Graphene Nanoplatelets Reinforced ABS Nanocomposite Films by Sonication-Assisted Cast Film Technique for Emission Shielding Application
Abstract
1. Introduction
2. Materials
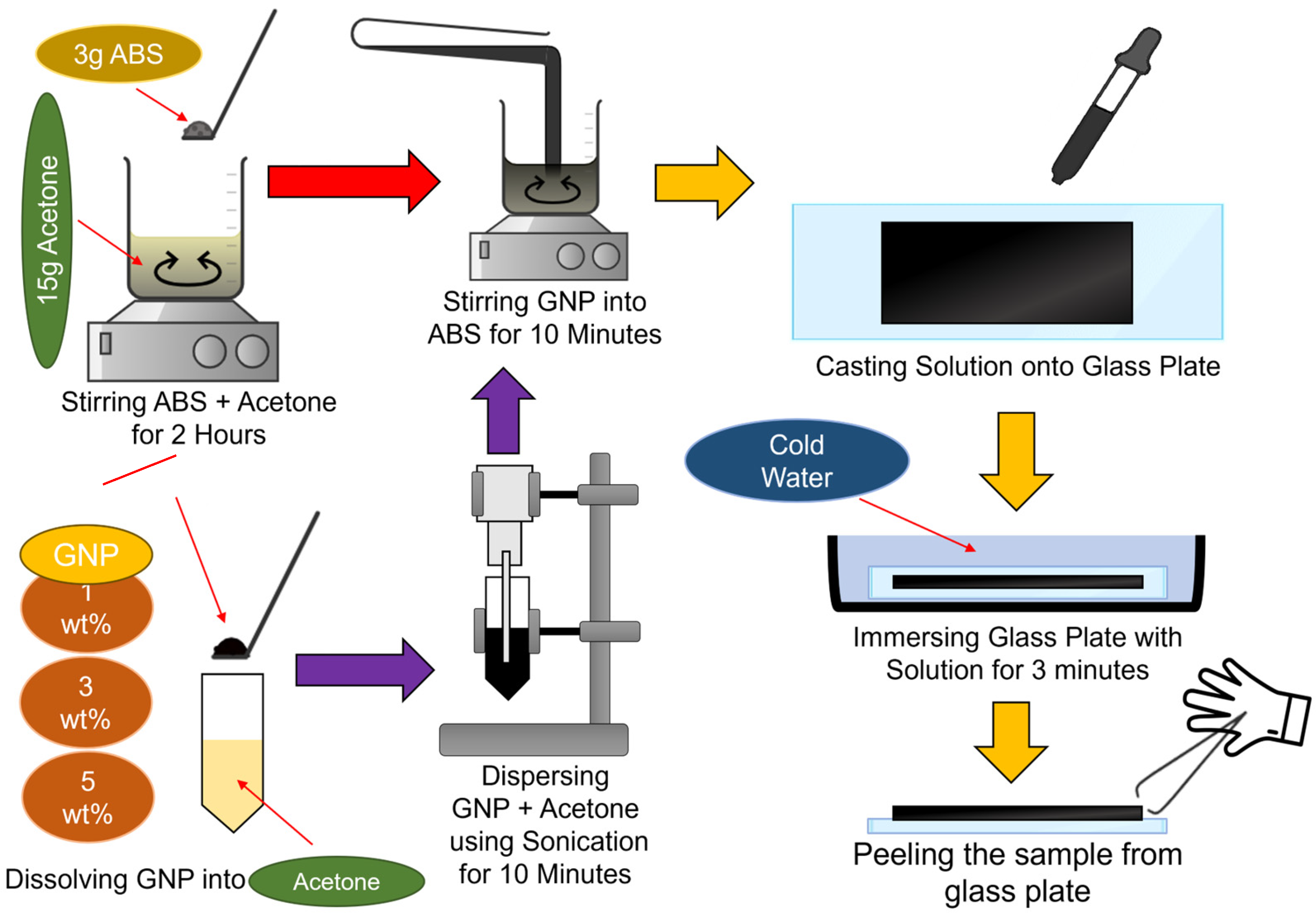
2.1. ABS/GNPs Film Nanocomposite Fabrication
2.2. The Contact Angle and Surface Energy
2.3. TGA Analyzer
2.4. DSC Analyzer
2.5. FTIR Spectroscopy
2.6. Dielectric Test
2.7. EMI Shielding Effectiveness (SE)
2.8. Field Emission Scanning Electron Microscope (FESEM)
3. Results and Discussion
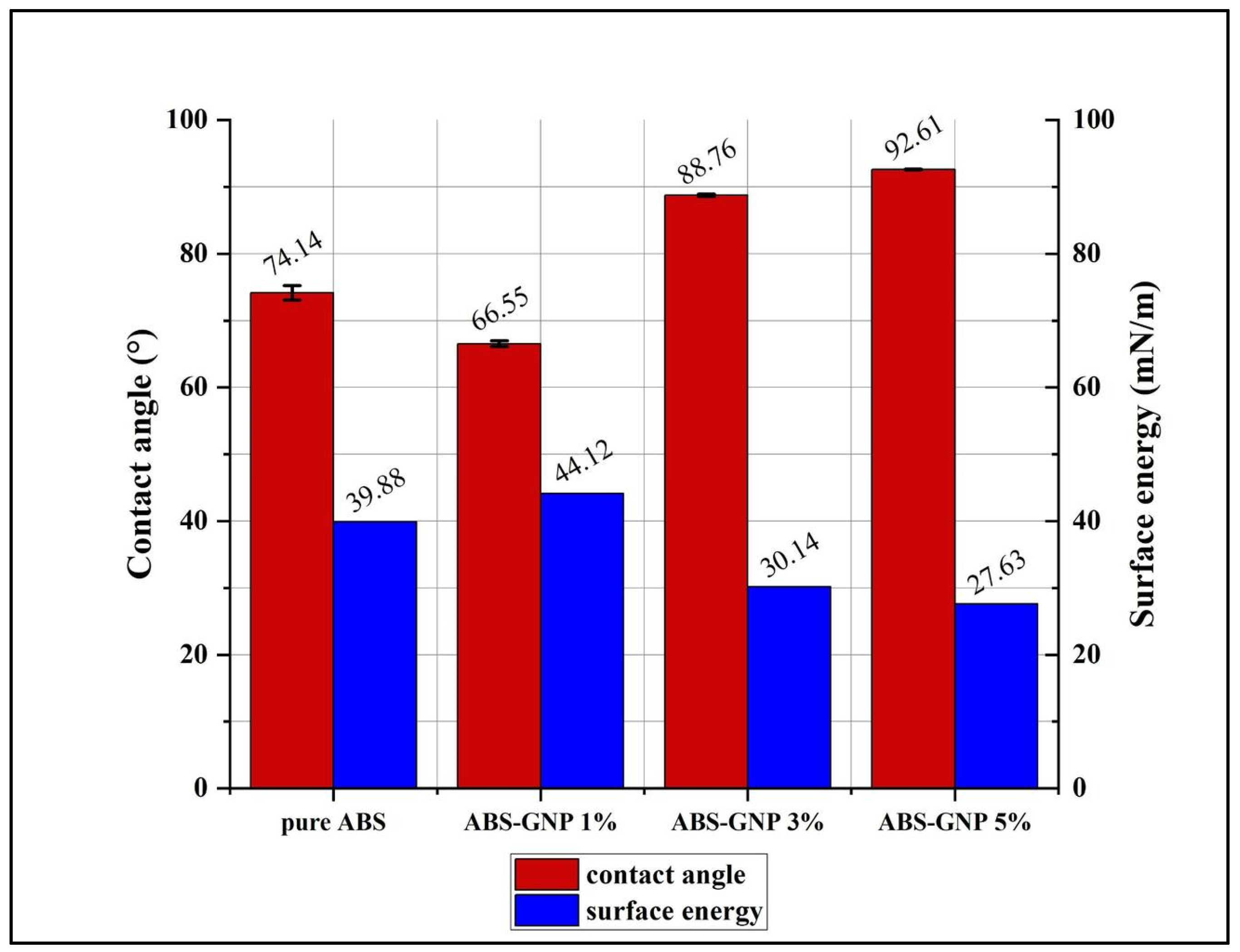
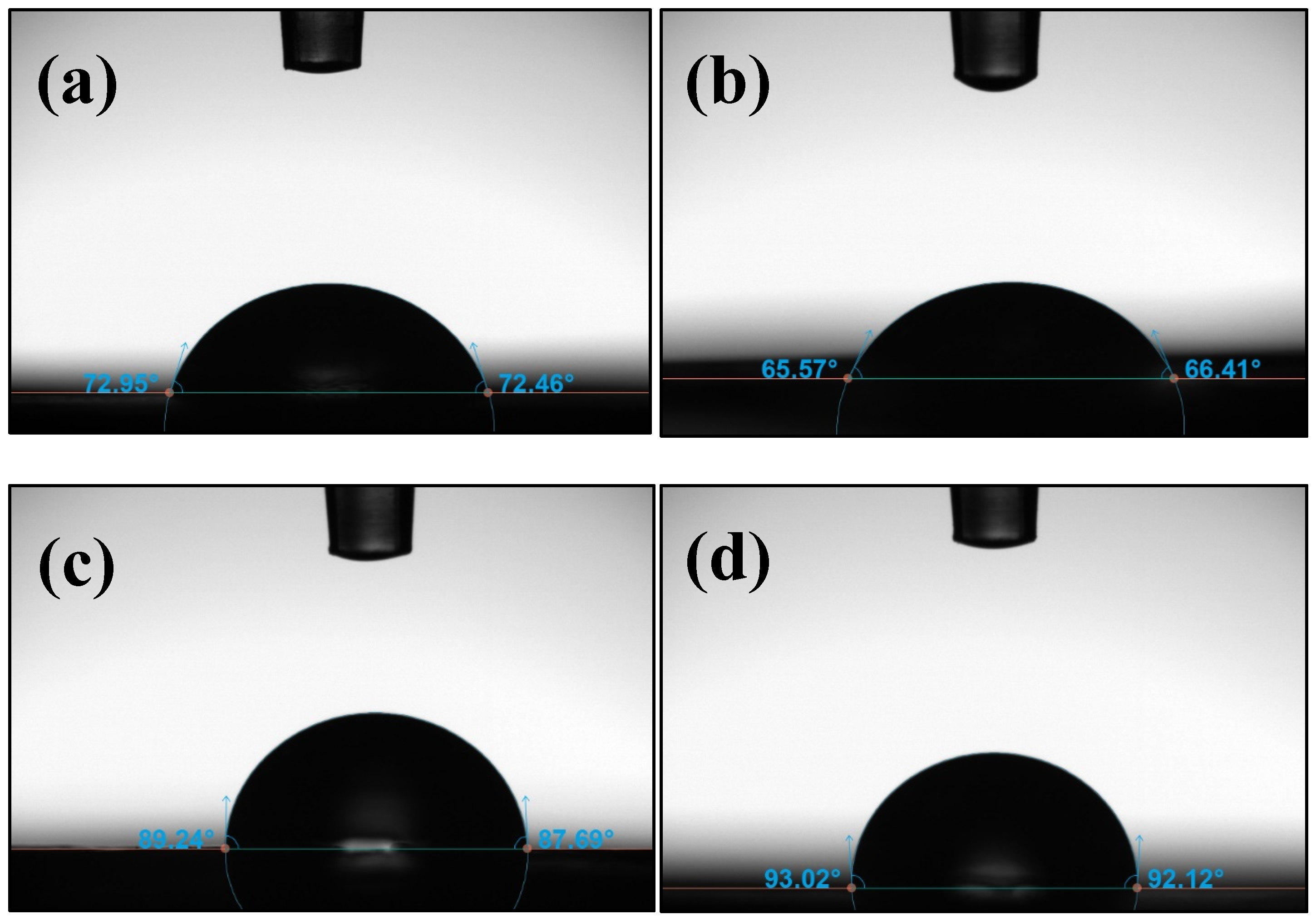
3.1. Optical Contact Angle (OCA)
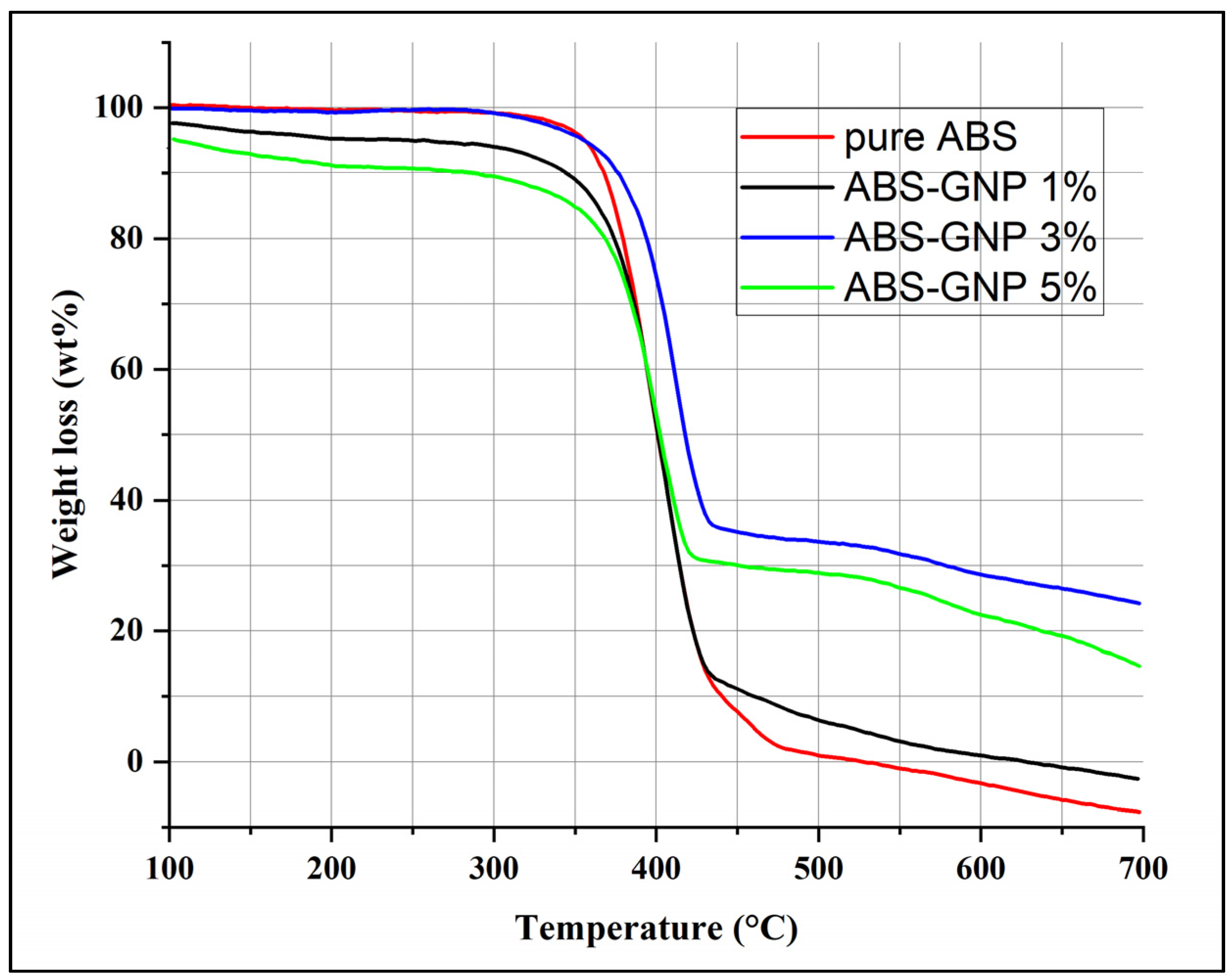
3.2. Thermogravimetric Analysis (TGA)
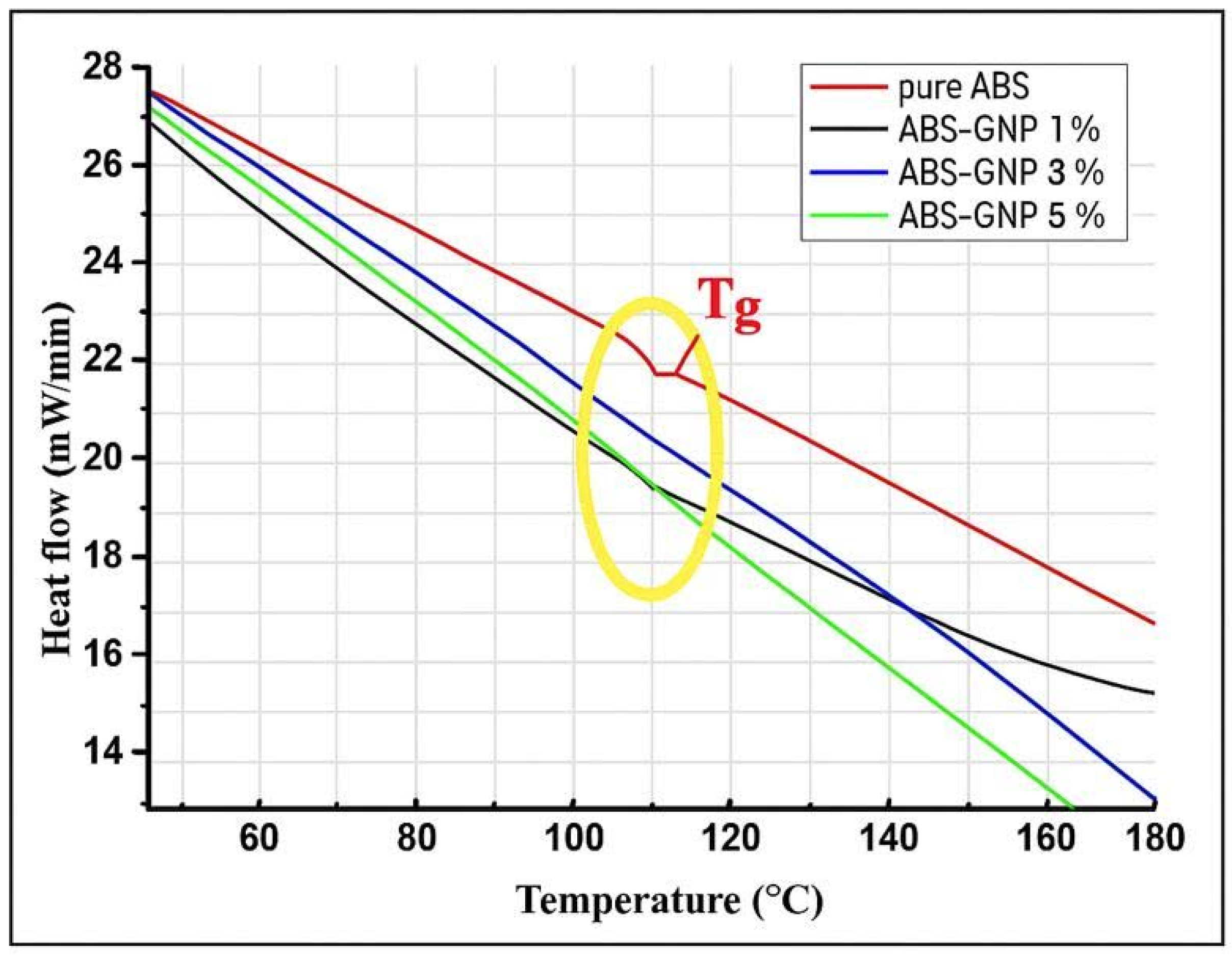
3.3. Differential Scanning Calorimetry (DSC)
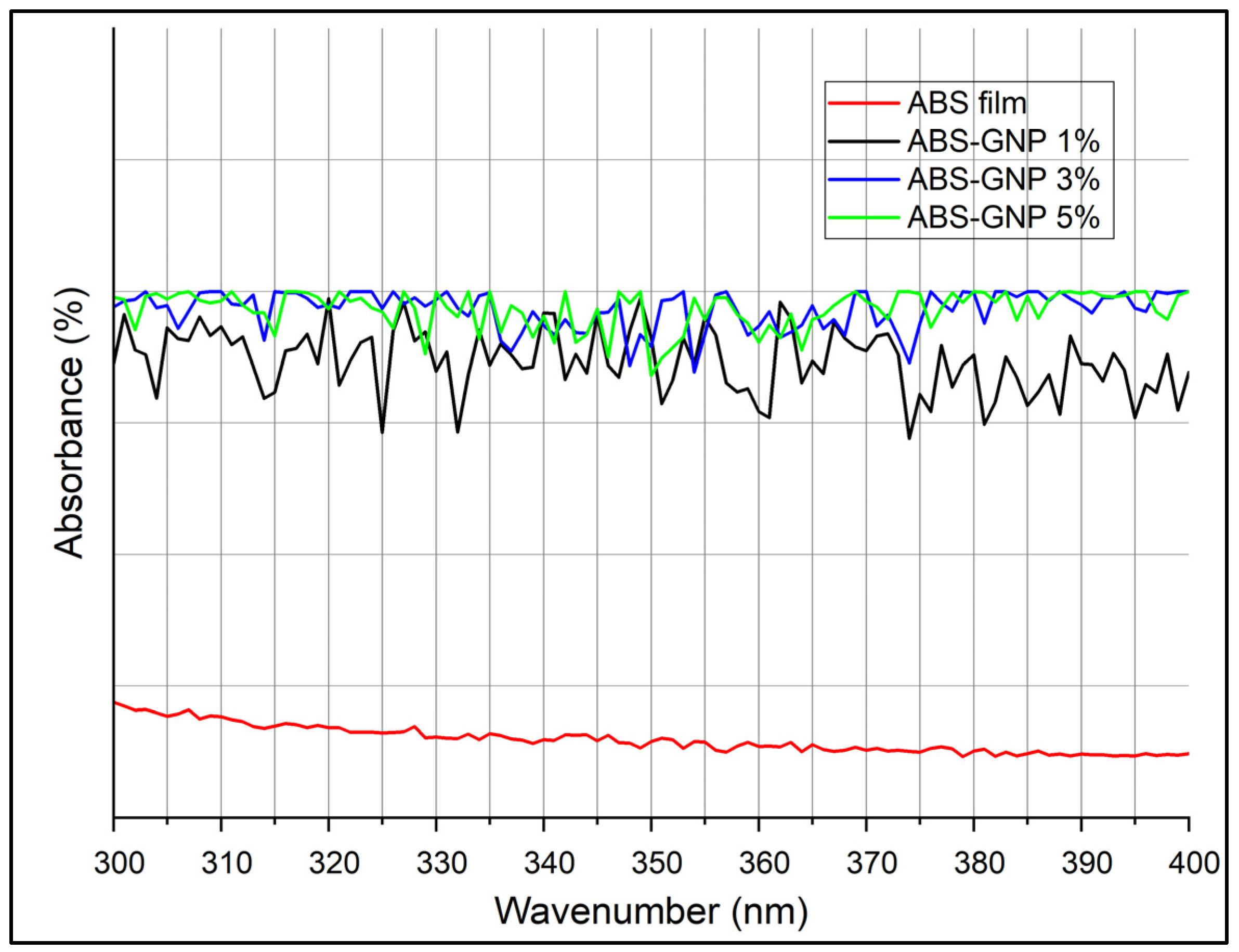
3.4. Fourier Transform Infrared Analysis (FTIR)
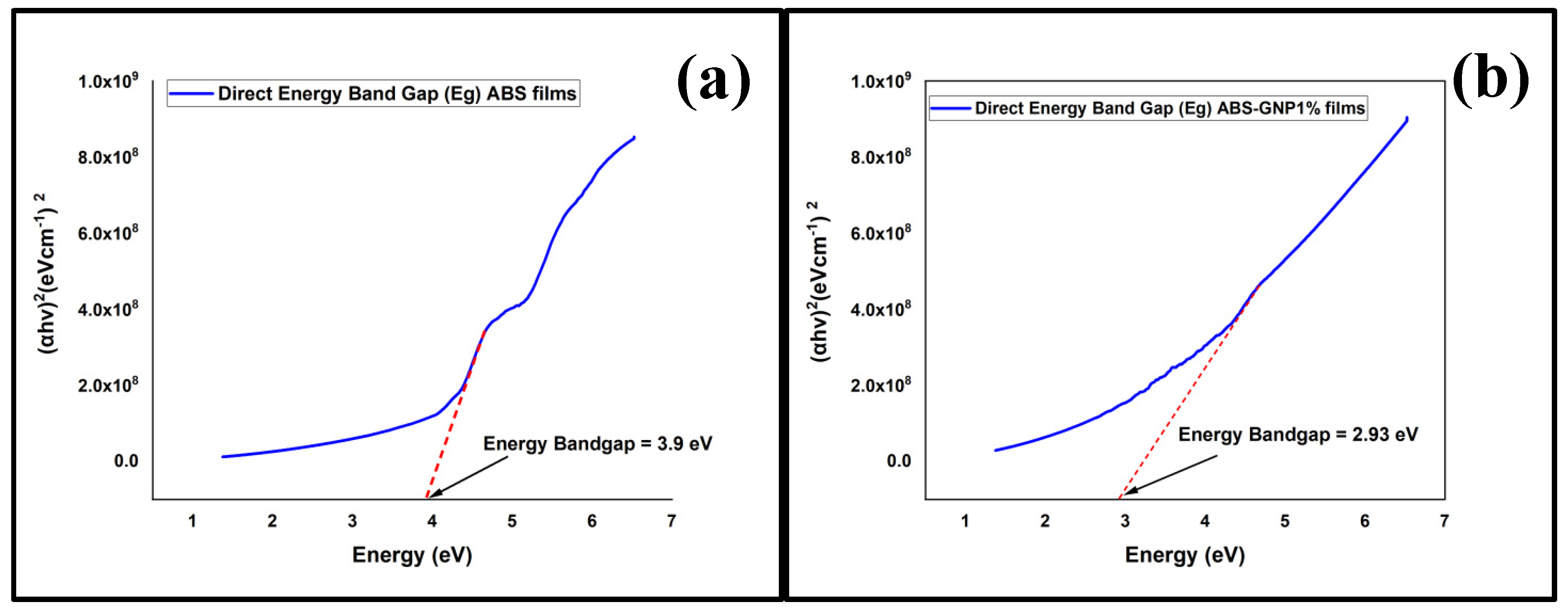
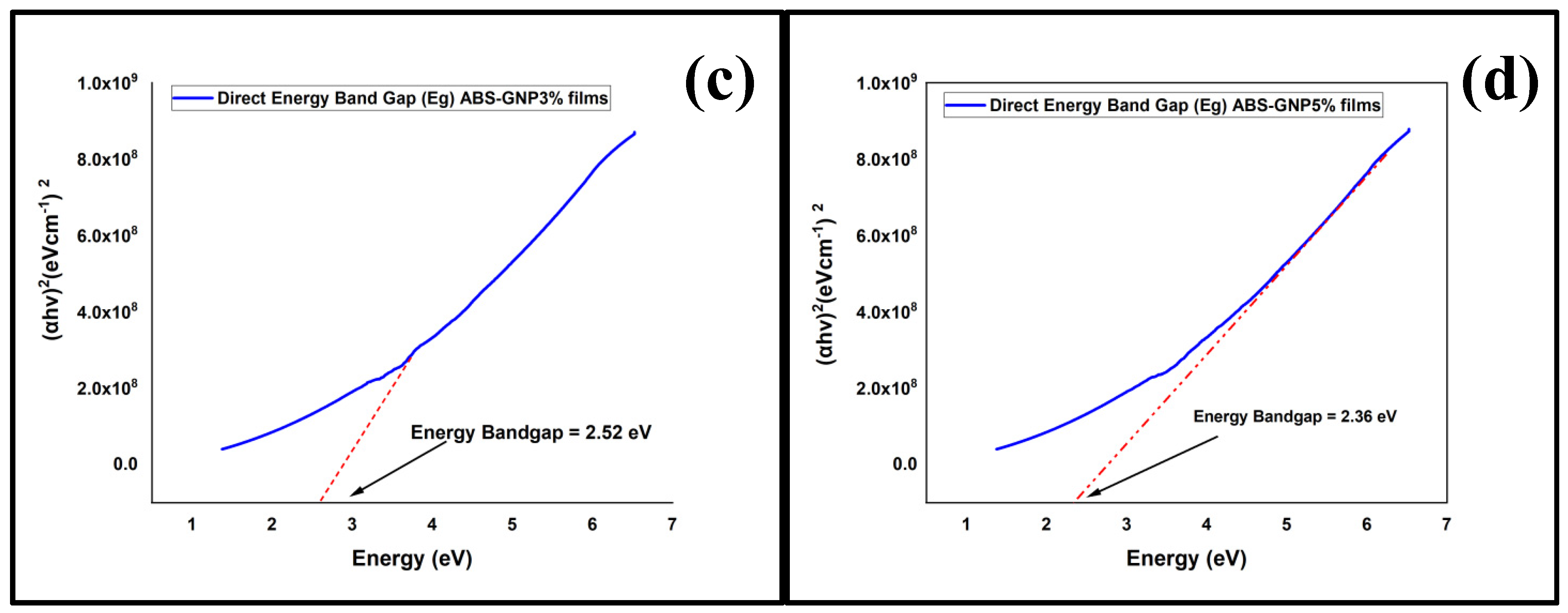
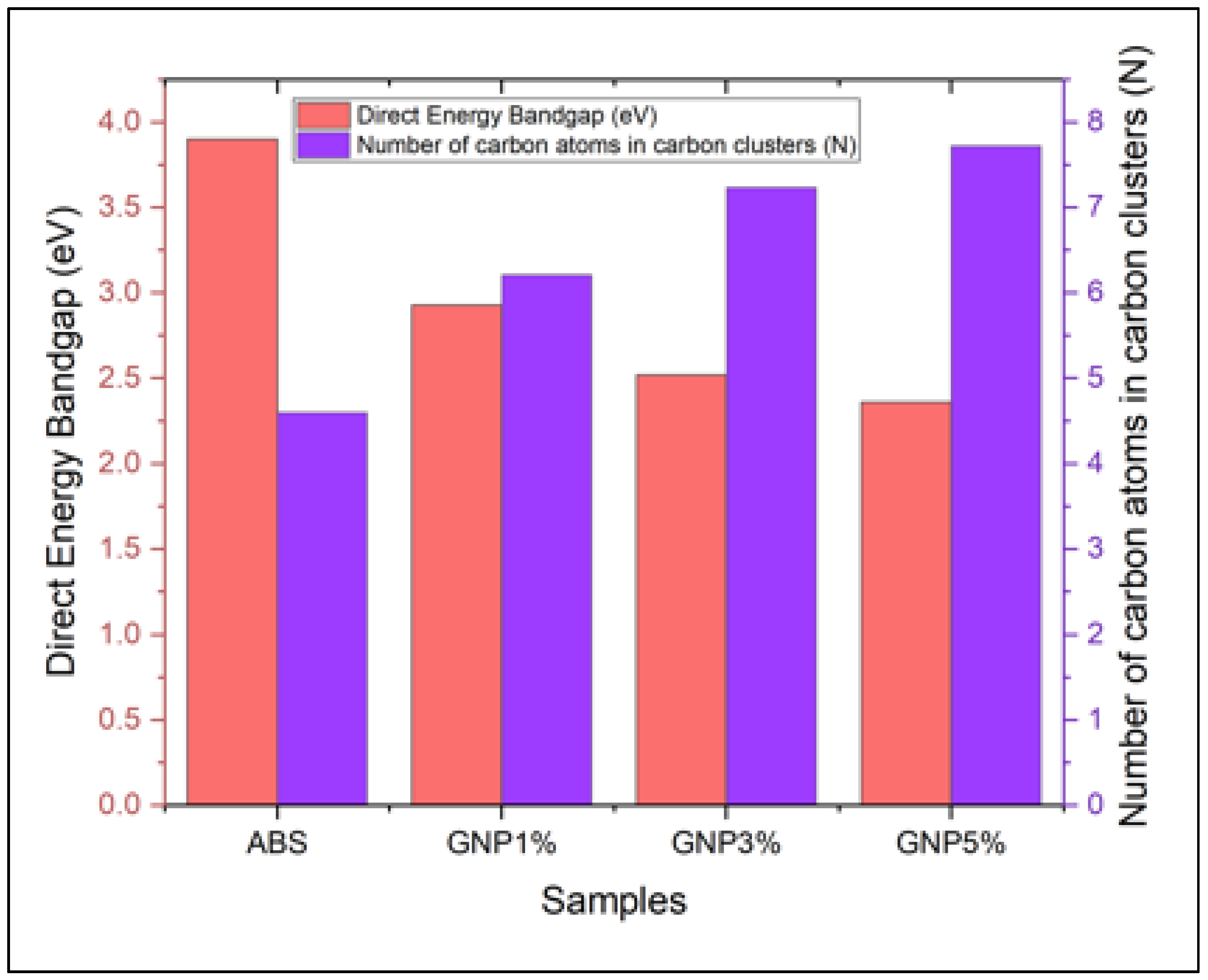
3.5. Ultraviolet-Visible Spectroscopy (UV-Vis)
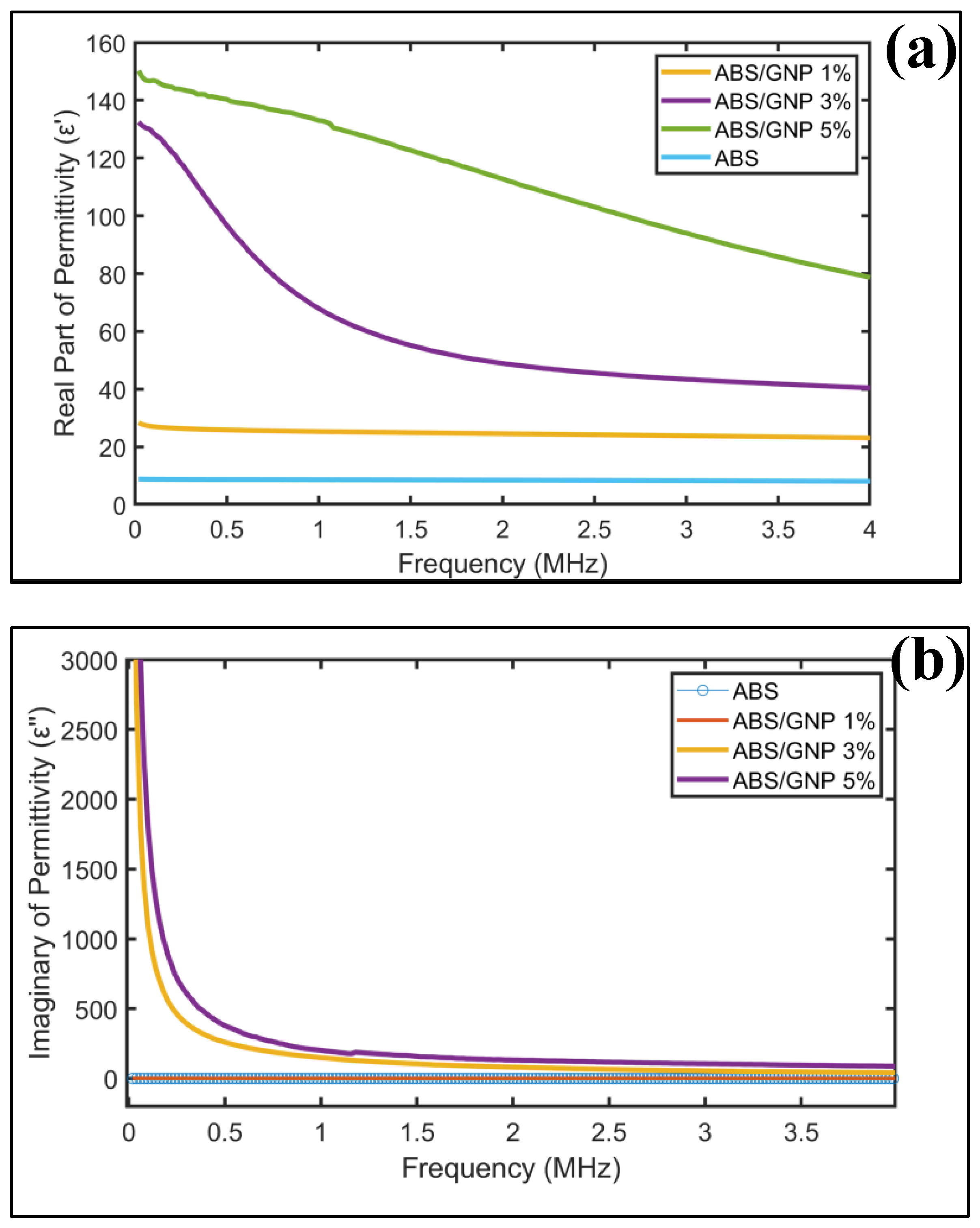
3.6. Dielectric
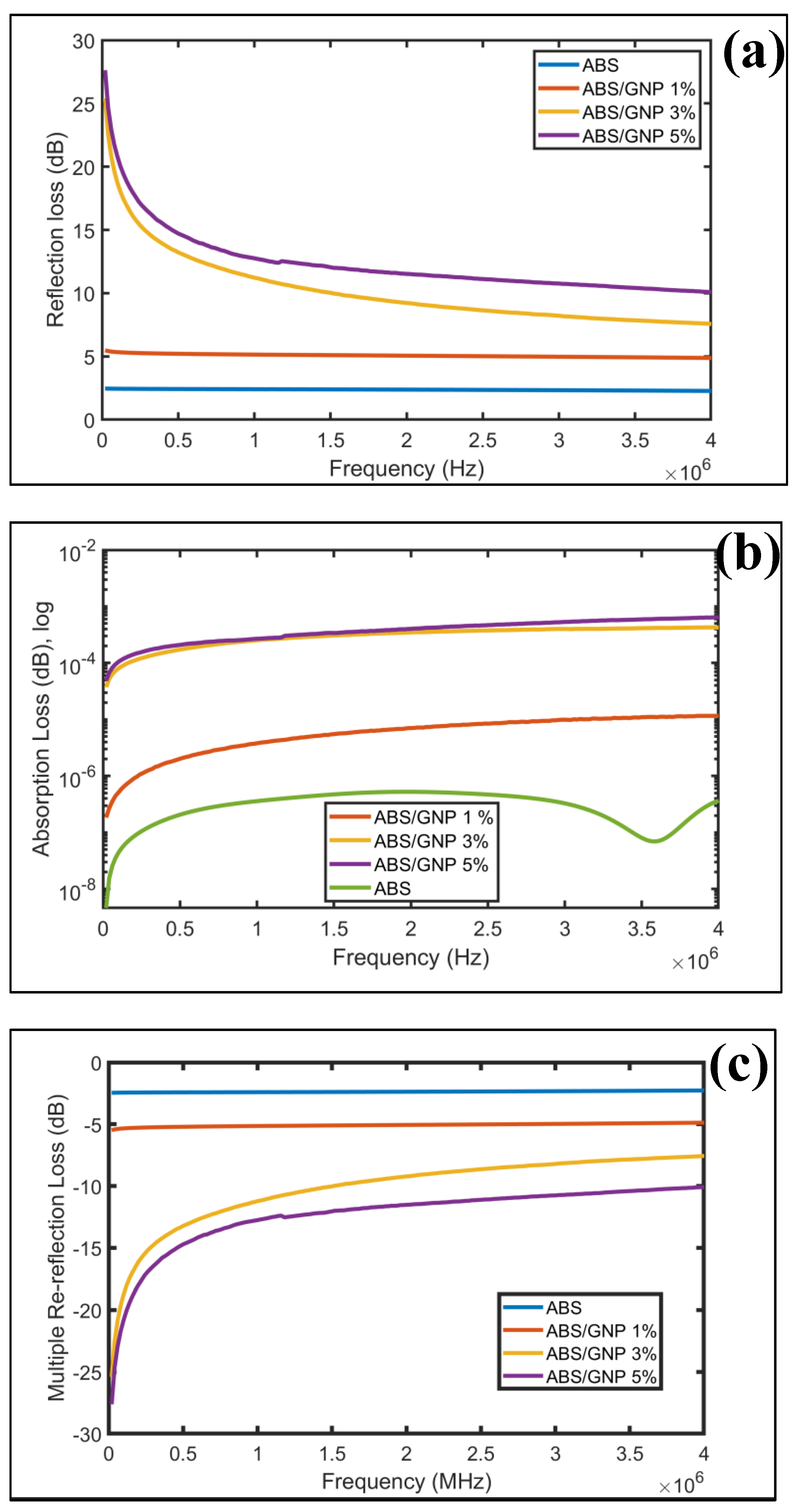
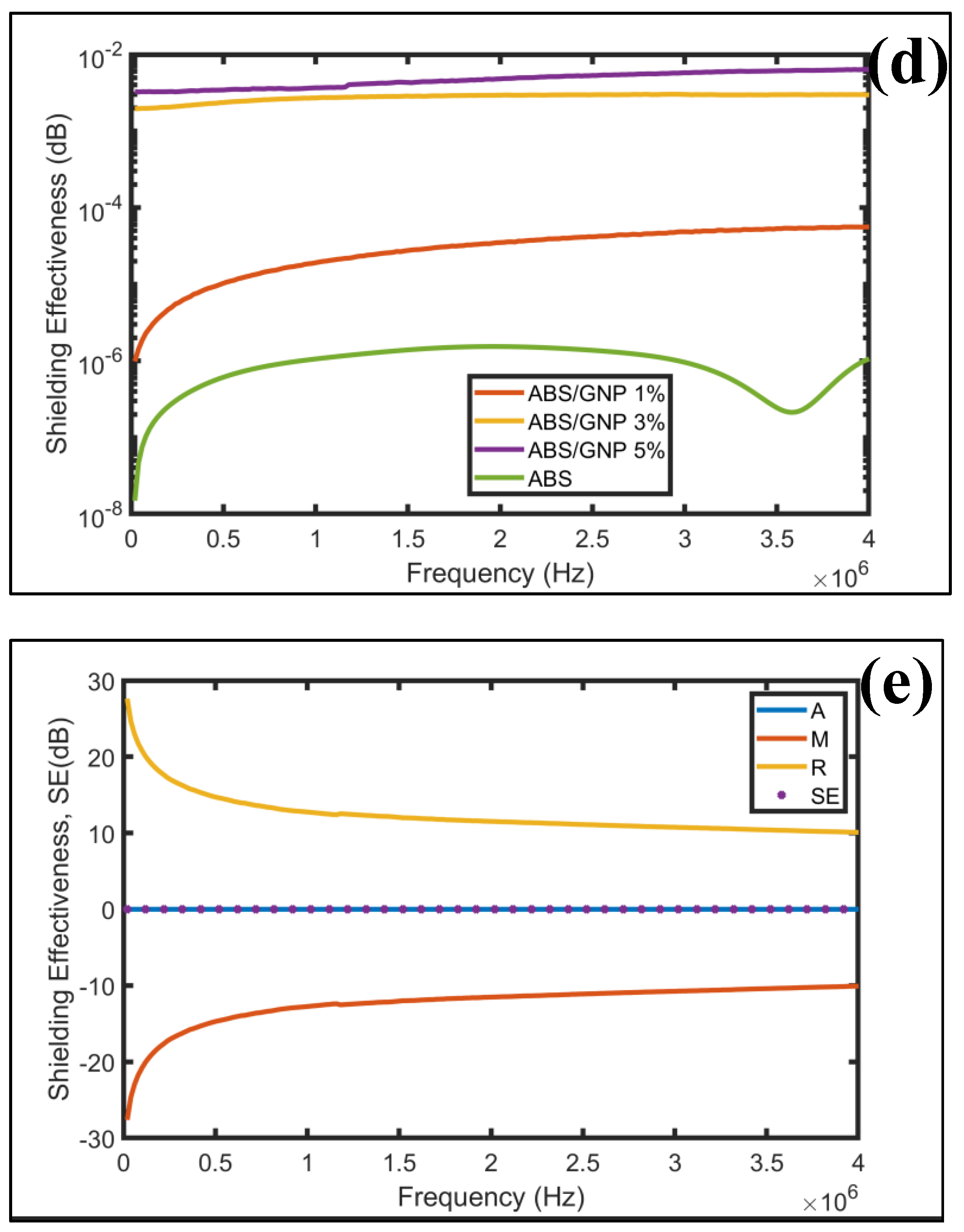
3.7. EMI Shielding
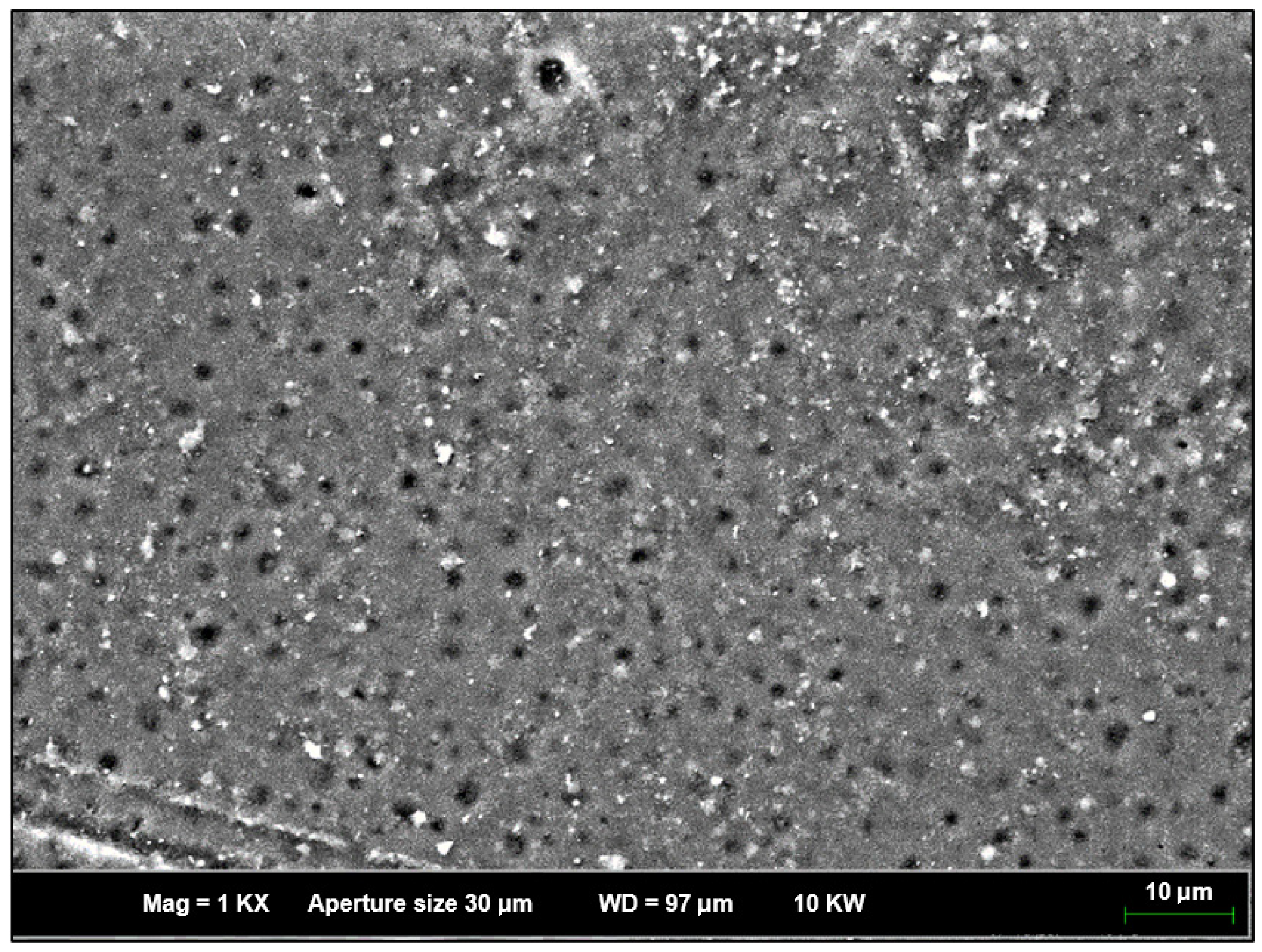
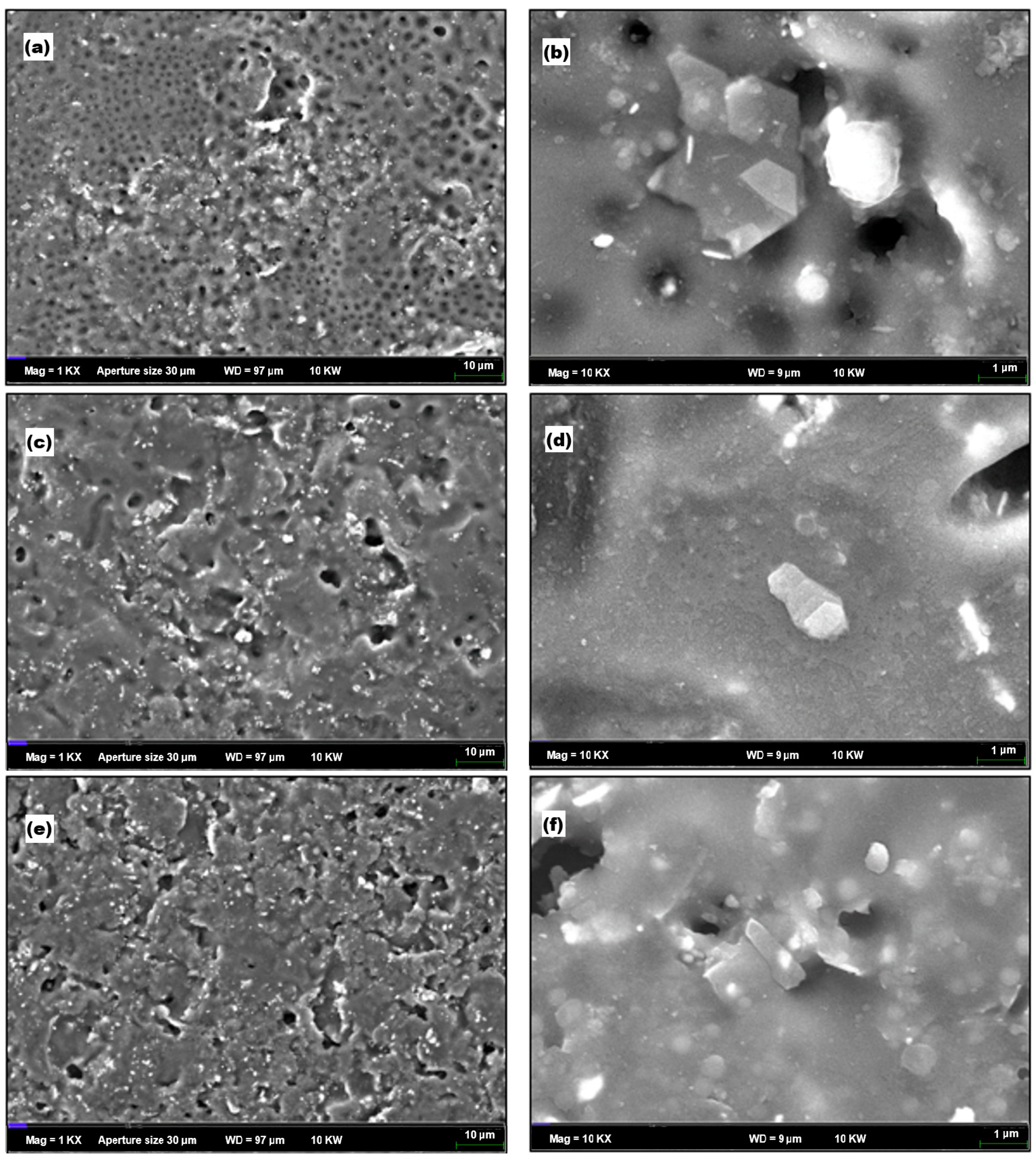
3.8. Field Emission Scanning Microscopy (FESEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bee, S.-T.; Sin, L.T.; Ratnam, C.T.; Chew, W.S.; Rahmat, A.R. Enhancement Effect of Trimethylopropane Trimethacrylate on Electron Beam Irradiated Acrylonitrile Butadiene Styrene (ABS). Polym. Bull. 2018, 75, 5015–5037. [Google Scholar] [CrossRef]
- Chandra, R.B.J.; Shivamurthy, B.; Kumar, M.S.; Prabhu, N.N.; Sharma, D. Mechanical and Electrical Properties and Electromagnetic-Wave-Shielding Effectiveness of Graphene-Nanoplatelet-Reinforced Acrylonitrile Butadiene Styrene Nanocomposites. J. Compos. Sci. 2023, 7, 117. [Google Scholar] [CrossRef]
- Sharma, V.; Kapoor, S.; Goyal, M.; Jindal, P. Enhancement of the Mechanical Properties of Graphene Based Acrylonitrile Butadiene Styrene (ABS) Nanocomposites. Mater. Today Proc. 2020, 28, 1744–1747. [Google Scholar] [CrossRef]
