Preparation of Magnetic Carbon Composite from Waste Amine-Oxime Resin and Its Adsorption Properties for Chromium
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Materials, and Apparatus
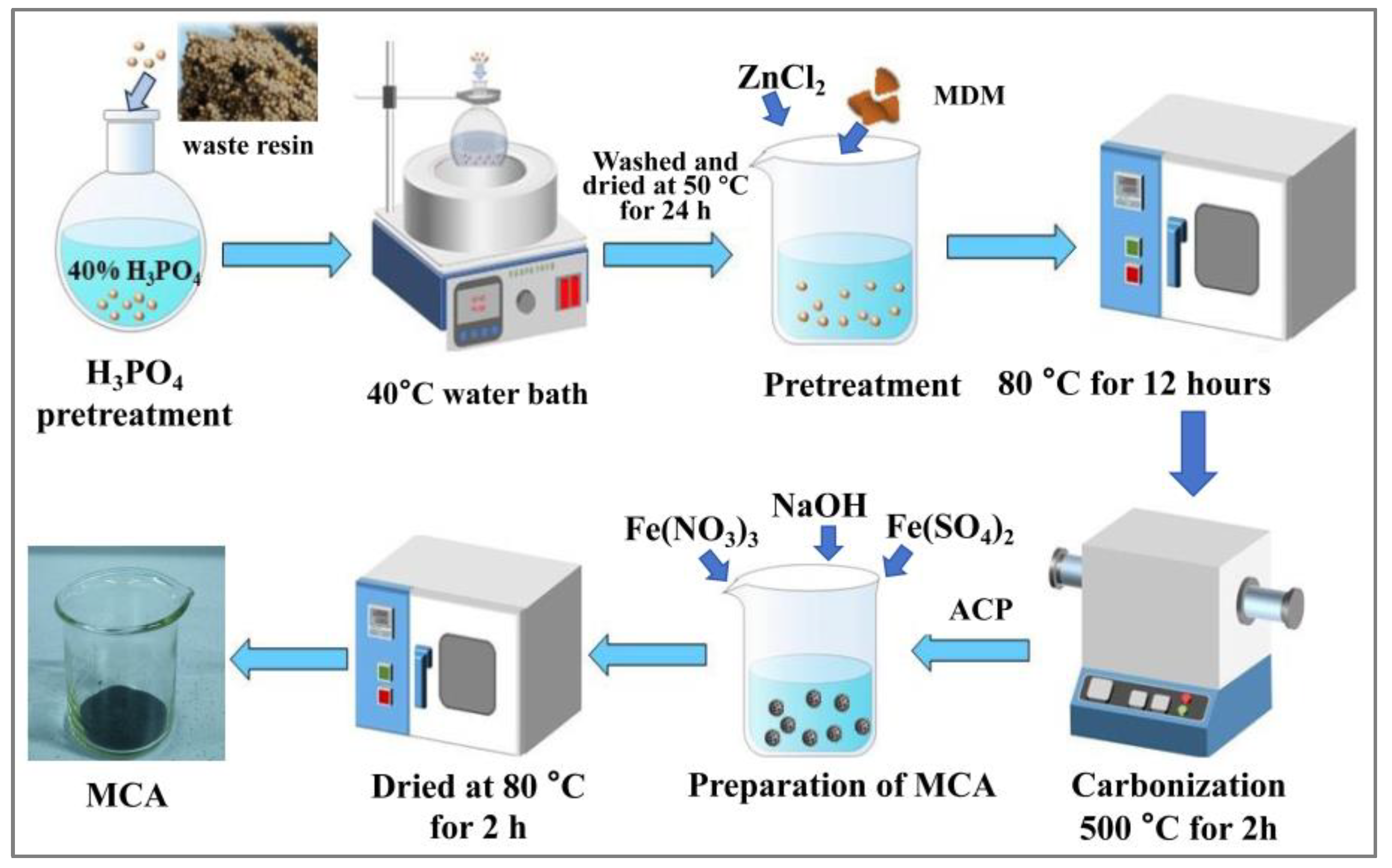
2.2. Preparation of Materials
2.2.1. Carbonization
2.2.2. Preparation of the MCA
2.3. Experimental
2.3.1. Adsorption Conditions and Experimental Parameters
2.3.2. The Model Fitted to the Data Used in the Experiment
2.3.3. Material Characterization and Mechanism Analysis Methods
3. Results and Discussion
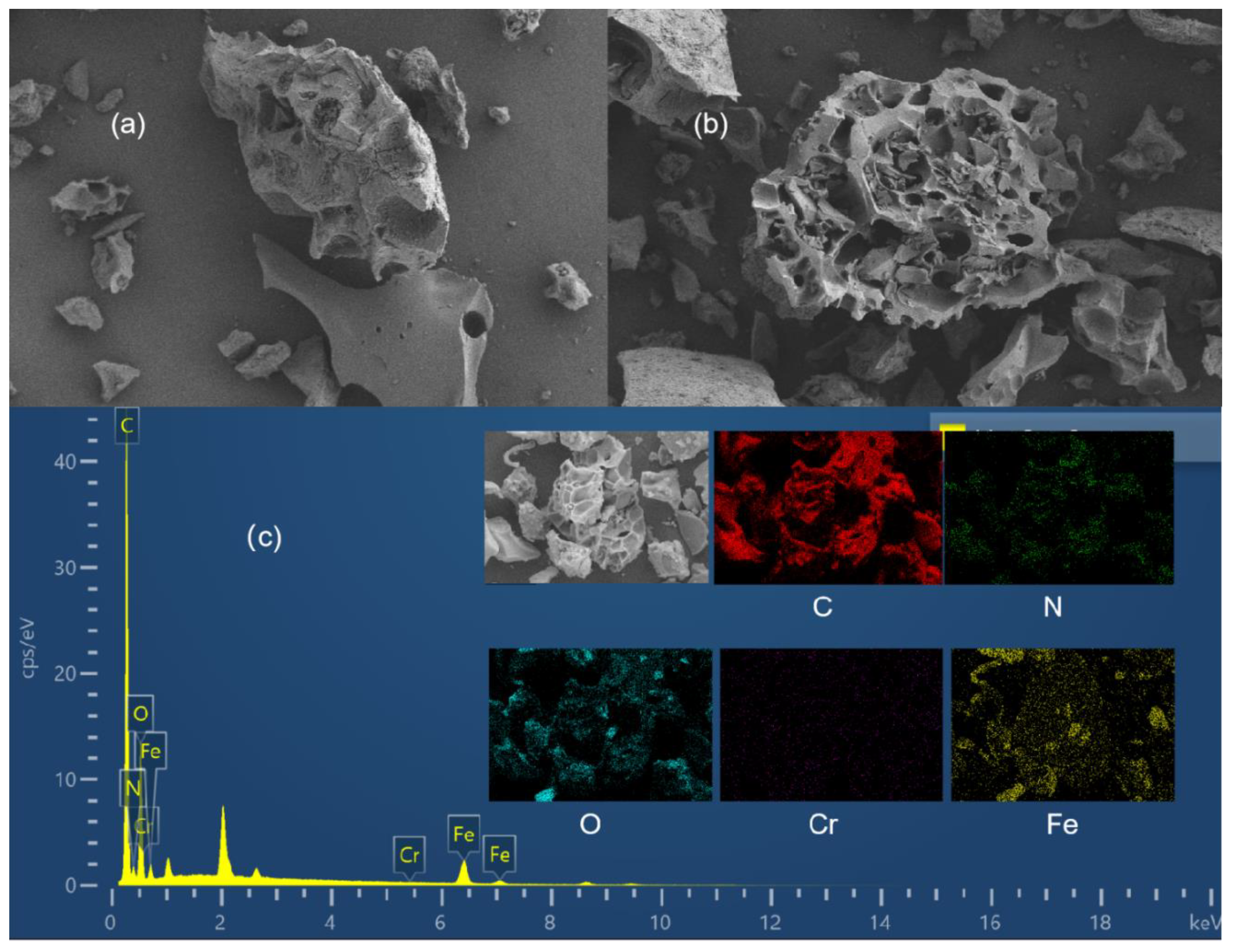
3.1. SEM-EDS
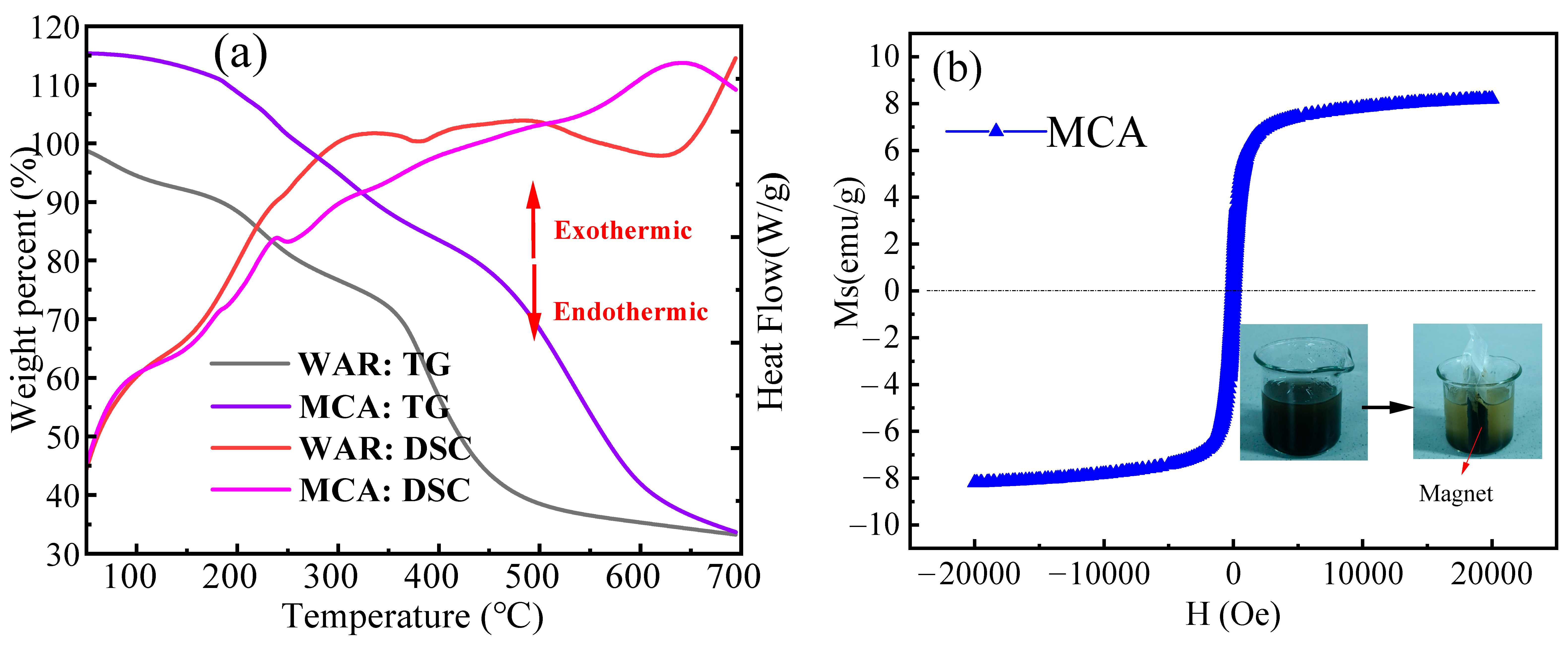
3.2. TG-DSC and Magnetism
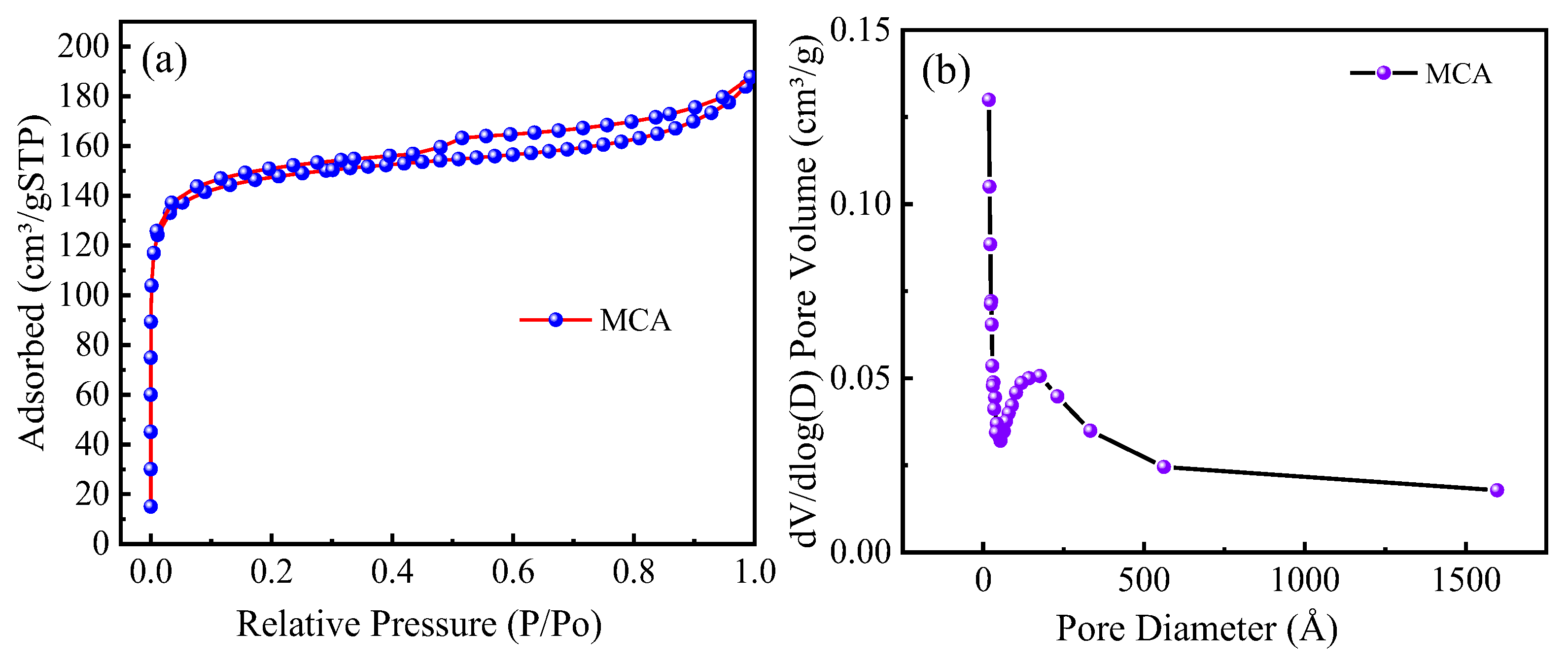
3.3. BET
3.4. Adsorption
3.4.1. Effect of Initial pH and Temperature on Adsorption
3.4.2. Adsorption Kinetic
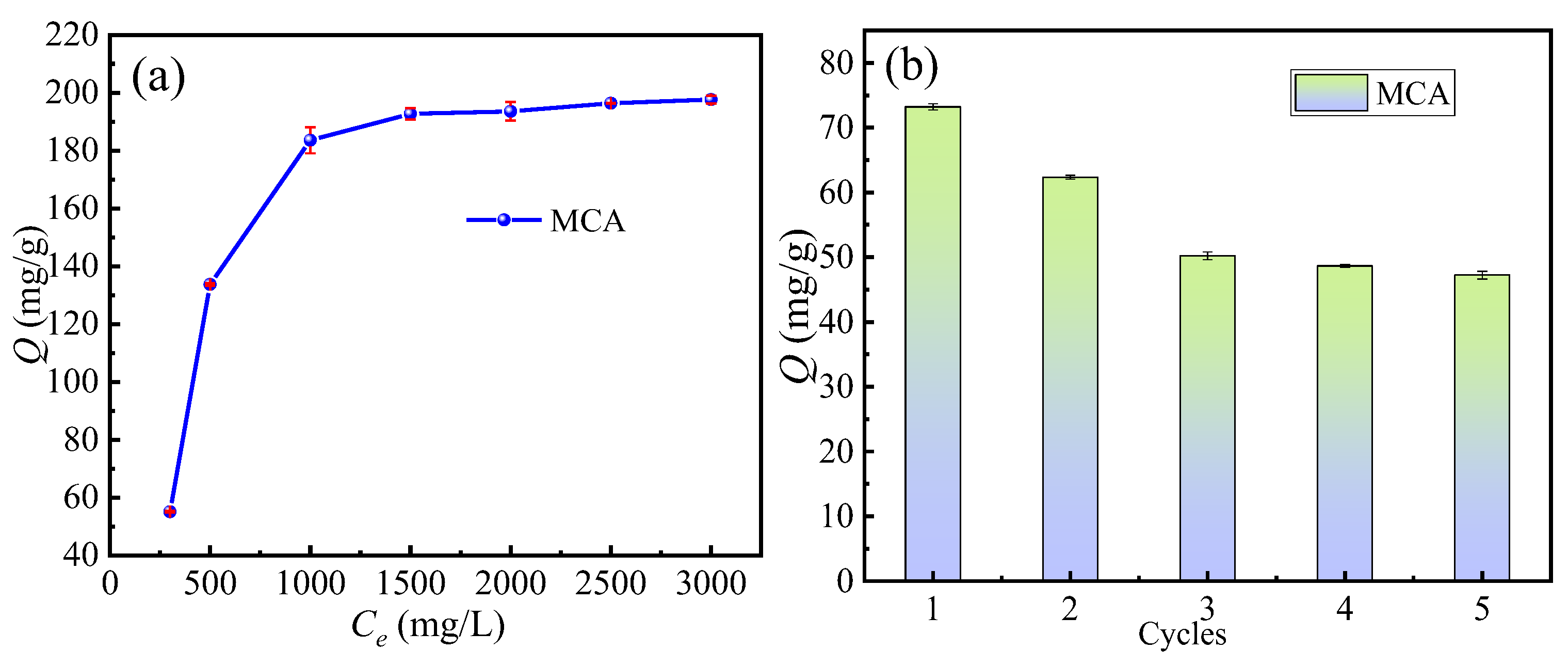
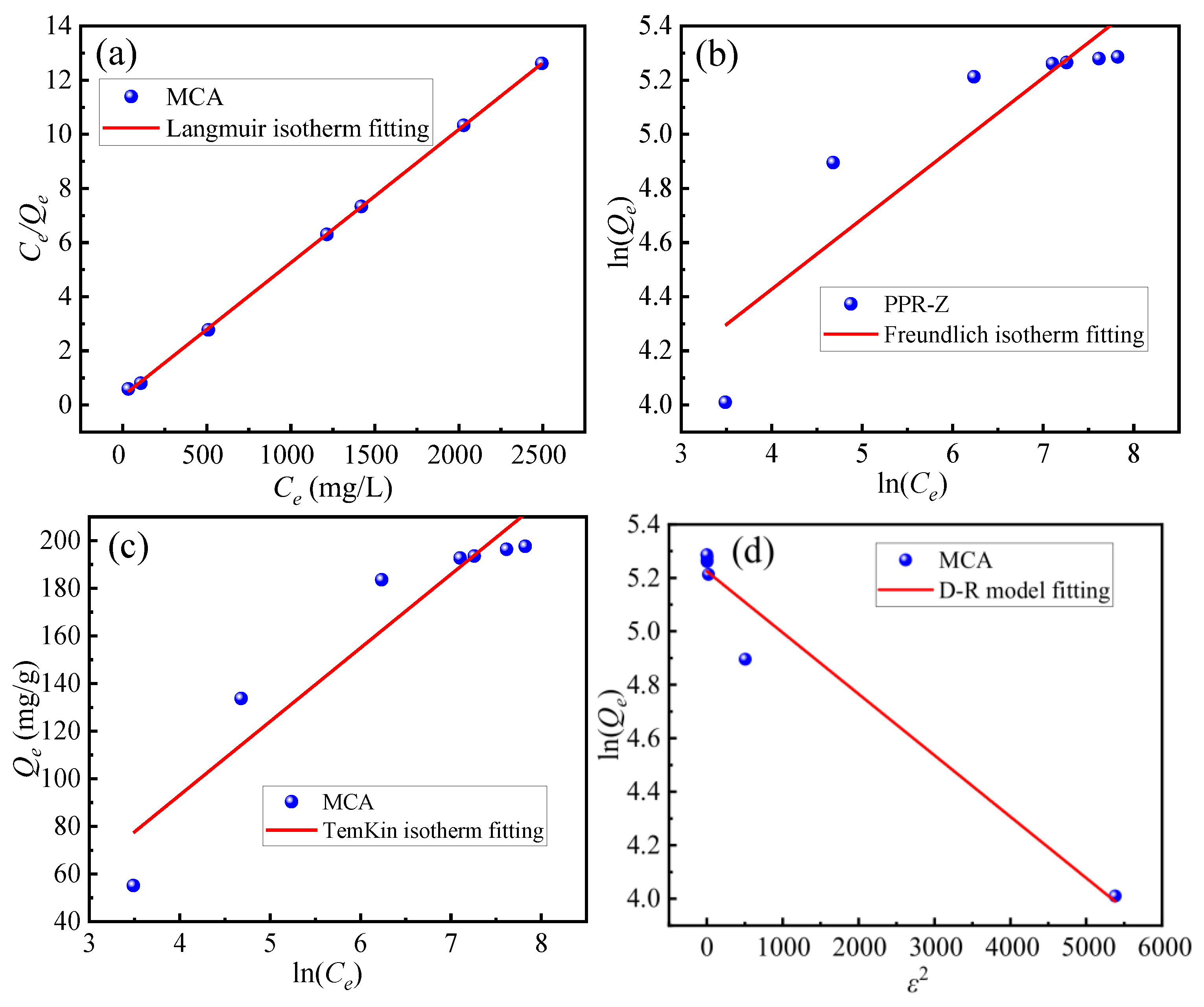
3.4.3. Saturation Adsorption, Reuse Performance, and Adsorption Isotherm Modeling
3.4.4. Adsorption Thermodynamics
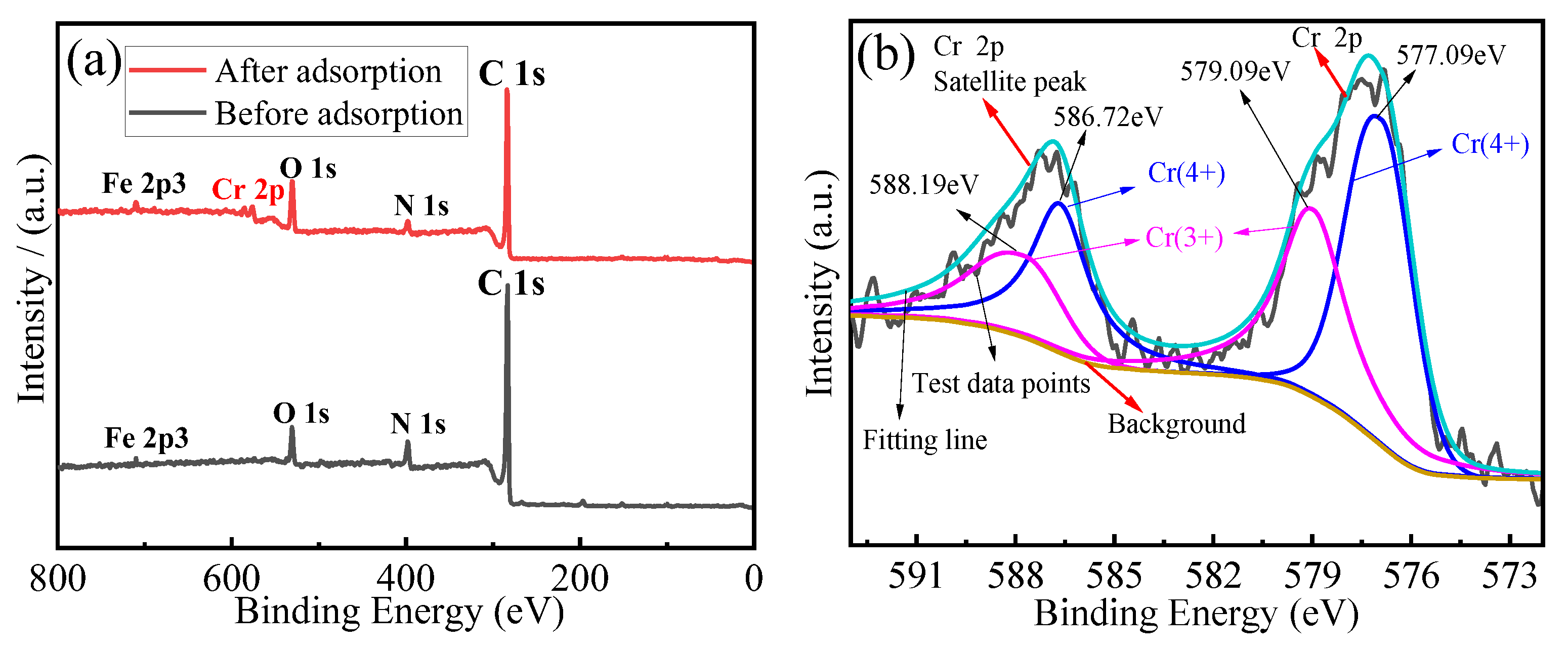
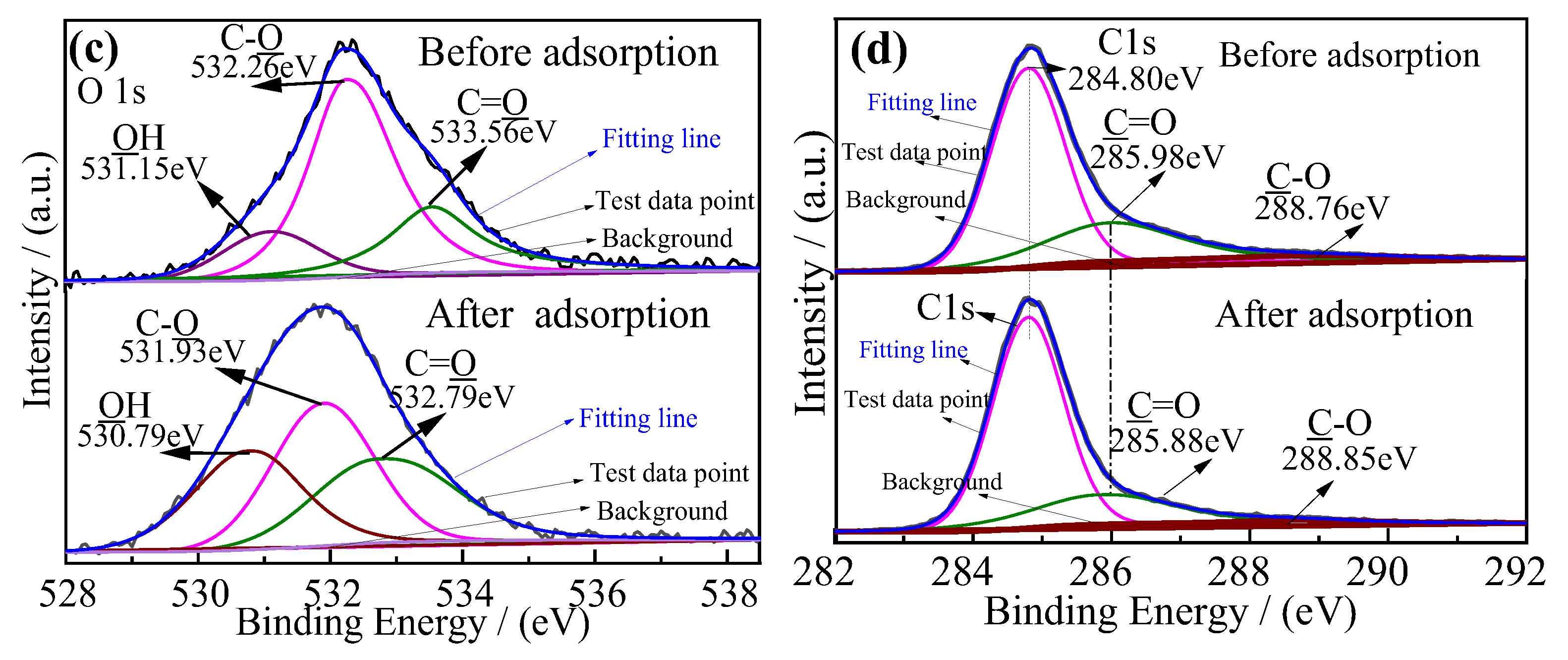
3.5. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Long, H.; Zhao, Z.; Chai, Y.; Li, X.; Hua, Z.; Xiao, Y.; Yang, Y. Binding mechanism of the amidoxime functional group on chelating resins toward gallium(III) in bayer liquor. Ind. Eng. Chem. Res. 2015, 54, 8025–8030. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Chai, Y.; Hua, Z.; Xiao, Y.; Yang, Y. Adsorption performances and mechanisms of amidoxime resin toward gallium(III) and vanadium(V) from bayer liquor. ACS Sustain. Chem. Eng. 2016, 4, 53–59. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarjadi, M.S.; Guerin, S.; Sarkar, S.M. Poly(Amidoxime) resins for efficient and eco-friendly metal extraction. ACS Appl. Polym. Mater. 2022, 4, 2216–2232. [Google Scholar] [CrossRef]
