Synthesis of Lanthanum-Modified Natural Magnetite: Characterization and Valorization for Phosphorus Recovery from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnetite Preparation
2.2. Synthesis of Lanthanum-Modified Magnetite Decorated with Ferrihydrite
2.3. Materials Physico-Chemical Characterization
2.4. Batch Phosphorus Recovery
2.4.1. Phosphorus Solutions’ Preparation and Analysis
2.4.2. Phosphorus Adsorption Protocol and Data Analysis
2.4.3. Phosphorus Recovery from Actual Wastewater
2.4.4. Adsorbent Regeneration
2.5. Statistical Analysis
3. Results and Discussion
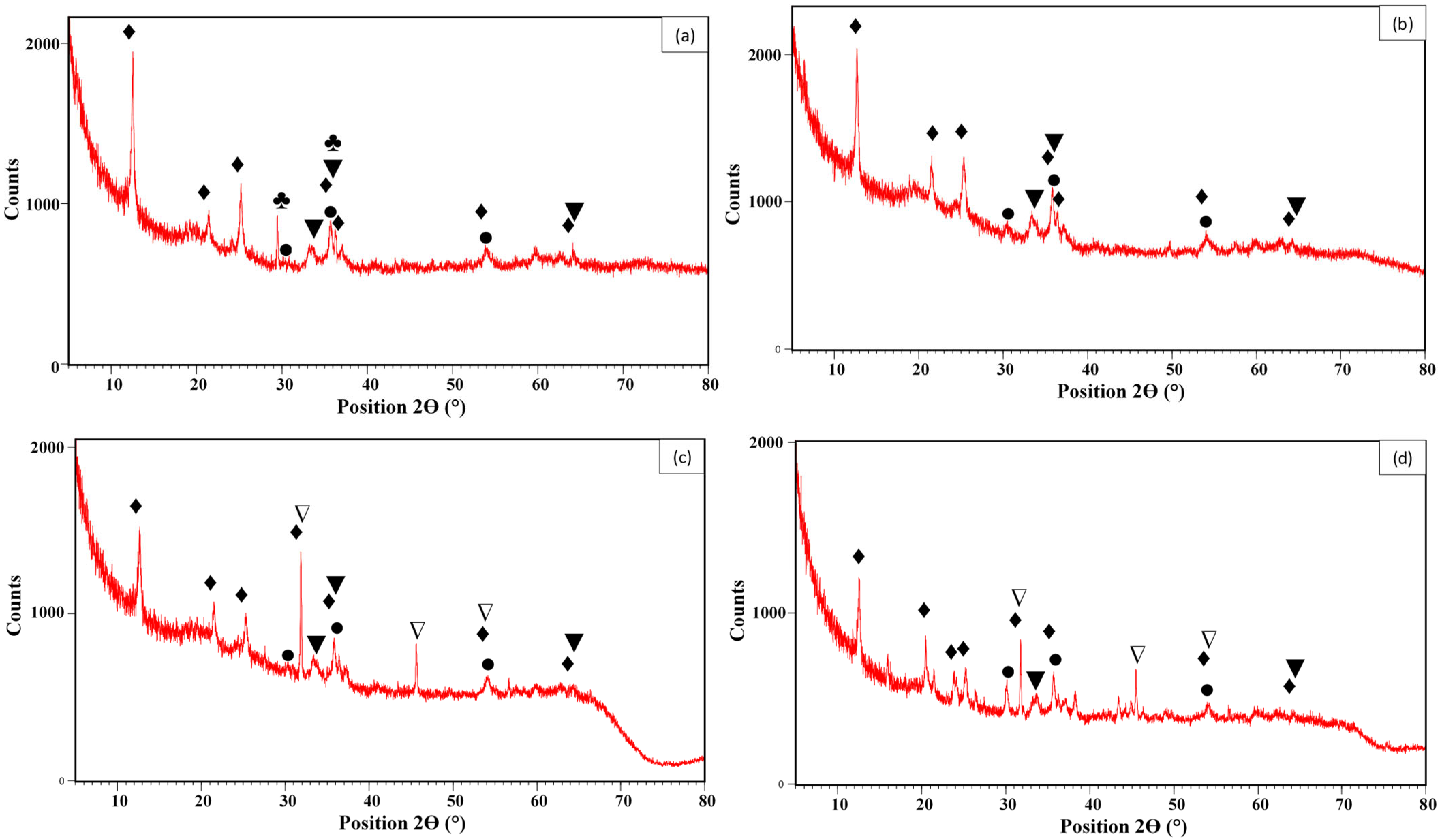
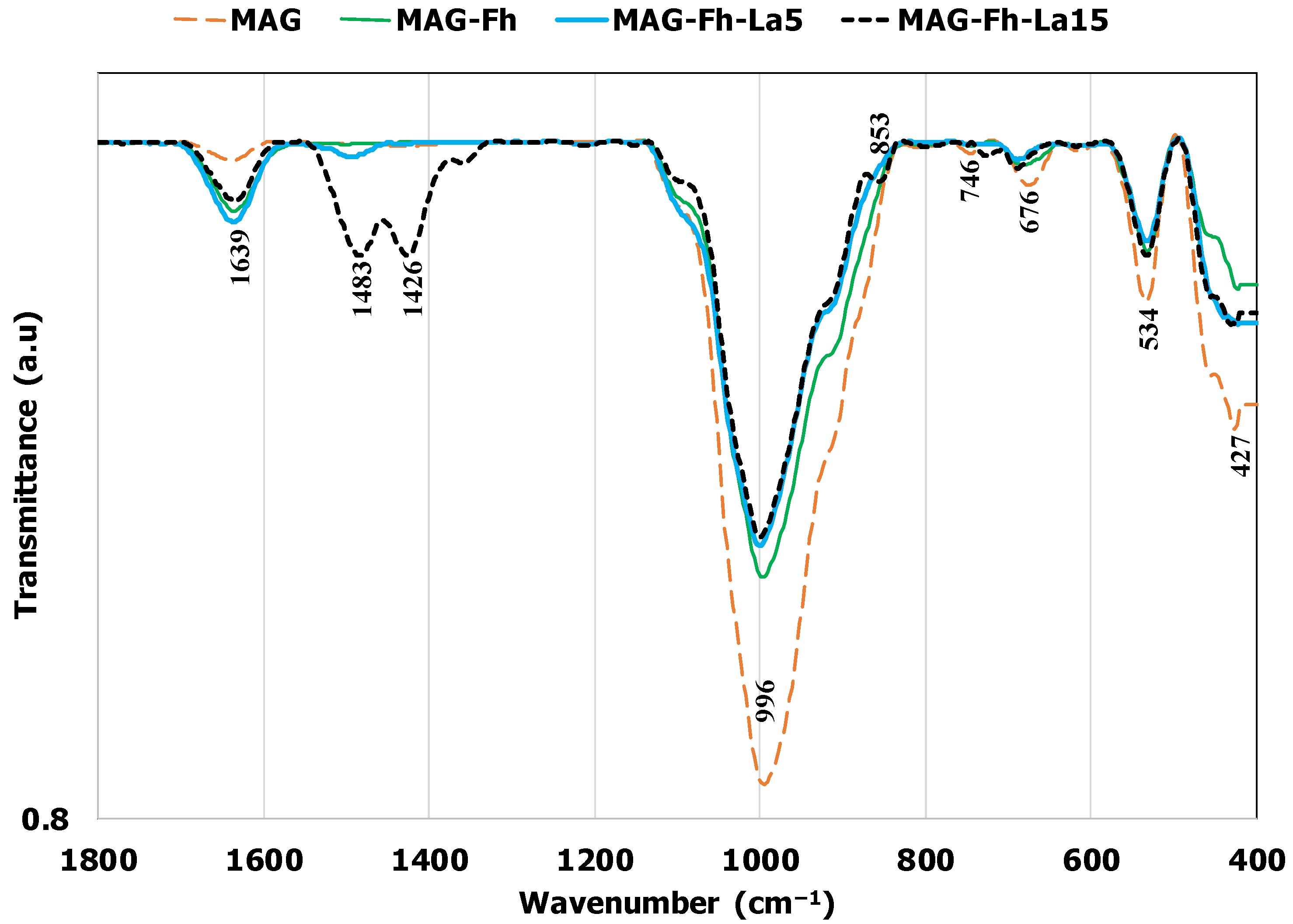
3.1. Material Characterization
3.2. Phosphorus Recovery Experimental Results
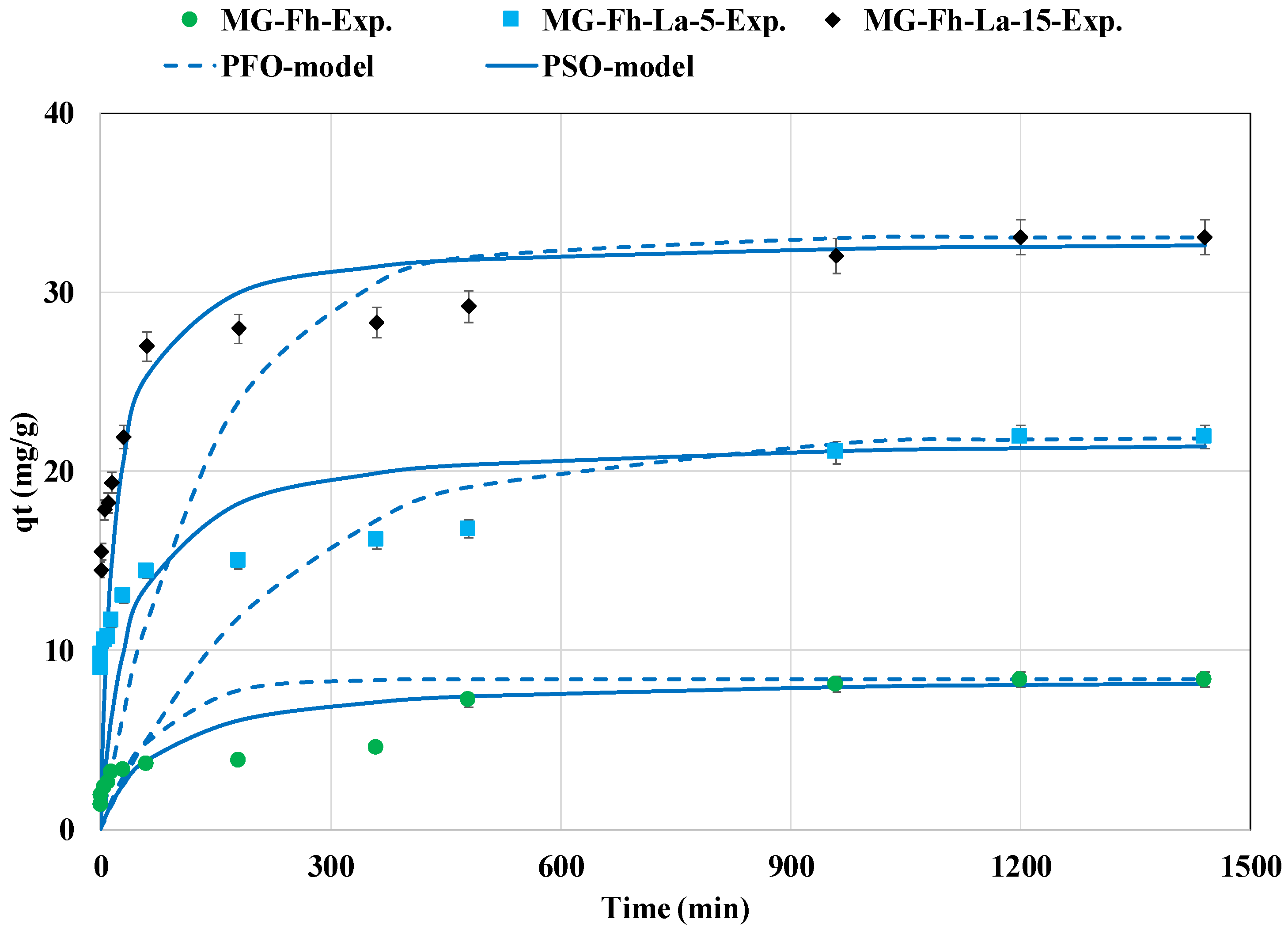
3.2.1. Effect of Contact Time Kinetic Study
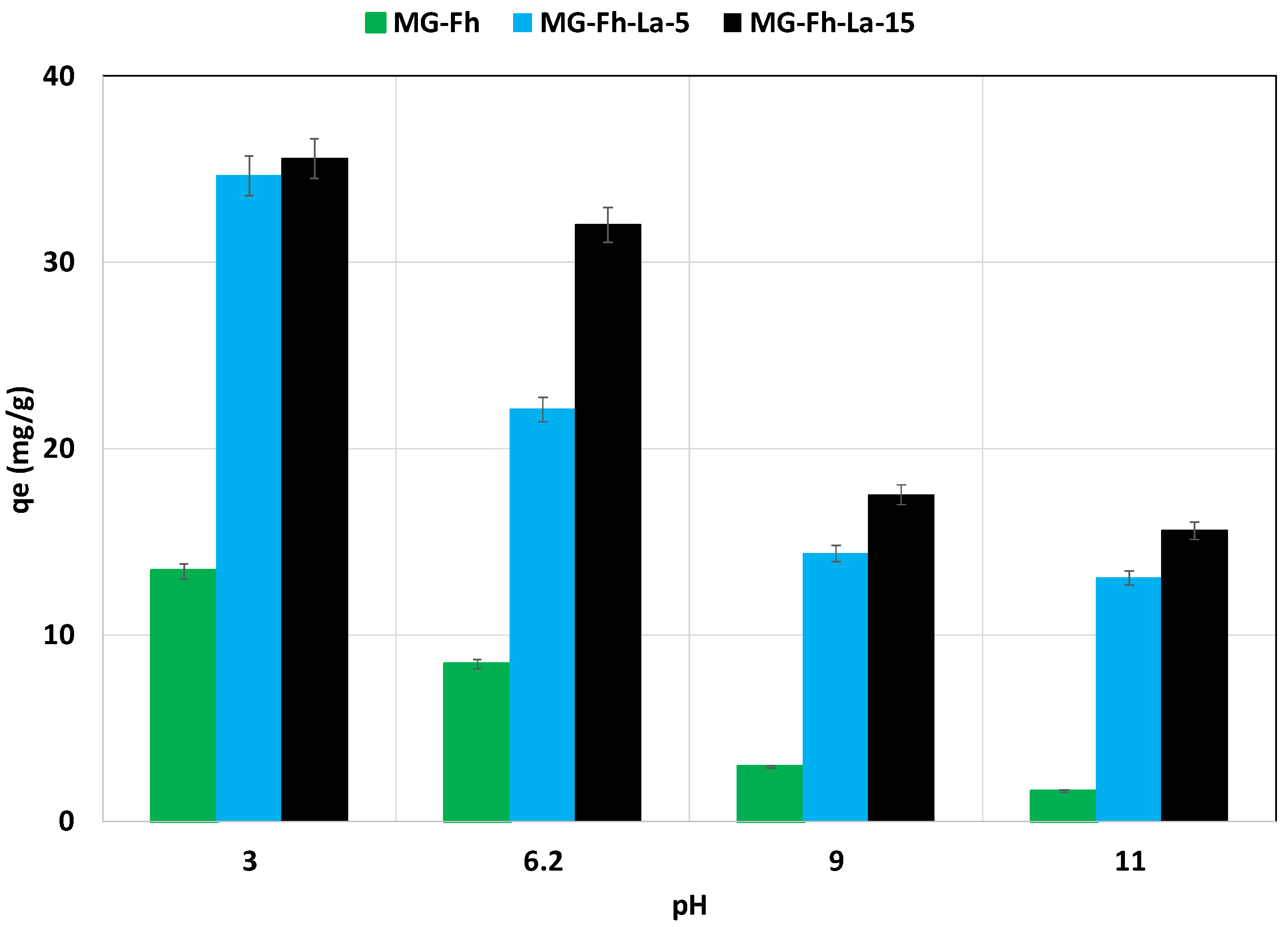
3.2.2. Effect of Initial Aqueous pH
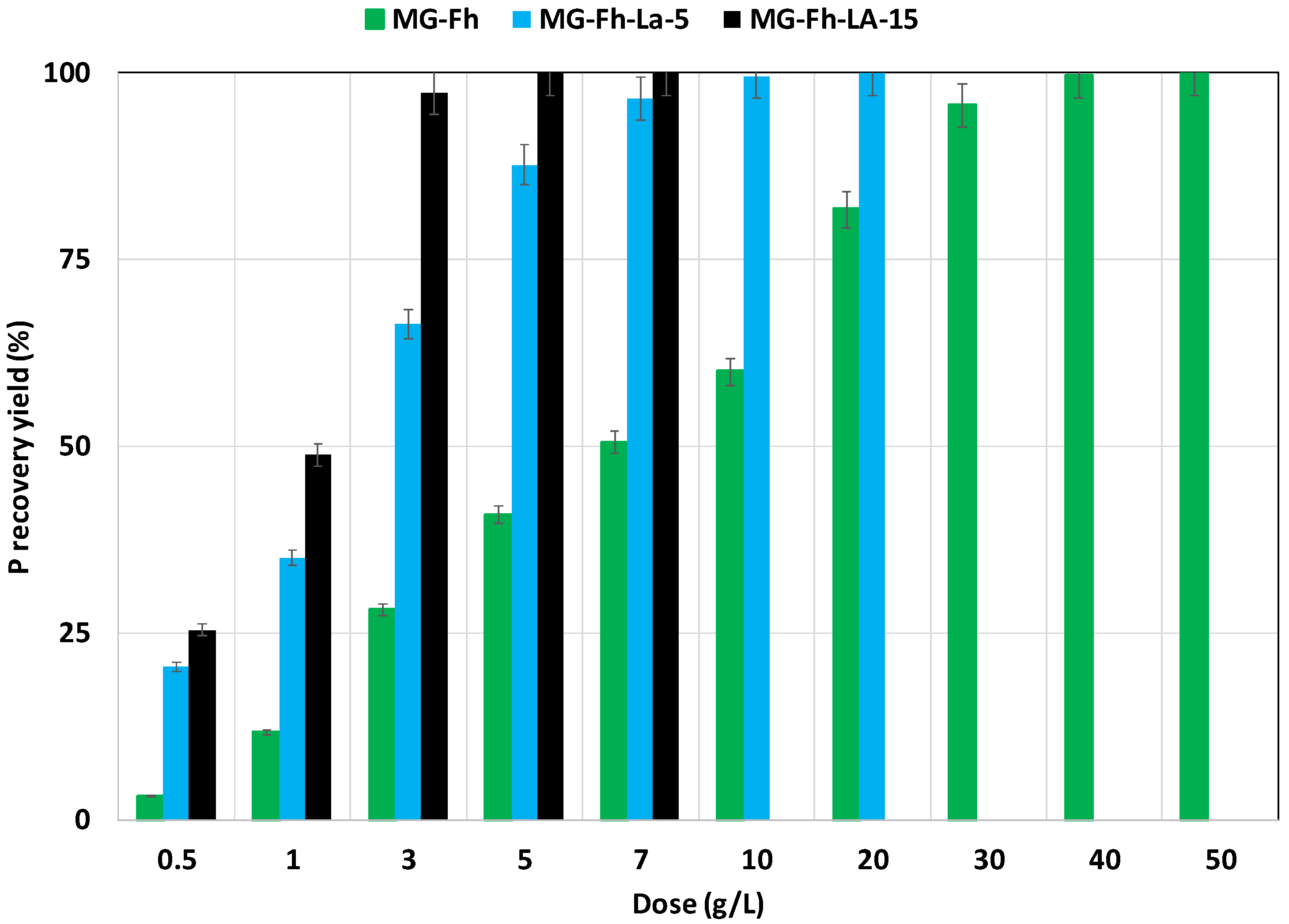
3.2.3. Effect of Adsorbent Doses
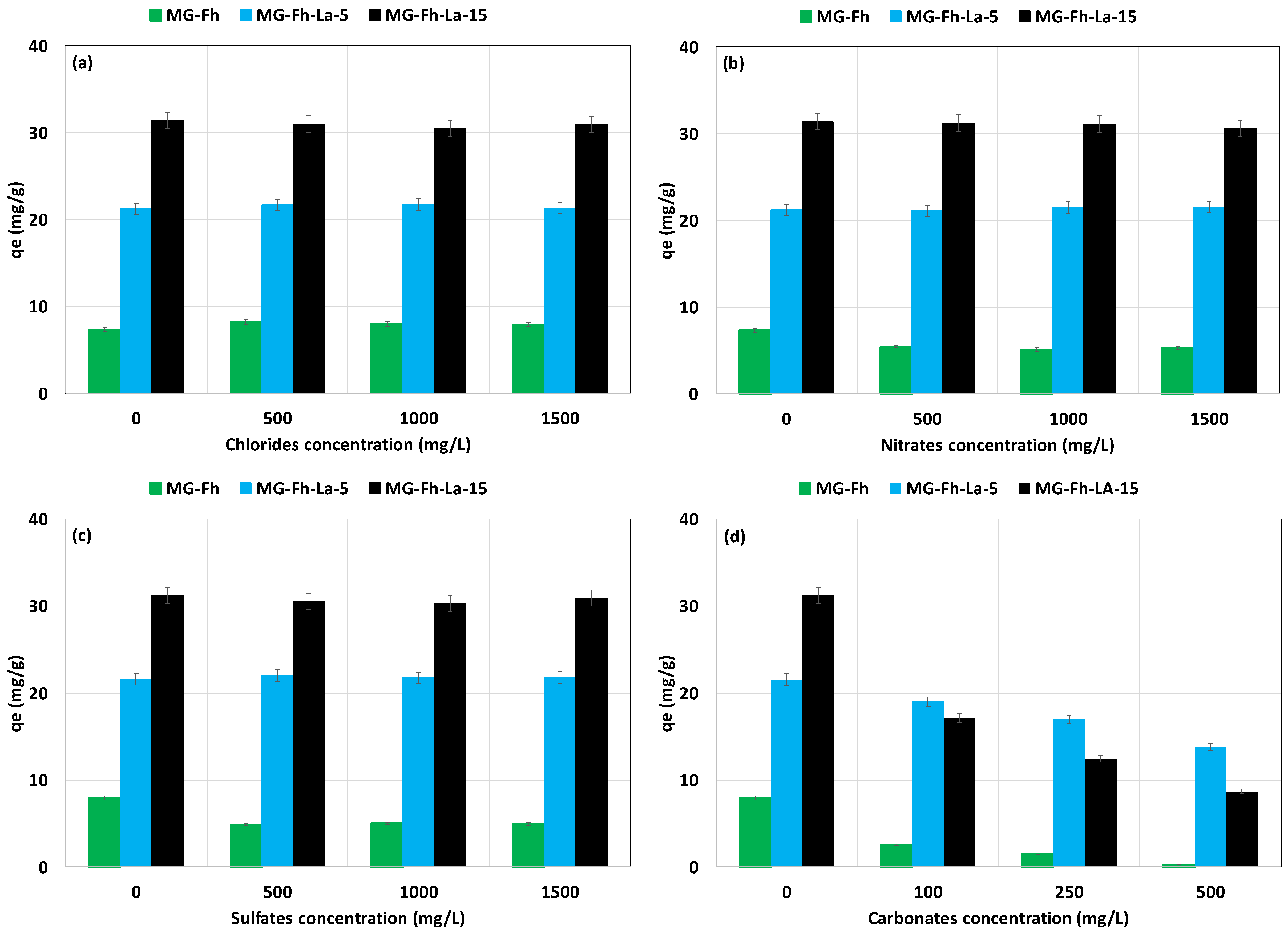
3.2.4. Effect of the Presence of Anions
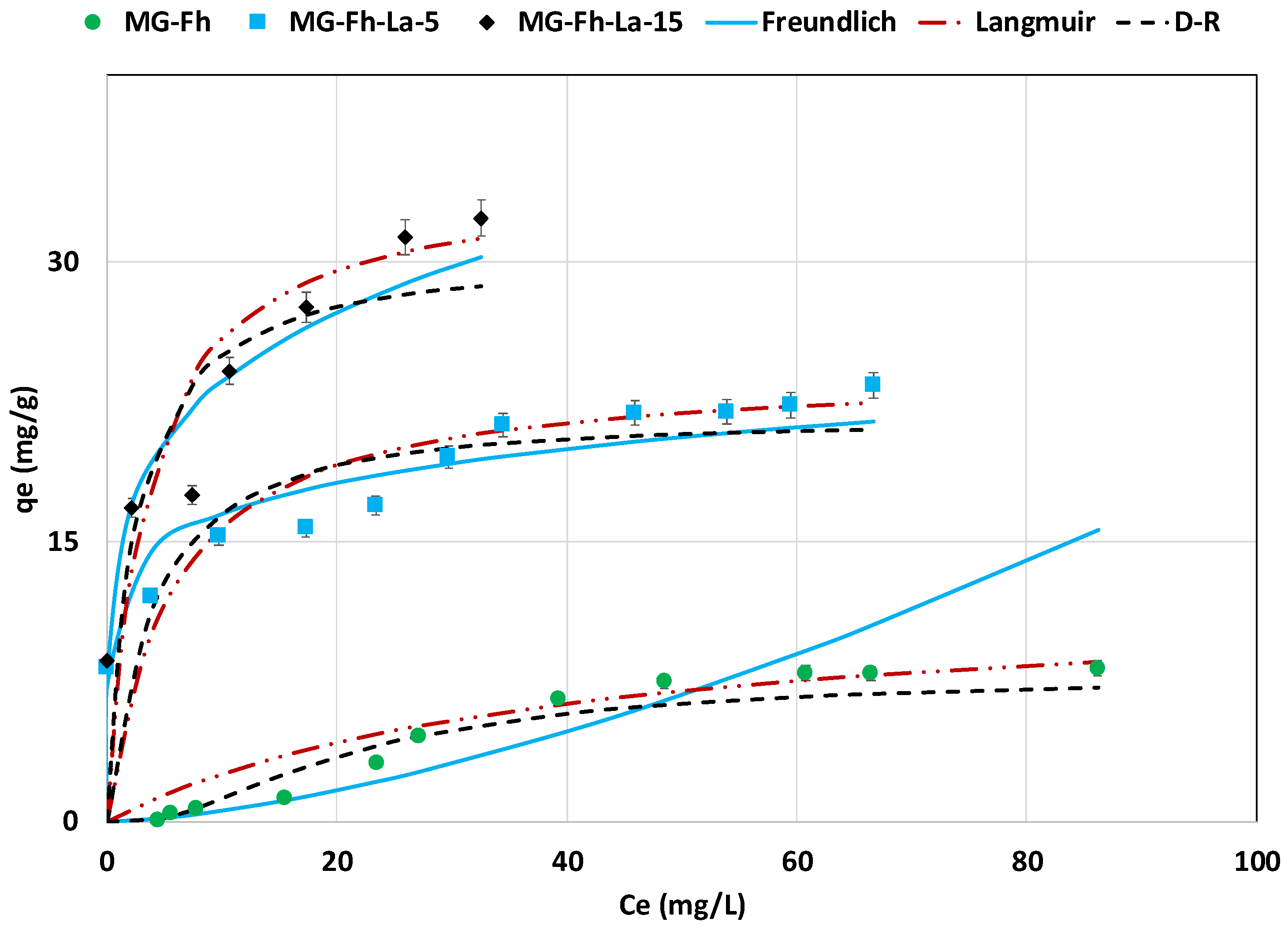
3.2.5. Effect of Initial Concentration Isotherm Study
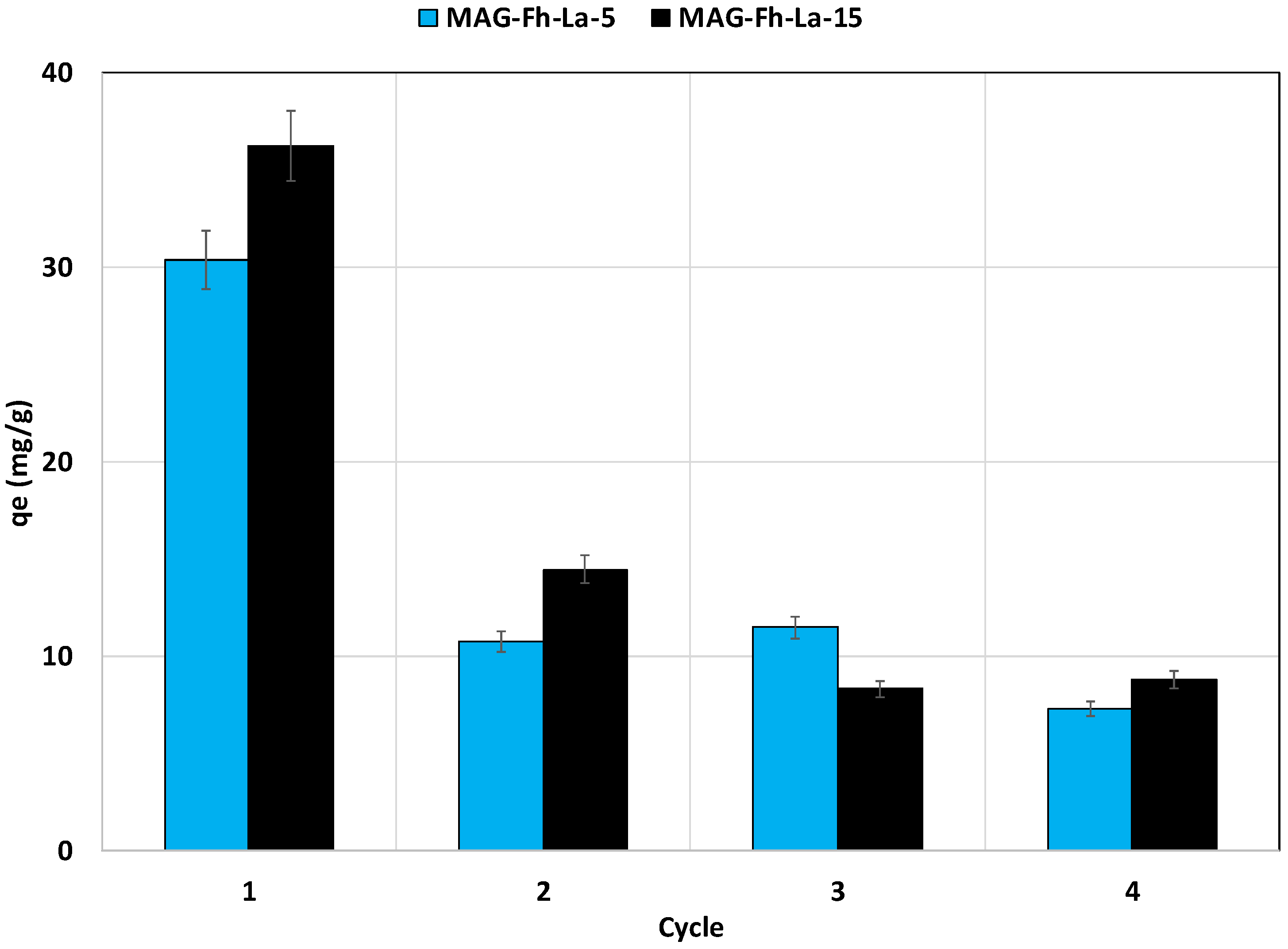
3.2.6. Regeneration Study
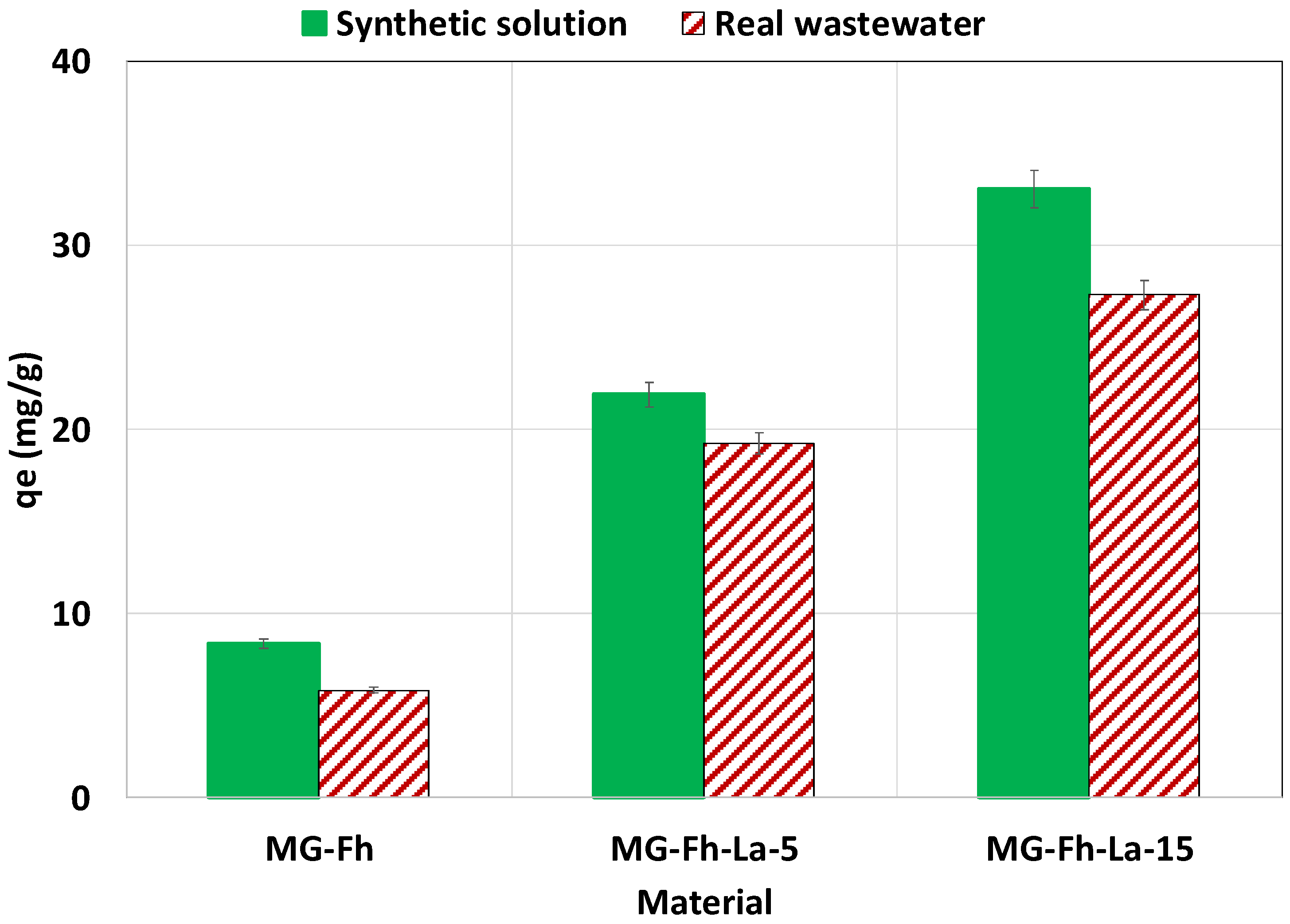
3.2.7. Effect of Using Real P-Doped Wastewater
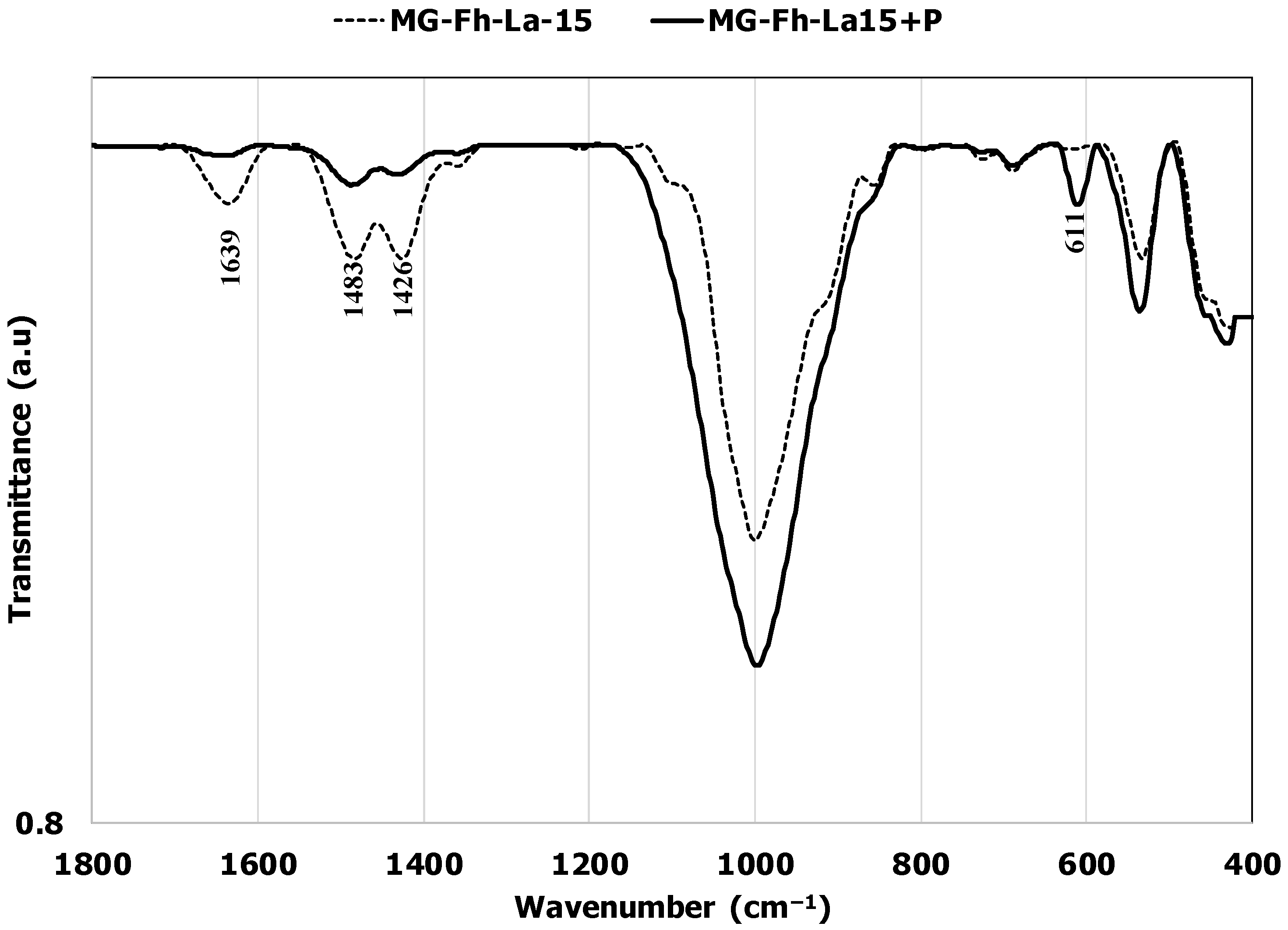
3.3. P Recovery Mechanisms Exploration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous Removal and Recovery from Urban Wastewater: Current Practices and New Directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef] [PubMed]
- van Puijenbroek, P.J.T.M.; Beusen, A.H.W.; Bouwman, A.F. Global Nitrogen and Phosphorus in Urban Waste Water Based on the Shared Socio-Economic Pathways. J. Environ. Manag. 2019, 231, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Akinnawo, S.O. Eutrophication: Causes, Consequences, Physical, Chemical and Biological Techniques for Mitigation Strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Pereira, C.A.; Mulligan, N.C. Practices for Eutrophic Shallow Lake Water Remediation and Restoration: A Critical Literature Review. Water 2023, 15, 2270. [Google Scholar] [CrossRef]
- McDowell, R.W.; Pletnyakov, P.; Haygarth, P.M. Phosphorus Applications Adjusted to Optimal Crop Yields Can Help Sustain Global Phosphorus Reserves. Nat. Food 2024, 5, 332–339. [Google Scholar] [CrossRef]
- Pratt, C.; Soares, A. New Opportunities for Biologically and Chemically mediated adsorption and precipitation of phosphorus from wastewater. Curr. Opin. Biotechnol. 2025, 92, 103261. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Luo, Z.; Qiu, J.; Zhao, L.; Zhang, J.; Xiao, X.; Lin, X.; Wang, X.; Cai, Q.; et al. Nitrogen and Phosphorus Recovery from Livestock Wastewater via Magnesium Ammonium Phosphate Precipitation: A Critical Review of Methods, Progress, and Insights. J. Water Process Eng. 2024, 67, 106139. [Google Scholar] [CrossRef]
