A Novel Hydro-Thermal Synthesis of Nano-Structured Molybdenum-Iron Intermetallic Alloys at Relatively Low Temperatures
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Materials and Characterizations
2.2. Reduction Procedure
3. Results and Discussion
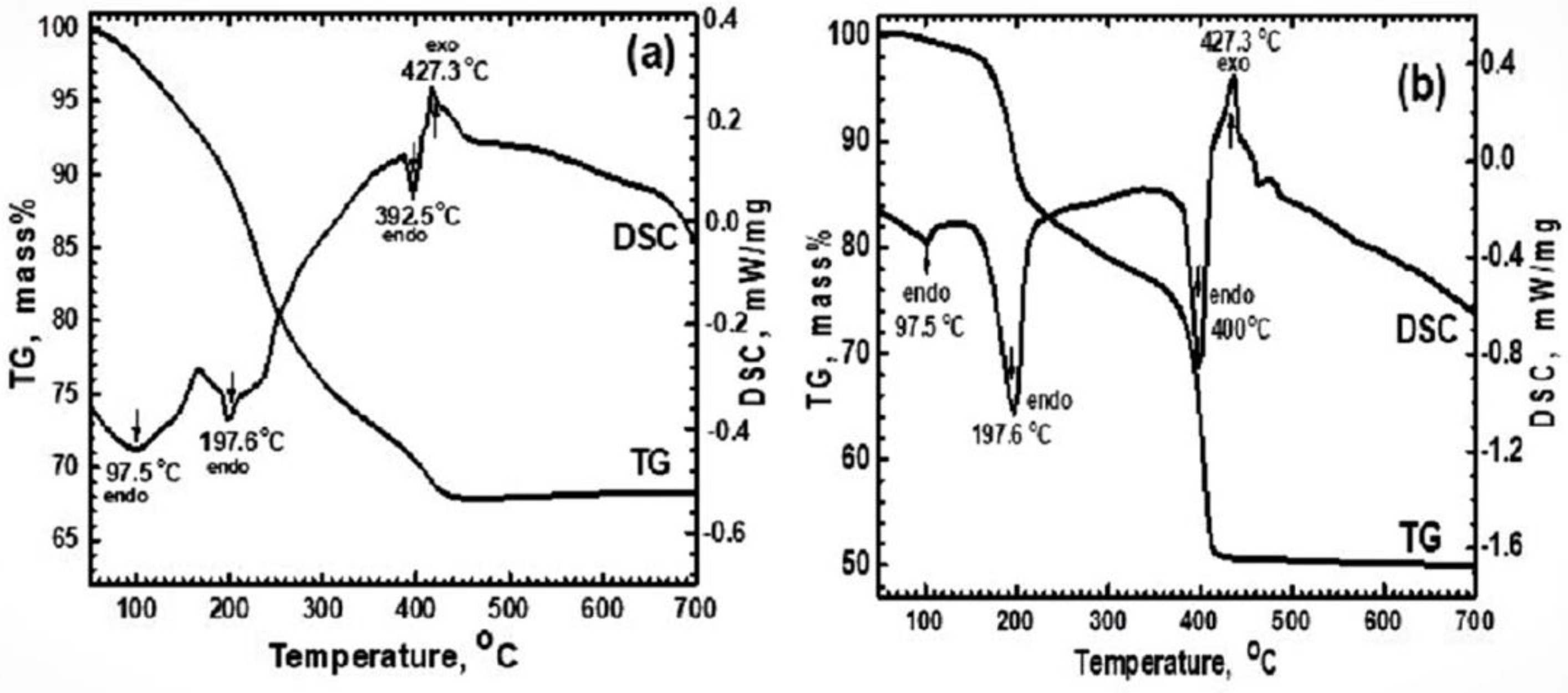
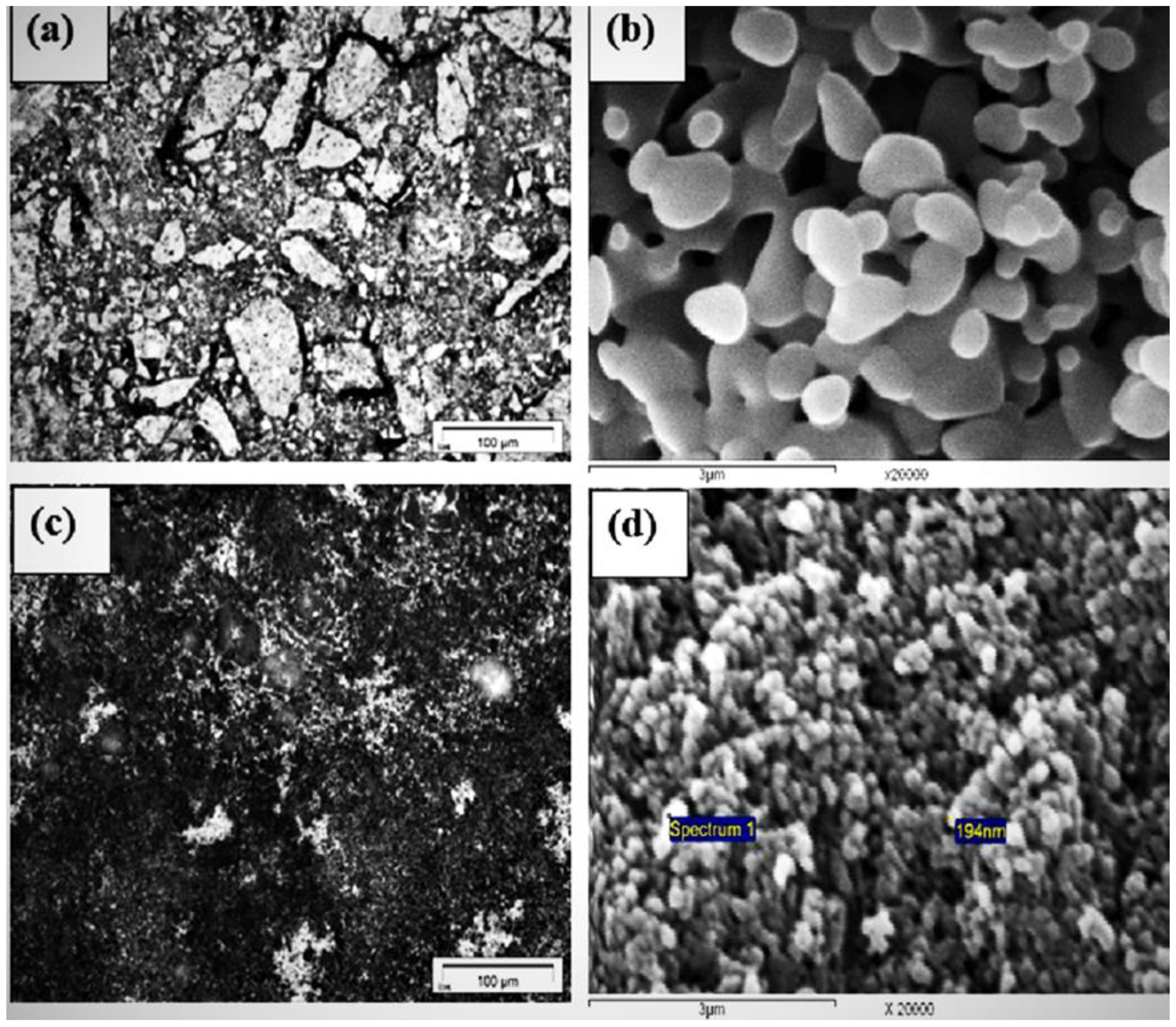
3.1. Characterization of Materials
3.2. Reduction Behavior
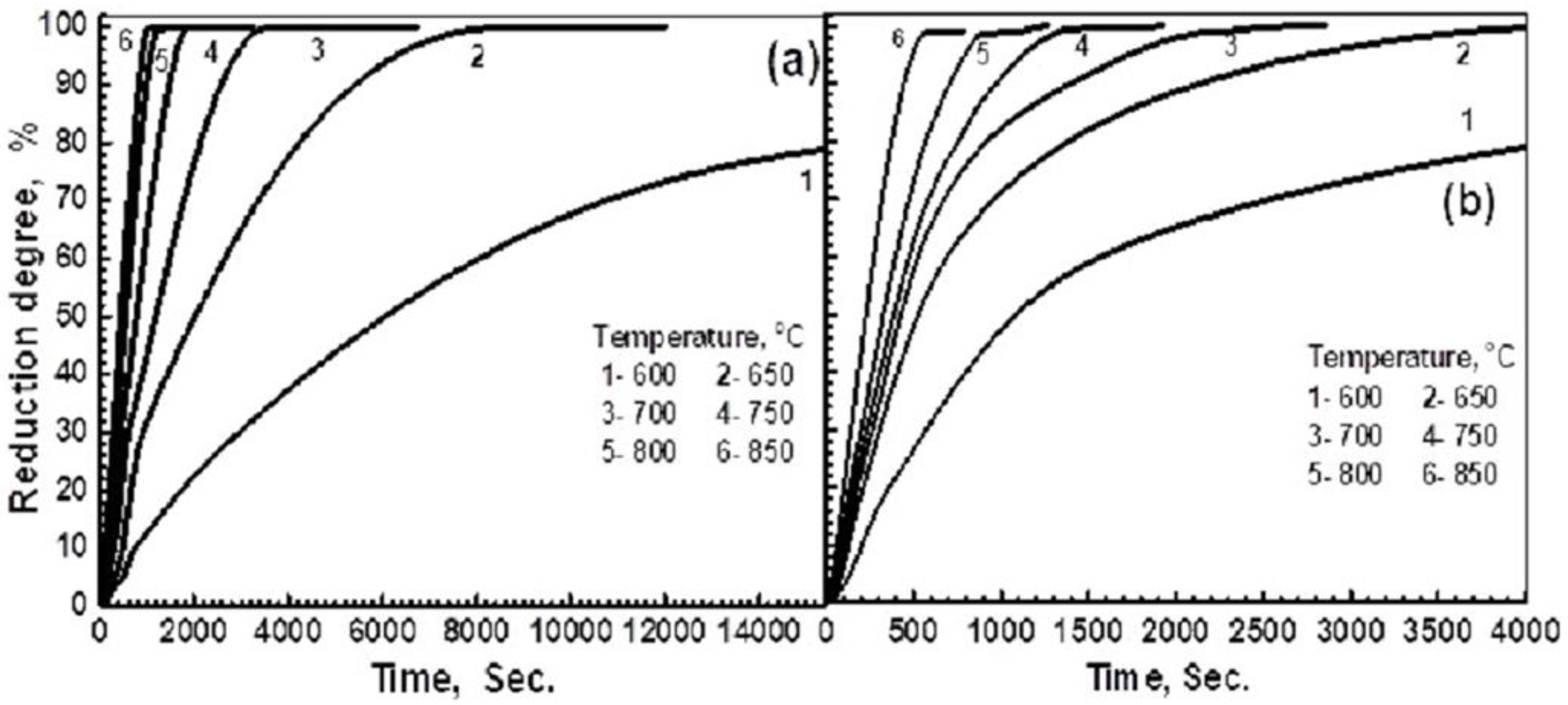
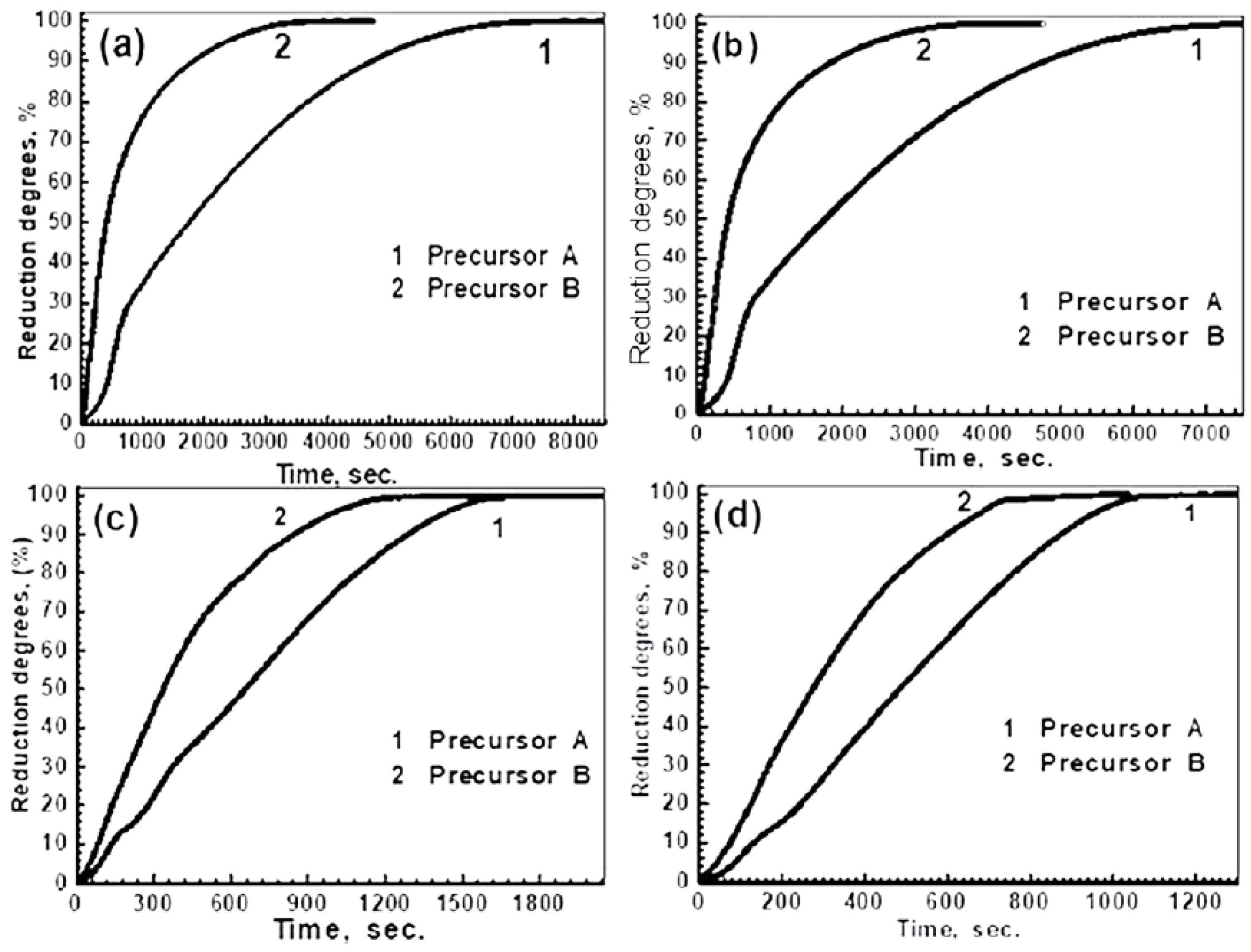
3.2.1. Influence of Reduction Temperature
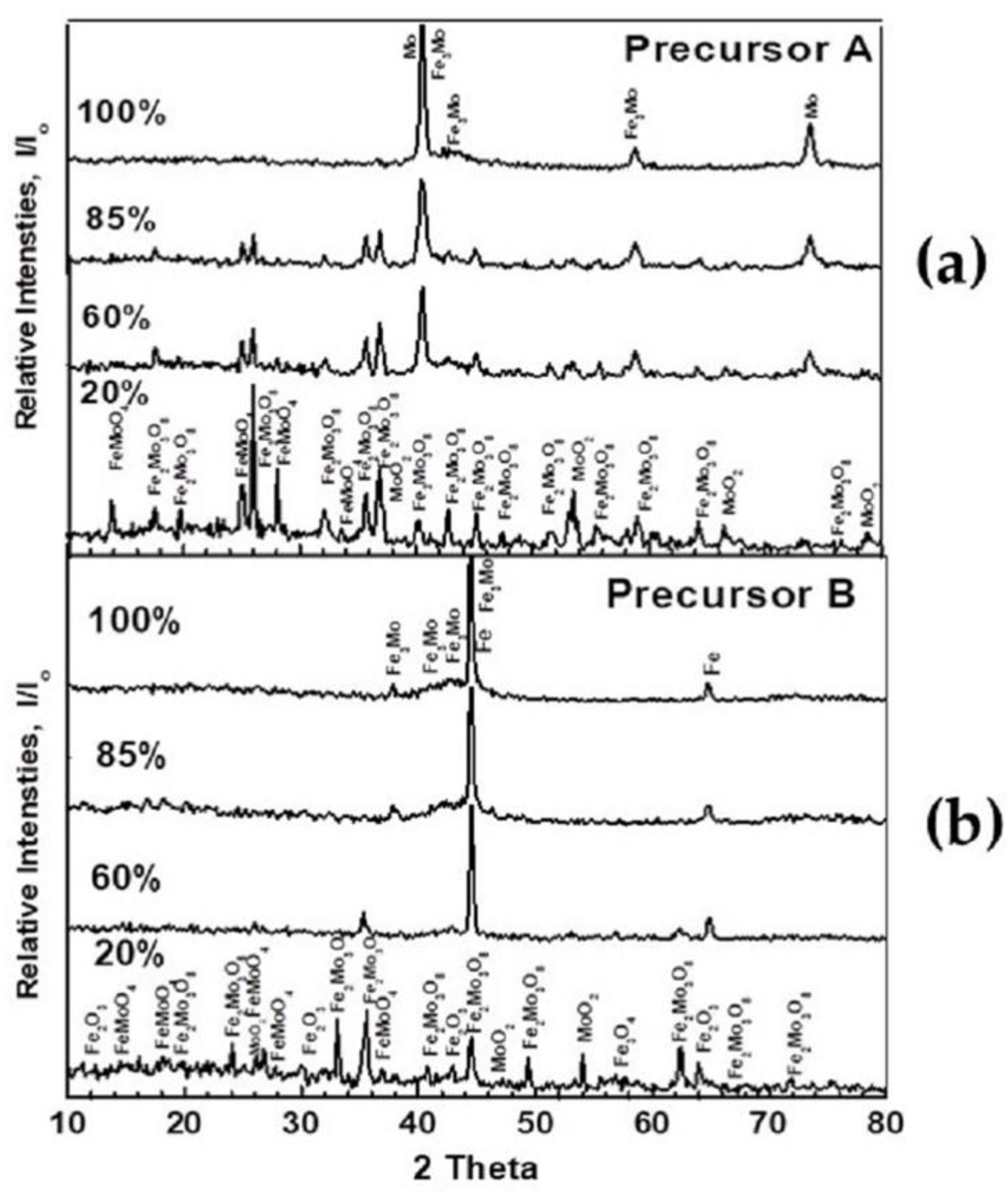
3.2.2. Influence of Precursor Composition
3.3. Chemistry of Intermetallics Formation
- The chemical reaction equations of precursor A, [100% Fe2(MoO4)3]
MoO2 + 2 H2 = Mo + 2 H2O
- 2.
- The chemical reaction equations of precursor (B), [46.68% Fe3(MoO4)3 + 53.32% Fe2O3];
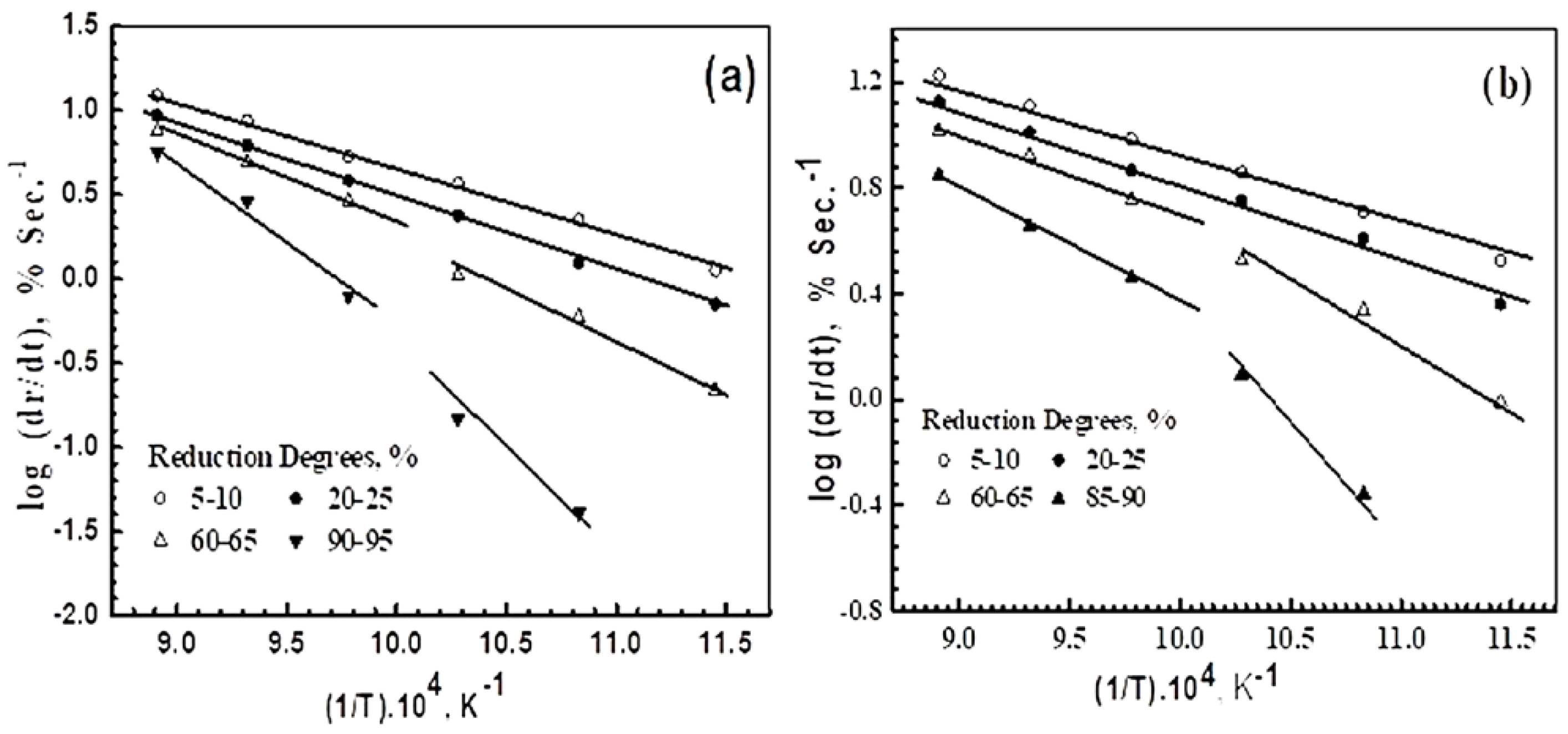
3.4. Reduction Kinetics and Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biserova-Tahchieva, A.; Biezma-Moraleda, M.V.; Llorca-Isern, N.; Gonzalez-Lavin, J.; Linhardt, P. Additive Manufacturing Processes in Selected Corrosion Resistant Materials: A State of Knowledge Review. Materials 2023, 16, 1893. [Google Scholar] [CrossRef] [PubMed]
- Bahgat, M.; Paek, M.K.; Pak, J.J. Hydrogen Reduction of Fe2O3/WO3 Mixture with Synthesis of Nanocrystalline Fe/W Composite. Mater. Trans. 2008, 49, 1480. [Google Scholar] [CrossRef]
