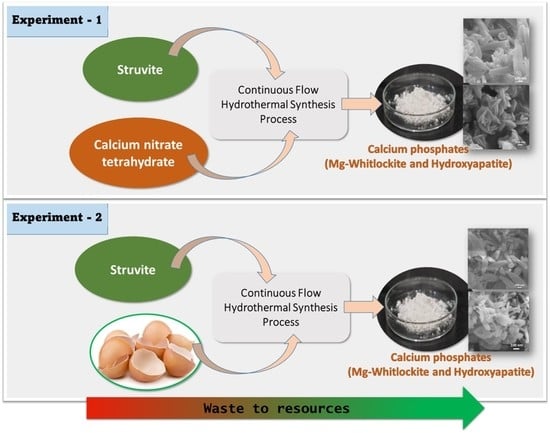
Production of Nano Hydroxyapatite and Mg-Whitlockite from Biowaste-Derived products via Continuous Flow Hydrothermal Synthesis: A Step towards Circular Economy
Abstract
1. Introduction
2. Materials and Methodologies
2.1. Materials
2.2. Methodologies
2.2.1. Calcium Nitrate Tetrahydrate (Ca(NO3)2.4H2O) Solution
2.2.2. Struvite (MgNH4PO4.6H2O) Solution
2.2.3. Eggshell Solution Preparation
2.2.4. Hydrothermal Synthesis Process
2.2.5. Sample Preparation Using Ca(NO3)2.4H2O as Ca and Struvite as P Sources
2.2.6. Sample Preparation Using Eggshells as Ca and Struvite as P Sources