- Jellali, S.; Hadroug, S.; Al-Wardy, M.; Al-Nadabi, H.; Nassr, N.; Jeguirim, M. Recent Developments in Metallic-Nanoparticles-Loaded Biochars Synthesis and Use for Phosphorus Recovery from Aqueous Solutions. A Critical Review. J. Environ. Manag. 2023, 342, 118307. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, J.; Hossain, M.F.; Lu, J.; Lu, Q.; Zhou, Y.; Zhou, Y. Recent Advances in the Removal and Recovery of Phosphorus from Aqueous Solution by Metal-Based Adsorbents: A Review. Resour. Conserv. Recycl. 2024, 204, 107464. [Google Scholar] [CrossRef]
- Wu, L.; Xu, D.; Li, B.; Wu, D.; Yang, H. Enhanced Removal Efficiency of Nitrogen and Phosphorus from Swine Wastewater Using MgO Modified Pig Manure Biochar. J. Environ. Chem. Eng. 2024, 12, 111793. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, X.; Wei, J.; Zhang, J.; Guo, Z.; Song, Y. High-Efficiency Lanthanum-Modified Zeolite Adsorbents for Phosphorus Control and Algal Suppression: Preparation, Characterization and Mechanistic Insights. Sep. Purif. Technol. 2025, 352, 128146. [Google Scholar] [CrossRef]
- Ahmed, S.; Lo, I.M.C. Phosphate Removal from River Water Using a Highly Efficient Magnetically Recyclable Fe3O4/La(OH)3 Nanocomposite. Chemosphere 2020, 261, 128118. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, J.O. Effect of Coexisting Components on Phosphate Adsorption Using Magnetite Particles in Water. Environ. Sci. Pollut. Res. 2019, 26, 1054–1060. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Li, J.; Xu, C.; Huang, L.Z.; Wang, D. Magnetite/Lanthanum Hydroxide for Phosphate Sequestration and Recovery from Lake and the Attenuation Effects of Sediment Particles. Water Res. 2018, 130, 243–254. [Google Scholar] [CrossRef]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile Preparation of Dual La-Zr Modified Magnetite Adsorbents for Efficient and Selective Phosphorus Recovery. Chem. Eng. J. 2021, 413, 127530. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Wu, H.; Yuan, P.; Bu, H.; Tan, X. Efficient and Sustainable Phosphate Removal and Recovery from Wastewater with Zinc-Substituted Magnetite. Sep. Purif. Technol. 2025, 360, 130642. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Wu, H.; Yuan, P. Efficient and Regenerative Phosphate Removal from Wastewater Using Stable Magnetite/Magnesium Iron Oxide Nanocomposites. Environ. Res. 2025, 264, 120268. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, J.; Zhan, Y. Interception of Phosphorus Release from Sediments Using Mg/Fe-Based Layered Double Hydroxide (MF-LDH) and MF-LDH Coated Magnetite as Geo-Engineering Tools. Sci. Total Environ. 2020, 739, 139749. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Zhan, Y.; Zhang, Z. Magnetite-Modified Activated Carbon Based Capping and Mixing Technology for Sedimentary Phosphorus Release Control. J. Environ. Manag. 2019, 248, 109287. