Study of New Glass–Ceramic and Dense Ceramic Containing Biogenic Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
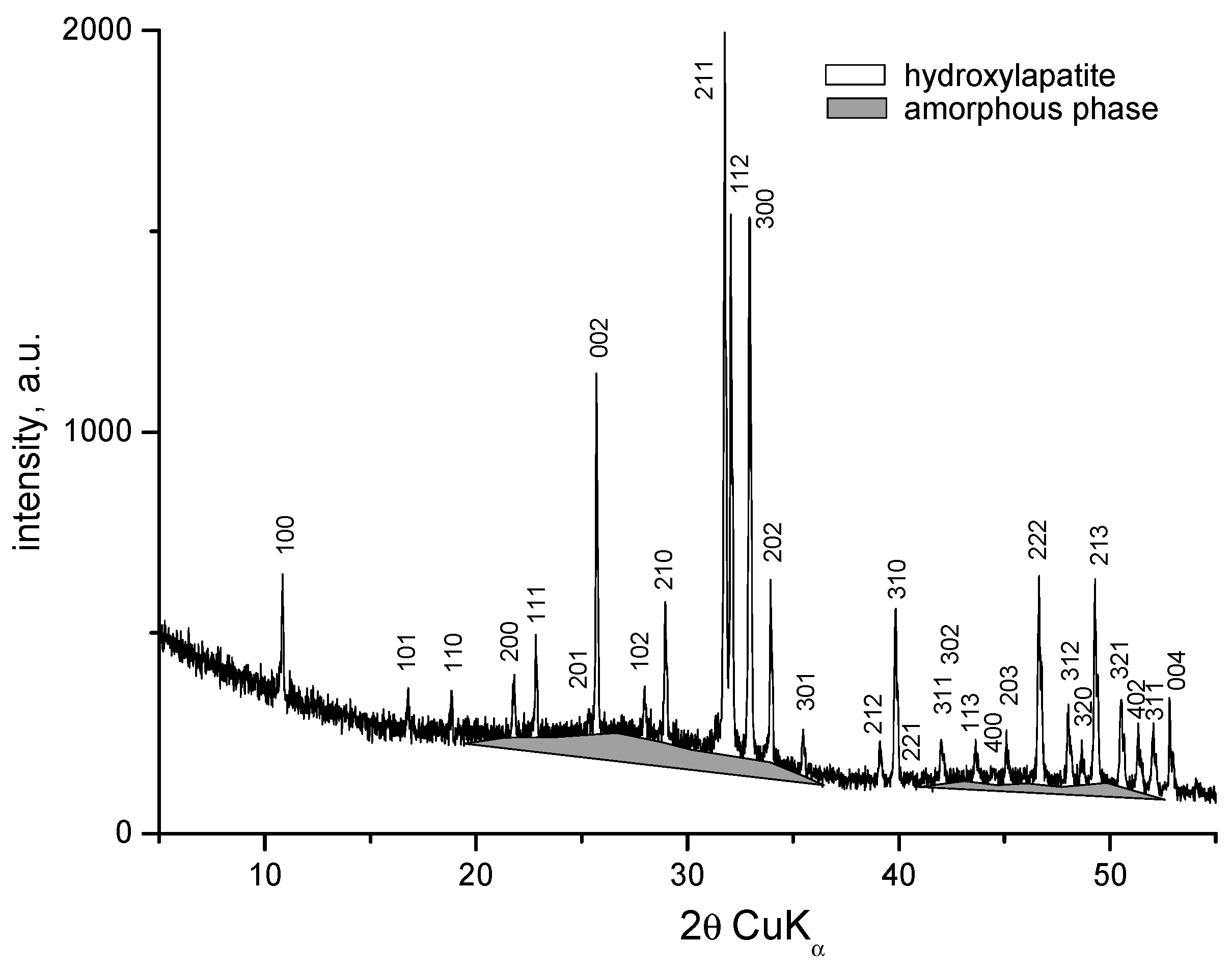
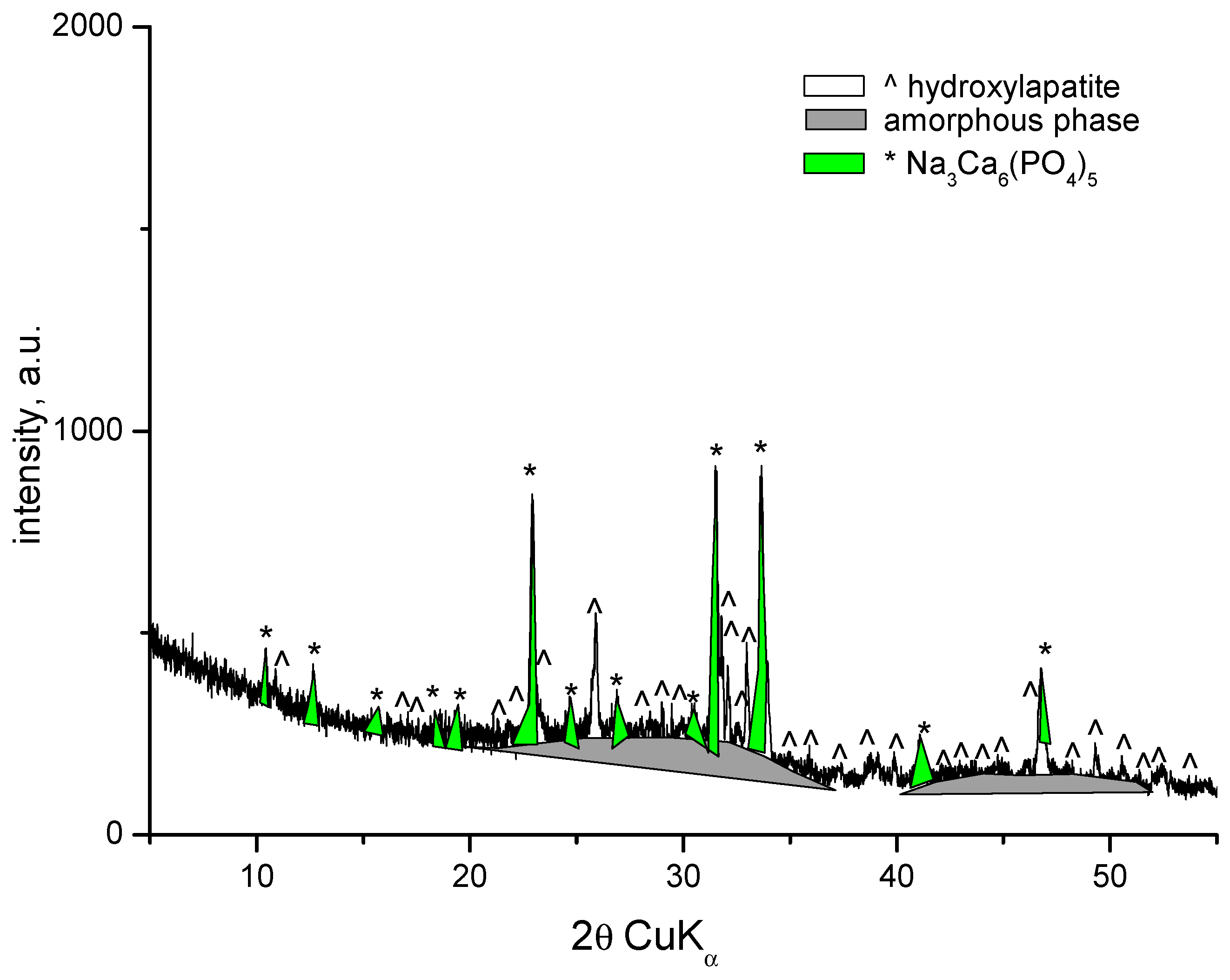
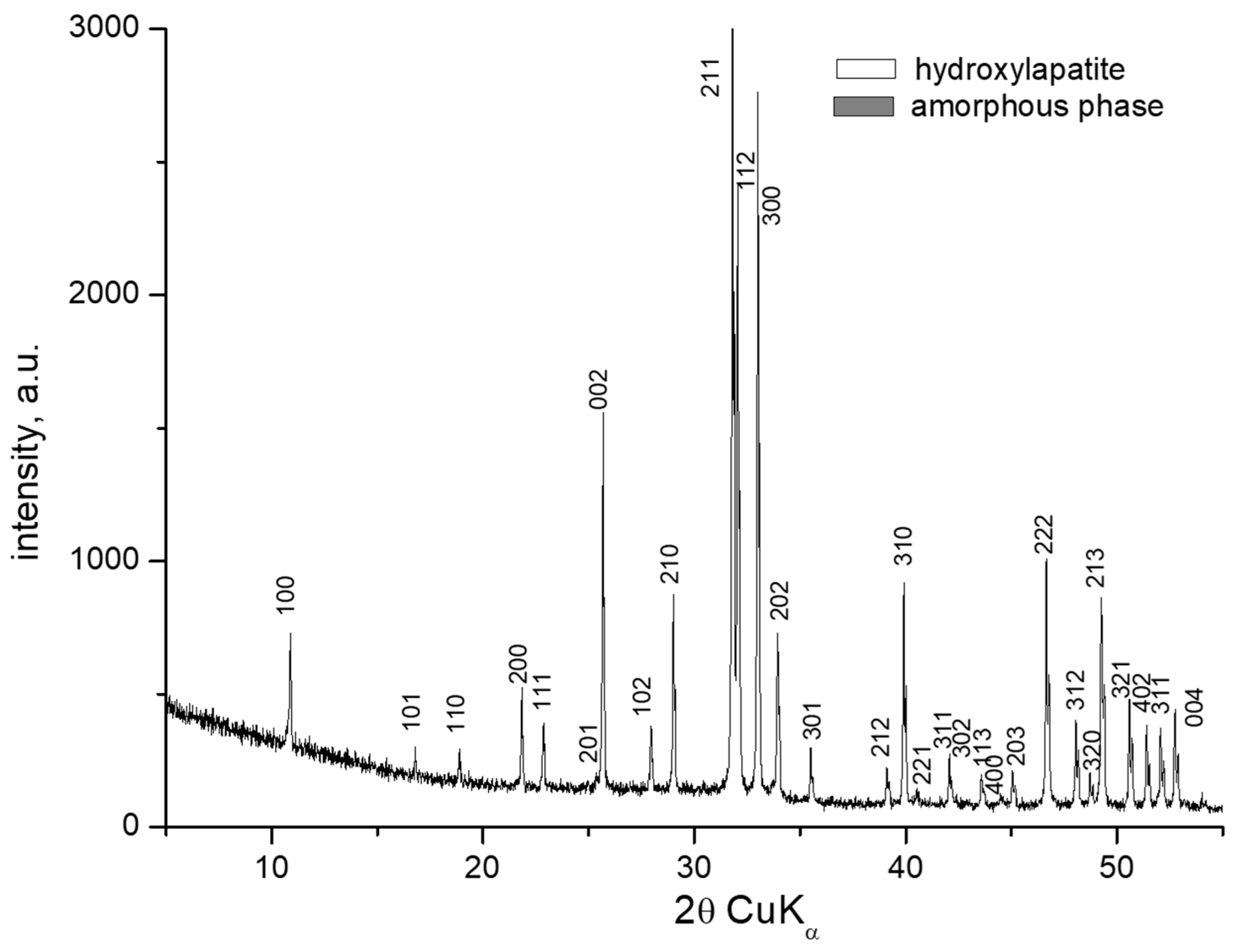
3.1. X-Ray Powder Diffraction
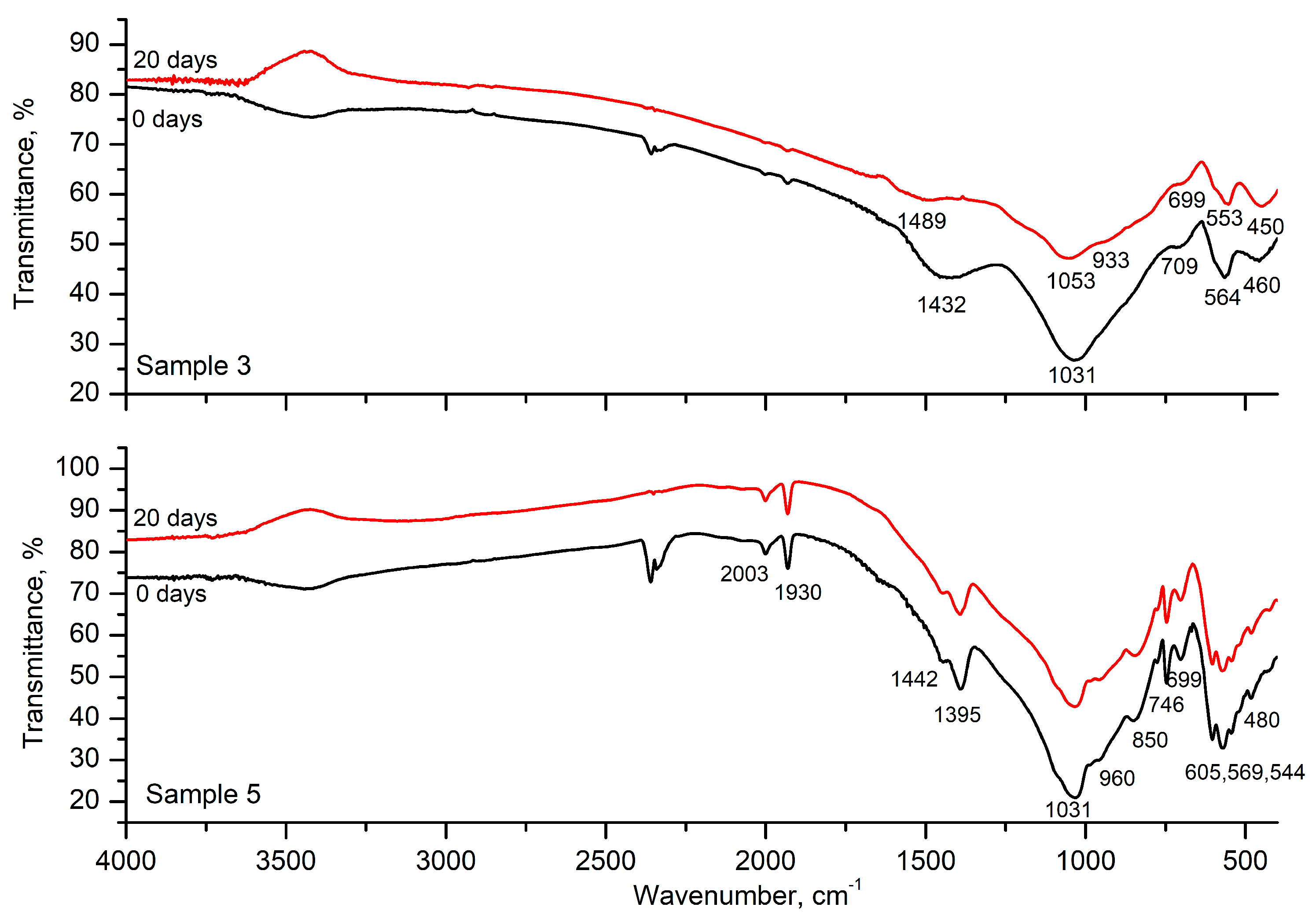
3.2. FTIR Spectroscopy
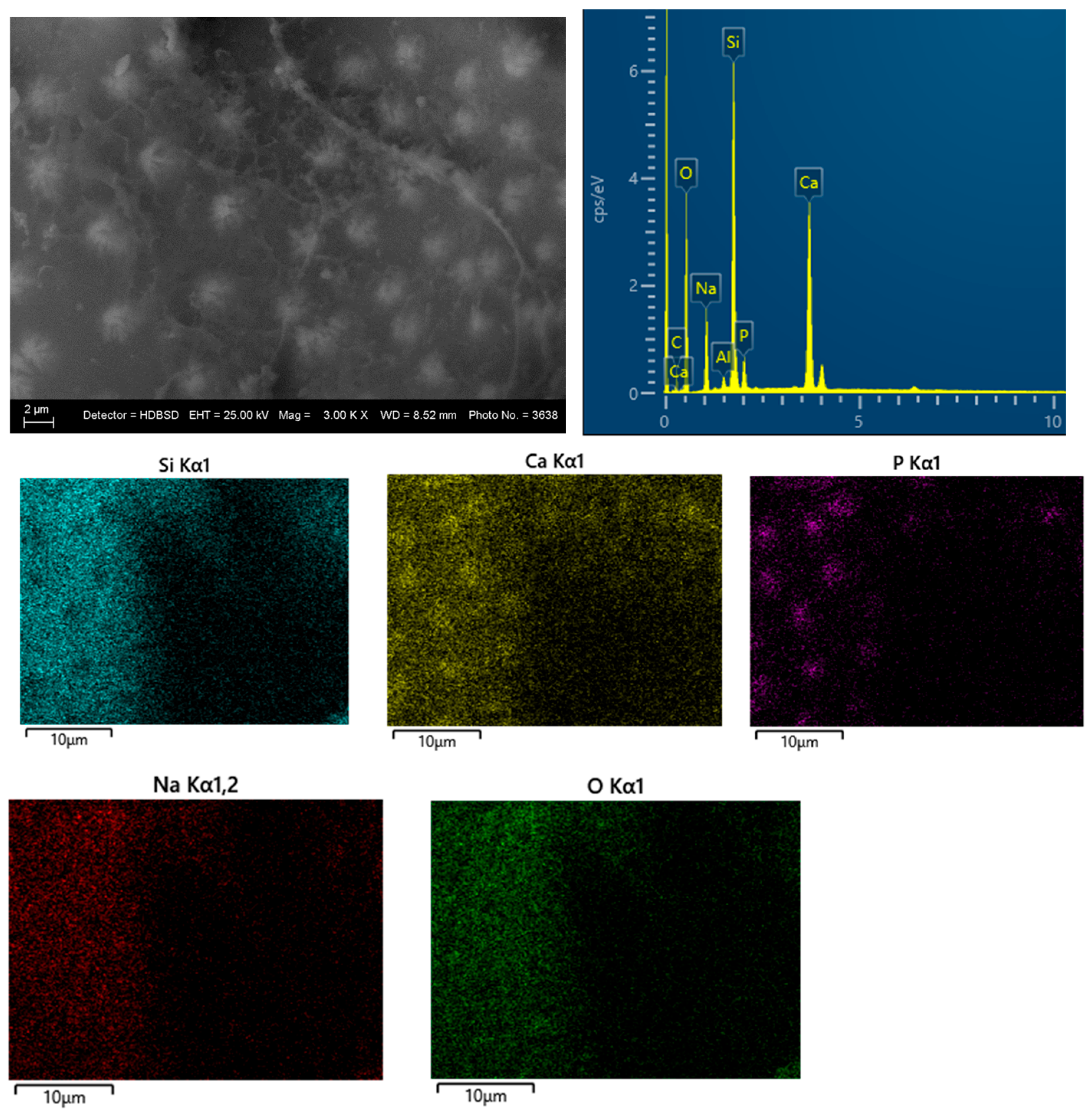
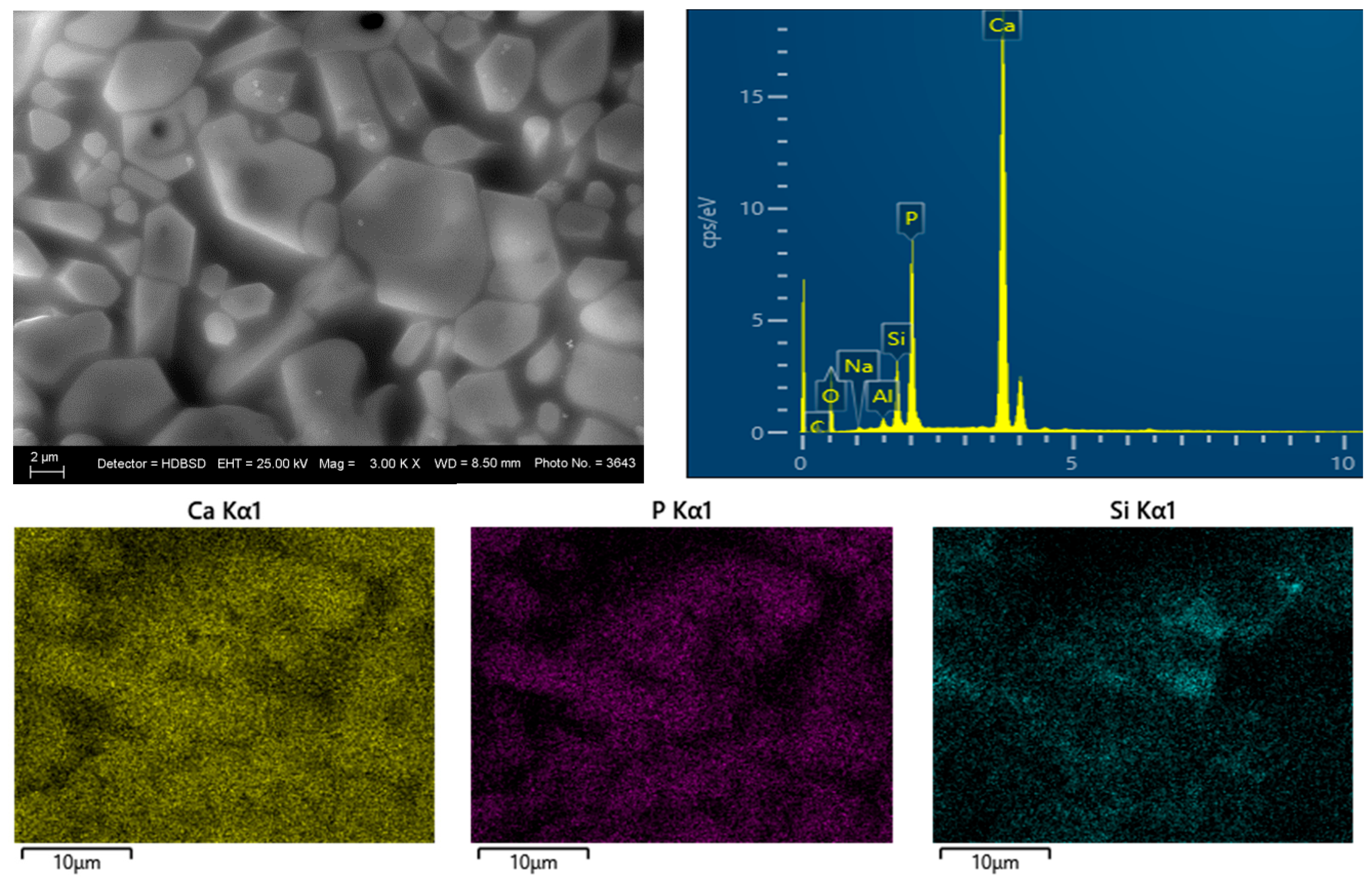
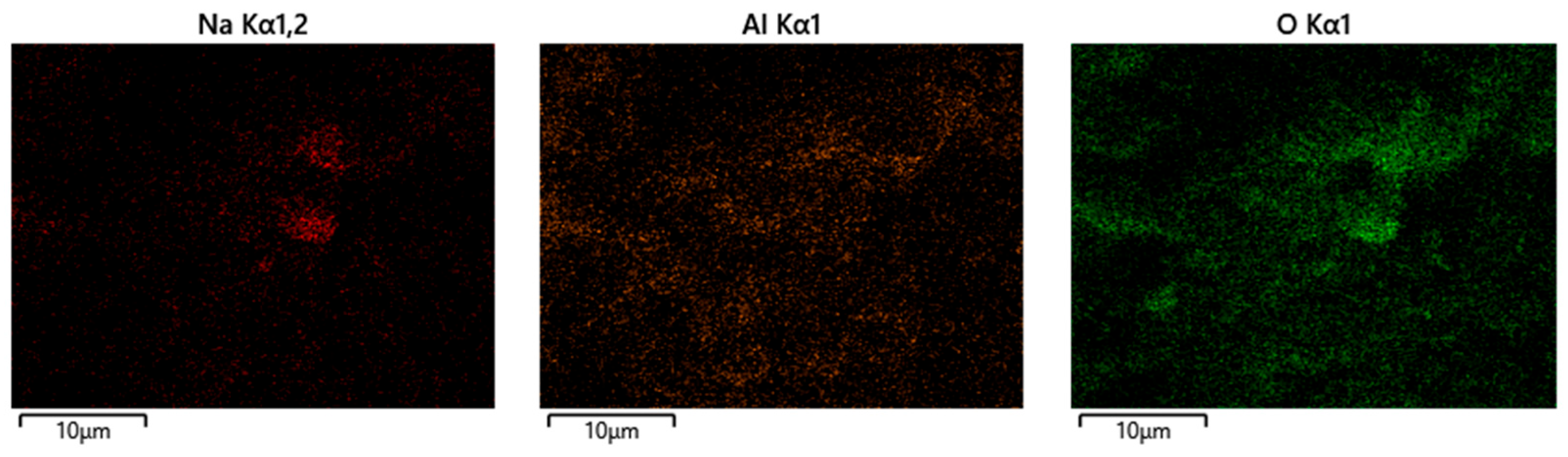
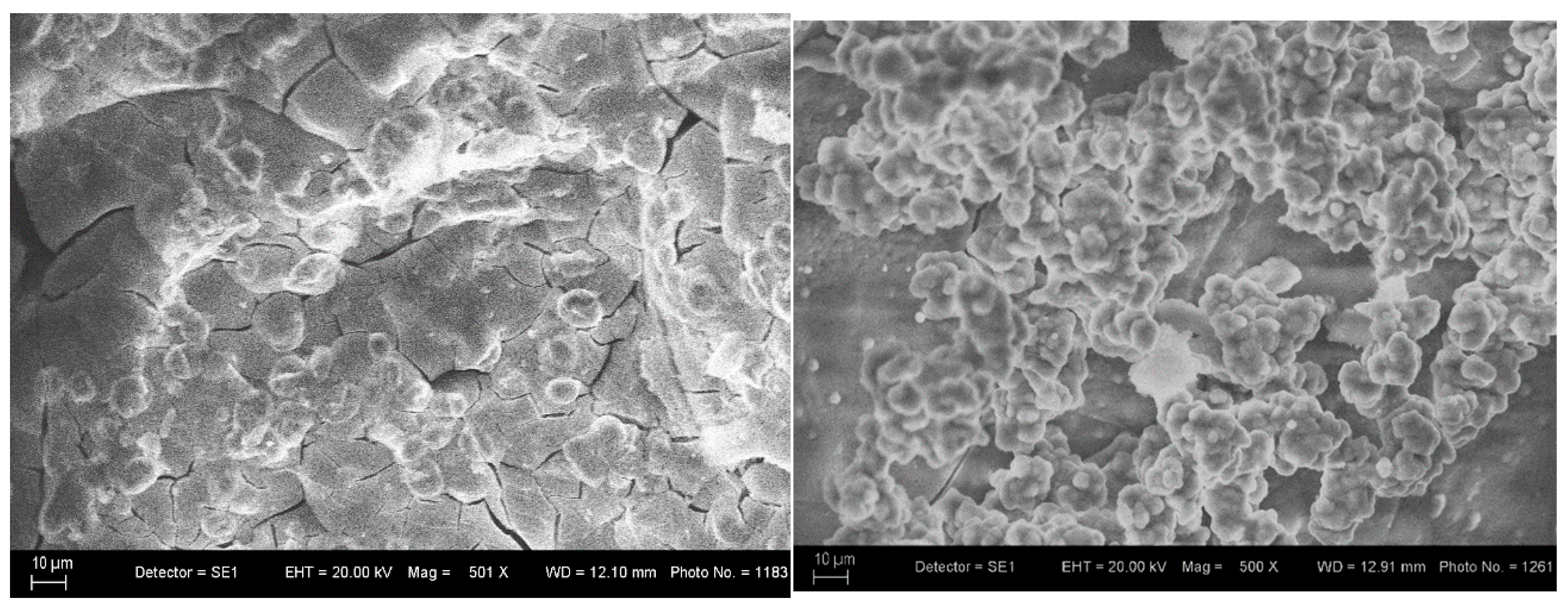
