Mitigation of Volume Changes of Alkali-Activated Materials by Using Limestone Filler
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositions
2.2.1. Reference Composition
2.2.2. Limestone Filler Substitution
2.3. Methods and Materials
2.3.1. Preparation
2.3.2. Slump Flow
2.3.3. Compressive Strength
2.3.4. Apparent Density
2.3.5. Isothermal Calorimetry
2.3.6. Autogenous Strains and Coefficient of Thermal Expansion
3. Results and Discussion
3.1. Preliminary Campaign
3.2. In Depth Investigation
3.2.1. Reaction Kinetics with Isothermal Calorimetry
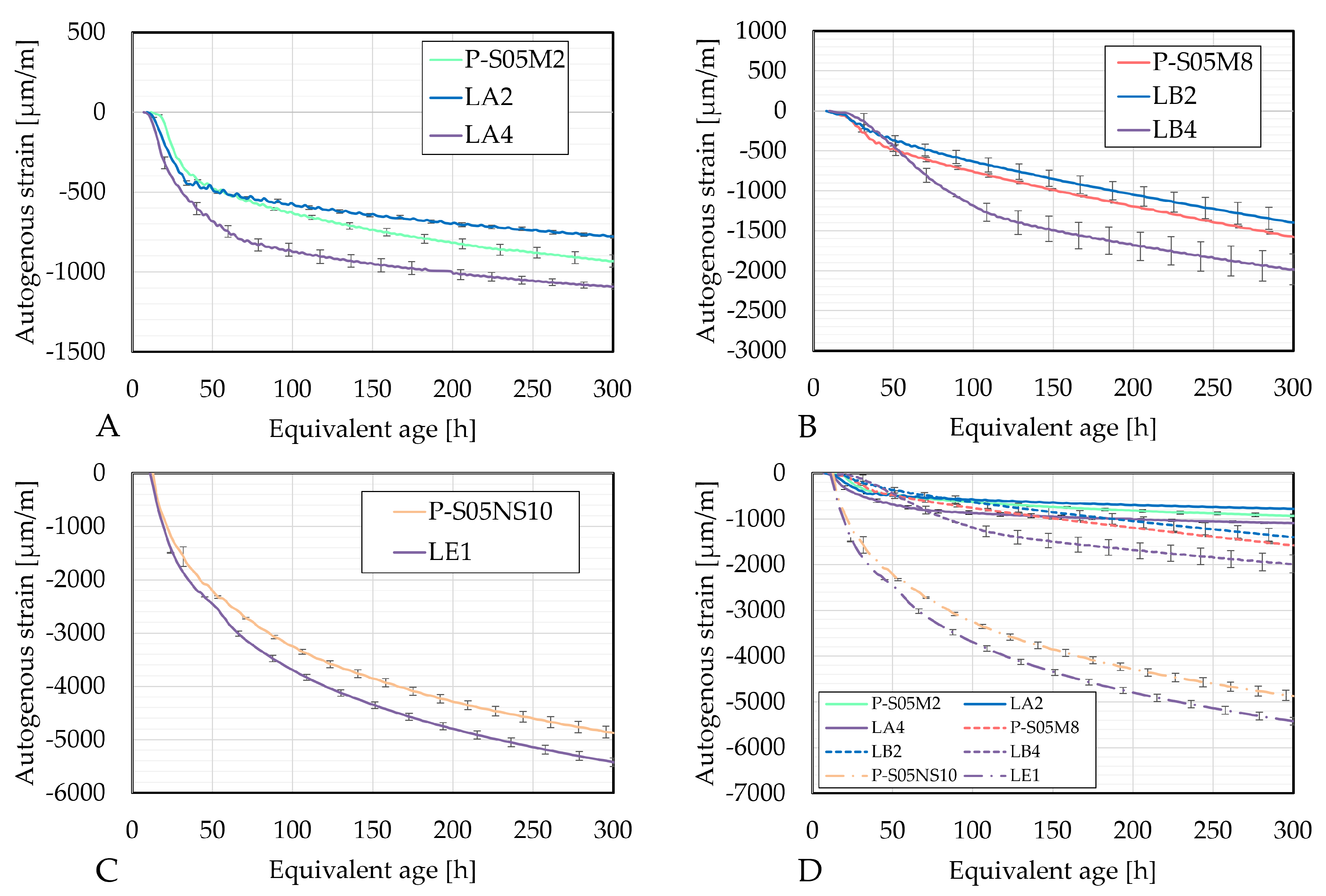
3.2.2. Autogenous Strains
3.2.3. Evolution of the Coefficient of Thermal Expansion
4. Conclusions and Perspectives
- The initial study considered the effect of different rates of replacement on the workability as well as on the compressive strength of the material. Five of the 24 initially proposed compositions were investigated in greater detail. At 7 days of age, LA2 and LB2 showed a reduction smaller than 13% for their compressive strength with a substitution of 15%, while LA4, LB4, and LE1 showed a decrease of less than 23% for a 30% substitution rate compared to their reference compositions. This resulted in compressive strengths at 7 days of 15.8 MPa, 30.8 MPa, 15.0 MPa, 28.4 MPa, and 81.8 MPa, respectively.
