Optimization of the Electrochemical Method of Obtaining Graphene Nanoplatelets (GNPs)
Abstract
1. Introduction
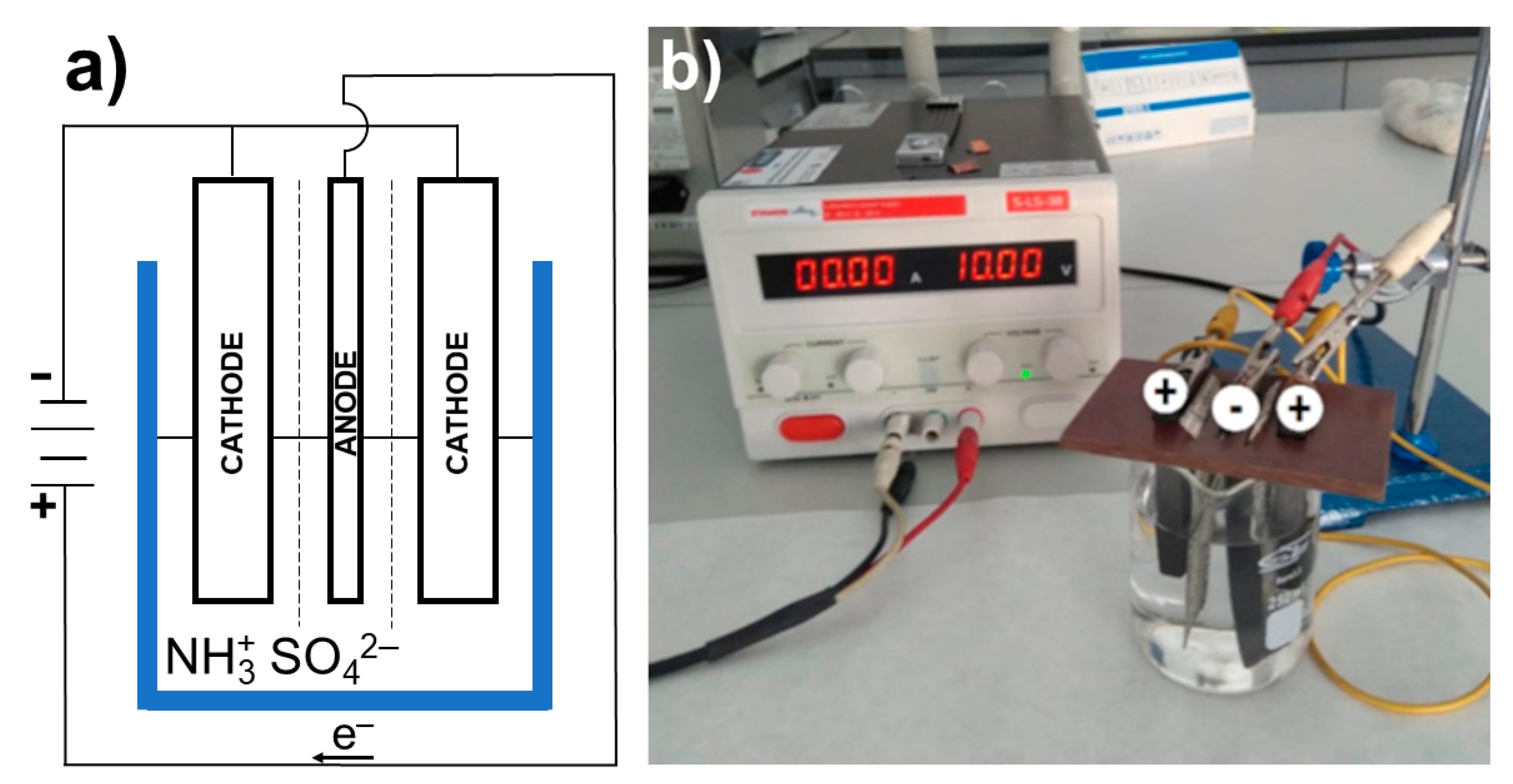
2. Materials and Methods
3. Results
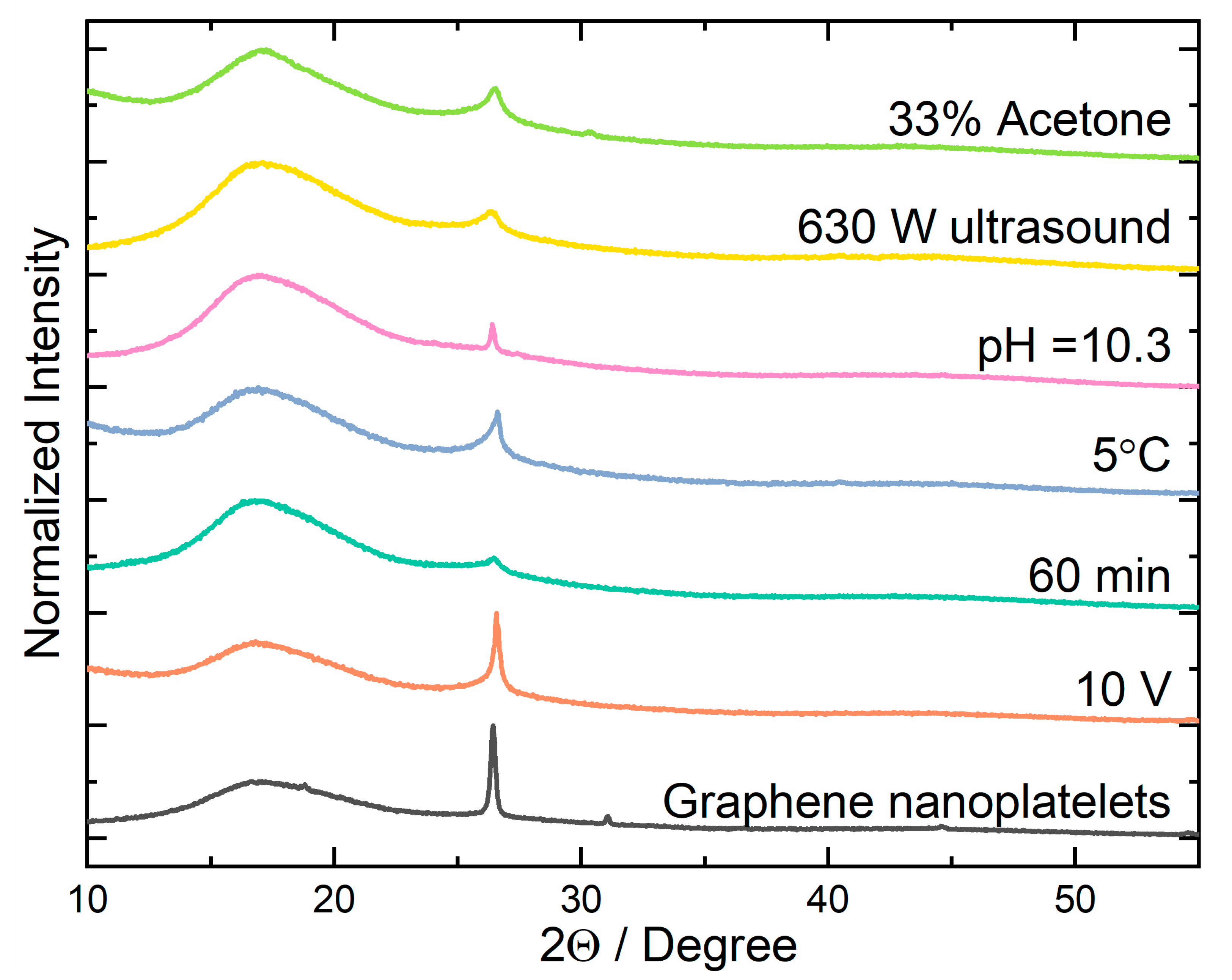
3.1. XRD Analysis
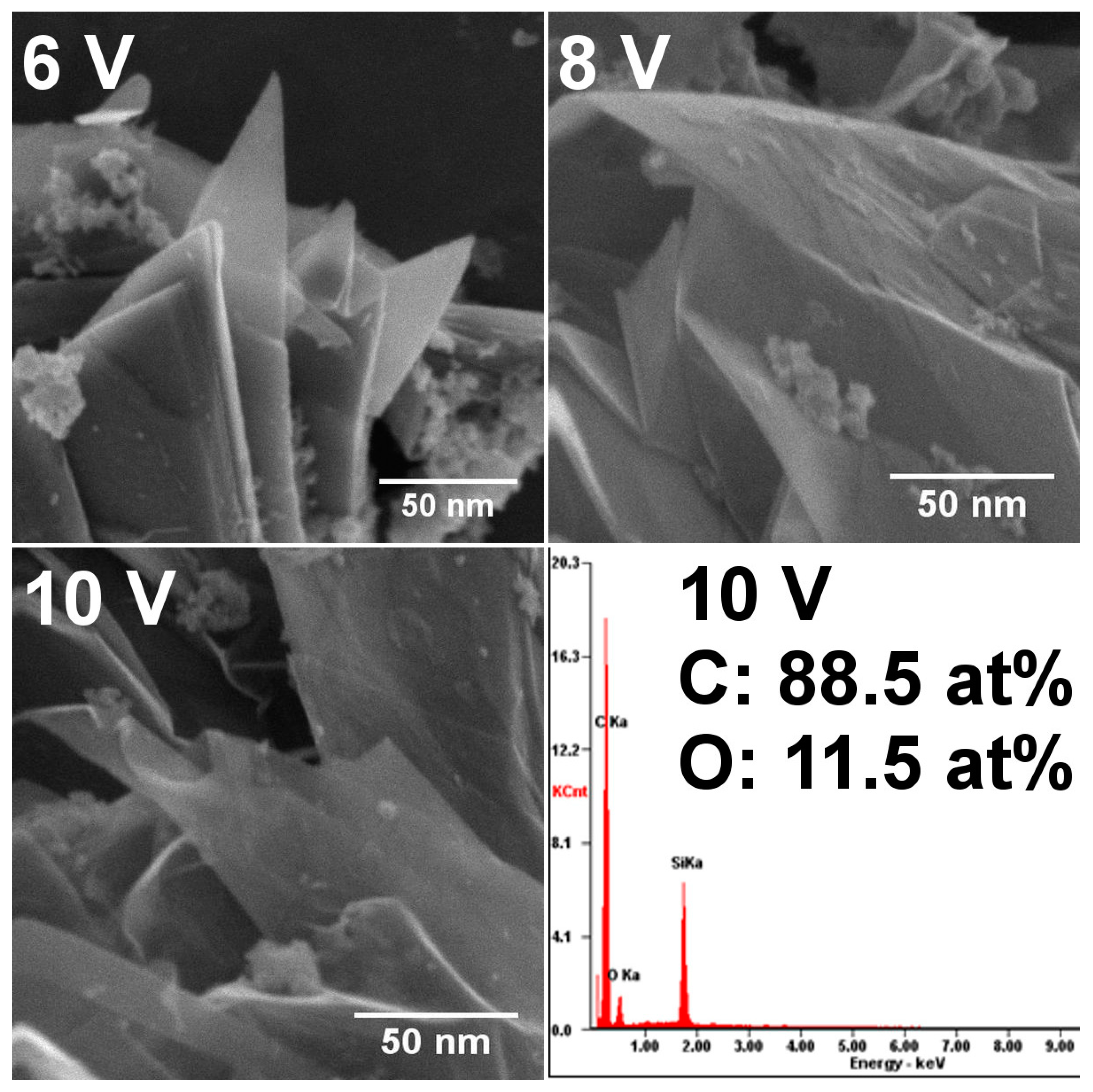
3.2. TEM and SEM Analysis
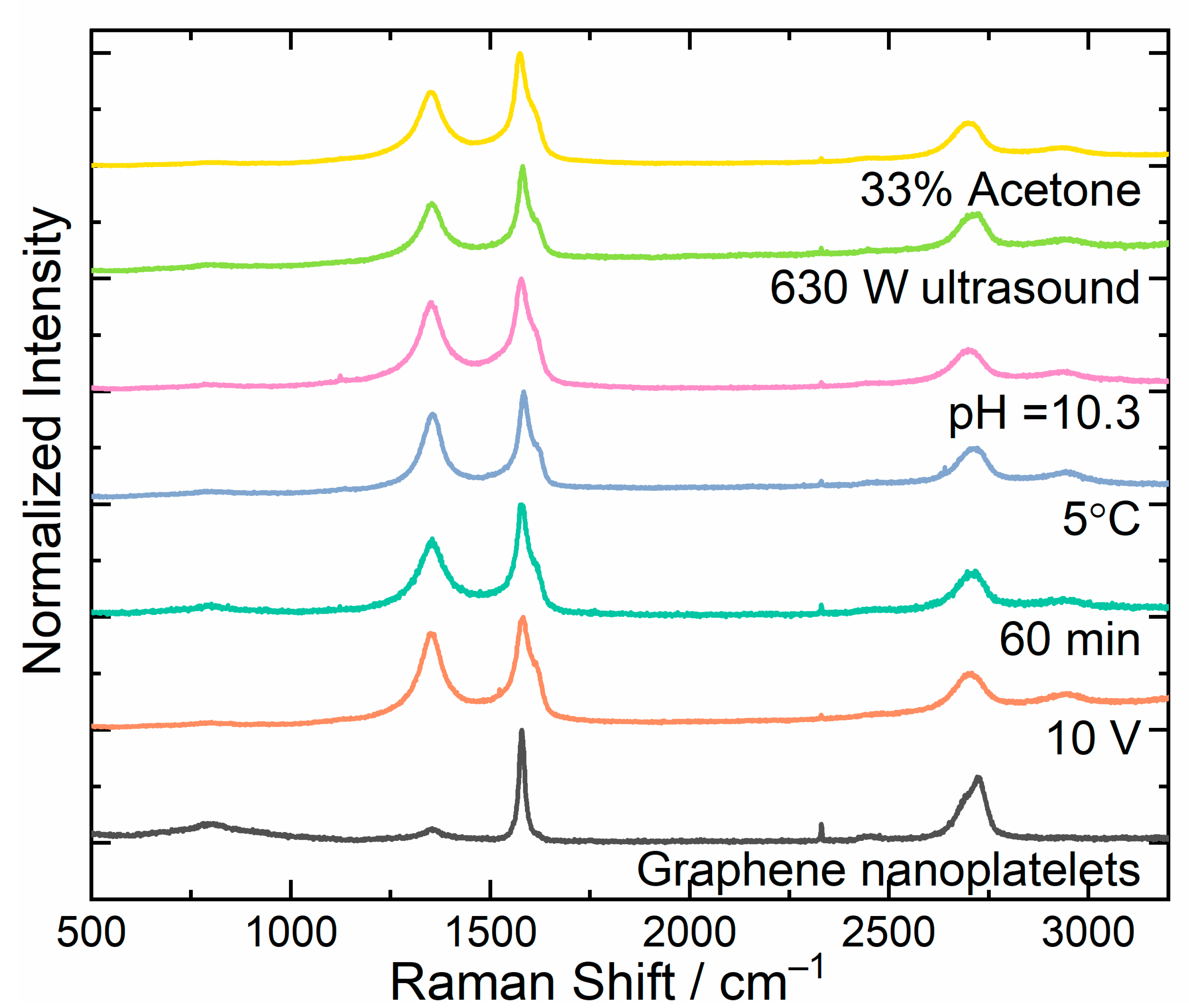
3.3. Raman Analysis
3.4. IR Analysis
3.5. Zeta Potential
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Shen, M.Y.; Liao, W.Y.; Wang, T.Q.; Lai, W.M. Characteristics and Mechanical Properties of Graphene Nanoplatelets-Reinforced Epoxy Nanocomposites: Comparison of Different Dispersal Mechanisms. Sustainability 2021, 13, 1788. [Google Scholar] [CrossRef]
- Kumar, V. Linear and Nonlinear Optical Properties of Graphene: A Review. J. Electron. Mater. 2021, 50, 3773–3799. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef]
- Rodríguez-Laguna, M.R.; Castro-Alvarez, A.; Sledzinska, M.; Maire, J.; Costanzo, F.; Ensing, B.; Pruneda, M.; Ordejón, P.; Sotomayor Torres, C.M.; Gómez-Romero, P.; et al. Mechanisms behind the enhancement of thermal properties of graphene nanofluids. Nanoscale 2018, 10, 15402–15409. [Google Scholar] [CrossRef]
- Kang, J.; Choi, Y.; Kim, J.H.; Choi, E.; Choi, S.E.; Kwon, O.; Kim, D.W. Functionalized nanoporous graphene membrane with ultrafast and stable nanofiltration. J. Memb. Sci. 2021, 618, 118635. [Google Scholar] [CrossRef]
