Synthesis of Mesoporous Alumina with High Specific Surface Area via Reverse Precipitation Method for Enhanced Adsorption and Regeneration of Congo Red
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
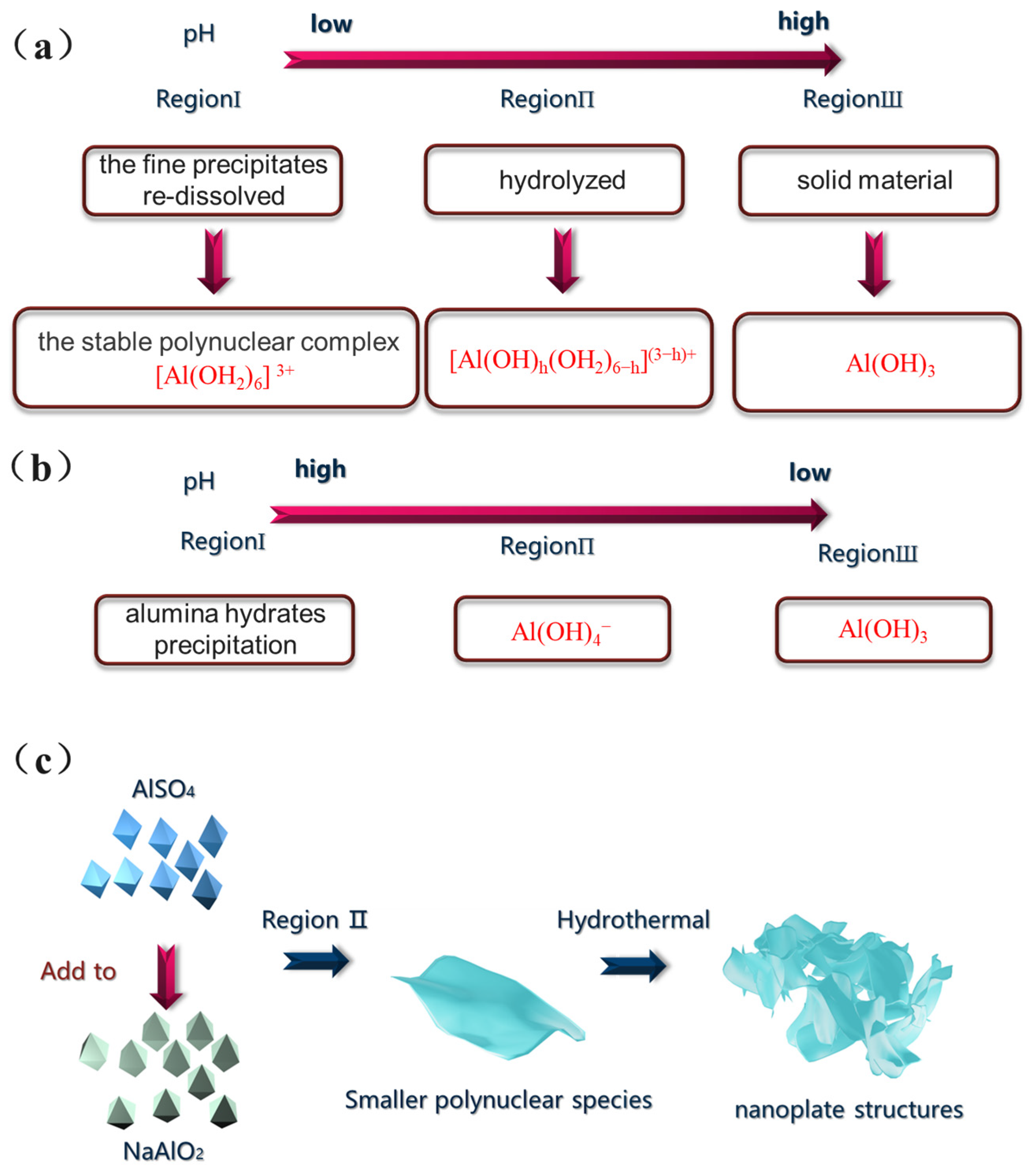
2.2. Synthesis
2.3. Analytical Procedures
2.4. Adsorption Studies
2.4.1. Adsorption Kinetics and Isotherm
2.4.2. Regeneration Properties of the Adsorbent
3. Results and Discussion
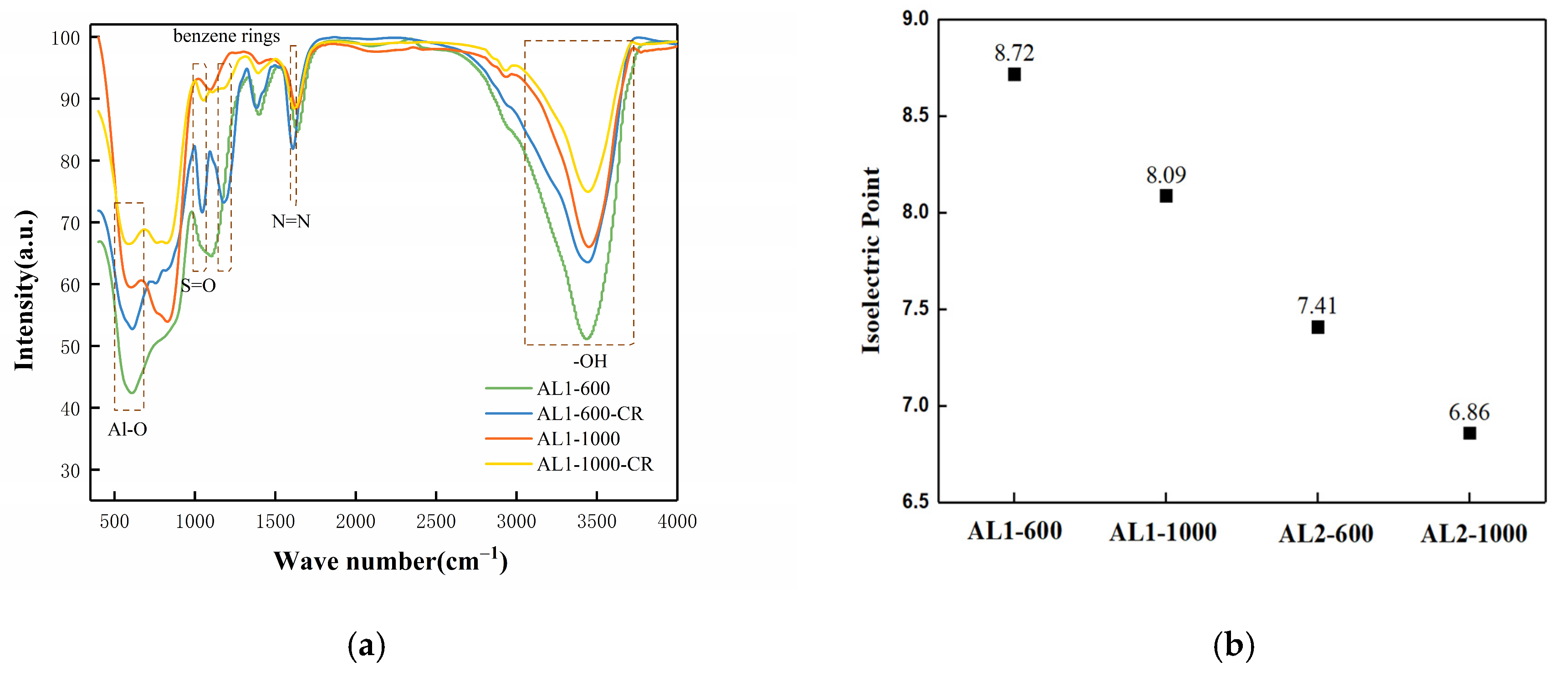
3.1. Characterization of Adsorbents
3.2. Adsorption Behavior
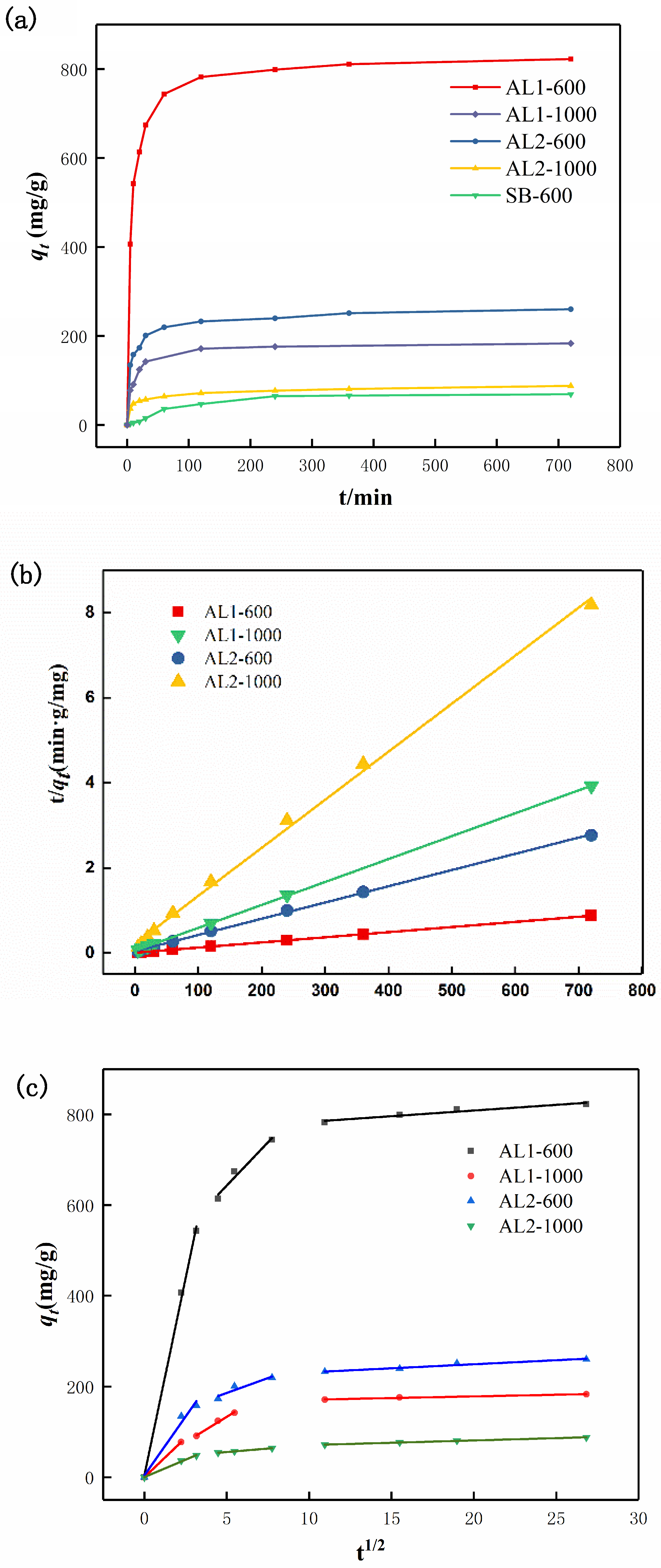
3.2.1. Kinetics of Adsorption
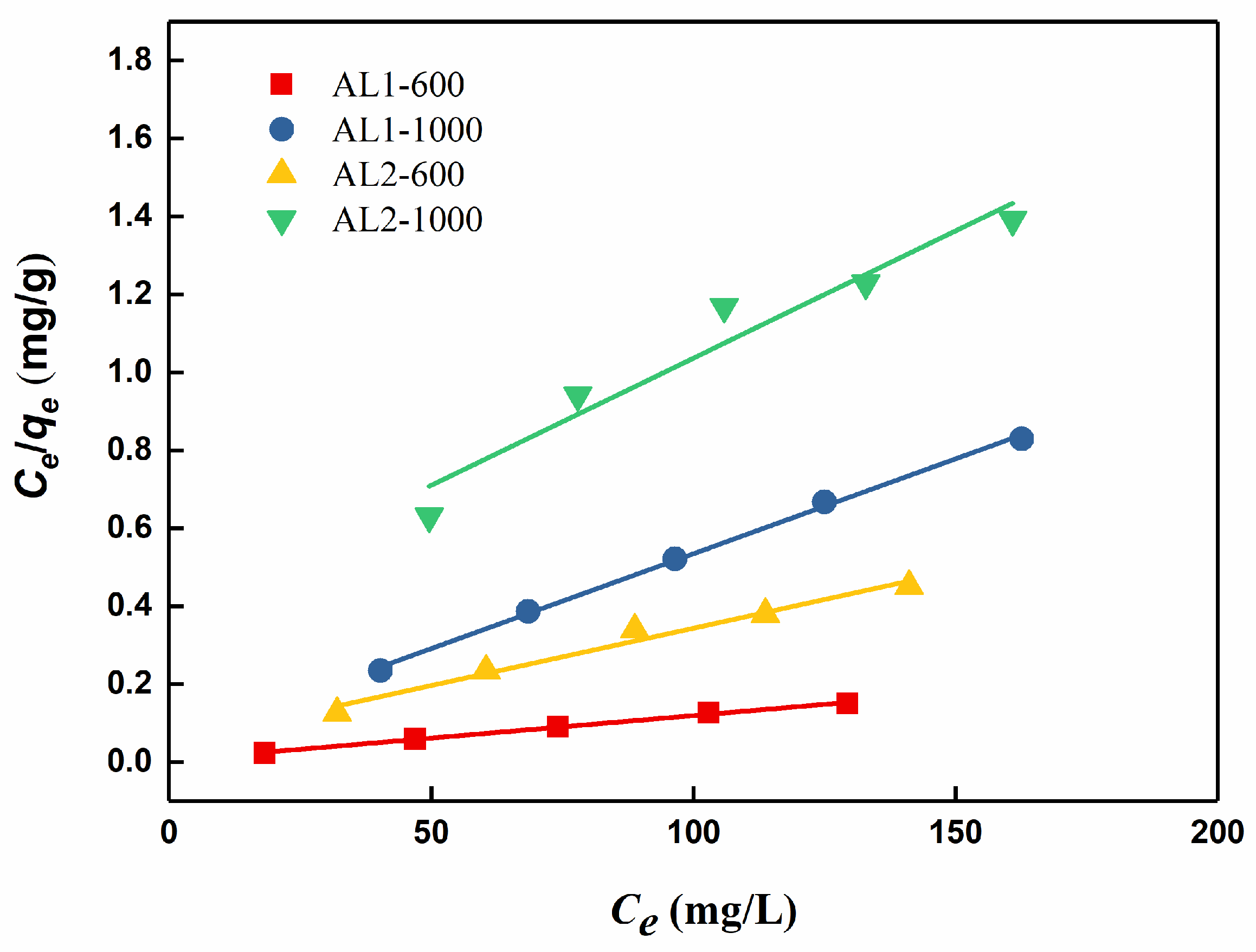
3.2.2. Adsorption Isotherm Models
3.2.3. Adsorption Mechanism
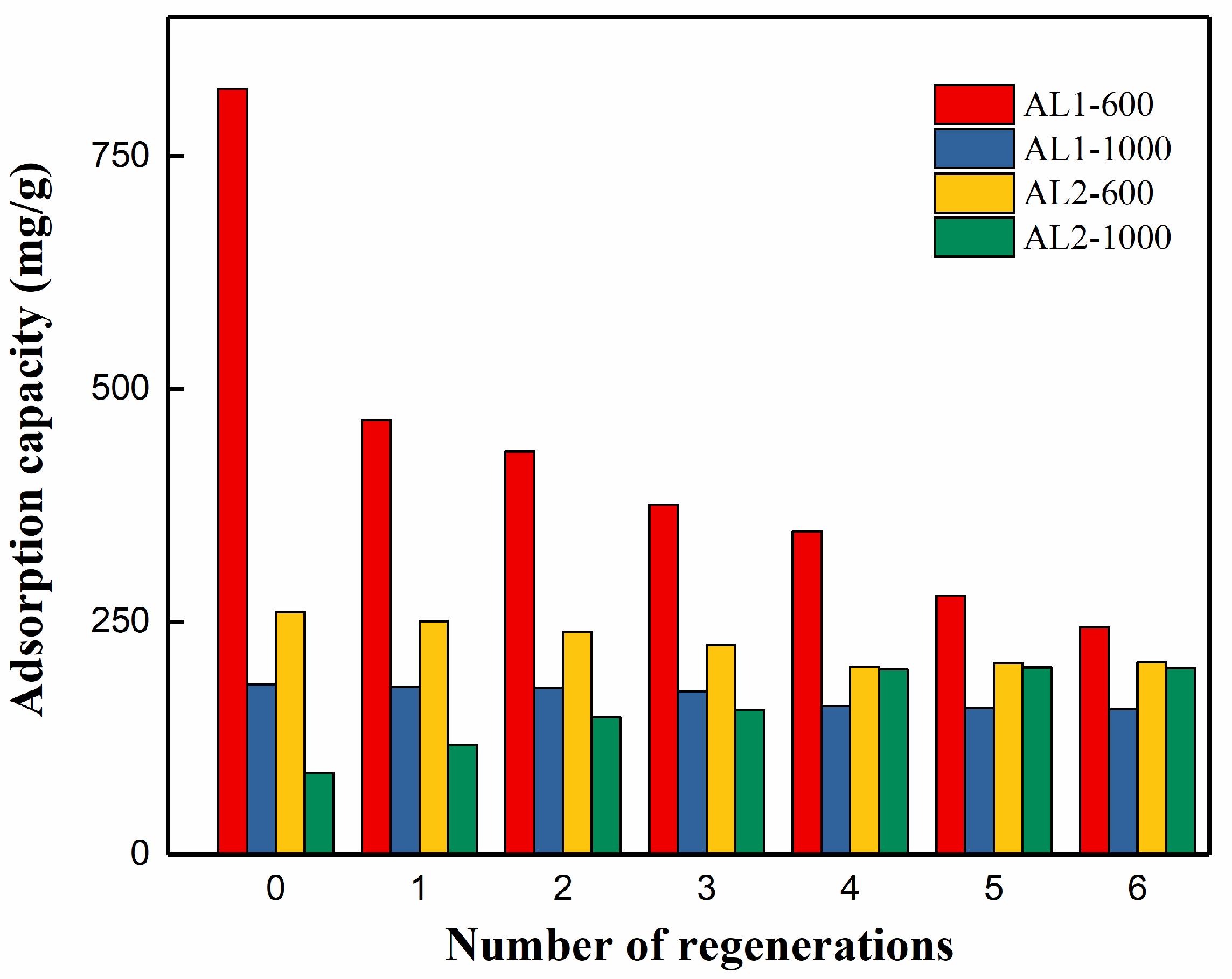
3.3. Recyclability
3.4. Comparison with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adam, A.M.A.; Saad, H.A.; Atta, A.A.; Alsawat, M.; Hegab, M.S.; Refat, M.S.; Altalhi, T.A.; Alosaimi, E.H.; Younes, A.A.O. Preparation and Characterization of New CrFeO3-Carbon Composite Using Environmentally Friendly Methods to Remove Organic Dye Pollutants from Aqueous Solutions. Crystals 2021, 11, 960. [Google Scholar] [CrossRef]
- Lata, H.; Garg, V.K.; Gupta, R.K. Adsorptive removal of basic dye by chemically activated Parthenium biomass: Equilibrium and kinetic modeling. Desalination 2008, 219, 250–261. [Google Scholar] [CrossRef]
- Greluk, M.; Hubicki, Z. Efficient removal of Acid Orange 7 dye from water using the strongly basic anion exchange resin Amberlite IRA-958. Desalination 2011, 278, 219–226. [Google Scholar] [CrossRef]