- Isothermal calorimetry results indicated that limestone filler plays a role in the reaction. An increased substitution rate raised the solution-to-slag ratio (dilution effect), leading to a higher reaction peak; while also slowing down the reaction. In addition, slag might react more in the presence of limestone filler because of the nucleation effect (more nucleation surface available). The addition of limestone filler might result in the formation of more reaction products and extended length chains.
- Autogenous shrinkage decreased with a 15% limestone filler substitution, likely due to the increased solution-to-slag ratio. This could reduce the self-desiccation shrinkage by decreasing capillary tension and delaying the reaction process. In contrast, higher autogenous shrinkage at increased substitution levels may be linked to the nucleation effect. In the end, the compositions containing limestone filler exhibited a reduced rate of autogenous shrinkage compared to the reference composition.
- The substitution of limestone filler primarily delayed the development of the coefficient of thermal expansion, resulting in slightly lower results at 300 h. Notably, LA4 exhibited a CTE that was 5 µm/m/°C lower than the reference.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAM | Alkali-activated materials |
| CTE | Coefficient of thermal expansion |
| DOR | Degree of reaction |
| LF | Limestone filler |
| PC | Portland cement |
| S/B | Solution-to-binder mass ratio |
Appendix A
| P-S05M2 | LA2 | LA4 | P-S05M8 | LB2 | LB4 | P-S05NS10 | LE1 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Per gram of slag | Q∞,1,slag | a | 2256.20 | 1290.20 | 2200.60 | 5103.60 | 4204.70 | 4632.00 | 2084.10 | 3601.20 |
| b | −1089.30 | −893.09 | −1219.90 | −1968.80 | −1709.10 | −1934.40 | −1160.90 | −1820.80 | ||
| c = Q∞,1,slag | 220.20 | 242.01 | 288.86 | 345.99 | 341.20 | 396.53 | 256.80 | 354.63 | ||
| R2 | 0.9984 | 0.9992 | 0.9986 | 0.9954 | 0.9967 | 0.9969 | 0.9992 | 0.9978 | ||
| Per gram of slag | Q∞,2,slag | Q1 | 63.68 | 142.34 | 158.21 | 217.51 | 226.74 | 278.77 | 59.05 | 70.55 |
| 1 | 5.26 | 8.42 | 7.86 | 5.65 | 5.73 | 6.87 | 15.33 | 18.58 | ||
| a1 | 1.03 | 0.81 | 0.85 | 0.64 | 0.72 | 0.71 | 2.45 | 2.53 | ||
| Q2 | 231.65 | 185.67 | 327.86 | 200.10 | 131.27 | 112.55 | 420.58 | 1026.31 | ||
| 2 | 156.89 | 442.07 | 1388.60 | 504.91 | 338.21 | 295.43 | 472.37 | 6548.13 | ||
| a2 | 0.32 | 0.28 | 0.25 | 0.60 | 0.72 | 0.86 | 0.22 | 0.17 | ||
| error | 31.82 | 45.61 | 31.82 | 50.06 | 51.76 | 104.84 | 426.88 | 608.90 | ||
| Q∞,2,slag | 295.33 | 328.02 | 486.07 | 417.61 | 358.01 | 391.32 | 479.62 | 1096.86 | ||
| Per gram of binder | Q∞,2,slag | a | 2256.20 | 1096.70 | 1540.40 | 5103.60 | 3574.00 | 3242.40 | 2084.10 | 2520.80 |
| b | −1089.30 | −759.12 | −853.94 | −1968.80 | −1452.70 | −1354.10 | −1160.90 | −1274.60 | ||
| c = Q∞,1,slag | 220.20 | 205.71 | 202.20 | 345.99 | 290.20 | 277.57 | 256.80 | 248.24 | ||
| R2 | 0.9984 | 0.9992 | 0.9986 | 0.9954 | 0.9967 | 0.9969 | 0.9992 | 0.9978 | ||
| Per gram of binder | Q∞,2,slag | Q1 | 63.68 | 110.91 | 111.16 | 217.51 | 192.73 | 195.14 | 59.05 | 49.33 |