- Sheheri, S.Z.A.; Al-Amshany, Z.M.; Sulami, Q.a.A.; Tashkandi, N.Y.; Hussein, M.A.; El-Shishtawy, R.M. The Preparation of Carbon Nanofillers and Their Role on the Performance of Variable Polymer Nanocomposites. Des. Monomers Polym. 2019, 22, 8–53. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Rodríguez-Fernández, O.; Acuña, P. Effect of Zinc Oxide Nanoparticles Concentration on the Mechanical Properties and UV Protection of in Situ Synthesized ABS Based Nanocomposites. Macromol. Symp. 2013, 325–326, 147–155. [Google Scholar] [CrossRef]
- Shueb, N.M.I.; Manaf, N.M.E.A.; Mohamed, N.M.; Mohamad, N.N.; Razak, N.J.A.; Sani, N.N.A.; Umar, N.K. nor K. Thermal Characterization of Non-Functionalized Low Content Graphene Nanoplatelets (GNP) Added Nylon 66 Polymer. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 89, 13–25. [Google Scholar] [CrossRef]
- Shueb, M.I.; Manaf, M.E.A.; Ratnam, C.T.; Mohamad, N.; Mohamed, M. Enhancement of Mechanical and Electrical Properties in Graphene Nanoplatelet Modified Nylon 66. Malays. J. Compos. Sci. Manuf. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Jiang, D.; Murugadoss, V.; Wang, Y.; Lin, J.; Ding, T.; Wang, Z.; Shao, Q.; Wang, C.; Liu, H.; Lu, N.; et al. Electromagnetic Interference Shielding Polymers and Nanocomposites—A Review. Polym. Rev. 2019, 59, 280–337. [Google Scholar] [CrossRef]
- Mohseni, K. Surface Tension, Capillarity and Contact Angle. In Encyclopedia of Microfluidics and Nanofluidics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1949–1956. [Google Scholar]
- Joynal Abedin, F.N.; Fizal, A.N.S.; Alkarkhi, A.F.M.; Khalil, N.A.; Ahmad Yahaya, A.N.; Hossain, M.S.; Safie, S.I.; Ismail, N.A.; Zulkifli, M. Synergistic Reinforcement with SEBS-g-MAH for Enhanced Thermal Stability and Processability in GO/rGO-Filled PC/ABS Composites. Polymers 2024, 16, 2554. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kwon, D.-J.; Lim, C.-S.; Seo, B.-K.; DeVries, K.L.; Park, J.