- Nidamanuri, N.; Li, Y.; Li, Q.; Dong, M. Graphene and graphene oxide-based membranes for gas separation. Eng. Sci. 2020, 9, 3–16. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Zandiatashbar, A.; Lee, G.H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef]
- García-Hernández, E.; Salazar-García, E.; Shakerzadeh, E.; Chigo-Anota, E. Effect of dehydrogenated hydrocarbon doping on the electronic properties of graphene-type nanosheets. Phys. Lett. A 2020, 384, 126702. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Wang, B.; Ruoff, R.S. Chemical vapor deposition of graphene on thin-metal films. Cell Rep. Phys. Sci. 2021, 2, 100372. [Google Scholar] [CrossRef]
- Wang, J.B.; Ren, Z.; Hou, Y.; Yan, X.L.; Liu, P.Z.; Zhang, H.; Zhang, H.X.; Guo, J.J. A review of graphene synthesis at low temperatures by CVD methods. New Carbon Mater. 2020, 35, 193–208. [Google Scholar] [CrossRef]
- Song, Y.; Zou, W.; Lu, Q.; Lin, L.; Liu, Z. Graphene Transfer: Paving the Road for Applications of Chemical Vapor Deposition Graphene. Small 2021, 17, 2007600. [Google Scholar] [CrossRef] [PubMed]
- Liu, F. Mechanical exfoliation of large area 2D materials from vdW crystals. Prog. Surf. Sci. 2021, 96, 100626. [Google Scholar] [CrossRef]
