Mitigation of Volume Changes in Alkali-Activated Slag by Using Metakaolin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositions
2.2.1. Reference Composition
2.2.2. Metakaolin Substitution
2.3. Methods and Devices
2.3.1. Slump Flow
2.3.2. Compressive Strength
2.3.3. Apparent Density
2.3.4. Isothermal Calorimetry
2.3.5. Autogenous Strains and Coefficient of Thermal Expansion
3. Results and Discussions
3.1. Preliminary Campaign
3.2. In-Depth Investigation
3.2.1. Isothermal Calorimetry
3.2.2. Autogenous Strain
3.2.3. Coefficient of Thermal Expansion
4. Conclusions and Perspectives
- A preliminary study investigated different metakaolin substitution rates in terms of compressive strength and workability. Five compositions were selected and examined in detail. MA1 and MB1 exhibited a compressive strength increase of 122% and 146%, respectively, at 7 days for a 10% substitution rate. In contrast, MD1 showed a decrease of 28%, already indicating the important role of the activator. In addition, MB2 and MB3 showed a remarkable 380 and 650% increase in the compressive strength for a 20% and 30% metakaolin substitution rate, compared to the reference composition. However, the workability decreased as the metakaolin substitution rate increased.
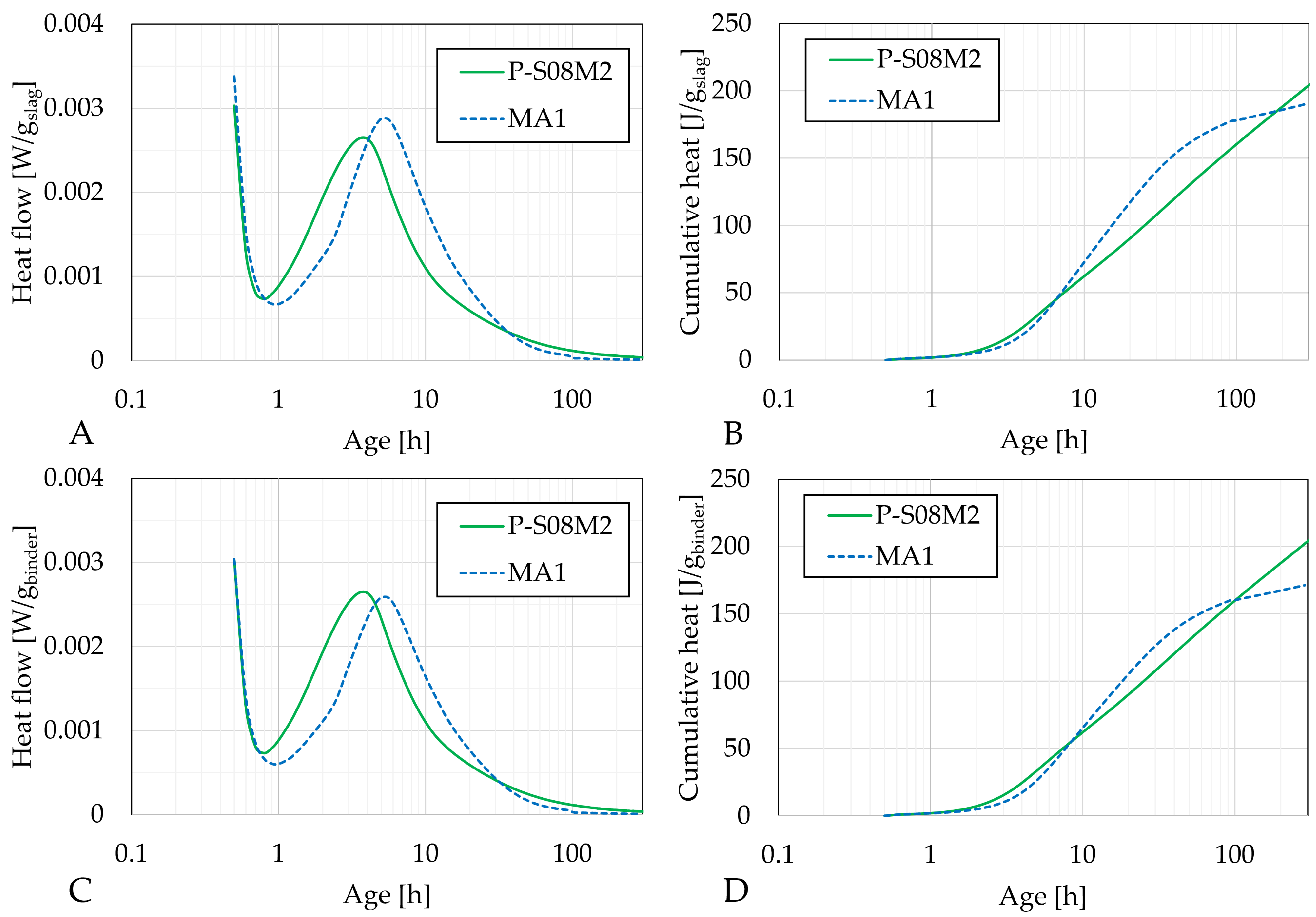
- Isothermal calorimetry results indicated that metakaolin does affect the heat release. At a 10% substitution rate, compositions activated with 2 M NaOH and 10 M sodium silicate had a slightly delayed second heat flow peak. In contrast, compositions activated with 8 M NaOH exhibited the appearance of a third peak. For the latter, as the substitution rate was increased, the second peak was decreased and delayed while the third peak increased. The reduction in and delay of the heat flow evolution might be related to the dilution effect caused by the presence of metakaolin in the mix. After this, aluminum-rich products are formed. Finally, the third peak might be related to the polymerization and reorganization of the previously formed entities.
- The autogenous swelling increased with a 10% metakaolin substitution. Later on, the subsequent shrinkage appeared at a constant rate. Compositions MB2 and MB3 exhibited very similar autogenous strain developments: no apparent swelling was observed and the autogenous shrinkage was significantly increased. The start of the increase in shrinkage rate coincides with the appearance of the third peak in the heat flow. At 300 h, only a 5% difference was observed between the two compositions. The effect of metakaolin on the autogenous strain development is strongly dependent on the activator: while a sodium hydroxide activation altered the behavior of the autogenous strain development, the autogenous train of sodium silicate activated composition was significantly reduced. This explains the mitigating effect of metakaolin observed in the literature with precursors activated with sodium hydroxide and sodium silicate.