-M. Interfacial Adhesion Evaluation via Wettability for Fiber Reinforced Polymer Composites: A Review. Compos. Interfaces 2023, 30, 283–299. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Baluschev, S.; Eftimov, P.; Bacheva, M.; Landfester, K. Addressing the Apparent Controversies Between the Contact Angle-Based Models for Estimation of Surface Free Energy: A Critical Review. Colloids Interfaces 2024, 8, 62. [Google Scholar] [CrossRef]
- Rohman, N.; Mohiuddin, T.; Al-Ruqeishi, M.S. Surface Free Energy of Graphene-Based Coatings and Its Component Elements. Inorg. Chem. Commun. 2023, 153, 110855. [Google Scholar] [CrossRef]
- Ford, J. Thermal Analysis—Fundamentals and Applications to Polymer Science, 2nd Edition, Edited by T. Hatakeyama and F.X. Quinn, Wiley, Chichester, 1999, Viii+180 Pages. ISBN 0-471-98362-4; £55.00. Talanta 2000, 51, 411. [Google Scholar] [CrossRef]
- Bifulco, A.; Varganici, C.; Rosu, L.; Mustata, F.; Rosu, D.; Gaan, S. Recent Advances in Flame Retardant Epoxy Systems Containing Non-Reactive DOPO Based Phosphorus Additives. Polym. Degrad. Stab. 2022, 200, 109962. [Google Scholar] [CrossRef]
- Ratwani, C.R.; Kamali, A.R.; Abdelkader, A.M. Self-healing by Diels-Alder cycloaddition in advanced functional polymers: A review. Prog. Mater. Sci. 2023, 131, 101001. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Ligand extension of aluminum fumarate metal-organic framework in transferring higher water for adsorption desalination. Desalination 2024, 592, 118135. [Google Scholar] [CrossRef]
- Han, B.; Gabriel, J.C.P. Thin-film nanocomposite (TFN) membrane technologies for the removal of emerging contaminants from wastewater. J. Clean. Prod. 2024, 480, 144043. [Google Scholar] [CrossRef]
- Szalóki, M.; Szabó, Z.; Martos, R.; Csík, A.; Szőllősi, G.J.; Hegedűs, C. The Surface Free Energy of Resin-Based Composite in Context of Wetting Ability of Dental Adhesives. Appl. Sci. 2023, 13, 12061. [Google Scholar] [CrossRef]
