Fruit Seed Biomass as an Alternative Material to Use in Recycling Processes of Metals from Industrial Waste
Abstract
1. Introduction
2. Materials and Methods
- Determination of the combustion heat of cherry stones.
- Thermogravimetric studies—determining the changes occurring in the biomass during heating in an inert and oxidizing atmosphere.
- High-temperature reductive melting of metallurgical slags using biomass as a reductant in the form of cherry stones.
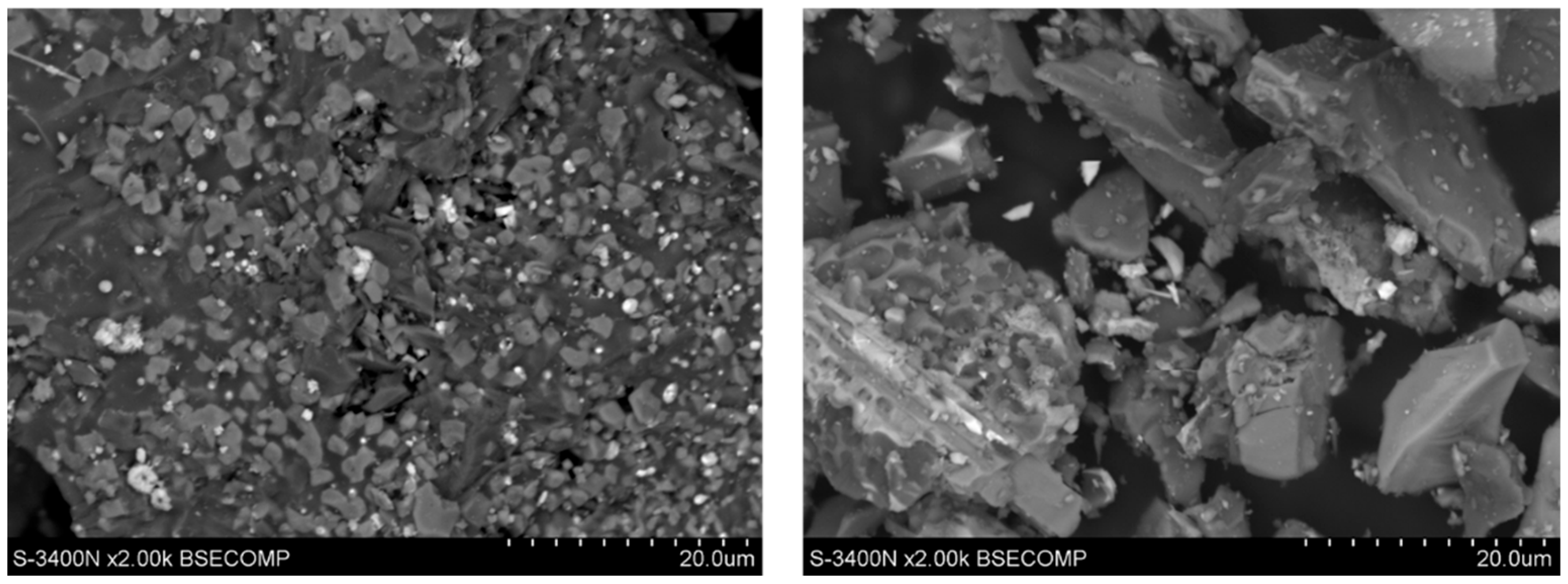
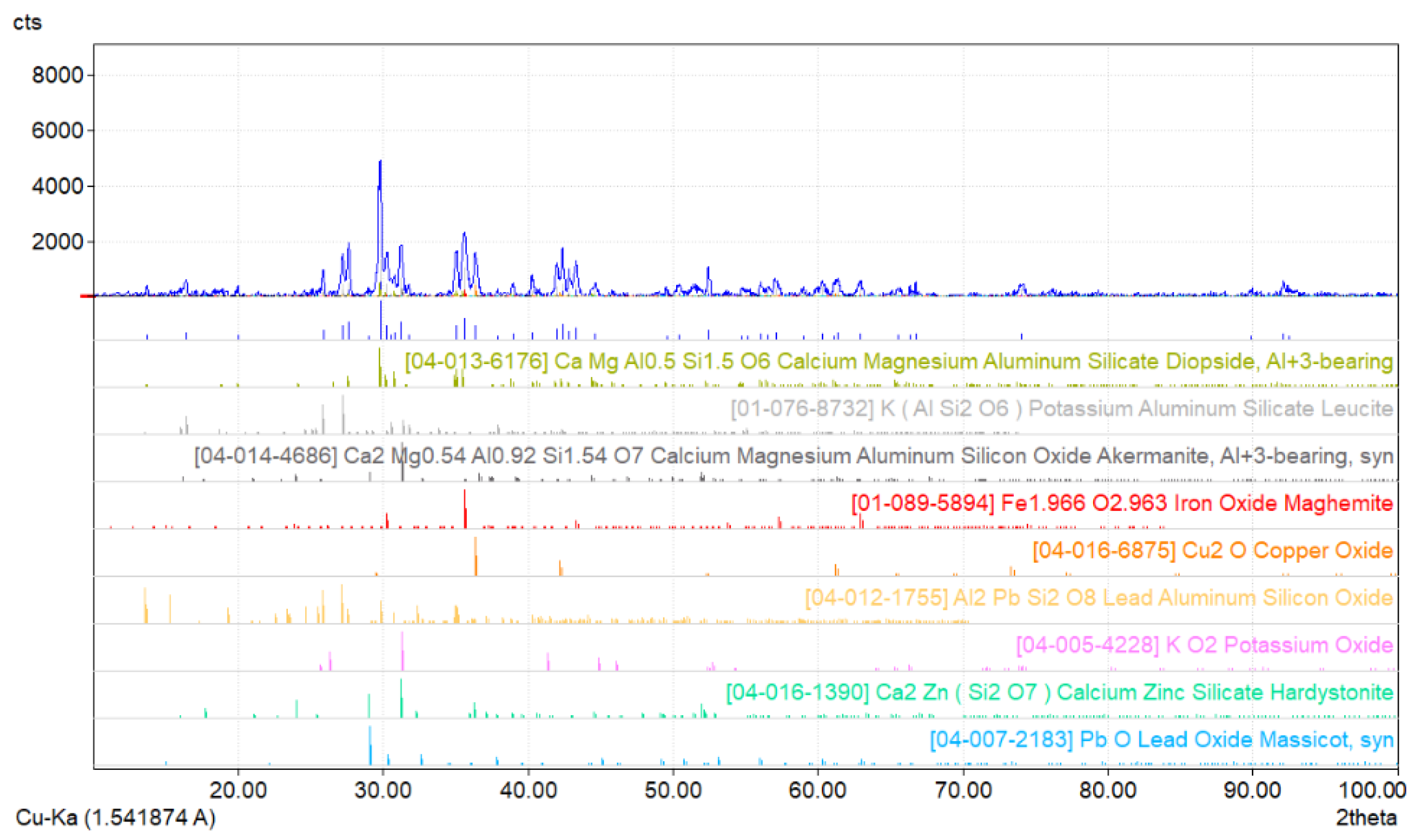
2.1. Research Materials
- Maghemite Fe1.966O2.963 with a tetragonal structure;
- Cu2O cuprite with a regular structure;
- Al2PbSi2O8 with a single-stranded structure;
- PbO masicot with a rhombic structure.