- The effect of the metakaolin substitution on the CTE was also dependent on the activator and the substitution rate. A 10% substitution rate caused a delay in the development of the CTE, possibly due to the dilution effect. In addition, these compositions stabilized higher than the reference composition when activated by sodium hydroxide. Substitution rates of 20% (MB2) and 30% (MB3) in the reference composition P-S08M8 led to a faster CTE increase with respect to age. However, MB3 slightly decreased again, while MB2 remained constant for the rest of the test. This fast increase started around the time the third peak in the heat flow appeared.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAMs | Alkali-activated materials |
| AAS | Alkali-activated slag |
| CTE | Coefficient of thermal expansion |
| DOR | Degree of reaction |
| M | Concentration in mol/L |
| MK | Metakaolin |
| PC | Portland cement |
| q | Heat flow |
| Q | Cumulative heat |
| S/B | Solution-to-binder mass ratio |
Appendix A
| P-S08M2 | MA1 | P-S08M8 | MB1 | MB2 | MB3 | P-S08NS10 | MD1 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Per of g of slag | Q∞,1,slag | a | 3071.20 | −345.42 | 4283.10 | 15,437.00 | 11,693.00 | 10,684.00 | 3762.60 | 2129.40 |
| b | −1490.30 | −325.90 | −1823.60 | −3935.10 | −5250.30 | −6792.70 | −1972.10 | −1825.20 | ||
| c = Q∞,1,slag | 278.59 | 211.65 | 368.35 | 494.60 | 770.22 | 993.76 | 376.00 | 421.18 | ||
| R2 | 0.9990 | 0.9936 | 0.9958 | 0.9214 | 0.9417 | 0.9856 | 0.9994 | 0.9994 | ||
| Per of g of slag | Q∞,2,slag | Q1 | 31.96 | 46.06 | 288.94 | 354.13 | 541.80 | 189.25 | 73.88 | 102.91 |
| 1 | 4.57 | 4.48 | 8.36 | 9.48 | 20.97 | 98.62 | 20.84 | 27.07 | ||
| a1 | 1.60 | 0.99 | 0.55 | 0.54 | 0.44 | 3.22 | 2.11 | 2.14 | ||
| Q2 | 295.36 | 148.71 | 185.32 | 105.23 | 97.93 | 727.83 | 1159.34 | 1052.72 | ||
| 2 | 62.94 | 12.42 | 816.50 | 98,887.57 | 111.95 | 51.62 | 9085.82 | 4229.70 | ||
| a2 | 0.39 | 1.07 | 0.62 | 0.64 | 4.52 | 0.39 | 0.16 | 0.17 | ||
| error | 31.50 | 213.87 | 46.82 | 143,420.47 | 310.17 | 5605.96 | 1035.46 | 1593.49 | ||
| Q∞,2,slag | 327.32 | 194.77 | 474.26 | 459.36 | 639.73 | 917.08 | 1233.22 | 1155.64 | ||
| Per of g of binder | Q∞,2,binder | a | 3071.20 | −310.88 | 4283.10 | 13,893.00 | 9354.60 | 7478.50 | 3762.60 | 1916.50 |
| b | −1490.30 | −293.31 | −1823.60 | −3541.60 | −4200.20 | −4754.90 | −1972.10 | −1642.70 | ||
| c = Q∞,1,binder | 278.59 | 190.49 | 368.35 | 445.05 | 616.18 | 695.63 | 376.00 | 379.06 | ||
| R2 | 0.9990 | 0.9936 | 0.9958 | 0.9417 | 0.9817 | 0.9856 | 0.9994 | 0.9994 | ||
| Per of g of binder | Q∞,2,binder | Q1 | 31.96 | 133.84 | 288.94 | 137.78 | 433.44 | 530.19 | 73.88 | 92.62 |
| 1 | 4.57 | 12.42 | 8.36 | 5.05 | 20.97 | 56.83 | 20.84 | 27.07 | ||
| a1 | 1.60 | 1.07 | 0.55 | 1.06 | 0.44 | 0.38 | 2.11 | 2.14 | ||
