Abstract
Lithium slag (LS), a by-product of lithium extraction processes, poses a significant disposal challenge during the rapid development of new energy technologies. In this study, LS was used to replace partially washed sand in the process of mortar production to compensate for the content of stone powder in sand. Five mortar mixes containing varying proportions of LS were prepared, and the macroscopic performance was evaluated. A comprehensive microscopic analysis, including microstructure observations, hydration product identification, and pore structure analysis, was conducted. The impact of LS on the chloride ion permeability of mortar was also investigated in this study. The results indicate that an increase in LS content gradually reduces the workability of the mortar, with a 39.29% decrease in fluidity when 40% of the sand is replaced with LS. Moreover, the compressive and flexural strengths of the mortar initially increase and then decrease with higher LS content. Microscopic tests reveal that 20% LS substitution significantly optimizes the pore structure of the mortar, resulting in a lower chloride ion permeability coefficient. Consequently, 20% LS substitution is recommended as the optimal dosage for use as fine aggregate in mortar.
1. Introduction
As the world economy thrives and environmental awareness escalates, the development of the new energy industry is vigorously promoted worldwide. Lithium, a crucial component in various sectors, such as electric vehicles, aviation technology, and energy storage for electronic products, has triggered accelerated mining of lithium salts [1,2,3]. The global annual production of lithium salts exceeds 1.2 million tons, with China contributing over 800,000 tons [4]. However, under the current production process, approximately 10 tons of lithium slag (LS) waste is generated for every ton of lithium carbonate produced, posing a pressing challenge in proper disposal [5,6].
The prevalent methods for handling LS include landfilling or open-air storage, which not only consumes significant land resources but also raises environmental risks [7,8]. The sulfur content in LS can contaminate surrounding soil and groundwater through water diffusion. Therefore, the safe disposal of LS has become a significant focus of research [9,10]. A large number of scholars have begun to explore the application value of LS in the fields of ceramic materials [6], construction materials [11], biological electrode materials, and molecular sieves [12]. In addition, LS also has application prospects in silica–aluminum aerogels, glass ceramics, and other fields.
A wide range of industrial waste residues, including steel slag, high titanium slag, and phosphorous slag, are extensively utilized in construction materials [13,14,15]. This is due to the substantial demand for building materials, leading to the exhaustion of natural aggregates and irreversible environmental degradation [16,17]. Meanwhile, construction materials demonstrate remarkable versatility, allowing for the production of materials with varying properties from diverse raw materials [18,19]. For example, Santillán improved the electrical conductivity and mechanical properties of concrete by 70% and 14%, respectively, through the combined action of steel slag and metal fiber [20]. Liu prepared a series of new low-calcium CO2 sequestration cementitious materials by sintering the mixture of waste concrete fine powder and calcium carbide slag [21]. The combined application of industrial waste residues with concrete not only reduces the reliance on natural materials but also realizes the recycling of discarded resources, aligning with the principles of low-carbon and sustainable development. These research results provide a theoretical basis for the utilization of LS in the field of construction materials.
LS exhibits a similar particle size distribution to ordinary Portland cement and it is rich in silicon, aluminum, and calcium oxides, resulting in potential utilization in building materials [22,23,24]. Some researchers have denoted efforts to replace cement with LS in concrete, aiming to reduce the amount of cementitious material, thus reducing carbon emissions and costs [25,26,27]. However, the irregular shape of LS particles leads to a decrease in the workability of the paste as the LS content increases, particularly in terms of air content and slump, limiting its application [28,29]. Despite this, the compressive strength and durability of concrete can be enhanced when LS is used as a partial cement replacement. Rahman evaluated the pozzolanic activity of LS through various chemical tests and discovered that LS contains 31.6% amorphous phases, primarily composed of aluminosilicate phases with Na, Ca, and Mg, consistent with other commonly used pozzolans [30]. This indicates its potential as a pozzolanic material. However, regardless of whether LS is used as a cement replacement or a supplementary cementitious material, the actual utilization rate of LS remains relatively low compared to the huge quantities of available LS.
Most of the previous studies focused on the pozzolanic activity of LS, aiming to use it as an auxiliary gelling material. However, in addition to cementing materials in building materials, sand is also an essential component, even higher than the content of cementing materials. Washed sand is widely used in engineering. However, due to the addition of flocculant to the washed sand, the partial stone powder and the mud in the sand are washed together. The lower content of stone powder is unfavorable to the working performance of concrete, such as the reduction in wrapping properties. Replacing part of washed sand with LS can increase the content of stone powder in the sand and then change the grading of the sand. However, little research has been reported in this area. In a small number of similar studies, it is not clear how LS as a substitute for sand affects the properties of building materials. The high SiO2 content in LS, coupled with its microscopic morphology resembling that of manufactured sand, presents a novel utilization path as a potential sand substitute, meriting further exploration [31]. Dong utilized LS as an ultrafine aggregate at different sand replacement levels in the preparation of mortar. The results indicate that LS can significantly reduce the structural deadweight and enhance the flexural and compressive strength and peak stress of the mortar [32,33]. Moreover, the utilization rate of LS as an ultrafine aggregate is five times higher than when it is used as a supplementary cementitious material. As an emerging industrial waste residue, LS has a relatively limited distribution, leading to processing challenges. The research on LS as a fine aggregate substitute remains insufficient and needs investigation. Previous studies have primarily focused on macroscopic-level observations, lacking in-depth analysis of the mechanisms governing how LS impacts the performance of mortar or concrete as a fine aggregate. This research is crucial for enhancing the resource utilization rate of LS and promoting its wider application.
