Evaluating Optical Properties of Mixed-Phase 2D MoSe2/Poly(vinyl alcohol) Nanocomposite Film
Abstract
1. Introduction
2. Materials
2.1. Synthesis of Graphene-like 2D MoSe2
2.2. Preparation of PVA/2D MoSe2 Nanocomposite Film
2.3. Characterization Technique
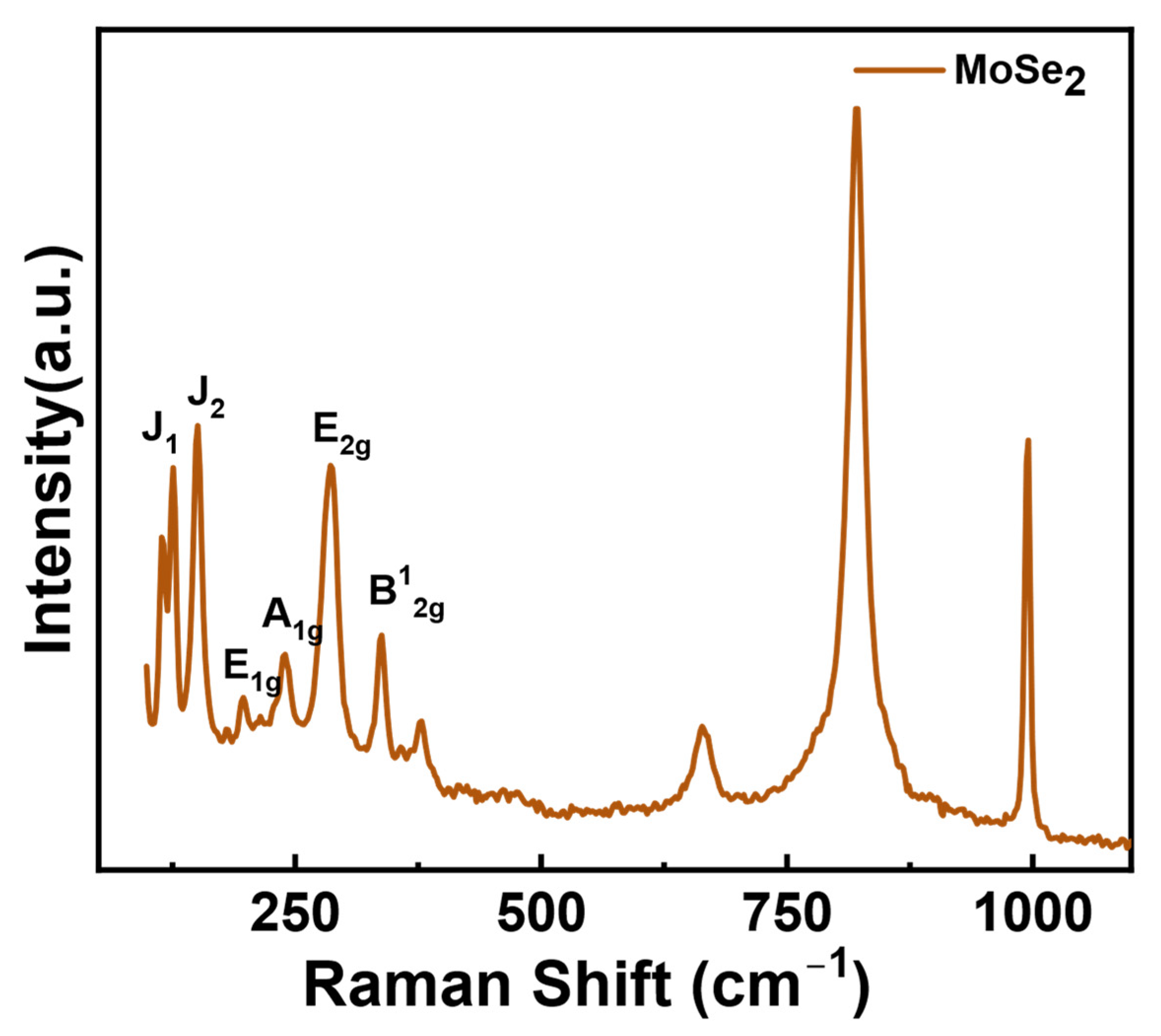
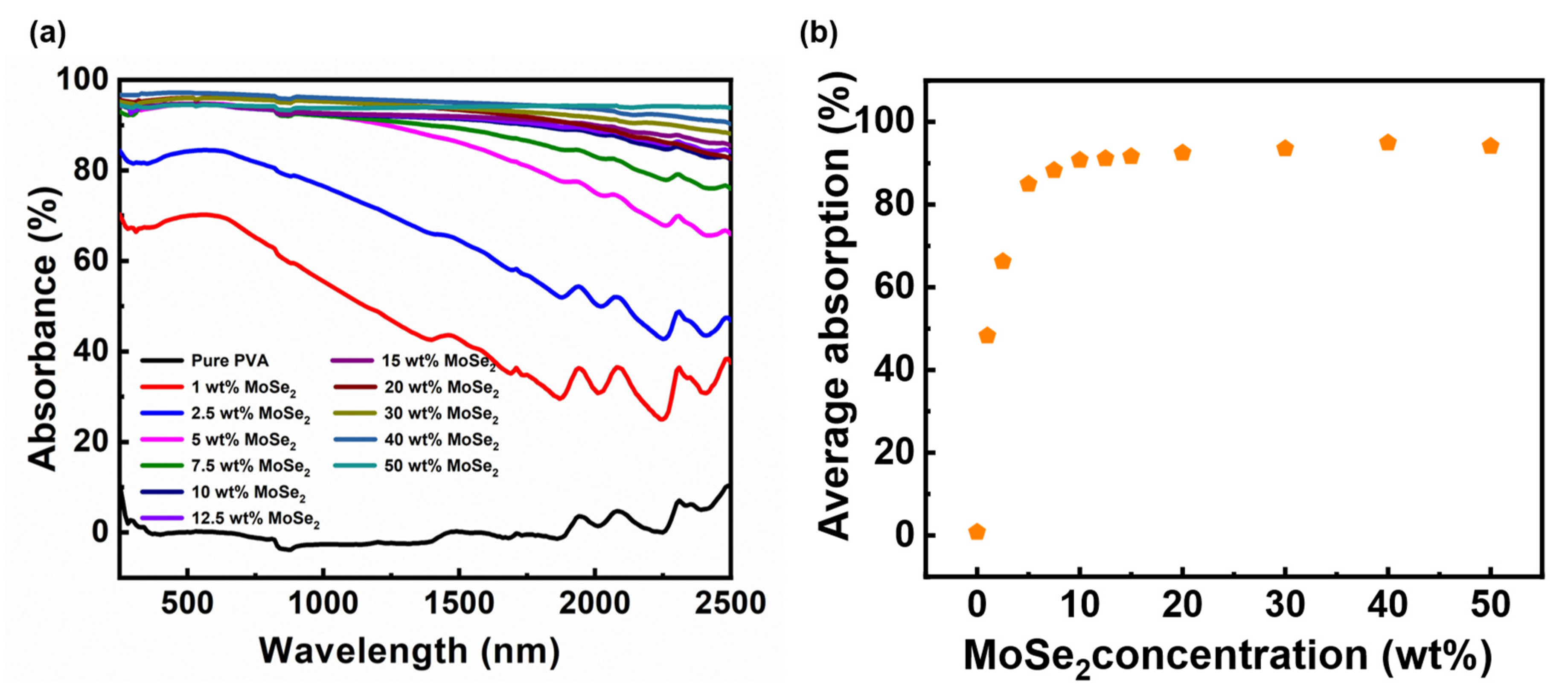
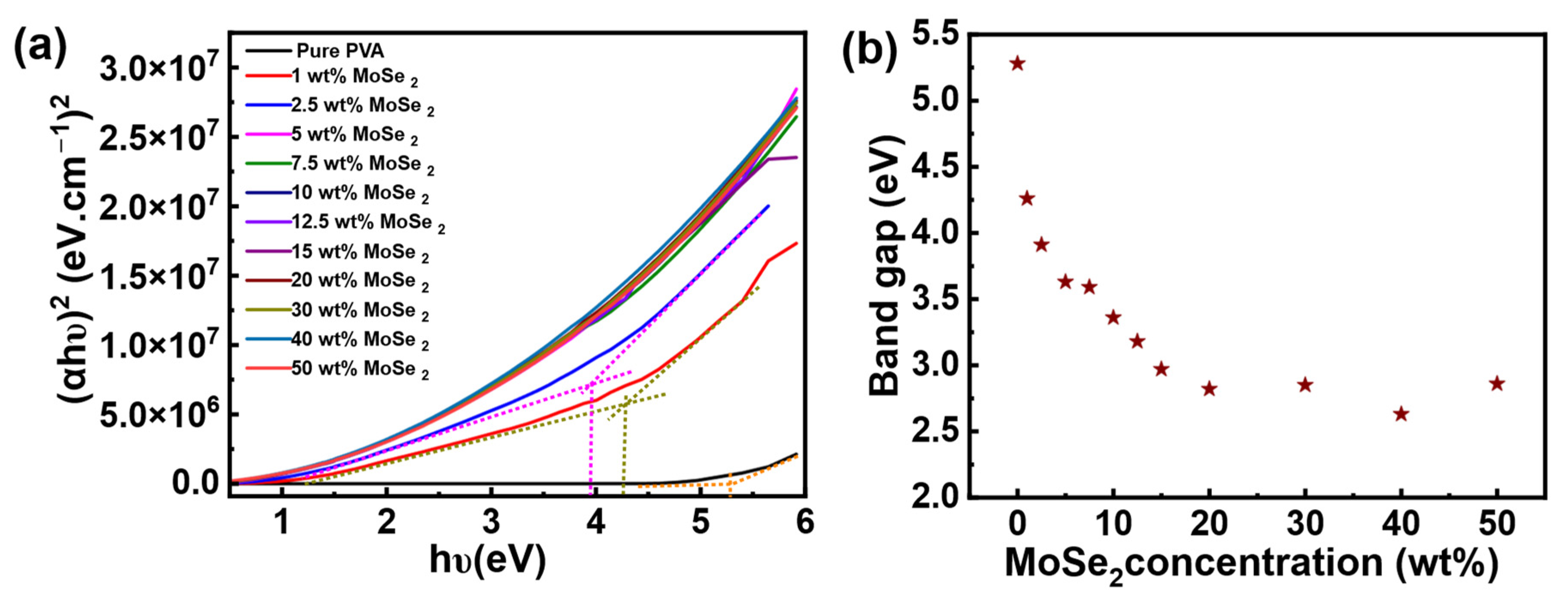
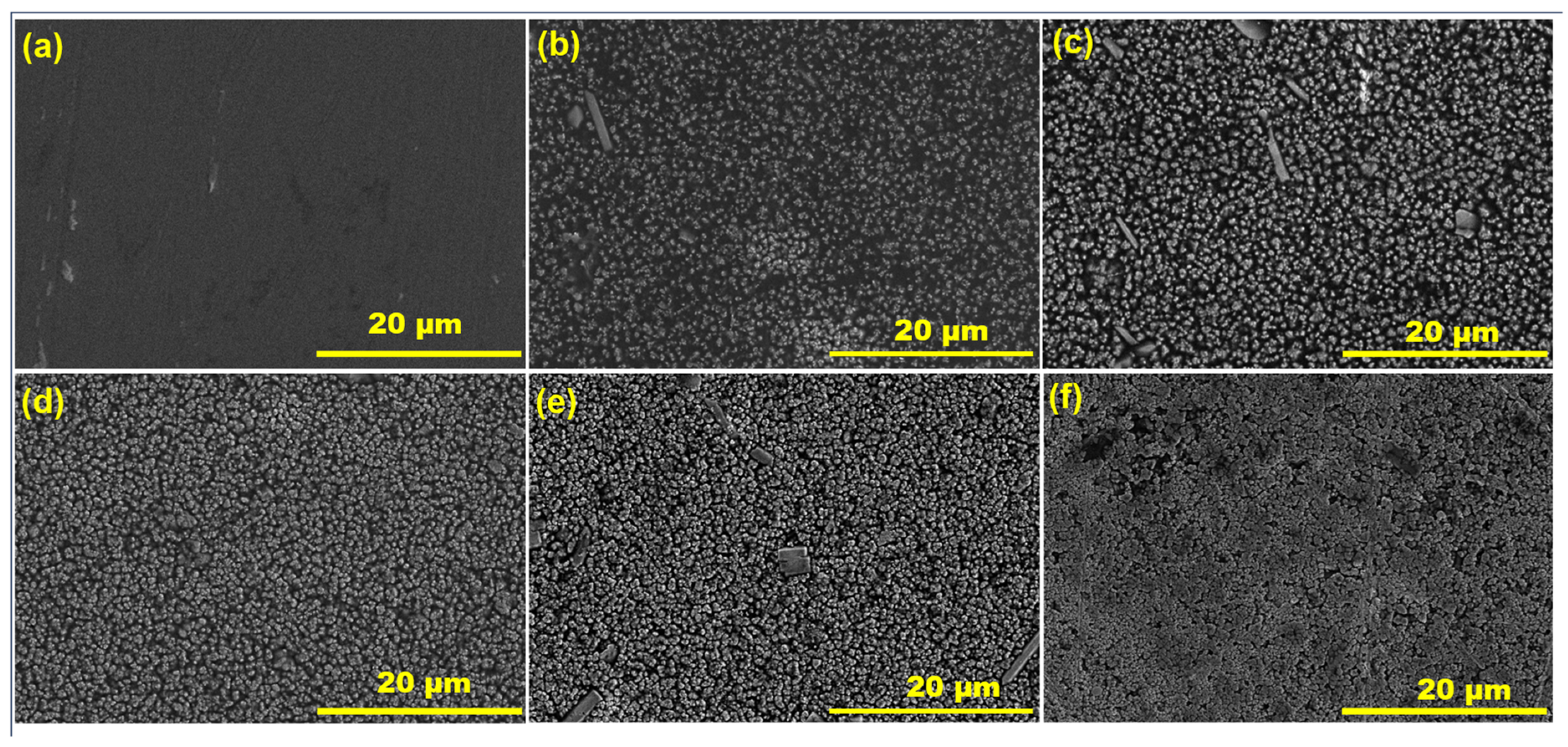
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hemalatha, K.S.; Rukmani, K. Synthesis, Characterization and Optical Properties of Polyvinyl Alcohol–Cerium Oxide Nanocomposite Films. RSC Adv. 2016, 6, 74354–74366. [Google Scholar] [CrossRef]
- Banerjee, M.; Jain, A.; Mukherjee, G.S. Microstructural and Optical Properties of Polyvinyl Alcohol/Manganese Chloride Composite Film. Polym. Compos. 2019, 40, E765–E775. [Google Scholar] [CrossRef]
- Ali, S.A.; Sau, S.; Kundu, S. In-Situ Fabrication of CuO Nanoparticles inside PVA Matrix via Heat Treatment with Modified Optical, Electrical and Mechanical Properties of the Composite Films. Colloid Surf. A Physicochem. Eng. Asp. 2023, 674, 131840. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Omer, K.M.; Salih, Y.M. Reducing the Optical Band Gap of Polyvinyl Alcohol (PVA) Based Nanocomposite. J. Mater. Sci. Mater. Electron. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Suma, G.R.; Subramani, N.K.; Shilpa, K.N.; Sachhidananda, S.; Satyanarayana, S.V. Siddaramaiah Effect of Ce0.5Zr0.5O2 Nano Fillers on Structural and Optical Behaviors of Poly(Vinyl alcohol). J. Mater. Sci. Mater. Electron. 2017, 28, 10707–10714. [Google Scholar] [CrossRef]