- Lakshmikanthan, A.; Angadi, S.; Malik, V.; Saxena, K.K.; Prakash, C.; Dixit, S.; Mohammed, K.A. Mechanical and Tribological Properties of Aluminum-Based Metal-Matrix Composites. Materials 2022, 15, 6111. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Olekšáková, D.; Kollár, P.; Neslušan, M.; Jakubčin, M.; Füzer, J.; Bureš, R.; Fáberová, M. Impact of the Surface Irregularities of NiFeMo Compacted Powder Particles on Irreversible Magnetization Processes. Materials 2022, 15, 8937. [Google Scholar] [CrossRef]
- Ahmed, H.M.; El-Geassy, A.A.; Seetheraman, S. Kinetic studies of hydrogen reduction of NiO-WO3 precursors in fluidized bed reactor. ISIJ Int. 2011, 51, 1359–1367. [Google Scholar] [CrossRef]
- Abdel Halim, K.S. Novel synthesis of porous Fe–Ni ferroalloy powder for energy applications. Mater. Lett. 2012, 68, 478. [Google Scholar] [CrossRef]
- Nasr, M.I.; Omar, A.A.; Khedr, M.H.; EL Geassy, A.A. Effect of nickel oxide iron oxide reduction doping on the kinetics and mechanism of iron oxide reduction. ISIJ Int. 1995, 35, 1043–1049. [Google Scholar] [CrossRef]
- Simić, L.; Stopic, S.; Friedrich, B.; Zadravec, M.; Jelen, Ž.; Bobovnik, R.; Anžel, I.; Rudolf, R. Synthesis of Complex Concentrated Nanoparticles by Ultrasonic Spray Pyrolysis and Lyophilisation. Metals 2022, 12, 1802. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Bram, M.; Buchkremer, H.P.; Bahgat, M. Synthesis of heavy tungsten alloy by thermal technique. Ind. Eng. Chem. Res. 2012, 51, 16354–16360. [Google Scholar] [CrossRef]