- Nilchi, A.; Babalou, A.A.; Rafiee, R.; Sid Kalal, H. Adsorption properties of amidoxime resins for separation of metal ions from aqueous systems. React. Funct. Polym. 2008, 68, 1665–1670. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Zeng, M.; Liu, Y. Preparation of PAMAM dendrimer modified amidoxime chelating resin and its adsorption for U(VI) in aqueous. Inorg. Chem. Commun. 2022, 144, 109909. [Google Scholar] [CrossRef]
- Rahman, M.; Aiman, M.; Sarjadi, M.; Arshad, S.; Sarkar, S.; Kumar, S. Poly(amidoxime) chelating ligand from pine wood cellulose for eco-friendly toxic metals extraction from water sources. Results Chem. 2025, 15, 102198. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Zheng, C.; Jiang, Y.; Liao, Y.; Huang, J.; Fujita, T.; Wei, Y.; Ma, S. Utilization of waste amine-oxime (WAO) Resin to Generate Carbon by Microwave and Its Removal of Pb(II) in Water. Toxics 2022, 10, 489. [Google Scholar] [CrossRef]
- Jiao, G.-J.; Ma, J.; Zhang, J.; Zhai, S.; Sun, R. Efficient Extraction of Uranium from Seawater by Reticular Polyamidoxime-Functionalized Oriented Holocellulose Bundles. Carbohydr. Polym. 2023, 300, 120244. [Google Scholar] [CrossRef]
- Zheng, Q.; Chunlin, H.; Jiejie, M.; Toyohisa, F.; Chunhui, Z.; Wei, D.; Yuezhou, W. Behaviours of adsorption and elution on amidoxime resin for gallium, vanadium and aluminium ions in alkaline aqueous solution. Solvent Extr. Ion. Exc 2021, 39, 373–398. [Google Scholar] [CrossRef]
- Zheng, C.; He, C.; Yang, Y.; Fujita, T.; Wang, G.; Yang, W. Characterization of Waste Amidoxime Chelating Resin and Its Reutilization Performance in Adsorption of Pb(II), Cu(II), Cd(II) and Zn(II) Ions. Metals 2022, 12, 149. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Qi, M.; Liu, Z.; Wei, Y.; Fujita, T.; Wang, G.; Ma, S.; Yang, W. A Functionalized activated carbon adsorbent prepared from waste amidoxime resin by modifying with H3PO4 and ZnCl2 and its excellent Cr(VI) adsorption. Int. J. Miner. Met. Mater. 2024, 31, 585–598. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, Y.; Qiu, X.; Qi, M.; Liu, Z.; Chen, H.; Yang, W.; Jiang, Z.; He, C. A novel adsorbent for separation of Cr(VI) derived from abandoned amine-oxime resin by the hydrolysis method combined with extractant. J. Water Process Eng. 2025, 71, 107377. