Effect of Cu Content on Corrosion Resistance of 3.5%Ni Weathering Steel in Marine Atmosphere of South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructural Analysis
2.3. Mechanical Property Tests
2.4. Periodic Immersion Wet–Dry Cyclic Corrosion Tests
2.5. Electrochemical Evaluation
2.6. X-Ray Diffraction (XRD) Analysis
2.7. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3. Results and Discussion
3.1. Initial Microstructure and Mechanical Properties
3.2. Analysis of Corrosion Resistance Mechanisms
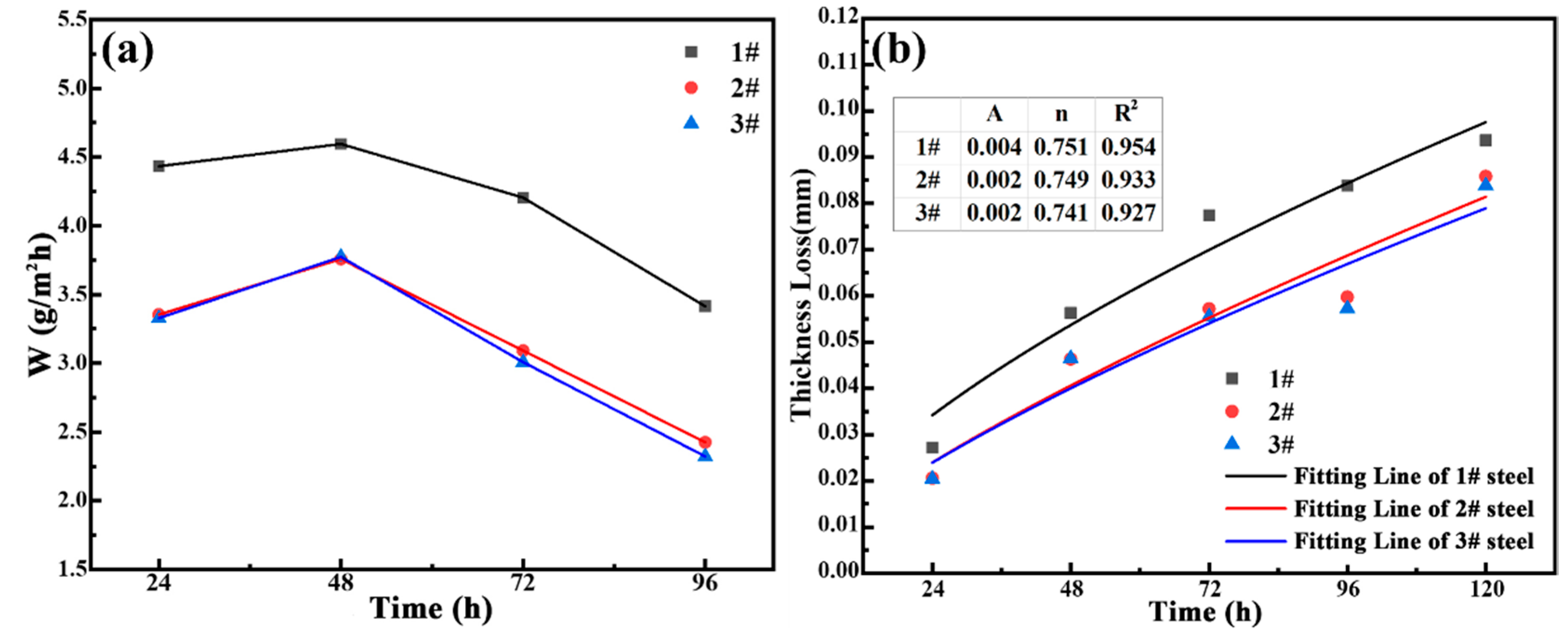
3.2.1. Accelerated Corrosion Rate Analysis