- Darwesh, A.H.; Aziz, S.B.; Hussen, S.A. Insights into Optical Band Gap Identification in Polymer Composite Films Based on PVA with Enhanced Optical Properties: Structural and Optical Characteristics. Opt. Mater. 2022, 133, 113007. [Google Scholar] [CrossRef]
- Ghanipour, M.; Dorranian, D. Effect of Ag-Nanoparticles Doped in Polyvinyl Alcohol on the Structural and Optical Properties of PVA Films. J. Nanomater. 2013, 2013, 897043. [Google Scholar] [CrossRef]
- Bulla, S.S.; Bhajantri, R.F.; Chavan, C. Optical and Structural Properties of Biosynthesized Silver Nanoparticle Encapsulated PVA (Ag–PVA) Films. J. Inorg. Organomet. Polym. 2021, 31, 2368–2380. [Google Scholar] [CrossRef]
- Venkata Subba Rao, C.; Ravi, M.; Raja, V.; Balaji Bhargav, P.; Sharma, A.K.; Narasimha Rao, V.V.R. Preparation and Characterization of PVP-Based Polymer Electrolytes for Solid-State Battery Applications. Iran. Polym. J. 2012, 21, 531–536. [Google Scholar] [CrossRef]
- Meng, N.; Zhu, X.; Lian, F. Particles in Composite Polymer Electrolyte for Solid-State Lithium Batteries: A Review. Particuology 2022, 60, 14–36. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, K.P.; Tripathi, S.K. Electrical, Linear and Non-Linear Optical Properties of MoSe2/PVA Nanocomposites as Charge Trapping Elements for Memory Device Applications. J. Alloys Compd. 2022, 905, 164103. [Google Scholar] [CrossRef]