This study is committed to investigating the impact of using LS as a substitute for fine aggregate on the macro-properties and micro-characteristics of mortar. By employing various replacement ratios (10%, 20%, 30%, 40%), LS is substituted for manufactured sand by weight. Firstly, this study explored the influence of different LS replacement ratios on macro-properties, such as mortar fluidity, compressive strength, and flexural strength. Subsequently, microscopic analysis was conducted to understand the mechanisms underlying the effects of LS on mortar, including microstructure, hydration products, hardened pore structure, and chloride ion permeability. The primary objective of this research is to determine the optimal replacement rate of LS as a substitute for fine aggregate in mortar. This study can provide a comprehensive understanding of the feasibility, optimal replacement rate, and mechanism of action of LS as a fine aggregate substitute in mortar, thus laying a solid theoretical foundation for conserving natural sand and broadening the application of LS.
2. Experimental Investigation
2.1. Raw Materials
The cement utilized in the experiments is class 42.5 Ordinary Portland cement manufactured by the China Conch Group, and the 3-day and 28-day compressive strengths are measured at 23.7 MPa and 47.6 MPa, respectively, in accordance with the GB 175-2023 [34] standard. The fly ash (FA) was sourced from Donglanxing New Materials Co., Ltd., in Chengdu, Sichuan Province. The discarded LS, which originated from the lithium salt plant in Mianyang, Sichuan Province, exhibits a natural water absorption rate of 22.6%. The primary chemical compositions of the cement, FA, and LS are presented in Table 1. The sand used in the experimental tests is manufactured sand with a fineness modulus of 3.64. The grading curve of sand is shown in Figure 1. It can be seen that the particle size of the sand used in the test is larger, which is to better highlight the grading adjustment effect of LS on sand. Figure 2 illustrates the microstructure and particle size distribution of the cement, FA, and LS. It can be seen that the particle size of LS is the largest, which is very close to the particle size of stone powder in sand.

Table 1.
The main chemical components of the raw materials.
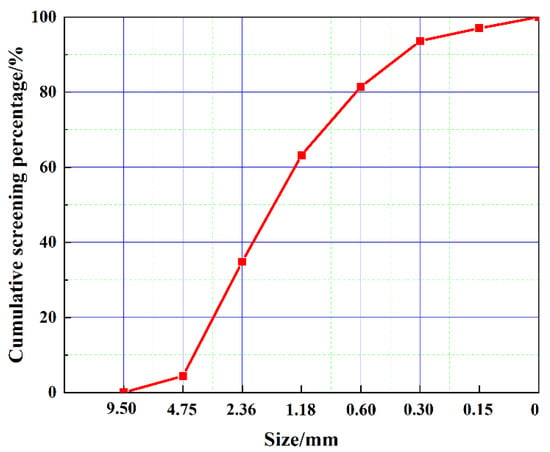
Figure 1.
Sand grading curve.
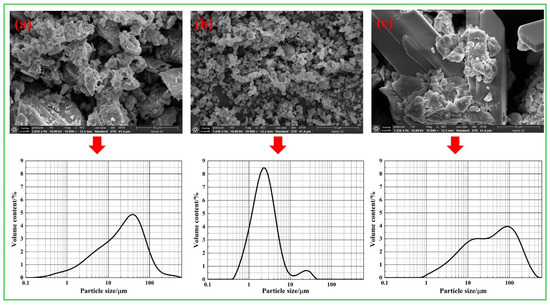
Figure 2.
Microstructures and particle size distribution curves of the raw materials: (a) cement; (b) fly ash; (c) LS.
2.2. Mix Proportions
Using cement and fly ash as binder materials and manufactured sand as a fine aggregate, a control group was developed and named SR0 for the preparation of mortar. Subsequently, four experimental groups with different LS contents were prepared to replace manufactured sand by weight. Based on the replacement levels, these groups were designated as SR10, SR20, SR30, and SR40. All five groups of the samples were cast with a water-to-binder ratio of 0.3. The workability of the mortar was adjusted using a superplasticizer. The polycarboxylic acid superplasticizer used in the experiment was provided by Xiamen Jibang New Materials Co., Ltd. (Xiamen, China), with a water reduction rate of 25%. Due to the larger specific surface area and water absorption rate of LS compared to manufactured sand, an increased dosage of superplasticizer was required to maintain a similar workability to the control group. The purpose is to avoid any impact on the performance of the mortar due to differences in fluidity. The mix design for five groups of samples is presented in Table 2.

Table 2.
Mix proportions of the mortar samples (kg/m3).
2.3. Sample Preparation
The preparation process for the mortar is outlined as follows: Firstly, the cement, FA, manufactured sand, and LS were placed in a mortar mixer and mixed for 1 min to ensure uniform distributions. Subsequently, water and superplasticizer were gradually added to the mixture during the mixing process. Once the addition of water and superplasticizer was complete, the mixture was further mixed for 2 min. After mixing, the mortar was subjected to a fluidity test. The mixture, following the fluidity test, was then poured into molds of varying sizes for molding, involving vibration to ensure the uniformity of the samples. To prevent water evaporation from the mortar, the top of the mold was covered with a film. After maintaining the samples in the mold for 24 h, they were removed and placed in a standard curing chamber (a temperature condition of 20 ± 2 °C and relative humidity condition of 95%). Figure 3 illustrates the sample preparation process. Prismatic specimens of 40 mm × 40 mm × 160 mm were used for compressive and flexural strength testing. Cubic specimens of 100 mm × 100 mm × 100 mm were used to characterize the pore structure of the samples. Cylindrical specimens with a diameter of 100 mm and a height of 50 mm were utilized for the chloride ion permeability test.

Figure 3.
Specimen preparation and curing process.