| 1 | 5.26 | 8.06 | 7.88 | 5.65 | 5.73 | 6.87 | 15.33 | 18.58 | ||
| a1 | 1.03 | 0.84 | 0.84 | 0.64 | 0.72 | 0.71 | 2.45 | 2.54 | ||
| Q2 | 231.65 | 147.72 | 231.36 | 200.10 | 111.58 | 78.79 | 420.58 | 716.87 | ||
| 2 | 156.89 | 208.06 | 1455.55 | 504.91 | 338.21 | 295.43 | 472.37 | 6463.27 | ||
| a2 | 0.32 | 0.31 | 0.25 | 0.60 | 0.72 | 0.86 | 0.22 | 0.17 | ||
| error | 31.82 | 32.74 | 15.59 | 50.06 | 37.40 | 51.37 | 426.88 | 298.38 | ||
| Q∞,2,slag | 295.33 | 258.64 | 342.52 | 417.61 | 304.31 | 273.92 | 479.62 | 766.20 |
References
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Oxon, UK, 2006. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. (Eds.) Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing, Elsevier: Cambridge, UK, 2015. [Google Scholar]
- Lacante, M.; Delsaute, B.; Staquet, S. Effect of Solution-to-Binder Ratio and Molarity on Volume Changes in Slag Binder Activated by Sodium Hydroxide at Early Age. Materials 2024, 17, 3308. [Google Scholar] [CrossRef]
- Lacante, M.; Delsaute, B.; Staquet, S. Effect of Curing Temperature on Volume Changes of Alkali-Activated Slag Pastes. Materials 2025, 18, 1073. [Google Scholar] [CrossRef] [PubMed]
- Carette, J. Towards Early Age Characterisation of Eco-Concrete Containing Blast-Furnace Slag and Limestone Filler. Ph.D. Thesis, Université libre de Bruxelles, Bruxelles, Belgium, 2015. [Google Scholar]
- Gleize, P.J.; Cyr, M.; Escadeillas, G. Effects of metakaolin on autogenous shrinkage of cement pastes. Cem. Concr. Compos. 2007, 29, 80–87. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Chen, Y.; Ye, G. Effect of metakaolin on the autogenous shrinkage of alkali-activated slag-fly ash paste. Constr. Build. Mater. 2021, 278, 122397. [Google Scholar] [CrossRef]
- Li, Z.; Nedeljković, M.; Chen, B.; Ye, G. Mitigating the autogenous shrinkage of alkali-activated slag by metakaolin. Cem. Concr. Res. 2019, 122, 30–41. [Google Scholar] [CrossRef]
- Elmaasrawy, M.; Lan, S.; Cao, H.; Li, S.; Gao, X. Microstructure and durability evaluation of slag-metakaolin-limestone ternary blended alkali activated UHPC. J. Build. Eng. 2025, 106, 112674. [Google Scholar] [CrossRef]
- Diederich, P.; Mouret, M.; de Ryck, A.; Ponchon, F.; Escadeillas, G. The nature of limestone filler and self-consolidating feasibility-Relationships between physical, chemical and mineralogical properties of fillers and the flow at different states, from powder to cement-based suspension. Powder Technol. 2012, 218, 90–101. [Google Scholar] [CrossRef]
- Xuan, M.Y.; Han, Y.; Wang, X.Y. The Hydration, Mechanical, Autogenous Shrinkage, Durability, and Sustainability Properties of Cement–Limestone–Slag Ternary Composites. Sustainability 2021, 13, 1881. [Google Scholar] [CrossRef]
- Michel, F.; Courard, L. Natural limestone filler: Physical and chemical properties with regard to cement based materials properties. In Proceedings of the ICDC International Congress on Durability of Concrete, Trondheim, Norway, 18–21 June 2012. [Google Scholar]
