Effects of Mellowing Practice on the Strength and Swelling Properties of Road Construction Materials: Case of Sulphate-Bearing Clay Soils Stabilised with Lime-Silica Fume Blended Binder
Abstract
1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Mix Compositions and Specimens Preparation
- Using water equivalent of 30 wt.% of the OMC, with the final compaction being carried out using the remainder of 70 wt.% of the OMC after mellowing.
- Using water equivalent of 40 wt.% of the OMC, with the final compaction being carried out using the remainder of 60 wt.% of the OMC after mellowing.
2.3. Experimental Testing Methods
2.3.1. Unconfined Compressive Strength (UCS)
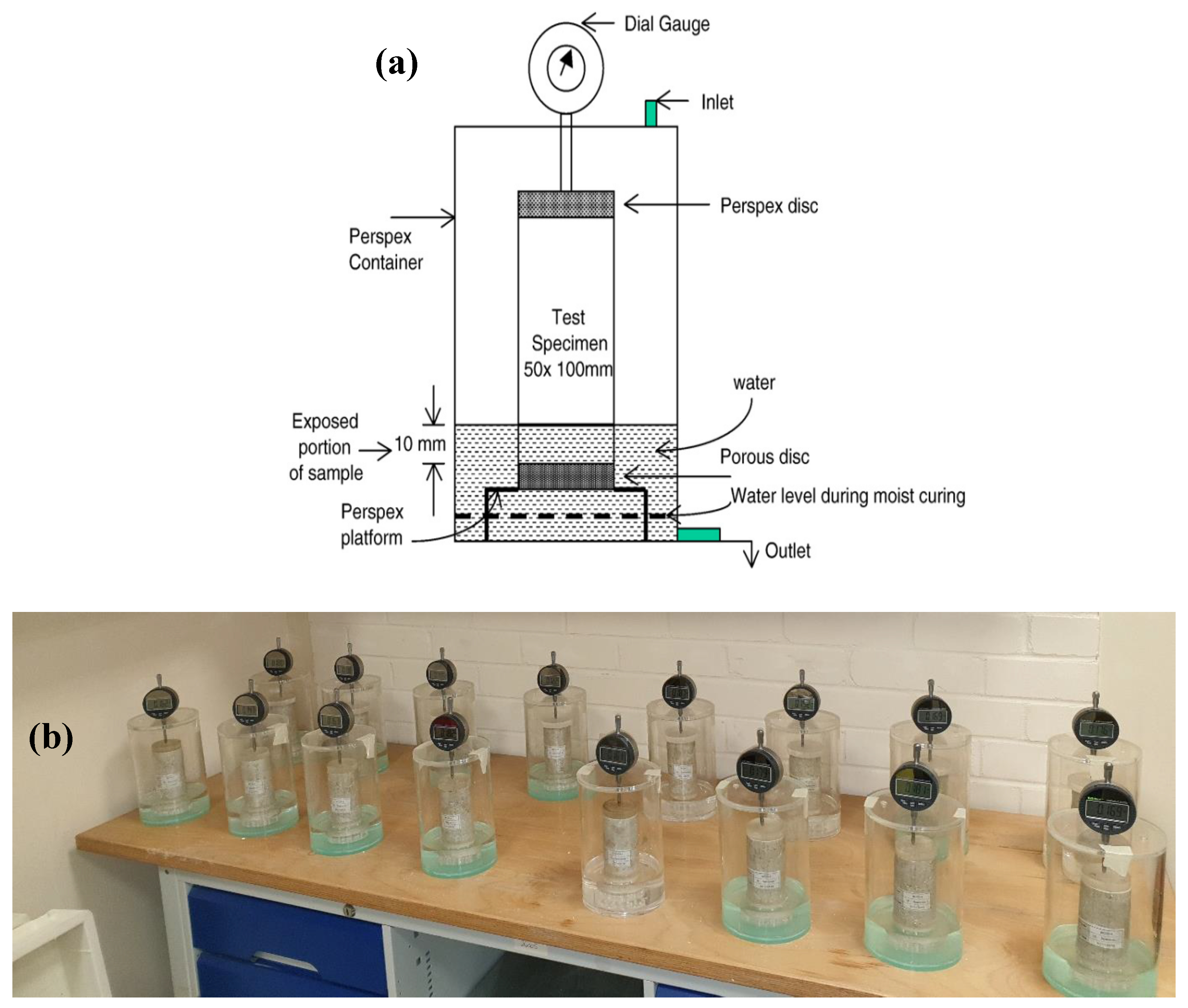
2.3.2. Linear Expansion
3. Results
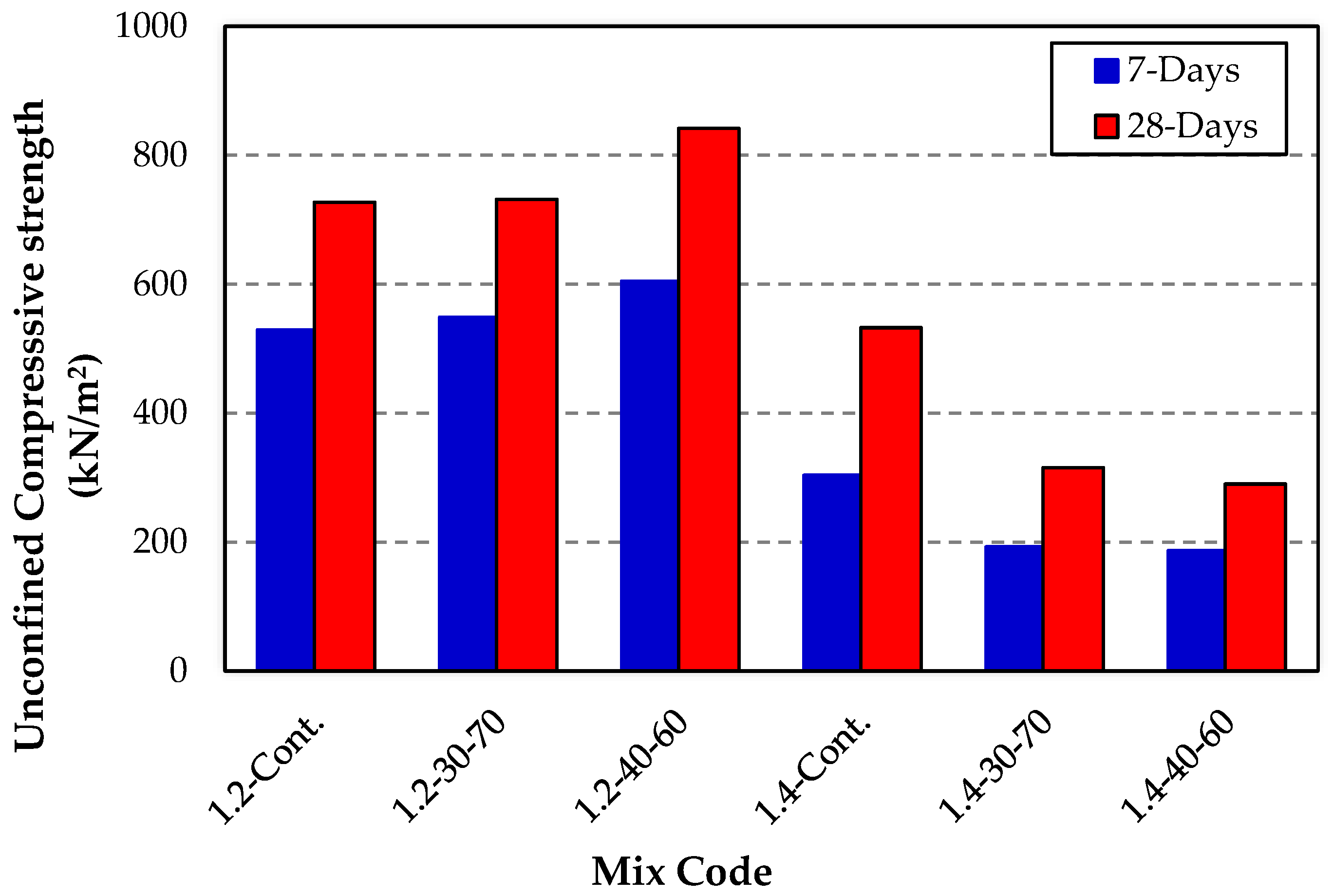
