Research on Preventing High-Density Materials from Settling in Liquid Resin
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Reagents and Instruments
2.2. Surface Modification of Magnetic Particles
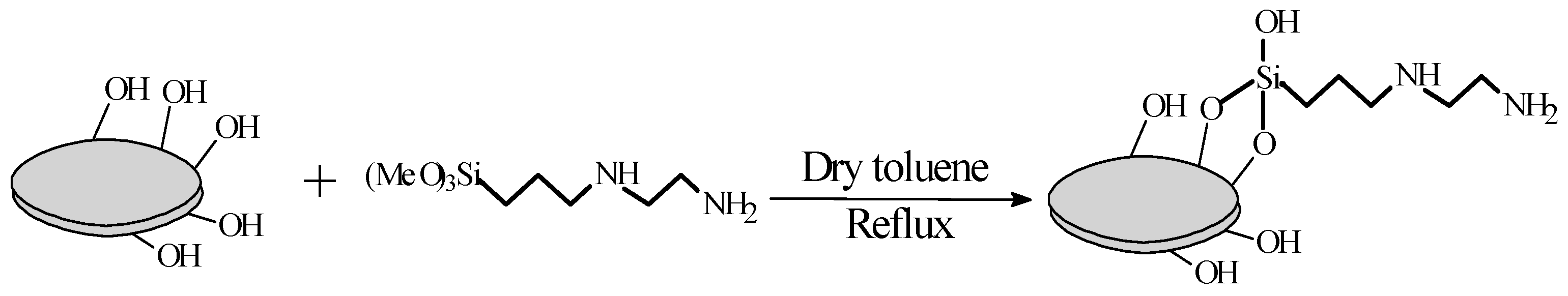
2.3. Surface Modification of Hollow Silica
2.4. Preparation of Low-Density Magnetic Particles
3. Results and Discussion
3.1. Surface Modification of Hollow Silica
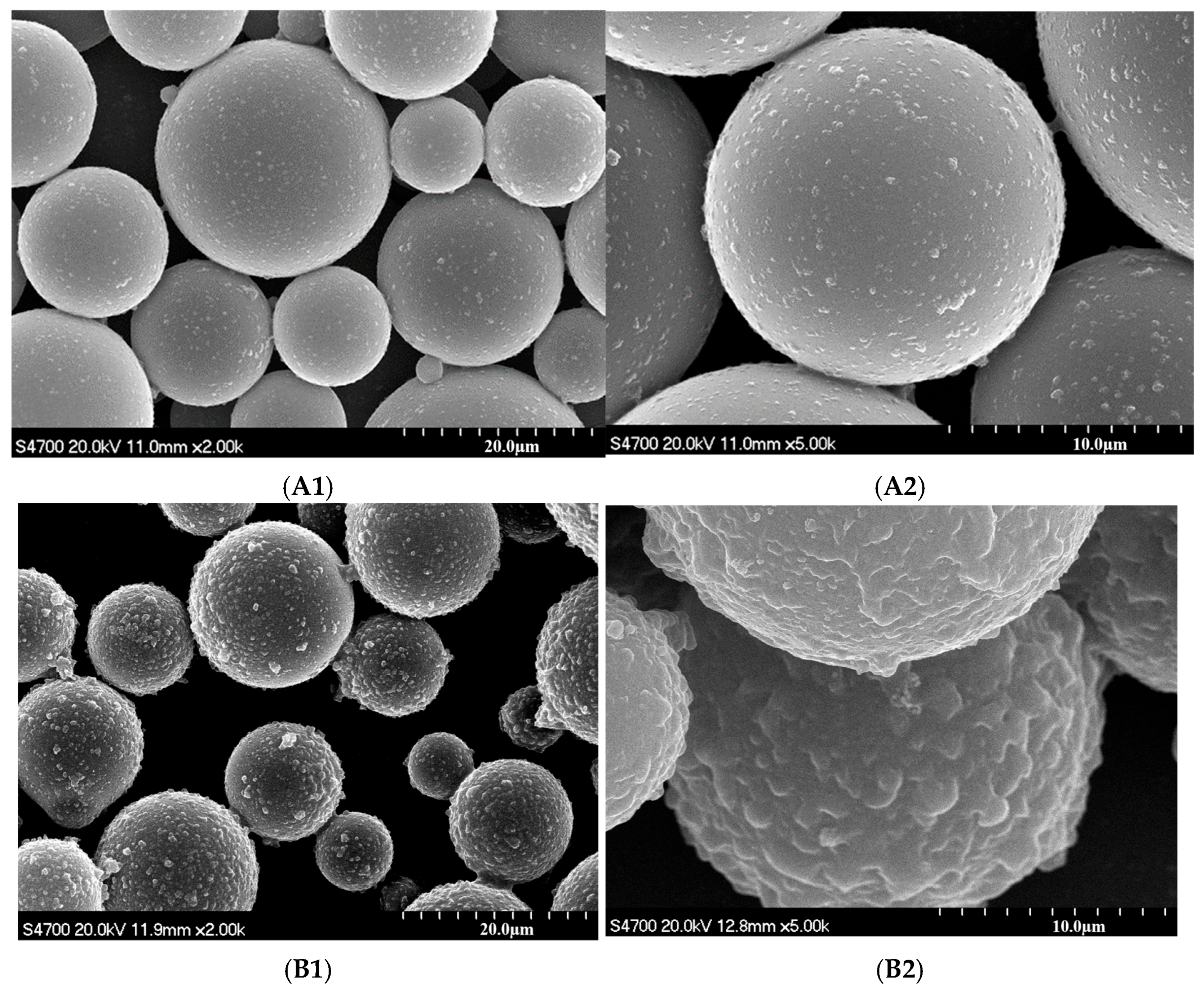
3.2. Characterization of Modified CIP
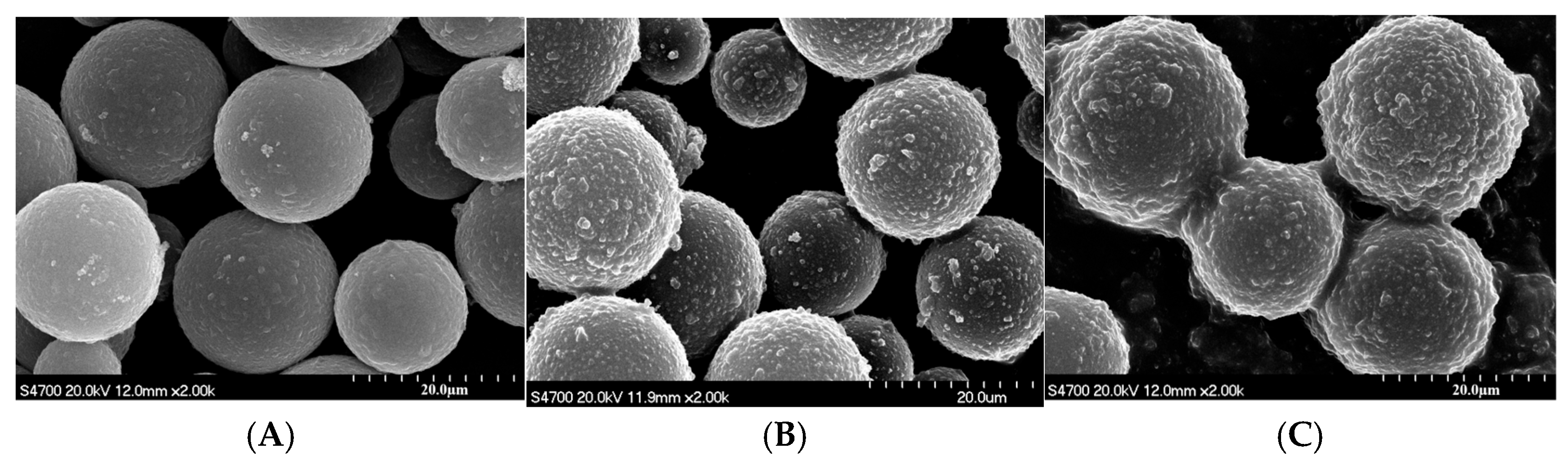
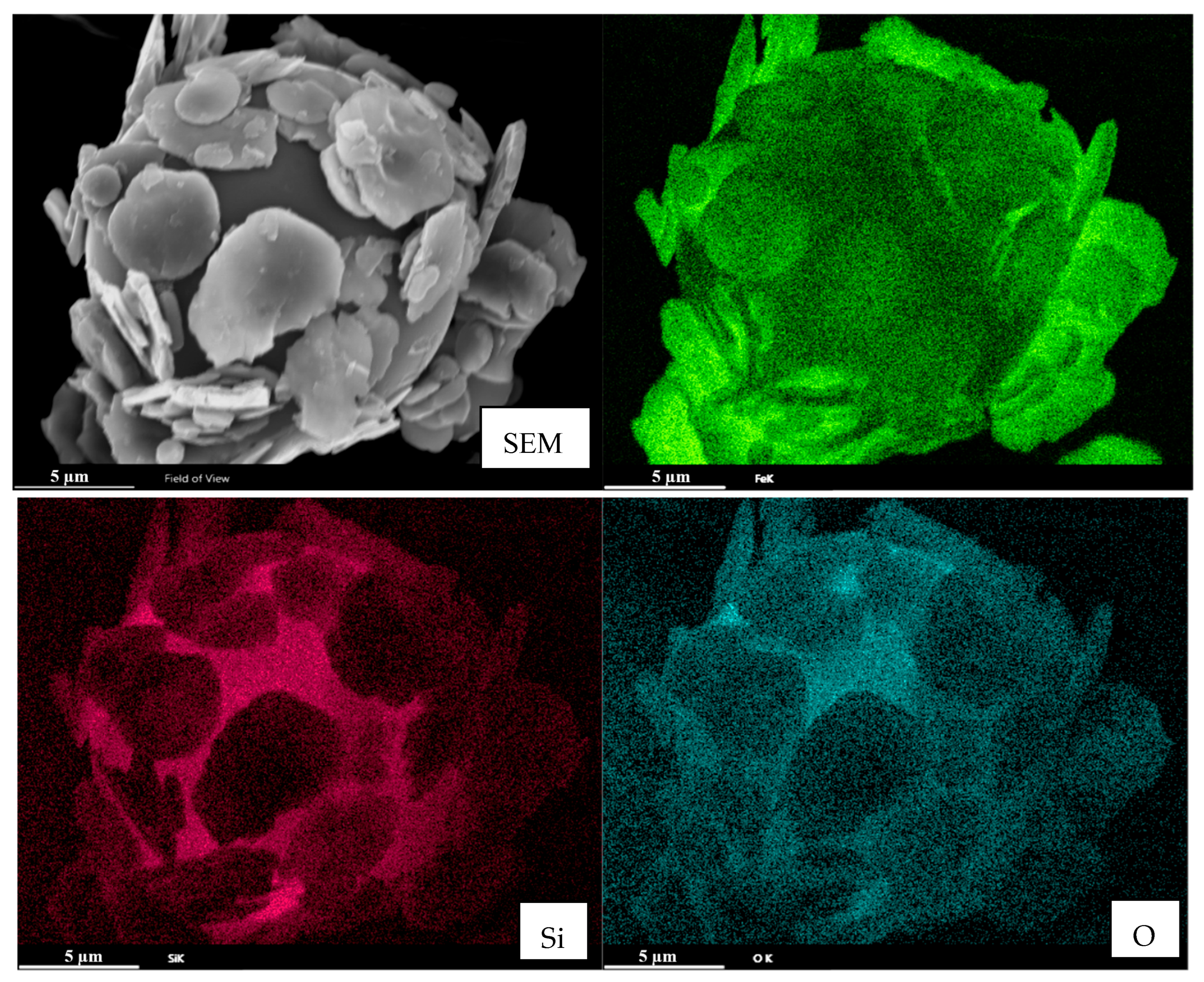
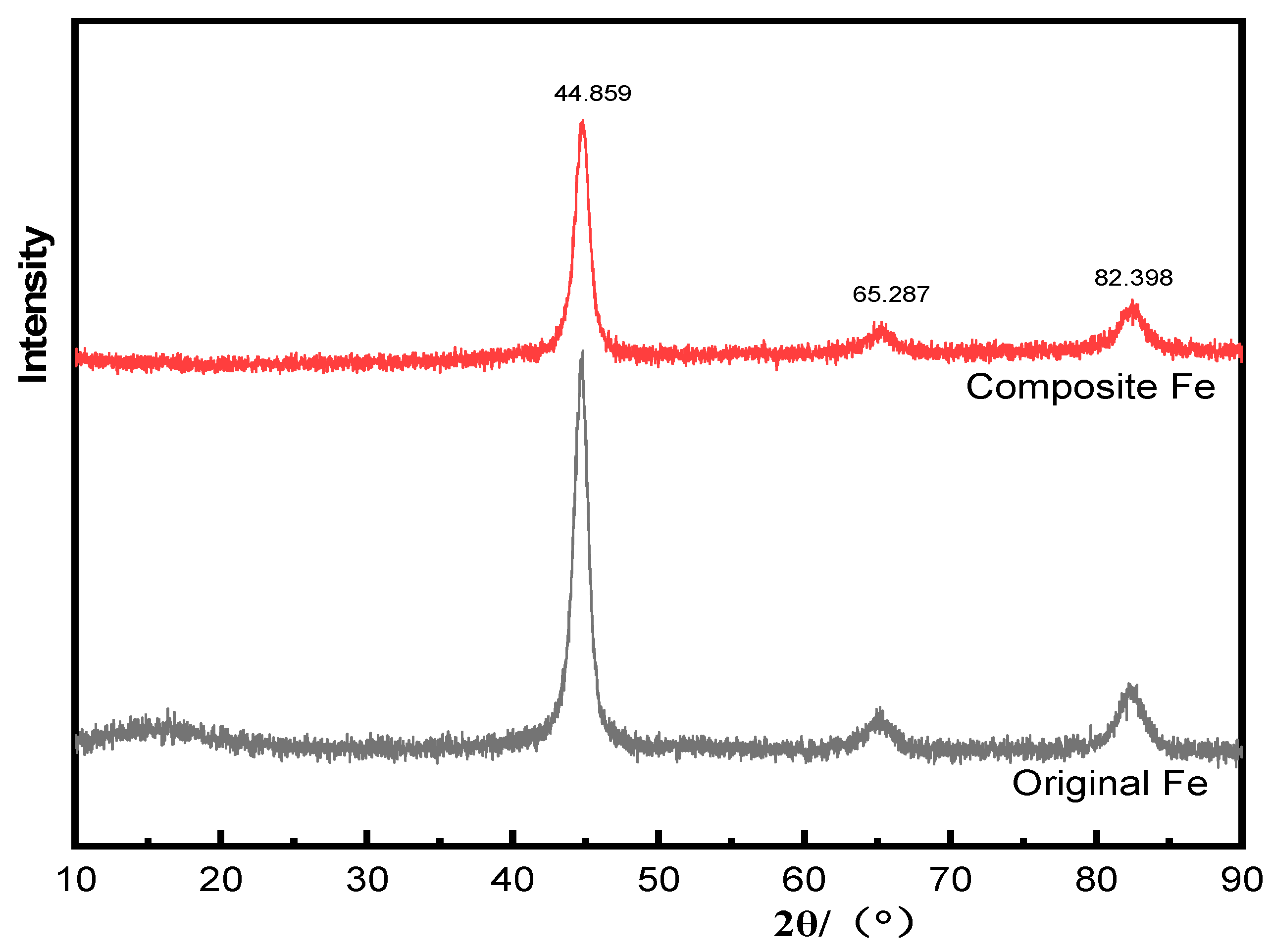
3.3. Morphological and Structural Characterization of Magnetic Composite Materials
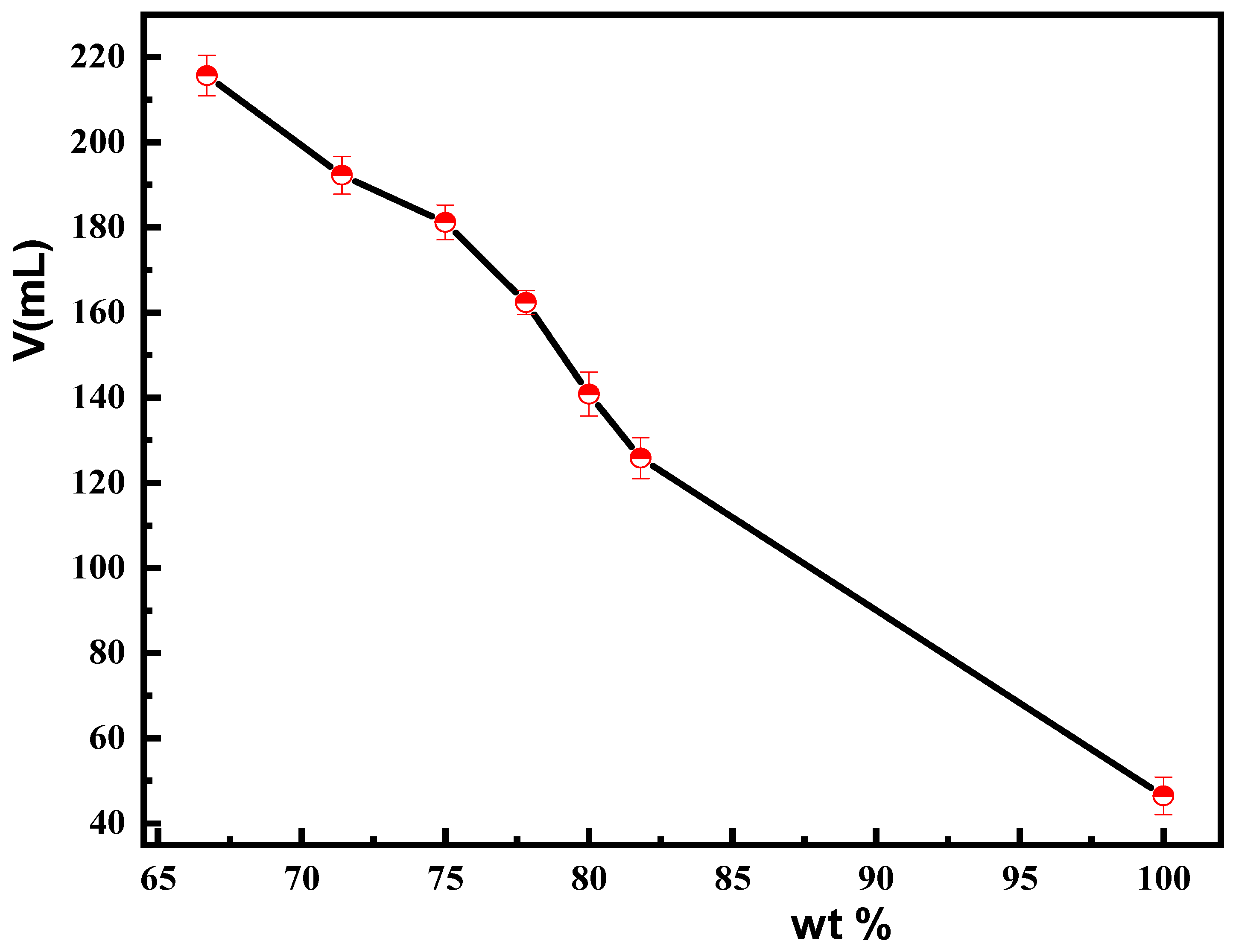
3.4. Characterization of Suspension Properties of Magnetic Composite Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutfleisch, O.; Willard, M.A.; Bruck, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Jeong, U.; Teng, X.; Wang, Y.; Yang, H.; Xia, Y. Superparamagnetic Colloids: Controlled Synthesis and Niche Applications. Adv. Mater. 