Advancements in Electrochromic Technology for Multifunctional Flexible Devices
Abstract
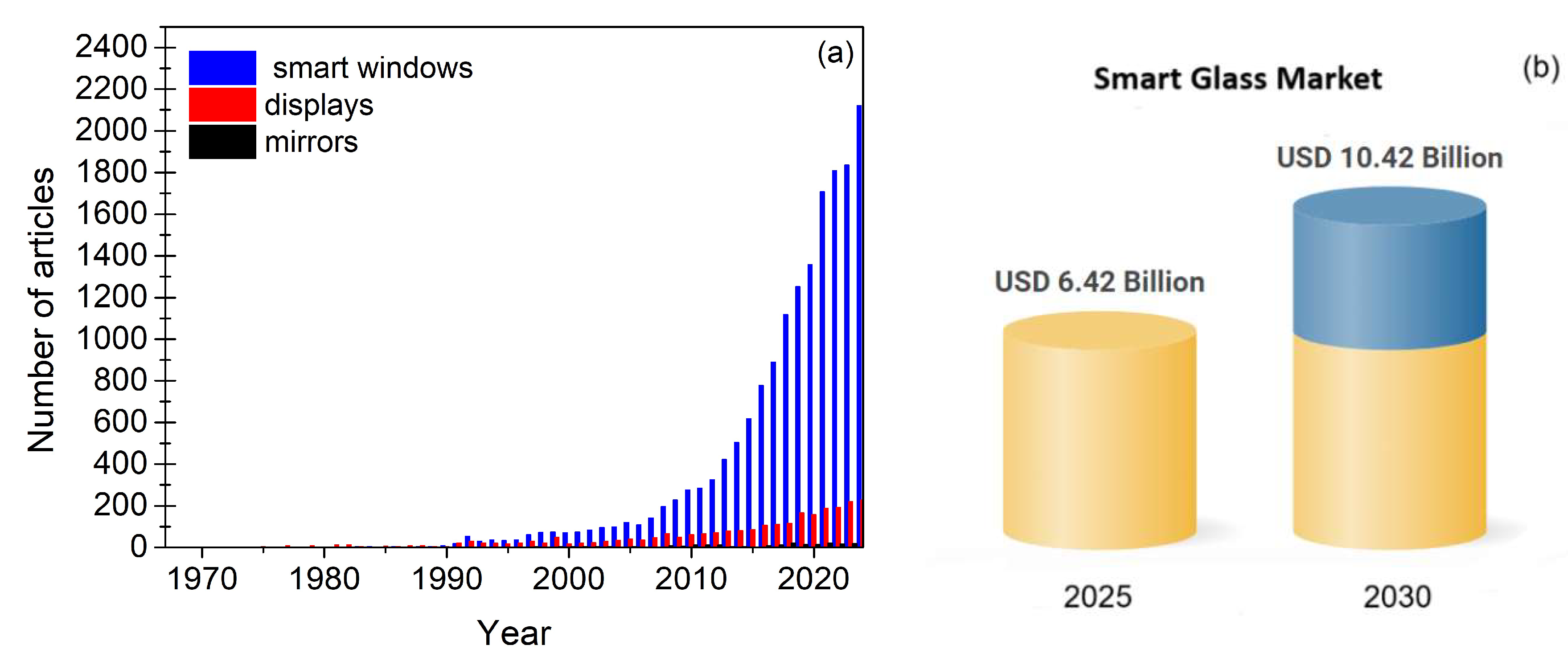
1. Introduction
2. Materials and Methods
2.1. Classification of Electrochromic Materials
- (I)
- Redox mode (electron transfer): electrochromic materials are classified as cathodic, which gains electrons and typically exhibits colouration, or anodic, which loses electrons and becomes coloured [115,116]. Some transition metal oxides, such as molybdenum trioxide (MoO3) and niobium pentoxide (Nb2O5), exhibit cathodic electrochromic behaviour, while nickel oxide (NiO) demonstrates anodic electrochromic behaviour.
- (II)
- Colour change: (a) materials that exhibit at least one coloured and one bleached state, such as MoO3; (b) materials that display two distinct colour states, for example, polythiophenes switching from red to blue; (c) multicoloured electrochromic materials, including or not, a bleached state, and this category typically comprises polymers and copolymers [117,118].
- (III)
- Solubility of the redox states: (a) materials where both the reduced state and oxidized state are soluble, some examples are organic molecules and metal complexes; (b) materials in which only one redox state is soluble, such as in the reversible electrodeposition of metals; (c) materials in which all redox states are solid (insoluble). In this type of electrochromic materials fall tungsten oxide (WO3), polymeric viologens, conducting polymers, etc. [119].
- (IV)
- Relationship between redox-active units and chromophores in a more recent classification: (a) direct redox mode and (b) indirect redox mode [120]. In electrochromic materials with direct redox mode, chromophores and redox-active units are the same entity, and the colour change is caused by the electrochemically driven redox process of such units. In electrochromic materials with indirect redox, chromophores and redox-active are different entities. In this case, the colour change in chromophores is induced by energy transfer resulting from the electrochemically driven redox process of the redox-active units [121,122].
- (V)
- Based on chemical composition and structure, electrochromic materials can be classified as inorganic, organic, composite/nanocomposite and hybrid materials [123].
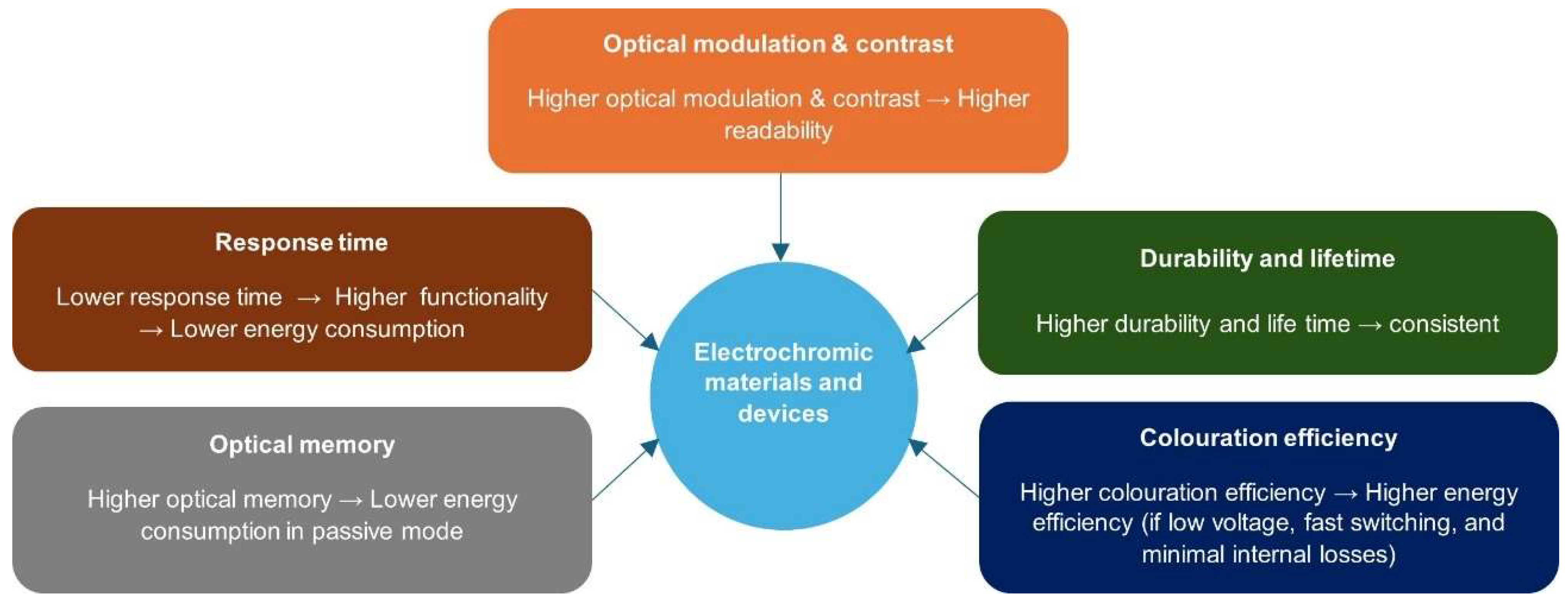
2.2. Performance Indexes of Electrochromic Materials and Devices
2.2.1. Optical Modulation (OM) and Contrast Ratio (CR)
2.2.2. Response Time
2.2.3. Optical Memory Effect
2.2.4. Colouration Efficiency
2.2.5. Durability and Lifetime
2.3. Conventional and Emerging Electrochromic Materials
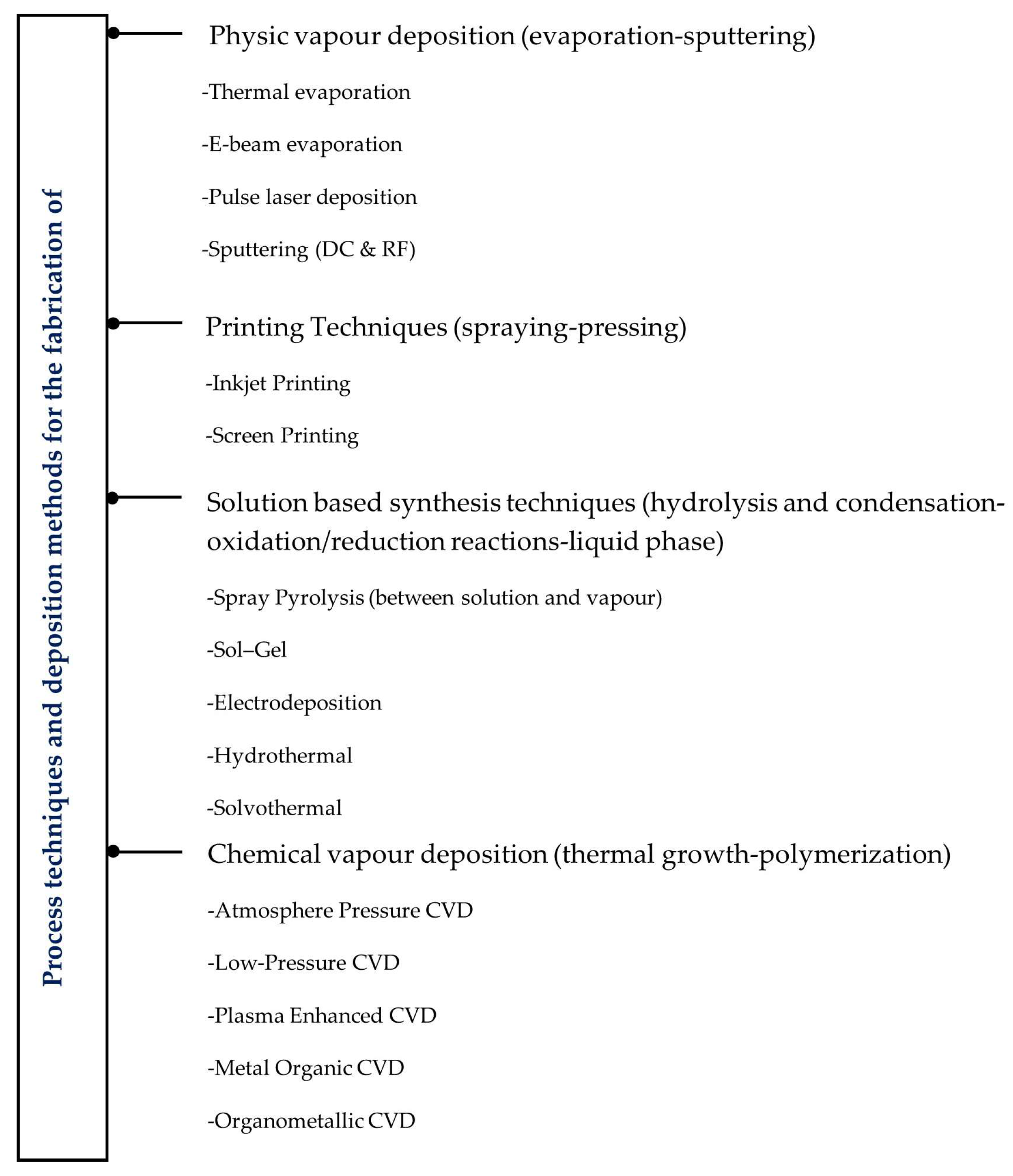
2.4. Process Techniques and Deposition Methods for the Fabrication of Electrochromic Materials
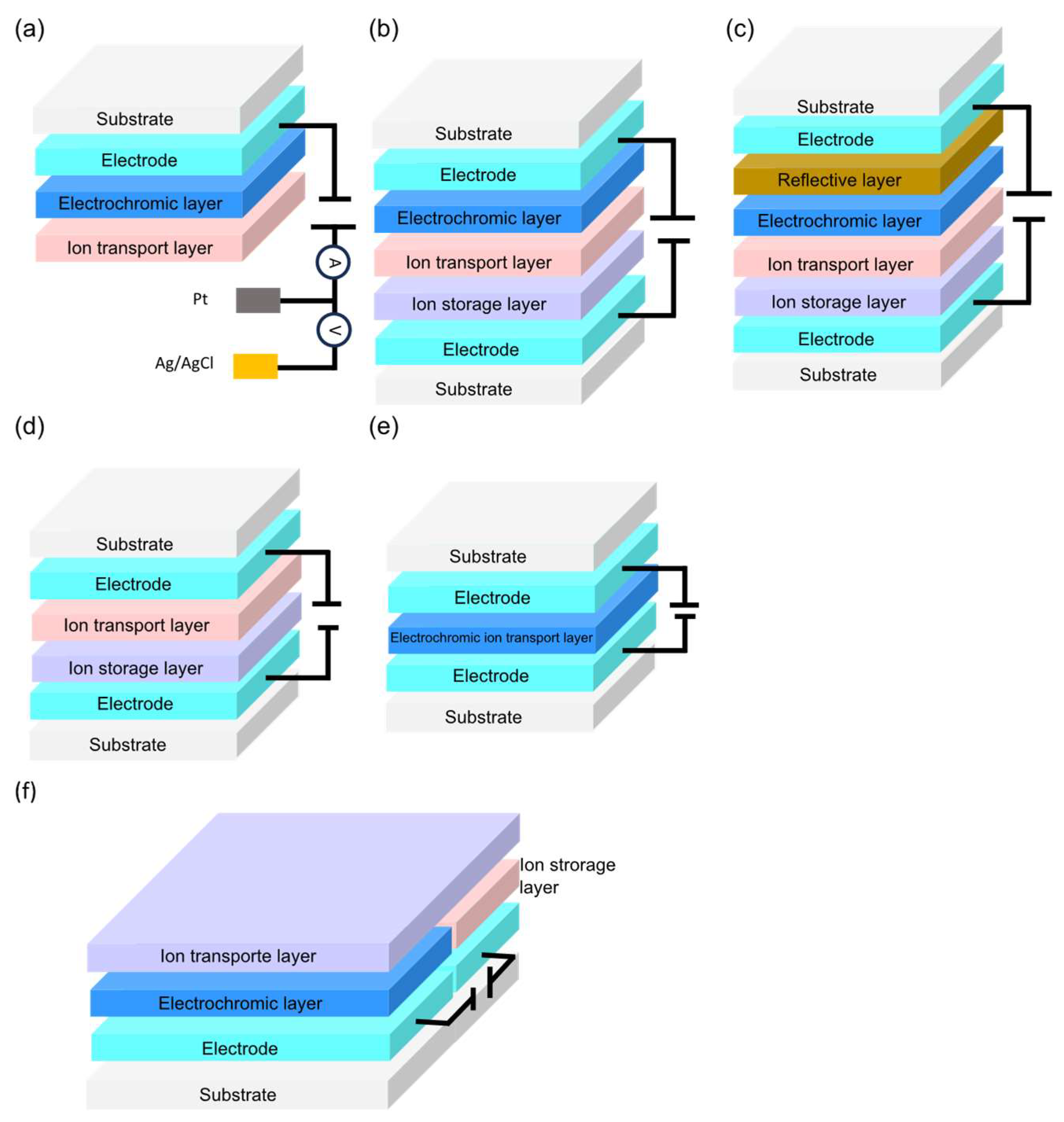
2.5. Conventional and Multifunctional Flexile Electrochromic Devices Architectures
3. Results
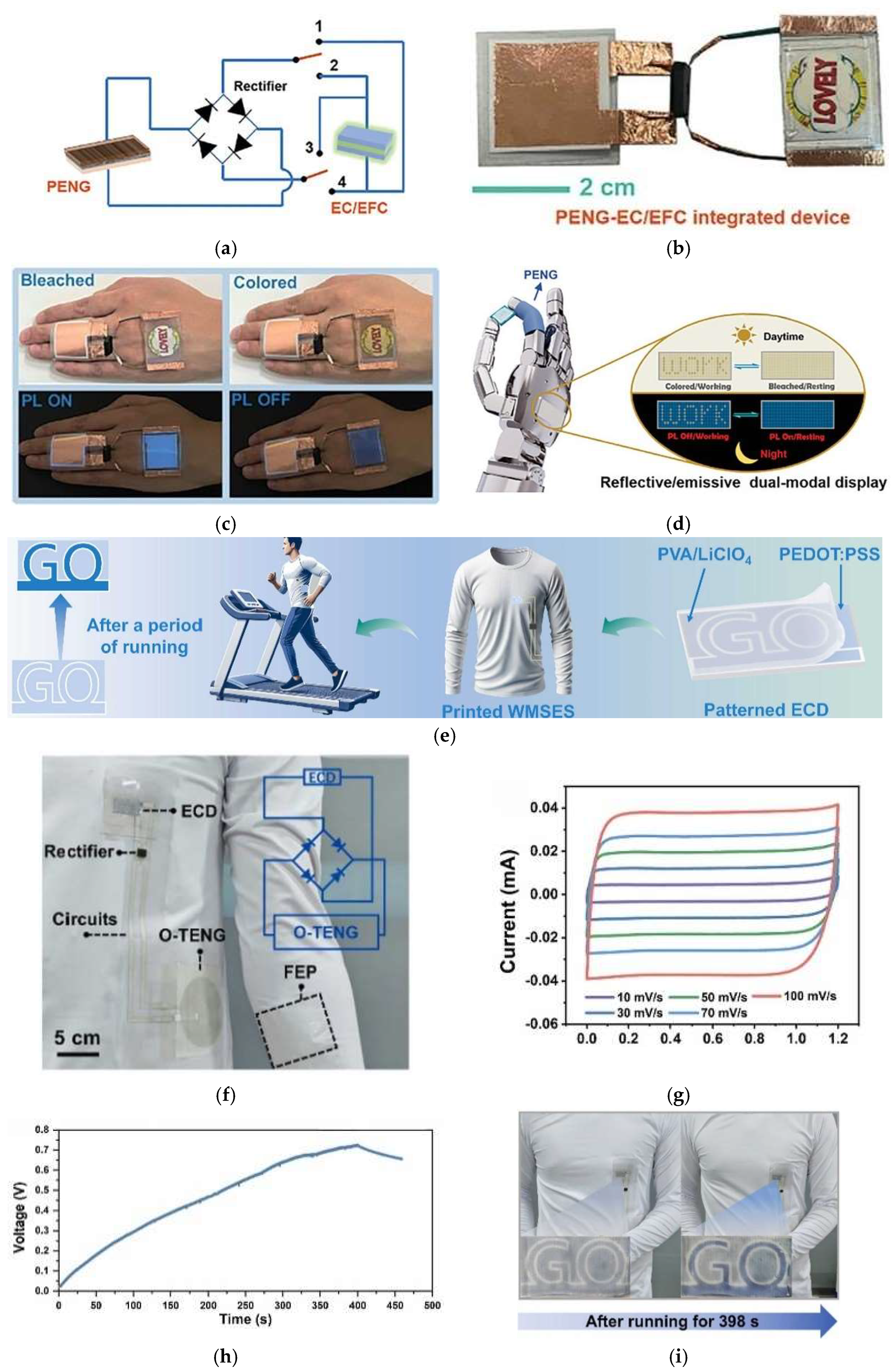
3.1. Self-Powered Electrochromic Devices
3.1.1. ECDs Powered by Nanogenerators
3.1.2. ECDs Powered by Solar Energy
3.2. Flexible Electrochromic Energy Storage Devices
3.2.1. Flexible Electrochromic Supercapacitors
3.2.2. Flexible Electrochromic Batteries
3.3. Flexible Multicolour Electrochromic Displays
3.4. Flexible Smart Windows
3.5. Other Types of Multifunctional and Flexible Electrochromic Devices
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granqvist, C.G. Electrochromic devices. J. Eur. Ceram. Soc. 2005, 25, 2907–2912. [Google Scholar] [CrossRef]
- Somani, P.; Radhakrishnan, S. Electrochromic Materials and Devices: Present and Future. Mater. Chem. Phys. 2003, 77, 117–133. [Google Scholar] [CrossRef]
- Deb, S. Opportunities and Challenges of Electrochromic Phenomena in Transition Metal Oxides. Sol. Energy Mater. Sol. Cells 1992, 25, 327–338. [Google Scholar] [CrossRef]
- Li, Y.; Sun, P.; Chen, J.; Zha, X.; Tang, X.; Chen, Z.; Zhang, Y.; Cong, S.; Geng, F.; Zhao, Z. Colorful Electrochromic Displays with High Visual Quality Based on Porous Metamaterials. Adv. Mater. 2023, 35, 2300116. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, T.; Okada, Y.; Naijoh, Y.; Hirano, S.; Sagisaka, T.; Gotoh, D.; Inoue, M.; Kim, S.; Tsuji, K.; Takahashi, H.; et al. Flexible Electrochromic Display. Int. Disp. Workshops 2013, IDW’13, 1300–1303. [Google Scholar]
- Gu, C.; Jia, A.; Zhang, Y.; Zhang, S. Emerging Electrochromic Materials and Devices for Future Displays. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef]
- Lynam, N. Electrochromic Automotive Day/Night Mirrors. In Proceedings of the SAE International Congress and Exposition, Detroit, MI, USA, 23–27 February 1987; SAE Technical Paper 870636. pp. 1–9. [Google Scholar]
- Wang, K.; Tao, K.; Jiang, R.; Zhang, H.; Liang, L.; Gao, J.; Cao, H. A Self-Bleaching Electrochromic Mirror Based on Metal Organic Frameworks. Materials 2021, 14, 2771. [Google Scholar] [CrossRef] [PubMed]
- Ranney, T.; Simmons, L.; Masalonis, A. The Immediate Effects of Glare and Electrochromic Glare-Reducing Mirrors in Simulated Truck Driving. Hum. Factors 2000, 42, 337–347. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Fisher, A.; Lee, J.Y. Al3+ Intercalation/de-Intercalation-Enabled Dual-Band Electrochromic Smart Windows with a High Optical Modulation, Quick Response and Long Cycle Life. Energy Environ. Sci. 2018, 11, 2884–2892. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.; Gustavsen, A. Properties, Requirements and Possibilities of Smart Windows for Dynamic Daylight and Solar Energy Control in Buildings: State-of-the-Art. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Hu, X.; Zhao, W.; Broer, D.J.; Zhou, G. Reverse Mode Polymer Dispersed Liquid Crystal-based Smart Windows: A Progress Report. Recent. Prog. Mater. 2021, 3, 44. [Google Scholar] [CrossRef]
- Macher, S.; Schott, M.; Dontigny, M.; Guerfi, A.; Zaghib, K.; Posset, U.; Löbmann, P. Large-Area Electrochromic Devices on Flexible Polymer Substrates with High Optical Contrast and Enhanced Cycling Stability. Adv. Mater. Technol. 2021, 6, 2000836. [Google Scholar] [CrossRef]
- Fan, H.; Wei, W.; Hou, C.; Zhang, Q.; Li, Y.; Li, K.; Wang, H. Wearable Electrochromic Materials and Devices: From Visible to Infrared Modulation. J. Mater. Chem. C 2023, 11, 7183–7210. [Google Scholar] [CrossRef]
- Fu, G.; Gong, H.; Bai, T.; Zhang, Q.; Wang, H. Progress and Challenges in Wearable Electrochromic Devices: A Review. J. Mater. Sci. Mater. Electron. 2023, 34, 1316. [Google Scholar] [CrossRef]
- Sheng, M.; Wang, W.; Li, L.; Zhang, L.; Fu, S. All-in-One Wearable Electronics Design: Smart Electrochromic Liquid-Crystal-Clad Fibers without External Electrodes. Colloids Surf. 2021, 630, 127535. [Google Scholar] [CrossRef]
- Yang, G.; Ding, J.; Yang, B.; Wang, X.; Gu, C.; Guan, D.; Yu, Y.; Zhang, Y.-M.; Zhang, S.X.-A. Highly stretchable electrochromic hydrogels for use in wearable electronic devices. J. Mater. Chem. C 2019, 7, 9481–9486. [Google Scholar] [CrossRef]
- Ranjbar, S.; Salavati, A.H.; Ashari Astani, N.; Naseri, N.; Davar, N.; Ejtehadi, M.R. Electrochromic Sensor Augmented with Machine Learning for Enzyme-Free Analysis of Antioxidants. ACS Sens. 2023, 8, 4281–4292. [Google Scholar] [CrossRef]
- Pellitero, M.; Campo, F. Electrochromic Sensors: Innovative Devices Enabled by Spectroelectrochemical Methods. Curr. Opin. Electrochem. 2019, 15, 66–72. [Google Scholar] [CrossRef]
- Celiesiute, R.; Ramanaviciene, A.; Gicevicius, M.; Ramanavicius, A. Electrochromic Sensors Based on Conducting Polymers, Metal Oxides, and Coordination Complexes. Crit. Rev. Anal. Chem. 2019, 49, 195–208. [Google Scholar] [CrossRef]
- Capoferri, D.; Diduk, R.; Carlo, M.; Compagnone, D.; Merkoçi, A. Electrochromic Molecular Imprinting Sensor for Visual and Smartphone-Based Detections. Anal. Chem. 2018, 90, 5850–5856. [Google Scholar] [CrossRef]
- Pellitero, M.; Guimerà, A.; Kitsara, M.; Villa, R.; Rubio, C.; Lakard, B.; Doche, M.-L.; Hihn, J.-Y.; del Campo, F.J. Quantitative Self-Powered Electrochromic Biosensors. Chem. Sci. 2017, 8, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Malagón, S.; Colín, D.; Azizkhani, H.; Aller-Pellitero, M.; Guirado, G.; del Campo, F.J. A Self-Powered Skin-Patch Electrochromic Biosensor. Biosens. Bioelectron. 2021, 175, 112879. [Google Scholar] [CrossRef]
- Chou, H.; Nguyen, A.; Chortos, A.; To, J.W.; Lu, C.; Mei, J.; Kurosawa, T.; Bae, W.-G.; Tok, J.B.-H.; Bao, Z. A Chameleon-Inspired Stretchable Electronic Skin with Interactive Colour Changing Controlled by Tactile Sensing. Nat. Commun. 2015, 6, 8011. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhang, L.; Dong, Y.; Zhang, C.; Li, W. Recent Advances in Electrochromic Materials and Devices for Camouflage Applications. Mater. Chem. Front. 2023, 7, 2337–2358. [Google Scholar] [CrossRef]
- Tong, Z.; Tian, Y.; Zhang, H.; Li, X.; Ji, J.; Qu, H.; Li, N.; Zhao, J.; Li, Y. Recent advances in multifunctional electrochromic energy storage devices and photoelectrochromic devices. Sci. China Chem. 2017, 60, 13–37. [Google Scholar] [CrossRef]
- Pathak, D.; Moon, H. Recent Progress in Electrochromic Energy Storage Materials and Devices: A Minireview. Mater. Horiz. 2022, 9, 2949–2975. [Google Scholar] [CrossRef]
- Li, H.; Elezzabi, A. Simultaneously Enabling Dynamic Transparency Control and Electrical Energy Storage: Via Electrochromism. Nanoscale Horiz. 2020, 5, 691–695. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid. Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Kubota, T.; Tajima, K.; Kitamura, M.; Imai, H. Complementary electrochromic devices based on acrylic substrates for smart window applications in aircrafts. Mater. Chem. Phys. 2022, 277, 125460. [Google Scholar] [CrossRef]
- Marciel, A.; Graça, M.; Bastos, A.; Pereira, L.; Kumar, J.S.; Borges, J.; Vaz, F.; Peres, M.; Magalhães, S.; Lorenz, K.; et al. Molybdenum Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates. Materials 2021, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sheng, S.; He, Z.; Wang, R.; Pan, Z.; Zhao, H.-Y.; Liu, J.-W.; Yu, S.-H. Self-Powered Flexible Electrochromic Smart Window. Nano Lett. 2021, 21, 9976–9982. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.; Macher, S.; Niklaus, L.; Schott, M.; Hillmer, H.; Hartung, M.; Heim, H.-P. Flexible Electrochromic Device on Polycarbonate Substrate with PEDOT:PSS and Color-Neutral TiO2 as Ion Storage Layer. Polymers 2023, 15, 1982. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Kang, W.; Wang, J.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Stretchable and wearable electrochromic devices. ACS Nano 2014, 8, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Amoli, V.; Kim, S.Y.; Lee, C.; Kim, J.; Park, S.-M.; Kim, J.; Ahn, J.M.; Jung, K.J.; Kim, D.H. Low-power, deformable, dynamic multicolor electrochromic skin. Nano Energy 2020, 78, 105199. [Google Scholar] [CrossRef]
