Modification of Silver-Loaded Biodegradable Polymer Nanoparticles with Bacterial Membrane Vesicles for Treating Intracellular Bacterial Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of NPs
2.3. Characterization of NPs
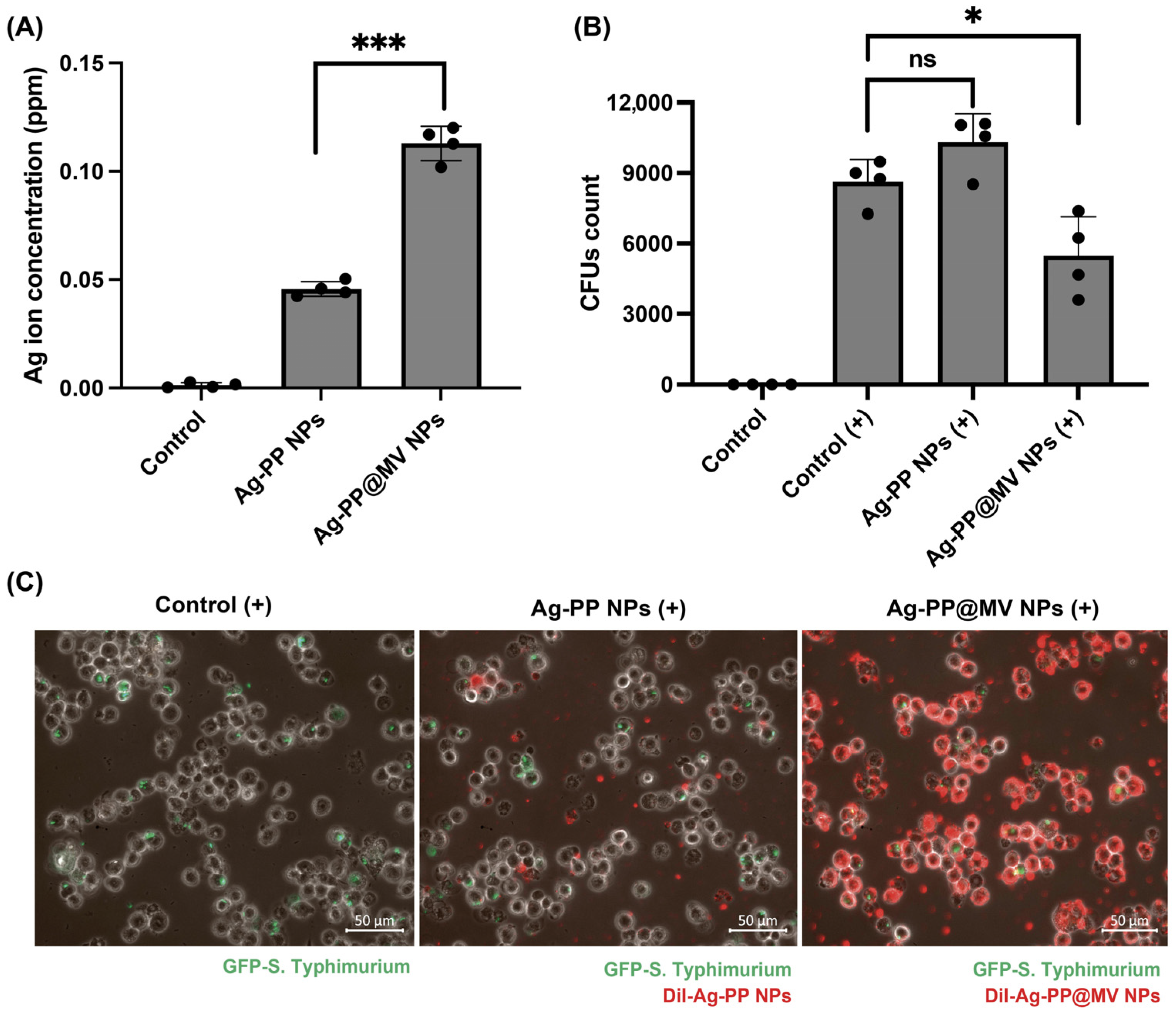
2.4. Silver Ions Released from NPs
2.5. Cytotoxicity Evaluation
2.6. Cellular Uptake of NPs by RAW 264.7 Cells
2.7. Antibacterial Activity of NPs Against Intracellular Bacteria
2.8. Statistical Analysis
3. Results and Discussion
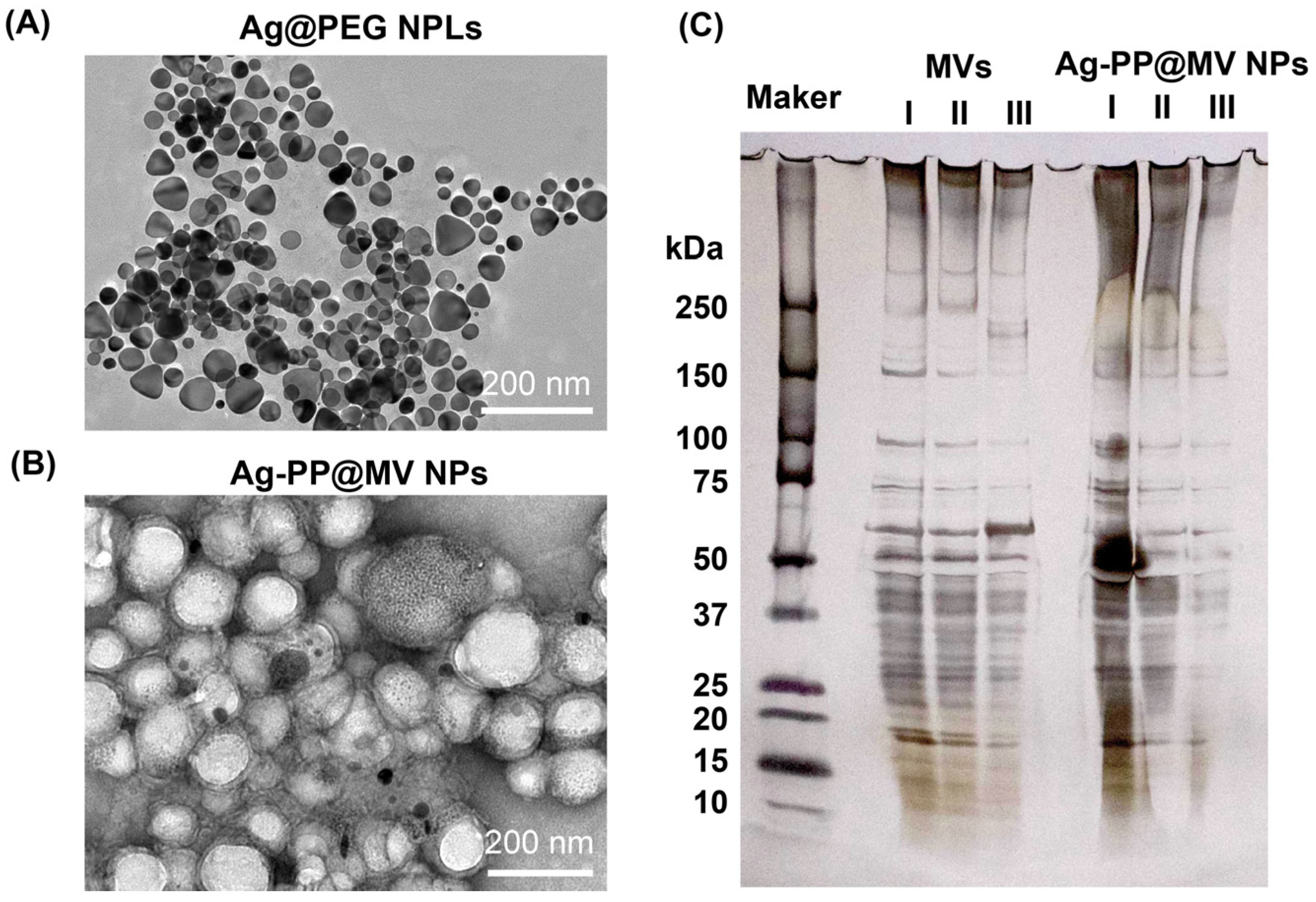
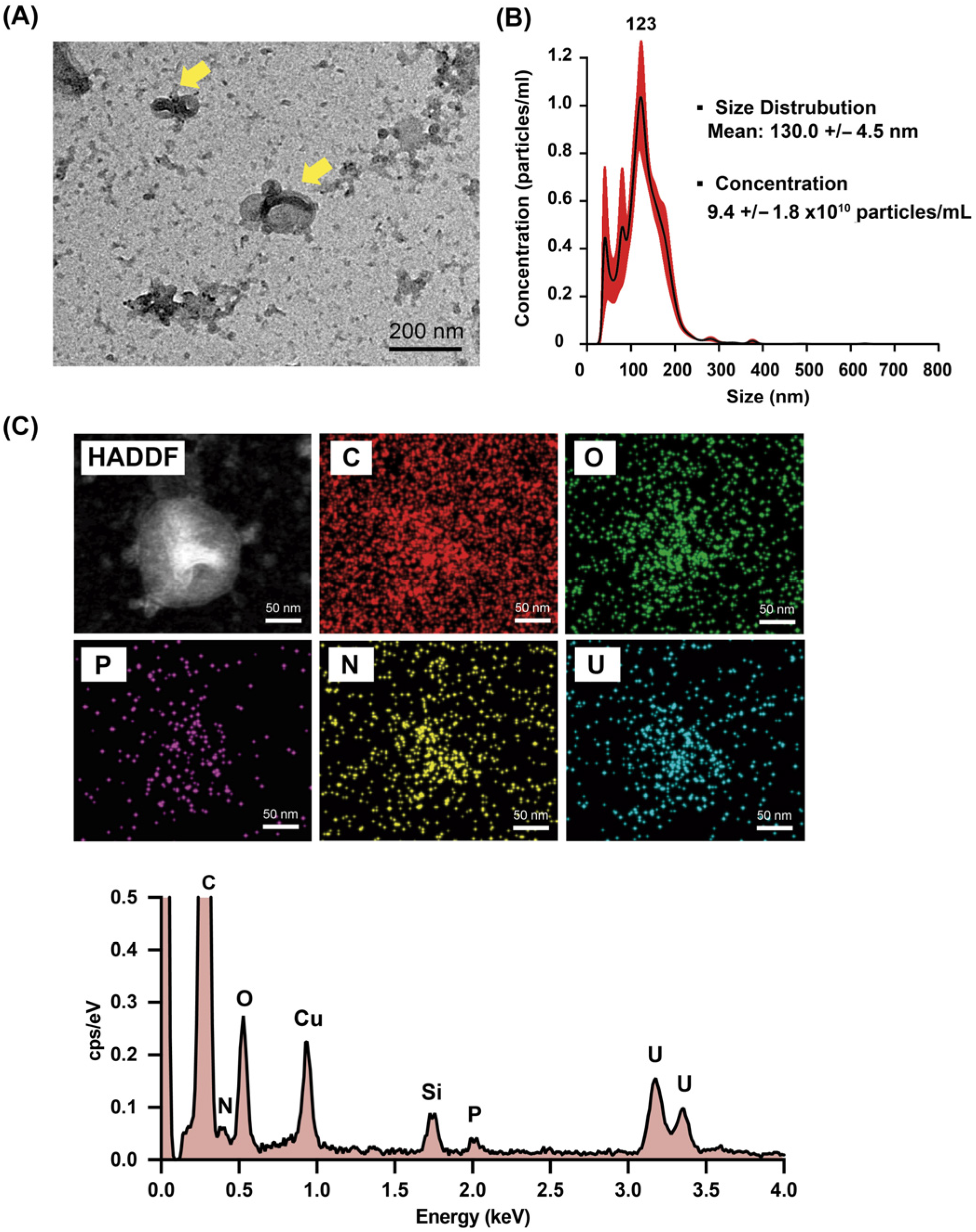
3.1. Characterization of NPs
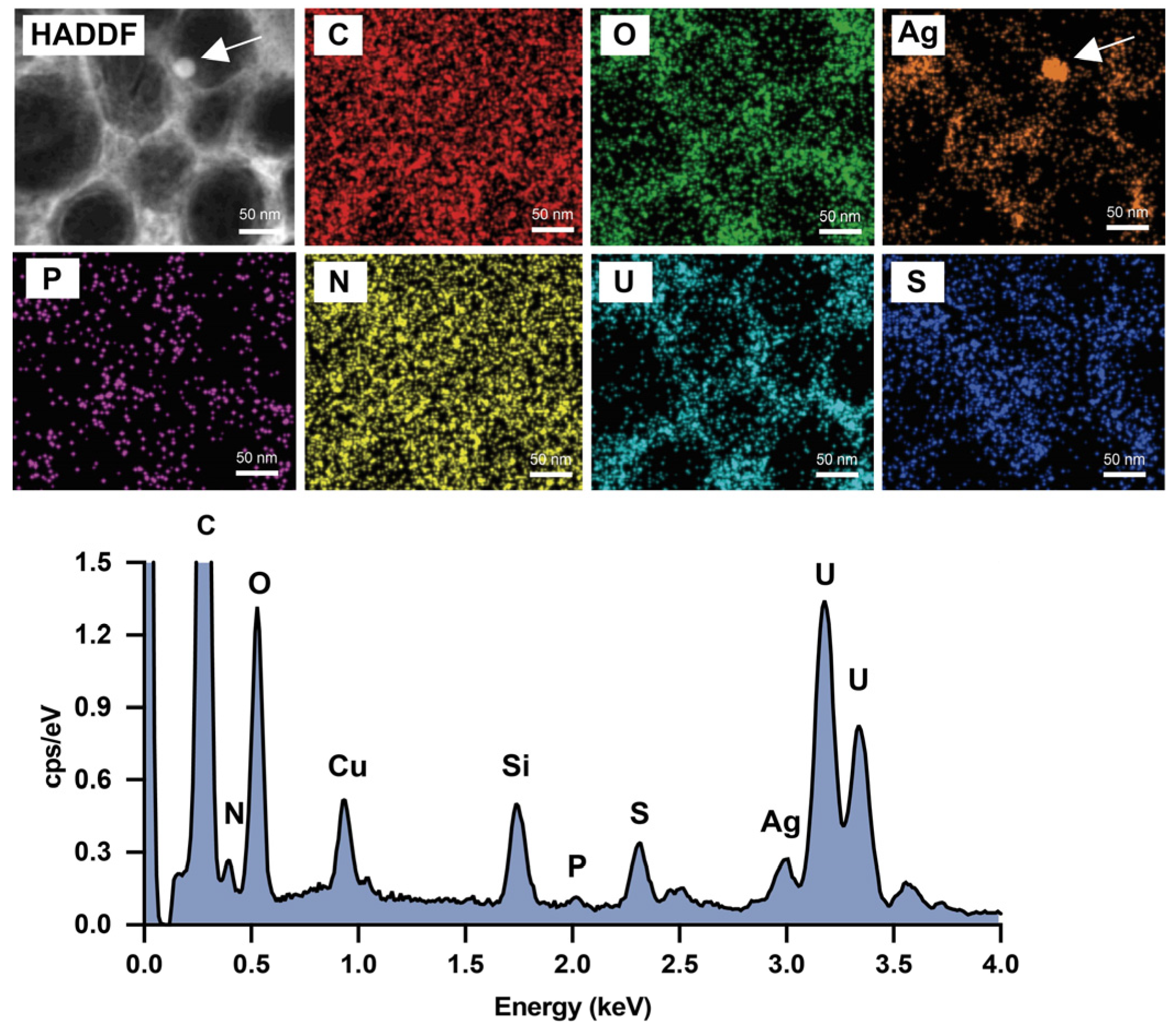
3.2. Morphology and Elemental Composition Analysis
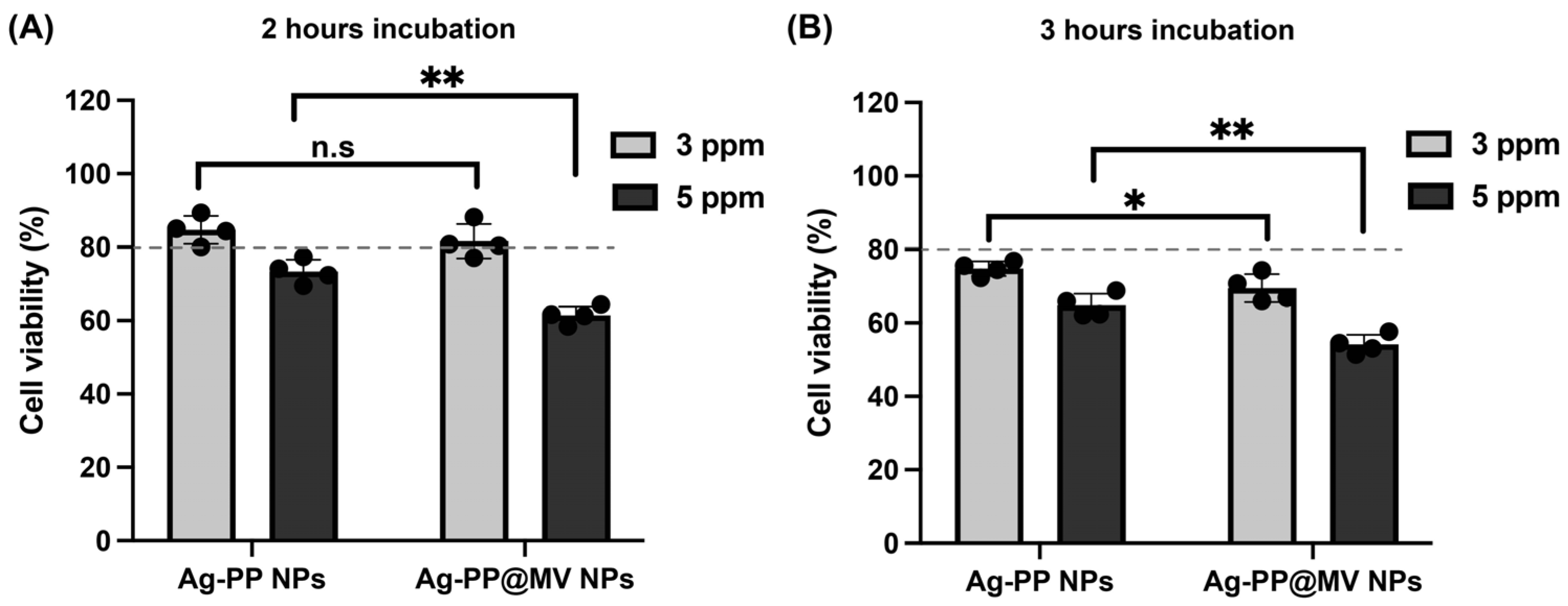
3.3. Silver Ions Released from NPs and Cytotoxicity Evaluation
3.4. Cellular Uptake of NPs by Macrophages and Antibacterial Activity of NPs Against Intracellular Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flannagan, R.; Cosío, G.; Grinstein, S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 2009, 7, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Reuter, T.; Vorwerk, S.; Liss Viktoria Chao, T.C.; Hensel, M.; Hansmeier, N. Proteomic analysis of Salmonella-modified membranes reveals adaptations to macrophage hosts. Mol. Cell. Proteom. 