2007, 19, 33–60. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.M.; Dong, X.L.; Duan, Y.P. Electromagnetic Wave Shielding and Absorbing Materials; Chemical Industry Press: Beijing, China, 2013; pp. 54–60. [Google Scholar]
- Chen, Q.; Li, L.; Wang, Z.; Ge, Y.; Zhou, C.; Yi, J. Synthesis and enhanced microwave absorption performance of CIP@SiO2@Mn0.6Zn0.4Fe2O4 ferrite composites. J. Alloys Compd. 2019, 779, 720–727. [Google Scholar] [CrossRef]
- Ding, X.; Huang, Y.; Wang, J.; Wu, H.; Liu, P. Excellent electromagnetic wave absorption property of quaternary composites consisting of reduced graphene oxide, polyaniline and FeNi3@SiO2 nanoparticles. Appl. Surf. Sci. 2015, 357, 908–914. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Bai, S.X. Research on Ferrite/Carbonyl Iron Powder Composite Absorbing Materials. Ordnance Mater. Sci. Eng. 2007, 30, 39. [Google Scholar]
- Gong, J.H.; Qiu, T.; Shen, C.Y. Study on the Physical Properties of Carbonyl Iron Powder/Silicone Rubber Composite System. J. Mater. Sci. Eng. 2006, 24, 718–721. [Google Scholar]
- Qing, Y.; Zhou, W.; Luo, F.; Zhu, D. Microwave-absorbing and mechanical properties of carbonyl-iron/epoxy-silicone resin coatings. J. Magn. Magn. Mater. 2009, 321, 25. [Google Scholar] [CrossRef]
- Song, Z.; Xie, J.; Zhou, P.; Wang, X.; Liu, T.; Deng, L. Toughened polymer composites with flake carbonyl iron powders and their electromagnetic/absorption properties. J. Alloys Compd. 2013, 551, 677–681. [Google Scholar] [CrossRef]
- Kim, I.; Bae, S.; Kim, J. Composition effect on high frequency properties of carbonyl-iron composites. Mater. Lett. 2008, 62, 3043–3046. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Y.; Liu, S.; Chen, L.; Guo, J. Microwave absorption properties of one thin sheet employing carbonyl-iron powder and chlorinated polyethylene. J. Magn. Magn. Mater. 