3.1. Unconfined Compressive Strength (UCS)
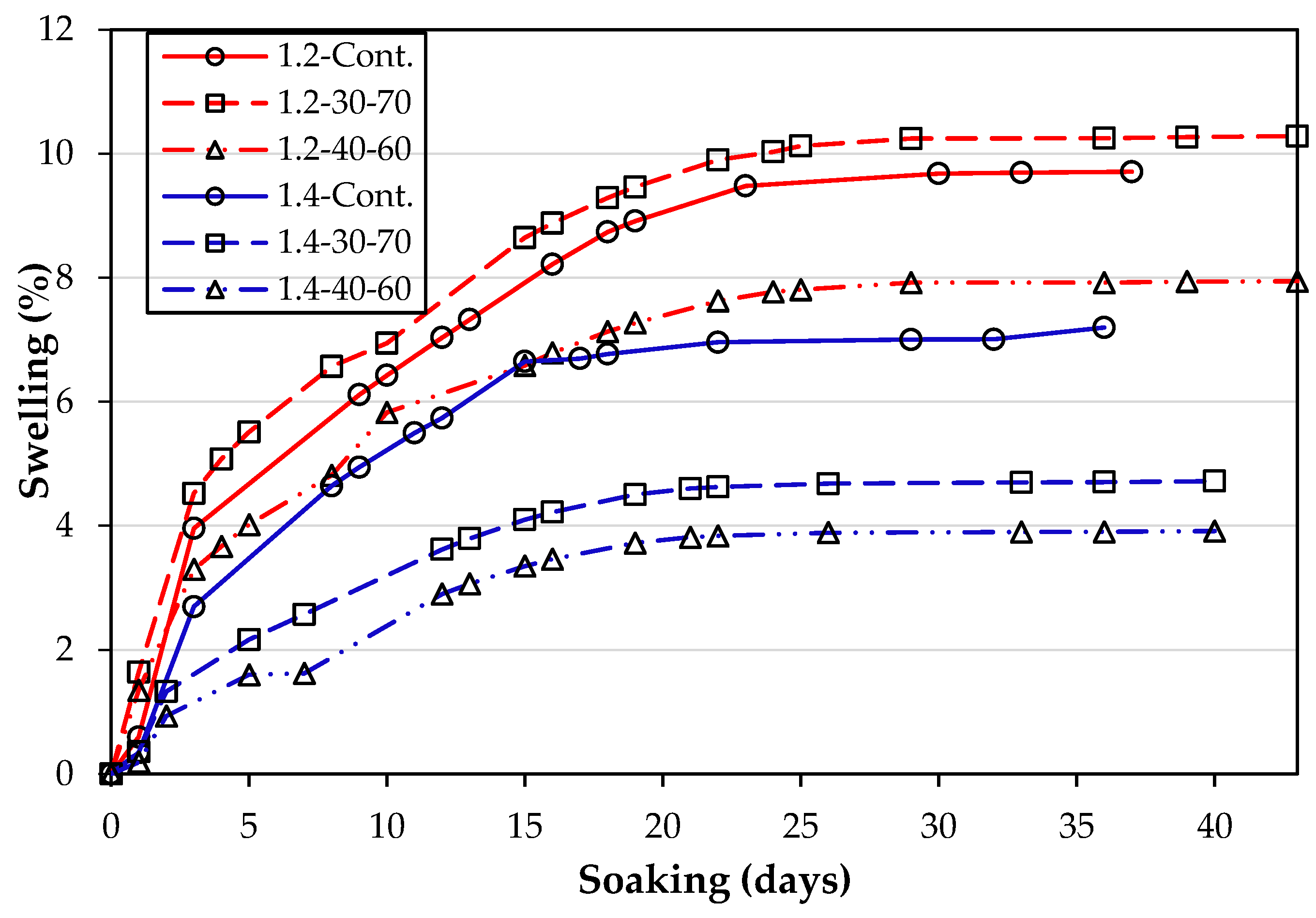
3.2. Swell Development of the Mellowed and Unmellowed Test Specimens
4. Discussion
5. Conclusions
- Overall, a trade-off between performance based on strength development and swelling potential was made. It was found more practical to aim for high strength and then to explore methods to mitigate against linear expansion.