2.2. Research Methodology
2.2.1. Determination of Cherry Stones’ Combustion Heat
- PCS—gross calorific value [MJ/kg];
- E—water equivalent of the calorimeter, bomb, and its equipment, and water introduced into the bomb, read from the standard (PN-EN ISO 1716, 2010 [20]) [MJ/K];
- Tm—maximum temperature [°C];
- Ti—initial temperature [°C];
- C—temperature correction taking into account exchange with the environment [°C]; in the method used, it was C = 0.0;
- bi—correction for the combustion heat of the ignition wire [MJ], which is the product of the combustion heat of the wire b [MJ/kg] and md [kg];
- m—mass of the test sample [kg].
2.2.2. Thermogravimetric Analysis
2.2.3. Slag Reduction
3. Test Results and Discussion
3.1. Combustion Heat
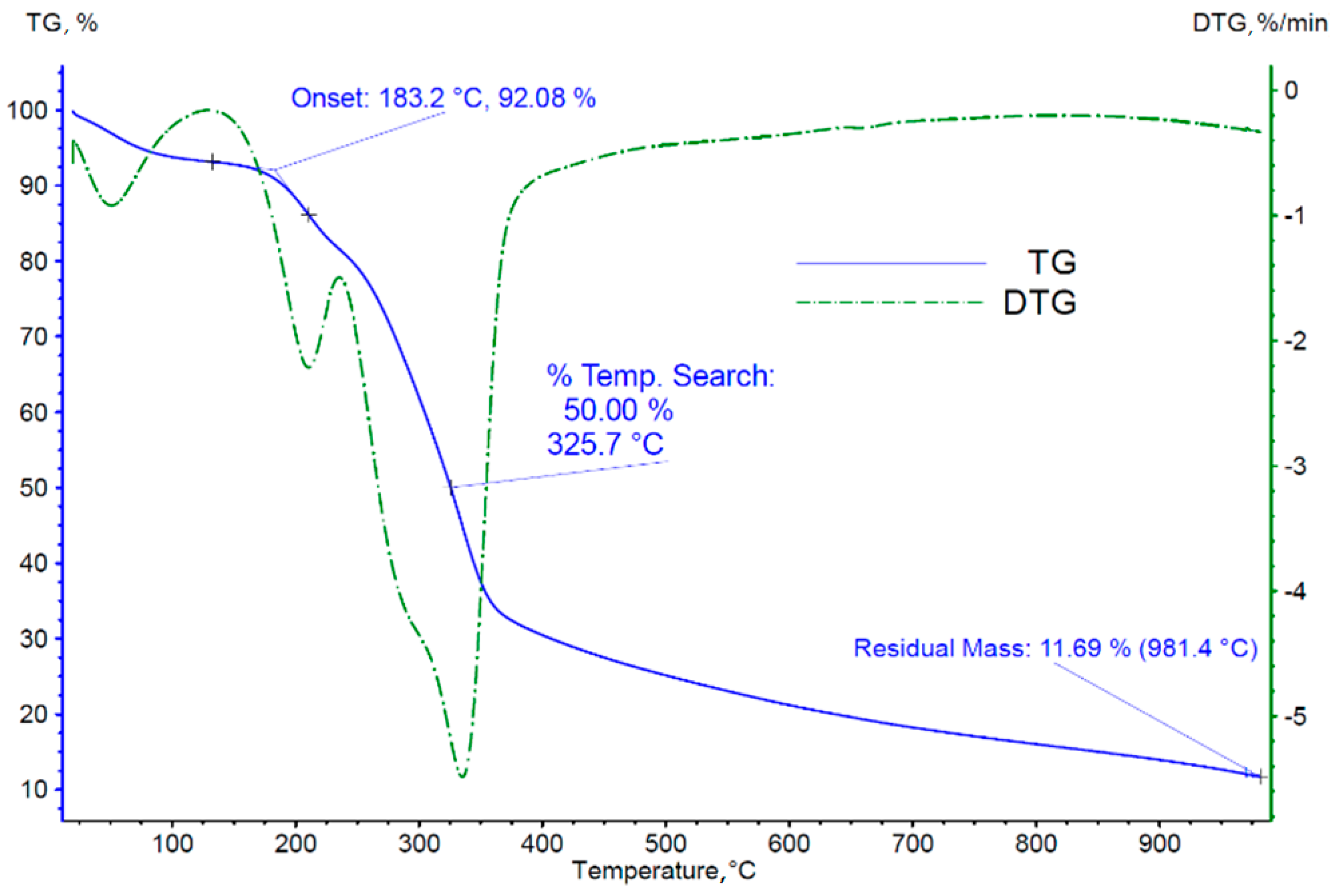
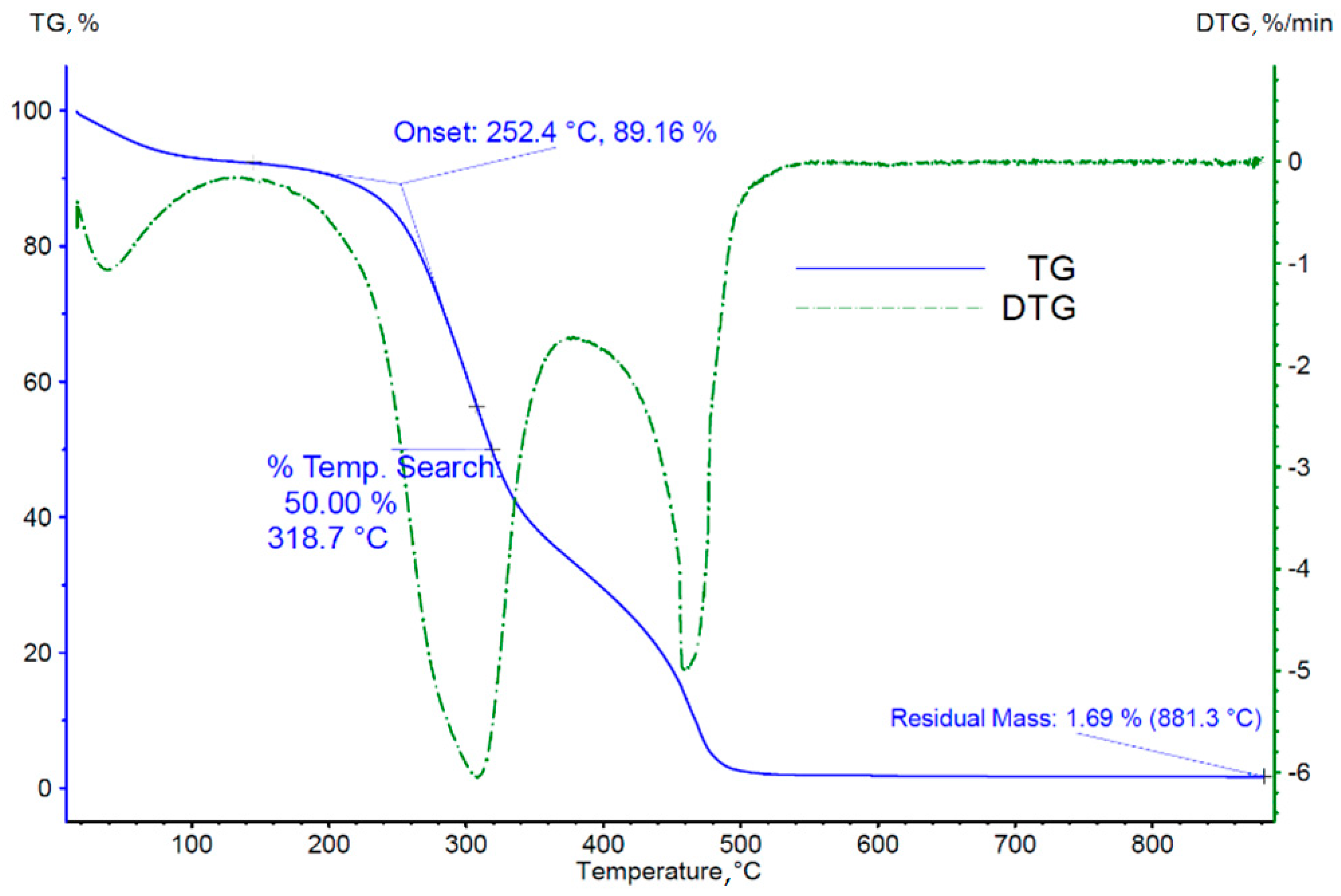
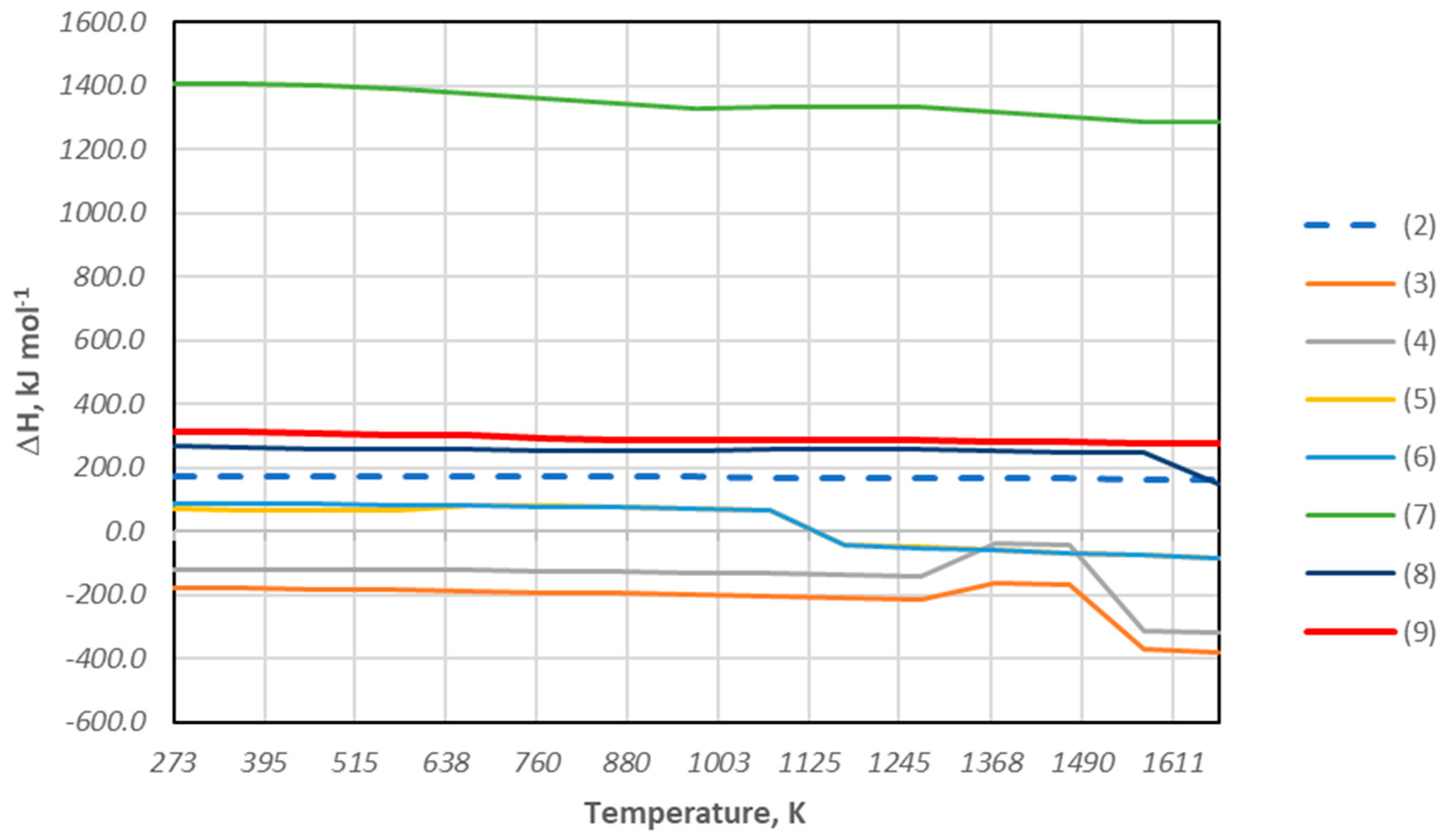
3.2. Thermogravimetric Analysis of Biomass
- (a)
- The temperature of onset of thermal decomposition;
- (b)
- The temperature at which 50% of the sample mass is lost;
- (c)
- The mass of the solid residue of the sample after reaching the set temperature;
- (d)
- The temperatures of occurrence of individual peaks of the derivative of the sample mass loss over time, identified with the successive phases of thermal decomposition/pyrolysis of the tested material.