2010, 322, 1736–1740. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, G.; Gu, S.; Li, S.; Ma, G. Study on microwave absorbing properties of carbonyl-iron composite coating based on PVC and Al sheet. Appl. Surf. Sci. 2012, 258, 5746–5752. [Google Scholar]
- Ma, Z.X.; Yang, B.B.; Yang, J.J. Surface coating preparation of spherical magnetic materials. RSC Adv. 2022, 12, 9836–9844. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Chen, Z.; Yang, J.J. Verification of Reinforced Surface Loose Layer of Zinc–Aluminum–Magnesium Steel Plate. Materials 2023, 16, 6221. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Shi, C.; Wang, T.; Li, X.; Ma, J. Recent progress in hollow silica: Template synthesis, morphologies and applications. Microporous Mesoporous Mater. 2016, 227, 121–136. [Google Scholar] [CrossRef]




| Drug Name | Chemical Formula | Purity | Manufacturer |
|---|---|---|---|
| Carbonyl iron | Fe | 99.95% | Henan Fluorine-based New Materials Industry Research Institute, Zhengzhou, China |
| Hollow silica | SiO2 | 99% | 3M Company, Maplewood, MN, USA |
| N-[3-(Trimethoxysilyl)propyl]ethylenediamine | NH2CH2CH2NHCH2CH2CH2Si(OCH3)3 | Analytical pure | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Vinyltrimethoxysilane | CH2=CHSi(OCH3)3 | Analytical pure | Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China |
| Toluene | C7H8 | Analytical pure | China National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China |
| Acetone | CH3COCH3 | Analytical pure | Beijing Chemical Plant Co., Ltd., Beijing, China |
| Maleic acid | HO2CCH=CHCO2H | Analytical pure | Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China |
| Acrylic acid | CH2=CHCO2H | Analytical pure | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Azobisisobutyronitrile | C8H18N4 | Analytical pure | Shanghai Aladdin Biochemical Technology Co., Ltd. |
| Ethanol | CH3CH2OH | Analytical pure | Beijing Chemical Plant Co., Ltd., Beijing, China |