- Arslan, I.; Balcioglu, I.A.; Bahnemann, D.W. Advanced chemical oxidation of reactive dyes in simulated dyehouse effluents by ferrioxalate-Fenton/UV-A and TiO_2/UV-A processes. Dyes Pigment. 2000, 47, 207–218. [Google Scholar] [CrossRef]
- Akbari, A.; Remigy, J.C.; Aptel, P. Treatment of textile dye effluent using a polyamide-based nanofiltration membrane. Chem. Eng. Proces. 2002, 41, 601–609. [Google Scholar] [CrossRef]
- Wang, C.; Yediler, A.; Lienert, D.; Wang, Z.; Kettrup, A. Ozonation of an azo dye C.I. Remazol Black 5 and toxicological assessment of its oxidation products. Chemosphere 2003, 52, 1225–1232. [Google Scholar] [CrossRef]
- Al-Bastaki, N. Removal of methyl orange dye and Na2SO4 salt from synthetic waste water using reverse osmosis. Chem. Eng. Proces. 2004, 43, 1561–1567. [Google Scholar] [CrossRef]
- Wu, R.; Qu, J.; Chen, Y. Magnetic powder MnO–Fe2O3 composite—A novel material for the removal of azo-dye from water. Water. Res. 2005, 39, 630–638. [Google Scholar] [CrossRef]
- Shi, B.; Li, G.; Wang, D.; Feng, C.; Tang, H. Removal of direct dyes by coagulation: The performance of preformed polymeric aluminum species. J. Hazard. Mater. 2007, 143, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.; Ahmed Basha, C. Chemical or electrochemical techniques, followed by ion exchange, for recycle of textile dye wastewater. J. Hazard. Mater. 2007, 149, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Abbasi, M. Synthesis and characterization of magnetic nanocomposite of chitosan/SiO2/carbon nanotubes and its application for dyes removal. J. Clean. Prod. 2017, 145, 105–113. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; DasGupta, S.; De, S. Removal of congo red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Gupta, V.K. Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J. Colloid. Interface 2009, 340, 16–26. [Google Scholar] [CrossRef]