- Al-Kelesh, H.; Abdel Halim, K.S.; Nasr, M.I. Synthesis of heavy tungsten alloys via powder reduction technique. J. Mater. Res. 2016, 31, 2977–2986. [Google Scholar] [CrossRef]
- El-Geassy, A.A. Gaseous reduction of pure Fe2O3 and MgO-doped Fe2O3 compacts with carbon monoxide at 1173–1473 K. J. Iron Steel Inst. Jpn. Int. 1996, 36, 1328–1337. [Google Scholar] [CrossRef]
- El-Geassy, A.A. Gaseous reduction of Fe2O3 compacts at 600–1050 °C. J. Mater. Sci. 1986, 21, 3889–3900. [Google Scholar] [CrossRef]
- Tremel, W.; Kleinke, H.; Derstroff, V.; Reisner, C. Transition metal chalcogenides: New views on an old topic. J. Alloy. Compd. 1995, 219, 73–82. [Google Scholar] [CrossRef]
- King, P.L.; Fegley, B.; Seward, T. High Temperature Gas-Solid Reactions in Earth and Planetary Processes. Rev. Mineral. Geochem. 2018, 84, 4. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Ramadan, M.; Shawabkeh, A.; Abufara, A. Synthesis and characterization of metallic materials for membrane technology. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 72–79. [Google Scholar] [CrossRef]
- Arun, R. Size-dependent fracture characteristics of intermetallic alloys. Exp. Mech. 2022, 62, 863–877. [Google Scholar] [CrossRef]