- George, E.; Joy, J.; Vijayan, P.P.; Sarath, P.S.; George, S.C.; Anas, S. Development, Characterization, and Tribological Behavior of Polymeric Carbon Nitride/Acrylonitrile Butadiene Styrene Nanocomposites. Polym. Compos. 2021, 43, 848–861. [Google Scholar] [CrossRef]
- Heo, C.; Moon, H.; Yoon, C.S.; Chang, J. ABS Nanocomposite Films Based on Functionalized-graphene Sheets. J. Appl. Polym. Sci. 2011, 124, 4663–4670. [Google Scholar] [CrossRef]
- Akrout, M.; Ben Difallah, B.; Kharrat, M.; Dammak, M.; Pereira, A.; Oliveira, F.J.; Duarte, I. On the Structural, Thermal, Micromechanical and Tribological Characterizations of Cu-Filled Acrylonitrile Butadiene Styrene Micro-Composites. Materials 2023, 16, 6428. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Recent Advances in Carbon-Based Polymer Nanocomposites for Electromagnetic Interference Shielding. Prog. Mater. Sci. 2019, 103, 319–373. [Google Scholar] [CrossRef]
- Ziąbka, M.; Dziadek, M.; Pielichowska, K. Surface and Structural Properties of Medical Acrylonitrile Butadiene Styrene Modified with Silver Nanoparticles. Polymers 2020, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.B.J.; Shivamurthy, B.; Kumar, M.S.; Thimmappa, B.H.S. Mechanical, Thermal and Electromagnetic Shielding Effectiveness of MWCN-ABS Films. Trans. Electr. Electron. Mater. 2021, 23, 228–236. [Google Scholar] [CrossRef]
- Pour, R.H.; Hassan, A.; Soheilmoghaddam, M.; Bidsorkhi, H.C. Mechanical, Thermal, and Morphological Properties of Graphene Reinforced Polycarbonate/Acrylonitrile Butadiene Styrene Nanocomposites. Polym. Compos. 2014, 37, 1633–1640. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization Is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, C.; Sousa, D.M.; Ferreira, A.R.; Moreira, J.A.; Gonçalves, I.C.; Magalhães, F.D. Smaller Particle Size and Higher Oxidation Improves Biocompatibility of Graphene-Based Materials. Carbon 2015, 99, 318–329. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Bourbigot, S.; Bras, M.L.