- Jain, A.; Banerjee, M.; Mukherjee, G.S. Effect of UV Irradiation on Optical Properties of Pristine and MnCl2 Doped PVA Films. Mater. Today Proc. 2021, 47, 697–703. [Google Scholar] [CrossRef]
- Naser, H.; Shanshool, H.M.; Mohammad, S.M.; Hassan, Z.; Abbas, A.M.A.; Abed, S.M.; Sifawa, A.A. The Role of the Polymer Matrix on the Energy Band Gap of Nanocomposites of Aluminium, Silver and Zinc Oxide. Plasmonics 2024, 1–15. [Google Scholar] [CrossRef]
- Vanitha, N.; Kanchana, S.K.; Basavaraj, R.B.; Watage, S.M. Structural and Optical Properties of Polyvinyl Alcohol/Zinc Oxide Nanocomposites. Phys. Status Solidi A 2023, 220, 2300052. [Google Scholar] [CrossRef]
- Anand, K.; Kaur, R.; Arora, A.; Tripathi, S.K. Tuning of Linear and Non-Linear Optical Properties of MoS2/PVA Nanocomposites via Ultrasonication. Opt. Mater. 2023, 137, 113523. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Yao, Y.; Shao, Y.; Wu, X. Graphene-like 2H/1T-MoSe2 with Superior Full Spectrum Absorption: Morphology and Phase Engineering. J. Alloys Compd. 2021, 877, 160317. [Google Scholar] [CrossRef]
- Nandihalli, N. Performance analysis of photodetectors based on 2D materials and heterostructures. Crit. Rev. Solid State Mater. Sci. 2024, 1–87. [Google Scholar] [CrossRef]