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D.C. Synthesis of Iron Oxide Nanoparticles under Oxidizing Environment and Their Stabilization in Aqueous and Non-Aqueous Media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Lin, J.; Li, Y.; Zhan, Y.; Wu, X. Combined Amendment and Capping of Sediment with Ferrihydrite and Magnetite to Control Internal Phosphorus Release. Water Res. 2023, 235, 119899. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, R.; Xu, T.; Xu, Y.; Ge, F.; Xi, Y.; Zhu, J.; He, H. Co-Adsorption of Phosphate and Zinc(II) on the Surface of Ferrihydrite. Chemosphere 2016, 144, 1148–1155. [Google Scholar] [CrossRef]
- Sassi, M.; Qafoku, O.; Bowden, M.E.; Pearce, C.I.; Latta, D.; Miller, Q.R.S.; Boamah, M.D.; N’Diaye, A.T.; Holliman, J.E.; Arenholz, E.; et al. Influence of Time and Ageing Conditions on the Properties of Ferrihydrite. Environ. Sci. Nano 2024, 11, 1682–1692. [Google Scholar] [CrossRef]
- Simeonidis, K.; Kalaitzidou, K.; Kaprara, E.; Mitraka, G.; Asimakidou, T.; Balcells, L.; Mitrakas, M. Uptake of Sb(V) by Nano Fe3O4-Decorated Iron Oxy-Hydroxides. Water 2019, 11, 181. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior Adsorption of Phosphate by Ferrihydrite-Coated and Lanthanum-Decorated Magnetite. J. Colloid Interface Sci. 2018, 530, 704–713. [Google Scholar] [CrossRef]
- Fu, H.; He, H.; Zhu, R.; Ling, L.; Zhang, W.; Chen, Q. Phosphate Modified Magnetite@ferrihydrite as an Magnetic Adsorbent for Cd(II) Removal from Water, Soil, and Sediment. Sci. Total Environ. 2021, 764, 142846. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Azzaz, A.A.; Al-Harrasi, M.; Charabi, Y.; Al-Sabahi, J.N.; Al-Raeesi, A.; Usman, M.; Al Nasiri, N.; Al-Abri, M.; Jeguirim, M. Conversion of Industrial Sludge into Activated Biochar for Effective Cationic Dye Removal: Characterization and Adsorption Properties Assessment. Water 2022, 14, 2206. [Google Scholar] [CrossRef]
- Jellali, S.; Hadroug, S.; Al-Wardy, M.; Hamdi, H.; Al-Sabahi, J.; Bekri, I.; Al-Raeesi, A.; Hamdi, W.; Jeguirim, M. Synthesis of Mg-, Al- and Mg/Al-Date Palm Fronds Modified Biochars: Characterization and Investigations on Phosphorus Adsorption Characteristics. Comptes Rendus Chim. 2024, 27, 83–96. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Issaoui, M.; Kwapinska, M.; Jeguirim, M.; Leahy, J.J. Poultry Manure Conversion into Eco-Friendly Materials: Synthesis of Mg-/Al-Based Biochars, Characterization and Application for Phosphorus Recovery from Aqueous Solutions. Biomass Convers. Biorefin. 2023, 14, 25379–25393. [Google Scholar] [CrossRef]
- Ma, Y.; Meng, H.; Shen, Y.; Zhou, H.; Zhu, M.; Geng, J.; Alengebawy, A.; Ding, J.; Zhang, D. Optimisation of Phosphorus Recovery Process from Biogas Slurry Using Straw-Derived Biochar Coupled with Mg/La Oxide as an Adsorbent. Chem. Eng. Res. Des. 2023, 191, 249–260. [Google Scholar] [CrossRef]