- Zhu, T.; Xiong, J.; Chen, J.; Zhou, X.; Cai, G.; Lai, Y.; Lee, P.S. Flexible electrochromic fiber with rapid color switching and high optical modulation. Nano Res. 2023, 16, 5473–5479. [Google Scholar] [CrossRef]
- Nuroldayeva, G.; Balanay, M.P. Flexing the Spectrum: Advancements and Prospects of Flexible Electrochromic Materials. Polymers 2023, 15, 2924. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent Conductive Electrodes for Electrochromic Devices: A Review. Appl. Phys. 1993, 57, 19–24. [Google Scholar] [CrossRef]
- Marciel, A.; Bastos, A.; Pereira, L.; Jakka, S.K.; Borges, J.; Vaz, F.; Peres, M.; Lorenz, K.; Bafti, A.; Pavić, L.; et al. Niobium Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates. Coatings 2024, 14, 1127. [Google Scholar] [CrossRef]
- Marciel, A.; Bastos, A.C.; Pereira, L.; Jakka, S.K.; Borges, J.; Vaz, F.; Peres, M.; Lorenz, K.; Alves, L.C.; Bafti, A.; et al. Niobium–Molybdenum Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates. ACS Appl. Energy Mater. 2025, 8, 4184–4199. [Google Scholar] [CrossRef]
- Yu, C.; Ma, D.; Wang, Z.; Zhu, L.; Guo, H.; Zhu, X.; Wang, J. Solvothermal growth of Nb2O5 films on FTO coated glasses and their electrochromic properties. Ceram. Int. 2021, 47, 9651–9658. [Google Scholar] [CrossRef]
- Khemasiri, N.; Klamchuen, A.; Jessadaluk, S.; Rattanawarinchai, P.; Borklom, P.; Rangkasikorn, A.; Rahong, S.; Saekung, C.; Horprathum, M.; Chananonnawathorn, C.; et al. Systematic investigations on morphological properties of aluminum-doped zinc oxide transparent electrode prepared from pulsed laser deposition and its electrochromic application. Vacuum 2023, 209, 111797. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Q.; Dong, G.; He, Y.; Diao, X. Influence of thickness on the structure, electrical, optical and electrochromic properties of AZO thin films and their inorganic all-solid-state devices. Electrochim. Acta 2017, 258, 1336–1347. [Google Scholar] [CrossRef]
- Scardaci, V. Copper nanowires for transparent electrodes: Properties, challenges and applications. Appl. Sci. 2021, 11, 8035. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly Stable Transparent Conductive Silver Grid/PEDOT:PSS Electrodes for Integrated Bifunctional Flexible Electrochromic Supercapacitors. Adv. Energy Mater. 2016, 6, 1501882. [Google Scholar] [CrossRef]
- Han, J.; Yang, J.; Gao, W.; Bai, H. Ice-Templated, Large-Area Silver Nanowire Pattern for Flexible Transparent Electrode. Adv. Funct. Mater. 2021, 31, 2010155. [Google Scholar] [CrossRef]
- Soo Choi, D.; Ho Han, S.; Kim, H.; Kang, S.H.; Kim, Y.; Yang, C.-M.; Kim, T.Y.; Yoon, D.H.; Yang, W.S. Flexible electrochromic films based on CVD-graphene electrodes. Nanotechnology 2014, 25, 395702. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, L.; Xu, Y.; Qiu, T.; Zhi, L.; Shi, G. Polyaniline electrochromic devices with transparent graphene electrodes. Electrochim. Acta 2009, 55, 491–497. [Google Scholar] [CrossRef]
- Lin, F.; Bult, J.B.; Nanayakkara, S.; Dillon, A.C.; Richards, R.M.; Blackburn, J.L.; Engtrakul, C. Graphene as an efficient interfacial layer for electrochromic devices. ACS Appl. Mater. Interfaces 2015, 7, 11330–11336. [Google Scholar] [CrossRef]
- Ghosh, D.S.; Chen, T.L.; Pruneri, V. High figure-of-merit ultrathin metal transparent electrodes incorporating a conductive grid. Appl. Phys. Lett. 2010, 96, 041109. [Google Scholar] [CrossRef]
- Schneider, J.; Rohner, P.; Thureja, D.; Schmid, M.; Galliker, P.; Poulikakos, D. Electrohydrodynamic NanoDrip Printing of High Aspect Ratio Metal Grid Transparent Electrodes. Adv. Funct. Mater. 2016, 26, 833–840. [Google Scholar] [CrossRef]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; van Haesendonck, C.; Van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The Origin of the High Conductivity of Poly(3,4-ethylenedioxythiophene)-Poly(styrenesulfonate) (PEDOT-PSS) Plastic Electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Singh, R.; Tharion, J.; Murugan, S.; Kumar, A. ITO-Free Solution-Processed Flexible Electrochromic Devices Based on PEDOT:PSS as Transparent Conducting Electrode. ACS Appl. Mater. Interfaces 2017, 9, 19427–19435. [Google Scholar] [CrossRef] [PubMed]
- Elsokary, A.; Soliman, M.; Abulfotuh, F.; Ebrahim, S.; Sadat-Shafai, T.; Karim, M. Fabrication of composite transparent conductive electrodes based on silver nanowires. Sci. Rep. 2024, 14, 3045. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Jafari, M.; Siaj, M.; Wang, H.; Xiao, S.; Ma, D. Highly Stable Ag–Au Core–Shell Nanowire Network for ITO-Free Flexible Organic Electrochromic Device. Adv. Funct. Mater. 2021, 31, 2010022. [Google Scholar] [CrossRef]
- Zhang, W.; Song, W.; Huang, J.; Huang, L.; Yan, T.; Ge, J.; Peng, R.; Ge, Z. Graphene:silver nanowire composite transparent electrode based flexible organic solar cells with 13.4% efficiency. J. Mater. Chem. A Mater. 2019, 7, 22021–22028. [Google Scholar] [CrossRef]
- Yang, T.S.; Lin, Z.R.; Wong, M.S. Structures and electrochromic properties of tungsten oxide films prepared by magnetron sputtering. Appl. Surf. Sci. 2005, 252, 2029–2037. [Google Scholar] [CrossRef]
- Ataalla, M.; Afify, A.S.; Hassan, M.; Abdallah, M.; Milanova, M.; Aboul-Enein, H.Y.; Mohamed, A. Tungsten-based glasses for photochromic, electrochromic, gas sensors, and related applications: A review. J. Non Cryst. Solids 2018, 491, 43–54. [Google Scholar] [CrossRef]
- Cheng, K.C.; Chen, F.R.; Kai, J.J. V2O5 nanowires as a functional material for electrochromic device. Sol. Energy Mater. Sol. Cells 2006, 90, 1156–1165. [Google Scholar] [CrossRef]
- Mjejri, I.; Manceriu, L.M.; Gaudon, M.; Rougier, A.; Sediri, F. Nano-vanadium pentoxide films for electrochromic displays. Solid. State Ion. 2016, 292, 8–14. [Google Scholar] [CrossRef]
- Turel, O.; Hacioglu, S.O.; Coskun, S.; Toppare, L.; Unalan, H.E. Sequential Deposition of Electrochromic MoO3 Thin Films with High Coloration Efficiency and Stability. J. Electrochem. Soc. 2017, 164, E565–E571. [Google Scholar] [CrossRef]
- Usha, K.S.; Lee, S.Y.; Sivakumar, R.; Sanjeeviraja, C. Ultra-fast switching of energy efficient electrochromic nickel oxide thin films for smart window applications. Ceram. Int. 2024, 50, 36651–36665. [Google Scholar] [CrossRef]
- Mjejri, I.; Grocassan, R.; Rougier, A. Enhanced Coloration for Hybrid Niobium-Based Electrochromic Devices. ACS Appl. Energy Mater. 2018, 1, 4359–4366. [Google Scholar] [CrossRef]
- Yao, D.; Rani, R.; Mullane, A.; Kalantar-Zadeh, K.; Ou, J.Z. High Performance Electrochromic Devices Based on Anodized Nanoporous Nb2O5. J. Phys. Chem. C 2013, 118, 476–481. [Google Scholar] [CrossRef]
- Assis, L.M.N.; Leones, R.; Kanicki, J.; Pawlicka, A.; Silva, M. Prussian blue for electrochromic devices. J. Electroanal. Chem. 2016, 777, 33–39. [Google Scholar] [CrossRef]
- Shah, K.; Wang, S.; Soo, D.; Xu, J. Viologen-Based Electrochromic Materials: From Small Molecules, Polymers and Composites to Their Applications. Polymers 2019, 11, 1839. [Google Scholar] [CrossRef]
- Wu, W.; Guo, S.; Bian, J.; Xu, J.W. Viologen-based flexible electrochromic devices. J. Energy Chem. 2024, 93, 453–470. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lee, K.M.; Huang, J.H.; Thomas, K.J.; Lin, J.T.; Ho, K.-C. A novel photoelectrochromic device with dual application based on poly(3,4-alkylenedioxythiophene) thin film and an organic dye. J. Power Sources 2008, 185, 1505–1508. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, C.; Wang, L.; Wang, T.; Yang, M.; Zhang, S.; Lv, Y. Multi-pattern polyaniline electrochromic device by controllable three-dimensional movement of ions. Opt. Mater. 2024, 147, 114605. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Zhuang, B.; Wang, X.; Zhang, Q.; Liu, J.; Jin, Y.; Wang, H. Nanoengineering of poly(3,4-ethylenedioxythiophene) for boosting electrochemical applications. Sol. Energy Mater. Sol. Cells 2021, 232, 111357. [Google Scholar] [CrossRef]
- Park, S.; Cho, H.; Faheem, A.B.; Song, S.; Van Tran, H.; Okwako, J.A.; Lee, K.-K.; Han, C.-H.; Park, Y.S.; Hong, S. Tailor-made anodically coloring organic-inorganic hybrid electrochromic materials derived from phenothiazine cores. Electrochim. Acta 2024, 507, 145156. [Google Scholar] [CrossRef]
- Mohanadas, D.; Sulaiman, Y. Recent advances in development of electroactive composite materials for electrochromic and supercapacitor applications. J. Power Sources 2022, 523, 231029. [Google Scholar] [CrossRef]
- Xiong, S.; Yin, S.; Wang, Y.; Kong, Z.; Lan, J.; Zhang, R.; Gong, M.; Wu, B.; Chu, J.; Wang, X. Organic/inorganic electrochromic nanocomposites with various interfacial interactions: A review. Mater. Sci. Eng. B 2017, 221, 41–53. [Google Scholar] [CrossRef]
- Chen, J.; Song, G.; Cong, S.; Zhao, Z. Resonant-Cavity-Enhanced Electrochromic Materials and Devices. Adv. Mater. 2023, 35, e2300179. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Cao, S.; Chen, J.; Zou, B.; Li, H. Plasmonic-based electrochromic materials and devices. Nanophotonics 2024, 13, 155–172. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Chen, Z.; Cong, S.; Zhao, Z. Fabry-Perot Cavity-Type Electrochromic Supercapacitors with Exceptionally Versatile Color Tunability. Nano Lett. 2020, 20, 1915–1922. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Tian, Y.; Pan, F.; Gould, T.; Zhang, S. Nanoarchitectonics of Two-Dimensional Electrochromic Materials: Achievements and Future Challenges. Adv. Mater. Technol. 2023, 8, 2200917. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, R.; Chen, C.; Yee, W.-A.; Kong, J.; Ding, G.; Lu, X. Non-volatile polymer electrolyte based on poly(propylene carbonate), Ionic liquid, and lithium perchlorate for electrochromic devices. J. Phys. Chem. B 2013, 117, 7783–7789. [Google Scholar] [CrossRef]
- Hallinan, D.T.; Balsara, N.P. Polymer electrolytes. Annu. Rev. Mater. Res. 2013, 43, 503–525. [Google Scholar] [CrossRef]
- Di Noto, V.; Lavina, S.; Giffin, G.A.; Negro, E.; Scrosati, B. Polymer electrolytes: Present, past and future. Electrochim. Acta 2011, 57, 4–13. [Google Scholar] [CrossRef]