- Han, R.; Ding, D.; Xu, Y.; Zou, W.; Wang, Y.; Li, Y.; Zou, L. Use of rice husk for the adsorption of congo red from aqueous solution in column mode. Bioresour. Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Removal of congo red from aqueous solution by bagasse fly ash and activated carbon: Kinetic study and equilibrium isotherm analyses. Chemosphere 2005, 61, 492–501. [Google Scholar] [CrossRef]
- Koli, D.K.; Agnihotri, G.; Purohit, R. A Review on Properties, Behaviour and Processing Methods for Al- Nano Al2O3 Composites. Procedia Mater. Sci. 2014, 6, 567–589. [Google Scholar] [CrossRef]
- Peng, L.; Xu, X.; Lv, Z.; Song, J.; He, M.; Wang, Q.; Yan, L.; Li, Y.; Li, Z. Thermal and morphological study of Al2O3 nanofibers derived from boehmite precursor. J. Therm. Anal. Calorim. 2012, 110, 749–754. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Xu, X.; He, M.; Li, Z.; Wang, Q.; Yan, L. Organic-free Synthesis of Boehmite Nanofibers by Al2(SO4)3·18H2O with High Pore Volume. Ind. Eng. Chem. Res. 2013, 52, 7752–7757. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Li, Z.; Lv, Z.; Song, J.; He, M.; Wang, Q.; Yan, L.; Li, Z. Thermal study of boehmite nanofibers with controlled particle size. J. Therm. Anal. Calorim. 2014, 115, 1111–1117. [Google Scholar] [CrossRef]
- Sun, J.C.; Wang, X.H.; Chen, S.; Liao, Y.; Gao, A.; Hu, Y.; Yang, T.; Xu, X.; Wang, Y.; Song, J. Defluoridation of Water Using Active Alumina Derived from Single-Layer Boehmite. Acta Phys.-Chim. Sin. 2021, 37, 1911009. [Google Scholar]