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.V.; Nguyen, T.N.; Doan, T.H.; Thi, T.A.D.; Nguyen, H.T.; Hoang, M.H.; Huong, P.T.L.; Nguyen, V.T. Preparation of Flame Retardant Polycarbonate-acrylonitrile Butadiene Styrene Composite Using Graphene Nanoplatelets and Potassium Perfluorobutane Sulfonate Additives. ChemistrySelect 2023, 8, e202300594. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Lai, M.; Xue, Y.; Zhao, J. Synergistic Flame Retardancy of Aluminum Diethylphosphinate and Piperazine Pyrophosphate/Β-cyclodextrin in Polylactic Acid. J. Appl. Polym. Sci. 2024, 141, e55698. [Google Scholar] [CrossRef]
- Zailan, F.D.; Chen, R.S.; Ahmad, S.H.; Flaifel, M.H.; Shahdan, D.; Busu, W.N.W.; Yu, L.J. Synergistic Improvement of Mechanical, Electrical and Thermal Properties by Graphene Nanoplatelets in Polyaniline Incorporated Rubbery Thermoplastic Composites. J. Mater. Res. Technol. 2024, 28, 4097–4109. [Google Scholar] [CrossRef]
- Abuoudah, C.K.; Greish, Y.E.; Abu-Jdayil, B.; El-said, E.M.; Iqbal, M.Z. Graphene/Polypropylene Nanocomposites with Improved Thermal and Mechanical Properties. J. Appl. Polym. Sci. 2020, 138, 50024. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, G.; Ding, Y.; Zheng, J. Construction of a Different Polymer Chain Structure to Study π-π Interaction between Polymer and Reduced Graphene Oxide. Polymers 2018, 10, 716. [Google Scholar] [CrossRef]
- Huang, G.; Huo, S.; Xu, X.; Chen, W.; Jin, Y.; Li, R.; Song, P.; Wang, H. Realizing Simultaneous Improvements in Mechanical Strength, Flame Retardancy and Smoke Suppression of ABS Nanocomposites from Multifunctional Graphene. Compos. Part B Eng. 2019, 177, 107377. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Zhu, Q.; Ong, P.J.; Goh, S.H.A.; Yeo, R.J.; Wang, S.; Liu, Z.; Loh, X.J. Recent Advances in Graphene-Based Phase Change Composites for Thermal Energy Storage and Management. Nano Mater. Sci. 2023, 6, 115–138. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical Methods for the Production of Graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent Advances in TiO2-Functionalized Textile Surfaces. Surf. Interfaces 2020, 22, 100890. [Google Scholar] [CrossRef]