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Zhao, X.; Du, R.; Xu, S.; Wang, Y.-Z. Multiple functional materials from crushing waste thermosetting resins. Mater. Horiz. 2021, 8, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Karasik, R.; Lauer, N.E.; Baker, A.-E.; Lisi, N.E.; Somarelli, J.A.; Eward, W.C.; Fürst, K.; Dunphy-Daly, M.M. Inequitable Distribution of Plastic Benefits and Burdens on Economies and Public Health. Front. Mar. Sci. 2023, 9, 1017247. [Google Scholar] [CrossRef]
- Wang, D.T.; Sun, Y.M. Impact of green finance incentive policies on China’s carbon neutrality capability: An evaluation based on the difference-in-differences model. Financ. Res. Lett. 2025, 77, 107066. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Wang, J.; Li, W. Fe@C carbonized resin for reroxymonosulfate activation and bisphenol S segradation. Environ. Pollut. 2019, 252, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liao, Y.; Xie, X.; Wang, Y.; Sun, Z. As2O3 removal from coal-fired flue gas by the carbon-based adsorbent: Effects of adsorption temperature and flue gas components. Chem. Eng. J. 2022, 450, 138023. [Google Scholar] [CrossRef]
- Wei, M.; Yu, Q.; Mu, T.; Hou, L.; Zuo, Z.; Peng, J. Preparation and characterization of waste ion-exchange resin-based activated carbon for CO2 capture. Adsorption 2016, 22, 385–396. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Leboda, R.; Skubiszewska-Zięba, J.; Charmas, B.; Oleszczuk, P. Carbon Adsorbents from waste ion-exchange resins. Carbon 2005, 43, 1143–1150. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Zheng, C.; Liao, Y.; Fujita, T.; Wei, Y.; Wang, G.; Ma, S. Utilization of an amidoxime resin waste to prepare solvent-impregnated resins (CA−12/SIRs) for the extraction of Ga(III) from acid solutions. J. Environ. Chem. Eng. 2023, 11, 110871. [Google Scholar] [CrossRef]