- Thakur, V.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X.H. Hybrid Materials and Polymer Electrolytes for Electrochromic Device Applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef]
- Bosque, A.; Muñoz, K.; Sánchez, M.; Ureña, A. Thermomechanically Robust Ceramic/Polymer Nanocomposites Modified with Ionic Liquid for Hybrid Polymer Electrolyte Applications. ACS Appl. Energy Mater. 2022, 5, 4247–4258. [Google Scholar] [CrossRef]
- He, J.; You, L.; Tran, D.T.; Mei, J. Low-Temperature Thermally Annealed Niobium Oxide Thin Films as a Minimally Color Changing Ion Storage Layer in Solution-Processed Polymer Electrochromic Devices. ACS Appl. Mater. Interfaces 2019, 11, 4169–4177. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Chen, K.; Zemlyanov, D.Y.; You, L.; Mei, J. Stabilizing Hybrid Electrochromic Devices through Pairing Electrochromic Polymers with Minimally Color-Changing Ion-Storage Materials Having Closely Matched Electroactive Voltage Windows. ACS Appl. Mater. Interfaces 2021, 13, 5312–5318. [Google Scholar] [CrossRef]
- He, J.; Mukherjee, S.; Zhu, X.; You, L.; Boudouris, B.W.; Mei, J. Highly Transparent Crosslinkable Radical Copolymer Thin Film as the Ion Storage Layer in Organic Electrochromic Devices. ACS Appl. Mater. Interfaces 2018, 10, 18956–18963. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Chang, C.T.; Kuo, P.H. Ionic Storage Materials for Anodic Discoloration in Electrochromic Devices. Energies 2023, 16, 8119. [Google Scholar] [CrossRef]
- Choi, D.; Son, M.; Im, T.; Ahn, S.-H.; Lee, C.S. Microstructure control of NiO-based ion storage layer with various sized NiO particles to evaluate the electrochromic performance. Mater. Chem. Phys. 2020, 249, 123121. [Google Scholar] [CrossRef]
- Avellaneda, C.O.; Berton, M.A.C.; Bulhões, L.O.S. Optical and electrochemical properties of CeO2 thin film prepared by an alkoxide route. Sol. Energy Mater. Sol. Cells 2008, 92, 240–244. [Google Scholar] [CrossRef]
- Ko, T.F.; Chen, P.W.; Li, K.M.; Young, H.-T.; Chang, C.-T.; Hsu, S.-C. High-performance complementary electrochromic device based on iridium oxide as a counter electrode. Materials 2021, 14, 1591. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Tam, B.; Pei, P.; Li, F.; Xie, A.; Cheng, W. Macroporous Vanadium Oxide Ion Storage Films Enable Fast Switching Speed and High Cycling Stability of Electrochromic Devices. ACS Appl. Mater. Interfaces 2022, 14, 30021–30028. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Xu, F.; Zhang, R.; Wang, X.; Zhai, L.; Li, X.; Qian, H.-J.; Wu, L.; Li, H. Hybrid Liquid-Crystalline Electrolytes with High-Temperature-Stable Channels for Anhydrous Proton Conduction. J. Am. Chem. Soc. 2021, 143, 21433–21442. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Zou, X.; Tan, Y.; Jia, C.; Weng, X.; Deng, L. Infrared Electrochromic Materials, Devices and Applications. Appl. Mater. Today 2021, 24, 101073. [Google Scholar] [CrossRef]
- Pugliese, M.; Scarfiello, R.; Prontera, C.T.; Giannuzzi, R.; Bianco, G.V.; Bruno, G.; Carallo, S.; Mariano, F.; Maggiore, A.; Carbone, L.; et al. Visible Light-Near-Infrared Dual-Band Electrochromic Device. ACS Sustain. Chem. Eng. 2023, 11, 9601–9612. [Google Scholar] [CrossRef]
- Gong, H.; Li, W.; Fu, G.; Zhang, Q.; Liu, J.; Jin, Y.; Wang, H. Recent progress and advances in electrochromic devices exhibiting infrared modulation. J. Mater. Chem. A Mater. 2022, 10, 6269–6290. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, X.; Li, D.; Ma, Z.; Liu, Z.; Bai, X.; Mao, Z. Predicting flow status of a flexible rectifier using cognitive computing. Expert. Syst. Appl. 2025, 264, 125878. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Hu, F.; He, M.; Mao, Z.; Huang, X.; Ding, J. Predictive Modeling of Flexible EHD Pumps using Kolmogorov-Arnold Networks. Biomim. Intell. Robot. 2024, 4, 100184. [Google Scholar] [CrossRef]
- Deb, S.K. A Novel Electrophotographic System. Appl. Opt. 1969, 8, 192. [Google Scholar] [CrossRef]
- Schoot, C.J.; Ponjee, J.J.; Van Dam, H.T.; van Doorn, R.A.; Bolwijn, P.T. New electrochromic memory display. Appl. Phys. Lett. 1973, 23, 64–65. [Google Scholar] [CrossRef]
- Bauer, F.T.; Bechtel, J.H. Automatic Rearview Mirror for Automotive Vehicles. US4443057A, 17 April 1984. pp. 1–18. [Google Scholar]
- Gentex Corporation. GENTEX-Annual Report 2015; Gentex Corporation: Zeeland, MI, USA, 2015. [Google Scholar]
- Svensson, J.S.E.M.; Granqvist, C.G. Electrochromic Tungsten Oxide Films for Energy Efficient Windows. Sol. Energy Mater. 1984, 11, 29–34. [Google Scholar] [CrossRef]
- Lampert, C.M. Electrochromic materials and devices for energy efficient windows. Sol. Energy Mater. 1984, 11, 1–27. [Google Scholar] [CrossRef]
- Argun, A.A.; Cirpan, A.; Reynolds, J.R. The First Truly All-Polymer Electrochromic Devices. Adv. Mater. 2003, 15, 1338–1341. [Google Scholar] [CrossRef]
- Granqvist, C. Electrochromic Materials Out of a niche. Nat. Mater. 2006, 5, 89–90. [Google Scholar] [CrossRef]
- PPG Aerospace. Alteos Interactive Window Systems. Aerospace; PPG Industries, Inc.: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Cai, G.; Wang, J.; Lee, P. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Gicevicius, M.; Ramanaviciene, A.; Shirsat, M.D.; Viter, R.; Ramanavicius, A. Hybrid electrochemical/electrochromic Cu(II) ion sensor prototype based on PANI/ITO-electrode. Sens. Actuators B Chem. 2017, 248, 527–535. [Google Scholar] [CrossRef]
- Yang, P.; Sun, P.; Mai, W. Electrochromic energy storage devices. Mater. Today 2016, 19, 394–402. [Google Scholar] [CrossRef]
- Argun, A. Electrochromic Laminates For Advanced Spascesuit Visors; Giner, Inc.: Newton, MA, USA, 2018; pp. 1–3. [Google Scholar]
- Fan, H.; Li, K.; Lui, X.; Xu, K.X.; Su, Y.; Hou, C.Y.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z. Continuously Processed Long Electrochromic Fibers with Multi-Environmental Stability. Appl. Mater. Interfaces 2020, 12, 28251–28460. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Cui, P.; Sheng, M.; Gong, X.; Zhang, L.; Fu, S. Multicolor and Multistage Response Electrochromic Color-Memory Wearable Smart Textile and Flexible Display. ACS Appl. Mater. Interfaces 2021, 13, 12313–12321. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials, 1995th ed.; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Shchegolkov, A.V.; Jang, S.H.; Shchegolkov, A.V.; Rodionov, Y.V.; Sukhova, A.O.; Lipkin, M.S. A brief overview of electrochromic materials and related devices: A nanostructured materials perspective. Nanomaterials 2021, 11, 2376. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, R.J. Electrochromic materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, H.; Han, J.; An, Y.; Zhang, Y.-M.; Zhang, S.X.-A. Green revolution in electronic displays expected to ease energy and health crises. Light Sci. Appl. 2021, 10, 33. [Google Scholar] [CrossRef]
- Nakamura, K.; Kanazawa, K.; Kobayashi, N. Electrochemically controllable emission and coloration by using europium(iii) complex and viologen derivatives. Chem. Commun. 2011, 47, 10064–10066. [Google Scholar] [CrossRef]
- Kanazawa, K.; Nakamura, K.; Kobayashi, N. Electroswitching of emission and coloration with quick response and high reversibility in an electrochemical cell. Chem. Asian J. 2012, 7, 2551–2554. [Google Scholar] [CrossRef]
- Kustov, L.M. New organic-inorganic hybrid molecular systems and highly organized materials in catalysis. Russ. J. Phys. Chem. A 2015, 89, 2006–2021. [Google Scholar] [CrossRef]
- Wang, J.L.; Lu, Y.R.; Li, H.H.; Liu, J.-W.; Yu, S.-H. Large Area Co-Assembly of Nanowires for Flexible Transparent Smart Windows. J. Am. Chem. Soc. 2017, 139, 9921–9926. [Google Scholar] [CrossRef]
- Baucke, F.G.K. Electrochromic mirrors with variable reflectance. Solar Energy Mater. 1987, 16, 67–77. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, R.; Nishii, J.; Fujioka, M.; Zhang, C.; Wang, J.; Wang, Y.; Zhao, G.; Yun, K. Preparation of an Inorganic All-Solid-State Electrochromic Device with Excellent Open-Circuit Memory. ACS Appl. Mater. Interfaces 2024, 16, 19094–19102. [Google Scholar] [CrossRef]
- Kortz, C.; Hein, A.; Ciobanu, M.; Walder, L.; Oesterschulze, E. Complementary hybrid electrodes for high contrast electrochromic devices with fast response. Nat. Commun. 2019, 10, 4874. [Google Scholar] [CrossRef]
- Lin, S.; Bai, X.; Wang, H.; Wang, H.; Song, J.; Huang, K.; Wang, C.; Wang, N.; Li, B.; Lei, M.; et al. Roll-to-Roll Production of Transparent Silver-Nanofiber-Network Electrodes for Flexible Electrochromic Smart Windows. Adv. Mater. 2017, 29, 1703238. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Li, Y.; Wang, J. The progress of electrochromic materials based on metal–organic frameworks. Coord. Chem. Rev. 2023, 475, 214891. [Google Scholar] [CrossRef]
- Kumar, A.; Prajapati, C.S.; Sahay, P.P. Results on the microstructural, optical and electrochromic properties of spray-deposited MoO3 thin films by the influence of W doping. Mater. Sci. Semicond. Process. 2019, 104, 104668. [Google Scholar] [CrossRef]
- Usha, N.; Sivakumar, R.; Sanjeeviraja, C. Electrochromic properties of radio frequency magnetron sputter deposited mixed Nb2O5:MoO3 (95:5) thin films cycled in H+ and Li+ ions. Mater. Sci. Semicond. Process. 2015, 30, 31–40. [Google Scholar] [CrossRef]
- Guo, X.; Jia, S.; Li, N.; Cai, G. Regulating the Ion Transport in the Layered V2O5 Electrochromic Films with Tunable Interlayer Spacing. Adv. Opt. Mater. 2024, 12, 2400459. [Google Scholar] [CrossRef]
- Santhosh, S.; Balamurugan, K.; Mathankumar, M.; Shankar, K.; Subramanian, B. Electrochromic and optical studies on Nb2O5–NiO mixed oxide films for smart window applications. Opt. Mater. 2023, 135, 113248. [Google Scholar] [CrossRef]
- Sonmez, G.; Meng, H.; Wudl, F. Organic Polymeric Electrochromic Devices: Polychromism with Very High Coloration Efficiency. Chem. Mater. 2004, 16, 574–580. [Google Scholar] [CrossRef]
- Hlguchi, M. Electrochromic organic-metallic hybrid polymers: Fundamentals and device applications. Polym. J. 2009, 41, 511–520. [Google Scholar] [CrossRef]
- ASTM E2141-21; Standard Test Method for Accelerated Aging of Electrochromic Devices in Sealed Insulating Glass Units. ASTM: West Conshohocken, PA, USA, 2012. [CrossRef]
- ISO 18543; Glass in Building–Electrochromic Glazing–Accelerated Ageing Test and Requirements. ISO: Geneva, Switzerland, 2021; pp. 1–13.