- Rakhimova, N.R.; Rakhimov, R.Z.; Naumkina, N.I.; Khuzin, A.F.; Osin, Y.N. Influence of limestone content, fineness, and composition on the properties and microstructure of alkali-activated slag cement. Cem. Concr. Compos. 2016, 72, 268–274. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, L.; Cui, X.; He, Y.; Zheng, G.; Shi, C. Effect of limestone on rheological, shrinkage and mechanical properties of alkali—Activated slag/fly ash grouting materials. Constr. Build. Mater. 2018, 191, 1285–1292. [Google Scholar] [CrossRef]
- Cousture, A.; Renault, N.; Gallias, J.L.; Ndiaye, K. Study of a binder based on alkaline activated limestone. Constr. Build. Mater. 2021, 311, 125323. [Google Scholar] [CrossRef]
- Rashad, A.M.; Morsi, W.M.; Khafaga, S.A. Effect of limestone powder on mechanical strength, durability and drying shrinkage of alkali-activated slag pastes. Innov. Infrastruct. Solut. 2021, 6, 127. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Chen, Y.T.; Shi, W.; Ghiassi, B. Role of limestone powder in alkali-activated slag paste with superabsorbent polymer. J. Build. Eng. 2023, 76, 107243. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Dainese, E.; Brouwers, H.J. Autogenous and drying shrinkage of sodium carbonate activated slag altered by limestone powder incorporation. Constr. Build. Mater. 2017, 153, 459–468. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yücel Yardimci, M.; Qiang, R.; Lesage, K.; De Schutter, G. Rheology, early-age hydration and microstructure of alkali-activated GGBFS-Fly ash-limestone mixtures. Cem. Concr. Compos. 2021, 124, 104244. [Google Scholar] [CrossRef]
- Oey, T.; Kumar, A.; Bullard, J.W.; Neithalath, N.; Sant, G. The filler effect: The influence of filler content and surface area on cementitious reaction rates. J. Am. Ceram. Soc. 2013, 96, 1978–1990. [Google Scholar] [CrossRef]
- Aqel, M.; Panesar, D. Physical and chemical effects of limestone filler on the hydration of steam cured cement paste and mortar. Rev. ALCONPAT 2020, 10, 191–205. [Google Scholar] [CrossRef]
- Lin Chan, C.; Zhang, M. Effect of limestone on engineering properties of alkali-activated concrete: A review. Constr. Build. Mater. 2023, 362, 129709. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Black, L.; Skocek, J.; Ben Haha, M.; Zajac, M. Effect of sulfate additions on hydration and performance of ternary slag-limestone composite cements. Constr. Build. Mater. 2018, 164, 451–462. [Google Scholar] [CrossRef]
- Carrasco, M.F.; Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength optimization of "tailor-made cement" with limestone filler and blast furnace slag. Cem. Concr. Res. 2005, 35, 1324–1331. [Google Scholar] [CrossRef]
- Afroz, S.; Zhang, Y.; Nguyen, Q.D.; Kim, T.; Castel, A. Effect of limestone in General Purpose cement on autogenous shrinkage of high strength GGBFS concrete and pastes. Constr. Build. Mater. 2022, 327, 126949. [Google Scholar] [CrossRef]
- Lacante, M.; Delsaute, B.; Aggelis, D.G.; Staquet, S. Volume changes and acoustic emission study of alkali-activated slag pastes in free and sealed conditions. Dev. Built Environ. 2025, submitted.