- Zhang, J. Physical insights into kinetic models of adsorption. Sep. Purif. Technol. 2019, 229, 115832. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and Non-Linear Regression Analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 137, 1538–1544. [Google Scholar] [CrossRef]
- Greluk, M.; Hubicki, Z. Sorption of SPADNS Azo Dye on polystyrene anion exchangers: Equilibrium and kinetic studies. J. Hazard. Mater. 2009, 172, 289–297. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Yang, W.B.; Wang, G.H.; Yu, H.Z. A Study on capillary actions of power-law fluids in porous fibrous materials via W-M function. Int. J. Heat. Mass. Transf. 2019, 129, 255–264. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Wang, X.; Lv, R.; Liu, H.; Lin, J.; Barakos, G.; Chang, P. A Fractal Langmuir adsorption equation on coal: Principle, methodology and implication. Chem. Eng. J. 2024, 488, 150869. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-second-order and modified pseudo-girst-order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A Review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Jahandar Lashaki, M.; Fayaz, M.; Niknaddaf, S.; Hashisho, Z. Effect of the adsorbate kinetic diameter on the accuracy of the Dubinin–Radushkevich equation for modeling adsorption of organic vapors on activated carbon. J. Hazard. Mater. 2012, 241–242, 154–163. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Mastron, J.N.; Tokmakoff, A. Fourier transform fluorescence-encoded infrared spectroscopy. J. Phys. Chem. A 2018, 122, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lian, S.; Li, R.; Yang, Y.; Zang, G.; Song, C. Fabricating leaf-like hierarchical ZIF-67 as intra-mixed matrix membrane microarchitecture for efficient intensification of CO2 separation. Sep. Purif. Technol. 2023, 305, 122460. [Google Scholar] [CrossRef]
- Curcio, D.; Sierda, E.; Pozzo, M.; Bignardi, L.; Sbuelz, L.; Lacovig, P.; Lizzit, S.; Alfè, D.; Baraldi, A. Unusual reversibility in molecular break-up of PAHs: The case of pentacene dehydrogenation on Ir(111). Chem. Sci. 2021, 12, 170–178. [Google Scholar] [CrossRef]