- He, H.; Zhong, Y.; Liang, X.; Tan, W.; Zhu, J.; Wang, C.Y. Natural Magnetite: An Efficient Catalyst for the Degradation of Organic Contaminant. Sci. Rep. 2015, 5, 10139. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Surface Area of Ferrihydrite Consistently Related to Primary Surface Charge, Ion Pair Formation, and Specific Ion Adsorption. Chem. Geol. 2020, 532, 119304. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, H.; Gan, J.; Ahmed, T.; Wang, T.; Liu, J.; Mei, M.; Chen, S.; Li, J. Simultaneous Removal of Phosphate and Tetracycline Using LaFeO3 Functionalised Magnetic Biochar by Obtained Ultrasound-Assisted Sol–Gel Pyrolysis: Mechanisms and Characterisation. Environ. Res. 2023, 239, 117227. [Google Scholar] [CrossRef]
- Yi, Y.; Fu, Y.; Wang, Y.; Xu, Z.; Diao, Z. Lanthanum/Iron Co-Modified Biochar for Highly Efficient Adsorption of Low-Concentration Phosphate from Aqueous Solution. J. Environ. Chem. Eng. 2024, 12, 111876. [Google Scholar] [CrossRef]
- Jellali, S.; Hadroug, S.; Al-Wardy, M.; Hamdi, H.; Al-Sabahi, J.; Zorpas, A.; Hamdi, W.; Al-Raeesi, A.; Jeguirim, M. Phosphorus Recovery from Aqueous Solutions by a Mg/Al-Modified Biochar from Date Palm Wastes in Column Mode: Adsorption Characteristics and Scale-up Design Parameters Assessment. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Hao, H.; Wang, Y.; Shi, B. NaLa(CO3)2 Hybridized with Fe3O4 for Efficient Phosphate Removal: Synthesis and Adsorption Mechanistic Study. Water Res. 2019, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, J.J.; Solache-Ríos, M.; Martínez-Gutiérrez, J.M.; Arteaga-Larios, N.V.; Ojeda-Escamilla, M.C.; Rodríguez-Torres, I. Modified Natural Magnetite with Al and La Ions for the Adsorption of Fluoride Ions from Aqueous Solutions. J. Fluor. Chem. 2016, 186, 115–124. [Google Scholar] [CrossRef]
- Saikia, N.J.; Bharali, D.J.; Sengupta, P.; Bordoloi, D.; Goswamee, R.L.; Saikia, P.C.; Borthakur, P.C. Characterization, Beneficiation and Utilization of a Kaolinite Clay from Assam, India. Appl. Clay Sci. 2003, 24, 93–103. [Google Scholar] [CrossRef]
- Sengyang, P.; Rangsriwatananon, K.; Chaisena, A. Preparation of Zeolite N from Metakaolinite by Hydrothermal Method. J. Ceram. Process. Res. 2015, 16, 111–116. [Google Scholar]
- Veerasingam, S.; Venkatachalapathy, R. Estimation of Carbonate Concentration and Characterization of Marine Sediments by Fourier Transform Infrared Spectroscopy. Infrared Phys. Technol. 2014, 66, 136–140. [Google Scholar] [CrossRef]
- Jellali, S.; Khiari, B.; Al-balushi, M.; Al-sabahi, J.; Hamdi, H.; Bengharez, Z.; Al-abri, M.; Al-nadabi, H.; Jeguirim, M. Use of Waste Marble Powder for the Synthesis of Novel Calcium-Rich Biochar: Characterization and Application for Phosphorus Recovery in Continuous Stirring Tank Reactors. J. Environ. Manag. 2024, 351, 119926. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Z.; Song, Z.; Cao, S.; Li, X.; Wang, Y.; Zhan, Z.; Du, M.; Teng, D.; Lv, D.; et al. Preparation of La/Mg Modified Sheep Dung Activated Carbon and Its Adsorption Characteristics for Phosphorus in Wastewater. Desalin. Water Treat. 2024, 317, 100013. [Google Scholar] [CrossRef]
- Mo, J.; Li, Q.; Sun, X.; Zhang, H.; Xing, M.; Dong, B.; Zhu, H. Capacity and Mechanisms of Phosphate Adsorption on Lanthanum-Modified Dewatered Sludge-Based Biochar. Water 2024, 16, 418. [Google Scholar] [CrossRef]