3.2.2. Macroscopic Corrosion Morphology
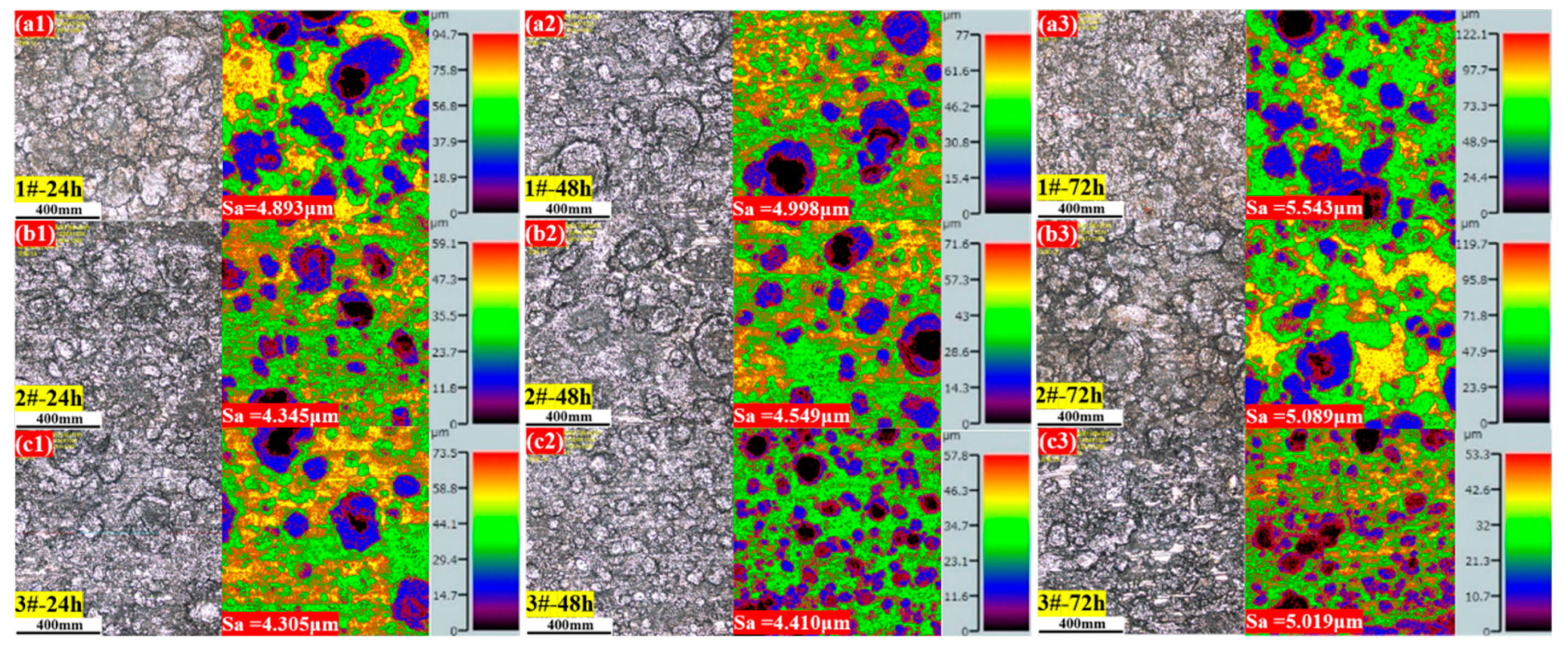
3.2.3. Surface Roughness (Sa) Analysis
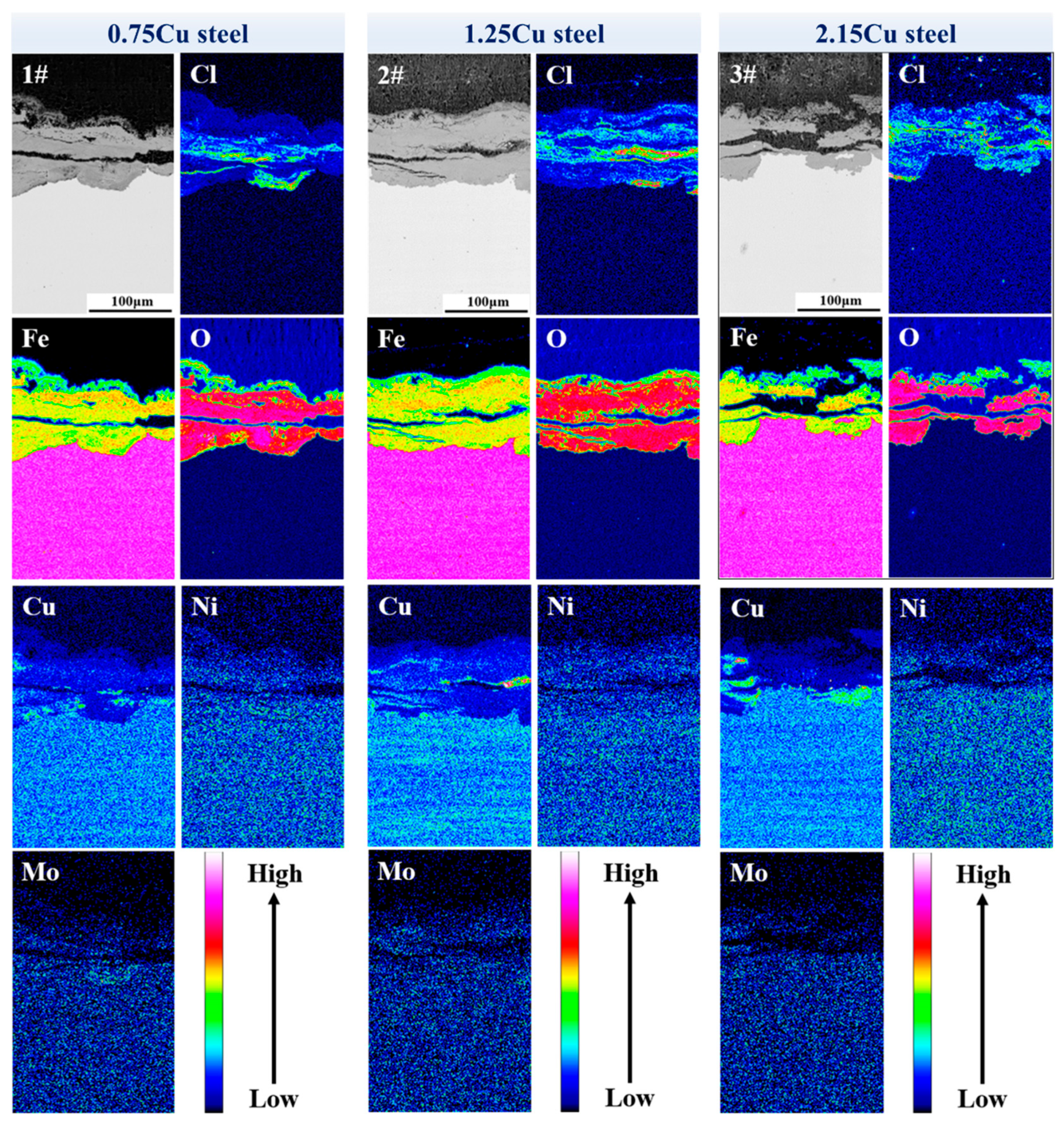
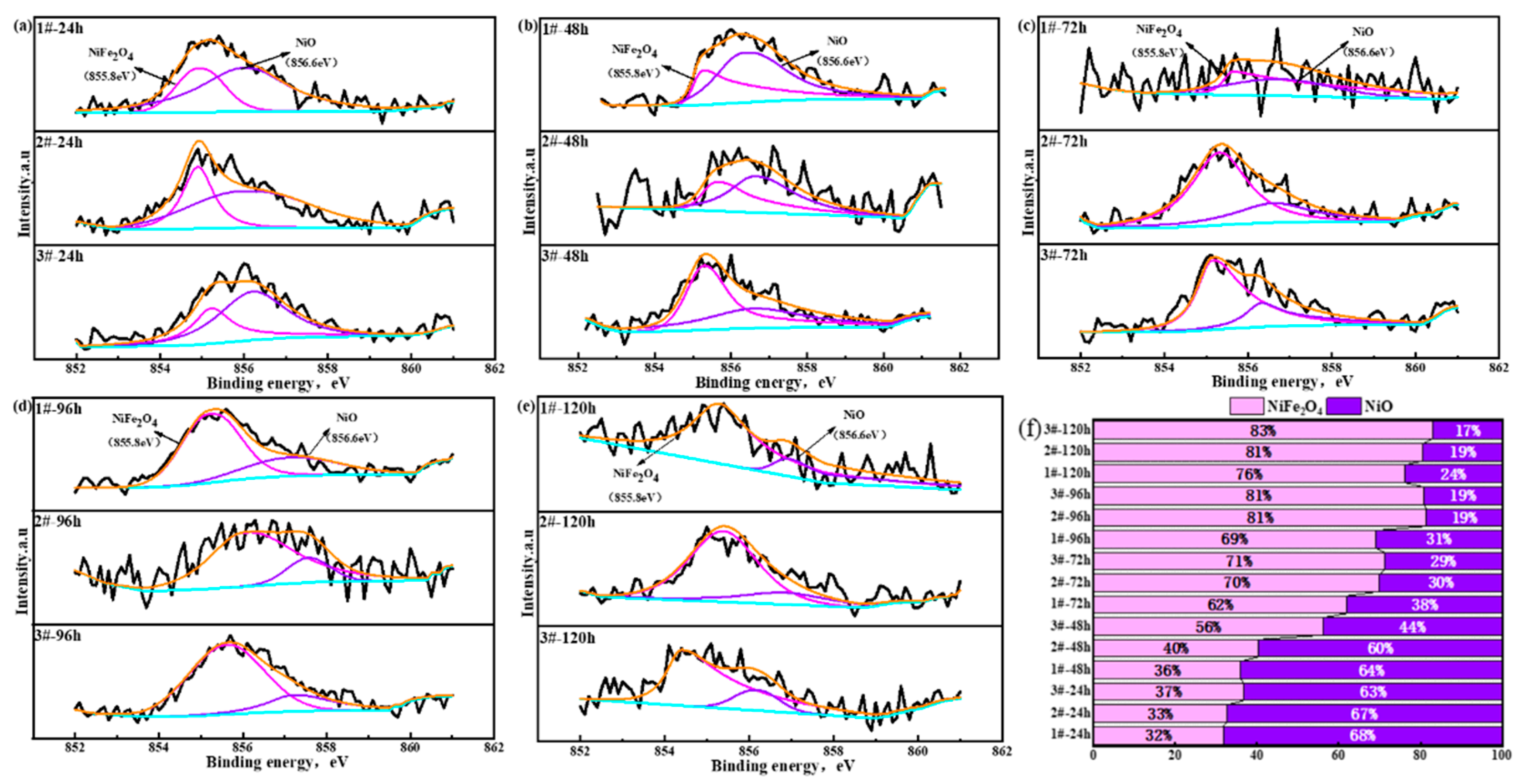
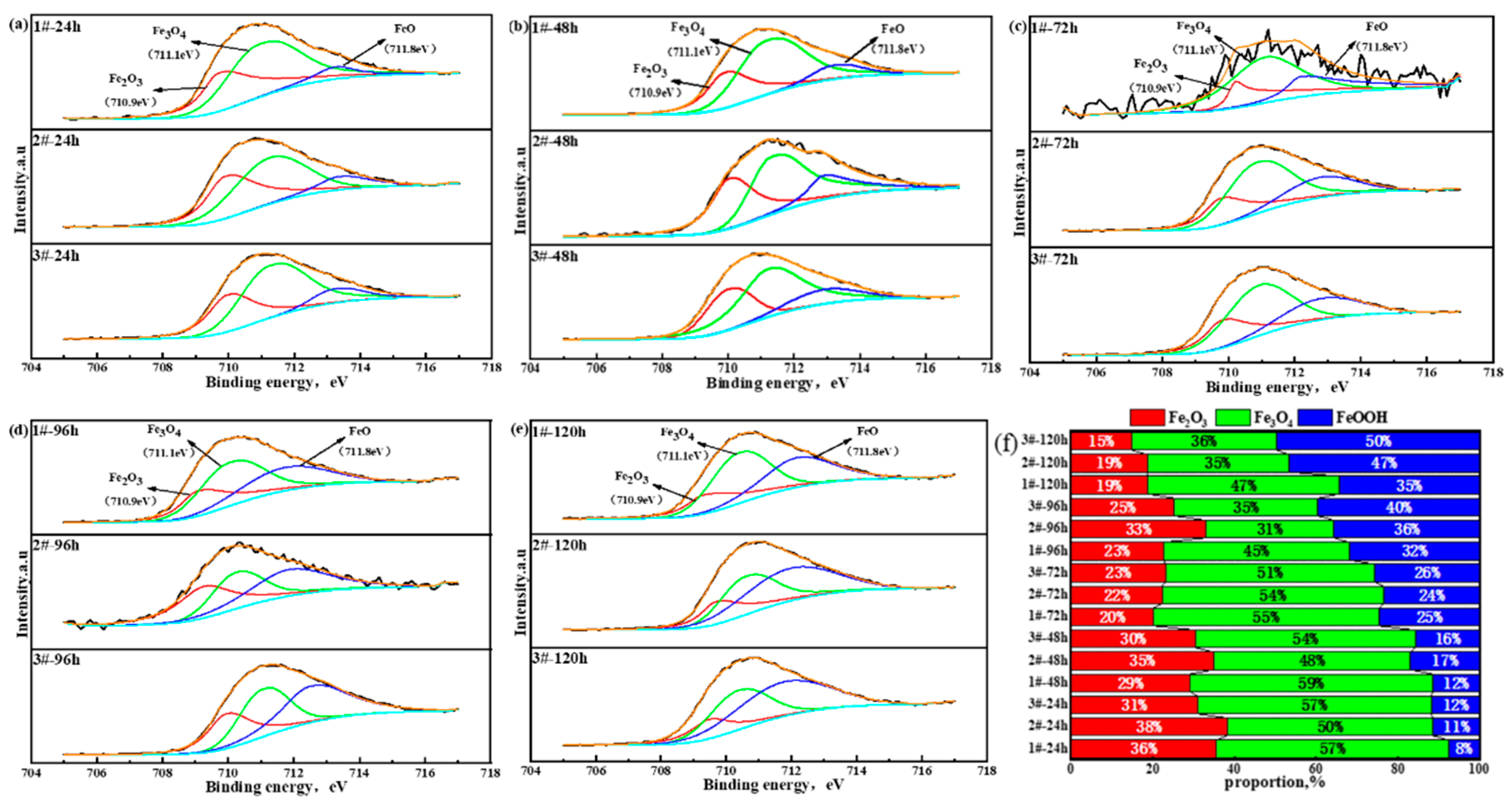
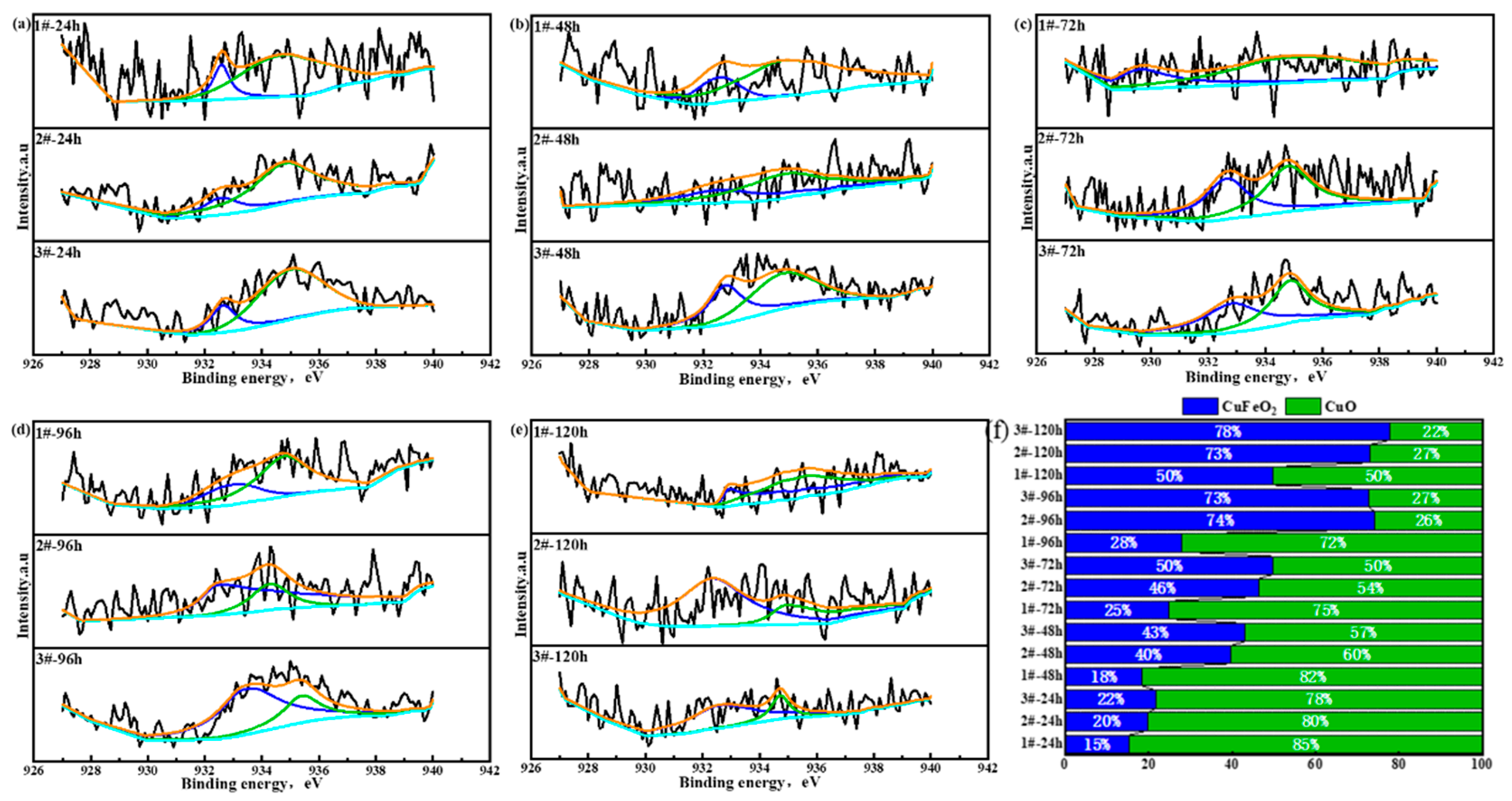