2020, 18, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J., II; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An overview of epidemiology, pathogenesis, and innovative approaches to mitigate the antimicrobial resistant infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, E.W.; Yang, Q. Clinical perspective of antimicrobial resistance in bacteria. Infect. Drug Resist. 2022, 15, 735–746. [Google Scholar] [CrossRef]
- Sridhar, S.; Forrest, S.; Pickard, D.; Cormie, C.; Lees, E.A.; Thomson, N.R.; Dougan, G.; Baker, S. Inhibitory Concentrations of Ciprofloxacin Induce an Adaptive Response Promoting the Intracellular Survival of Salmonella enterica Serovar Typhimurium. mBio 2021, 12, 1128. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef]
- Nawata, R.; Maruyama, S.; Xu, W.; Niidome, T. Encapsilation of silver nanoplates into poly(lactic-co-glycolic) acid nanoparticles and the antibacterial activity against intracellular bacteria. Chem. Lett. 2024, 54, upae084. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am. J. Transl. Res. 2016, 8, 749–764. [Google Scholar] [PubMed] [PubMed Central]
- Nam, Y.S.; Kang, H.S.; Park, J.Y.; Park, T.G.; Han, S.H.; Chang, I.S. New micelle-like polymer aggregated made from PEI-PLGA deblock copolymers: Micellar characteristics and cellular uptake. Biomaterials 2003, 24, 2053–2059. [Google Scholar] [CrossRef]