- Elkhlifi, Z.; Kamran, M.; Maqbool, A.; El-Naggar, A.; Ifthikar, J.; Parveen, A.; Bashir, S.; Rizwan, M.; Mustafa, A.; Irshad, S.; et al. Phosphate-Lanthanum Coated Sewage Sludge Biochar Improved the Soil Properties and Growth of Ryegrass in an Alkaline Soil. Ecotoxicol. Environ. Saf. 2021, 216, 112173. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, E.; Xiang, H.; Zhao, R.; Yang, H.; He, W.; Zhang, R. Structural Design of La2(CO3)3 Loaded Magnetic Biochar for Selective Removal of Phosphorus from Wastewater. Environ. Pollut. 2024, 345, 123510. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Khiari, B.; Al-Harrasi, M.; Charabi, Y.; Al-Sabahi, J.; Al-Abri, M.; Usman, M.; Al-Raeesi, A.; Jeguirim, M. Industrial Sludge Conversion into Biochar and Reuse in the Context of Circular Economy: Impact of Pre-Modification Processes on Pharmaceuticals Removal from Aqueous Solutions. Sustain. Chem. Pharm. 2023, 33, 101114. [Google Scholar] [CrossRef]
- Haddad, K.; Jellali, S.; Jeguirim, M.; Ben Hassen Trabelsi, A.; Limousy, L. Investigations on Phosphorus Recovery from Aqueous Solutions by Biochars Derived from Magnesium-Pretreated Cypress Sawdust. J. Environ. Manag. 2018, 216, 305–314. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate Adsorption on Lanthanum Loaded Biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Huang, W.; Jin, Z.; Yang, H.; Qu, Y.; Che, F.; Xu, Z.; Dong, J.; Wang, K. Interception of Phosphorus Release from Sediment by Magnetite/Lanthanum Carbonate Co Modified Activated Attapulgite Composite: Performance and Mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 664, 131139. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, L.; Yu, H.; Yan, T.; Li, X. Adsorption of Phosphate from Aqueous Solution by Vegetable Biochar/Layered Double Oxides: Fast Removal and Mechanistic Studies. Bioresour. Technol. 2019, 284, 65–71. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, T.; Li, W.; Li, M.; Su, K.; Chen, J.; Yang, H. Lanthanum-Loaded Peanut Shell Biochar Prepared via One-Step Pyrolysis Method for Phosphorus Removal and Immobilization. Environ. Technol. 2023, 44, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater Using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Yuan, Y.; Si, Y.; Xu, J.; Li, M.; Peng, X. La(OH)3 Loaded Magnetic Nanocomposites Derived from Sugarcane Bagasse Cellulose for Phosphate Adsorption: Characterization, Performance and Mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127060. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, X.; Zhou, Y.; Li, F.; Liu, W.; Huang, Y.; Zhang, H.; Ma, J.; Hu, G. Tri-Functional Lanthanum-Based Biochar for Efficient Phosphorus Recovery, Bacterial Inhibition, and Soil Fertility Enhancement. Biochar 2023, 5, 16. [Google Scholar] [CrossRef]