- The findings of this study showed a significant drop in compressive strength when the targeted materials were mellowed at 1.4 OMC. Therefore, it is recommended to compact the materials at lower moisture condition, such as 1.2 OMC (as opposed to say 1.4 OMC), but mellow at the higher initial moisture condition of approximately 40% OMC (as opposed to say at 30% OMC).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmat, M.N.; Kinuthia, J.M. Effects of mellowing sulfate-bearing clay soil stabilized with wastepaper sludge ash for road construction. Eng. Geol. 2011, 117, 170–179. [Google Scholar] [CrossRef]
- Jones, L.D.; Jefferson, J. ICE Manual of Geotechnical Engineering Volume 1: Geotechnical Engineering Principles, Problematic Soils and Site Investigation; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Wild, S.; Kinuthia, J.M.; Jones, G.I.; Higgins, D.D. Suppression of swelling associated with ettringite formation in lime stabilized sulphate bearing clay soils by partial substitution of lime with ground granulated blastfurnace slag. Eng. Geol. 1999, 51, 257–277. [Google Scholar] [CrossRef]
- Seco, A.; del Castillo, J.M.; Espuelas, S.; Marcelino, S.; García, B. Sulphate soil stabilisation with magnesium binders for road subgrade construction. Int. J. Pavement Eng. 2022, 23, 1840–1850. [Google Scholar] [CrossRef]
- Wild, S.; Kinuthia, J.M.; Jones, G.I.; Higgins, D.D. Effects of partial substitution of lime with ground granulated blast furnace slag (GGBS) on the strength properties of lime-stabilised sulphate-bearing clay soils. Eng. Geol. 1998, 51, 37–53. [Google Scholar] [CrossRef]
- Kinuthia, J.M.; Wild, S.; Jones, G.I. Effects of monovalent and divalent metal sulphates on consistency and compaction of lime-stabilised kaolinite. Appl. Clay Sci. 1999, 14, 27–45. [Google Scholar] [CrossRef]
- Seco, A.; Miqueleiz, L.; Prieto, E.; Marcelino, S.; García, B.; Urmeneta, P. Sulfate soils stabilization with magnesium-based binders. Appl. Clay Sci. 2017, 135, 457–464. [Google Scholar] [CrossRef]
- Seco, A.; Ramírez, F.; Miqueleiz, L.; García, B. Stabilization of expansive soils for use in construction. Appl. Clay Sci. 2011, 51, 348–352. [Google Scholar] [CrossRef]
- Puppala, A.J. Advances in ground modification with chemical additives: From theory to practice. Transp. Geotech. 2016, 9, 123–138. [Google Scholar] [CrossRef]
- Oti, J.E.; Kinuthia, J.M.; Bai, J. Unfired clay bricks: From laboratory to industrial production. Proc. Inst. Civ. Eng. Eng. Sustain. 2009, 162, 229–237. [Google Scholar] [CrossRef]
- Aldaood, A.; Bouasker, M.; Al-Mukhtar, M. Free swell potential of lime-treated gypseous soil. Appl. Clay Sci. 2014, 102, 93–103. [Google Scholar] [CrossRef]
- Alberici, S.; de Beer, J.; van der Hoorn, I.; Staats, M. Fly Ash and Blast Furnace Slag for Cement Manufacturing; BEIS Research Paper, no.19; Department for Business, Energy and Industrial Strategy: London, UK, 2017; Volume 19, pp. 1–34. [Google Scholar]
- Saygili, A.; Dayan, M. Freeze-thaw behavior of lime stabilized clay reinforced with silica fume and synthetic fibers. Cold Reg. Sci. Technol. 2019, 161, 107–114. [Google Scholar] [CrossRef]
- GhavamShirazi, S.; Bilsel, H. Characterization of volume change and strength behavior of micro-silica and lime-stabilized Cyprus clay. Acta Geotech. 2021, 16, 827–840. [Google Scholar] [CrossRef]
- Moayyeri, N.; Oulapour, M.; Haghighi, A. Study of geotechnical properties of a Gypsiferous soil treated with lime and silica fume. Geomech. Eng. 2019, 17, 195–206. [Google Scholar] [CrossRef]
- Behnood, A. Soil and clay stabilization with calcium- and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Department of Transport. Specification for Highway Works, 7th ed.; Amendment August 1994; HMSO: London, UK, 1994.