2.4. Test Procedures
2.4.1. Fluidity Test
The fluidity test of the mortar was conducted in accordance with Chinese Standard GB/T 2419-2005 [35]. To ensure the accuracy of the tests, the fluidity of each sample was measured three times. To investigate the impact of LS content on mortar fluidity, the same amount of superplasticizer as in the control group (SR0) was added to the mortars with varying LS substitution rates. Considering the fluidity of the control group as a reference, the fluidity of the mortars with different LS substitution rates was adjusted to a similar level by increasing the dosage of superplasticizer.
2.4.2. Compressive and Flexural Strength Test
The compressive and flexural strength tests for the mortar were performed in accordance with the “Test Method of Cement Mortar Strength” (GB/T 17671-2021 [36]). These tests are conducted at 3-day, 7-day, and 28-day curing ages to measure the compressive and flexural strengths of the mortar. The flexural and compressive strengths of each mortar group were determined as the average of 3 and 6 samples, respectively.
2.4.3. Scanning Electron Microscope (SEM) Test
The microstructures of mortar with varying LS substitution rates were observed using a Scanning Electron Microscope (SEM). During the test, the equipment voltage remained at 5 kV, and the microscopic features of the sample were observed through different amplification factors. The test samples are intact fragments remaining after the compressive strength testing, ensuring minimal disturbance to their original structures. The test samples were dried in an oven at a temperature of 108 °C.
2.4.4. X-Ray Diffraction (XRD) Test
The test results for indicators such as the type and quantity of hydration products can provide a microscopic perspective on the influence of LS on the mechanical properties of mortar. Since sand does not affect the hydration process of cement but may introduce substances, like silica, that could compromise the accuracy of hydration product testing, the samples in Table 1 were re-prepared without sand for hydration product testing. After 28 days of curing, the prepared samples were dried and grinded. X-ray diffraction (XRD) analysis was then conducted to determine the type and content of crystalline phases in the samples with different LS contents. The mineral composition of the mortar was analyzed using the DX-2800 X-ray diffraction instrument produced by China Dandong Haoyuan Instrument Co., Ltd. (Dandong, China).
2.4.5. Pore Structures
Cubic specimens of 100 mm × 100 mm × 100 mm were prepared to investigate the pore structure of mortar under different LS substitution rates. After 28 days of curing, the specimens were sliced into 15 mm × 100 mm × 100 mm samples using a cutting machine. For each test piece, three central slices were selected for further experimentation. These slices were then grinded using silicon carbide abrasive papers of varying fineness (50, 100, 300, 800, 1500, and 3000 grit) with the assistance of a grinding machine. Each abrasive paper was used for 3 min of grinding. The slices were then cleaned using an ultrasonic cleaner. The cleaned samples were coated with a black marker to darken their surfaces and enhance comparison by filling them with 20 nm white barium sulfate powder. Finally, the samples were scanned using a hardened concrete pore structure analyzer (HC-457), which is produced by Hangzhou Guanli Intelligent Technology Co., Ltd. (Hangzhou, China). Figure 4 illustrates the detailed testing procedure.
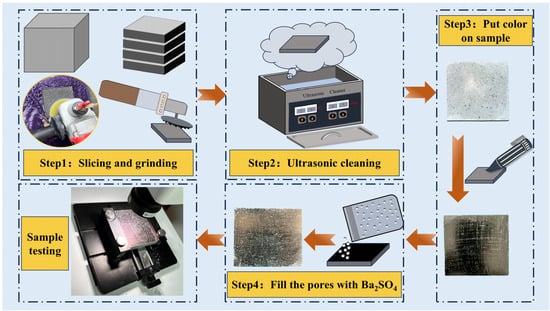
Figure 4.
Test procedure for pore structure analysis of mortar.
2.4.6. Chloride Ion Permeability Resistance Test
Drawing inspiration from Chinese Standard GB/T 50082-2009 [37], this study explores the impact of LS substitution for varying sand types on the chloride ion penetration resistance of mortar. After the completion of the test, the chloride ion penetration depth at 42 points was measured on the sample, and the average penetration depth was calculated. The chloride ion permeability coefficient of different mortars was calculated using Equation (1):
where is the unsteady-state chloride ion migration coefficient, expressed with a precision of 0.1 × 10−12 m2/s; is the absolute value of the applied voltage (V); is the average of the initial and final temperatures of the anode solution (°C); is the thickness of the specimen (mm), expressed with a precision of 0.1 mm; is the average chloride ion penetration depth (mm), expressed with a precision of 0.1 mm; and is the duration of the test (h).
3. Experimental Results and Discussion
3.1. Workability
The influence of LS content on mortar workability can be better represented without altering the dosage of superplasticizer, as presented in Figure 5. An increase in the proportion of LS substitution in the mortar results in a significant decrease in its workability. As the substitution rate rises from 0% to 40%, the workability drops from 280 mm to 170 mm, representing a reduction of 39.29%. This phenomenon is attributed to the finer particle size of LS, leading to a larger specific surface area compared to sand, enabling it to adsorb more water. Additionally, LS exhibits a higher water absorption capacity. These two factors contribute to a decrease in free water content and an increase in frictional forces within the mortar, ultimately reducing its workability. It is noteworthy that the addition of a small amount of superplasticizer can mitigate this issue, as evident from Table 1, where varying admixture dosages enable each mortar mix to achieve similar workability.