- Jellali, S.; Hamdi, W.; Al-Harrasi, M.; Al-Wardy, M.; Al-Sabahi, J.; Al-Nadabi, H.; Al-Raeesi, A.; Jeguirim, M. Investigations on Amoxicillin Removal from Aqueous Solutions by Novel Calcium-Rich Biochars: Adsorption Properties and Mechanisms Exploration. Processes 2024, 12, 1552. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; He, S.; Zhan, Y.; Zhang, Z.; Wang, D.; Zhang, Z. Synthesis and Evaluation of Zirconia/Magnetite/Zeolite Composite for Controlling Phosphorus Release from Sediment: A Laboratory Study. Ecol. Eng. 2020, 151, 105874. [Google Scholar] [CrossRef]
- Muñoz-Garcia, A.; Montoro-Leal, P.; López Guerrero, M.d.M.; Vereda-Alonso, C.; Vereda Alonso, E. Green Chemistry: Advancing Planetary Phosphorus Sustainability through the Synergy of Graphene Oxide Modified with Magnetic Nanoparticles (M@GO) for Extracting Tertiary Effluent Phosphorus in Sewage Treatment Plants. Environ. Sci. Nano 2024, 11, 2607–2619. [Google Scholar] [CrossRef]
- Wei, X.; Miao, J.; Lv, Z.; Wan, X.; Zhang, N.; Zhang, R.; Peng, S. Phosphate Adsorption onto an Al-Ti Bimetal Oxide Composite in Neutral Aqueous Solution: Performance and Thermodynamics. Appl. Sci. 2022, 12, 2309. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Hamdi, N.; Ben Ali, M.; Jellali, S.; Srasra, E. Enhanced Adsorptive Removal of Cationic and Anionic Dyes from Aqueous Solutions by Olive Stone Activated Carbon. Comptes Rendus Chim. 2020, 11, 689–704. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, S.; Zhang, X.; Zheng, S. Phosphorus Removal and Recovery from Secondary Effluent in Sewage Treatment Plant by Magnetite Mineral Microparticles. Powder Technol. 2017, 306, 68–73. [Google Scholar] [CrossRef]
- Riddle, M.; Cederlund, H.; Schmieder, F.; Bergström, L. Magnetite-Coated Biochar as a Soil Phosphate Filter: From Laboratory to Field Lysimeter. Geoderma 2018, 327, 45–54. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Y.; Zhang, Z.; Zhan, Y.; Zhang, Z.; Wang, Y.; Yu, Y.; Wu, X. Immobilization of Mobile and Bioavailable Phosphorus in Sediments Using Lanthanum Hydroxide and Magnetite/Lanthanum Hydroxide Composite as Amendments. Sci. Total Environ. 2019, 687, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.S.S.; Lam, K.H.; Lee, J.M.N.; Lau, T.C. Removal of Phosphate from Water by a Highly Selective La(III)-Chelex Resin. Chemosphere 2007, 69, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ru, J.; Yin, W.; Liu, X.; Wang, J.; Zhang, W. Removal of Phosphate from Polluted Water by Lanthanum Doped Vesuvianite. J. Hazard. Mater. 2009, 168, 326–330. [Google Scholar] [CrossRef]
- Yan, L.G.; Yang, K.; Shan, R.R.; Yan, T.; Wei, J.; Yu, S.J.; Yu, H.Q.; Du, B. Kinetic, Isotherm and Thermodynamic Investigations of Phosphate Adsorption onto Core-Shell Fe3O4@LDHs Composites with Easy Magnetic Separation Assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Souissi, R.; Ergaieg, K.; Bousselmi, L. Alkaline-Treated Sawdust as an Effective Material for Cationic Dye Removal from Textile Effluents under Dynamic Conditions: Breakthrough Curve Prediction and Mechanism Exploration. Environ. Sci. Pollut. Res. 2017, 24, 18240–18256. [Google Scholar] [CrossRef]
