioglass-ceramic. J. Mater. Sci. Mater. Med. 2021, 32, 76. [Google Scholar] [CrossRef]
- Grau-Benítez, M.; Silvestre, F.J.; Pascual, A.; Albero, A.; Silvestre-Rangil, J. In vivo study of the behaviour of glass ionomer restorations in patients with special needs. Med. Oral Patol. Oral Cir. Bucal 2024, 29, e559–e567. [Google Scholar] [CrossRef]
- Moheet, I.A.; Luddin, N.; Rahman, I.A.; Masudi, S.M.; Kannan, T.P.; Ghani, N.R.N.A. Analysis of ionic-exchange of selected elements between novel nano-hydroxyapatite-silica added glass ionomer cement and natural teeth. Polymers 2021, 13, 3504. [Google Scholar] [CrossRef]
- Kim, H.J.; Bae, H.E.; Lee, J.E.; Park, I.S.; Kim, H.G.; Kwon, J.; Kim, D.-S. Effects of bioactive glass incorporation into glass ionomer cement on demineralised dentin. Sci. Rep. 2021, 11, 7016. [Google Scholar] [CrossRef]
- Morales-Valenzuela, A.A.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Garcia-Contreras, R.; Hegazy-Hassan, W.; Toral-Rizo, V.H.; Salmerón-Valdés, E.N. Enhancement of fluoride release in glass ionomer cements modified with titanium dioxide nanoparticles. Medicine 2022, 101, e31434. [Google Scholar] [CrossRef]
- Tuygunov, N.; Khairunnisa, Z.; Yahya, N.A.; Aziz, A.A.; Zakaria, M.N.; Israilova, N.A.; Cahyanto, A. Bioactivity and remineralisation potential of modified glass ionomer cement: A systematic review of the impact of calcium and phosphate ion release. Dent. Mater. J. 2024, 43, 1–10. [Google Scholar] [CrossRef]
- Imazato, S.; Nakatsuka, T.; Kitagawa, H.; Sasaki, J.I.; Yamaguchi, S.; Ito, S.; Takeuchi, H.; Nomura, R.; Nakano, K. Multiple-ion releasing bioactive surface pre-reacted glass-ionomer (S-PRG) filler: Innovative technology for dental treatment and care. J. Funct. Biomater. 2023, 14, 236. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Hasan, A.M.H.R.; Sidhu, S.K.; Nicholson, J.W. Fluoride release and uptake in enhanced bioactivity glass ionomer cement (“glass carbomer™”) compared with conventional and resin-modified glass ionomer cements. J. Appl. Oral Sci. 2019, 27, e20180230. [Google Scholar] [CrossRef]
- Ghilotti, J.; Fernández, I.; Sanz, J.L.; Melo, M.; Llena, C. Remineralisation potential of three restorative glass ionomer cements: An in vitro study. J. Clin. Med. 2023, 12, 2434. [Google Scholar] [CrossRef]
- Puttipanampai, O.; Panpisut, P.; Sitthisettapong, T. Assessment of fluoride-releasing materials in remineralisation of adjacent demineralised enamel. Appl. Sci. 2025, 15, 2077. [Google Scholar] [CrossRef]
- Myanmar, K.; Inoue, G.; Chen, X.; Shimada, Y. Remineralisation effects of zinc-containing glass ionomer cement restoratives on demineralised enamel under pH cycling conditions. Crystals 2025, 15, 329. [Google Scholar] [CrossRef]
- Viana, C.S.; Maske, T.T.; Signori, C.; van de Sande, F.H.; Oliveira, E.F.; Cenci, M.S. Influence of caries activity and number of saliva donors: Mineral and microbiological responses in a microcosm biofilm model. J. Appl. Oral Sci. 2021, 29, e20200778. [Google Scholar] [CrossRef]
- Conti, G.; Veneri, F.; Amadori, F.; Garzoni, A.; Majorana, A.; Bardellini, E. Evaluation of antibacterial activity of a bioactive restorative material versus a glass-ionomer cement on Streptococcus mutans: In vitro study. Dent. J. 2023, 11, 149. [Google Scholar] [CrossRef]
- Dcruz, M.M.; Tapashetti, S.; Naik, B.; Shah, M.A.; Mogi, P.; Horatti, P. Comparative evaluation of fluoride release profiles in new glass ionomer cements and conventional type II GIC: Implications for cariostatic efficacy. Bioinformation 2024, 20, 2009–2014. [Google Scholar] [CrossRef]
- 3M ESPE. Ketac™ Universal Aplicap™ Glass Ionomer Restorative Technical Product Profile. Available online: https://multimedia.3m.com/mws/media/1090408O/ketac-universal-aplicap-technical-product-profile-pdf.pdf (accessed on 1 July 2025).