- Guan, X.; Yu, X.; Periyanagounder, D.; Benzigar, M.R.; Huang, J.-K.; Lin, C.-H.; Kim, J.; Singh, S.; Hu, L.; Liu, G.; et al. Recent Progress in Short- to Long-Wave Infrared Photodetection Using 2D Materials and Heterostructures. Adv. Opt. Mater. 2021, 9, 2001708. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Pan, H.; Chu, H.; Li, Y.; Zhao, S.; Li, D. Comprehensive Study on the Nonlinear Optical Properties of Few-Layered MoSe2 Nanosheets at 1 Μm. J. Alloys Compd. 2019, 806, 52–57. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, D.; Ouyang, B.; Raja, A.; Song, J.; Heinz, T.F.; Brus, L.E. Probing the Dynamics of the Metallic-to-Semiconducting Structural Phase Transformation in MoS2 Crystals. Nano Lett. 2015, 15, 5081–5088. [Google Scholar] [CrossRef]
- Thomas, M.; Veeralingam, S.; Badhulika, S. MoSe2/PVA-Based Wearable Multi-Functional Platform for Pulse Rate Monitoring, Skin Hydration Sensor, and Human Gesture Recognition Utilizing Electrophysiological Signals. J. Appl. Phys. 2022, 132, 224303. [Google Scholar] [CrossRef]
- Bi, D.; Li, Y.; Yao, Y.; Tao, T.; Liang, B.; Lu, S. Preparation and Characterizations of Flexible Photothermal Ti2O3-PVA Nanocomposites. J. Alloys Compd. 2020, 825, 153998. [Google Scholar] [CrossRef]
- Shen, X.; Ji, M.; Zhang, S.; Qin, Y.; Zhang, P.; Wang, Y.; Guo, Z.-X.; Pan, M.; Zhang, Z. Fabrication of Multi-Walled Carbon- Nanotube-Grafted Polyvinyl-Chloride Composites with High Solar-Thermal-Conversion Performance. Compos. Sci. Technol. 2019, 170, 77–84. [Google Scholar] [CrossRef]