- Pirzado, A.A.; Le Normand, F.; Romero, T.; Paszkiewicz, S.; Papaefthimiou, V.; Ihiawakrim, D.; Janowska, I. Few-Layer Graphene from Mechanical Exfoliation of Graphite-Based Materials: Structure-Dependent Characteristics. ChemEngineering 2019, 3, 37. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide—A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Bing, C.; Jiahao, Y.; Xiaoying, L.; Qi, J.; Guoping, W.; Linghua, J. Effects of reduction method on reduced graphene oxide and its electrochemical energy storage performance. Diam. Relat. Mater. 2021, 114, 108305. [Google Scholar] [CrossRef]
- Vacacela Gomez, C.; Guevara, M.; Tene, T.; Villamagua, L.; Usca, G.T.; Maldonado, F.; Tapia, C.; Cataldo, A.; Bellucci, S.; Caputi, L.S. The liquid exfoliation of graphene in polar solvents. Appl. Surf. Sci. 2021, 546, 149046. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008 39 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, S.; Wee, A.T.S.; Chen, W. Epitaxial growth of graphene on silicon carbide (SiC). In Graphene: Properties, Preparation, Characterisation and Devices; Woodhead Publishing: Cambridge, UK, 2014; pp. 3–26. [Google Scholar]
- Zhou, Q.; Lu, Y.; Xu, H. High-yield production of high-quality graphene by novel electrochemical exfoliation at air-electrolyte interface. Mater. Lett. 2019, 235, 153–156. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, C.K.; Sarkar, C.K.; Roy, S. Facile synthesis of multi-layer graphene by electrochemical exfoliation using organic solvent. Nanotechnol. Rev. 2018, 7, 497–508. [Google Scholar] [CrossRef]