- Metzger, F.; Rienzi, V.; Mascetti, C.; Nguyen, T.; Pimputkar, S. Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures. Materials 2022, 15, 5270. [Google Scholar] [CrossRef]
- Panel on Intermetallic Development. Intermetallic Alloy Development: A Program Evaluation; Panel on Intermetallic Development, National Research Council (U.S.) Publication NMAB-487-1; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Song, C.; Wang, S.; Gui, Y.; Cheng, Z.; Ni, G. Microstructure and Tribological Properties of Mo–40Ni–13Si Multiphase Intermetallic Alloy. Materials 2016, 9, 986. [Google Scholar] [CrossRef]
- Mendiratta, M.G.; Dimiduk, D.M.; Kim, Y.W.; Miracle, D.B. Recent progress on intermetallic alloys for advanced aerospace systems. ISIJ Int. 2008, 31, 1223–1234. [Google Scholar]
- Gostishchev, V.V.; Astapov, I.A.; Khimukhin, S.N. Exothermic synthesis of cast nickel aluminide alloys with tungsten and molybdenum Carbides. Inorg. Mater. 2017, 53, 160–163. [Google Scholar] [CrossRef]
- Pöttgen, R.; Johrendt, D. Intermetallics: Synthesis, Structure, Function; De Gruyter Textbook Oldenbourg: Berlin, Germany, 2014. [Google Scholar]
- Lopatin, S.; Saji, I.; Viswanathan, S. Molybdenum and Its Compounds: Applications, Electrochemical Properties and Geological Implications, Chemistry Research and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014. [Google Scholar]
- Considine, G.D. Van Nostrand’s Encyclopedia of Chemistry, 5th ed.; Wiley-Inter Science: New York, NY, USA, 2005; pp. 1038–1040. ISBN 978-0-471-61525-5. [Google Scholar]