- Mergen, Ö.B.; Arda, E. Electrical, Optical and Dielectric Properties of Polyvinylpyrrolidone/Graphene Nanoplatelet Nanocomposites. Opt. Mater. 2023, 139, 113823. [Google Scholar] [CrossRef]
- Amiri, P.; Shirazi, M.; Aliakbari, A.; Salehi, H. A Gap Opening in Graphene Covered by a Conducting Polymer: The Role of Many-Body Effects. Phys. B Condens. Matter 2021, 614, 413036. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-Reduction of Graphite- and Graphene Oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Gao, L.; Liu, B.; Jiang, C.; Cheng, H.-M. Synthesis of High-Quality Graphene with a Pre-Determined Number of Layers. Carbon 2008, 47, 493–499. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-Based Nanostructured Materials and Their Composites as Supercapacitor Electrodes. J. Mater. Chem. 2011, 22, 767–784. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, Rippled and Crumpled Graphene: An Overview of Formation Mechanism, Electronic Properties, and Applications. Mater. Today 2015, 19, 197–212. [Google Scholar] [CrossRef]
- Wang, J.; Song, F.; Ding, Y.; Shao, M. The Incorporation of Graphene to Enhance Mechanical Properties of Polypropylene Self-Reinforced Polymer Composites. Mater. Des. 2020, 195, 109073. [Google Scholar] [CrossRef]
- Maruzhenko, O.; Mamunya, Y.; Boiteux, G.; Pusz, S.; Szeluga, U.; Pruvost, S. Improving the Thermal and Electrical Properties of Polymer Composites by Ordered Distribution of Carbon Micro- and Nanofillers. Int. J. Heat Mass Transf. 2019, 138, 75–84. [Google Scholar] [CrossRef]
- Jyoti, J.; Arya, A.K. EMI Shielding and Dynamic Mechanical Analysis of Graphene Oxide-Carbon Nanotube-Acrylonitrile Butadiene Styrene Hybrid Composites. Polym. Test. 2020, 91, 106839. [Google Scholar] [CrossRef]
- Badia, J.D.; Teruel-Juanes, R.; Echegoyen, Y.; Torres-Giner, S.; Lagarón, J.M.; Ribes-Greus, A. Effect of Graphene Nanoplatelets on the Dielectric Permittivity and Segmental Motions of Electrospun Poly(Ethylene-Co-Vinyl Alcohol) Nanofibers. Polym. Degrad. Stab. 2020, 183, 109404. [Google Scholar] [CrossRef]