- GC Corporation. Fuji IX GP Product Brochure. Available online: https://www.gc.dental (accessed on 1 July 2025).
- GC Corporation. EQUIA Forte Fil Brochure. Available online: https://www.gc.dental (accessed on 1 July 2025).
- Butera, A.; Pascadopoli, M.; Gallo, S.; Lelli, M.; Tarterini, F.; Giglia, F.; Scribante, A. SEM/EDS evaluation of the mineral deposition on a polymeric composite resin of a toothpaste containing biomimetic Zn-carbonate hydroxyapatite (microRepair®) in oral environment: A randomised clinical trial. Polymers 2021, 13, 2740. [Google Scholar] [CrossRef]
- Martín-Vacas, A.; Vera-González, V.; Ramírez-Castellanos, J.; González-Gil, D.; de Nova, M.J.G. SEM/EDS analysis of tubules and mineral deposition in the dentine of children with osteogenesis imperfecta. Appl. Sci. 2023, 13, 12451. [Google Scholar] [CrossRef]
- Kam Hepdeniz, Ö.; Gürdal, O. The effect of titanium dioxide nanoparticles on microhardness and SEM-EDS analysis of glass ionomer cement and amalgomer. Selcuk Dent. J. 2021, 8, 623–628. [Google Scholar] [CrossRef]
- Durrant, L.; Mutahar, M.; Daghrery, A.A.; Albar, N.H.; Alwadai, G.S.; Alqahtani, S.A.; Al Dehailan, L.A.; Abogazalah, N.N.; Alamoudi, N.A.; Al Moaleem, M.M. Clinical performance of glass ionomer cement in load-bearing restorations: A systematic review. Med. Sci. Monit. 2024, 30, e943489. [Google Scholar] [CrossRef]
- Nicholson, J.W. Maturation processes in glass-ionomer dental cements. Acta Biomater. Odontol. Scand. 2018, 4, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Palani, H.; Paulraj, J.; Maiti, S.; Ganesh, M.K. Evaluating the biocompatibility of novel green-synthesised nano-modified glass ionomer cement: A biochemical and histopathological analysis study in Wistar albino rats. J. Contemp. Dent. Pract. 2025, 26, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.K.; Wong, A.W.; Chu, C.H.; Yu, O.Y. Update on dental luting materials. Dent. J. 2022, 10, 208. [Google Scholar] [CrossRef]
- Zainuddin, N.; Karpukhina, N.; Law, R.V.; Hill, R.G. Characterisation of a remineralising Glass Carbomer® ionomer cement by MAS-NMR spectroscopy. Dent. Mater. 2012, 28, 1051–1058. [Google Scholar] [CrossRef]
- Magalhães, G.A.P.; Thomson, J.J.; Smoczer, C.; Young, L.A.; Matos, A.O.; Pacheco, R.R.; Souza, M.T.; Zanotto, E.D.; Puppin Rontani, R.M. Effect of Biosilicate® addition on physical-mechanical and biological properties of dental glass ionomer cements. J. Funct. Biomater. 2023, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.; Galankar, K.; Varadarajan, S.; Gupta, A.A. Evaluating fluoride uptake of dentine from different restorative materials at various time intervals—In vitro study. J. Oral Biol. Craniofac. Res. 2022, 12, 216–222. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Coleman, N.J.; Sidhu, S.K. Kinetics of ion release from a conventional glass-ionomer cement. J. Mater. Sci. Mater. Med. 2021, 32, 30. [Google Scholar] [CrossRef]