3.3. SEM and EDS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, properties, and applications of dense hydroxyapatite: A review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [PubMed]
- Workie, A.B.; Shih, S.J. A study of bioactive glass–ceramic’s mechanical properties, apatite formation, and medical applications. RSC Adv. 2022, 12, 23143–23152. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, N.; Parkhomey, O.; Sych, O. In vitro investigation of bioactive glass-ceramic composites based on biogenic hydroxyapatite or synthetic calcium phosphates. Nanoscale Res. Lett. 2017, 12, 111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forero-Sossa, P.A.; Olvera-Alvarez, I.U.; Salazar-Martinez, J.D.; Espinosa-Arbelaez, D.G.; Segura-Giraldo, B.; Giraldo-Betancur, A.L. Biogenic hydroxyapatite powders: Effects of source and processing methodologies on physicochemical properties and bioactive response. Mater. Charact. 2021, 173, 110950–110964. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Vernéand, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Antoniac, I.; Lesci, I.G.; Blajan, A.I.; Vitioanu, G.; Antoniac, A. Bioceramics and biocomposites from marine sources. Key Eng. Mater. 2015, 672, 276–292. [Google Scholar] [CrossRef]
- Cursaru, L.M.; Iota, M.; Piticescu, R.M.; Tarnita, D.; Savu, S.V.; Savu, I.D.; Dumitrescu, G.; Popescu, D.; Hertzog, R.-G.; Calin, M. Hydroxyapatite from Natural Sources for Medical Applications. Materials 2022, 15, 5091. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. Bioactive glass/hydroxyapatite composites: Mechanical properties and biological evaluation. Mater. Sci. Eng. C 2015, 51, 196–205. [Google Scholar] [CrossRef]