| Q2 | 295.36 | 41.45 | 185.32 | 405.66 | 78.34 | 128.39 | 1159.34 | 947.45 | ||
| 2 | 62.94 | 4.48 | 816.50 | 373.21 | 111.95 | 98.59 | 9085.82 | 4229.70 | ||
| a2 | 0.39 | 0.99 | 0.62 | 0.23 | 4.52 | 3.31 | 0.16 | 0.17 | ||
| error | 31.50 | 173.24 | 46.82 | 12,458.33 | 198.51 | 2756.02 | 1035.46 | 1290.73 | ||
| Q∞,2,binder | 327.32 | 175.29 | 474.26 | 543.44 | 511.79 | 658.58 | 1233.22 | 1040.07 |
Appendix B

References
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Oxon, UK, 2006. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. (Eds.) Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Cambridge, UK; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Lacante, M.; Delsaute, B.; Gambacorta, J.; Königsberger, M.; Staquet, S. Development of early age autogenous and thermal strains of alkali-activated slag-fly ash pastes. Front. Built Environ. 2022, 8, 1085912. [Google Scholar] [CrossRef]
- Lacante, M.; Delsaute, B.; Staquet, S. Effect of Solution-to-Binder Ratio and Molarity on Volume Changes in Slag Binder Activated by Sodium Hydroxide at Early Age. Materials 2024, 17, 3308. [Google Scholar] [CrossRef]
- Lacante, M.; Delsaute, B.; Aggelis, D.G.; Staquet, S. Volume changes and acoustic emission study of alkali-activated slag pastes in free and sealed conditions. Dev. Built Environ. 2025; submitted. [Google Scholar]
- Lacante, M.; Delsaute, B.; Staquet, S. Effect of Curing Temperature on Volume Changes of Alkali-Activated Slag Pastes. Materials 2025, 18, 1073. [Google Scholar] [CrossRef]
- Carette, J. Towards Early Age Characterisation of Eco-Concrete Containing Blast-Furnace Slag and Limestone Filler. Ph.D. Thesis, Université libre de Bruxelles, Brussels, Belgium, 2015. [Google Scholar]
- Gleize, P.J.; Cyr, M.; Escadeillas, G. Effects of metakaolin on autogenous shrinkage of cement pastes. Cem. Concr. Compos. 2007, 29, 80–87. [Google Scholar] [CrossRef]
- Lacante, M.; Delsaute, B.; Staquet, S. Mitigation of volume changes of alkali-activated materials by using limestone filler. Materials, 2025; submitted. [Google Scholar]
- Rosa, L.M.; Utzig, J.; Meier, H.F. Towards Green Cements: The Metakaolin Route. Asp. Min. Miner. Sci. 2020, 5, 602. [Google Scholar] [CrossRef]
- San Nicolas, R.; Cyr, M.; Escadeillas, G. Characteristics and applications of flash metakaolins. Appl. Clay Sci. 2013, 83–84, 253–262. [Google Scholar] [CrossRef]
- Salvador, S.; Pons, O. A semi-mobile flash dryerrcalciner unit to manufacture pozzolana from raw clay soils application to soil stabilisation. Constr. Build. Mater. 2000, 14, 109–117. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Bernal, S.A. Microstructural Changes Induced by CO2 Exposure in Alkali-Activated Slag/Metakaolin Pastes. Front. Mater. 2016, 3, 43. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Shi, C.; Li, N.; Jiao, D.; Yuan, Q. Rheology of alkali-activated materials: A review. Cem. Concr. Compos. 2021, 121, 104061. [Google Scholar] [CrossRef]