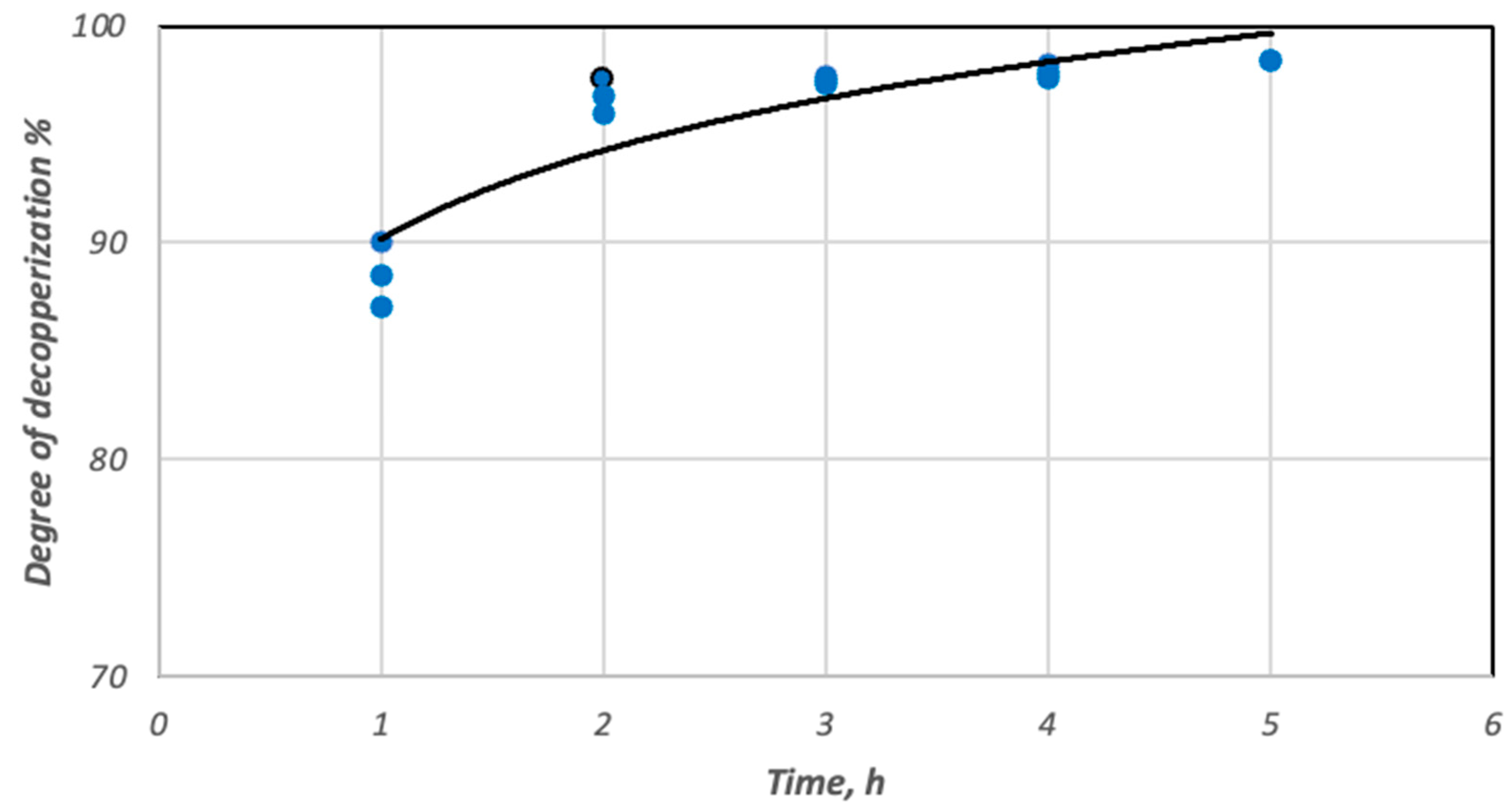
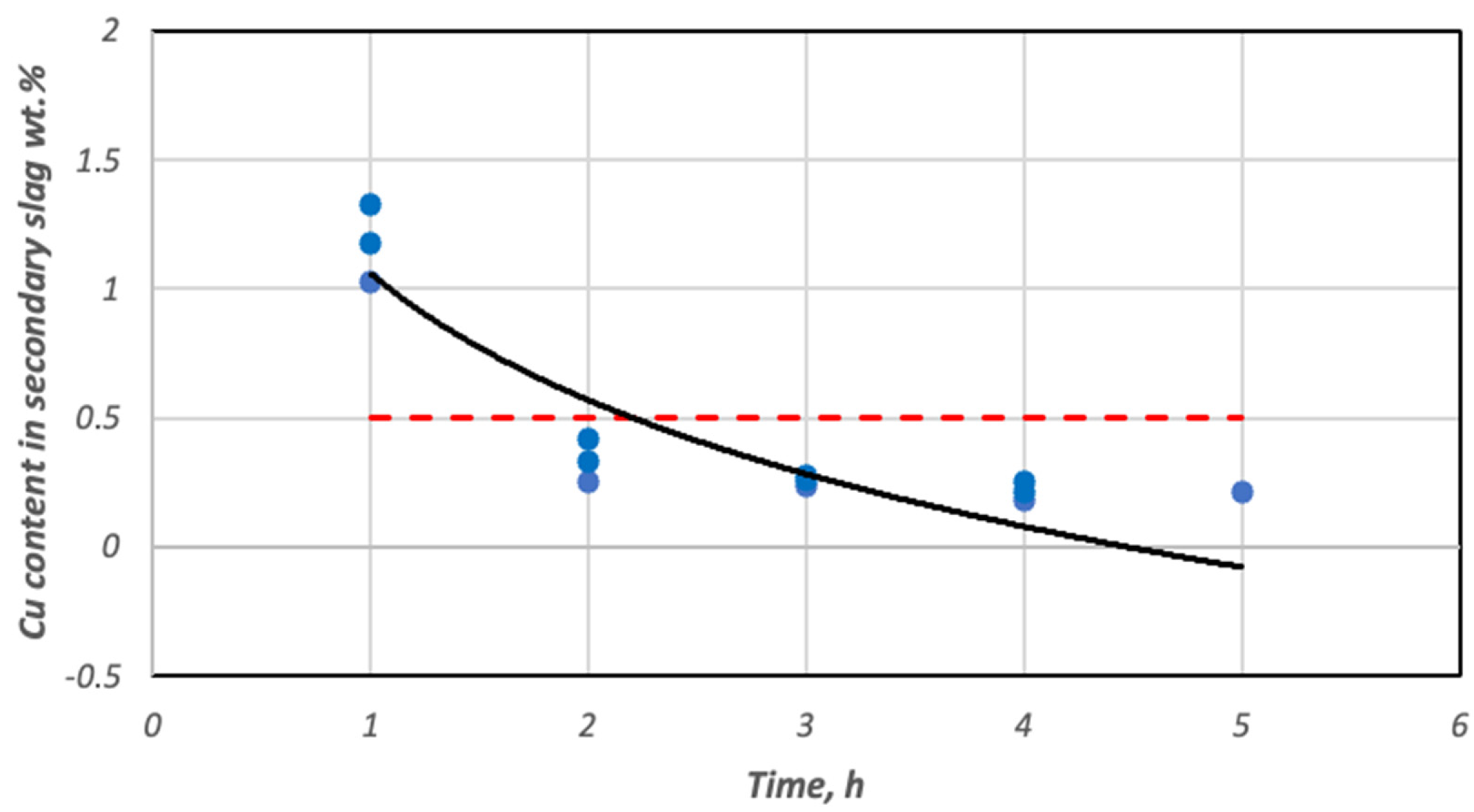
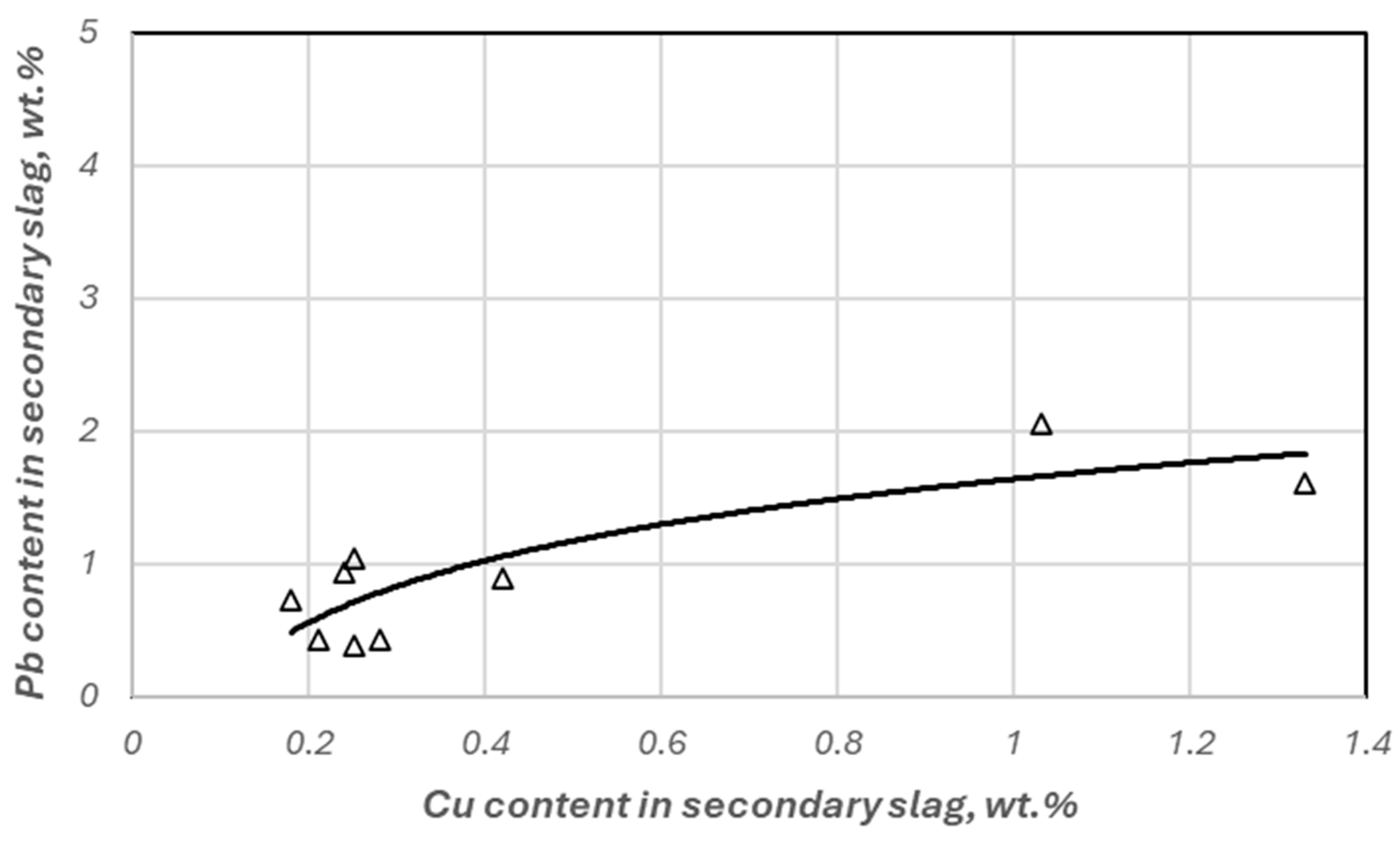