- Niklasson, G.A.; Wen, R.-T.; Qu, H.-Y.; A Arvizu, M.; Granqvist, C.-G. Durability of Electrochromic Films: Aging Kinetics and Rejuvenation. ECS Trans. 2017, 77, 1659–1669. [Google Scholar] [CrossRef]
- Wu, W.; Wang, M.; Ma, J.; Cao, Y.; Deng, Y. Electrochromic Metal Oxides: Recent Progress and Prospect. Adv. Electron. Mater. 2018, 4, 1800185. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Gong, H.; Zhang, Q.; Yan, L.; Wang, H. Recent progress in Prussian blue electrode for electrochromic devices. Front. Energy 2024, 18, 160–186. [Google Scholar] [CrossRef]
- Jensen, J.; Madsen, M.V.; Krebs, F.C. Photochemical stability of electrochromic polymers and devices. J. Mater. Chem. C Mater. 2013, 1, 4826–4835. [Google Scholar] [CrossRef]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C Mater. 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Striepe, L.; Baumgartner, T. Viologens and Their Application as Functional Materials. Chem. Eur. J. 2017, 23, 16924–16940. [Google Scholar] [CrossRef]
- Duan, J.; Li, Y.; Pan, Y.; Behera, N.; Jin, W. Metal-organic framework nanosheets: An emerging family of multifunctional 2D materials. Coord. Chem. Rev. 2019, 395, 25–45. [Google Scholar] [CrossRef]
- Patel, M.; Ghosh, S.; Cho, S.; Kim, J. Highly Transparent Spectral Tunable Electrochromic Window Based on Solid-State WO3 Thin Films. Int. J. Energy Res. 2025, 2025, 8585226. [Google Scholar] [CrossRef]
- Welsh, T.A.; Draper, E.R. Water soluble organic electrochromic materials. RSC Adv. 2021, 11, 5245–5264. [Google Scholar] [CrossRef]
- Ding, M.; Li, W.; Li, A.; Wang, Y.; Liu, J.; Zhang, Q.; Wang, H. Electrochromic fabrics with improved cycling stability via modified polyaniline towards environmentally adaptive camouflage. J. Mater. Chem. C Mater. 2025, 13, 4673–4682. [Google Scholar] [CrossRef]
- An, F.H.; Yuan, Y.Z.; Liu, J.Q.; He, M.D.; Zhang, B. Enhanced electrochromic properties of WO3/ITO nanocomposite smart windows. RSC Adv. 2023, 13, 13177–13182. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Y.; Lu, Z.; Tian, M.; Wang, F.; Wang, M.; Diao, X.; Zhong, X. A dual-function device with high coloring efficiency based on a highly stable electrochromic nanocomposite material. Chem. Eng. J. 2023, 456, 141075. [Google Scholar] [CrossRef]
- Fan, X.; Pan, M.; Li, X.; Kong, L.; Kuchmizha, A.; Xu, H. Research progress of MOF electrochromic materials. Resour. Chem. Mater. 2024, 3, 230–245. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Li, J.; Inge, A.K.; Ott, S. Electrochromism in Isoreticular Metal-Organic Framework Thin Films with Record High Coloration Efficiency. ACS Nano 2023, 17, 21595–21603. [Google Scholar] [CrossRef]
- Wang, K.; Meng, Q.; Wang, Q.; Ott, S. Advances in Energy-Efficient Plasmonic Electrochromic Smart Windows Based on Metal Oxide Nanocrystals. Adv. Energy Sustain. Res. 2021, 2, 2100117. [Google Scholar] [CrossRef]
- Kim, Y.; Cha, S.; Kim, J.H.; Oh, J.-W.; Nam, J.-M. Electrochromic response and control of plasmonic metal nanoparticles. Nanoscale 2021, 13, 9541–9552. [Google Scholar] [CrossRef]
- Roman, B.J.; Zuleta, S.; Milliron, D. Tunable optical response of plasmonic metal oxide nanocrystals. MRS Bull. 2024, 49, 1032–1044. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Lee, J.Y. Plasmonic Oxygen-Deficient TiO2−x Nanocrystals for Dual-Band Electrochromic Smart Windows with Efficient Energy Recycling. Adv. Mater. 2020, 32, e2004686. [Google Scholar] [CrossRef] [PubMed]
- Mohanadas, D.; Azman, N.H.N.; Sulaiman, Y. A bifunctional asymmetric electrochromic supercapacitor with multicolor property based on nickel oxide/vanadium oxide/reduced graphene oxide. J. Energy Storage 2022, 48, 103954. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Tiwari, S.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Sen, S.; Kundu, S. Robust Dual-Color Electrochromism of Vanadium Oxide Nanorods Embedded on Reduced Graphene Oxide: Unraveling the Mechanism. J. Electrochem. Soc. 2024, 171, 093504. [Google Scholar] [CrossRef]
- Yu, F.; Liu, W.; Ke, S.W.; Kurmoo, M.; Zuo, J.-L.; Zhang, Q. Electrochromic two-dimensional covalent organic framework with a reversible dark-to-transparent switch. Nat. Commun. 2020, 11, 5534. [Google Scholar] [CrossRef] [PubMed]
- Bessinger, D.; Muggli, K.; Beetz, M.; Auras, F.; Bein, T. Fast-Switching Vis-IR Electrochromic Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 7351–7357. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Bao, B.; Li, R.; Li, Y.; Zhang, Q.; Li, K.; Wang, H. Facilitating Charge Transfer via Ti-Knot Pathway in Electrochromic Three-Dimensional Metalated Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2024, 16, 57571–57579. [Google Scholar] [CrossRef]
- Takada, K.; Sakamoto, R.; Yi, S.T.; Katagiri, S.; Kambe, T.; Nishihara, H. Electrochromic Bis(terpyridine)metal Complex Nanosheets. J. Am. Chem. Soc. 2015, 137, 4681–4689. [Google Scholar] [CrossRef]
- Bera, M.K.; Mohanty, S.; Kashyap, S.; Sarmah, S. Electrochromic coordination nanosheets: Achievements and future perspective. Coord. Chem. Rev. 2022, 454, 214353. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, C. Interfacial Coordination Nanosheet Based on Nonconjugated Three-Arm Terpyridine: A Highly Color-Efficient Electrochromic Material to Converge Fast Switching with Long Optical Memory. ACS Appl. Mater. Interfaces 2020, 12, 35181–35192. [Google Scholar] [CrossRef]
- Salles, P.; Pinto, D.; Hantanasirisakul, K.; Maleski, K.; Shuck, C.E.; Gogotsi, Y. Electrochromic Effect in Titanium Carbide MXene Thin Films Produced by Dip-Coating. Adv. Funct. Mater. 2019, 29, 1809223. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Jimmy, J.; Kandasubramanian, B. Mxene functionalized polymer composites: Synthesis and applications. Eur. Polym. J. 2020, 122, 109367. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef] [PubMed]
- Ghamsarizade, R.; Ramezanzadeh, B.; Mohammadloo, H.E. A review on recent advances in 2D-transition metal carbonitride-MXenes nano-sheets/polymer composites’ electromagnetic shields, mechanical and thermal properties. J. Taiwan. Inst. Chem. Eng. 2023, 144, 104740. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Huang, Q. MXenes: Two-Dimensional Building Blocks for Future Materials and Devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Singh, M.K.; Bansal, L.; Rath, D.K.; Rai, D.K.; Kumar, R. Ti3C2Tx-MXene-Based Color-Indicative All-Organic Electrochromic Supercapacitors. Adv. Eng. Mater. 2024, 24, 2401295. [Google Scholar] [CrossRef]
- Vinh Quy, V.H.; Kim, K.W.; Yeo, J.; Tang, X.; In, Y.R.; Jung, C.; Oh, S.M.; Kim, S.J.; Lee, S.W.; Moon, H.C.; et al. Tunable electrochromic behavior of biphenyl poly(viologen)-based ion gels in all-in-one devices. Org. Electron. 2022, 100, 106395. [Google Scholar] [CrossRef]
- Zhang, T.; Mu, X.; Li, Y.; Cong, S.; Zheng, S.; Huang, R.; Geng, F.; Zhao, Z. Optical-cavity-Incorporated colorful all-solid-state electrochromic devices for dual anti-counterfeiting. Adv. Mater. 2024, 36, 2402670. [Google Scholar] [CrossRef]
- Bunshah, R. Handbook of Deposition Technologies for Films and Coatings, 2nd ed.; Noyes Publications: Los Angeles, CA, USA, 1994. [Google Scholar]
- Çarpan, M.; Şentürk, O.; Tokgöz, S.; Sarsıcı, S.; Akay, S.; Peksöz, A. Ag decorated V2O5 electrodes as a promising option for electrochromic, photovoltaic, and energy-saving applications. Ceram. Int. 2024, 50, 33111–33122. [Google Scholar] [CrossRef]
- El-Nahass, M.; Saadeldin, M.; Ali, H.; Zaghllol, M. Electrochromic properties of amorphous and crystalline WO3 thin films prepared by thermal evaporation technique. Mater. Sci. Semicond. Process. 2015, 29, 201–205. [Google Scholar] [CrossRef]
- Pereira, S.; Gonçalves, A.; Correia, N.; Pinto, J.; Pereira, L.; Martins, R.; Fortunato, E. Electrochromic behavior of NiO thin films deposited by e-beam evaporation at room temperature. Sol. Energy Mater. Sol. Cells 2014, 120, 109–115. [Google Scholar] [CrossRef]
- Evecan, D.; Zayim, E. Highly uniform electrochromic tungsten oxide thin films deposited by e-beam evaporation for energy saving systems. Curr. Appl. Phys. 2019, 19, 198–203. [Google Scholar] [CrossRef]
- Santhosh, S.; Kumar, A.; Kennedy, J.; Subramanian, B. Electrochromic response of pulsed laser deposited oxygen deficient monoclinic β-MoO3 thin films. Electrochim. Acta 2020, 354, 136745. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, N.; Liu, Y.; Cui, D.; Yu, C.-F.; Liu, H.; Li, Z. Effect of laser power density on the electrochromic properties of WO3 films obtained by pulsed laser deposition. Ceram. Int. 2021, 47, 22416–22423. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Zhao, F.; Li, H.; Chen, J.; Wang, H.; Yu, W.W. Inkjet printing for smart electrochromic devices. FlexMat 2024, 1, 23–45. [Google Scholar] [CrossRef]