3.2.4. Element Distribution in Oxide Layer of Surface Layer
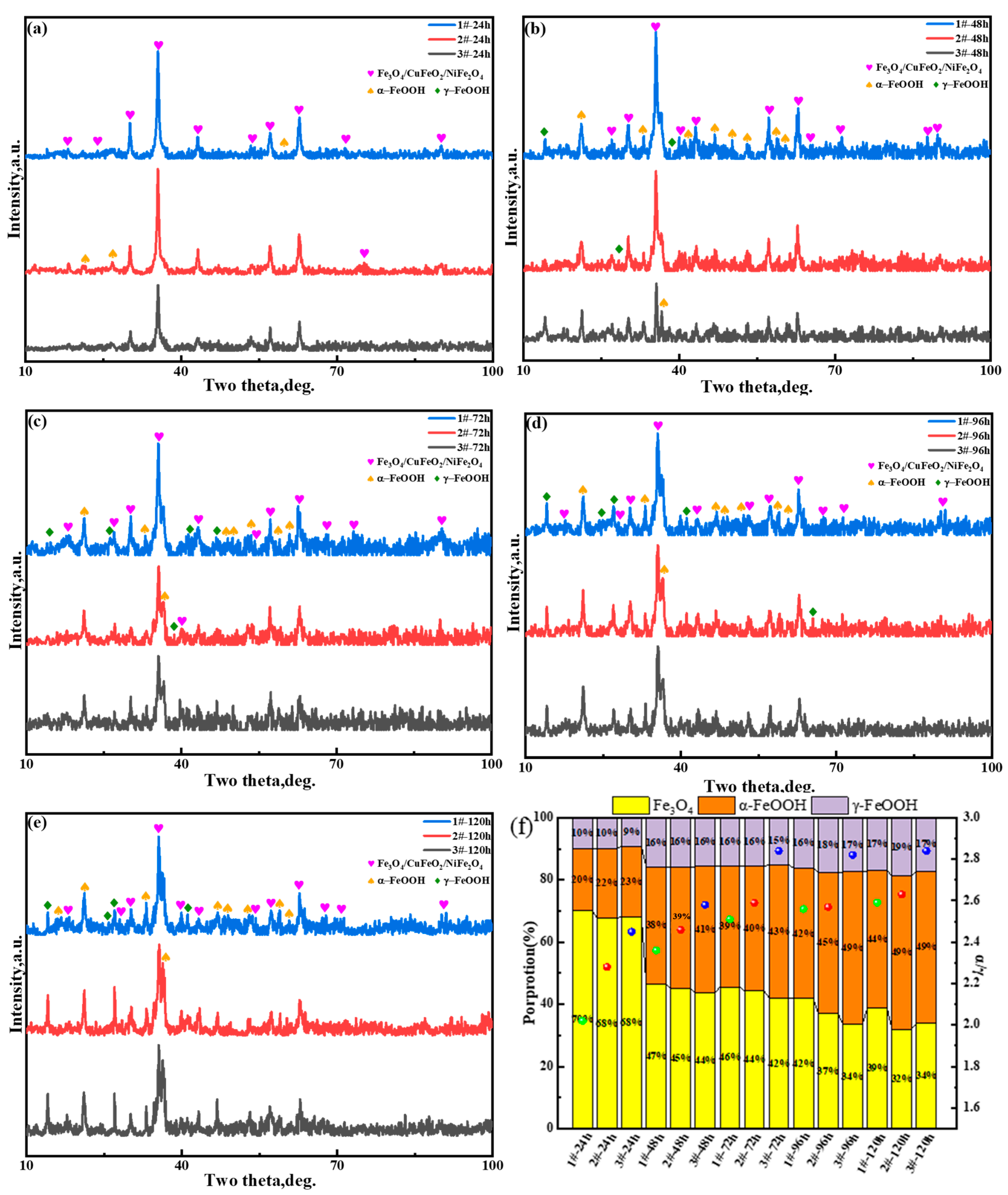
3.2.5. Rust Structure Analysis
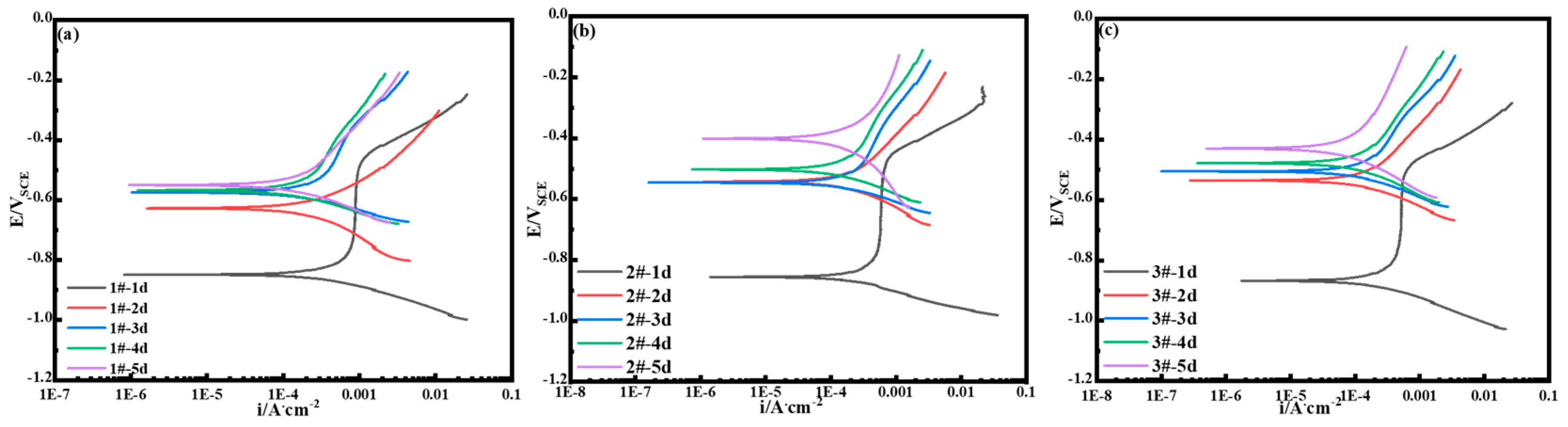
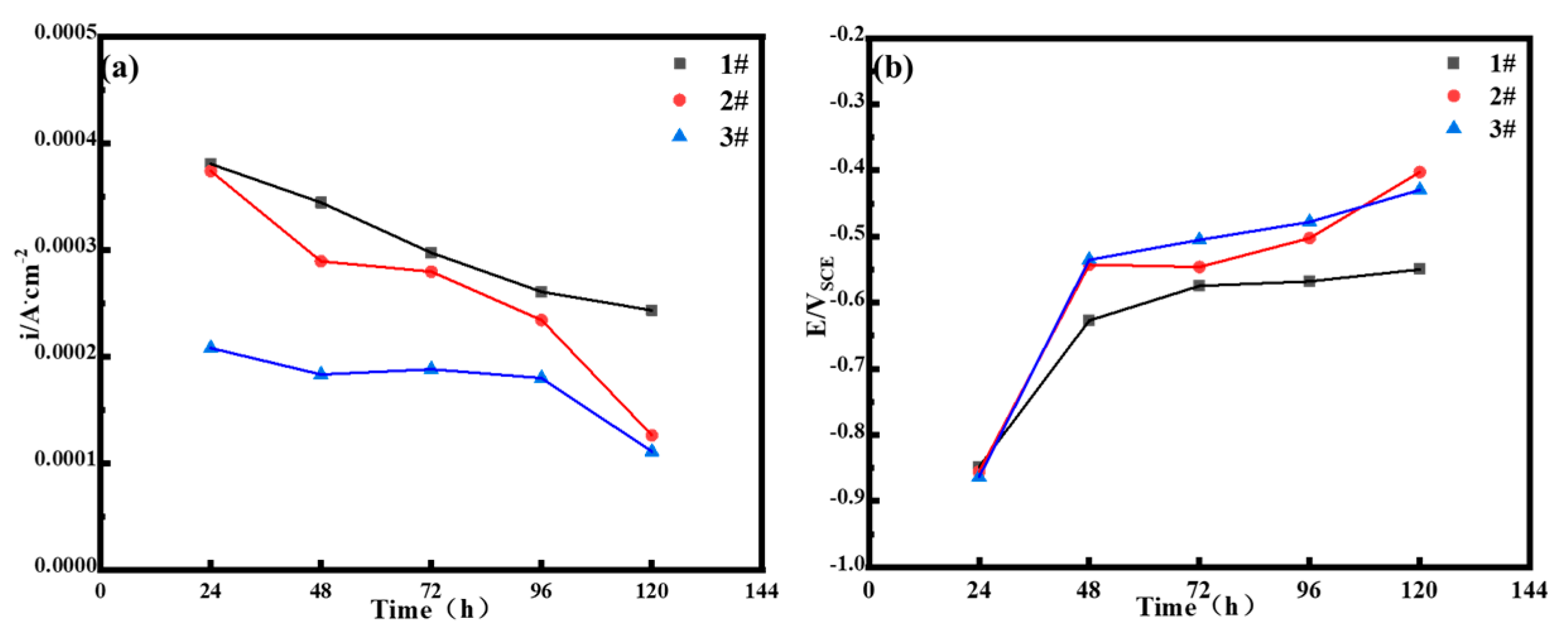
3.2.6. Electrochemical Test Analysis (Polarization Curves)
4. Discussions
5. Conclusions
- (1)
- The microstructure of 0.75Cu steel involves bainite plus ferrite, while the microstructure of 1.25Cu and 2.15Cu steels involves bainite. With the increase in the Cu content, the strength of 3.5%Ni steel increased, while the impact toughness at −20 °C decreased from 96 J (0.75Cu steel) to 13 J (2.15Cu steel).