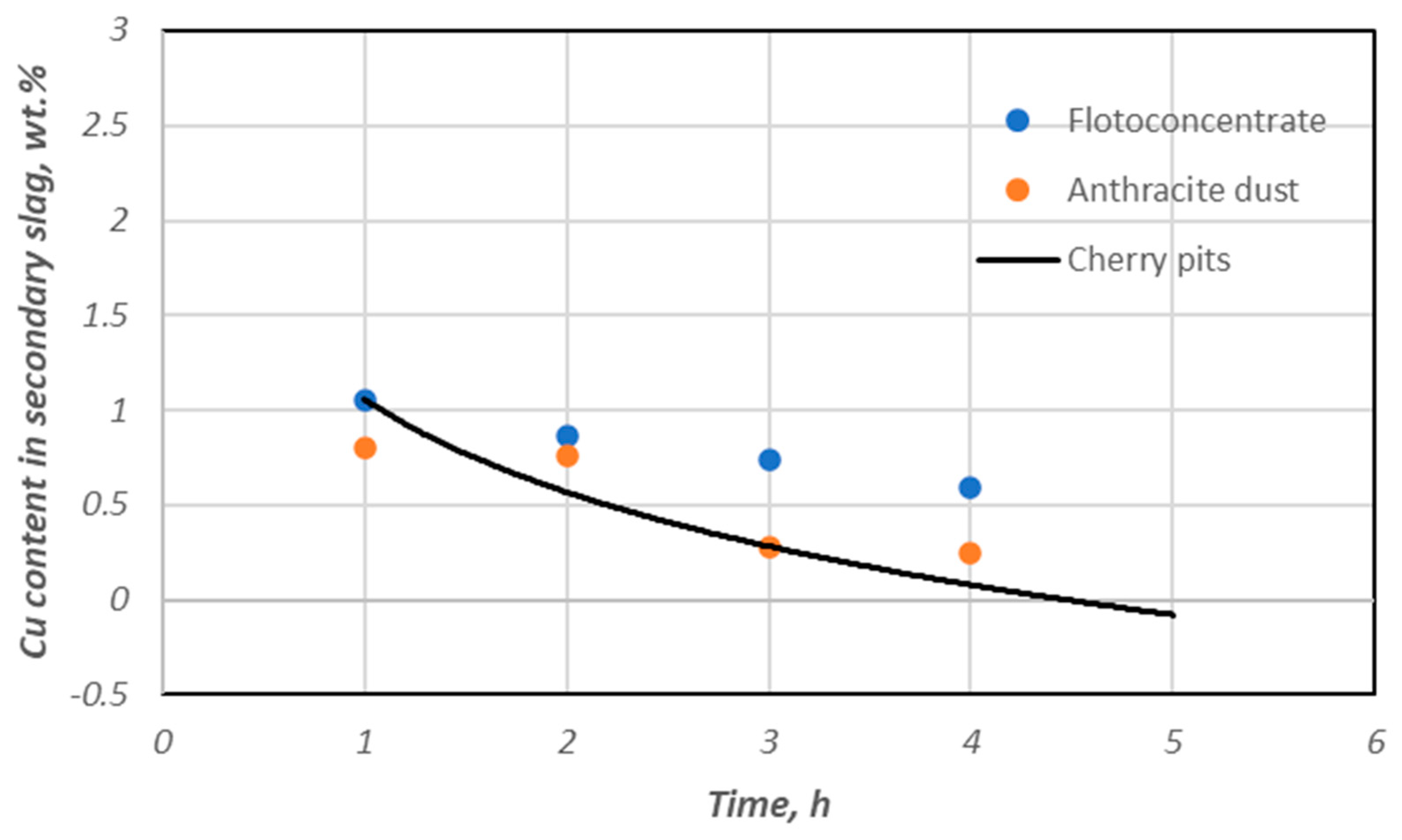
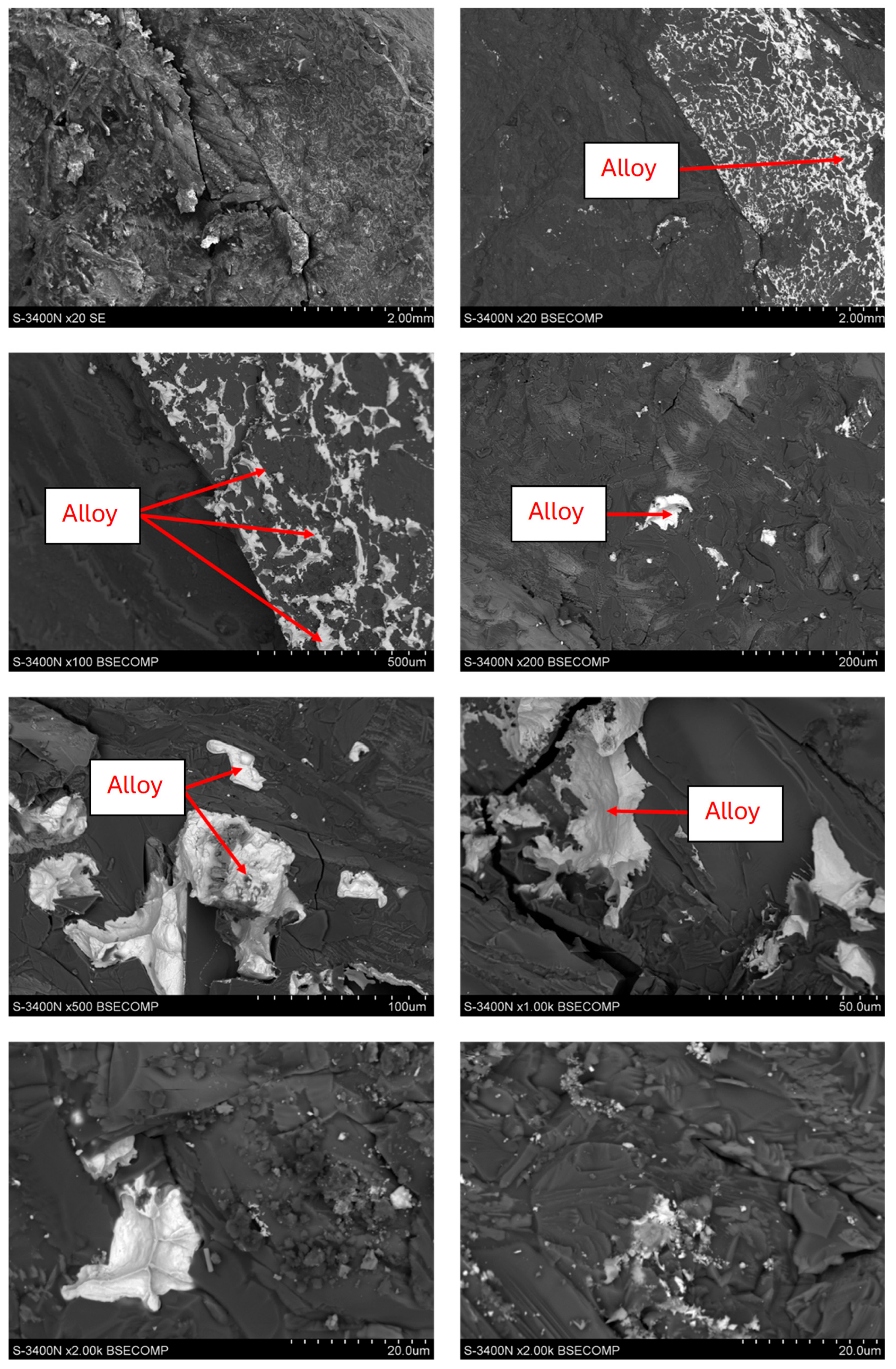
3.3. Copper Recovery Studies in the Process of Reductive Slag Remelting
- mkCu—weight of copper in secondary slag, [g];
- m0Cu—mass of copper in slag undergoing a reduction process, [g].