- Wongphattarakul, S.; Kuson, R.; Sastraruji, T.; Suttiat, K. Fluoride release and rechargeability of poly(lactic acid) composites with glass ionomer cement. Polymers 2023, 15, 4041. [Google Scholar] [CrossRef]
- Aydın, N.; Karaoğlanoğlu, S.; Aybala-Oktay, E.; Çetinkaya, S.; Erdem, O. Investigation of water sorption and aluminium releases from high-viscosity and resin-modified glass ionomer. J. Clin. Exp. Dent. 2020, 12, e844–e851. [Google Scholar] [CrossRef] [PubMed]
- Kuru, E.; Eronat, N.; Türkün, M.; Çoğulu, D. Comparison of remineralisation ability of tricalcium silicate and of glass ionomer cement on residual dentine: An in vitro study. BMC Oral Health 2024, 24, 732. [Google Scholar] [CrossRef]
- Saxena, K.; Ann, C.M.; Azwar, M.A.B.M.; Banavar, S.R.; Matinlinna, J.; Peters, O.A.; Daood, U. Effect of strontium fluoride on mechanical and remineralisation properties of enamel: An in-vitro study on a modified orthodontic adhesive. Dent. Mater. 2024, 40, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Yazid, F.; Nasruddin, N.S.; Anuar, N.N.M. Effects of nanohydroxyapatite incorporation into glass ionomer cement (GIC). Minerals 2022, 12, 9. [Google Scholar] [CrossRef]
- Lopes, C.M.C.F.; Galvan, J.; Chibinski, A.C.R.; Wambier, D.S. Fluoride release and surface roughness of a new glass ionomer cement: Glass Carbomer. Rev. Odonto Ciênc. 2018, 33, 1–6. [Google Scholar] [CrossRef][Green Version]
- Bezerra, A.C.; Novaes, R.C.; Faber, J.; Frencken, J.E.; Leal, S.C. Ion concentration adjacent to glass-ionomer restorations in primary molars. Dent. Mater. 2012, 28, e259–e263. [Google Scholar] [CrossRef] [PubMed]
- Bilić-Prcić, M.; Šalinović, I.; Gürgan, S.; Koç Vural, Ü.; Krmek, S.J.; Miletić, I. Effects of incorporation of marine derived hydroxyapatite on the microhardness, surface roughness, and fluoride release of two glass-ionomer cements. Appl. Sci. 2021, 11, 11027. [Google Scholar] [CrossRef]
- Elshenawy, E.A.; El-Ebiary, M.A.; Kenawy, E.R.; El-Olimy, G.A. Modification of glass-ionomer cement properties by quaternized chitosan-coated nanoparticles. Odontology 2023, 111, 328–341. [Google Scholar] [CrossRef]
- Tromp, R.M. Energy-dispersive X-ray spectroscopy in a low energy electron microscope. Ultramicroscopy 2024, 259, 113935. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Leniart, A.; Lapińska, B.; Skrzypek, S.; Łukomska-Szymanska, M. Selected spectroscopic techniques for surface analysis of dental materials: A narrative review. Materials 2021, 14, 2624. [Google Scholar] [CrossRef] [PubMed]