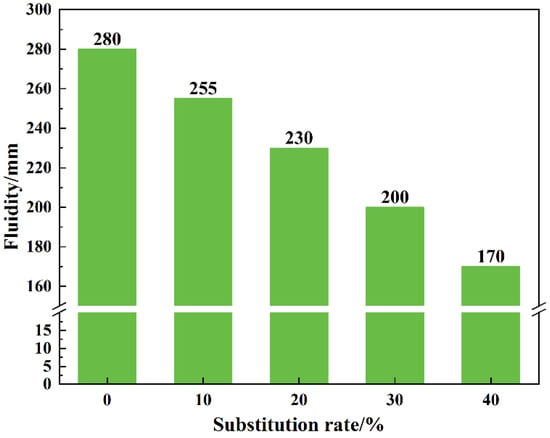
Figure 5.
The impact of lithium slag dosage on the workability of mortar.
3.2. Mechanical Properties
Figure 6 depicts the compressive and flexural strengths of mortar at 3 d, 7 d, and 28 d curing ages with varying LS dosages. It is evident from Figure 6 that the trend in compressive and flexural strengths for all three curing ages follows a similar pattern: an initial increase followed by a decrease. Specifically, mortar with an LS dosage of 20% exhibits the most superior mechanical properties. This is attributed to the fine-grained LS effectively filling the internal pores of the mortar. However, as the dosage exceeds 30%, the strength of the mortar begins to decline, falling below the control group. This observation can be explained by three main factors: Firstly, with a high binder content and fewer internal pores, there is less LS available for pore filling. Secondly, the remaining lithium slag functions as aggregate, but its strength is significantly lower than that of natural fine aggregates. Thirdly, the reduced content of natural fine aggregates provides support and contributes to the decline. Furthermore, when compared to the control group, the compressive strengths of the mortar with a dosage of 40% at 3 d, 7 d, and 28 d decreased by 19.17%, 12.47%, and 7.74%, respectively. Similarly, the flexural strengths of SR40 at 3 d, 7 d, and 28 d are reduced by 28.27%, 22.98%, and 18.64% compared to SR0. This phenomenon indicates that high LS dosages primarily impact the early-age strength of mortar because LS slows down the early hydration reactions of cement.
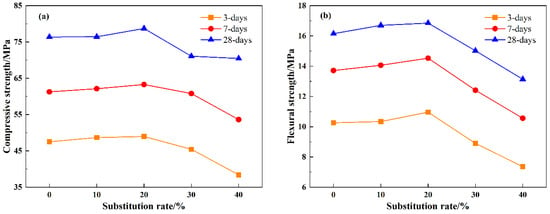
Figure 6.
The impact of different slag admixtures on the compressive strength and flexural strength of mortar at different ages: (a) compressive strength, (b) flexural strength.
3.3. Microstructure Analysis
A comparative analysis of the microstructure of mortars with 0%, 20%, and 40% lithium slag content is presented in Figure 7. Mortar without LS exhibited a relatively dense internal structure, containing only a minimal amount of cracks. Additionally, it demonstrated a high degree of hydration, where most calcium hydroxide (CH) transformed into hydration products, such as calcium silicate hydrate (C-S-H). Upon the substitution of 20% sand with LS, the microstructure became the most compact, practically devoid of cracks. The degree of hydration remained high within the mortar. However, with a 40% LS mixture, the microstructural density of the mortar significantly decreased, resulting in the appearance of cracks and even voids. Moreover, the surface of the hydration products exhibited a substantial amount of needle-like ettringite (AFt) crystals. Previous studies have indicated that the introduction of substances with high SO3 content into cement paste promotes the formation of AFt [38,39]. As AFt is a crystal with extremely high water content, its formation consumes a significant amount of free water, thereby reducing the amount of water available for cement hydration and subsequently diminishing the quantity of hydration products. Furthermore, the expansive nature of AFt also contributes to the reduction in mortar structural density, leading to an increase in cracks and voids [40,41].
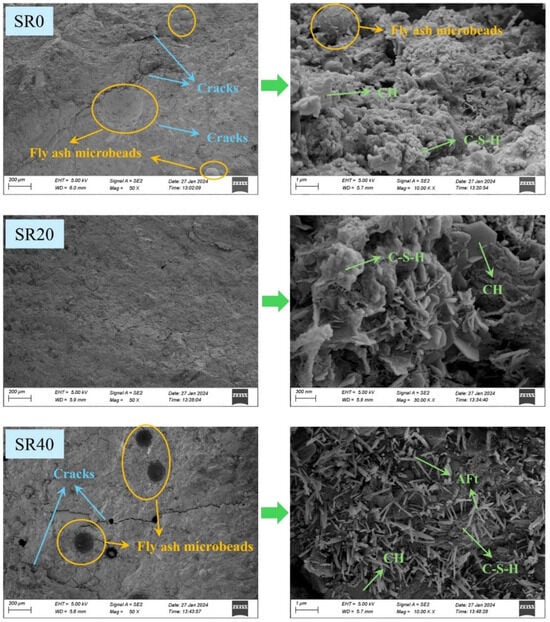
Figure 7.
Microstructures of mortar with different LS content.
3.4. Hydration Products
The composition of mortar with different lithium slag content was analyzed using XRD testing, as shown in Figure 8. The crystalline components in the five groups of mortars primarily comprised unreacted or insufficiently reacted silica (SiO2), dicalcium silicate (C2S), tricalcium silicate (C3S), and calcium hydroxide (CH) formed after hydration. As the LS content increased, the diffraction peaks of calcium hydroxide in the mortar gradually weakened, indicating a decrease in the quantity of calcium hydroxide and a lower degree of hydration within the mortar. This explains the reduction in mortar strength and the decreased compactness of its microstructure. Additionally, the diffraction peaks of spodumene (LiAlSi2O6) and gypsum (CaSO4∙2H2O) became more prominent as the LS increased. This phenomenon suggests that the presence of SO3 in the LS leads to the formation of new substances. According to previous research, a small amount of SO3 can have a positive impact on mortar performance, while an excessive amount can have a negative impact [9]. This is a significant reason why the mortar performance rapidly deteriorated when the LS exceeded 30% due to high SO3 content.