- Obuzor, G.N.; Kinuthia, J.M.; Robinson, R.B. Enhancing the durability of flooded low-capacity soils by utilizing lime-activated ground granulated blastfurnace slag (GGBS). Eng. Geol. 2011, 123, 179–186. [Google Scholar] [CrossRef]
- Harris, J.P.; Sebesta, S.; Scullion, T. Hydrated lime stabilization of sulfate-bearing vertisols in Texas. Transp. Res. Rec. 2004, 1868, 31–39. [Google Scholar] [CrossRef]
- Ali, H.; Mohamed, M. The effects of compaction delay and environmental temperature on the mechanical and hydraulic properties of lime-stabilized extremely high plastic clays. Appl. Clay Sci. 2017, 150, 333–341. [Google Scholar] [CrossRef]
- Puppala, A.J.; Talluri, N.; Chittoori, B.C.S. Calcium-based stabiliser treatment of sulfate-bearing soils. Proc. Inst. Civ. Eng.-Ground Improv. 2014, 167, 162–172. [Google Scholar] [CrossRef]
- BS EN 15309:2007; Characterization of Waste and Soil. Determination of Elemental Composition by X-ray Fluorescence. BSI Standards Limited: London, UK, 2007.
- BS ISO 18227:2014; Soil Quality. Determination of Elemental Composition by X-ray Fluorescence. BSI Standards Limited: London, UK, 2014.
- BS EN ISO 17892-4:2016; Geotechnical Investigation and Testing—Laboratory Testing of Soil. Part 4: Determination of Particle Size Distribution (ISO 17892-4:2016). BSI Standards Limited: London, UK, 2016.
- BS EN ISO 787-9-2019; General Methods of Test for Pigments and Extenders. Part 9: Determination of pH Value of an Aqueous Suspension. BSI Standards Limited: London, UK, 2019.
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.E. Strength and Swell Performance of High-Sulphate Kaolinite Clay Soil. Sustainability 2020, 12, 10164. [Google Scholar] [CrossRef]
- BS 1377-4:1990; Methods of Test for Soils for Civil Engineering Purposes. Part 4: Compaction-Related Tests. British Standards Institution: London, UK, 2015.
- BS 1924-2; Hydraulically Bound and Stabilized Materials for Civil Engineering Purposes—Part 2: Sample Preparation and Testing of Materials During and After Treatment. British Standards Institution: London, UK, 2018.
- BS EN ISO 17892-7; Geotechnical Investigation and Testing-Laboratory Testing of Soil—Part 7: Unconfined Compression Test. British Standards Institution: London, UK, 2017.
- BS EN 13286-49; Unbound and Hydraulically Bound Mixtures—Part 49: Accelerated Swelling Test for Soil Treated by Lime and/or Hydraulic Binder. British Standards Institution: London, UK, 2004.