- Nedeljković, M.; Li, Z.; Ye, G. Setting, Strength, and Autogenous Shrinkage of Alkali-Activated Fly Ash and Slag Pastes: Effect of Slag Content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V.; Mounanga, P.; Khelidj, A.; Loukili, A.; Rafaï, N. Autogenous deformations of cement pastes. Cem. Concr. Res. 2006, 36, 123–136. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement-Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- ASTM C230/C230M-08; Standard Specification for Flow Table for Use in Tests of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM C109/C109M-16a; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2016.
- EN 196-11:2018; Methods of Testing Cement-Part 11: Heat of Hydration-Isothermal Conduction Calorimetry Method. European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- TA Instruments. TAM Air Calorimeter Operator’s Manual; Technical Report; TA Instruments: New Castle, DE, USA, 2006. [Google Scholar]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Early age reaction, rheological properties and pore solution chemistry of NaOH-activated slag mixtures. Cem. Concr. Compos. 2022, 133, 104715. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; de Schutter, G. Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Brouwers, H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A review on alkali-activated binders: Materials composition and fresh properties of concrete. Constr. Build. Mater. 2020, 260, 119788. [Google Scholar] [CrossRef]
- ASTM C 1698-09; Test Method for Autogenous Strain of Cement Paste and Mortar. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Delsaute, B.; Staquet, S. Decoupling Thermal and Autogenous Strain of Concretes with Different Water/Cement Ratios During the Hardening Process. Adv. Civ. Eng. Mater. 2017, 6, 20160063. [Google Scholar] [CrossRef]
- Serdar, M.; Gabrijel, I.; Schlicke, D.; Staquet, S.; Azenha, M. (Eds.) Advanced Techniques for Testing of Cement-Based Materials; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Belov, V.; Kuliaev, P. Limestone filler as one of the cheapest and best additive to concrete. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2018; Volume 365. [Google Scholar] [CrossRef]
- Abdul Rahi, R.; Azizli, K.; Man, Z.; Rahmiati, T.; Nuruddin, M. Effect of Sodium Hydroxide Concentration on the Mechanical Property of Non Sodium Silicate Fly Ash Based Geopolymer. J. Appl. Sci. 2014, 14, 3381–3384. [Google Scholar] [CrossRef]
- Newman, J.; Choo, B.S. (Eds.) Advanced Concrete Technology; Elsevier: Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Khatib, J.M. (Ed.) Sustainability of Construction Materials; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cement Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J. Properties of alkali activated slag-fly ash blends with limestone addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Naqi, A. Early Age Characterization of Viscoelastic Properties of Alkali-Activated Binders. Ph.D. Thesis, Université libre de Bruxelles, Bruxelles, Belgium, 2023. [Google Scholar]