- Viculis, L.M.; Mack, J.J.; Mayer, O.M.; Hahn, H.T.; Kaner, R.B. Intercalation and exfoliation routes to graphite nanoplatelets. J. Mater. Chem. 2005, 15, 974–978. [Google Scholar] [CrossRef]
- Alshamkhani, M.T.; Lahijani, P.; Lee, K.T.; Mohamed, A.R. Electrochemical exfoliation of graphene using dual graphite electrodes by switching voltage and green molten salt electrolyte. Ceram. Int. 2022, 48, 22493–22505. [Google Scholar] [CrossRef]
- Raj, C.J.; Manikandan, R.; Thondaiman, P.; Sivakumar, P.; Savariraj, A.D.; Cho, W.J.; Kim, B.C.; Jung, H. Sonoelectrochemical exfoliation of graphene in various electrolytic environments and their structural and electrochemical properties. Carbon N. Y. 2021, 184, 266–276. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.; Feng, X.; Müllen, K. Exfoliation of graphene via wet chemical routes. Synth. Met. 2015, 210, 123–132. [Google Scholar] [CrossRef]
- Orooji, Y.; Khojasteh, H.; Amiri, O.; Amiri, M.; Hasanifard, S.; Khanahmadzadeh, S.; Salavati-Niasari, M. A combination of hydrothermal, intercalation and electrochemical methods for the preparation of high-quality graphene: Characterization and using to prepare graphene-polyurethane nanocomposite. J. Alloys Compd. 2020, 848, 156495. [Google Scholar] [CrossRef]
- Saha, U.; Jaiswal, R.; Goswami, T.H.; Maji, P.K. Role and effect of electrolytes selection on supercapacitance behaviour of aminated graphenes. Electrochim. Acta 2022, 435, 141400. [Google Scholar] [CrossRef]