- Ke, K.; McMaster, M.; Christopherson, W.; Singer, K.D.; Manas-Zloczower, I. Effects of Branched Carbon Nanotubes and Graphene Nanoplatelets on Dielectric Properties of Thermoplastic Polyurethane at Different Temperatures. Compos. Part B Eng. 2019, 166, 673–680. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Ibnaouf, K.H.; Eisa, M.H.; Lam, T.D. Graphene Nanocomposites for Electromagnetic Interference Shielding—Trends and Advancements. J. Compos. Sci. 2023, 7, 384. [Google Scholar] [CrossRef]
- Yan, W.; Qin, S.; Guo, J.; Zhang, M.; He, M.; Yu, J. Morphology and Mechanical Properties of Acrylonitrile-Butadiene-Styrene (ABS)/Polyamide 6 (PA6) Nanocomposites Prepared via Melt Mixing. J. Macromol. Sci. Part B 2011, 51, 70–82. [Google Scholar] [CrossRef]
- Li, Y.; Pan, D.; Chen, S.; Wang, Q.; Pan, G.; Wang, T. In Situ Polymerization and Mechanical, Thermal Properties of Polyurethane/Graphene Oxide/Epoxy Nanocomposites. Mater. Des. 2013, 47, 850–856. [Google Scholar] [CrossRef]
- Suresha, R.; Sachidananda, H.K.; Shivamurthy, B.; Swamy, N.K.; Parasuram, S. Mechanical and Electromagnetic Shielding Properties of Carbon Fabric with Graphene Nanoplatelets Reinforced Epoxy Composites. Sci. Rep. 2025, 15, 634. [Google Scholar] [CrossRef]
- Salehiyan, R.; El-Samak, A.A.; Kamkar, M.; Erfanian, E.; Hodge, S.A.; Sundararaj, U.; McNally, T. Unveiling the Significance of Graphene Nanoplatelet (GNP) Localization in Tuning the Performance of PP/HDPE Blends. Materials 2024, 17, 5673. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K. Structure and Properties of Poly(Vinyl Chloride)/Graphene Nanocomposites. Polym. Test. 2019, 81, 106282. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution States of Graphene in Polymer Nanocomposites: A Review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
- Mathur, R.B.; Pande, S.; Singh, B.P.; Dhami, T.L. Electrical and Mechanical Properties of Multi-walled Carbon Nanotubes Reinforced PMMA and PS Composites. Polym. Compos. 2008, 29, 717–727. [Google Scholar] [CrossRef]