- Aytar Çelik, P.; Erdogan-Gover, K.; Barut, D.; Enuh, B.M.; Amasya, G.; Sengel-Türk, C.T.; Derkus, B.; Çabuk, A. Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy. Pharmaceutics 2023, 15, 1052. [Google Scholar] [CrossRef]
- Laughlin, R.C.; Mickum, M.; Bowin, K.; Adams, G.; Alaniz, R.C. Altered host immune responses to membrane vesicles from Salmonella and Gram-negative pathogens. Vaccine 2015, 33, 5012–5019. [Google Scholar] [CrossRef] [PubMed]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane vesicles are immunogenic facsimiles of salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses and stimulate protective immunity in vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, M.; Zeng, Y.; Miao, T.; Luo, H.; Tong, Y.; Zhao, M.; Mu, R.; Gu, J.; Yang, S.; et al. Biomimetic lipopolysaccharide-free bacterial outer membrane-functionalized nanoparticles for brain-targeted drug delivery. Adv. Sci. 2022, 9, 2105854. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Ansong, C.; Adkins, J.N.; Heffron, F. Discovery of Salmonella Virulence Factors Translocated via Outer Membrane Vesicles to Murine Macrophages. Infect. Immun. 2011, 79, 2182–2192. [Google Scholar] [CrossRef]