- Ruse, E.; Larboni, M.; Lavi, A.; Pyrikov, M.; Leibovitch, Y.; Ohayon-Lavi, A.; Vradman, L.; Regev, O. Molten salt in-situ exfoliation of graphite to graphene nanoplatelets applied for energy storage. Carbon N. Y. 2021, 176, 168–177. [Google Scholar] [CrossRef]
- Komoda, M.; Nishina, Y. Electrochemical Production of Graphene Analogs from Various Graphite Materials. Chem. Lett. 2020, 50, 503–509. [Google Scholar] [CrossRef]
- Xia, Z.; Bellani, V.; Sun, J.; Palermo, V. Electrochemical exfoliation of graphite in H2SO4, Li2SO4 and NaClO4 solutions monitored in situ by Raman microscopy and spectroscopy. Faraday Discuss. 2021, 227, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Niu, Y.; Hou, W.; Liu, R. Highly efficient dual-electrode exfoliation of graphite into high-quality graphene via square-wave alternating currents. Chem. Eng. J. 2023, 456, 140977. [Google Scholar] [CrossRef]
- Park, S.W.; Jang, B.; Kim, H.; Lee, J.; Park, J.Y.; Kang, S.O.; Choa, Y.H. Highly Water-Dispersible Graphene Nanosheets From Electrochemical Exfoliation of Graphite. Front. Chem. 2021, 9, 519. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Roh, J.S. Analysis of Activation Process of Carbon Black Based on Structural Parameters Obtained by XRD Analysis. Crystals 2021, 11, 153. [Google Scholar] [CrossRef]
- Takagi, H.; Maruyama, K.; Yoshizawa, N.; Yamada, Y.; Sato, Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. [Google Scholar] [CrossRef]