- Naqi, A.; Delsaute, B.; Königsberger, M.; Staquet, S. Monitoring early age elastic and viscoelastic properties of alkali-activated slag mortar by means of repeated minute-long loadings. Dev. Built Environ. 2023, 16, 100275. [Google Scholar] [CrossRef]
- Freiesleben Hansen, P.; Pedersen, E.J. Måleinstrument til control af betons hærdning. Nord. Betong 1977, 1, 21–25. [Google Scholar]
- Nielsen, C.V.; Kaasgaard, M. Activation Energy for the Concrete Maturity Model – Part 1: Compressive Strength Tests at Different Curing Temperatures. Nord. Concr. Res. 2020, 62, 87–106. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Chen, Z. Hydration kinetics, strength, autogenous shrinkage, and sustainability of cement pastes incorporating ultrafine limestone powder. Case Stud. Constr. Mater. 2024, 20, e03149. [Google Scholar] [CrossRef]
- Tenório Filho, J.R.; Pereira Gomes de Araújo, M.A.; Snoeck, D.; De Belie, N. Discussing Different Approaches for the Time-Zero as Start for Autogenous Shrinkage in Cement Pastes Containing Superabsorbent Polymers. Materials 2019, 12, 2962. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Provis, J.L.; Cizer, O.; Ye, G. Autogenous shrinkage of alkali-activated slag: A critical review. Cem. Concr. Res. 2023, 172, 107244. [Google Scholar] [CrossRef]
- Uppalapati, S. Early-Age Structural Development and Autogenous Shrinkage of Alkali-Activated Slag/Fly Ash Cements. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2020. [Google Scholar]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Sirotti, M. Drying Shrinkage and Deformation Mechanisms in NaOH-Activated Slags. Ph.D. Thesis, Université libre de Bruxelles, Bruxelles, Belgium, 2023. [Google Scholar]













| Material | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | TiO2 | SO3 | Na2O | BaO | MnO | SrO | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 34.20 | 12.86 | 0.35 | 39.95 | 0.62 | 7.91 | 1.13 | 1.88 | 0.50 | 0.13 | 0.30 | / | / | / |
| LF | 0.31 | 0.11 | 0.09 | 55.03 | 0.02 | 0.42 | 0.01 | 0.09 | 0.09 | / | / | 0.03 | 0.01 | 43.78 |
| Ref Compositions | Alkaline Solution | S/B Ratio [-] | Concentration [mol/L] | Water/Alkaline Solution [-] |
|---|---|---|---|---|
| P-S05M2 | NaOH | 0.5 | 2 | 0 |
| P-S05M8 | ||||
| P-S08M2 | NaOH | 0.8 | 8 | 0 |
| P-S08M8 | ||||
| P-S05NS10 | Na2SiO3 | 0.5 | 10 | 1/1 |
| P-S08NS10 | 0.8 | 1/1 |
| ID | Compositions | Alkaline Solution | Limestone Filler Ratio [%] | S/B Ratio [-] | Concentration [mol/L] | Water/Alkaline Solution [-] |
|---|---|---|---|---|---|---|
| LA1 | LF10S05M2 | NaOH | 10 | 0.5 | 2 | 0 |
| LA2 | LF15S05M2 | 15 | ||||
| LA3 | LF20S05M2 | 20 | ||||
| LA4 | LF30S05M2 | 30 | ||||
| LA5 | LF40S05M2 | 40 | ||||
| LA6 | LF50S05M2 | 50 | ||||
| LB1 | LF10S05M8 | NaOH | 10 | 0.5 | 8 | 0 |
| LB2 | LF15S05M8 | 15 | ||||
| LB3 | LF20S05M8 | 20 | ||||
| LB4 | LF30S05M8 | 30 | ||||
| LB5 | LF40S05M8 | 40 | ||||
| LB6 | LF50S05M8 | 50 | ||||
| LC1 | LF10S08M2 | NaOH | 10 | 0.8 | 2 | 0 |