- Wu, Y.-S.; Ma, J.; Hu, F. Thermal stabilization of γ-Al2O3 with modification of polyethylene glycol by reverse precipitation method. Adv. Mater. Res. 2012, 560–561, 169–173. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Y.; Lai, X.; Wu, Y.; Du, F.; Wang, C. Facile synthesis of mesoporous Si-containing γ-Al2O3 nanofiber with enhanced thermal stability. Chem. Res. Chin. Univ. 2015, 31, 156–159. [Google Scholar] [CrossRef]
- Al-Salihi, S.; Jasim, A.M.; Fidalgo, M.M.; Xing, Y. Removal of Congo red dyes from aqueous solutions by porous γ-alumina nanoshells. Chemosphere 2022, 286, 131769. [Google Scholar] [CrossRef]
- Li, F.; Wan, L.S.; Wang, Y.Q.; Wang, Y.J. Template-free method for the synthesis of high-pore-volume γ-Al2O3 nanofibers in a membrane dispersion microreactor. Nanotechnology 2021, 32, 185601. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, X.; Zhang, X.; Lin, Q. Facile preparation of ultrathin-wall graphitic mesoporous carbon containing graphene sheets with desirable adsorption performance for organic dyes. J. Mol. Liq. 2020, 319, 114306. [Google Scholar] [CrossRef]
- Bai, P.; Wu, P.; Yan, Z.; Zhao, X.S. A reverse cation–anion double hydrolysis approach to the synthesis of mesoporous γ-Al2O3 with a bimodal pore size distribution. Micropor. Mesopor. Mat. 2009, 118, 288–295. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Shi, M.; Qiu, R.R.; Li, Q.Z.; Liao, Q.; Yang, W.C.; Si, M.Y.; Wang, Q.W.; Yang, Z.H. The mechanism of cobalt enrichment by biogenic manganese oxides with different average oxidation states. J. Environ. Chem. Eng. 2025, 13, 115412. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Feng, L.J.; Li, J.L.; Ou, Y.Y.; Liu, A.R. Study on the Pb(II) adsorption mechanism on acid-modified ceramsite made from sewage sludge and industrial solid wastes. Int. J. Appl. Ceram. Technol. 2024, 21, 3389–3400. [Google Scholar] [CrossRef]
- Li, Z.; Du, Y.; Zhang, S.; Chen, Z.; Yang, K.; Lv, X.; Zhu, C. Synthesis and characterization of hierarchical γ-AlOOH and γ-Al2O3 microspheres with high adsorption performance for organic dyes. RSC Adv. 2016, 6, 89699–89707. [Google Scholar] [CrossRef]
- Adesina, A.O.; Elvis, O.A.; Mohallem, N.D.S.; Olusegun, S.J. Adsorption of Methylene blue and Congo red from aqueous solution using synthesized alumina–zirconia composite. Environ. Technol. 2021, 42, 1061–1070. [Google Scholar] [CrossRef]
- Zhao, S.; Wen, Y.; Du, C.; Tang, T.; Kang, D. Introduction of vacancy capture mechanism into defective alumina microspheres for enhanced adsorption of organic dyes. Chem. Eng. J. 2020, 402, 126180. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Xie, F.; Chen, X.; Zhang, Y.; Zhang, Q. Magnetic Biochar Prepared with Rosa roxburghii Residue as Adsorbents for Congo Red Removal. Materials 2025, 18, 1306. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Lv, F.; Chen, J.; Wu, C.; Li, Y.; Shen, J.; Li, Y. Synthesis and Characterization of High-Purity Mesoporous Alumina with Excellent Adsorption Capacity for Congo Red. Materials 2022, 15, 970. [Google Scholar] [CrossRef]
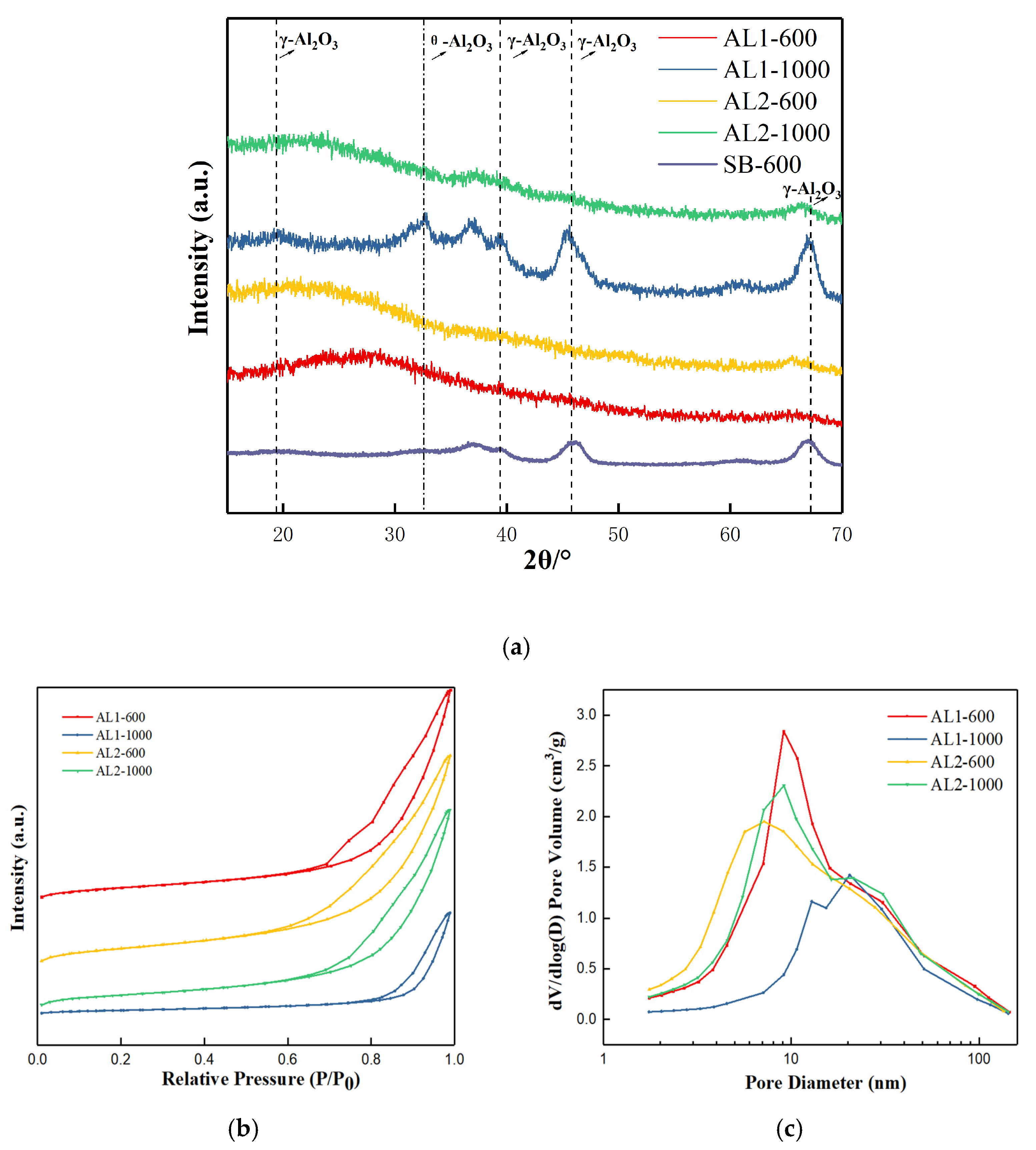
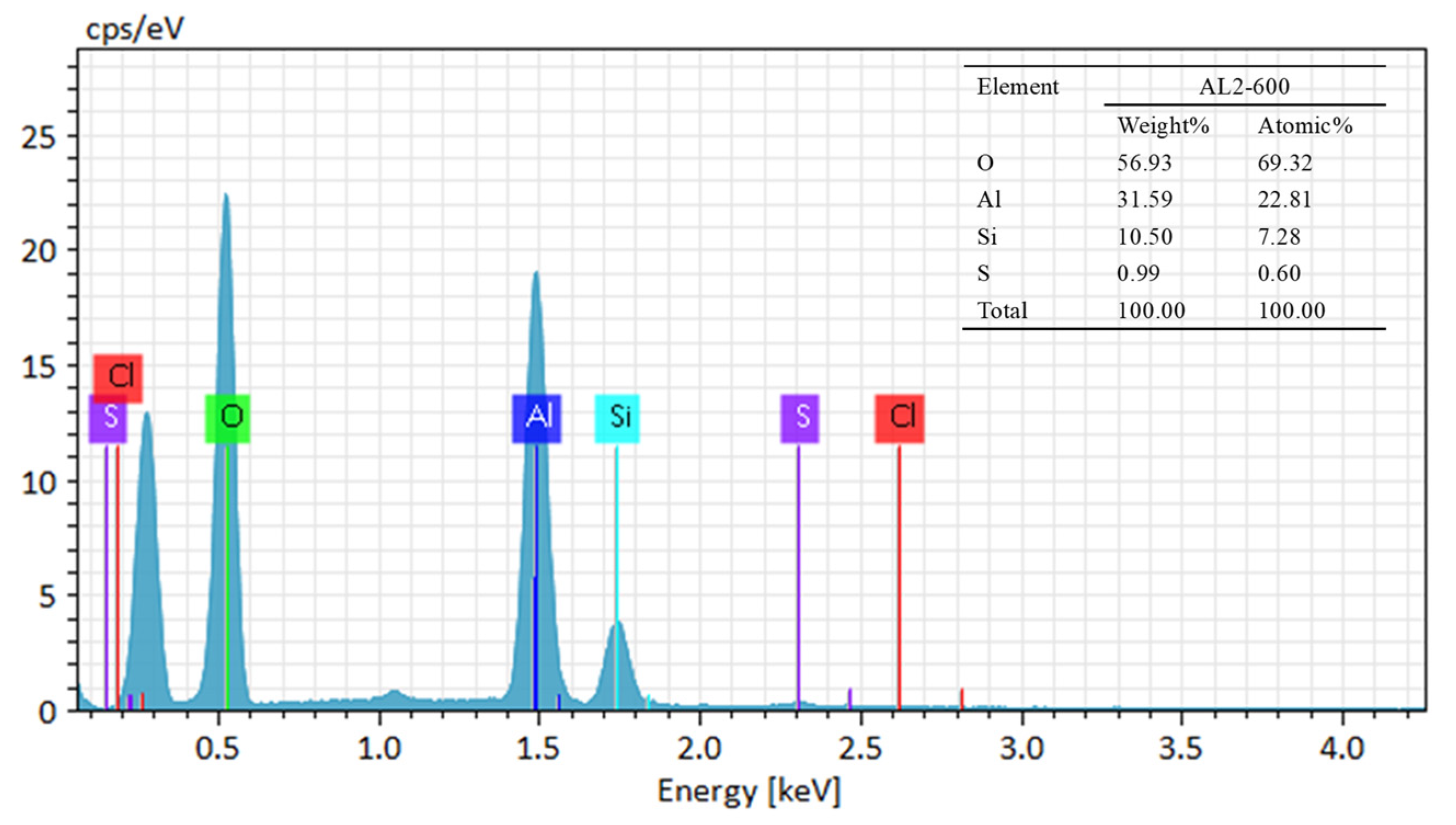