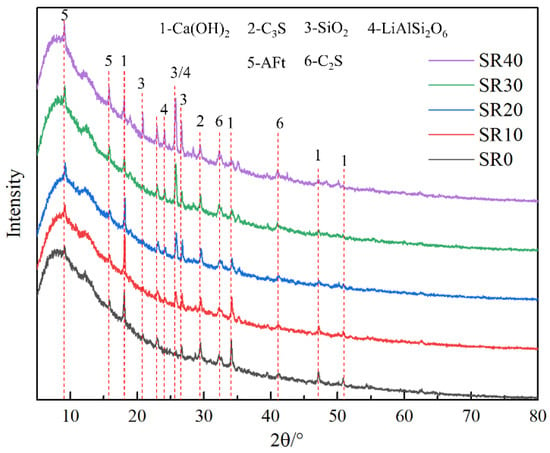
Figure 8.
Analysis of crystal components in mortar with different LS content.
3.5. Air Content Properties
An analysis of the air content properties of three mortar mixtures, SR0, SR20, and SR40, after 28 days of curing is presented in Figure 9. The results reveal that the air content in the mortar initially decreased and then increased with an increasing amount of LS. Specifically, the air contents of SR0, SR20, and SR40 were 3.74%, 3.51%, and 4.92%, respectively. A moderate amount of LS as a sand substitute can reduce the internal air content of the mortar. However, an excessive amount of LS leads to an increase in internal air content. Two main reasons account for this phenomenon. Firstly, a high LS admixture can affect the hydration of cement, reducing the compactness of the mortar. Secondly, as the internal relative humidity decreases, LS with high water absorption releases water, leading to the formation of pores. Many industrial wastes with similar physical and chemical properties to LS exhibit the same phenomenon when they are used as substitutes for sand [42,43,44].
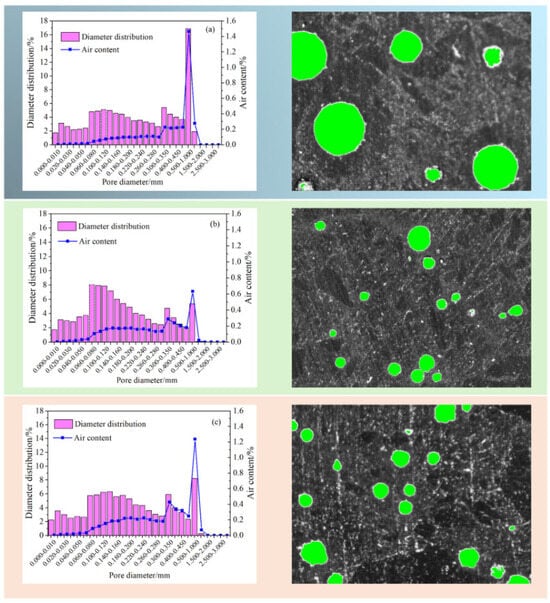
Figure 9.
Air content and the spacing factor of hardened mortar. (a) SR0; (b) SR20; (c) SR40.
Similar to the air content, the air bubble spacing factor exhibited a trend of first decreasing and then increasing with an increasing lithium slag admixture. However, interestingly, SR40 exhibited a smaller bubble spacing compared to SR0. This is attributed to the addition of LS, reducing the number of large air bubbles and increasing the number of small air bubbles in the mortar [45,46].
The distribution of pore diameters, air content, and pore distribution characteristics in mortar are illustrated in Figure 10. With variations in the LS replacement, the proportion of pores with different diameters within the mortar underwent significant changes. Overall, the addition of lithium slag noticeably reduced the proportion of large-diameter pores and increased the proportion of small-diameter pores, owing to the filling effect of LS. However, this modification effect tended to weaken as the LS increased. The pore distribution characteristics further validate these observations. In SR0, the number of pores was small, but their diameters were significant. The incorporation of LS reduced the pore diameters but increased the number of pores. The increase in large-diameter pores in SR40 is attributed to the impact of LS on the workability.
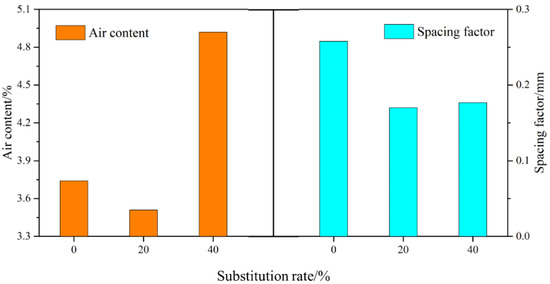
Figure 10.
Pore diameter distribution, air content, and pore distribution characteristics of mortar.
3.6. Chloride Ion Permeability Resistance
The chloride ion penetration depths at 42 distinct locations on three mortar mixtures, including SR0, SR20, and SR40, were measured, as shown in Figure 11. The average penetration depths and permeability coefficients were calculated, as presented in Figure 12. Evidently, the overall trend in chloride ion penetration depths across different locations in the three mortar mixtures was consistent, indicating the scientific agreement of the results. An appropriate amount of LS significantly reduced the chloride ion penetration depth. Conversely, an excessive amount of lithium slag weakened this effect. The average chloride ion penetration depths of SR0, SR20, and SR40 were 9.91 mm, 7.28 mm, and 8.44 mm, respectively. Compared to the control group (SR0), the chloride ion penetration depths of SR20 and SR40 were reduced by 26.54% and 14.84%, respectively. This advantage is attributed to the optimization of the internal pore structure by LS [47,48]. The calculated chloride ion permeability coefficients for SR0, SR20, and SR40 were 0.546 × 10−12 m2/s, 0.394 × 10−12 m2/s, and 0.456 × 10−12 m2/s, respectively. By combining the trend of the strength values of S0, S20, and S40, it can be observed that the rule of the mortar’s resistance to chloride ion penetration is similar to the variation pattern of the strength values.