- Arora, E.; Sharma, V.; Ravi, A.; Shahi, A.; Jagtap, S.; Adhikari, A.; Dash, J.K.; Kumar, P.; Patel, R. Polyaniline-Based Ink for Inkjet Printing for Supercapacitors, Sensors, and Electrochromic Devices. Energies 2023, 16, 6716. [Google Scholar] [CrossRef]
- Linderhed, U.; Petsagkourakis, I.; Ersman, P.; Beni, V.; Tybrandt, K. Fully screen printed stretchable electrochromic displays. Flex. Print. Electron. 2021, 6, 045014. [Google Scholar] [CrossRef]
- Bakacak, P.; Kovalska, E.; Tüzemen, S. Graphene for switchable flexible smart windows application. Opt. Mater. 2024, 151, 115302. [Google Scholar] [CrossRef]
- Park, Y.; Park, W.; Lee, K. Durability improvement of electrochromic WO3 thin films by deposition of an ultra-thin Al2O3 layer via atomic layer deposition. J. Alloys Compd. 2025, 1010, 177210. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, L.; Liu, X.; Zhang, J. Atomic layer deposition of SnO2 enables fast-dynamics in electrochromic vanadium oxide/polyaniline films. Next Mater. 2025, 6, 100328. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Liu, S.; Zhang, B. Synthesis, optimization, electrochemical and electrochromic properties of Zr-doped NiO films by chemical spray pyrolysis. Electrochim. Acta 2024, 506, 145057. [Google Scholar] [CrossRef]
- Lei, Y.; Feng, K.; Zeng, A.; Yang, H.; Zhang, L.; Liu, Z.; Chen, Z. Sol-gel deposited ZnO substrate for the modulation of electrodeposited PEDOT nanostructures and enhancement of electrochromic stability. Appl. Surf. Sci. 2025, 681, 161480. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, J.; Gao, G.; Gao, X.; Wang, Q.; Sun, J.; Li, Y.; Huang, H.; Yan, Y.; Sun, D.; et al. Preparation of tunable magnesium atom-doped nickel oxide films with short response time and high coloring efficiency by sol-gel method. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134879. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Wu, R.; Wu, S.; Wang, X.; Xie, H.; Yan, D.; Liu, Y.; Ye, W.; Wang, C.; et al. Robust MnO2-WO3 Complementary Electrochromic Device Enabled by Reversible Electrodeposition of MnO2. Nano Lett. 2024, 52, 16360–16367. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Wang, Z.; Wang, M.; Guo, X.; Tang, X.; Wang, X.; Lai, W.; Tong, M.; Wang, C.; et al. Electrochromism via reversible electrodeposition of solid iodine. Nat. Commun. 2025, 16, 724. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhang, Y.; Zhang, W.; Yang, N.; Lv, F.; Guo, J.; Cui, X.; Fang, K.; Chen, M.; Wang, C.; et al. Solvothermal Synthesis and Growth of Covalent Organic Framework Electrochromic Film with Triphenylamine Active Unit. J. Electron. Mater. 2024, 53, 2656–2665. [Google Scholar] [CrossRef]
- Kamath, K.; Kumar, K.N.; Reddy, G.V.A.; Shaik, H.; Prabhu, S.G.; Jafri, R.I.; Shetty, H.D.; Manjunatha, K.B. Synthesis and Characterization of WO3 Nanostructures by the Solvothermal Method for Electrochromic Applications. J. Electron. Mater. 2024, 53, 4564–4574. [Google Scholar] [CrossRef]
- Soltani, S.; Ardyanian, M.; Shahidi, M. Enhancement of electrochromic efficiency of TiO2 nanorods. Opt. Mater. 2024, 152, 115484. [Google Scholar] [CrossRef]
- Wang, X.; Ma, M.; Zhang, N.; Zuo, K.; Ma, Y.; Wu, L.; Li, M. The nano-sheet structure adjustment and long-term stability of Zn-doped NiO electrochromic films. Electrochim. Acta 2024, 492, 144342. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, M.A.K.; Khan, A.; Bibi, S.; Bououdina, M.; Usman, M.; Khan, N.A.; Pirzado, A.A.A.; Abumousa, R.A.; Humayun, M. State-of-the-art electrochromic thin films devices, fabrication techniques and applications: A review. Nanocomposites 2023, 10, 1–40. [Google Scholar] [CrossRef]
- Magdassi, S.; Kamyshny, A. Nanomaterials for 2D and 3D Printing; John Wiley & Sons, Inc.: Weinheim, Germany, 2017. [Google Scholar]
- Choy, K. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Workie, A.; Ningsih, H.; Shih, S. An comprehensive review on the spray pyrolysis technique: Historical context, operational factors, classifications, and product applications. J. Anal. Appl. Pyrolysis. 2023, 170, 105915. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, N.; Bahtt, M.; Mishra, N.K. Sol-Gel Derived Nanomaterials and It’s Applications: A Review. Res. J. Chem. Sci. 2015, 5, 98–105. [Google Scholar]
- Devaraju, M.; Honma, I. Hydrothermal and solvothermal process towards development of LiMPO4 (M = Fe, Mn) nanomaterials for lithium-ion batteries. Adv. Energy Mater. 2012, 2, 284–297. [Google Scholar] [CrossRef]
- Huo, Y.; Xiu, S.; Meng, L.Y.; Quan, B. Solvothermal synthesis and applications of micro/nano carbons: A review. Chem. Eng. J. 2023, 451, 138572. [Google Scholar] [CrossRef]
- Bretos, I.; Jiménez, R.; Ricote, J.; Rivas, A.Y.; Echániz-Cintora, M.; Sirera, R.; Calzada, M.L. Low-temperature sol–gel methods for the integration of crystalline metal oxide thin films in flexible electronics. J. Solgel Sci. Technol. 2023, 107, 269–277. [Google Scholar] [CrossRef]
- Patel, K.J.; Bhatt, G.G.; Ray, J.R.; Suryavanshi, P.; Panchal, C.J. All-inorganic solid-state electrochromic devices: A review. J. Solid. State Electrochem. 2017, 21, 337–347. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Hu, F.; Xu, R.; Yan, B.; Jiang, M.; Gu, Y.; Yang, F.; Cao, Y. Spray-processable, large-area, patterned and all-solid-state electrochromic device based on silica/polyaniline nanocomposites. Sol. Energy Mater. Sol. Cells 2019, 200, 109951. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, X.; Li, W.; Wang, L.; Li, Z.; Zhao, J.; Endres, F.; Li, Y. Preparation of Sn-NiO films and all-solid-state devices with enhanced electrochromic properties by magnetron sputtering method. Electrochim. Acta 2021, 367, 137457. [Google Scholar] [CrossRef]
- Moon, H.C.; Kim, C.H.; Lodge, T.P.; Frisbie, C.D. Multicolored, Low-Power, Flexible Electrochromic Devices Based on Ion Gels. ACS Appl. Mater. Interfaces 2016, 8, 6252–6260. [Google Scholar] [CrossRef]
- Alesanco, Y.; Viñuales, A.; Rodriguez, J.; Tena-Zaera, R. All-in-one gel-based electrochromic devices: Strengths and recent developments. Materials 2018, 11, 414. [Google Scholar] [CrossRef]
- Primiceri, V.; Pugliese, M.; Prontera, C.T.; Monteduro, A.G.; Esposito, M.; Maggiore, A.; Cannavale, A.; Giannuzzi, R.; Gigli, G.; Maiorano, V. Low-cost gel polymeric electrolytes for electrochromic applications. Solar Energy Mater. Sol. Cells 2022, 240, 111657. [Google Scholar] [CrossRef]
- Yang, B.; Yang, G.; Zhang, Y.M.; Zhang, S.X.A. Recent advances in poly(ionic liquid)s for electrochromic devices. J. Mater. Chem. C 2021, 9, 4730–4741. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.M.; Moon, H.C. Asymmetric molecular modification of viologens for highly stable electrochromic devices. RSC Adv. 2019, 10, 394–401. [Google Scholar] [CrossRef]
- Ai, X.; Zhao, Q.; Duan, Y.; Chen, Z.; Zhang, Z.; Liu, Y.; Gao, Y. Zinc polyacrylamide hydrogel electrolyte for quasi-solid-state electrochromic devices with low-temperature tolerance. Cell Rep. Phys. Sci. 2022, 3, 101148. [Google Scholar] [CrossRef]
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A Review on Recent Advances in Electrochromic Devices: A Material Approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Bange, K.; Gambke, T. Electrochromic Materials for Optical Switching Devices **. Adv. Mater. 1990, 2, 10–16. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Yin, M.; Zhu, Y.; Wen, R.-T. Recent progress in transmissive and reflective electrochromic devices for multi-color modulation. Sol. Energy Mater. Sol. Cells 2024, 267, 112706. [Google Scholar] [CrossRef]
- Brooke, R.; Mitraka, E.; Sardar, S.; Sandberg, M.; Sawatdee, A.; Berggren, M.; Crispin, X.; Jonsson, M.P. Infrared electrochromic conducting polymer devices. J. Mater. Chem. C Mater. 2017, 5, 5824–5830. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, X.; Sun, B.; Huang, R.; Wang, C.; Chao, D. A Piezoelectric-Driven Electrochromic/Electrofluorochromic Dual-Modal Display Device. Small 2023, 19, e2301886. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Meng, Q.; Jiang, X.; Li, X.; Liu, H.; Li, Z. Bending the straight into curved: A tree-ring-inspired fully printed omnidirectional triboelectric nanogenerator with ring-nested structure for all-in-one wearable self-powered systems and IoT smart packaging. Nano Energy 2025, 135, 110631. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wang, F.; Cheng, Z.; Fang, Y.; Chang, Q.; Zhu, J.; Wang, L.; Wang, J.; Huang, W.; et al. Full-frame and high-contrast smart windows from halide-exchanged perovskites. Nat. Commun. 2021, 12, 3360. [Google Scholar] [CrossRef]