- Choi, I.-H.; Kim, J.-S. Glass Transition temperature of styrene-based ionomer controlled over a very wide temperature range by nonpolar plasticizer content and ion vontent. J. Appl. Polym. Sci. 2023, 140, e54550. [Google Scholar] [CrossRef]
- Jankovská, Z.; Matějová, L.; Tokarský, J.; Peikertová, P.; Dopita, M.; Gorzolková, K.; Habermannová, D.; Vaštyl, M.; Bělík, J. microporous carbon prepared by microwave pyrolysis of dcrap tyres and the effect of K in its Structure on xylene adsorption. Carbon 2024, 216, 118581. [Google Scholar] [CrossRef]
- Bai, L.; Su, X.; Feng, J.; Ma, S. Preparation of sugarcane bagasse biochar/nano-iron oxide composite and mechanism of its Cr (VI) adsorption in wate. J. Clean. Prod. 2021, 320, 128723. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, J.; Yu, Z.; Shi, L.; Meng, X. Preparation of hierarchically porous carbon spheres derived from waste resins and its application in water purification. J. Porous Mater. 2018, 26, 163–174. [Google Scholar] [CrossRef]
- Yang, K.; Xing, J.; Xu, P.; Chang, J.; Zhang, Q.; Usman, K.M. Activated carbon microsphere from sodium lignosulfonate for Cr(VI) adsorption evaluation in wastewater treatment. Polymers 2020, 12, 236. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Zan, Y.; Miao, G.; Wang, H.; Kong, L. Hydrothermal carbonization of microalgae (Chlorococcum) for porous carbons with high Cr(VI) adsorption performance. Appl. Biochem. Biotechnol. 2018, 186, 414–424. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Liang, H.; Gao, C.; Qin, J.; You, L.; Wang, R.; Li, J.; Yang, S. Adsorption of Cr(VI) from aqueous solutions using novel activated carbon spheres derived from glucose and sodium dodecylbenzene sulfonate. Sci. Total Env. 2021, 759, 143457. [Google Scholar] [CrossRef]
- Huang, R.; Yang, B.; Liu, Q.; Liu, Y. Multifunctional activated carbon/chitosan composite preparation and its simultaneous adsorption of phenol and Cr(VI) from aqueous solutions. Environ. Prog. Sustain. Energy 2014, 33, 814–823. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magne-tite coated biomass: A comparative kinetic and diffusion study. S. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar]