- Eyo, E.U.; Abbey, S.J.; Ngambi, S.; Ganjian, E.; Coakley, E. Incorporation of a nanotechnology-based product in cementitious binders for sustainable mitigation of sulphate-induced heaving of stabilised soils. Eng. Sci. Technol. Int. J. 2021, 24, 436–448. [Google Scholar] [CrossRef]
- Al-Mukhtar, M.; Lasledj, A.; Alcover, J.F. Lime consumption of different clayey soils. Appl. Clay Sci. 2014, 95, 133–145. [Google Scholar] [CrossRef]
- Vitale, E.; Deneele, D.; Russo, G.; Ouvrard, G. Short-term effects on physical properties of lime treated kaolin. Appl. Clay Sci. 2016, 132–133, 223–231. [Google Scholar] [CrossRef]
- Al-Mukhtar, M.; Lasledj, A.; Alcover, J.F. Behaviour and mineralogy changes in lime-treated expansive soil at 20 °C. Appl. Clay Sci. 2010, 50, 191–198. [Google Scholar] [CrossRef]
- Konan, K.L.; Peyratout, C.; Smith, A.; Bonnet, J.P.; Rossignol, S.; Oyetola, S. Comparison of surface properties between kaolin and metakaolin in concentrated lime solutions. J. Colloid Interface Sci. 2009, 339, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Choobbasti, A.J.; Kutanaei, S.S. Microstructure characteristics of cement-stabilized sandy soil using nanosilica. J. Rock Mech. Geotech. Eng. 2017, 9, 981–988. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Z.; Du, P.; Cheng, X. Effects of nano-silica on hydration properties of tricalcium silicate. Constr. Build. Mater. 2016, 125, 1169–1177. [Google Scholar] [CrossRef]
- Aldaood, A.; Bouasker, M.; Al-Mukhtar, M. Mechanical Behavior of Gypseous Soil Treated with Lime. Geotech. Geol. Eng. 2021, 39, 719–733. [Google Scholar] [CrossRef]
- Wild, S.; Abdi, M.R.; Leng-Ward, G. Sulphate Expansion of Lime-Stabilized Kaolinite: II. Reaction Products and Expansion. Clay Miner. 1993, 28, 569–583. [Google Scholar] [CrossRef]
- Thomas, B. Stabilisation of Sulphide Rich Soil: Problems and Solutions. Ph.D. Thesis, University of Glamorgan, Pontypridd, UK, 2001. [Google Scholar]
- Holt, C.C.; Freer-Hewish, R.J.; Ghataora, G.S. Use of lime-treated British clays in pavement construction. Part 2: The effect of mellowing on the stabilization process. Proc. Inst. Civ. Eng. Transp. 2000, 141, 207–216. [Google Scholar] [CrossRef]
- Holt, C.C.; Freer-Hewish, R.J. The use of lime-treated British clays in pavement construction. Part 1: The effect of mellowing on the modification process. Proc. Inst. Civ. Eng. Transp. 1998, 129, 228–239. [Google Scholar] [CrossRef]
- Ho, L.S.; Nakarai, K.; Ogawa, Y.; Sasaki, T.; Morioka, M. Strength development of cement-treated soils: Effects of water content, carbonation, and pozzolanic reaction under drying curing condition. Constr. Build. Mater. 2017, 134, 703–712. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Miura, N.; Nagaraj, T.S. Assessment of strength development in cement-admixed high water content clays with Abrams’ law as a basis. Geotechnique 2003, 53, 439–444. [Google Scholar] [CrossRef]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, C.; Gao, L. Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilisation. Eng. Geol. 2015, 195, 53–62. [Google Scholar] [CrossRef]
| Oxides | Composition (%) | ||
|---|---|---|---|