- Salverda, M.; Thiruppathi, A.R.; Pakravan, F.; Wood, P.C.; Chen, A. Electrochemical Exfoliation of Graphite to Graphene-Based Nanomaterials. Molecules 2022, 27, 8643. [Google Scholar] [CrossRef]
- Liu, W.W.; Aziz, A. Review on the Effects of Electrochemical Exfoliation Parameters on the Yield of Graphene Oxide. ACS Omega 2022, 7, 33719–33731. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Keong Koo, C.; Shen, Z.; Thong, J.T.; Thong, J.T.; et al. Probing Layer Number and Stacking Order of Few-Layer Graphene by Raman Spectroscopy**. Small 2010, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mondragon, R.; Julia, J.E.; Barba, A.; Jarque, J.C. Characterization of silica–water nanofluids dispersed with an ultrasound probe: A study of their physical properties and stability. Powder Technol. 2012, 224, 138–146. [Google Scholar] [CrossRef]


| Synthesis Factor | Unit | |
|---|---|---|
| Voltage | V | 6, 8, 10, 12 |
| Electrolysis duration | min | 45, 60, 90 |
| Temperature during electrolysis | °C | 5, 15, 25 |
| pH of the electrolyte | - | 3.2, 4.2, 5.3, 6.1, 7.7, 8.7, 9.6, 10.3 |
| Acetone content in the electrolyte | % | 0, 33, 50 |
| Ultrasound power | W | 230, 630 |
| Ultrasound duration | min | 15, 30, 45, 60, 90 |
| Temperature during sonification | °C | 5, 20, 30 |
| FWHM [cm−1] | Number of Graphene Layers |
|---|---|
| 27.5 ± 3.8 | 1 |
| 51.7 ± 1.7 | 2 |