- Cánovas-Saura, A.; Ruiz, R.; López-Vicente, R.; Abad, J.; Urbina, A.; Padilla, J. Portable Photovoltaic-Self-Powered Flexible Electrochromic Windows for Adaptive Envelopes. Electron. Mater. 2021, 2, 174–185. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, R.; Chen, J.; Liu, W.; Zhang, Y.; Li, J.; Li, H. MXene-based all-solid flexible electrochromic microsupercapacitor. Microsyst. Nanoeng. 2024, 10, 89. [Google Scholar] [CrossRef]
- Chen, H.; Fang, P.; Yang, M.; Yu, J.; Ma, X.; Hu, Y.; Yan, F. Janus Gel Electrolyte Enabled High-Performance Quasi-Solid-State Electrochromic Zn-Ion Batteries. ACS Appl. Polym. Mater. 2025, 7, 3718–3727. [Google Scholar] [CrossRef]
- Bera, M.K.; Ninomiya, Y.; Higuchi, M. Constructing Alternated Heterobimetallic [Fe(II)/Os(II)] Supramolecular Polymers with Diverse Solubility for Facile Fabrication of Voltage-Tunable Multicolor Electrochromic Devices. ACS Appl. Mater. Interfaces 2020, 12, 14376–14385. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Li, H.; Wang, C.; Sheng, M.; Zhang, L.; Fu, S. Bistable Elastic Electrochromic Ionic Gels for Energy-Saving Displays. ACS Appl. Mater. Interfaces 2021, 13, 27200–27208. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Yan, Y.; He, J.; Pu, D.; Chen, L.; Zhang, Y.-M.; Zhang, S.X.-A. Transparent and energy-efficient electrochromic AR display with minimum crosstalk using the pixel confinement effect. Device 2023, 1, 100126. [Google Scholar] [CrossRef]
- Srivastava, S.; Sahu, B.; Mishra, D.; Bansal, L.; Ahlawat, N.; Rath, D.K.; Rout, P.S.; Kumar, S.; Singh, S.; Pandey, P.; et al. Polymer–MXene–Viologen-Based Suprahybrid Electrochromic Device: Flexible Smart Window with Visible and Near-Infrared Switchability. ACS Appl. Opt. Mater. 2025, 3, 889–897. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Ma, Y.; Xie, W.; Dong, J.; Dong, Y.; Li, W.; Zhang, C. Construction of diverse adaptive camouflage nets based on soluble yellow-to-green switching electrochromic materials. Chem. Eng. J. 2024, 498, 155278. [Google Scholar] [CrossRef]
- Wei, J.; Sha, J.; Di, K.; Chen, S.; Liu, W.; Long, L.; Ding, L.; Zhou, Y.; Wang, X.; Wang, K. Reusable Self-Powered Electrochromic Sensor Patch Based on Enzymatic Biofuel Cells for On-Site Visualized Monitoring of Lactic Acid. Anal. Chem. 2025, 97, 2604–2609. [Google Scholar] [CrossRef]
- Jafari, A.; Al-Ostaz, A.; Nouranian, S. Versatile, Adaptable, and Stretchable Electrochromic Energy Storage Systems. Polym. Adv. Technol. 2025, 36, e70144. [Google Scholar] [CrossRef]
- Halder, S.; Chakraborty, C. Evolving trends in electrochromic energy storage devices: Insights from the nanoarchitectonics of metallo-supramolecular polymers. Nano Energy 2024, 131, 110243. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Zhang, Y.; Wei, Z. Integrated energy storage and electrochromic function in one flexible device: An energy storage smart window. Energy Environ. Sci. 2012, 5, 8384–8389. [Google Scholar] [CrossRef]
- Lu, Z.; Zhong, X.; Liu, X.; Wang, J.; Diao, X. Energy storage electrochromic devices in the era of intelligent automation. Phys. Chem. Chem. Phys. 2021, 23, 14126–14145. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Ma, X.; Zhang, C.; Han, J.; He, S.; Liu, K.; Jiang, S. Nanocellulose-based nanogenerators for sensor applications: A review. Int. J. Biol. Macromol. 2024, 259, 129268. [Google Scholar] [CrossRef]
- Rani, S.; Khandelwal, G.; Kumar, S.; Pillai, S.C.; Stylios, G.K.; Gadegaard, N.; Mulvihill, D.M. Flexible self-powered supercapacitors integrated with triboelectric nanogenerators. Energy Storage Mater. 2025, 74, 103977. [Google Scholar] [CrossRef]
- Chen, X.; Luo, L.; Zeng, Z.; Jiao, J.; Shehzad, M.; Yuan, G.; Luo, H.; Wang, Y. Bio-inspired flexible vibration visualization sensor based on piezo-electrochromic effect. J. Mater. 2020, 6, 643–650. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, H.; Xu, B. Advanced design of triboelectric nanogenerators for future eco-smart cities. Adv. Compos. Hybrid Mater. 2024, 7, 102. [Google Scholar] [CrossRef]
- Wang, J.; Meng, C.; Gu, Q.; Tseng, M.C.; Tang, S.T.; Kwok, H.S.; Cheng, J.; Zi, Y. Normally Transparent Tribo-Induced Smart Window. ACS Nano 2020, 14, 3630–3639. [Google Scholar] [CrossRef]
- Fan, F.R.; Tian, Z.Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Kim, W.G.; Kim, D.W.; Tcho, I.W.; Kim, J.-K.; Kim, M.-S.; Choi, Y.-K. Triboelectric Nanogenerator: Structure, Mechanism, and Applications. ACS Nano 2021, 15, 258–287. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, A. Pyroelectric nanogenerators (PyNGs) in converting thermal energy into electrical energy: Fundamentals and current status. Nano Energy 2021, 84, 105888. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Li, Y.; Tan, J.; Zhang, J.; Zhang, H.; Liang, J.; Li, T.; Liu, Y.; Jiang, H.; et al. Flexible Piezoelectric and Pyroelectric Nanogenerators Based on PAN/TMAB Nanocomposite Fiber Mats for Self-Power Multifunctional Sensors. ACS Appl. Mater. Interfaces 2022, 14, 46789–46800. [Google Scholar] [CrossRef] [PubMed]
- Dokouzis, A.; Bella, F.; Theodosiou, K.; Gerbaldi, C.; Leftheriotis, G. Photoelectrochromic devices with cobalt redox electrolytes. Mater Today Energy 2020, 15, 100365. [Google Scholar] [CrossRef]
- Lee, S.I.; Okwako, J.A.; Song, S.H.; Park, S.; Dao, T.T.; Van Tran, H.; Aduda, B.O.; Waita, S.; Hong, Y.-S.; Kwak, K.; et al. Performance Comparison of Different Methyl Group Positioning on Salicylic acid Sensitizers for Photoelectrochromic Device. In Proceedings of the 2023 IEEE 23rd International Conference on Nanotechnolog, IEEE Computer Society, Jeju Island, Republic of Korea, 2–5 July 2023; pp. 255–260. [Google Scholar]
- Syrrokostas, G.; Leftheriotis, G.; Yannopoulos, S.N. Lessons learned from 25 years of development of photoelectrochromic devices: A technical review. Renew. Sustain. Energy Rev. 2022, 162, 112462. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chiang, Y.J.; Yu, H.F.; Hsiao, L.-Y.; Yeh, C.-L.; Chang, L.-Y.; Ho, K.-C.; Yeh, M.-H. Designing a hybrid type photoelectrochromic device with dual coloring modes for realizing ultrafast response/high optical contrast self-powered smart windows. Nano Energy 2021, 90, 106575. [Google Scholar] [CrossRef]
- Ling, H.; Wu, J.; Su, F.; Tian, Y.; Liu, Y.J. Automatic light-adjusting electrochromic device powered by perovskite solar cell. Nat. Commun. 2021, 12, 1010. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Leftheriotis, G.; Huang, H.; Xia, Y.; Gan, Y.; Zhang, W.; Zhang, J. A solar-powered multifunctional and multimode electrochromic smart window based on WO3/Prussian blue complementary structure. Sustain. Mater. Technol. 2022, 31, e00372. [Google Scholar] [CrossRef]
- Krishna Prasad, A.; Kim, J.Y.; Kang, S.H.; Ahn, K.S. Molybdenum induced defective WO3 multifunctional nanostructure as an electrochromic energy storage device: Novel assembled photovoltaic-electrochromic Mo–WO3 film. J. Ind. Eng. Chem. 2024, 135, 388–396. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Cong, S.; Geng, F.; Zhao, Z. Fusing electrochromic technology with other advanced technologies: A new roadmap for future development. Mater. Sci. Eng. R Rep. 2020, 140, 100524. [Google Scholar] [CrossRef]
- Na, Z.; Liang, X.; Wang, H.; Yu, L.; Fan, C.; Wang, Q.; Wang, X.; Yang, H. Broadband Modulation, Self-Driven, and Self-Cleaning Smart Photovoltaic Windows for High Efficiency Energy Saving Buildings. Adv. Funct. Mater. 2024, 34, 2308312. [Google Scholar] [CrossRef]
- Bechinger, C.; Ferrere, S.; Zaban, A.; Sprague, J.; Gregg, B.A. Photoelectrochromic windows and displays. Nature 1996, 383, 608–610. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Hu, M.; Zeng, J.; Mu, Y.; Guo, Y.; Yu, J.; Ma, X.; Qiu, Y.; Huang, Y. A flexible, electrochromic, rechargeable Zn//PPy battery with a short circuit chromatic warning function. J. Mater. Chem. A Mater. 2018, 6, 11113–11118. [Google Scholar] [CrossRef]
- Manjakkal, L.; Pereira, L.; Kumi Barimah, E.; Grey, P.; Franco, F.F.; Lin, Z.; Jose, G.; Hogg, R.A. Multifunctional flexible and stretchable electrochromic energy storage devices. Prog. Mater. Sci. 2024, 142, 101244. [Google Scholar] [CrossRef]
- Kiruthika, S.; Kulkarni, G.U. Smart Electrochromic Supercapacitors Made of Metal Mesh Electrodes with Polyaniline as Charge Storage Indicator. Energy Technol. 2020, 8, 1901364. [Google Scholar] [CrossRef]