| Kaolin | Lime | Silica Fume | |
| CaO | <0.01 | 71.56 | 0.2 |
| MgO | 0.21 | 0.58 | 0.1 |
| Al2O3 | 47.32 | 0.67 | 98.4 |
| SiO2 | 35.96 | 0.07 | 0.2 |
| Na2O | 0.07 | <0.02 | - |
| P2O5 | 0.12 | 0.03 | 0.03 |
| Fe2O3 | 0.69 | 0.05 | 0.01 |
| Mn2O3 | 0.02 | 0.02 | - |
| K2O | 1.8 | <0.01 | 0.2 |
| TiO2 | 0.02 | <0.01 | - |
| V2O5 | <0.01 | 0.02 | - |
| BaO | 0.07 | <0.01 | - |
| SO3 | 0.01 | 0.19 | 0.1 |
| SO4 | 0.1 | - | - |
| S | 0.1 | - | - |
| Properties | Kaolin | Lime | Silica Fume |
|---|---|---|---|
| Particle size distribution | |||
| D60 (mm) | 0.012 | 0.35 | 0.1 |
| D50 (mm) | 0.0085 | 0.25 | 0.04 |
| D30 (mm) | 0.0062 | 0.06 | 0.025 |
| D10 (mm) | 0.0018 | <0.01 | 0.01 |
| Other properties | |||
| Linear shrinkage (%) | 10.8 | - | - |
| Linear expansion (%) | 6.2 | - | - |
| Swelling pressure (kPa) | 1.3 | - | - |
| Bulk density (kg/m3) | - | 480 | 300 |
| Specific gravity (mg/m3) | 2.14 | 2.82 | 3.15 |
| Alkalinity value (pH) | 5.37 | 12.62 | 7 |
| Colour | White | White | Grey |
| Loss on ignition (LOI) | 13.1 | 27.4 | 0.5 |
| Mix Code | Elaborated Abbreviation | Mix Ingredients (g) per Specimen | Moisture Content (MC) | |||||
|---|---|---|---|---|---|---|---|---|
| Kaolin | Gypsum | Lime | SF | Total MC | MC Pre-Mellowing | MC After-Mellowing | ||
| 1.2-Cont. | K6G-2L2S-1.2OMC-Control | 283 | 18.9 | 6.3 | 6.3 | 100.6 | 100.6 | 100.6 |
| 1.2-30-70 | K6G-2L2S-1.2OMC (30%70%M) | 283 | 18.9 | 6.3 | 6.3 | 100.6 | 30.2 | 70.4 |
| 1.2-40-60 | K6G-2L2S-1.2OMC (40%60%M) | 283 | 18.9 | 6.3 | 6.3 | 100.6 | 40.2 | 60.4 |
| 1.4-Cont. | K6G-2L2S-1.4OMC-Control | 270.7 | 18 | 6 | 6 | 114.3 | 114.3 | 114.3 |
| 1.4-30-70 | K6G-2L2S-1.4OMC (30%70%M) | 270.7 | 18 | 6 | 6 | 114.3 | 34.3 | 80 |
| 1.4-40-60 | K6G-2L2S-1.4OMC (40%60%M) | 270.7 | 18 | 6 | 6 | 114.3 | 45.7 | 68.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Waked, Q.; Kinuthia, J.M.; Adeleke, B.O.; Oti, J.; Khalifa, A. Effects of Mellowing Practice on the Strength and Swelling Properties of Road Construction Materials: Case of Sulphate-Bearing Clay Soils Stabilised with Lime-Silica Fume Blended Binder. Materials 2023, 16, 2187. https://doi.org/10.3390/ma16062187
Al-Waked Q, Kinuthia JM, Adeleke BO, Oti J, Khalifa A. Effects of Mellowing Practice on the Strength and Swelling Properties of Road Construction Materials: Case of Sulphate-Bearing Clay Soils Stabilised with Lime-Silica Fume Blended Binder. Materials. 2023; 16(6):2187. https://doi.org/10.3390/ma16062187
Chicago/Turabian StyleAl-Waked, Qusai, John M. Kinuthia, Blessing O. Adeleke, Jonathan Oti, and Ahmed Khalifa. 2023. "Effects of Mellowing Practice on the Strength and Swelling Properties of Road Construction Materials: Case of Sulphate-Bearing Clay Soils Stabilised with Lime-Silica Fume Blended Binder" Materials 16, no. 6: 2187. https://doi.org/10.3390/ma16062187
APA StyleAl-Waked, Q., Kinuthia, J. M., Adeleke, B. O., Oti, J., & Khalifa, A. (2023). Effects of Mellowing Practice on the Strength and Swelling Properties of Road Construction Materials: Case of Sulphate-Bearing Clay Soils Stabilised with Lime-Silica Fume Blended Binder. Materials, 16(6), 2187. https://doi.org/10.3390/ma16062187