- Gong, W.; Qi, C.; Huang, L.; Tian, Z.; Huang, Z.; Tao, C.; Lin, H.; Guo, L.; Yu, Z. Adsorption of Phosphorus in Wastewater by Lanthanum-Modified Magnetic Sewage Sludge Biochar. Desalin. Water Treat. 2024, 320, 100603. [Google Scholar] [CrossRef]
- Thomas, S.S.; Viswanathan, N.; Periyasamy, S.; Al-Asbahi, B.A. Enriched Phosphate Adsorption Using Lanthanum Organic Frameworks Decorated Papaya Biochar Supported Alginate Composite. J. Mol. Liq. 2025, 426, 127286. [Google Scholar] [CrossRef]


| Material | Mineral Contents (%) | pHpzc | BET Analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Fe | Si | Al | Mg | Cr | Ca | Ni | Mn | Cl | Zn | Cd | P | Pb | Hg | SA (m2 g−1) | TPV (cm3 g−1) | ||
| MAG | 69.10 | 12.40 | 7.91 | 6.50 | 1.25 | 1.09 | 0.93 | 0.30 | 0.04 | 0.02 | 0.01 | 0.001 | ND | ND | ND | - | 44.3 | 0.045 |
| MG-Fh | 75.40 | 10.20 | 6.22 | 5.14 | ND | 0.94 | 0.20 | 0.24 | 0.03 | 1.43 | 0.01 | 0.001 | ND | ND | ND | 7.02 | 87.0 | 0.084 |
| MG-Fh-La-5 | 80.50 | 6.72 | 4.96 | 4.11 | ND | 0.93 | 0.16 | 0.16 | 0.07 | 2.00 | 0.004 | 0.001 | ND | ND | ND | 7.14 | 88.8 | 0.142 |
| MG-Fh-La-15 | 84.90 | 4.13 | 3.81 | 3.21 | ND | 0.97 | 0.12 | 0.11 | 0.07 | 1.95 | 0.002 | <0.001 | ND | ND | ND | 6.31 | 71.5 | 0.103 |
| Parameter | MG-Fh | MG-Fh-La-5 | MG-Fh-La-15 | |
|---|---|---|---|---|
| qe,exp (mg g−1) | 8.36 | 21.89 | 33.05 | |
| PFO model | k1 (min−1) | 0.0144 | 0.0043 | 0.0071 |
| R2 | 0.749 | 0.905 | 0.909 | |
| MAPD (%) | 47.6 | 51.1 | 48.1 | |
| PSO model | k2 (g mg−1 min−1) | 0.0016 | 0.0012 | 0.0016 |
| qe,pred (mg g −1) | 8.57 | 21.91 | 33.0 | |
| R2 | 0.840 | 0.854 | 0.941 | |
| MAPD (%) | 41.4 | 36.2 | 26.7 | |
| Diffusion model | Df (×10−13 m2 s−1) | 0.80 | 0.68 | 1.14 |
| R2 | 0.883 | 0.983 | 0.982 | |
| Dip (×10−13 m2 s−1) | 2.34 | 1.67 | 1.34 | |
| R2 | 0.928 | 0.903 | 0.978 |
| Isotherm | Parameter | MG-Fh | MG-Fh-La-5 | MG-Fh-La-15 |
|---|---|---|---|---|
| Freundlich | n | 0.66 | 7.22 | 4.72 |
| KF | 0.017 | 11.993 | 14.464 | |
| R2 | 0.777 | 0.902 | 0.934 | |
| MAPE (%) | 57.3 | 8.6 | 7.5 | |
| Langmuir | KL (L mg−1) | 0.026 | 0.184 | 0.297 |
| qm,L,calc (mg g−1) | 12.4 | 24.3 | 34.5 | |
| R2 | 0.964 | 0.874 | 0.866 | |
| MAPD (%) | 331.4 | 14.8 | 23.8 | |
| D-R | qm,D-R,calc (mg g−1) | 7.8 | 21.4 | 29.9 |
| E (kJ mol−1) | 1.788 | 4.729 | 5.687 | |
| R2 | 0.960 | 0.806 | 0.827 | |
| MAPD (%) | 43.6 | 16.4 | 22.7 |
| Adsorbent | Adsorption Experimental Conditions | qm,L (mg g−1) | Reference |
|---|---|---|---|
| Magnetite mineral microparticles (average particle size: 34 μm) | C0 = 0.5–9 mg L−1; pH = 7.0; D = 2 g L−1; t = 24 h; T = 25 °C | 0.6 | [60] |
| La-modified commercial resin at a percentage of 6% | C0 = 155 mg L−1; pH= 7.0; D = - g L−1; t = - h; T = RT °C | 1.3 | [63] |
| Synthetic magnetite-coated commercial biochar | C0 = 25–500 mg L−1; pH = 6.5; D = 10 g L−1; t = 24 h; T = - | 3.4 | [61] |