- Cai, R.; Tian, Z.; Ye, H.; He, Z.; Tang, S. The role of metakaolin in pore structure evolution of Portland cement pastes revealed by an impedance approach. Cem. Concr. Compos. 2021, 119, 103999. [Google Scholar] [CrossRef]
- Kishore, K.; Gupta, N. Mechanical characterization and assessment of composite geopolymer concrete. Mater. Today Proc. 2021, 44, 58–62. [Google Scholar] [CrossRef]
- Jithendra, C.; Dalawai, V.N.; Elavenil, S. Effects of metakaolin and sodium silicate solution on workability and compressive strength of sustainable Geopolymer mortar. Mater. Today Proc. 2022, 51, 1580–1584. [Google Scholar] [CrossRef]
- Tognonvi, T.M.; Balaguer Pascual, A.; Tagnit-Hamou, A. Physico-chemistry of geopolymers based on recycled glass powder and metakaolin: Effect of metakaolin content. Mater. Today Proc. 2022, 58, 1508–1514. [Google Scholar] [CrossRef]
- Alanazi, H.; Yang, M.; Zhang, D.; Gao, Z.J. Bond strength of PCC pavement repairs using metakaolin-based geopolymer mortar. Cem. Concr. Compos. 2016, 65, 75–82. [Google Scholar] [CrossRef]
- Lu, K.; Wang, B.; Han, Z.; Ji, R. Experimental study of magnesium ammonium phosphate cements modified by fly ash and metakaolin. J. Build. Eng. 2022, 51, 104137. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.d.; Matos, P.R.d.; Monteiro, S.N.; Vieira, C.M.F. Rheological and the Fresh State Properties of Alkali-Activated Mortars by Blast Furnace Slag. Materials 2021, 14, 2069. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, X.; Chen, Y.; Ye, G. Effect of metakaolin on the autogenous shrinkage of alkali-activated slag-fly ash paste. Constr. Build. Mater. 2021, 278, 122397. [Google Scholar] [CrossRef]
- Kocak, Y. Effects of metakaolin on the hydration development of Portland—Composite cement. J. Build. Eng. 2020, 31, 101419. [Google Scholar] [CrossRef]
- Abbas, M.S.; Mahfoud, T.; Zenati, A. Apparent activation energy determination of metakaolin-cement binders. In Proceedings of the 10th ACI/RILEM International Conference of Cementitious Materials and Alternative Binders for Sustainable Concrete, Montreal, QC, Canada, 2–4 October 2017. [Google Scholar]
- Lagier, F.; Kurtis, K.E. Influence of Portland cement composition on early age reactions with metakaolin. Cem. Concr. Res. 2007, 37, 1411–1417. [Google Scholar] [CrossRef]
- Wild, S.; Khatib, J.M.; Roose, L.J. Chemical shrinkage and autogenous shrinkage of Portland cement-metakaolin pastes. Adv. Cem. Res. 1998, 10, 109–119. [Google Scholar] [CrossRef]
- Akcay, B.; Tasdemir, M.A. Autogenous Shrinkage, Pozzolanic Activity and Mechanical Properties of Metakaolin Blended Cementitious Materials. KSCE J. Civ. Eng. 2019, 23, 4727–4734. [Google Scholar] [CrossRef]
- EN 196-6:2018; Methods of Testing Cement—Part 6: Determination of Fineness. European Committee for Standardization: Brussels, Belgium, 2018.