- Morales, E.R. Hydrogen Reduction Route towards the Production of Nano-Grained Alloys. Synthesis and Characterization of Fe2Mo Powder. Ph.D. Thesis, Institutionen för Materialvetenskap, KTH, Stockholm, Sweden, 2002. Available online: http://kth.diva-portal.org/smash/get/diva2:9200/FULLTEXT01.pdf (accessed on 20 February 2023).
- Mazumder, S.; Pantawane, M.V.; Joshi, S.S.; Dahotre, N.B. Electrochemical and thermal-induced degradation of additively manufactured titanium alloys: A review. Crit. Rev. Solid State Mater. Sci. 2022, 47, 915–954. [Google Scholar] [CrossRef]
- Boey, F.Y.C.; Khor, K.A.; Srivatsan, T.S.; Wang, M.; Zhou, W. Processing and Fabrication of Advanced Materials VIII, Proceedings of a Symposium Organized by School of Mechanical & Production Engineering, Nanyang Technological University, Singapore, Singapore, 8–10 September 1999; World Scientific Publishing: Singapore, 1999. [Google Scholar]
- Abel Halim, K.S.; El-Geassy, A.A.; Ramadan, M.; Nasr, M.I.; Hussein, A.; Fathy, N.; Alghamdi, A.S. Reduction Behavior and Characteristics of Metal Oxides in the Nanoscale. Metals 2022, 12, 2182. [Google Scholar] [CrossRef]
- Qu, Y.; Xing, L.; Shao, L.; Luo, Y.; Zou, Z. Microstructural characterization and gas-solid reduction kinetics of iron ore fines at high temperature. Powder Technol. 2019, 355, 26–36. [Google Scholar] [CrossRef]