- m0, mk—initial and final mass of the slag after the process, respectively, g;
- CMe(0), CMe(k)—slag’s initial and final metal content, wt. %.
| Reducer | Time, h | Metal Mass, g | Secondary Slag Mass, g |
|---|---|---|---|
| Cherry stones | 1 | 9.35 | 68.22 |
| 1 | 9.74 | 66.37 | |
| 2 | 10.78 | 64.92 | |
| 2 | 10.18 | 63.74 | |
| 3 | 12.42 | 63.11 | |
| 3 | 12.59 | 62.66 | |
| 4 | 12.51 | 62.35 | |
| 4 | 12.44 | 62.88 | |
| 5 | 12.77 | 61.53 |
| Reducer | Time, h | Metal Content in Secondary Slag, wt. % | The Degree of Metal Transition from Slag to Alloy, % | Degree of Decopperization, % | ||||
|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Fe | Cu | Pb | Fe | |||
| Cherry stones | 1 | 1.03 | 2.05 | 10.96 | 90.25 | 24.41 | 18.15 | 90.00 |
| 1 | 1.33 | 1.61 | 10.72 | 88.98 | 38.98 | 17.71 | 87.08 | |
| 2 | 0.25 | 1.04 | 10.48 | 98.03 | 62.49 | 25.64 | 97.57 | |
| 2 | 0.42 | 0.89 | 10.99 | 96.75 | 68.48 | 21.18 | 95.92 | |
| 3 | 0.24 | 0.94 | 10.18 | 98.17 | 67.27 | 28.23 | 97.66 | |
| 3 | 0.28 | 0.43 | 10.29 | 97.85 | 84.93 | 26.93 | 97.28 | |
| 4 | 0.18 | 0.72 | 10.53 | 98.63 | 75.06 | 26.13 | 98.25 | |
| 4 | 0.25 | 0.39 | 10.89 | 98.09 | 86.37 | 22.95 | 97.57 | |
| 5 | 0.21 | 0.43 | 9.98 | 98.13 | 85.19 | 25.91 | 98.43 | |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- JSW Raport Zintegrowany 2021. Available online: https://www.jsw.pl/raportroczny-2020/#Istotne-dane (accessed on 23 June 2025).
- Paulo, A.; Krzak, M. Iron and steel complementary raw materials market at the beginning of the 21st century. Przegląd Geol. 2022, 70, 172–179. [Google Scholar] [CrossRef]
- Ozbayoglu, G. 3.19 Energy Production from Coal. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 788–821, ISBN 978-0-12-814925-6. [Google Scholar]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.; Alvear, G.R.F.; Schlesinger, M.E. Extractive Metallurgy of Copper, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-821903-4. [Google Scholar]
- Kowalczyk, R.; Piwnicki, Ł. Pips of fruit as a valuable raw material of the food industry. Postęp. Tech. Przetwórstwa Spoż. 2007, 2, 61–66. [Google Scholar]
- Yılmaz, C.; Gökmen, V. Compositional Characteristics of Sour Cherry Kernel and Its Oil as Influenced by Different Extraction and Roasting Conditions. Ind. Crops Prod. 2013, 49, 130–135. [Google Scholar] [CrossRef]
- Rzeźnik, W.; Mielcarek, P.; Rzeźnik, I. Assessment of Energetic Potential of Cherry Stones in Poland. J. Res. Appl. Agric. Eng. 2016, 61, 84–87. [Google Scholar]
- Durán-Valle, C.J.; Gómez-Corzo, M.; Gómez-Serrano, V.; Pastor-Villegas, J.; Rojas-Cervantes, M.L. Preparation of Charcoal from Cherry Stones. Appl. Surf. Sci. 2006, 252, 5957–5960. [Google Scholar] [CrossRef]
- González, J.F.; Encinar, J.M.; Canito, J.L.; Sabio, E.; Chacón, M. Pyrolysis of Cherry Stones: Energy Uses of the Different Fractions and Kinetic Study. J. Anal. Appl. Pyrolysis 2003, 67, 165–190. [Google Scholar] [CrossRef]
- Purgał, P.; Pasternak, J. Mixed Biomass as an Alternative Energy Fuel. Environment 2016, 8, 266–272. [Google Scholar]
- Dołżyńska, M.; Obidziński, S.; Kowczyk-Sadowy, M.; Krasowska, M. Densification and Combustion of Cherry Stones. Energies 2019, 12, 3042. [Google Scholar] [CrossRef]
- Lussier, M.G.; Shull, J.C.; Miller, D.J. Activated Carbon from Cherry Stones. Carbon 1994, 32, 1493–1498. [Google Scholar] [CrossRef]
- Michałek, T.; Wojtaszek, K.; Małecki, S.; Kornaus, K.; Wandor, S.; Druciarek, J.; Fitzner, K.; Wojnicki, M. Recovery of Pd(II) Ions from Aqueous Solutions Using Activated Carbon Obtained in a Single-Stage Synthesis from Cherry Seeds. C 2023, 9, 46. [Google Scholar] [CrossRef]
- Nowicka, P.; Gładzik, M.; Wojdyło, A.; Oszmiański, J. Pestka Wiśni—Surowiec Odpadowy o Wysokim Potencjale prozdrowotnym=Cherry Seed—A Waste Material with a High pro-Health Potential. In Bioactive Components of Plant Raw Materials and Products; Słupski, J., Tarko, T., Drożdż, I., Eds.; Polish Society of Food Technologists: Kraków, Poland, 2018; pp. 154–164. [Google Scholar]
- Vidrih, R.; Hribar, J.; Sekse, L. Cherry Seeds as a Source of Nutritionally Important Fatty Acids. Acta Hortic. 2014, 1020, 165–172. [Google Scholar] [CrossRef]