- (2)
- Under the simulation of marine atmospheric corrosion in the South China Sea by periodic dry–wet cycle accelerated corrosion tests, the corrosion resistance of 3.5%Ni weathering steel increased accordingly with the increase in the Cu content. The polarization curves of the rusted samples show that corrosion potential shifts positively and corrosion current density decreases.
- (3)
- As the Cu content increased, the surface roughness of the 3.5%Ni weathering steel after corrosion decreased, and the corrosion degree was lower. With a higher Cu content, the corrosion behavior was more uniform, which is beneficial to the formation of a uniform oxide layer at the surface layer.
- (4)
- After the 72 h periodic immersion dry–wet cycle accelerated corrosion test, there were different Cl-rich areas and the Cu-rich areas in the oxide layer of the surface layer. The Cu-rich areas involved CuO and CuFeO2, significantly inhibiting the penetration of Cl−.
- (5)
- After accelerated corrosion tests, the phase structures of the oxide layer of the surface layer mainly included two categories: iron hydroxide oxide (α-FeOOH and γ-FeOOH) and spinel phases (Fe3O4, CuFeO2, and NiFe2O4). With the increase in the Cu content, the fraction of α-FeOOH significantly increased, and the value of the α/γ protective factor of the oxide layer of the surface layer became larger.
6. Limitations and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, X.-M.; Zhang, M.-Q.; Liu, Y.; Shao, C.-L.; Hu, Y.; Wang, X.-Y.; Su, L.-R.; Wang, N.; Wang, C.-Y. Protective exploitation of marine bioresources in China. Ocean Coast. Manag. 2018, 163, 192–204. [Google Scholar] [CrossRef]
- Xia, R.; Jia, C.; Garbatov, Y. Deterioration of marine offshore structures and subsea installations subjected to severely corrosive environment: A review. Mater. Corros. 2025, 76, 758–775. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, Y.; Ji, P.; Li, B.; Xia, C.; Zhang, S.; Zhang, J.; Zhang, X.; Liu, R. Optimizing the corrosion performance of rust layers: Role of Al and Mn in lightweight weathering steel. NPJ Mater. Degrad. 2024, 8, 32. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Liao, T.; Wang, S.; Lv, B.; Guo, H.; Huang, Y.; Yong, Q.; Mao, X. Revealing the precipitation kinetics and strengthening mechanisms of a 450 MPa grade Nb-bearing HSLA steel. Mater. Sci. Eng. A 2023, 884, 145506. [Google Scholar] [CrossRef]
- Liu, G.; Liao, T.; Wang, S.; Li, Y.; Guo, H.; Wu, H.; Huang, Y.; Yong, Q.; Mao, X. Revealing the precipitation kinetics of multi-stage and multi-scale Ti-bearing precipitation in a 460 MPa grade HSLA steel. Mater. Sci. Eng. A 2024, 890, 145941. [Google Scholar] [CrossRef]
- Liu, G.; Guo, H.; Wang, S.; Wu, H.; Ruan, X.; Huang, Y.; Li, X.; Mao, X. Unveiling the kinetics of interphase and random quaternary NbTi precipitations, and strengthening mechanism of HSLA steel. Mater. Charact. 2024, 215, 114238. [Google Scholar] [CrossRef]
- Shaw, P.-T.; Chao, S.-Y.; Fu, L.-L. Sea surface height variations in the South China Sea from satellite altimetry. Oceanol. Acta 1999, 22, 1–17. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Wang, J.; Pan, X. Steel Structure Construction Industrialization in China: Literature Review and Future Direction. In Proceedings of the International Conference on Construction and Real Estate Management 2017, Guangzhou, China, 10–12 November 2017; pp. 224–233. [Google Scholar]
- Sun, F.; Ren, S.; Li, Z.; Liu, Z.; Li, X.; Du, C. Comparative study on the stress corrosion cracking of X70 pipeline steel in simulated shallow and deep sea environments. Mater. Sci. Eng. A 2017, 685, 145–153. [Google Scholar] [CrossRef]
- Zuo-Jiang, S.; Tian, Z.; Jiang, X.; Yu, H.; Sun, D. Research on pitting corrosion of 316 stainless steel in the deep-sea water and sediments of the South China Sea. Mater. Charact. 2025, 225, 115176. [Google Scholar] [CrossRef]
- Ding, K.; Guo, W.; Qiu, R.; Hou, J.; Fan, L.; Xu, L. Corrosion Behavior of Q235 Steel Exposed in Deepwater of South China Sea. J. Mater. Eng. Perform. 2018, 27, 4489–4496. [Google Scholar] [CrossRef]
- Sun, M.; Pang, Y.; Du, C.; Li, X.; Wu, Y. Optimization of Mo on the corrosion resistance of Cr-advanced weathering steel designed for tropical marine atmosphere. Constr. Build. Mater. 2021, 302, 124346. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, W.; Yang, W.; Zhao, Y.; Dong, B.; Chen, L.; Zhang, T. Optimizing the Corrosion Resistance of Ni-Containing Low-Alloy Steels with Mo Addition in Tropical Marine Atmosphere. J. Mater. Eng. Perform. 2023, 32, 7814–7830. [Google Scholar] [CrossRef]
- Díaz, I.; Cano, H.; Lopesino, P.; de la Fuente, D.; Chico, B.; Jiménez, J.A.; Medina, S.F.; Morcillo, M. Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments. Corros. Sci. 2018, 141, 146–157. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Xie, J.; Sun, Y.; Chen, L.; Li, H.; Wang, F.; Hou, B. Substitution of Ni with Cu and Its Impact on the Corrosion Resistance of Ni-Advanced Weathering Steels in the Simulated Tropical Marine Atmosphere. Met. Mater. Int. 2024, 30, 3030–3044. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Hou, H.; Liu, B.; Li, X. Insight into the product film formed on Ni-advanced weathering steel in a tropical marine atmosphere. Appl. Surf. Sci. 2018, 436, 80–89. [Google Scholar] [CrossRef]
- Tanaka, H.; Miyafuji, A.; Kandori, K.; Ishikawa, T.; Nakayama, T. Effect of Cu(II) on the formation, morphology and molecular adsorption properties of α-FeOOH rust particles prepared from acidic Fe(III) solutions. Corros. Sci. 2013, 66, 337–342. [Google Scholar] [CrossRef]