- Chhetri, S.; Nguyen, A.T.; Song, S.; Gaillard, N.; Severa, G.; Ma, T.; Yoon, S.-H.; Lee, W. Flexible Graphite Nanoflake/Polydimethylsiloxane Nanocomposites with Promising Solar-Thermal Conversion Performance. ACS Appl. Energy Mater. 2023, 6, 2582–2593. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, B.; Cheng, H.; Zhang, Q.; Zhang, B. Hierarchical 2H/1T-MoSe2@graphene on Cotton Fabric for Multifunctional and Flexible Microwave Absorption. Carbon 2023, 205, 562–572. [Google Scholar] [CrossRef]
- Atta, A.; Abdeltwab, E. Influence of Ion Irradiation on the Surface Properties of Silver-Coated Flexible PDMS Polymeric Films. Braz. J. Phys. 2022, 52, 3. [Google Scholar] [CrossRef]
- Borah, B.; Rajitha, G.; Dash, R.K. Correlation between the Thickness and Properties of the Ethanol Treated GO–PDMS Based Composite Materials. J. Mater. Sci. Mater. Electron. 2018, 29, 20216–20224. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc Method for Optical Absorption Edge Determination: ZnO Thin Films as a Model System: Tauc Method for Optical Absorption Edge Determination. Phys. Status Solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Jubu, P.R.; Yam, F.K.; Igba, V.M.; Beh, K.P. Tauc-Plot Scale and Extrapolation Effect on Bandgap Estimation from UV–Vis–NIR Data—A Case Study of β-Ga2O3. J. Solid State Chem. 2020, 290, 121576. [Google Scholar] [CrossRef]