- Wu, N.; Han, Z.; Lv, W.; Huang, Y.; Zhu, J.; Deng, J.; Xue, Q. Reprogramming peritoneal macrophages with outer membrane vesicles-coated PLGA nanoparticles for endometriosis prevention. Biomaterials 2025, 319, 123198. [Google Scholar] [CrossRef]
- Xu, W.; Maruyama, S.; Sato, A.; Niidome, T. Bacterial membrane vesicles combined with nanoparticles for bacterial vaccines and cancer immunotherapy. Colloids Surf. B Biointerfaces 2024, 243, 114125. [Google Scholar] [CrossRef]
- Chen, S.; Carroll, D.L. Silver nanoplates: Size control in two dimensions and formation mechanisms. J. Phys. Chem. B 2004, 108, 5500–5506. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Emwas, A.H.; Jaremko, M.; Hussien, M.A. Complexation of uranyl (UO2)2+ with bidentate ligands: XRD, spectroscopic, computational, and biological studies. PLoS ONE 2021, 16, e0256186. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]
- Dumontel, B.; Susa, F.; Limongi, T.; Vighetto, V.; Debellis, D.; Canta, M.; Cauda, V. Nanotechnological engineering of extracellular vesicles for the development of actively targeted hybrid nanodevices. Cell Biosci. 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, M.; Zhang, J.; Zhang, X.; Shen, K.; Wang, R.; Miron, R.J.; Xiao, Y.; Zhang, Y. Autologous versatile vesicles-incorporated biomimetic extracellular matrix induces biomineralization. Adv. Funct. Mater. 