- Yin, L.; Sampson, E.; Nakano, J.; Sridhar, S. The Effects of Nickel/Tin Ratio on Cu Induced Surface Hot Shortness in Fe. Oxid. Met. 2011, 76, 367–383. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Khan, H.I.; Dong, B.; Yang, W.; Sun, Y.; Zhang, B.; Chen, L.; Li, H. Effects of Cu on the corrosion resistance of heat-treated weathering steel in a marine environment. Mater. Today Phys. 2023, 36, 101160. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Sun, Y.; Dong, B.; Yang, W.; Chen, L. Investigating the corrosion resistance of Cu-doped Ni-Mo low alloy steel through electrochemical tests. Corros. Commun. 2023, 10, 10–26. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phases in Steels; Press of Metallurgy Industry: Beijing, China, 2006. [Google Scholar]
- Shen, X.; Görzen, D.; Xu, Z.; Blinn, B.; Bleck, W.; Beck, T.; Krupp, U.; Song, W. Nano-sized Cu precipitation and microstructural evolution in aged ultralow and medium carbon steels. Materialia 2022, 26, 101626. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, Y.; Zhan, D.; Song, Y.; Pan, X.; Hu, X.; Li, J.; Jiang, H.; Rong, L. Effect of Cu addition on the precipitation behavior of M2C carbides in maraging steel. Mater. Charact. 2024, 210, 113846. [Google Scholar] [CrossRef]
- Cheng, Z.; Wen, Q.; Zhong, B.; Liang, Y.; Zhang, X.; Liu, J. Coherent Cu-rich nanoprecipitates: Achieving both high strength and superior magnetic properties in non-oriented silicon steels. J. Mater. Sci. Technol. 2025, 233, 319–333. [Google Scholar] [CrossRef]
- Han, G.; Xie, Z.J.; Li, Z.Y.; Lei, B.; Shang, C.J.; Misra, R.D.K. Evolution of crystal structure of Cu precipitates in a low carbon steel. Mater. Des. 2017, 135, 92–101. [Google Scholar] [CrossRef]
- Cano, H.; Neff, D.; Morcillo, M.; Dillmann, P.; Diaz, I.; de la Fuente, D. Characterization of corrosion products formed on Ni 2.4wt%–Cu 0.5wt%–Cr 0.5wt% weathering steel exposed in marine atmospheres. Corros. Sci. 2014, 87, 438–451. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, G.; Chen, Y.; Zhao, P.; Tian, Y. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Liu, Z.; Cheng, X.; Du, C.; Li, X. Development and optimization of Ni-advanced weathering steel: A review. Corros. Commun. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, D.; Liu, W.; Li, F.; Lu, Q. Effect of Cu content on the precipitation behavior of Cu-rich and NiAl phases in steel. Mater. Charact. 2022, 187, 111849. [Google Scholar] [CrossRef]
- GB/T 229-2020; Metallic Materials—Charpy Pendulum Impact Test Method. The State Administration for Market Regulation and the National Standardization Administration Committee: Beijing, China, 2020.
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. General Administration of Market Regulation and Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- Cheng, P.; Hu, B.; Liu, S.L.; Guo, H.; Enomoto, M.; Shang, C.J. Influence of retained austenite and Cu precipitates on the mechanical properties of a cold-rolled and intercritically annealed medium Mn steel. Mater. Sci. Eng. A 2019, 746, 41–49. [Google Scholar] [CrossRef]
- Mai, H.L.; Cui, X.-Y.; Scheiber, D.; Romaner, L.; Ringer, S.P. The segregation of transition metals to iron grain boundaries and their effects on cohesion. Acta Mater. 2022, 231, 117902. [Google Scholar] [CrossRef]
- Singh, D.D.N.; Yadav, S.; Saha, J.K. Role of climatic conditions on corrosion characteristics of structural steels. Corros. Sci. 2008, 50, 93–110. [Google Scholar] [CrossRef]
- Hussain, Z.; Lin, Z.; Pan, H.; Huang, Y.; Tang, F.; Jiang, L. Synergizing empirical and AI methods to examine nano-silica’s microscale contribution to epoxy coating corrosion resistance. Ceram. Int. 2024, 50, 47172–47191. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Guo, H.; Zhang, Y.; Li, D.; Ye, F.; Tong, Y.; Wang, Z. Effects of particle size and modifier amount on hydrophobicity of as-synthesized and modified nano-silica spheres. Ceram. Int. 2024, 50, 21511–21518. [Google Scholar] [CrossRef]
- Arroyave, C.; Morcillo, M. Atmospheric corrosion products in iron and steels. Trends Corros. Res. 1997, 2, 1–16. [Google Scholar]
- De la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takeuchi, K.; Kandori, K.; Nakayama, T. Transformation of γ-FeOOH to α-FeOOH in acidic solutions containing metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 266, 155–159. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Yin, Z.; Dong, B.; Zhao, Y.; Fan, Y.; Wu, J.; Zhang, Z.; Li, X. Effects of the Addition of Cu and Ni on the Corrosion Behavior of Weathering Steels in Corrosive Industrial Environments. J. Mater. Eng. Perform. 2020, 29, 2531–2541. [Google Scholar] [CrossRef]
- Yamashita, M.; Misawa, T. Recent progress in the study of protective rust-layer formation on weathering steel. In Proceedings of the Corrosion 98, San Diego, CA, USA, 22–27 March 1998. NACE-98357. [Google Scholar]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Dillmann, P.; Mazaudier, F.; Hœrlé, S. Advances in understanding atmospheric corrosion of iron. I. Rust characterisation of ancient ferrous artefacts exposed to indoor atmospheric corrosion. Corros. Sci. 2004, 46, 1401–1429. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Montoya, P.; Díaz, I.; Granizo, N.; de la Fuente, D.; Morcillo, M. An study on accelerated corrosion testing of weathering steel. Mater. Chem. Phys. 2013, 142, 220–228. [Google Scholar] [CrossRef]
- Liu, G.; Guo, H.; Wang, S.; Liao, T.; Wu, H.; Ruan, X.; Huang, Y.; Li, X.; Mao, X. New route for fabricating low-carbon ductile martensite to optimize the stretch-flangeability of dual-phase steel. J. Mater. Res. Technol. 2024, 33, 8049–8062. [Google Scholar] [CrossRef]
- Jia, J.; Wu, W.; Cheng, X.; Zhao, J. Ni-advanced weathering steels in Maldives for two years: Corrosion results of tropical marine field test. Constr. Build. Mater. 2020, 245, 118463. [Google Scholar] [CrossRef]
- Fundamental XPS Data from Pure Elements, Pure Oxides, and Chemical Compounds. 2005. Available online: https://www.doc88.com/p-9932917207160.html (accessed on 6 July 2025).