- Xing, L.Y.; Zou, Z.S.; Qu, Y.X.; Shao, L.; Zou, J.Q. Gas–Solid Reduction Behavior of In-flight Fine Hematite Ore Particles by Hydrogen. Steel Res. Int. 2019, 90, 1800311. [Google Scholar] [CrossRef]
- Sohn, H.Y. Suspension Ironmaking Technology with Greatly Reduced Energy Requirement and CO2 Emissions. Steel Times Int. 2007, 31, 68–72. [Google Scholar]
- Abdel Halim, K.S.; Nasr, M.I.; El-Geassy, A.A. Developed model for reduction mechanism of iron ore pellets under load. Ironmak. Steelmak. 2011, 38, 189–196. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Abdel Halim, K.S.; Bahgat, M.; Mousa, E.A.; El-Shereafy, E.E.; El-Tawil, A.A. Carbothermic reduction of Fe2O3/C compacts: Comparative approach to kinetics and mechanism. Ironmak. Steelmak. 2013, 40, 534–544. [Google Scholar] [CrossRef]
- Cavus, H.; Kahruman, C.; Yusufoglu, I. Thermal decomposition kinetics of the thermal decomposition product of ammonium heptamolybdate tetrahydrate in air and inert gas atmospheres. In T.T Chen Hoonary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization; TMS: Pittsburgh, PA, USA, 2012; pp. 785–796. [Google Scholar]
- Kovács, T.N.; Hunyadi, D.; de Lucena, A.L.A.; Szilágyi, I.M. Thermal decomposition of ammonium molybdates. J. Therm. Anal. Calorim. 2016, 124, 1013–1021. [Google Scholar] [CrossRef]