- Nedeljković, M.; Li, Z.; Ye, G. Setting, Strength, and Autogenous Shrinkage of Alkali-Activated Fly Ash and Slag Pastes: Effect of Slag Content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V.; Mounanga, P.; Khelidj, A.; Loukili, A.; Rafaï, N. Autogenous deformations of cement pastes. Cem. Concr. Res. 2006, 36, 123–136. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Abdullah, M.M.A.B.; Cheng-Yong, H.; Hussin, K. Effect of Sodium Hydroxide Molarity on Physical, Mechanical and Thermal Conductivity of Metakaolin Geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 343, 012015. [Google Scholar] [CrossRef]
- Abdul Rahi, R.; Azizli, K.; Man, Z.; Rahmiati, T.; Nuruddin, M. Effect of Sodium Hydroxide Concentration on the Mechanical Property of Non Sodium Silicate Fly Ash Based Geopolymer. J. Appl. Sci. 2014, 14, 3381–3384. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- ASTM C230/C230M-08; Standard Specification for Flow Table for Use in Tests of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2008.
- Naqi, A.; Delsaute, B.; Königsberger, M.; Staquet, S. Effect of Solution-to-Binder Ratio and Alkalinity on Setting and Early-Age Properties of Alkali-Activated Slag-Fly Ash Binders. Materials 2022, 16, 373. [Google Scholar] [CrossRef] [PubMed]
- Delsaute, B. New Approach for Monitoring and Modelling of the Creep and Shrinkage Behaviour of Cement Pastes, Mortars and Concretes Since Setting Time. Ph.D. Thesis, Université libre de Bruxelles, BATir, Brussels, Belgium, Université Paris-Est, Ifsttar, France, 2016. [Google Scholar]
- ASTM C109/C109M-16a; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2016.
- EN 196-11:2018; Methods of Testing Cement—Part 11: Heat of Hydration—Isothermal Conduction Calorimetry Method. European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- TA Instruments. TAM Air Calorimeter Operator’s Manual; Technical Report; TA Instruments: New Castle, DE, USA, 2006. [Google Scholar]
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A review on alkali-activated binders: Materials composition and fresh properties of concrete. Constr. Build. Mater. 2020, 260, 119788. [Google Scholar] [CrossRef]
- EN 196-3:2016; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- ASTM C 1698-09; Test Method for Autogenous Strain of Cement Paste and Mortar. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Delsaute, B.; Staquet, S. Decoupling Thermal and Autogenous Strain of Concretes with Different Water/Cement Ratios During the Hardening Process. Adv. Civ. Eng. Mater. 2017, 6, 20160063. [Google Scholar] [CrossRef]
- Serdar, M.; Gabrijel, I.; Schlicke, D.; Staquet, S.; Azenha, M. (Eds.) Advanced Techniques for Testing of Cement-Based Materials; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Elmaasrawy, M.; Lan, S.; Cao, H.; Li, S.; Gao, X. Microstructure and durability evaluation of slag-metakaolin-limestone ternary blended alkali activated UHPC. J. Build. Eng. 2025, 106, 112674. [Google Scholar] [CrossRef]
- Li, Z.; Nedeljković, M.; Chen, B.; Ye, G. Mitigating the autogenous shrinkage of alkali-activated slag by metakaolin. Cem. Concr. Res. 2019, 122, 30–41. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Ren, J. Utilisation of municipal solid waste incinerator (MSWI) fly ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 123451. [Google Scholar] [CrossRef]