- Pardi, M.; da Cunha, B.M.; Cunha, H.M.; Marques, M.E.S.; Ribeiro, K.L.; Cruz, C.E.; Costa, C.R.; Lepri, C.P.; de Castro, D.T. Correlation between fluoride release, surface hardness and diametral tensile strength of restorative glass ionomer cements. J. Clin. Exp. Dent. 2024, 16, e610–e615. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.F.; Pires, P.M.; Alambiaga-Caravaca, A.M.; Spagnuolo, G.; Hibbitts, A.; Salvatore, S. Remineralisation of mineral-deficient dentine induced by experimental ion-releasing materials in combination with a biomimetic dual-analogue primer. J. Dent. 2024, 152, 105468. [Google Scholar] [CrossRef]
- Venugopal, K.; Krishnaprasad, L.; V P, P.S.; Ravi, A.B.; Haridas, K.; Soman, D. A comparative evaluation of microleakage between resin-modified glass ionomer, flowable composite, and Cention-N in class V restorations: A confocal laser scanning microscope study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 1), S132–S136. [Google Scholar] [CrossRef]



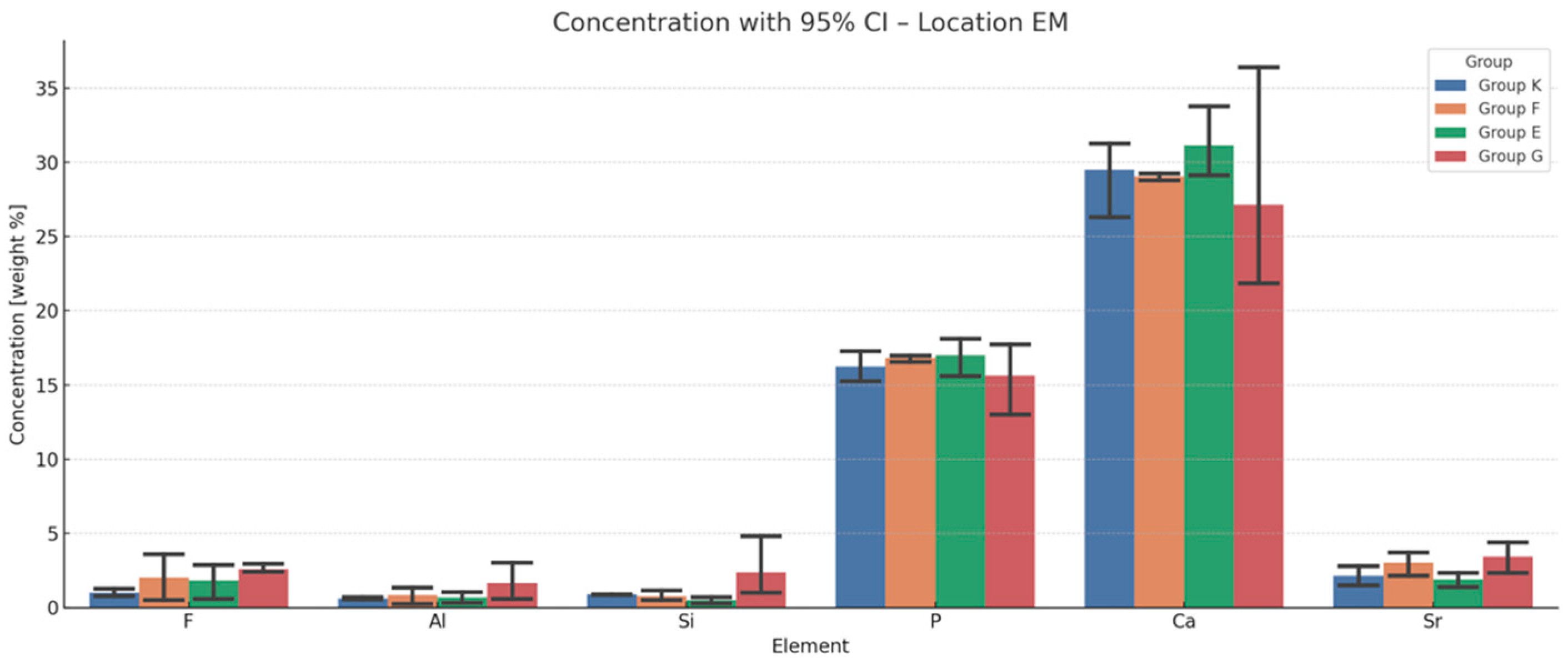

| DM vs. EM | F | Al | Si | Sr | ||||
|---|---|---|---|---|---|---|---|---|
| p Value | Mean Differences | p Value | Mean Differences | p Value | Mean Differences | p Value | Mean Differences | |
| K | 0.001 | +1.98 | 0.027 | +2.47 | 0.059 | +1.45 | 0.046 | +2.00 |
| F | 0.027 | +2.43 | 0.508 | +0.07 | 1.000 | +0.51 | 0.137 | +0.22 |
| E | 0.003 | +5.30 | 0.010 | +2.62 | 0.020 | +2.23 | 0.002 | +3.12 |
| G | 0.600 | +0.37 | 0.183 | −0.05 | 0.656 | +0.49 | 0.008 | +2.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turjanski, D.; Jakovljević, S.; Lisjak, D.; Bučević Sojčić, P.; Glavina, F.; Goršeta, K.; Glavina, D. Bioactivity of Glass Carbomer Versus Conventional GICs in Sound Enamel and Dentine: A 12-Month SEM-EDS Study. Materials 2025, 18, 3580. https://doi.org/10.3390/ma18153580
Turjanski D, Jakovljević S, Lisjak D, Bučević Sojčić P, Glavina F, Goršeta K, Glavina D. Bioactivity of Glass Carbomer Versus Conventional GICs in Sound Enamel and Dentine: A 12-Month SEM-EDS Study. Materials. 2025; 18(15):3580. https://doi.org/10.3390/ma18153580
Chicago/Turabian StyleTurjanski, Dubravka, Suzana Jakovljević, Dragutin Lisjak, Petra Bučević Sojčić, Fran Glavina, Kristina Goršeta, and Domagoj Glavina. 2025. "Bioactivity of Glass Carbomer Versus Conventional GICs in Sound Enamel and Dentine: A 12-Month SEM-EDS Study" Materials 18, no. 15: 3580. https://doi.org/10.3390/ma18153580
APA StyleTurjanski, D., Jakovljević, S., Lisjak, D., Bučević Sojčić, P., Glavina, F., Goršeta, K., & Glavina, D. (2025). Bioactivity of Glass Carbomer Versus Conventional GICs in Sound Enamel and Dentine: A 12-Month SEM-EDS Study. Materials, 18(15), 3580. https://doi.org/10.3390/ma18153580