- Al-Qodah, Z.; Dweiri, R.; Khader, M.; Al-Sabbagh, S.; Al-Shannag, M.; Qasrawi, S.; Al-Halawani, M. Processing and characterization of magnetic composites of activated carbon, fly ash, and beach sand as adsorbents for Cr(VI) removal. Case Stud. Chem. Environ. Eng. 2023, 7, 100333. [Google Scholar] [CrossRef]
- Rajput, M.K.; Hazarika, R.; Sarma, D. Zerovalent iron decorated tea waste derived porous biochar [ZVI@TBC] as an efficient adsorbent for Cd(II) and Cr(VI) removal. J. Environ. Chem. Eng. 2023, 11, 110279. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, B.; Li, M.; Li, C.; Zheng, S. Carboxyl-rich carbon nanocomposite based on natural diatomite as adsorbent for efficient removal of Cr (VI). J. Mater. Res. Technol. 2020, 9, 948–959. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, D.; Hu, Z.; Xiao, Y.; He, C.; Jiang, F.; Zhao, N.; Zhao, C.; Zhang, W.; Qiu, R. The adsorption and reduction of anionic Cr(VI) in groundwater by novel iron carbide loaded on N-doped carbon nanotubes: Effects of Fe-confinement. Chem. Eng. J. 2023, 452, 139357. [Google Scholar] [CrossRef]
- Schreiber, B.; Schmalz, V.; Brinkmann, T.; Worch, E. The Effect of water temperature on the adsorption equilibrium of dissolved organic matter and atrazine on granular activated carbon. Environ. Sci. Technol. 2007, 41, 6448–6453. [Google Scholar] [CrossRef]




| Reagent Name | Molecular Formula | Reagent Grade | Company |
|---|---|---|---|
| Sodium hydroxide | NaOH | Analytical Reagent | Guangdong Guanghua Technology Co., Ltd., (Guangzhou, China) |
| Zinc chloride | ZnCl2 | Analytical Reagent | Guangdong Guanghua Technology Co., Ltd., (Guangzhou, China) |
| Ferric nitrate | Fe(NO3)3 | Analytical Reagent | Guangdong Guanghua Technology Co., Ltd., (Guangzhou, China) |
| Ethanol absolute | C2H5OH | Analytical Reagent | Guangdong Guanghua Technology Co., Ltd., (Guangzhou, China) |