- Thomazeau, C.; Martin, V.; Afanasiev, P. Effect of support on the thermal decomposition of (NH4)6Mo7O24·4H2O in the inert gas atmosphere. Appl. Catal. A Gen. 2000, 199, 61–72. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Szekely, J. A Structural Model for Gas-Solid Reactions with a Moving Boundary-III. A Generalized Dimensionless Representation of the Irreversible Reaction between a Porous Solid and a Reactant Gas. Chem. Eng. Sci. 1972, 27, 763. [Google Scholar] [CrossRef]
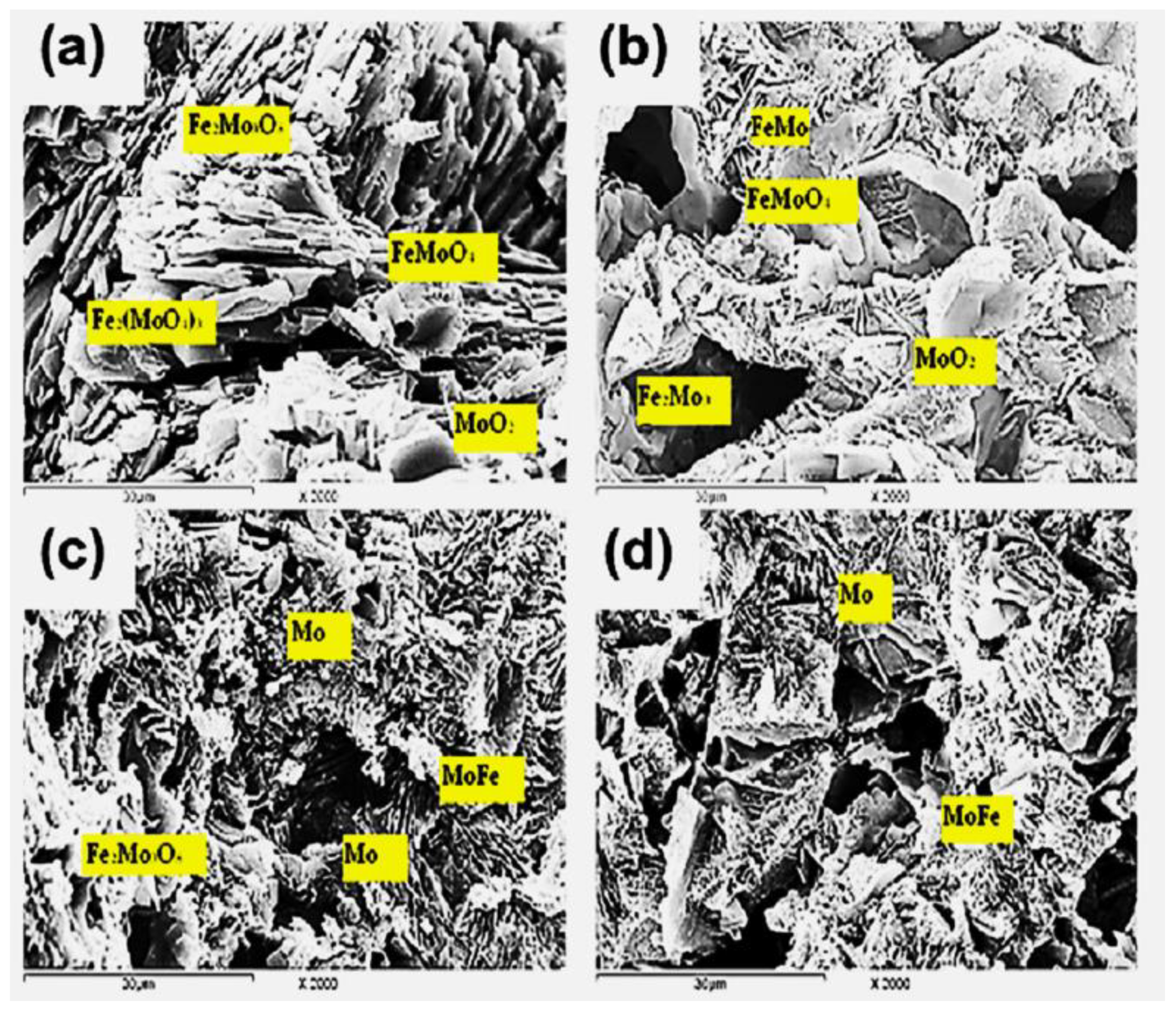
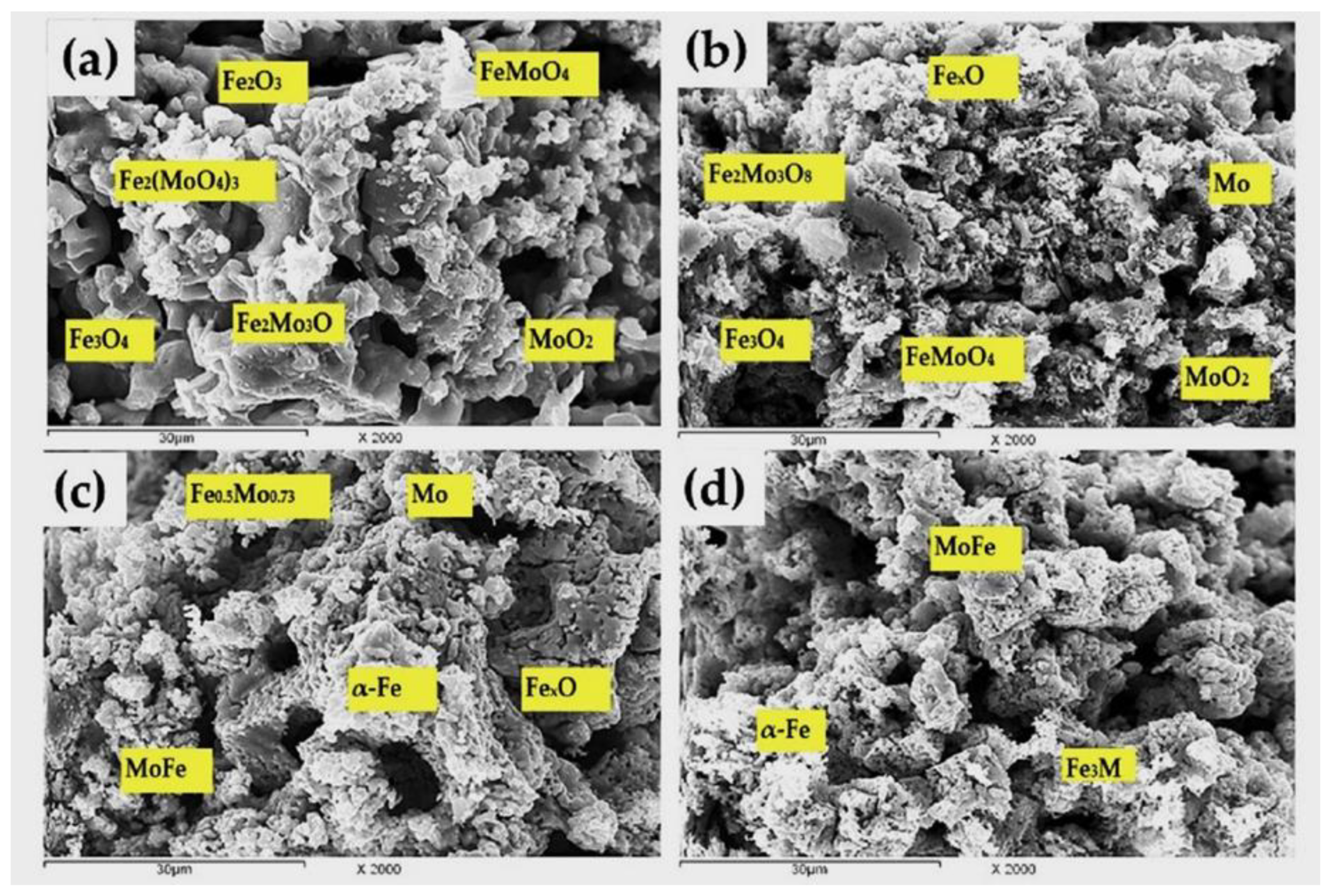

| Precursor | Composition | Phases Identified | Molar Ratio (%) | Total Porosity (%) | Apparent Density (g/mL) | |
|---|---|---|---|---|---|---|
| Mo % | Fe % | |||||
| (A) | 72 | 28 | Fe2(MoO4)3 | 100 | 59.87 | 6.43 |
| (B) | 30 | 70 | Fe2(MoO4)3 α-Fe2O3 | 46.68 53.32 | 48.39 | 5.35 |
| Precursor Composition | Phases Identified at Different Reduction Extents | |||
|---|---|---|---|---|
| 20% | 60% | 85% | 100% | |
| Precursor A 100% Fe2(MoO4)3 | Fe2(MoO4)3, Fe2Mo3O8 FeMoO4, MoO2 | Fe2Mo3O8, FeMoO4, FeMo, MoO2 | Fe2Mo3O8, MoFe, Mo | MoFe, Mo |
| Precursor B 46.68% Fe2(MoO4)3 +53.32% Fe2O3 | Fe2(MoO4)3, Fe2Mo3O8 FeMoO4, MoO2, Fe2O3, Fe3O4 | Fe2Mo3O8, FeMoO4 Mo, Fe3O4, FexO, MoO2 | Fe0.5Mo0.73, MoFe, Mo, FexO, α-Fe | MoFe, Fe3Mo, α-Fe |
| Reduction Degree, % | Ea, kJ mole−1 | Rate Controlling Mechanism According to the Reference Values of Activation Energy, [29] | |||
|---|---|---|---|---|---|
| Precursor (A) | Precursor (B) | ||||