- Jalal, N.I.; Ibrahim, R.I.; Oudah, M.K. A Review on Supercapacitors: Types and Components. J. Phys. Conf. Ser. 2021, 1973, 012015. [Google Scholar] [CrossRef]
- Dharmasiri, B.; Stojcevski, F.; Usman, K.A.S.; Qin, S.A.; Razal, J.M.; Doeven, E.H.; Francis, P.S.; Connell, T.U.; Yin, Y.; Andersson, G.G.; et al. Flexible carbon fiber based structural supercapacitor composites with solvate ionic liquid-epoxy solid electrolyte. Chem. Eng. J. 2023, 455, 140778. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, J.; Wang, L.; Li, X.; Jiang, Y. Facile WO3@PANI composite film for applications in double-layer photoelectrochromic supercapacitors. Mater. Lett. 2023, 335, 133809. [Google Scholar] [CrossRef]
- Dong, D.; Benhaddouch, T.E.; Metler, C.L.; Marcial, J.; Zhao, Y.; Venkadesh, V.; Thundat, T.; Bhansali, S. Pseudocapacitive Charge Storage in Electrochromic Transition-Metal Oxide Thin Films. J. Electrochem. Soc. 2022, 169, 080511. [Google Scholar] [CrossRef]
- Sahu, B.; Bansal, L.; Ghosh, T.; Kandpal, S.; Rath, D.K.; Rani, C.; Wesemann, C.; Bigall, N.C.; Kumar, R. Metal oxide-mixed polymer-based hybrid electrochromic supercapacitor: Improved efficiency and dual band switching. J. Phys. D Appl. Phys. 2024, 57, 245110. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; He, Z.; Wang, M.; Luo, Z.; Liu, Q.; Huang, J.; Zhong, X.; Du, K.; Diao, X. All-solid-state electrochromic Li-ion hybrid supercapacitors for intelligent and wide-temperature energy storage. Chem. Eng. J. 2021, 414, 128892. [Google Scholar] [CrossRef]
- Xie, J.; Yang, P.; Wang, Y.; Qi, T.; Lei, Y.; Li, C.M. Puzzles and confusions in supercapacitor and battery: Theory and solutions. J. Power Sources 2018, 401, 213–223. [Google Scholar] [CrossRef]
- Kreysa, G.; Ota, K.; Savinell, R. Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 1–2198. [Google Scholar]
- Liu, Q.; Ou, X.; Niu, Y.; Li, L.; Xing, D.; Zhou, Y.; Yan, F. Flexible Zn-ion Electrochromic Batteries with Multiple-color Variations. Angew. Chem.-Int. Ed. 2024, 63, e202317944. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, B.; Wang, J.; Zhao, H.; Ma, W.; Xu, L.; Zhang, W.; Zhou, K.; Du, Y.; He, G. Electrochromic Poly(chalcogenoviologen)s as Anode Materials for High-Performance Organic Radical Lithium-Ion Batteries. Angew. Chem.-Int. Ed. 2019, 58, 8468–8473. [Google Scholar] [CrossRef]
- Chen, Z.; Mei, S.; Li, W.; Xu, N.; Dong, Y.; Jin, Y.; Ouyang, M.; Zhang, C. Study of multi-electron redox mechanism: Via electrochromic behavior in hexaazatrinaphthylene-based polymer as the cathode of lithium-organic batteries. J. Mater. Chem. A Mater. 2021, 9, 27010–27018. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Shi, H.; Yang, Y.; Zhang, C.; Yu, Y.; Liu, W. High-performance Complementary Electrochromic Batteries using Nb18W16O93 by the Synergistic Effects of Aqueous Al3+/K+ Dual-ion. Angew. Chem. Int. Ed. 2024, 64, e202415050. [Google Scholar] [CrossRef]
- Tong, Z.; Zhu, X.; Xu, H.; Li, Z.; Li, S.; Xi, F.; Kang, T.; Ma, W.; Lee, C. Multivalent-Ion Electrochromic Energy Saving and Storage Devices. Adv. Funct. Mater. 2024, 35, 2308989. [Google Scholar] [CrossRef]
- Yu, X.; Chang, M.; Chen, W.; Liang, D.; Lu, X.; Zhou, G. Colorless-to-Black Electrochromism from Binary Electrochromes toward Multifunctional Displays. ACS Appl. Mater. Interfaces 2020, 12, 39505–39514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Wang, Y.; Yang, G.; Gu, C.; Zheng, W.; Zhang, Y.-M.; Li, M.; Zhang, S.X.-A. Bio-inspired ultra-high energy efficiency bistable electronic billboard and reader. Nat. Commun. 2019, 10, 1559. [Google Scholar] [CrossRef]
- DeConto, R.M.; Pollard, D.; Alley, R.B.; Velicogna, I.; Gasson, E.; Gomez, N.; Sadai, S.; Condron, A.; Gilford, D.M.; Ashe, E.L.; et al. The Paris Climate Agreement and future sea-level rise from Antarctica. Nature 2021, 593, 83–89. [Google Scholar] [CrossRef]
- Zarco-Soto, F.J.; Zarco-Soto, I.M.; Ali, S.S.S.; Zarco-Periñán, P.J. Energy consumption in buildings: A compilation of current studies. Energy Rep. 2025, 13, 1293–1307. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Mohd Abdah, M.A.A.; Numan, A.; Moreno-Rangel, A.; Radwan, A.; Khalid, M. Smart window technology and its potential for net-zero buildings: A review. Renew. Sustain. Energy Rev. 2023, 181, 113355. [Google Scholar] [CrossRef]
- Cannavale, A.; Ayr, U.; Fiorito, F.; Martellotta, F. Smart electrochromic windows to enhance building energy efficiency and visual comfort. Energies 2020, 13, 1449. [Google Scholar] [CrossRef]
- Reynisson, H.E.; Guðmundsson, K. Energy Performance of Dynamic Windows in Different Climates. Master’s Thesis, School of Architecture and Built Environment, Stockholm, Sweden, 2015. [Google Scholar]
- Research and Markets. Available online: https://www.researchandmarkets.com/ (accessed on 28 March 2025).
- Hwang, Y.; Pyun, S.; Choi, M.; Kim, J.H.; Cho, E.C. Multi-Stimuli-Responsive and Multi-functional Smart Windows. ChemNanoMat 2022, 8, 2–13. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Fu, J. Multifunctional thermochromic smart windows for building energy saving. J. Mater. Chem. A Mater. 2024, 12, 12960–12982. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.H.; Kang, H.W.; Nahm, S.; Kim, B.H.; Kim, H.; Han, S.H. Flexible electrochromic and thermochromic hybrid smart window based on a highly durable ITO/graphene transparent electrode. Chem. Eng. J. 2021, 416, 129028. [Google Scholar] [CrossRef]
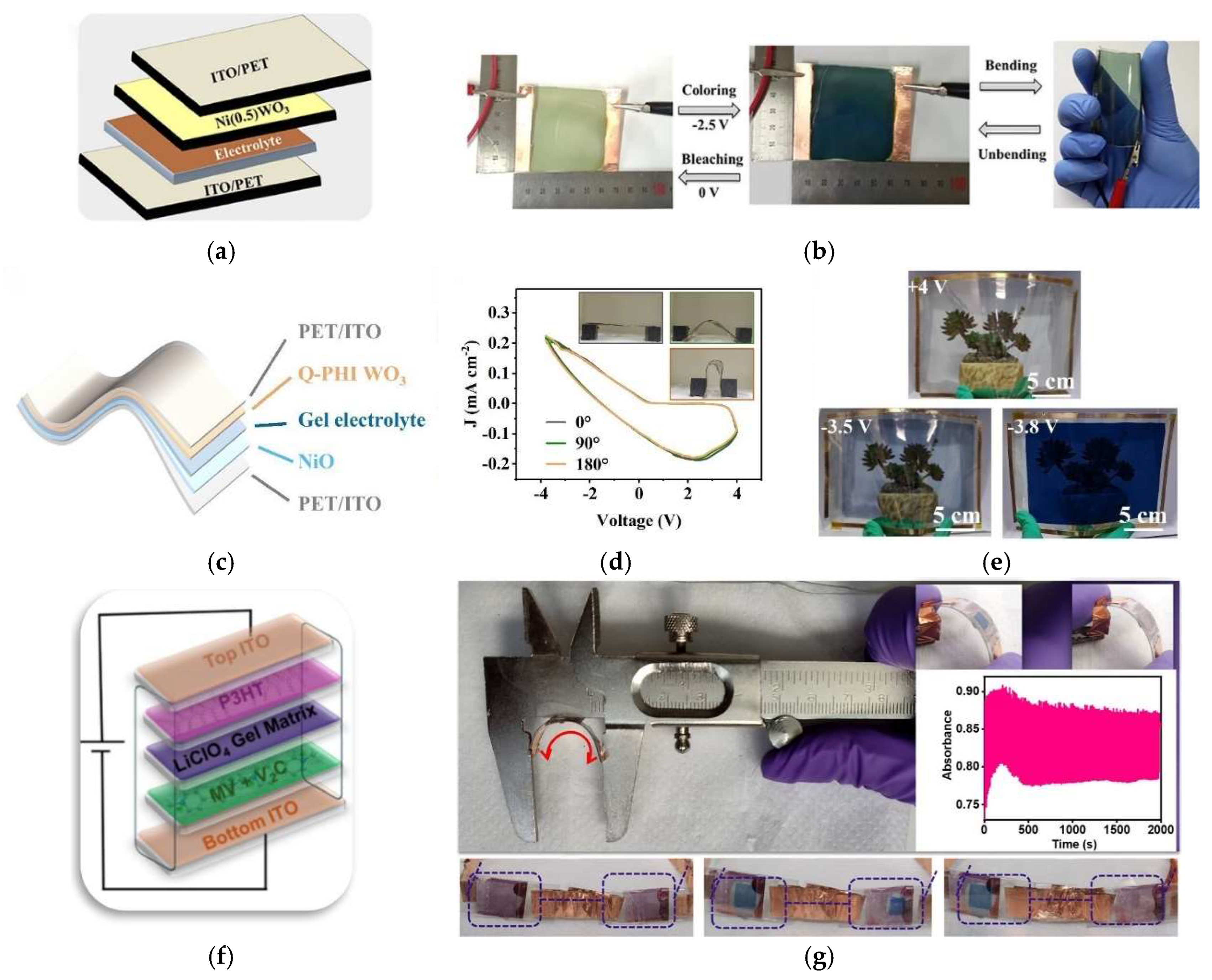
- Li, P.; Liu, X.; Lv, Y.; You, X.; Li, X.; Guo, X.; Wang, T.; Liu, X. Nanostructured Quasiplanar Heterointerface for a Highly Stable and Ultrafast Switching Flexible Inorganic Electrochromic Smart Window. Nano Lett. 2025, 25, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Kim, H. Fabrication of Nickel-Doped Tungsten Trioxide Thin Film-Based Highly Stable Flexible Electrochromic Devices for Smart Window Applications. ACS Sustain. Chem. Eng. 2023, 11, 10746–10754. [Google Scholar] [CrossRef]
- Matthews, J.P.; Bell, J.M.; Skryabin, I.L. Effect of temperature on electrochromic device switching voltages. Electrochim. Acta 1999, 44, 3245–3250. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Jiang, X.; Zhang, Z.; Wang, Y.; Liu, H.; Li, Z.; Gane, P. Coupling of high ion transport efficiency in hydrogel electrolytes and interfacial fusion for performance enhancement in all-solid-state paper-based self-powered electrochromic devices with low-temperature tolerance. J. Mater. Chem. A Mater. 2025, 13, 10060–10076. [Google Scholar] [CrossRef]
- Wu, W.; Fang, H.; Wu, L.; Ma, H.; Wang, H. Temperature-Dependent Electrochromic Devices for Energy-Saving Dual-Mode Displays. ACS Appl. Mater. Interfaces 2023, 15, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, H.; Wang, W.; Zhang, D.; Zhu, J.; Chen, K.; Sun, Y.; Wang, P.; Zhou, Q.; Qi, C.; et al. 3D printing of flexible batteries for wearable electronics. J. Power Sources 2024, 602, 234350. [Google Scholar] [CrossRef]
- Hakola, L.; Jansson, E.; Futsch, R.; Happonen, T.; Thenot, V.; Depres, G.; Rougier, A.; Smolander, M. Sustainable roll-to-roll manufactured multi-layer smart label. Int. J. Adv. Manuf. Technol. 2021, 117, 2921–2934. [Google Scholar] [CrossRef]
- Luo, X.; Wan, R.; Zhang, Z.; Song, M.; Yan, L.; Xu, J.; Yang, H.; Lu, B. 3D-Printed Hydrogel-Based Flexible Electrochromic Device for Wearable Displays. Adv. Sci. 2024, 11, e2404679. [Google Scholar] [CrossRef] [PubMed]
- Thai, L.H.; Nhi, L.T.T.; Giang, T.C.; Hiep, N.M.; Trung, T.Q.; Hung, T.Q.; Sinh, L.H. 3D printed multicolor Prussian blue-viologen hybrid electrochromic devices: Toward high contrast ratio and fast switching electrochromic devices. Appl. Mater. Today 2024, 40, 102369. [Google Scholar] [CrossRef]
- Song, M.; Gong, C.; Liu, X. 3D Printed PEDOT:PSS Flexible Electrochromic Devices for Patterned Displays. J. Polym. Mater. 2025, 42, 111–123. [Google Scholar] [CrossRef]
- Li, W.; Bai, T.; Fu, G.; Zhang, Q.; Liu, J.; Wang, H.; Sun, Y.; Yan, H. Progress and challenges in flexible electrochromic devices. Sol. Energy Mater. Sol. Cells 2022, 240, 111709. [Google Scholar] [CrossRef]
- Han, J.; Sung, C.; Shin, C.; Kim, Y.-S.; Kim, T.-Y. Optimization of oxide materials in oxide-metal-oxide(OMO) electrodes for flexible electrochromic devices. Sol. Energy Mater. Sol. Cells 2023, 249, 112035. [Google Scholar] [CrossRef]


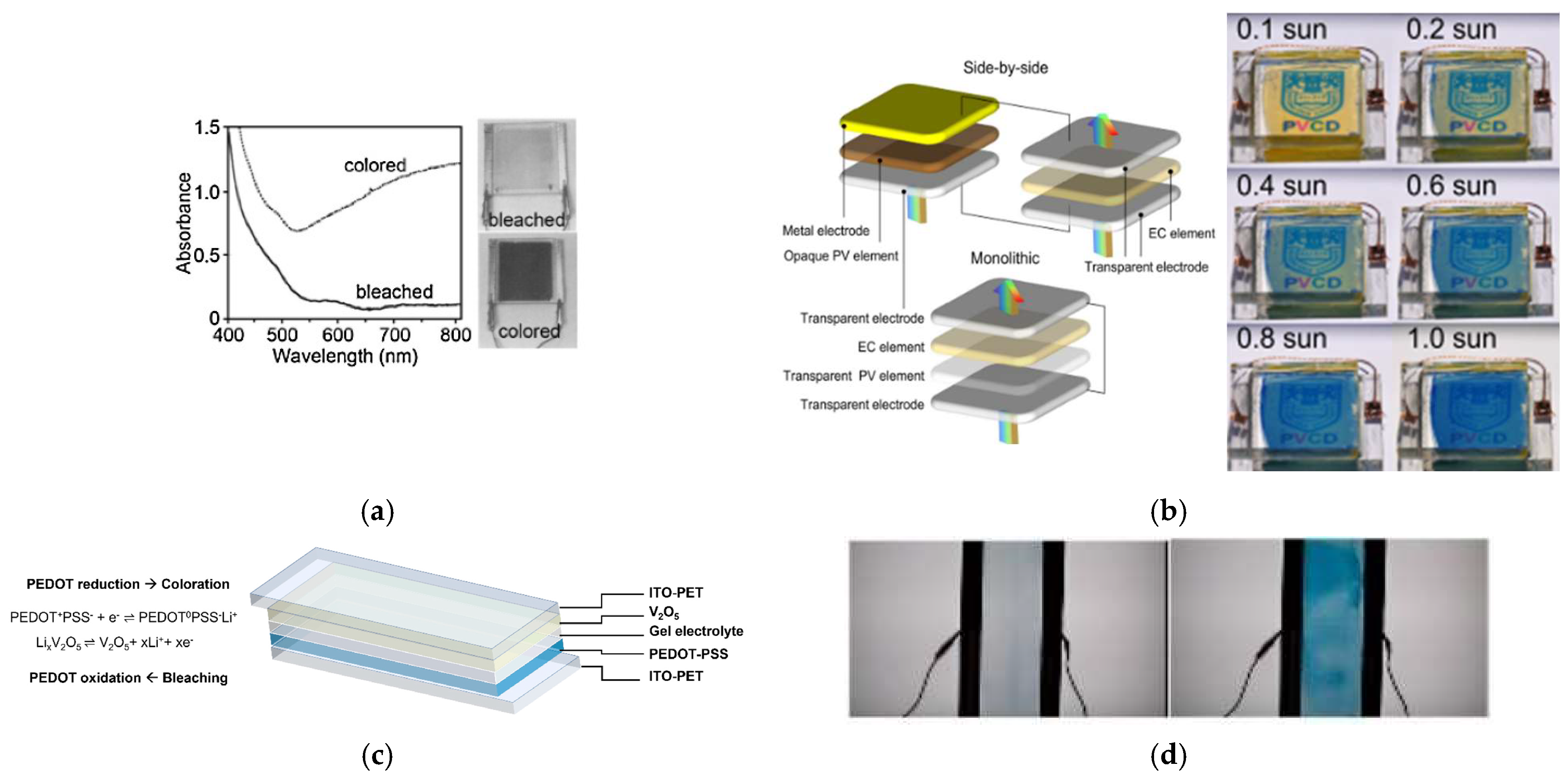
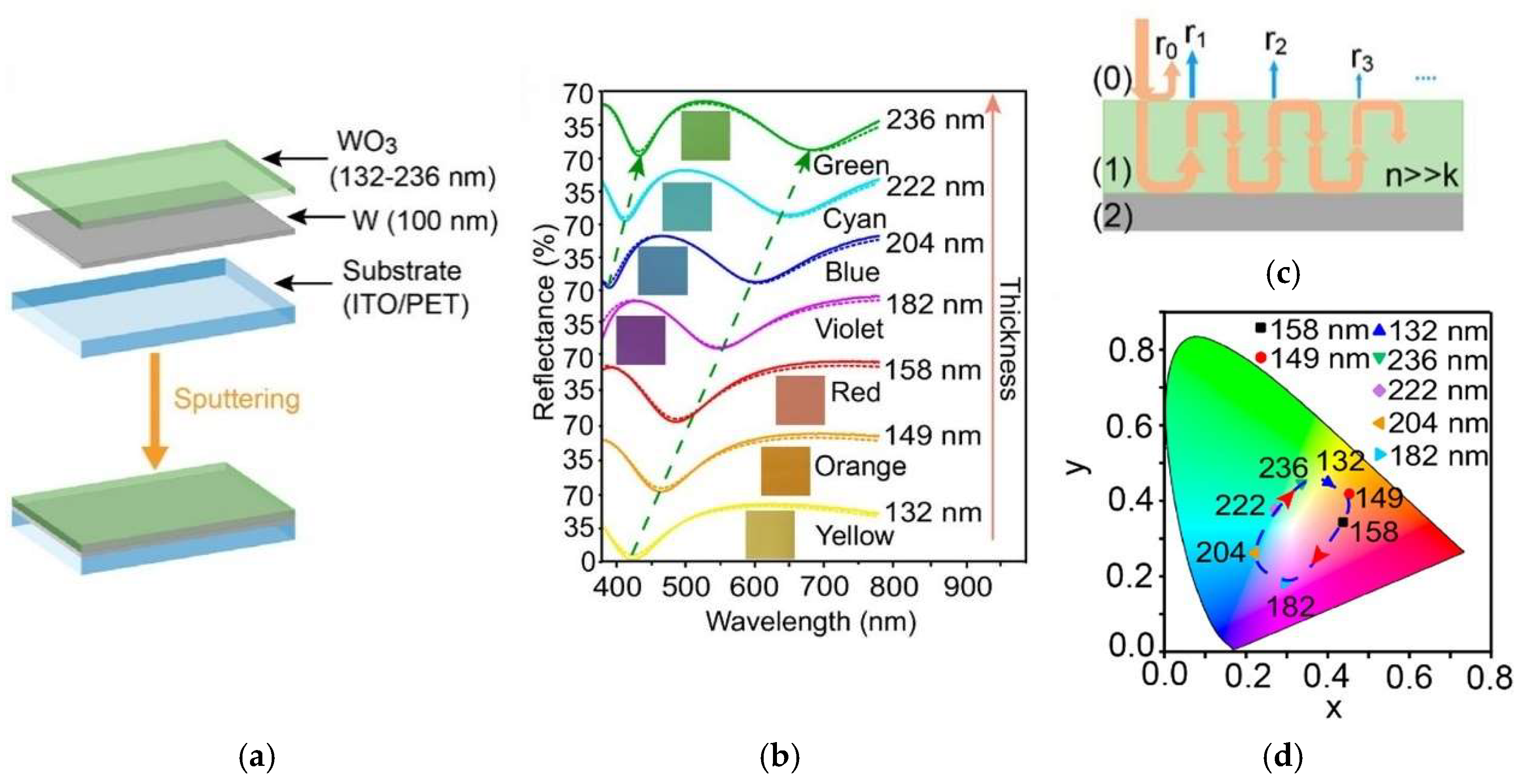
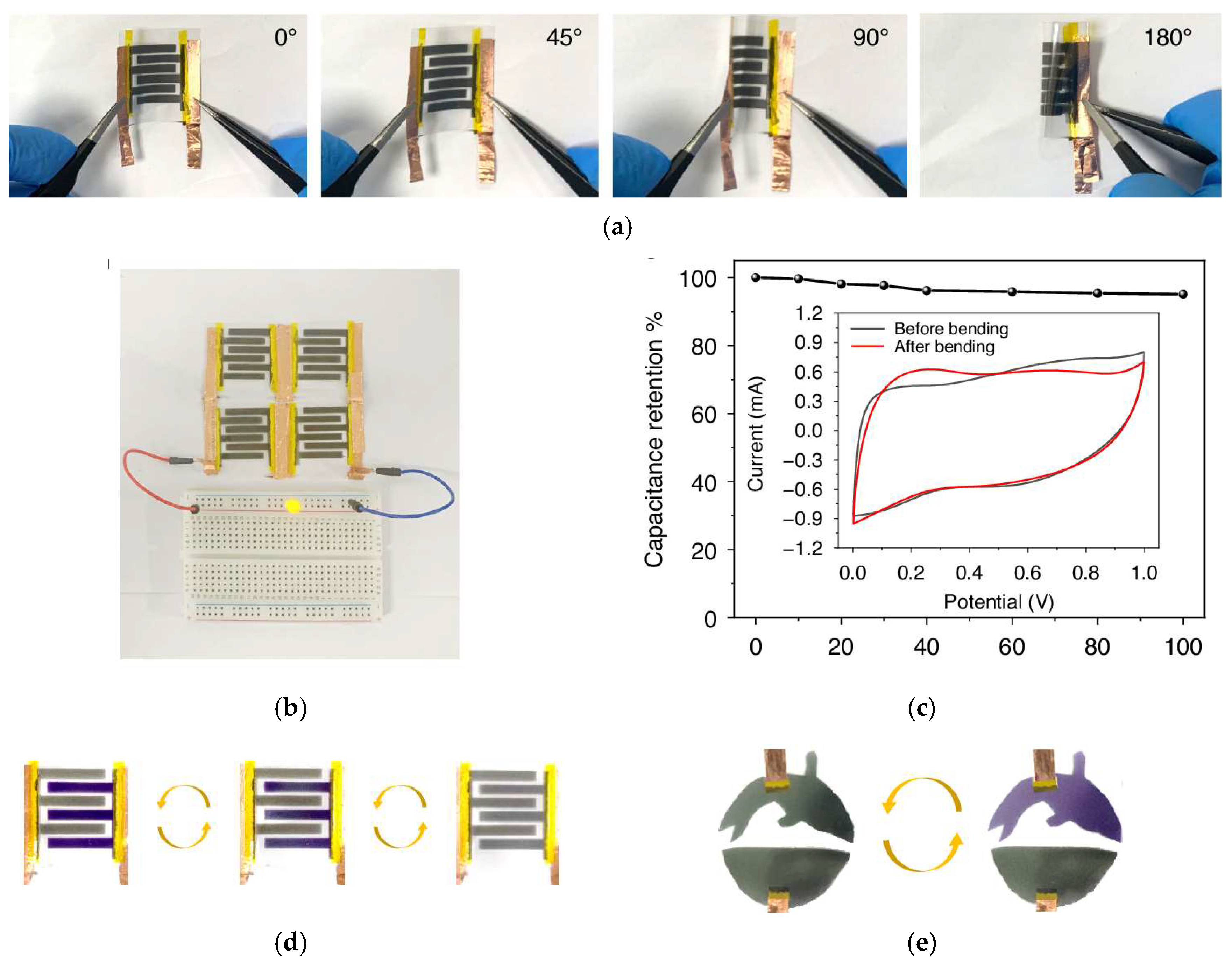
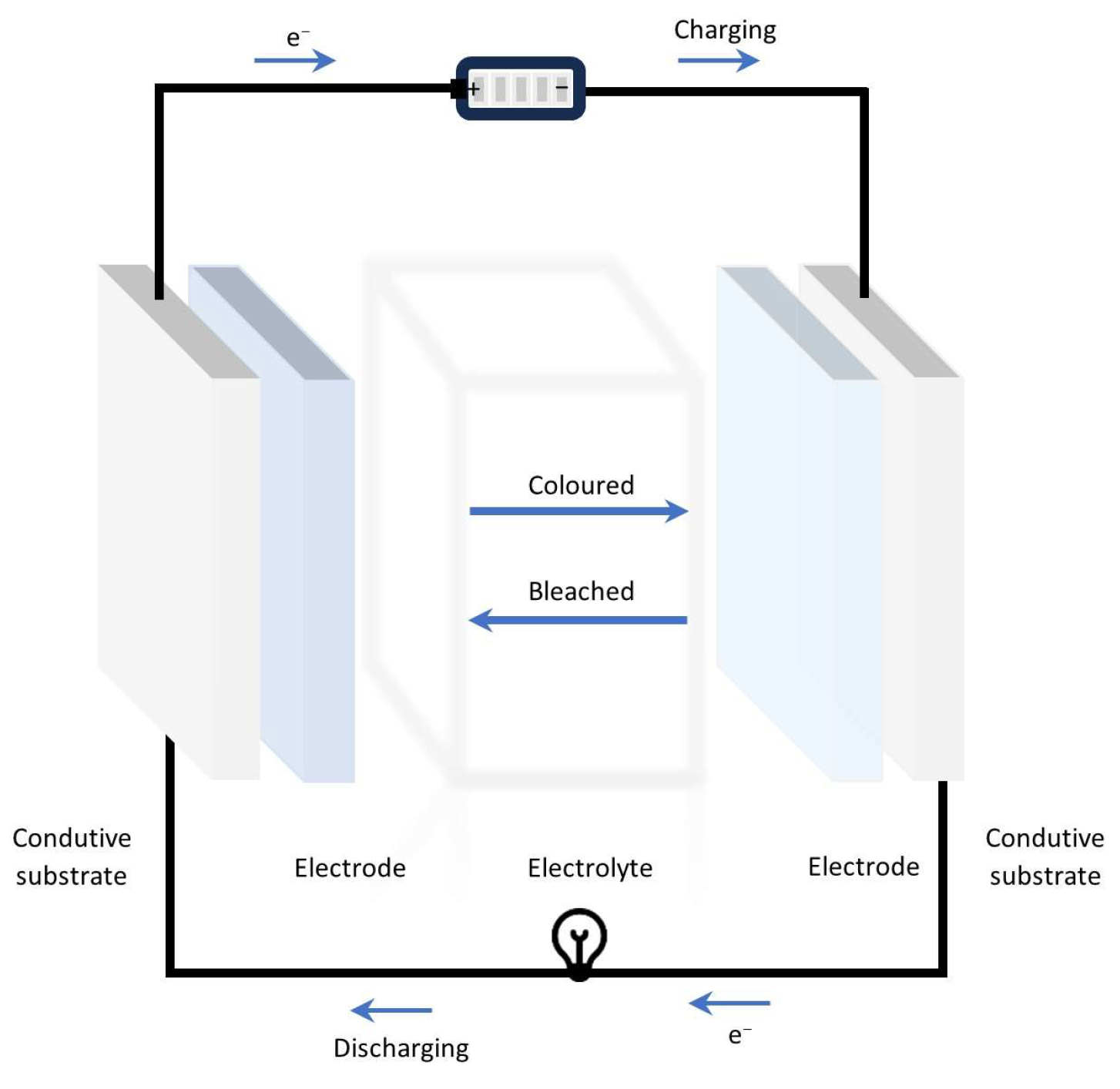
| Electrochromic Materials | Advantages | Challenges | |
|---|---|---|---|
| Conventional electrochromic materials | Inorganic