| Potassium dichromate | K2Cr2O7 | Analytical Reagent | Guangdong Guanghua Technology Co., Ltd., (Guangzhou, China) |
| Hydrochloric acid | HCl | Analytical Reagent | Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China) |
| Phosphoric acid | H3PO4 | Analytical Reagent | Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China) |
| Nitric acid | HNO3 | Analytical Reagent | Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China) |
| Instrument | Instrument Model | Manufacturer |
|---|---|---|
| Fourier Transform Infrared Spectroscopy | IR Tracer 100 | SHIMADZU (Kyoto, Japan) |
| SEM-EDS Phenom XL | Phenom Pro 800-07334 | Phenom (Eindhoven, the Netherlands) |
| Differential Heat-Thermogravimetric Simultaneous Analyzer | STA449F 3 | Netzsch (Selb, Germany) |
| Electronic Analytical Balance | AUY220 | SHIMADZU (Kyoto, Japan) |
| Specific Surface Area Analyzer | TriStarII 3020 | Micromeritics (Norcross, GA, USA) |
| Constant Temperature Drying Box | DG-410C | Chongqing Daho Technology Co., Ltd. (Chongqing, China) |
| Constant Temperature Water Bath Oscillator | NTS-4000BH | EYELA (Tokyo, Japan) |
| Thermostatic Water Bath | HCJ-4E | Changzhou Enpei Instrument Manufacturing Co., Ltd. (Changzhou, Jiangsu, China) |
| Pipette | Finnpipette | Thermo Fisher Scientific (Waltham, MA, USA) |
| Laboratory Water Purifier | Master-S15UV | Shanghai Hetai Instrument Co., Ltd. (Shanghai, China) |
| Tube Furnace | GXL-1700X | Hefei Kejing Instrument Co., Ltd. (Hefei, Anhui, China) |
| Ultrasonic Cleaner | XO-5200DTD | Nanjing Xianou Instrument Manufacturing Co., Ltd. (Nanjing, China) |
| Element (%) | Magnetic Activated Carbon Adsorbent (MCA) | |
|---|---|---|
| Original | Adsorption | |
| C | 71.19 | 71.06 |
| N | 8.15 | 8.36 |
| O | 19.39 | 18.91 |
| Cr | 0 | 1.43 |
| Fe | 1.26 | 0.23 |
| Adsorbent | Langmuir (m2/g) | BET (m2/g) | Pore Volume (m3/g) | Pore Size (nm) |
|---|---|---|---|---|
| MCA | 569.88 | 569.89 | 0.2904 | 20.38 |
| Adsorbent | PFO | PSO | W-M | |||||