| LC2 | LF20S08M2 | 20 | ||||
| LC3 | LF30S08M2 | 30 | ||||
| LC4 | LF40S08M2 | 40 | ||||
| LC5 | LF50S08M2 | 50 | ||||
| LD1 | LF10S08M8 | NaOH | 10 | 0.8 | 8 | 0 |
| LD2 | LF20S08M8 | 20 | ||||
| LD3 | LF30S08M8 | 30 | ||||
| LD4 | LF40S08M8 | 40 | ||||
| LD5 | LF50S08M8 | 50 | ||||
| LE1 | LF30S05NS10 | Na2SiO3 | 30 | 0.5 | 10 | 1/1 |
| LE2 | LF50S08NS10 | 50 | 0.8 |
| LF ratio | P-S05M2 | P-S05M8 | P-S08M2 | P-S08M8 | P-S05NS10 | P-S08NS10 |
|---|---|---|---|---|---|---|
| 10% | 1.88 | 5.42 | −10.29 | −12.02 | / | / |
| 15% | 10.06 | 12.37 | / | / | / | / |
| 20% | 26.95 | 9.02 | −3.88 | −2.17 | / | / |
| 30% | 15.05 | 19.25 | 22.50 | −11.93 | 22.64 | / |
| 40% | 42.58 | 30.29 | 32.45 | 12.20 | / | / |
| 50% | 48.86 | 52.23 | 51.78 | 41.30 | / | 51.20 |
| LF Ratio | P-S05M2 | P-S05M8 | P-S08M2 | P-S08M8 | P-S05NS10 | P-S08NS10 |
|---|---|---|---|---|---|---|
| 10% | 9.02 | 5.09 | 22.54 | 24.47 | / | / |
| 15% | 5.81 | 3.09 | / | / | / | / |
| 20% | −8.69 | 13.73 | 29.85 | 27.72 | / | / |
| 30% | 21.36 | 15.35 | 10.72 | 59.91 | 10.52 | / |
| 40% | −4.30 | 16.18 | 12.58 | 46.33 | / | / |
| 50% | 2.27 | −4.47 | −3.55 | 17.39 | / | −2.40 |
| P-S05M2 | LA2 | LA4 | P-S05M8 | LB2 | LB4 | P-S05NS10 | LE1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Per gram of slag | Q∞,1,slag | 220.20 | 242.01 | 288.86 | 345.99 | 341.20 | 396.53 | 256.80 | 354.63 |
| Q∞,2,slag | 295.33 | 328.02 | 486.07 | 417.61 | 358.01 | 391.32 | 479.62 | 1096.86 | |
| Per gram of binder | Q∞,1,binder | 220.20 | 205.71 | 202.20 | 345.99 | 290.20 | 277.57 | 256.80 | 248.24 |
| Q∞,2,binder | 295.33 | 258.64 | 342.52 | 417.61 | 304.31 | 273.92 | 479.62 | 766.20 |
| Composition | [h] | DOR∞,1,slag [-] | DOR∞,2,slag [-] | DOR∞,1,binder [-] | DOR∞,2,binder [-] |
|---|---|---|---|---|---|
| P-S05M2 | 11.47 | 0.287 | 0.214 | 0.287 | 0.214 |
| LA2 | 8.80 | 0.267 | 0.197 | 0.268 | 0.213 |
| LA4 | 6.98 | 0.212 | 0.126 | 0.212 | 0.125 |
| P-S05M8 | 9.89 | 0.314 | 0.260 | 0.314 | 0.260 |
| LB2 | 7.97 | 0.303 | 0.288 | 0.302 | 0.288 |
| LB4 | 9.90 | 0.325 | 0.330 | 0.325 | 0.330 |
| P-S05NS10 | 12.75 | 0.217 | 0.116 | 0.217 | 0.116 |
| LE1 | 11.06 | 0.157 | 0.050 | 0.157 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacante, M.; Delsaute, B.; Staquet, S. Mitigation of Volume Changes of Alkali-Activated Materials by Using Limestone Filler. Materials 2025, 18, 2963. https://doi.org/10.3390/ma18132963
Lacante M, Delsaute B, Staquet S. Mitigation of Volume Changes of Alkali-Activated Materials by Using Limestone Filler. Materials. 2025; 18(13):2963. https://doi.org/10.3390/ma18132963
Chicago/Turabian StyleLacante, Maïté, Brice Delsaute, and Stéphanie Staquet. 2025. "Mitigation of Volume Changes of Alkali-Activated Materials by Using Limestone Filler" Materials 18, no. 13: 2963. https://doi.org/10.3390/ma18132963
APA StyleLacante, M., Delsaute, B., & Staquet, S. (2025). Mitigation of Volume Changes of Alkali-Activated Materials by Using Limestone Filler. Materials, 18(13), 2963. https://doi.org/10.3390/ma18132963