- Rattanasak, U.; Pankhet, K.; Chindaprasirt, P. Effect of chemical admixtures on properties of high-calcium fly ash geopolymer. Int. J. Miner. Metall. Mater. 2011, 18, 364–369. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Ma, B.; Wu, B. Effect of MSWI fly ash and incineration residues on cement performances. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010, 25, 312–315. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Li, Z.; Alfredo Flores Beltran, I.; Chen, Y.; Šavija, B.; Ye, G. Early-age properties of alkali-activated slag and glass wool paste. Constr. Build. Mater. 2021, 291, 123326. [Google Scholar] [CrossRef]
- Chen, W.; Xie, Y.; Li, B.; Li, B.; Wang, J.; Thom, N. Role of aggregate and fibre in strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2021, 299, 124002. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Ruengsillapanun, K.; Udtaranakron, T.; Pulngern, T.; Tangchirapat, W.; Jaturapitakkul, C. Mechanical properties, shrinkage, and heat evolution of alkali activated fly ash concrete. Constr. Build. Mater. 2021, 299, 123954. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Fernández-jiménez, A.; Krivenko, P.V.; Kavalerova, E.; Palacios, M.; Shi, C. Alkali Activated Materials; RILEM State-of-the-Art Reports; Springer Netherlands: Dordrecht, The Netherlands, 2014; Volume 13, pp. 59–91. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Early age reaction, rheological properties and pore solution chemistry of NaOH-activated slag mixtures. Cem. Concr. Compos. 2022, 133, 104715. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; de Schutter, G. Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Kadri, E.H.; Kenai, S.; Ezziane, K.; Siddique, R.; De Schutter, G. Influence of metakaolin and silica fume on the heat of hydration and compressive strength development of mortar. Appl. Clay Sci. 2011, 53, 704–708. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Dainese, E.; Brouwers, H.J. Autogenous and drying shrinkage of sodium carbonate activated slag altered by limestone powder incorporation. Constr. Build. Mater. 2017, 153, 459–468. [Google Scholar] [CrossRef]
- Li, R.; Lei, L.; Plank, J. Impact of metakaolin content and fineness on the behavior of calcined clay blended cements admixed with HPEG PCE superplasticizer. Cem. Concr. Compos. 2022, 133, 104654. [Google Scholar] [CrossRef]
- Li, Z.; Ye, G. Experimental Study of the Chemical Deformation of Metakaolin Based Geopolymer. In Proceedings of the SynerCrete’18: Interdisciplinary Approaches for Cement-based Materials and Structural Concrete: Synergizing Expertise and Bridging Scales of Space and Time Vol. 1 & 2, Funchal, Portugal, 24–26 October 2018; Volume 1, pp. 443–448. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Zuo, Y.; Chen, W.; Ye, G. Chemical deformation of metakaolin based geopolymer. Cem. Concr. Res. 2019, 120, 108–118. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Freiesleben Hansen, P.; Pedersen, E.J. Måleinstrument til control af betons hærdning. Nord. Betong 1977, 1, 21–25. [Google Scholar]