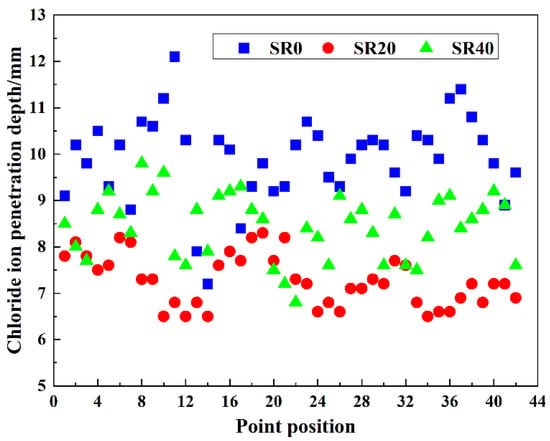
Figure 11.
Chloride ion penetration depth at different points of mortar with different LS content.
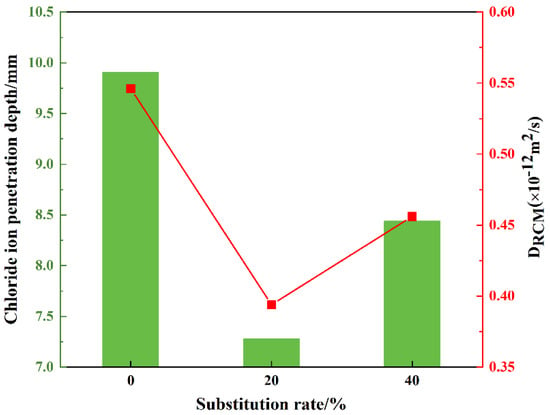
Figure 12.
The average chloride ion penetration depth and chloride ion permeability coefficient of mortar with different LS content.
4. Conclusions
This study comprehensively analyzes the feasibility of utilizing LS as a sand substitute in mortar, examining both macro and micro perspectives. Based on experimental results, the optimal substitution rate of LS and its impact mechanism on mortar performance were determined. The key findings are summarized as follows:
- (1)
- Due to the fine particle size and high water absorption capacity of LS, an increase in its admixture led to a gradual decrease in the fluidity of the mortar. Specifically, the fluidity of SR40 was reduced by 39.29% compared to SR0.
- (2)
- The addition of LS first enhanced and then diminished the compressive and flexural strengths of the mortar. The initial strength enhancement is attributed to the filling effect of LS, while the subsequent decrease is caused by its inferior supporting capability compared to fine aggregates. Furthermore, the 3 d and 28 d compressive strengths of SR40 were reduced by 19.17% and 7.74%, respectively, compared to SR0.
- (3)
- Microstructure, pore characteristics, and chloride ion permeability test results suggest that a 20% substitution rate of LS significantly optimizes the pore structure of the mortar, leading to a reduction in the chloride ion permeability coefficient. Consequently, a 20% substitution rate of LS is recommended as the optimal replacement for fine aggregates.
- (4)
- Further research is recommended to fully understand the impact of LS as a sand substitute on the durability of mortar. It can be speculated that the optimized pore structure resulting from the incorporation of lithium slag will positively influence the durability of mortar.
Author Contributions
X.Z. (Xianliang Zhou): writing—original draft, validation. X.Z. (Xi Zhu): conceptualization, methodology. W.D.: supervision, resources. X.Z. (Xiaojun Zhou): writing—review and editing, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Natural Science Foundation of Sichuan Province] grant number [2024NSFSC0163]. And The APC was funded by Natural Science Foundation of Sichuan Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author (s).
Conflicts of Interest
Author Xianliang Zhou was employed by the company Jinhua Xinsheng Zeolite Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, Z.; Wang, J.X.; Jiang, Q.K.; Cheng, G.D.; Li, L.; Kang, Y.X.; Wang, D.M. A green route to sustainable alkali-activated materials by heat and chemical activation of lithium slag. J. Clean. Prod. 2019, 225, 1184–1193. [Google Scholar] [CrossRef]
- He, Z.H.; Du, S.G.; Chen, D. Microstructure of ultra high performance concrete containing lithium slag. J. Hazard. Mater. 2018, 353, 35–43. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.S.; Qi, C.C.; Zhang, Q.L.; Xiao, C.C. Lithium slag and fly ash-based binder for cemented fine tailings backfill. J Environ. Manag. 2019, 248, 109282. [Google Scholar] [CrossRef]
- Luo, X.F.; Huang, L.; Li, Y.; Chen, Z.J. Preparation of geopolymers from thermally activated lithium slag as sole precursor: Mechanical properties and microstructure. Case Stud. Constr. Mater. 2024, 20, e03248. [Google Scholar] [CrossRef]
- Wang, X.G.; Yu, Y.; Zou, F.B.; Luo, H.Y.; Zhou, Z.H.; Zhu, J.L.; Guo, G.J.; Zhong, Y.W. High performance C-A-S-H seeds from fly ash-carbide slag for activating lithium slag towards a low carbon binder. J. Environ. Manag. 2023, 345, 118658. [Google Scholar] [CrossRef]
- Li, C.B.; Zhang, G.F.; Liu, D.Z.; Wang, M.T. Preparation of lightweight ceramsite from solid waste lithium slag and fly ash. Constr. Build. Mater. 2023, 398, 132419. [Google Scholar] [CrossRef]
- Li, J.Z.; Huang, S.W. Recycling of lithium slag as a green admixture for white reactive powder concrete. J. Mater. Cycles Waste 2020, 22, 1818–1827. [Google Scholar] [CrossRef]
- Wang, J.X.; Han, L.; Liu, Z.; Wang, D.M. Setting controlling of lithium slag-based geopolymer by activator and sodium tetraborate as a retarder and its effects on mortar properties. Cem. Concr. Compos. 2020, 110, 103598. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Yan, J.H.; Fan, J.Y.; Xu, Y.; Lu, Y.W.; Duan, P.; Zhu, Y.C.; Zhang, Z.H.; Lu, Z. Insight to workability, compressive strength and microstructure of lithium slag-steel slag based cement under standard condition. J. Build. Eng. 2023, 75, 107076. [Google Scholar] [CrossRef]