2020, 30, 2000015. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Liu, Q.; Jiang, G. Rethinking stability of silver sulfide nanoparticles (Ag2S-NPs) in the aquatic environment: Photoinduced transformation of Ag2S-NPs in the presence of Fe(III). Environ. Sci. Technol. 2016, 50, 188–196. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Hu, B.; Zhang, X.; Kong, Q. Outer membrane vesicles from flagellin-deficient Salmonella enterica serovar Typhimurium induce cross-reactive immunity and provide cross-protection against heterologous Salmonella challenge. Sci. Rep. 2016, 6, 34776. [Google Scholar] [CrossRef]
- Gnopo, Y.; Misra, A.; Hsu, H.L.; DeLisa, M.P.; Daniel, S.; Putnam, D. Induced fusion and aggregation of bacterial outer membrane vesicles: Experimental and theoretical analysis. Colloids Surf. B Biointerfaces 2020, 578, 522–532. [Google Scholar] [CrossRef]
- Dehinwal, R.; Gopinath, T.; Smith, R.D.; Ernst, R.K.; Schifferli, D.M.; Waldor, M.K.; Marassi, F.M. A pH-sensitive motif in an outer membrane protein activates bacterial membrane vesicle production. Nat. Commun. 2024, 15, 6958. [Google Scholar] [CrossRef]