| La-modified natural vesuvianite at a percentage of 14%, China | C0 = 1–5 mg L−1; pH = 7.1; D = 0.3 g L−1; t = 40 h; T = 20 °C | 6.7 | [64] |
| La-modified synthetic magnetite at a percentage of 5% | C0 = 1–10 mg L−1; pH = 7.0; D = 0.1 g L−1; t = 2 h; T = 25 °C | 13.4 | [14] |
| La-modified synthetic magnetite at a percentage of 10% | 12.3 | ||
| La-modified synthetic magnetite at a percentage of 20% | 25.4 | ||
| La-modified synthetic magnetite | C0 = 5–30 mg L−1; pH = 7.0; D = 0.4 g L−1; t = 24 h; T = 25 °C | 17.3 | [62] |
| La-modified natural magnetite decorated with ferrihydrite at a percentage of 20%, China | C0 = 2–120 mg L−1; pH = 6.28; D = 1 g L−1; t = 24 h; T = 25 °C | 44.8 | [25] |
| La/Zr-modified synthetic magnetite at La:MG and Zr:MG percentages of 64% and 40%, respectively | C0 = 2.5–50 mg L−1; pH = 2.0; D = 0.25 g L−1; t = 24 h; T = 25 °C | 49.1 | [15] |
| La-modified synthetic magnetite and attapulgite at a percentage of 28% | C0 = 1–300 mg L−1; pH = 7.0; D = 1 g L−1; t = 24 h; T = 25 °C | 51.7 | [49] |
| La-modified synthetic magnetite at a percentage of 128% | C0 = 0.5–250 mg L−1; pH = 7.0; D = 0.1 g L−1; t = 5 h; T = 23 °C | 253.8 | [12] |
| La-modified natural magnetite decorated with ferrihydrite at a percentage of 0%, Oman | C0 = 5–100 mg L−1; pH = natural; D = 1 g L−1; t = 24 h; T = RT | 12.4 | This study |
| La-modified natural magnetite decorated with ferrihydrite at a percentage of 5%, Oman | 24.3 | ||
| La-modified natural magnetite decorated with ferrihydrite at a percentage of 15%, Oman | 34.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nadabi, H.; Jellali, S.; Hamdi, W.; Al-Tamimi, A.; Al-Raeesi, A.; Al-Sidairi, A.; Al-Busaidi, W.; Al-Hanai, A.; Al-Zeidi, K.; Al-Wardy, M.; et al. Synthesis of Lanthanum-Modified Natural Magnetite: Characterization and Valorization for Phosphorus Recovery from Aqueous Solutions. Materials 2025, 18, 2283. https://doi.org/10.3390/ma18102283
Al-Nadabi H, Jellali S, Hamdi W, Al-Tamimi A, Al-Raeesi A, Al-Sidairi A, Al-Busaidi W, Al-Hanai A, Al-Zeidi K, Al-Wardy M, et al. Synthesis of Lanthanum-Modified Natural Magnetite: Characterization and Valorization for Phosphorus Recovery from Aqueous Solutions. Materials. 2025; 18(10):2283. https://doi.org/10.3390/ma18102283
Chicago/Turabian StyleAl-Nadabi, Hamed, Salah Jellali, Wissem Hamdi, Afrah Al-Tamimi, Ahmed Al-Raeesi, Ahmed Al-Sidairi, Waleed Al-Busaidi, Ahlam Al-Hanai, Khalifa Al-Zeidi, Malik Al-Wardy, and et al. 2025. "Synthesis of Lanthanum-Modified Natural Magnetite: Characterization and Valorization for Phosphorus Recovery from Aqueous Solutions" Materials 18, no. 10: 2283. https://doi.org/10.3390/ma18102283
APA StyleAl-Nadabi, H., Jellali, S., Hamdi, W., Al-Tamimi, A., Al-Raeesi, A., Al-Sidairi, A., Al-Busaidi, W., Al-Hanai, A., Al-Zeidi, K., Al-Wardy, M., & Jeguirim, M. (2025). Synthesis of Lanthanum-Modified Natural Magnetite: Characterization and Valorization for Phosphorus Recovery from Aqueous Solutions. Materials, 18(10), 2283. https://doi.org/10.3390/ma18102283