- Gu, X.W.; Wang, H.Y.; Zhu, Z.G.; Liu, J.P.; Xu, X.C.; Wang, Q. Synergistic effect and mechanism of lithium slag on mechanical properties and microstructure of steel slag-cement system. Constr. Build. Mater. 2023, 396, 131768. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Pan, Y.N.; Xu, K.C.; Bi, L.P.; Chen, M.C.; Han, B.G. Corrosion behavior of concrete fabricated with lithium slag as corrosion inhibitor under simulated acid rain corrosion action. J. Clean. Prod. 2022, 377, 134300. [Google Scholar] [CrossRef]
- Al-Fakih, A.; Al-Awsh, W.; Ahmed Al-Koshab, M.Q.; Al-Shugaa, M.A.; Al-Osta, M.A.; Drmosh, Q.A.; Musa, A.E.S.; Abdulqader, M.A.; Elgzoly, M.A.A.; Onaizi, S.A. Effects of zeolitic imidazolate framework-8 nanoparticles on physicomechanical properties and microstructure of limestone calcined clay cement mortar. Constr. Build. Mater. 2023, 366, 130236. [Google Scholar] [CrossRef]
- Zhan, P.M.; Zhang, X.X.; He, Z.H.; Shi, J.Y.; Gencel, O.; Hai Yen, N.T.; Wang, G.C. Strength, microstructure and nanomechanical properties of recycled aggregate concrete containing waste glass powder and steel slag powder. J. Clean. Prod. 2022, 341, 130892. [Google Scholar] [CrossRef]
- Chen, G.; Huang, Y.; Yang, R.; Yu, R.; Xiao, R.; Wang, Z.; Ke, X.; Xie, G.; Cheng, J.; Bao, M. Comparative study on mechanical properties and microstructure development of ultra-high performance concrete incorporating phosphorous slag under different curing regimes. Constr. Build. Mater. 2023, 392, 131963. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Shen, J.; Guo, P. High titanium heavy slag powder as a sustainability filler and its influence on the performance of asphalt mortar. J. Mater. Res. Technol. 2023, 25, 5586–5599. [Google Scholar] [CrossRef]
- Adomako, S.; Engelsen, C.J.; Døssland, L.T.; Danner, T.; Thorstensen, R.T. Technical and environmental properties of recycled aggregates produced from concrete sludge and excavation materials. Case Stud. Constr. Mater. 2023, 19, e02498. [Google Scholar] [CrossRef]
- Ahmad, S.; Upadhyay, S.; Umar, A.; Al-Osta, M.A. Effect of recycled crushed glass and recycled coarse aggregate on the properties of self-compacting concrete. Case Stud. Constr. Mater. 2023, 19, e02532. [Google Scholar] [CrossRef]
- Aliyah, F.; Kambali, I.; Setiawan, A.F.; Radzi, Y.M.; Rahman, A.A. Utilization of steel slag from industrial waste for ionizing radiation shielding concrete: A systematic review. Constr. Build. Mater. 2023, 382, 131360. [Google Scholar] [CrossRef]
- Ozturk, M.; Sevim, U.K.; Akgol, O.; Unal, E.; Karaaslan, M. Investigation of the mechanic, electromagnetic characteristics and shielding effectiveness of concrete with boron ores and boron containing wastes. Constr. Build. Mater. 2020, 252, 119058. [Google Scholar] [CrossRef]
- Santillán, N.; Speranza, S.; Torrents, J.M.; Segura, I. Evaluation of conductive concrete made with steel slag aggregates. Constr. Build. Mater. 2022, 360, 129515. [Google Scholar] [CrossRef]
- Liu, S.H.; Rong, P.J.; Zhang, C.; Lu, J.X.; Guan, X.M.; Shi, C.J.; Zhu, J.P. Preparation and carbonation hardening of low calcium CO2 sequestration materials from waste concrete powder and calcium carbide slag. Cem. Concr. Comp. 2023, 141, 105151. [Google Scholar] [CrossRef]
- Dong, J.L.; Chen, L.W.; Li, L.H.; Zhou, P.; Shi, Z.H.; Cai, J.P.; Zhang, T.T. Investigation into the alkali-activation of lithium slag: A sustainable alternative to conventional cement with optimized mechanical properties. Constr. Build. Mater. 2024, 416, 135022. [Google Scholar] [CrossRef]
- Guo, C.C.; Wang, R. Utilizing lithium slag to improve the physical-chemical properties of alkali-activated metakaolin-slag pastes: Cost and energy analysis. Constr. Build. Mater. 2023, 403, 133164. [Google Scholar] [CrossRef]
- Javed, U.; Shaikh, F.U.A.; Sarker, P.K. Microstructural investigation of lithium slag geopolymer pastes containing silica fume and fly ash as additive chemical modifiers. Cem. Concr. Comp. 2022, 134, 104736. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, B.G.; Tan, H.B.; Liu, X.H.; Chen, P.; Luo, Z.T. Effect of TIPA on mechanical properties and hydration properties of cement-lithium slag system. J. Environ. Manag. 2020, 276, 111274. [Google Scholar] [CrossRef]
- Zhai, M.Y.; Zhao, J.H.; Wang, D.M.; Wang, Y.R.; Wang, Q.B.a. Hydration properties and kinetic characteristics of blended cement containing lithium slag powder. J. Build. Eng. 2021, 39, 102287. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.H.; Hooton, R.D.; Zhang, X.; He, S. Effects of TEA on rheological property and hydration performance of lithium slag-cement composite binder. Constr. Build. Mater. 2022, 318, 125757. [Google Scholar] [CrossRef]