- Nielsen, C.V.; Kaasgaard, M. Activation Energy for the Concrete Maturity Model—Part 1: Compressive Strength Tests at Different Curing Temperatures. Nord. Concr. Res. 2020, 62, 87–106. [Google Scholar] [CrossRef]
- Delsaute, B.; Boulay, C.; Staquet, S. Creep testing of concrete since setting time by means of permanent and repeated minute-long loadings. Cem. Concr. Compos. 2016, 73, 75–88. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Tenório Filho, J.R.; Pereira Gomes de Araújo, M.A.; Snoeck, D.; De Belie, N. Discussing Different Approaches for the Time-Zero as Start for Autogenous Shrinkage in Cement Pastes Containing Superabsorbent Polymers. Materials 2019, 12, 2962. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Provis, J.L.; Cizer, O.; Ye, G. Autogenous shrinkage of alkali-activated slag: A critical review. Cem. Concr. Res. 2023, 172, 107244. [Google Scholar] [CrossRef]
- Tang, S.; Huang, D.; He, Z. A review of autogenous shrinkage models of concrete. J. Build. Eng. 2021, 44, 103412. [Google Scholar] [CrossRef]
- Uppalapati, S. Early-Age Structural Development and Autogenous Shrinkage of Alkali-Activated Slag/Fly Ash Cements. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2020. [Google Scholar]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Lolli, F.; Thomas, J.J.; Kurtis, K.E.; Cucinotta, F.; Masoero, E. Early age volume changes in metakaolin geopolymers: Insights from molecular simulations and experiments. Cem. Concr. Res. 2021, 144, 106428. [Google Scholar] [CrossRef]
- Canciam, C.A. Estudo do coefficiente de expansão térmica de soluções aquosas de soda cáustica. Rev. Eng. E Tecnol. 2014, 6, 72–79. [Google Scholar]
- Yeon, J.H.; Choi, S.; Won, M.C. Effect of Relative Humidity on Coefficient of Thermal Expansion of Hardened Cement Paste and Concrete. Transp. Res. Rec. J. Transp. Res. Board 2009, 2113, 83–91. [Google Scholar] [CrossRef]
- Sellevold, E.J.; Bjontegaard, O. Coefficient of thermal expansion of cement paste and concrete: Mechanisms of moisture interaction. Mater. Struct. 2006, 39, 809–815. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Shen, X.; Liu, S.; Li, H.; Li, B. Insights into autogenous shrinkage of alkali-activated slag under elevated curing temperature. Cem. Concr. Res. 2025, 190, 107803. [Google Scholar] [CrossRef]
- Dai, X.; Tao, Y.; Zhang, Y.; Ding, L.; Van Tittelboom, K.; De Schutter, G. Development of 3D printable alkali-activated slag-metakaolin concrete. Constr. Build. Mater. 2024, 444, 137775. [Google Scholar] [CrossRef]













| Material | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | TiO2 | SO3 | Na2O | BaO | MnO | SrO | V2O5 | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 34.20 | 12.86 | 0.35 | 39.95 | 0.62 | 7.91 | 1.13 | 1.88 | 0.50 | 0.13 | 0.30 | / | / | / | / |
| MK | 51.04 | 45.66 | 0.57 | 0.06 | 0.14 | 0.16 | 0.85 | 0.03 | 0.30 | / | / | 0.004 | 0.03 | 0.12 | 0.98 |
| Compositions | S/B Ratio [-] | Alkaline Solution | Concentration [mol/L] | Water/Alkaline Solution [-] |
|---|---|---|---|---|
| P-S05M2 | 0.5 | NaOH | 2 | 0 |
| P-S08M2 P-S08M8 | 0.8 | NaOH | 8 | 0 |
| P-S08NS10 | 0.8 | Na2SiO3 | 10 | 1/1 |