- Fan, Y.; Liu, W.; Sun, Z.; Chowwanonthapunya, T.; Zhao, Y.; Dong, B.; Zhang, T.; Banthukul, W. Effect of chloride ion on corrosion resistance of Ni-advanced weathering steel in simulated tropical marine atmosphere. Constr. Build. Mater. 2021, 266, 120937. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. A study of the evolution of rust on Mo–Cu-bearing fire-resistant steel submitted to simulated atmospheric corrosion. Corros. Sci. 2012, 54, 244–250. [Google Scholar] [CrossRef]
- Liu, R.; Chen, X.-P.; Wang, X.; Shi, Q.; Mi, F.; Li, Y. Effect of Nickel on Corrosion Resistance of Weathering Steels in a Simulated Marine Atmosphere Environment. Corros. Sci. Protetion Technol. 2016, 28, 122–128. [Google Scholar]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments. Corros. Sci. 2011, 53, 4187–4192. [Google Scholar] [CrossRef]
- Romano, P.; Brito, P.S.; Rodrigues, L. Monitoring of the degradation of concrete structures in environments containing chloride ions. Constr. Build. Mater. 2013, 47, 827–832. [Google Scholar] [CrossRef]
- Yue, X.; Ren, Y.; Huang, L.; Zou, S.; Zhang, L.; Hua, Y. The role of Cl- in the formation of the corrosion products and localised corrosion of 15Cr martensite stainless steel under an CO2-containing extreme oilfield condition. Corros. Sci. 2022, 194, 109935. [Google Scholar] [CrossRef]


| Specimen | C | Si | Mn | P | S | Ni | Mo | Cu |
|---|---|---|---|---|---|---|---|---|
| 1# (0.75Cu) | 0.020 | 0.19 | 0.74 | <0.01 | <0.001 | 3.56 | 0.25 | 0.75 |
| 2# (1.25Cu) | 0.019 | 0.21 | 0.73 | <0.01 | <0.001 | 3.55 | 0.25 | 1.25 |
| 3# (2.15Cu) | 0.022 | 0.2 | 0.73 | <0.01 | <0.001 | 3.55 | 0.25 | 2.15 |
| Specimens | Impact Energy at RT/J | Impact Energy at −20 °C/J | TS/MPa | YS/MPa | TE/% | RA/% |
|---|---|---|---|---|---|---|
| 1# | 99 | 96 | 680 | 560 | 21 | 77 |
| 2# | 104 | 68 | 724 | 651 | 21 | 72 |
| 3# | 25 | 13 | 822 | 713 | 20 | 75 |
| Main Compounds in Oxide Layer of Surface Layer and Binding Energy (eV) | ||||
|---|---|---|---|---|
| Ni 2p3/2 | Peak | NiFe2O4 | NiO | |
| EB/eV | 855.8 | 856.6 | ||
| Fe 2p3/2 | Peak | Fe2O3 | Fe3O4 | FeOOH |
| EB/eV | 710.9 | 711.1 | 711.8 | |
| Cu 2p3/2 | Peak | CuFeO2 | CuO | |
| EB/eV | 932.6 | 934.8 | ||
| Samples | 24 h | 48 h | 72 h | 96 h | 120 h |
|---|---|---|---|---|---|
| 1# | 6.8039 | 3.4432 | 2.9724 | 2.6072 | 2.4321 |
| 2# | 3.7376 | 2.8933 | 2.7949 | 2.3413 | 1.2609 |
| 3# | 2.079 | 1.8313 | 1.8802 | 1.7967 | 1.1080 |
| Samples | 24 h | 48 h | 72 h | 96 h | 120 h |
|---|---|---|---|---|---|
| 1# | −0.84813 | −0.62675 | −0.5745 | −0.56769 | −0.54944 |
| 2# | −0.85544 | −0.54231 | −0.5460 | −0.50237 | −0.40243 |
| 3# | −0.86328 | −0.53506 | −0.5050 | −0.47775 | −0.42931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Guo, Z.; Fu, T.; Sha, S.; Wang, B.; Chen, X.; Jia, S.; Liu, Q. Effect of Cu Content on Corrosion Resistance of 3.5%Ni Weathering Steel in Marine Atmosphere of South China Sea. Materials 2025, 18, 3496. https://doi.org/10.3390/ma18153496
Li Y, Guo Z, Fu T, Sha S, Wang B, Chen X, Jia S, Liu Q. Effect of Cu Content on Corrosion Resistance of 3.5%Ni Weathering Steel in Marine Atmosphere of South China Sea. Materials. 2025; 18(15):3496. https://doi.org/10.3390/ma18153496
Chicago/Turabian StyleLi, Yuanzheng, Ziyu Guo, Tianle Fu, Sha Sha, Bing Wang, Xiaoping Chen, Shujun Jia, and Qingyou Liu. 2025. "Effect of Cu Content on Corrosion Resistance of 3.5%Ni Weathering Steel in Marine Atmosphere of South China Sea" Materials 18, no. 15: 3496. https://doi.org/10.3390/ma18153496
APA StyleLi, Y., Guo, Z., Fu, T., Sha, S., Wang, B., Chen, X., Jia, S., & Liu, Q. (2025). Effect of Cu Content on Corrosion Resistance of 3.5%Ni Weathering Steel in Marine Atmosphere of South China Sea. Materials, 18(15), 3496. https://doi.org/10.3390/ma18153496