- Amin, M.T.E.; Sarker, P.K.; Shaikh, F.U.A. Transport properties of concrete containing lithium slag. Constr. Build. Mater. 2024, 416, 135073. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Yang, B.H.; Gu, X.W.; Han, D.; Wang, Q.J. Improving the performance of ultra-high performance concrete containing lithium slag by incorporating limestone powder. J. Build. Eng. 2023, 72, 106610. [Google Scholar] [CrossRef]
- Rahman, S.A.; Dodd, A.; Khair, S.; Shaikh, F.U.A.; Sarker, P.K.; Hosan, A. Assessment of lithium slag as a supplementary cementitious material: Pozzolanic activity and microstructure development. Cem. Concr. Comp. 2023, 143, 105262. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Gu, X.W.; Wang, S.Y.; Xu, X.C.; Wang, H.Y.; Liu, J.P.; Zhan, K. Investigation of synergistic effects of lithium slag and granulated blast furnace slag from the perspectives of physics and hydration. J. Build. Eng. 2024, 89, 109337. [Google Scholar] [CrossRef]
- Dong, S.K.; Tu, S.W.; Chen, L.L.; Chen, L.L.; Wu, F.F.; Xie, L.L.; Zhuo, Q.; Yu, S.H. Investigation of the performance of cement mortar incorporating lithium slag as a super-fine aggregate. Front. Mater. 2023, 10, 129979. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.S.; Hong, S.X.; Xing, F.; Dong, B.Q. Properties and microstructure of lithium-slag-based geopolymer by one-part mixing method, Constr. Build. Mater. 2021, 273, 121723. [Google Scholar] [CrossRef]
- GB 175-2023; Common Portland Cement. China Standards Press: Beijing, China, 2023.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. China Standards Press: Beijing, China, 2005.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). China Standards Press: Beijing, China, 2021.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Standards Press: Beijing, China, 2009.
- Zhang, S.; Ding, J.W.; Liu, J.Y.; Gao, M.Y.; Xue, C.R. Al2O3/SO3 molar ratio of PG-CAC-based binders on the macro-micro properties of cement-treated waste dredged clay. Constr. Build. Mater. 2024, 424, 135950. [Google Scholar] [CrossRef]
- Wang, Y.L.; He, H.; He, F.X. Effect of slaked lime and aluminum sulfate on the properties of dry-mixed masonry mortar. Constr. Build. Mater. 2018, 180, 117–123. [Google Scholar] [CrossRef]
- Liang, S.M.; Liu, Y.H.; Song, G.F.; Yan, H.; Song, B.C.; Liu, J.K. Effect of sulfoaluminate expansive additive on mechanical properties, internal relative humidity, and shrinkage of early-age mortar. Case Stud. Constr. Mater. 2023, 19, e02226. [Google Scholar] [CrossRef]
- Snehal, K.; Das, B.B.; Barbhuiya, S. Synergistic effect of nano silica on carbonation resistance of multi-blended cementitious mortar. Cem. Concr. Comp. 2023, 141, 105125. [Google Scholar] [CrossRef]
- Alwaeli, M.; Gołaszewski, J.; Niesler, M.; Pizoń, J.; Gołaszewska, M. Recycle option for metallurgical sludge waste as a partial replacement for natural sand in mortars containing CSA cement to save the environment and natural resources. J. Hazard. Mater. 2020, 398, 123101. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, B.; Liu, S.; He, J.; Hernandez, A.G. Role of recycled concrete powder as sand replacement in the properties of cement mortar. J. Clean. Prod. 2022, 371, 133424. [Google Scholar] [CrossRef]
- Darvish, P.; Johnson Alengaram, U.; Soon Poh, Y.; Ibrahim, S.; Yusoff, S. Performance evaluation of palm oil clinker sand as replacement for conventional sand in geopolymer mortar. Constr. Build. Mater. 2020, 258, 120352. [Google Scholar] [CrossRef]
- Lu, L.; Yang, Z.X.; Lin, Y.; Dong, S.L. Partial replacement of manufactured sand with homologous granite powder in mortar: The effect on porosity and capillary water absorption. Constr. Build. Mater. 2023, 376, 131031. [Google Scholar] [CrossRef]
- Sharma, R.; Das, K.K.; Pei, J.J.; Jang, J.G. Influence of sillimanite sand and corundum sand on the mechanical property, durability and pore structure of cement mortar. J. Build. Eng. 2023, 80, 108091. [Google Scholar] [CrossRef]
- Hemkemeier, T.A.; Almeida, F.C.R.; Sales, A.; Klemm, A.J. Repair mortars with water treatment plant sludge (WTPS) and sugarcane bagasse ash sand (SBAS) for more eco-efficient and durable constructions. J. Clean. Prod. 2023, 386, 135750. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Xiao, J.Z.; Duan, Z.H.; Hubert, J.; Grigoletto, S.; Courard, L. Performance and durability of self-compacting mortar with recycled sand from crushed brick. J. Build. Eng. 2022, 57, 104867. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).