| BET Surface Area (m2/g) | BET Pore Volume (cm3/g) | |
|---|---|---|
| AL1-600 | 540.5 | 2.41 |
| AL1-1000 | 173.5 | 1.15 |
| AL2-600 | 686.2 | 2.33 |
| AL2-1000 | 496.0 | 2.25 |
| SB-600 | 180.4 | 0.53 |
| Adsorbent | qt-6 h (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/(mg∙min)) | R2 | ||
| AL1-600 | 822.6 | 228.8 | 9.189 × 10−4 | 0.95529 | 826.4 | 1.803 × 10−4 | 0.99997 |
| AL1-1000 | 183.4 | 78.5 | 0.01119 | 0.93887 | 185.5 | 5.609 × 10−4 | 0.99879 |
| AL2-600 | 260.3 | 88.3 | 0.006633 | 0.93092 | 261.8 | 3.454 × 10−5 | 0.99995 |
| AL2-1000 | 306.4 | 38.3 | 0.005090 | 0.92107 | 88.6 | 6.016 × 10−4 | 0.99941 |
| Adsorbent | Elovich | Intraparticle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | R2 | Kt1 (g/mg·min−1/2) | R2 | Kt2 (g/mg·min−1/2) | R2 | Kt3 (g/mg·min−1/2) | R2 | |
| AL1-600 | 358.82 | 79.86 | 0.89094 | 173.45 | 0.99896 | 38.17 | 0.96825 | 2.50 | 0.94357 |
| AL1-1000 | 51.15 | 22.43 | 0.91613 | 34.90 | 0.99999 | 22.32 | 0.99035 | 0.73 | 0.96650 |
| AL2-600 | 102.51 | 25.53 | 0.96166 | 51.88 | 0.97742 | 13.14 | 0.89520 | 1.78 | 0.95641 |
| AL2-1000 | 23.51 | 9.85 | 0.99423 | 15.37 | 0.99579 | 3.08 | 0.99999 | 1.02 | 0.99092 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF | 1/n | R2 | |
| AL1-600 | 869.5 | 0.2584 | 0.99734 | 684.2 | 0.0414 | 0.70632 |
| AL1-1000 | 205.3 | 0.01009 | 0.99830 | 151.1 | 0.1385 | 0.71412 |
| AL2-600 | 340.1 | 0.05909 | 0.97875 | 120.5 | 0.0934 | 0.96141 |
| AL2-1000 | 153.1 | 0.01701 | 0.94443 | 19.8 | 0.3406 | 0.89287 |
| Temkin | Dubinin–Radushkevich | ||||||
|---|---|---|---|---|---|---|---|
| bT (J/mol) | KT (L/mg) | R2 | qm (mg/g) | KD (mol2/kJ2) | E (kJ/mol) | R2 | |