- Elbadawi, M.; Wally, Z.J.; Reaney, I.M. Porous hydroxyapatite-bioactive glass hybrid scaffolds fabricated via ceramic honeycomb extrusion. J. Am. Ceram. Soc. 2018, 101, 3541–3556. [Google Scholar] [CrossRef]
- Romez-Vega, J.M.; Saiz, E.; Tomsia, A.P. Glass–hydroxyapatite coatings on titanium-based implants. In Bioceramics: Materials and Applications III, 1st ed.; George, L., Rusin, R.P., Fischman, G.S., Janas, V., Eds.; Wiley-American Ceramic Society: Hoboken, NJ, USA, 2000; Volume III, pp. 15–21. [Google Scholar]
- Queiroz, A.C.; Santos, J.D.; Monteiro, F.J.; Prado da Silva, M.H. Dissolution studies of hydroxyapatite and glass-reinforced hydroxyapatite ceramics. Mater. Charact. 2003, 50, 197–202. [Google Scholar] [CrossRef]
- Rey, C. Calcium phosphate biomaterials and bone mineral. Difference in composition, structure, and properties. Biomaterials 2000, 11, 5–13. [Google Scholar]
- Georgiou, G.; Knowles, J.C. Glass-reinforced hydroxyapatite for hard tissue surgery—Part 1. Biomaterials 2001, 22, 2811–2815. [Google Scholar] [CrossRef]
- Vargas-Becerril, N.; Sánchez-Téllez, D.A.; Zarazúa-Villalobos, L.; González-García, D.M.; Álvarez-Pérez, M.A.; de León-Escobedo, C.; Téllez-Jurado, L. Structure of biomimetic apatite grown on hydroxyapatite (HA). Ceram. Int. 2020, 46, 28806–28813. [Google Scholar] [CrossRef]
- Bhatt, H.A. Synthesis and Characterization of Nanocrystalline Hydroxyapatite Powder; and the Effects of Oxide-Based Sintering Additives on Tricalcium Phosphate. Master’s Thesis, University of Central Florida Orlando, Orlando, FL, USA, 2005. Available online: https://stars.library.ucf.edu/etd/432 (accessed on 24 June 2025).