|---|---|---|---|---|---|---|---|---|
| Qe | k1 | R2 | Qe | k2 | R2 | R12 | R22 | |
| MCA | 77.6 | 0.022 | 0.68 | 77.6 | 0.58 | 0.99 | 0.99 | 0.81 |
| Adsorbents | Q/(mg/g) | Reference |
|---|---|---|
| MCA | 197.63 | This work |
| KSAC-CeO2 | 14.00 | [36] |
| SLACM | 227.70 | [37] |
| AC-NH3-900 | 95.60 | [38] |
| ACSs | 230.15 | [39] |
| chitosan | 82.78 | [40] |
| NTP | 6.48 | [41] |
| M-AC | 58.39 | [42] |
| ZVI@TBC | 186.20 | [43] |
| C-DE@S | 142.83 | [44] |
| MU-CNTs/Fe-700 | 23.70 | [45] |
| Metal ion | Langmuir model | Freundlich model | ||||
| Qm | KL | R2 | Kf | n | R2 | |
| Cr(VI) | 198.94 | 0.015 | 0.99 | 29.55 | 3.84 | 0.82 |
| Metal ion | TemKin model | Dubin Radushkevich model | ||||
| Kt | A | R2 | β | R2 | ||
| Cr(VI) | 0.37 | 30.9 | 0.91 | 2.29 | 0.96 | |
| Temperature (K) | Thermodynamic Parameters | ||
|---|---|---|---|
| ∆G (kJ·mol−1) | ∆H (kJ·mol−1) | ∆S (J·mol−1·K−1) | |
| 298 | 2.802 | 7.324 | 15.172 |
| 303 | 2.727 | ||
| 308 | 2.651 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Su, X.; Yu, H.; Yuan, Y.; Wu, J.; Yang, W.; He, C. Preparation of Magnetic Carbon Composite from Waste Amine-Oxime Resin and Its Adsorption Properties for Chromium. Materials 2025, 18, 3066. https://doi.org/10.3390/ma18133066
Wang H, Su X, Yu H, Yuan Y, Wu J, Yang W, He C. Preparation of Magnetic Carbon Composite from Waste Amine-Oxime Resin and Its Adsorption Properties for Chromium. Materials. 2025; 18(13):3066. https://doi.org/10.3390/ma18133066
Chicago/Turabian StyleWang, Haoyu, Xianzhuo Su, Hongdan Yu, Yuhang Yuan, Jing Wu, Wenchao Yang, and Chunlin He. 2025. "Preparation of Magnetic Carbon Composite from Waste Amine-Oxime Resin and Its Adsorption Properties for Chromium" Materials 18, no. 13: 3066. https://doi.org/10.3390/ma18133066
APA StyleWang, H., Su, X., Yu, H., Yuan, Y., Wu, J., Yang, W., & He, C. (2025). Preparation of Magnetic Carbon Composite from Waste Amine-Oxime Resin and Its Adsorption Properties for Chromium. Materials, 18(13), 3066. https://doi.org/10.3390/ma18133066