| AL1-600 | 73.51 | 4.72·108 | 0.69493 | 822.8 | 0.000003 | 408.2 | 0.37262 |
| AL1-1000 | 145.22 | 525.59 | 0.954529 | 190.5 | 0.000032 | 125.0 | 0.75629 |
| AL2-600 | 63.85 | 16.27 | 0.70575 | 289.6 | 0.000027 | 136.1 | 0.40977 |
| AL2-1000 | 76.60 | 5.14 | 0.87057 | 107.9 | 0.000150 | 57.7 | 0.65687 |
| Number of Regenerations | AL1-600 | AL1-1000 | ||||
|---|---|---|---|---|---|---|
| Adsorption Capacity (mg/g) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Adsorption Capacity (mg/g) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | |
| 0 | 822.6 | 540.5 | 2.41 | 183.3 | 161.7 | 1.09 |
| 1 | 466.7 | 451.8 | 1.76 | 180.0 | 158.6 | 1.09 |
| 2 | 432.9 | -- | -- | 179.0 | -- | -- |
| 3 | 376.1 | 348.0 | 1.38 | 175.6 | 162.9 | 1.06 |
| 4 | 347.2 | -- | -- | 159.7 | -- | -- |
| 5 | 278.1 | -- | -- | 157.9 | -- | -- |
| 6 | 244.4 | 303.8 | 1.25 | 156.0 | 152.1 | 1.05 |
| Number of Regenerations | AL2-600 | AL2-1000 | ||||
|---|---|---|---|---|---|---|
| Adsorption Capacity (mg/g) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Adsorption Capacity (mg/g) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | |
| 0 | 260.3 | 686.2 | 2.33 | 88.0 | 496.0 | 2.25 |
| 1 | 250.7 | 593.7 | 2.22 | 118.2 | 452.2 | 2.01 |
| 2 | 239.8 | -- | -- | 147.5 | -- | -- |
| 3 | 225.6 | 575.4 | 2.31 | 155.8 | 464.5 | 2.04 |
| 4 | 202.2 | -- | -- | 199.0 | -- | -- |
| 5 | 205.8 | -- | -- | 201.1 | -- | -- |
| 6 | 206.3 | 530.8 | 2.20 | 200.4 | 477.3 | 2.11 |
| Adsorbent | Adsorption Capacity mg/g | References |
|---|---|---|
| highly porous γ-Al2O3 nanoshells | 370.4 | [24] |
| mesoporous γ-Al2O3 nanofibers | 1323.7 | [25] |