- Yoleva, A.; Mihailova, I.; Djambazov, S. Solid-state synthesis of hydroxyapatite from Black Sea Rapana venosa shells. J. Chem. Technol. Metall. 2023, 58, 385–393. [Google Scholar] [CrossRef]
- Mateeva, J.; Yoleva, A.; Djambazov, S. Phase study of Rapana venosa shells from the Black Sea. J. Chem. Technol. Metall. 2023, 58, 38–43. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Demirkiran, H.; Mohandas, A.; Dohi, M.; Fuentes, A.; Nguyen, K.; Aswath, P. Bioactivity and mineralization of hydroxyapatite with bioglass as sintering aid and bioceramics with Na3Ca6(PO4)5 and Ca5(PO4)2SiO4 in a silicate matrix. Mater. Sci. Eng. C 2010, 30, 263–272. [Google Scholar] [CrossRef]
- Wojciech, S.; Masatomo, Y.; Masato, K.; Masahiro, Y. Hydroxyapatite ceramics with selected sintering additives. Biomoteriols 1997, 18, 923–933. [Google Scholar]
- Dimitrov, V.; Dimitriev, Y. Structural Analysis (Spectroscopy), 1st ed.; UCTM: Sofia, Bulgaria, 2010. (In Bulgarian) [Google Scholar]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Mačković, M.; Hoppe, A.; Detsch, R.; Mohn, D.; Stark, W.J.; Spiecker, E.; Boccaccini, A.R. Bioactive glass (type 45S5) nanoparticles: In vitro reactivity on nanoscale and biocompatibility. J. Nanopart. Res. 2012, 14, 966. [Google Scholar] [CrossRef]
- Yoleva, A.; Tasheva, T.; Djambazov, S.; Batsova, A. Development of multicomponent glasses for application as a glazing layer on dental zirconia. Int. J. Appl. Glass Sci. 2025, 16, e16684. [Google Scholar] [CrossRef]
- Tasheva, T.; Valova, G. FT-IR and Raman spectroscopy of ZnO and MgO containing glasses in the B2O3/Na2O/CaO/P2O5 system. J. Chem. Technol. Metall. 2024, 59, 1391–1398. [Google Scholar] [CrossRef]



| Sample No. | Composition, Mass % | Density, g/cm3 | |||
|---|---|---|---|---|---|
| SiO2 | Na2O | B2O3 | BHA Powder | ||
| 1 | - | 20 | 50 | 30 | 2.48 ± 0.04 |
| 2 | 20 | 15 | 35 | 30 | 2.50 ± 0.04 |
| 3 | 35 | 10 | 25 | 30 | 2.54 ± 0.04 |
| 4 | 50 mass % powdered glass with the following composition in mass %: 35 B2O3, 45 SiO2, 10 Al2O3, 10 Na2O | 50 | 2.60 ± 0.04 | ||
| 5 | 25 mass % powdered glass with the following composition in mass %: 35 B2O3, 45 SiO2,10 Al2O3, 10 Na2O | 75 | 2.70 ± 0.04 | ||
| Peak, cm−1 | Assignment | References |
|---|---|---|
| 2003, 1930 | 2.ν3 harmonic overtone or to a combination mode, ν1 + ν3 | [23] |
| 1468–1442 | Stretching mode (ν1) of the CO32− group in A-type CAP or bending mode (ν4 or ν3) of the CO32− group in A and B-type CAP | [25] |
| 1354–1395 | Asymmetric B–O stretching vibration of borate triangular units BO3 | [24] |
| 1021–1031 | Triple degenerate stretching antisymmetric vibration νdas(F) of PO4 | [17,22,23] |
| 960 | Symmetric stretching vibration νs(A1) of PO4 | [22,23] |
| 850 | Stretching mode of the CO32− group in CAP | [23] |
| 709 699, 746 | Overlapping of bending vibrations δ of B–O–B in [BO3] and symmetric stretching vibrations νs of Si–O–Al | [24,25,26] |
| 564 600, 564 605, 569, 544 | Triple degenerative bending δd(F) of PO4 | [17,22,23] |
| 470–480 | Overlapping of double degenerated bending vibrations δd(E) of PO4 and SiO4 tetrahedra | [23,24] |
| 454 | Bending vibration δ of Si–O–Si of connected [SiO4] units | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasheva, T.; Yoleva, A.; Mateeva, J.; Georgiev, H. Study of New Glass–Ceramic and Dense Ceramic Containing Biogenic Hydroxyapatite. Materials 2025, 18, 3059. https://doi.org/10.3390/ma18133059
Tasheva T, Yoleva A, Mateeva J, Georgiev H. Study of New Glass–Ceramic and Dense Ceramic Containing Biogenic Hydroxyapatite. Materials. 2025; 18(13):3059. https://doi.org/10.3390/ma18133059
Chicago/Turabian StyleTasheva, Tina, Albena Yoleva, Janna Mateeva, and Hristo Georgiev. 2025. "Study of New Glass–Ceramic and Dense Ceramic Containing Biogenic Hydroxyapatite" Materials 18, no. 13: 3059. https://doi.org/10.3390/ma18133059
APA StyleTasheva, T., Yoleva, A., Mateeva, J., & Georgiev, H. (2025). Study of New Glass–Ceramic and Dense Ceramic Containing Biogenic Hydroxyapatite. Materials, 18(13), 3059. https://doi.org/10.3390/ma18133059