| Nanoparticles | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| Ag@PEG NPLs | 4.0; 56.8 | −27.8 |
| MVs | 132.9 | −25.3 |
| Ag-P NPs | 245.9 | −27.6 |
| Ag-PP NPs | 218.5 | +26.8 |
| Ag-PP@MVs NPs | 250.1 | −16.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Maruyama, S.; Niidome, T. Modification of Silver-Loaded Biodegradable Polymer Nanoparticles with Bacterial Membrane Vesicles for Treating Intracellular Bacterial Infections. Materials 2025, 18, 3470. https://doi.org/10.3390/ma18153470
Xu W, Maruyama S, Niidome T. Modification of Silver-Loaded Biodegradable Polymer Nanoparticles with Bacterial Membrane Vesicles for Treating Intracellular Bacterial Infections. Materials. 2025; 18(15):3470. https://doi.org/10.3390/ma18153470
Chicago/Turabian StyleXu, Wei, Sayo Maruyama, and Takuro Niidome. 2025. "Modification of Silver-Loaded Biodegradable Polymer Nanoparticles with Bacterial Membrane Vesicles for Treating Intracellular Bacterial Infections" Materials 18, no. 15: 3470. https://doi.org/10.3390/ma18153470
APA StyleXu, W., Maruyama, S., & Niidome, T. (2025). Modification of Silver-Loaded Biodegradable Polymer Nanoparticles with Bacterial Membrane Vesicles for Treating Intracellular Bacterial Infections. Materials, 18(15), 3470. https://doi.org/10.3390/ma18153470