| ultrathin-walled graphitic mesoporous carbon | 738.9 | [26] |
| γ-Al2O3 | 416.1 | [32] |
| alumina/zirconia composites | 60.0 | [33] |
| alumina microspheres | 1000.0 | [34] |
| magnetic biochar (MBC1:1) | 172.9 | [35] |
| HPMA nanopowder | 492.19 (at concentration of 250 mg/L) | [36] |
| non-modified alumina | 822.6 | This study |
| silicon-modified alumina | 260.3 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhao, Z.; Jiang, B.; Zhang, Y.; Wang, X.; Xu, X.; Song, J. Synthesis of Mesoporous Alumina with High Specific Surface Area via Reverse Precipitation Method for Enhanced Adsorption and Regeneration of Congo Red. Materials 2025, 18, 2656. https://doi.org/10.3390/ma18112656
Chen S, Zhao Z, Jiang B, Zhang Y, Wang X, Xu X, Song J. Synthesis of Mesoporous Alumina with High Specific Surface Area via Reverse Precipitation Method for Enhanced Adsorption and Regeneration of Congo Red. Materials. 2025; 18(11):2656. https://doi.org/10.3390/ma18112656
Chicago/Turabian StyleChen, Shuaiqi, Ziqiang Zhao, Boning Jiang, Yuanchao Zhang, Xuhui Wang, Xiangyu Xu, and Jiaqing Song. 2025. "Synthesis of Mesoporous Alumina with High Specific Surface Area via Reverse Precipitation Method for Enhanced Adsorption and Regeneration of Congo Red" Materials 18, no. 11: 2656. https://doi.org/10.3390/ma18112656
APA StyleChen, S., Zhao, Z., Jiang, B., Zhang, Y., Wang, X., Xu, X., & Song, J. (2025). Synthesis of Mesoporous Alumina with High Specific Surface Area via Reverse Precipitation Method for Enhanced Adsorption and Regeneration of Congo Red. Materials, 18(11), 2656. https://doi.org/10.3390/ma18112656