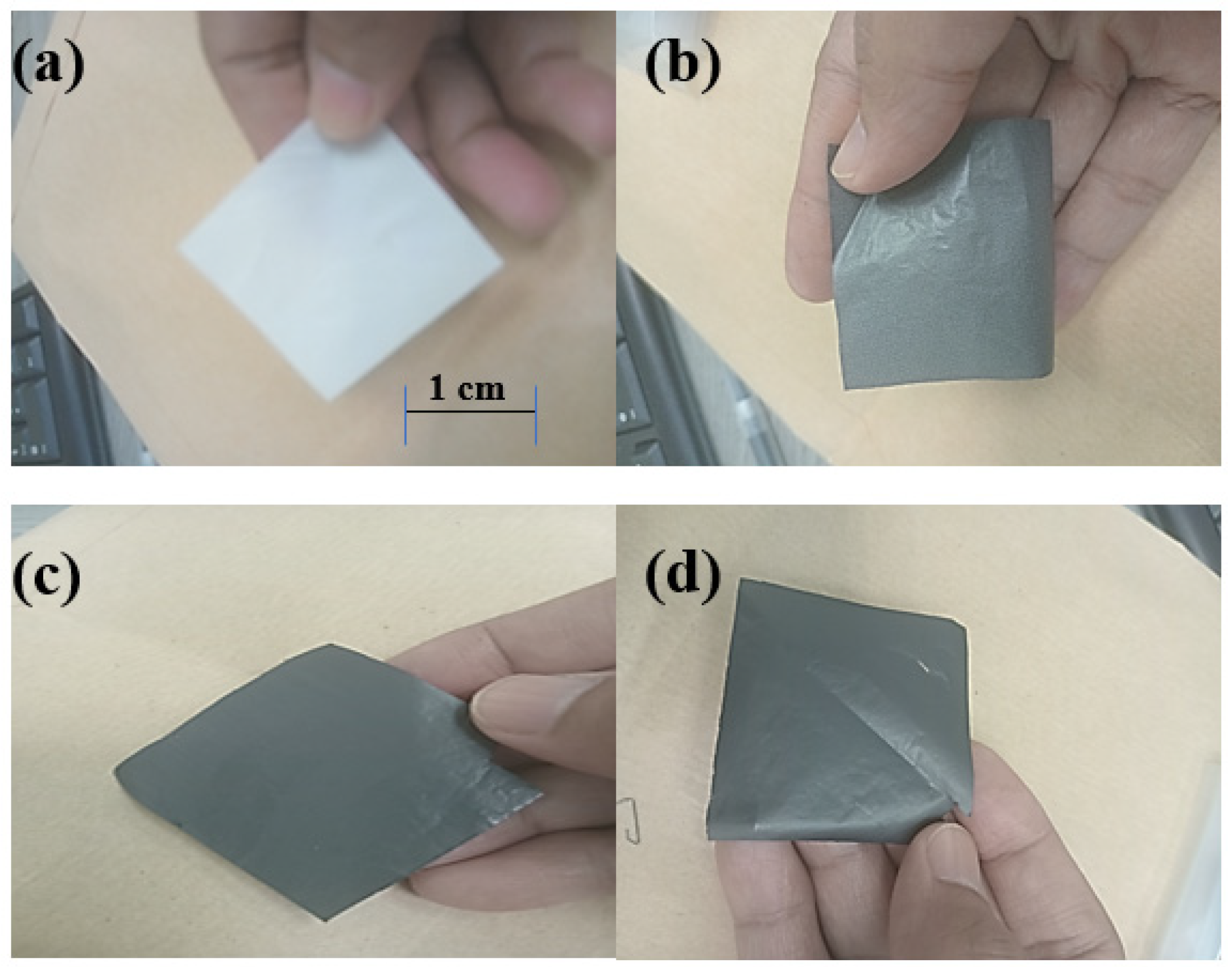

| Sample | Formulation (wt%) | |
|---|---|---|
| GNPs | ABS | |
| Control sample | 0 | 100 |
| ABS-GNP 1 | 1 | 99 |
| ABS-GNP 3 | 3 | 97 |
| ABS-GNP 5 | 5 | 95 |
| Samples | Contact Angle (°) | Surface Energy (mN/m) |
|---|---|---|
| pure ABS | 74.14 | 39.87 |
| ABS-GNP 1% | 66.54 | 44.12 |
| ABS-GNP 3% | 88.76 | 30.14 |
| ABS-GNP 5% | 92.61 | 27.63 |
| Samples | T20 (°C) | T50 (°C) | T80 (°C) |
|---|---|---|---|
| Pure ABS | 345 | 396.67 | 472.53 |
| ABS-GNP 1% | 354.17 | 402.47 | 431.69 |
| ABS-GNP 3% | 372.50 | 407.51 | 432.51 |
| ABS-GNP 5% | 362.47 | 397.47 | 422.47 |
| Samples | Tg (°C) |
|---|---|
| Pure ABS | 109.82 |
| ABS-GNP 1% | 110.75 |
| ABS-GNP 3% | 109.94 |
| ABS-GNP 5% | 123.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shueb, M.I.; Mohamad, N.; Sapuan, S.Z.; Khee, Y.S.; Che Halin, D.S.; Sandu, A.V.; Vizureanu, P. Graphene Nanoplatelets Reinforced ABS Nanocomposite Films by Sonication-Assisted Cast Film Technique for Emission Shielding Application. Materials 2025, 18, 2645. https://doi.org/10.3390/ma18112645
Shueb MI, Mohamad N, Sapuan SZ, Khee YS, Che Halin DS, Sandu AV, Vizureanu P. Graphene Nanoplatelets Reinforced ABS Nanocomposite Films by Sonication-Assisted Cast Film Technique for Emission Shielding Application. Materials. 2025; 18(11):2645. https://doi.org/10.3390/ma18112645
Chicago/Turabian StyleShueb, Mohammed Iqbal, Noraiham Mohamad, Syarfa Zahirah Sapuan, Yee See Khee, Dewi Suriyani Che Halin, Andrei Victor Sandu, and Petrica Vizureanu. 2025. "Graphene Nanoplatelets Reinforced ABS Nanocomposite Films by Sonication-Assisted Cast Film Technique for Emission Shielding Application" Materials 18, no. 11: 2645. https://doi.org/10.3390/ma18112645
APA StyleShueb, M. I., Mohamad, N., Sapuan, S. Z., Khee, Y. S., Che Halin, D. S., Sandu, A. V., & Vizureanu, P. (2025). Graphene Nanoplatelets Reinforced ABS Nanocomposite Films by Sonication-Assisted Cast Film Technique for Emission Shielding Application. Materials, 18(11), 2645. https://doi.org/10.3390/ma18112645