| 56.2 ± 1.6 | 3 |
| 63.1 ± 1.6 | 4 |
| 66.1 ± 1.4 | 5 |
| Sample | Zeta Potential/mV |
|---|---|
| 10 V | −28.9 |
| 60 min | −28.3 |
| 5 °C | −35.3 |
| pH 10.3 | −37.5 |
| 630 W ultrasound | −32.3 |
| 33% acetone | −42.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowska, A.; Kowalczyk, J.; Tomala, R.; Ptak, M.; Małecka, M.; Wędzyńska, A.; Stefanski, M.; Stręk, W.; Głuchowski, P. Optimization of the Electrochemical Method of Obtaining Graphene Nanoplatelets (GNPs). Materials 2023, 16, 2188. https://doi.org/10.3390/ma16062188
Grabowska A, Kowalczyk J, Tomala R, Ptak M, Małecka M, Wędzyńska A, Stefanski M, Stręk W, Głuchowski P. Optimization of the Electrochemical Method of Obtaining Graphene Nanoplatelets (GNPs). Materials. 2023; 16(6):2188. https://doi.org/10.3390/ma16062188
Chicago/Turabian StyleGrabowska, Adrianna, Jerzy Kowalczyk, Robert Tomala, Maciej Ptak, Małgorzata Małecka, Anna Wędzyńska, Mariusz Stefanski, Wiesław Stręk, and Paweł Głuchowski. 2023. "Optimization of the Electrochemical Method of Obtaining Graphene Nanoplatelets (GNPs)" Materials 16, no. 6: 2188. https://doi.org/10.3390/ma16062188
APA StyleGrabowska, A., Kowalczyk, J., Tomala, R., Ptak, M., Małecka, M., Wędzyńska, A., Stefanski, M., Stręk, W., & Głuchowski, P. (2023). Optimization of the Electrochemical Method of Obtaining Graphene Nanoplatelets (GNPs). Materials, 16(6), 2188. https://doi.org/10.3390/ma16062188