- Ptak, S.; Półka, M. Wpływ Biomasy na Parametry Palności i Wybuchowości Pyłu Węgla Kamiennego; Szkoła Główna Służby Pożarniczej: Warsaw, Poland, 2018; pp. 60–70. [Google Scholar]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An Overview of Fast Pyrolysis of Biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Kortyka, L.; Labaj, J.; Ptak, S.; Smalcerz, A.; Blacha, L.; Mycka, L.; Matula, T.; Findorak, R. A Study on the Potential for the Application of Peanut Shells as a Reducer in the Process of Metal Recovery from Metallurgical Slags. Sustainability 2024, 16, 9261. [Google Scholar] [CrossRef]
- Smalcerz, A.; Matula, T.; Slusorz, M.; Wojtasik, J.; Chaberska, W.; Kluska, S.; Kortyka, L.; Mycka, L.; Blacha, L.; Labaj, J. The Use of PCB Scrap in the Reduction in Metallurgical Copper Slags. Materials 2023, 16, 625. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 1716; Reaction to Fire Tests for Products—Determination of the Gross Heat of Combustion (Calorific Value). ISO: Geneva, Switzerland, 2018.
- PNEN15309:2010; Characterization of Waste and Soil—Determination of Elemental Composition by X-ray Fluorescence. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Bryś, A.; Zielińska, J.; Głowacki, S.; Tulej, W.; Bryś, J. Analysis of Possibilities of Using Biomass from Cherry and Morello Cherry Stones for Energy Purposes. E3S Web Conf. 2020, 154, 01005. [Google Scholar] [CrossRef]
- Kaynak, B.; Topal, H.; Atimtay, A. Peach and apricot stone combustion in a bubbling fluidized bed. Fuel Process. Technol. 2005, 86, 1175–1193. [Google Scholar] [CrossRef]
- Atimtay, A.; Kaynak, B. Co-combustion of peach and apricot stone with coal in a bubbling fluidized bed. Fuel Process. Technol. 2008, 89, 183–197. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stahl, R.; Yanik, J. The Slow and Fast Pyrolysis of Cherry Seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef] [PubMed]


| Material | Element Content, % by Mass | |||||
|---|---|---|---|---|---|---|
| C | S | Cl | N | H | O2 | |
| Cherry stones | 49.11–53.8 | 0.02–0.08 | - | 4.34 | 7.05 | 37.6–39.1 |
| Slag Component | Cu | Pb | Fe | SiO2 | CaO |
| Component Content, % by Weight | 10.30 | 2.25 | 11.11 | 34.50 | 14.12 |
| Sample | Qsp, kJ/kg | Qsr, kJ/kg | Comments |
|---|---|---|---|
| Cherry stones | 19,535 | 19,995 | Own research |
| 20,211 | |||
| 20,238 | |||
| Cherry stones | - | 19,540–22,020 | [5,7,22] |
| Apricot kernels | 20,390–20,656 | - | [23] |
| Peach stones | 20,820–22,082 | - | [23,24] |
| Plum stones | 20,760–22,460 | - | [11] |
| Material | Element Content, % by Mass | |||||
|---|---|---|---|---|---|---|
| Fe | K | Ca | Mg | Cu | Na | |
| Cherry stones ash | 15.65 | 13.01 | 12.68 | 4.98 | 0.62 | 0.49 |
| Sample Mass Loss | Nitrogen | Air | ||||
|---|---|---|---|---|---|---|
| Loss I, % | Loss II, % | Loss III, % | Loss I, % | Loss II, % | Loss III, % | |
| 8 | 13 | 47 | 5 | 55 | 15 | |
| The temperature range for the particular loss, °C | ≤130 | 130–240 | 240–400 | ≤150 | 240–380 | 380–485 |
| Maximum mass loss rate, %/min | 0.95 | 2.21 | 5.52 | 1.82 | 6.04 | 5.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kortyka, L.; Labaj, J.; Mycka, L.; Matula, T.; Ptak, S.; Babilas, D.; Wojtal, T.; Blacha, L.; Smalcerz, A.; Findorak, R.; et al. Fruit Seed Biomass as an Alternative Material to Use in Recycling Processes of Metals from Industrial Waste. Materials 2025, 18, 3063. https://doi.org/10.3390/ma18133063
Kortyka L, Labaj J, Mycka L, Matula T, Ptak S, Babilas D, Wojtal T, Blacha L, Smalcerz A, Findorak R, et al. Fruit Seed Biomass as an Alternative Material to Use in Recycling Processes of Metals from Industrial Waste. Materials. 2025; 18(13):3063. https://doi.org/10.3390/ma18133063
Chicago/Turabian StyleKortyka, Lukasz, Jerzy Labaj, Lukasz Mycka, Tomasz Matula, Szymon Ptak, Dorota Babilas, Tomasz Wojtal, Leszek Blacha, Albert Smalcerz, Robert Findorak, and et al. 2025. "Fruit Seed Biomass as an Alternative Material to Use in Recycling Processes of Metals from Industrial Waste" Materials 18, no. 13: 3063. https://doi.org/10.3390/ma18133063
APA StyleKortyka, L., Labaj, J., Mycka, L., Matula, T., Ptak, S., Babilas, D., Wojtal, T., Blacha, L., Smalcerz, A., Findorak, R., & Chmiela, B. (2025). Fruit Seed Biomass as an Alternative Material to Use in Recycling Processes of Metals from Industrial Waste. Materials, 18(13), 3063. https://doi.org/10.3390/ma18133063