| ID | Compositions | S/B Ratio [-] | Metakaolin Ratio [%] | Alkaline Solution | Concentration [mol/L] | Water/Alkaline Solution [-] |
|---|---|---|---|---|---|---|
| MA1 | MK10S08M2 | 10 | ||||
| MA2 | MK20S08M2 | 0.8 | 20 | NaOH | 2 | 0 |
| MA3 | MK30S08M2 | 30 | ||||
| MB1 | MK10S08M8 | 10 | ||||
| MB2 | MK20S08M8 | 0.8 | 20 | NaOH | 8 | 0 |
| MB3 | MK30S08M8 | 30 | ||||
| MC1 | MK10S08M10 | 10 | ||||
| MC2 | MK20S08M10 | 0.8 | 20 | NaOH | 10 | 0 |
| MC3 | MK30S08M10 | 30 | ||||
| MD1 | MK10S08NS10 | 10 | ||||
| MD2 | MK20S08NS10 | 0.8 | 20 | Na2SiO3 | 10 | 1/1 |
| MD3 | MK30S08NS10 | 30 | ||||
| ME1 | MK10S05M2 | 0.5 | 10 | 2 | 0 | |
| ME2 | MK20S06M2 | 0.6 | 20 | NaOH | 0 | |
| ME3 | MK10S06M8 | 0.6 | 10 | 8 | 0 | |
| ME4 | MK10S07M8 | 0.7 | 10 | 0 |
| MK Substitution | P-S08M2 | P-S08M8 | P-S08NS10 | P-S05M2 |
|---|---|---|---|---|
| 10% | 121.8 | 146.2 | −17.4 | 12.5 |
| 20% | −27.5 | 379.5 | −21.8 | / |
| 30% | / | 649.8 | −62.8 | / |
| MK Substitution | P-S08M2 | P-S08M8 | P-S08NS10 | P-S05M2 |
|---|---|---|---|---|
| 10% | 146.4 | 173.6 | −8.2 | 25.0 |
| 20% | −9.4 | 499.4 | −2.3 | / |
| 30% | / | 971.1 | −46.9 | / |
| Reference Composition | MK Substitution | 24 h | 168 h | 300 h |
|---|---|---|---|---|
| P-S08M2 | 10% | 17.3 | −8.6 | −15.4 |
| P-S08M8 | 10% | −35.9 | −11.6 | 5.2 |
| 20% | −39.6 | 27.7 | 41.8 | |
| 30% | −54.7 | 37.7 | 55.6 | |
| P-S08NS10 | 10% | −5.7 | 7.3 | 5.5 |
| P-S08M2 | MA1 | P-S08M8 | MB1 | MB2 | MB3 | P-S08NS10 | MD1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Per of g of slag | Q∞,1,slag | 278.59 | 211.65 | 368.35 | 494.60 | 770.22 | 993.76 | 376.00 | 421.18 |
| Q∞,2,slag | 327.32 | 194.77 | 474.26 | 459.36 | 639.73 | 917.08 | 1233.22 | 1155.64 | |
| Per of g of binder | Q∞,1,binder | 278.59 | 190.49 | 368.35 | 445.05 | 616.18 | 695.63 | 376.00 | 379.06 |
| Q∞,2,binder | 327.32 | 175.29 | 474.26 | 543.44 | 511.79 | 658.58 | 1233.22 | 1040.07 |
| Composition | Time Zero [h] | DOR1,slag [-] | DOR2,slag [-] | DOR1,binder [-] | DOR2,binder [-] |
|---|---|---|---|---|---|
| P-S08M2 | 10.89 | 0.235 | 0.200 | 0.235 | 0.200 |
| MA1 | 11.55 | 0.387 | 0.421 | 0.387 | 0.421 |
| P-S08M8 | 15.08 | 0.381 | 0.296 | 0.381 | 0.296 |
| MB1 | 3.48 | 0.124 | 0.134 | 0.124 | 0.102 |
| MB2 | 12.09 | 0.198 | 0.239 | 0.198 | 0.239 |
| MB3 | 15.70 | 0.146 | 0.158 | 0.146 | 0.154 |
| P-S08NS10 | 5.78 | 0.116 | 0.035 | 0.116 | 0.035 |
| MD1 | 2.25 | 0.084 | 0.031 | 0.084 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacante, M.; Delsaute, B.; Staquet, S. Mitigation of Volume Changes in Alkali-Activated Slag by Using Metakaolin. Materials 2025, 18, 2644. https://doi.org/10.3390/ma18112644
Lacante M, Delsaute B, Staquet S. Mitigation of Volume Changes in Alkali-Activated Slag by Using Metakaolin. Materials. 2025; 18(11):2644. https://doi.org/10.3390/ma18112644
Chicago/Turabian StyleLacante, Maïté, Brice Delsaute, and Stéphanie Staquet. 2025. "Mitigation of Volume Changes in Alkali-Activated Slag by Using Metakaolin" Materials 18, no. 11: 2644. https://doi.org/10.3390/ma18112644
APA StyleLacante, M., Delsaute, B., & Staquet, S. (2025). Mitigation of Volume Changes in Alkali-Activated Slag by Using Metakaolin. Materials, 18(11), 2644. https://doi.org/10.3390/ma18112644