- Coulter, J.B.; Birnie, D.P. Assessing Tauc Plot Slope Quantification: ZnO Thin Films as a Model System. Phys. Status Solidi B 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Khairy, Y.; Yahia, I.S.; Elhosiny Ali, H. Facile Synthesis, Structure Analysis and Optical Performance of Manganese Oxide-Doped PVA Nanocomposite for Optoelectronic and Optical Cut-off Laser Devices. J. Mater. Sci. Mater. Electron. 2020, 31, 8072–8085. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhetri, S.; Nguyen, A.T.; Gaillard, N.; Lee, W. Evaluating Optical Properties of Mixed-Phase 2D MoSe2/Poly(vinyl alcohol) Nanocomposite Film. Materials 2024, 17, 4178. https://doi.org/10.3390/ma17174178
Chhetri S, Nguyen AT, Gaillard N, Lee W. Evaluating Optical Properties of Mixed-Phase 2D MoSe2/Poly(vinyl alcohol) Nanocomposite Film. Materials. 2024; 17(17):4178. https://doi.org/10.3390/ma17174178
Chicago/Turabian StyleChhetri, Suman, Anh Tuan Nguyen, Nicolas Gaillard, and Woochul Lee. 2024. "Evaluating Optical Properties of Mixed-Phase 2D MoSe2/Poly(vinyl alcohol) Nanocomposite Film" Materials 17, no. 17: 4178. https://doi.org/10.3390/ma17174178
APA StyleChhetri, S., Nguyen, A. T., Gaillard, N., & Lee, W. (2024). Evaluating Optical Properties of Mixed-Phase 2D MoSe2/Poly(vinyl alcohol) Nanocomposite Film. Materials, 17(17), 4178. https://doi.org/10.3390/ma17174178