| -Long term stability -High optical contrast | -Slow switching time -Limited colour tunability |
Organic
| -Rapid switching times -Multi and bright colours | -Insufficient long-term stability -Flammability and toxicity risks | |
| Emerging advanced electrochromic materials | Composite/nanocomposite
| Comprising the advantages/disadvantages of both organic and inorganic materials | |
Hybrid (organic–organic/inorganic–inorganic and organic–inorganic)
| |||
Optical resonators
| -High optical contrast -Ultrafast switching times (milliseconds) -Multi colours | -Poor biostability -Inhomogeneous colours -Poor lifetime | |
Emerging 2D materials
| -Diversified structures -Customized functions -Multifunctionality | -Early-stage research | |
| Structure EC Device EC Film | Applied Potential (V) | Colour Change | Optical Modulation | Response Time (s) tc/tb | Colouration Efficiency (cm2/C) | Durability and Lifetime (Cycles) | Working Temperature Range (°C) | Year|Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Inorganic electrochromic devices | WO3/FTO/glass | −2.5 to 1 | Transparent-Blue | 68.5% at 550 nm | 10 24 | 96.96 at 550 nm | 1000 (100% electrochemical stability retained) | 0 to 50 | 2025 [145] |
| Nb-Mo-O 0.62-Mo/Nb/ITO/PET | −2 to 2 | Light golden Dark golden | 75% at 630 nm | 15.3 7.5 | 10.30 at 630 nm | >15 (100% electrochemical stability retained) | Room temperature | 2025 [41] | |
| Organic electrochromic devices | pTSA-PANI/ITO/glass | −0.8 to 1.2 | Yellow-Green | 73.9% at 660 nm | 1.5 1.2 | 66.65 at 660 nm | 6000 (78.49% electrochemical stability retained) | - | 2025 [147] |
| EC5-H2Q/BP-poly(viologen)/ITO/glass | −1.4 | Green | 79% at 617 nm | 46 11.5 | 67.2 at 617 nm | 500 (92.6% initial contrast retained) | - | 2022 [174] | |
| −1.9 | Purple | 64% at 534 nm | 15 27 | 62.1 at 534 nm | 500 (43.6% initial contrast retained) | ||||
| Composite /Nanocomposite electrochromic devices | Y24-ITO-WO3 nanosheets/ITO/glass | −0.7 to 0.7 | Transparent-Blue | 77.69% at 633 nm | 16.7 12.9 | 196.5 at 633 nm | 180 | - | 2023 [148] |
| EESD1-PB/MnO2/ITO/glass | −1 to 1.8 | Green-Blue | 32% at 480 | 2.98 3.62 | 2019.57 at 480 nm | 1500 (99.62% electrochemical stability retained) | - | 2023 [149] | |
| Hybrids electrochromic devices | MeO-2EPT/ATO/FTO/glass | 0 to +1.5 | Transparent-Greenish blue | 24.81% at 630 nm | <1 | 470 at 630 nm | 2000 | - | 2024 [73] |
| Zn-XDI-MOFs/FTO/glass | −1.9 to −0.6 −1.4 to −0.7 −0.9 to −04 | Multicolour | 96.4% at 746 nm | 1.6 2.6 | 941 at 746 nm | 150 (98% electrochemical stability retained) | - | 2023 [152] | |
| Resonant cavity electrochroMic devices | TiO2-x NCs/ITO/glass | 3.5 to 1.5 | Blue (bright/cool/dark) | 95.5% at 633 nm | 35.1 9.6 | 38.2 at 633 nm | 2000 (95.6% capability retention) | - | 2020 [156] |
| 77.5 at 1600 nm | 15.5 3.4 | 112.7 at 1600 nm | |||||||
| ITO/Cu/ITO/CeO2/LiNbO3/WO3/Al/ITO/ Glass | −4 to 4 | Multilcolour | - | 2.6 2.8 | 64.02 at 590 nm | 7200 (84% capability retention) | - | 2024 [175] | |
| 2D materials electrochromic devices | V2O5 in rGO/ITO/glass | −1 to 1 | Yellow-Green | 54% at 632 nm | 6.2 4.8 | 347 at 632 nm | 5000 | - | 2024 [160] |
| 3D Ti-DHTA-PyM COFs/FTO/Glass | −0.33- to 0.33 | Orange red-Olive green | 38% at 700 nm | 2.5 0.5 | 423 at 700 nm | 500 (93.6% electrochemical stability retained) | - | 2024 [163] | |
| 3tpy−Fe CONASH/ITO glass | 3 to −2 | Pink–Colourless | 53.2% at 556 nm | 1.49 2.49 | 470.16 at 556 nm | 1000 (90.7% electrochemical stability retained) | - | 2020 [166] | |
| Ti3C2Tx-MXene/ITO/glass | 0.2 to −1.8 | Magenta-Blue | 13.5% at 515 nm | ~1 | 340 at 515 | 100 (100% electrochemical stability retained) | - | 2024 [173] |
| Device Type | Characteristics | Electrochromic Material/Material with Additional Function/ Multifunctional Material | Driving Voltage (V) | Colour Change | Optical Modulation | Switching Time (s) Tc/Tb | Durability and Life Time | Year|Ref. |
|---|---|---|---|---|---|---|---|---|
| Piezoelectric-driven electrochromic/electrofluorochromic dual-mode display device | Interactive colour/fluorescence change for human motion indication | EFIL-TPA—electroactive fluorescent ionic liquid based on triphenylamine (TPA) and imidazole PENG-based on PVDF/BaTiO3 | 0 1 | Transparent-Blue | 62% at 474 nm | T-0.58/0.70 (500 cycles) F-0.57/1.8 (500 cycles) | 10,000 cycles (96% retained/91% fluorescence on/off ratio retention) | 2023| [219] |
| All-in-one wearable self-powered system | Wearable Motion-interactive self-powered Arial capacitance 1.1 mF/cm2 | PEDOT: PSS (poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) Tribolectric generator-WPU/BaTiO3 | 0 1.2 | Light blue-Dark blue | - | 6.27 9.09 | 6000 s (94.2% current retention) | 2025| [220] |
| Photovoltachromic smart window | Self-powered | (HV(TF-SI)2) heptyl viologen bis(trifluoromethylsulfonyl) imide PV-component | ~0.6 | Transparent-Blue | ~40% contrast ratio at 600 nm | 200 300 | 10,000 cycles (40% initial contrast retained) | 2021| [221] |
| Portable photovoltaic-self-powered flexible electrochromic windows | Portable Self-powered | PEDOT-PSS/V2O5 Organic solar modules | −4 0.5 | Transparent-Blue | 25% contrast ratio at 650 nm | <30 | - | 2021| [222] |
| Fabry–Perot cavity type electrochromic supercapacitors | Display of multicolour states Energy storage capacity Arial capacitance 22.6–68.4 mF/cm2 | Tungsten oxide (WO3) | −0.5 0 | Multicolour | - | Several seconds | 3000 cycles (92% capacitance retained) | 2020| [78] |
| Flexible and wearable electrochromic microsupercapacitor (EMS2) | Camouflage Anticounterfeiting Display Arial capacitance 12.5 mF/cm2 | Ethyl viologen dibromide (EVB) -2D Ti3C2MXene | 0 1 | Colorless-Deep purple | - | 2.6 2.5 | 100 cycles (100% capacitance retained) | 2024| [223] |
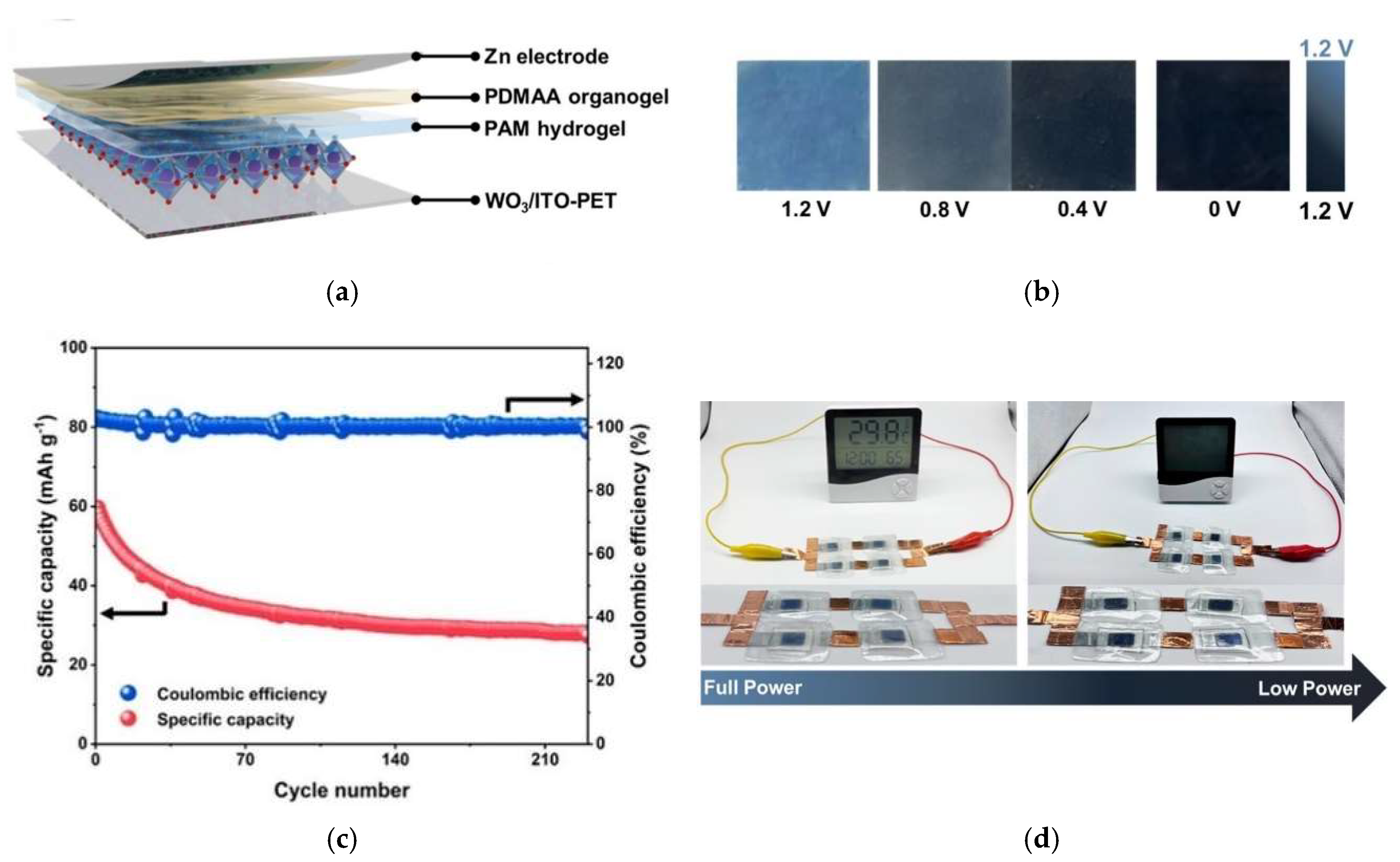
| Electrochromic Zn–ion batteries | Energy storage Powering electronic devices with real-time energy monitoring Specific capacity 43.64 mAh/g | Tungsten oxide (WO3) | 0 1.2 | Sky blue-Black | - | - | 160 cycles (60.84% capacitance retained) | 2025| [224] |
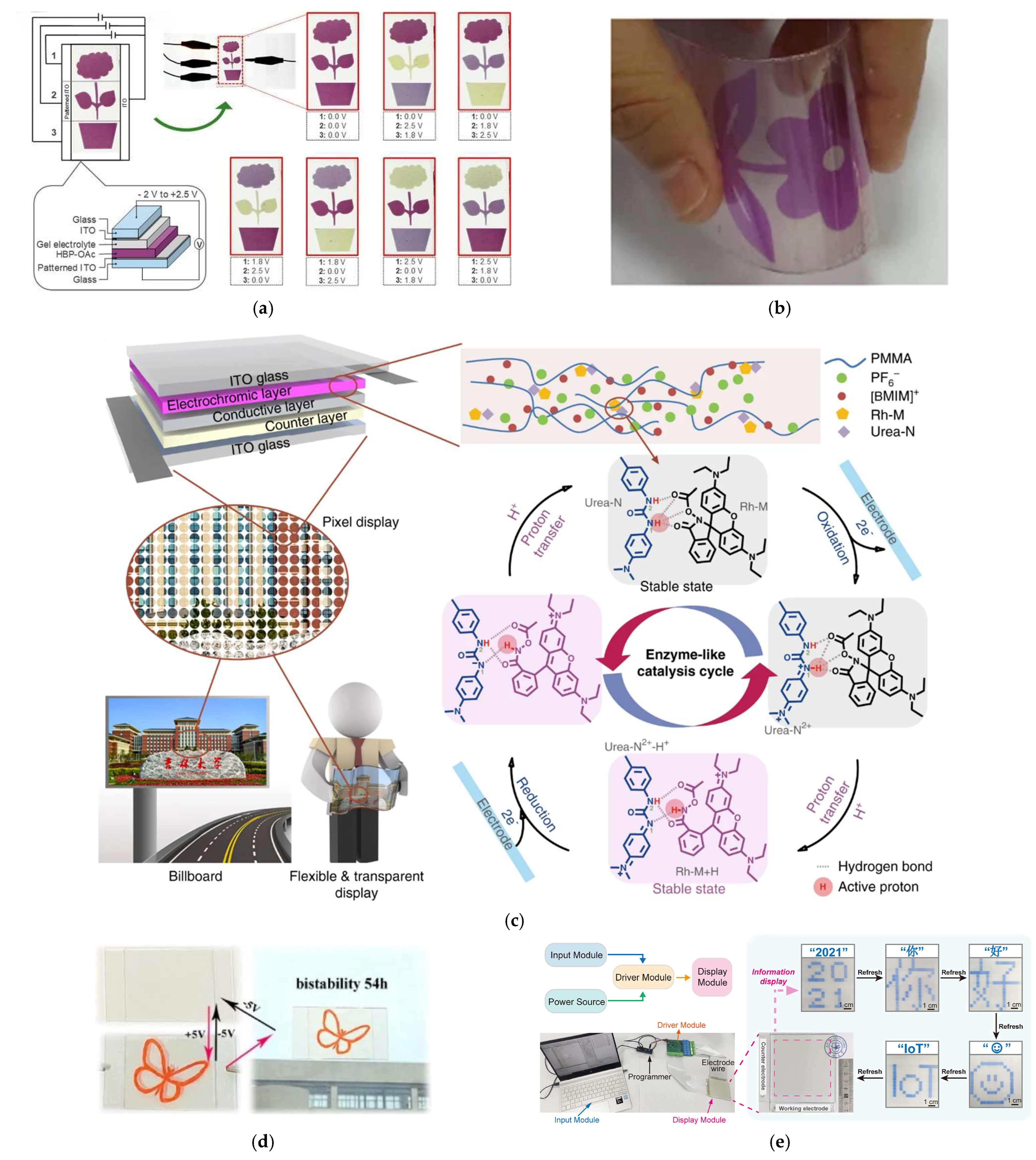
| Tunable multicolour electrochromic devices | Tunable multicolour display | HBP-OAc (Fe(II)/Os(II) polymer | −2 2.5 | Purple violet greenish yellow | at 575 nm | 0.98 1.45 | 100 cycles (95% retained) | 2020| [225] |
| Bistable energy-saving flexible displays | Bistablility electrochromic modulation (>54 h) | Poly(hydroxypropyl acrylate) (PHPA)-PMMA ionic gels | −5 5 | Colourless-red | 80% at 501 nm | 24.3 at 501 nm | >500 cycles | 2021| [226] |
| Transparent non-emissive electrochromic pixelated display | Augmented reality application Bistability (30 days) Energy consumption at 9.5 μW/cm2 | Rhodamine (RhNNE) | −1 3 | blue-Magenta-yellow-greenish black | at 580 nm | 0.9 1.2 | >20,000 cycles | 2023| [227] |
| Flexible smart window/3D vision goggles | Switchable colour and NIR modulation | Methyl viologen dichloride (MV) -2D V2C MXene | −1.5 1.5 | Magenta-blue | 34% of colour contrast at 520 nm 12.4% at 850 nm | 4.2 0.7 5.8 0.2 | 200 cycles (100% retained) | 2025| [228] |
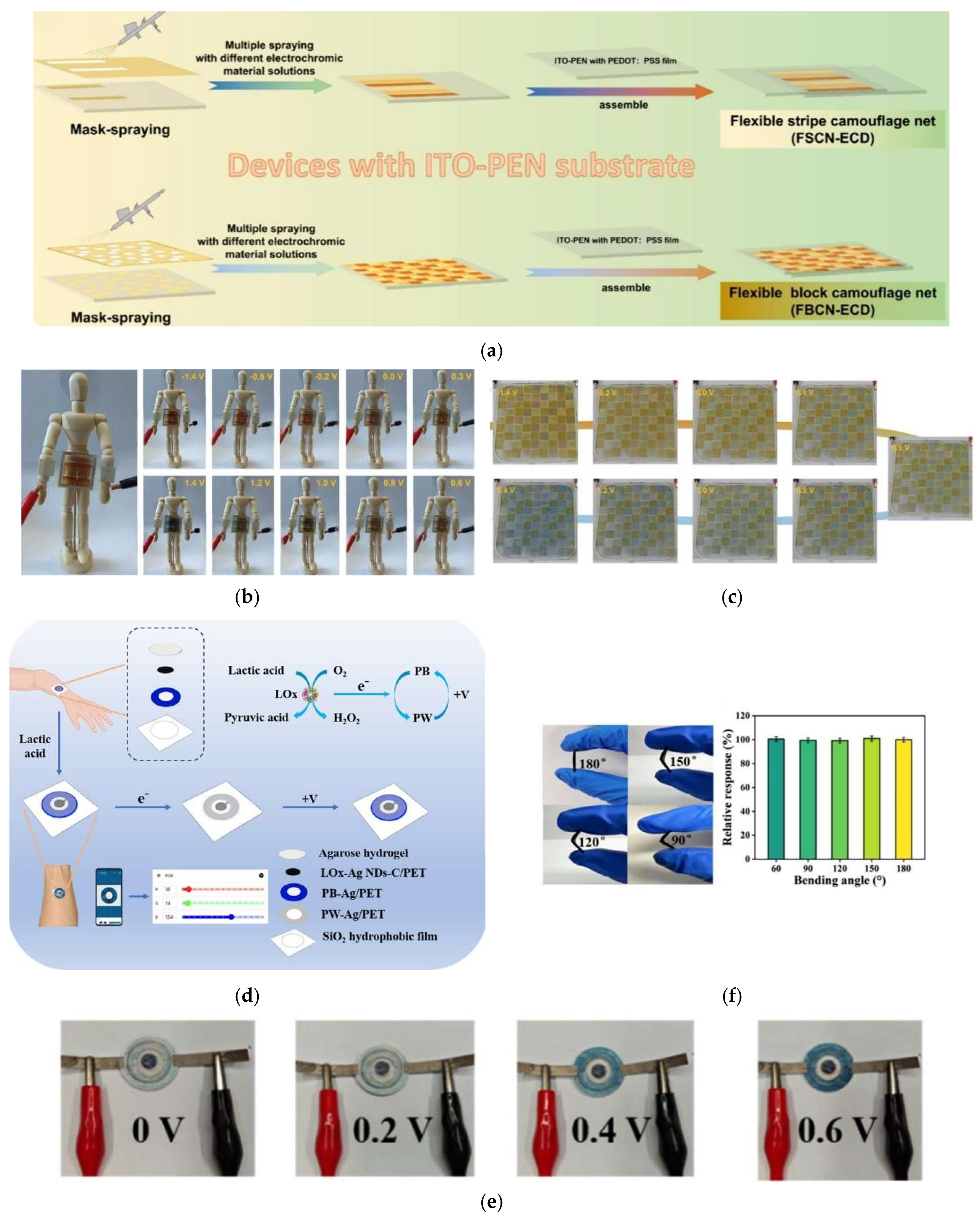
| Adaptative camouflage nets | Dynamic environmental adaptation through voltage-controlled colour-switching | PEDOT-PSS FEP electrochromic polymer | −1.4 1.4 | Yellow-green | at 650 nm | 1.15 2.09 | 1200 cycles (78% contrast retained) | 2024| [229] |
| Reusable self-power electrochromic sensor patch for on-site visualization monitoring of lactic acid | Portable Flexible self-powered Biofuel cell power density of 5.2 μW/cm2 Detection range: 1 to 45 mmol/L (colour based) 0.25 to 45 mmol/L (current based) | Prussian blue (PB) | −0.2 0.6 | Blue-blue fade | - | 400 | ≥50 cycles | 2024| [230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciel, A.; Borges, J.; Pereira, L.; Silva, R.F.; Graça, M. Advancements in Electrochromic Technology for Multifunctional Flexible Devices. Materials 2025, 18, 2964. https://doi.org/10.3390/ma18132964
Marciel A, Borges J, Pereira L, Silva RF, Graça M. Advancements in Electrochromic Technology for Multifunctional Flexible Devices. Materials. 2025; 18(13):2964. https://doi.org/10.3390/ma18132964
Chicago/Turabian StyleMarciel, Alice, Joel Borges, Luiz Pereira, Rui F. Silva, and Manuel Graça. 2025. "Advancements in Electrochromic Technology for Multifunctional Flexible Devices" Materials 18, no. 13: 2964. https://doi.org/10.3390/ma18132964
APA StyleMarciel, A., Borges, J., Pereira, L., Silva, R. F., & Graça, M. (2025). Advancements in Electrochromic Technology for Multifunctional Flexible Devices. Materials, 18(13), 2964. https://doi.org/10.3390/ma18132964