| 5–10% | 25.63 | 15.87 | Ea, (kJ mole−1) | Controlling mechanism | |
| 20–25% | 48.09 | 32.31 | 8–16 | Gas diffusion | |
| 60–70% | 65.23 | 48.98 | 29–42 | Gas diffusion and interfacial chemical reaction | |
| 90–95% | at ≥750 °C | 85.32 | 65.85 | 60–70 | Interfacial chemical reaction |
| at ≤700 °C | 125.79 | 94.85 | >90 | Solid-state diffusion | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Geassy, A.A.; Abdel Halim, K.S.; Alghamdi, A.S. A Novel Hydro-Thermal Synthesis of Nano-Structured Molybdenum-Iron Intermetallic Alloys at Relatively Low Temperatures. Materials 2023, 16, 2736. https://doi.org/10.3390/ma16072736
El-Geassy AA, Abdel Halim KS, Alghamdi AS. A Novel Hydro-Thermal Synthesis of Nano-Structured Molybdenum-Iron Intermetallic Alloys at Relatively Low Temperatures. Materials. 2023; 16(7):2736. https://doi.org/10.3390/ma16072736
Chicago/Turabian StyleEl-Geassy, A. A., K. S. Abdel Halim, and Abdulaziz S. Alghamdi. 2023. "A Novel Hydro-Thermal Synthesis of Nano-Structured Molybdenum-Iron Intermetallic Alloys at Relatively Low Temperatures" Materials 16, no. 7: 2736. https://doi.org/10.3390/ma16072736
APA StyleEl-Geassy, A. A., Abdel Halim, K. S., & Alghamdi, A. S. (2023). A Novel Hydro-Thermal Synthesis of Nano-Structured Molybdenum-Iron Intermetallic Alloys at Relatively Low Temperatures. Materials, 16(7), 2736. https://doi.org/10.3390/ma16072736