| Experimental Instrument Name | Instrument Model | Producer |
|---|---|---|
| Electronic balance | AL204 | Mettler-Toledo International Trading (Shanghai) Co., Ltd., Shanghai, China |
| pH meter | FE28 | Mettler-Toledo International Trading (Shanghai) Co., Ltd. China |
| CNC ultrasonic cleaner | KQ-400DB | KunShan Ultrasonic Instruments Co., Ltd., Kunshan, China |
| Electric blast constant temperature | 101-0B | Shaoxing Yuanmore Machine and Electrical Equipment Co., Ltd., Shaoxing, China |
| Field emission scanning electron microscope | HITACHI S-4700 | Hitachi, Tokyo, Japan |
| Fourier Transform Infrared Spectroscopy | Nicolet8700 | Thermo Nicolet Co., Ltd., Madison, WI, USA |
| X-ray diffractometer | XRD-6000 | Shimadzu, Kyoto, Japan |
| X-ray photoelectron spectrometer | ESCALAB 250 | ThermoFisher Scientific, Waltham, MA, USA |
| Element Name | Unmodified CIP (wt%) | Modified CIP (wt%) |
|---|---|---|
| Si | 0.25 | 4.71 |
| C | 5.67 | 20.38 |
| N | 0.17 | 3.21 |
| O | 12.89 | 25.46 |
| Fe | 81.02 | 46.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, L.; Wang, Z.; Yang, X.; Wu, X.; Yang, J.; Zheng, S. Research on Preventing High-Density Materials from Settling in Liquid Resin. Materials 2025, 18, 3469. https://doi.org/10.3390/ma18153469
Xuan L, Wang Z, Yang X, Wu X, Yang J, Zheng S. Research on Preventing High-Density Materials from Settling in Liquid Resin. Materials. 2025; 18(15):3469. https://doi.org/10.3390/ma18153469
Chicago/Turabian StyleXuan, Lixin, Zhiqiang Wang, Xuan Yang, Xiao Wu, Junjiao Yang, and Shijun Zheng. 2025. "Research on Preventing High-Density Materials from Settling in Liquid Resin" Materials 18, no. 15: 3469. https://doi.org/10.3390/ma18153469
APA StyleXuan, L., Wang, Z., Yang, X., Wu, X., Yang, J., & Zheng, S. (2025). Research on Preventing High-Density Materials from Settling in Liquid Resin. Materials, 18(15), 3469. https://doi.org/10.3390/ma18153469