3. Characterisation Techniques
3.1. Elemental Analysis of Eggshells Via ICP-MS
3.2. X-Ray Diffraction (XRD)
3.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Analysis (EDX)
3.4. Transmission Electron Microscopy (TEM)
3.5. Particle Size Analysis
3.6. FTIR Analysis
4. Results
4.1. Elemental Analysis
4.2. Hydroxyapatite Synthesis Using Calcium Nitrate Tetra-Hydrate—Struvite
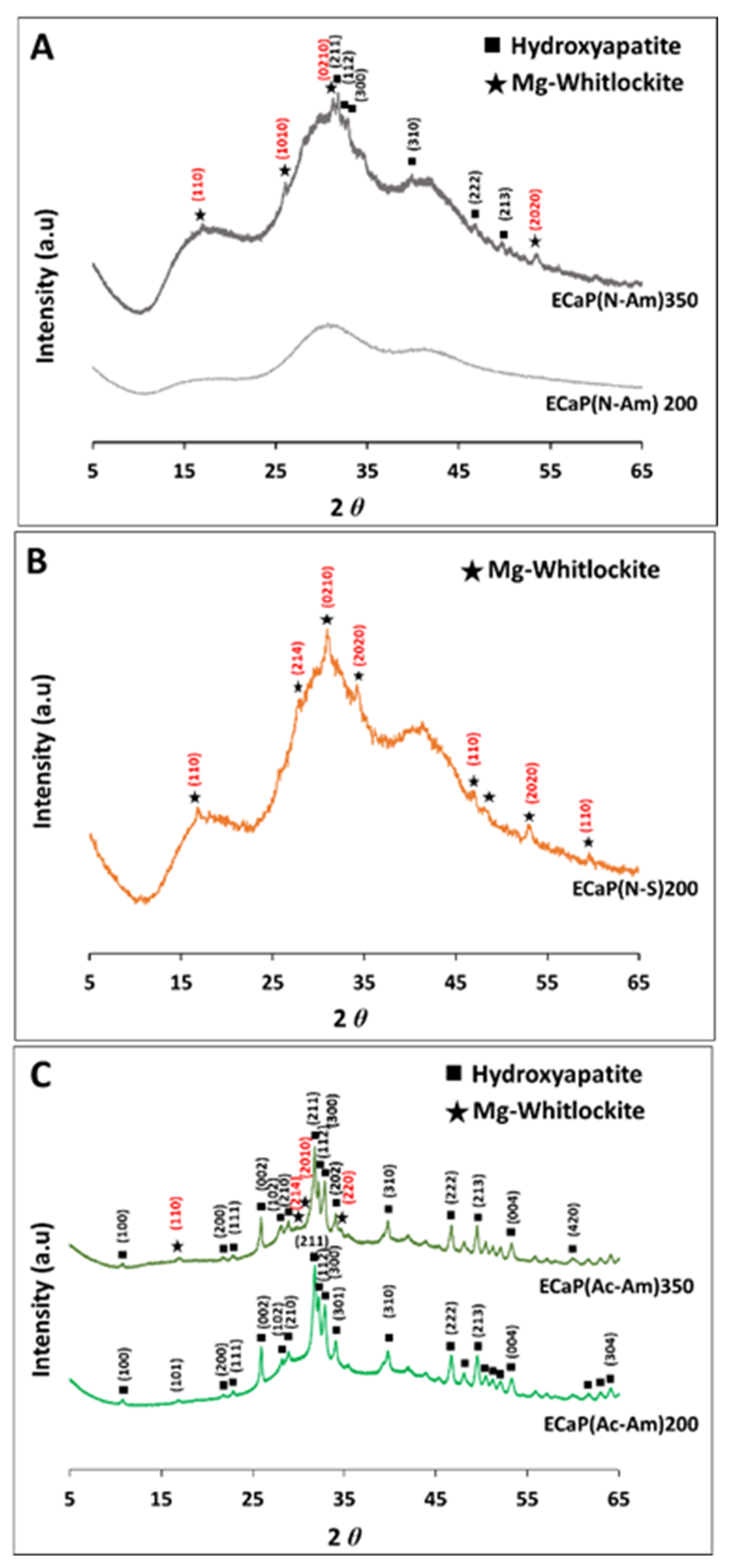
4.2.1. Investigating Phase Changes Using XRD
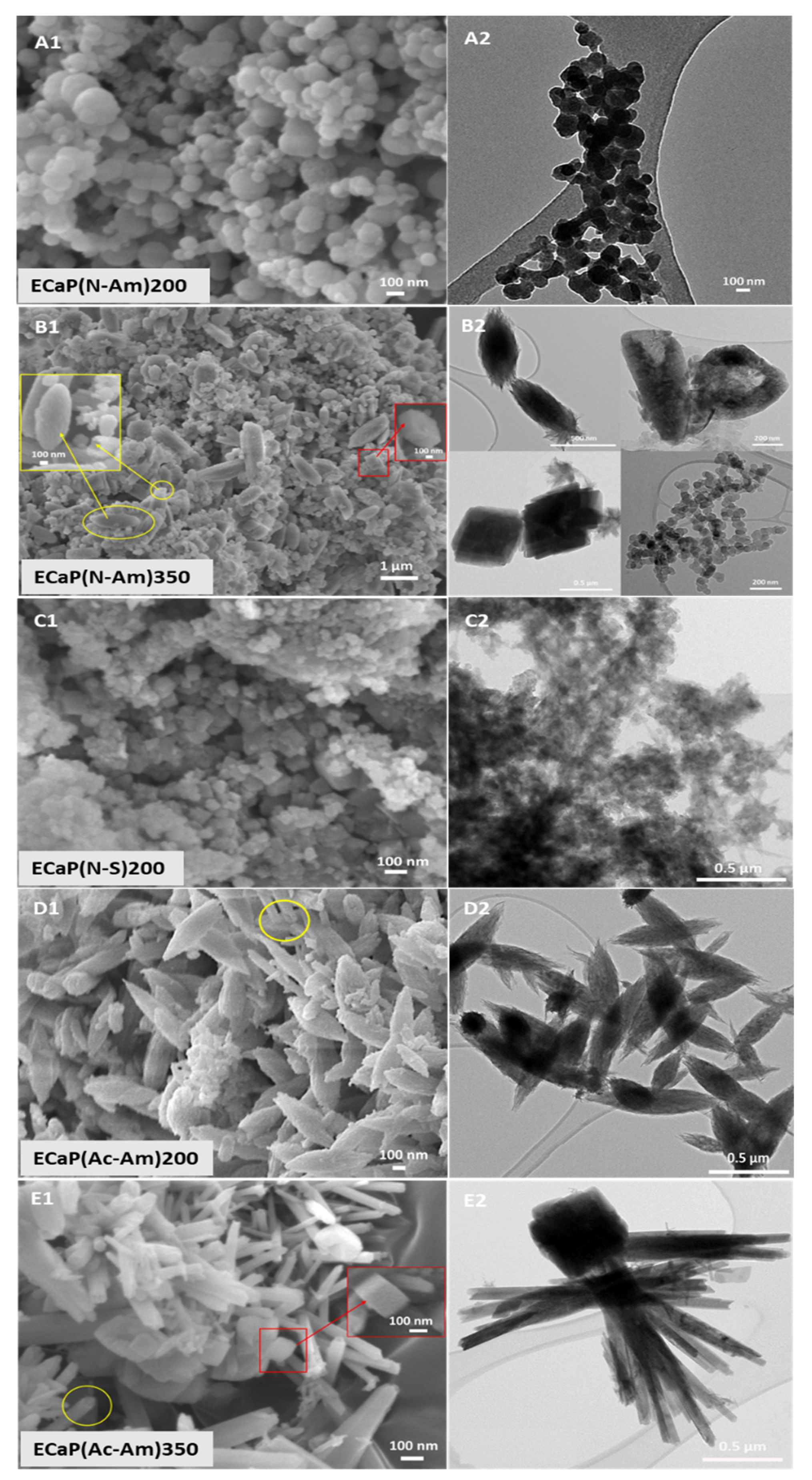
4.2.2. Morphology Characterisation Using FEG-SEM-EDX and TEM
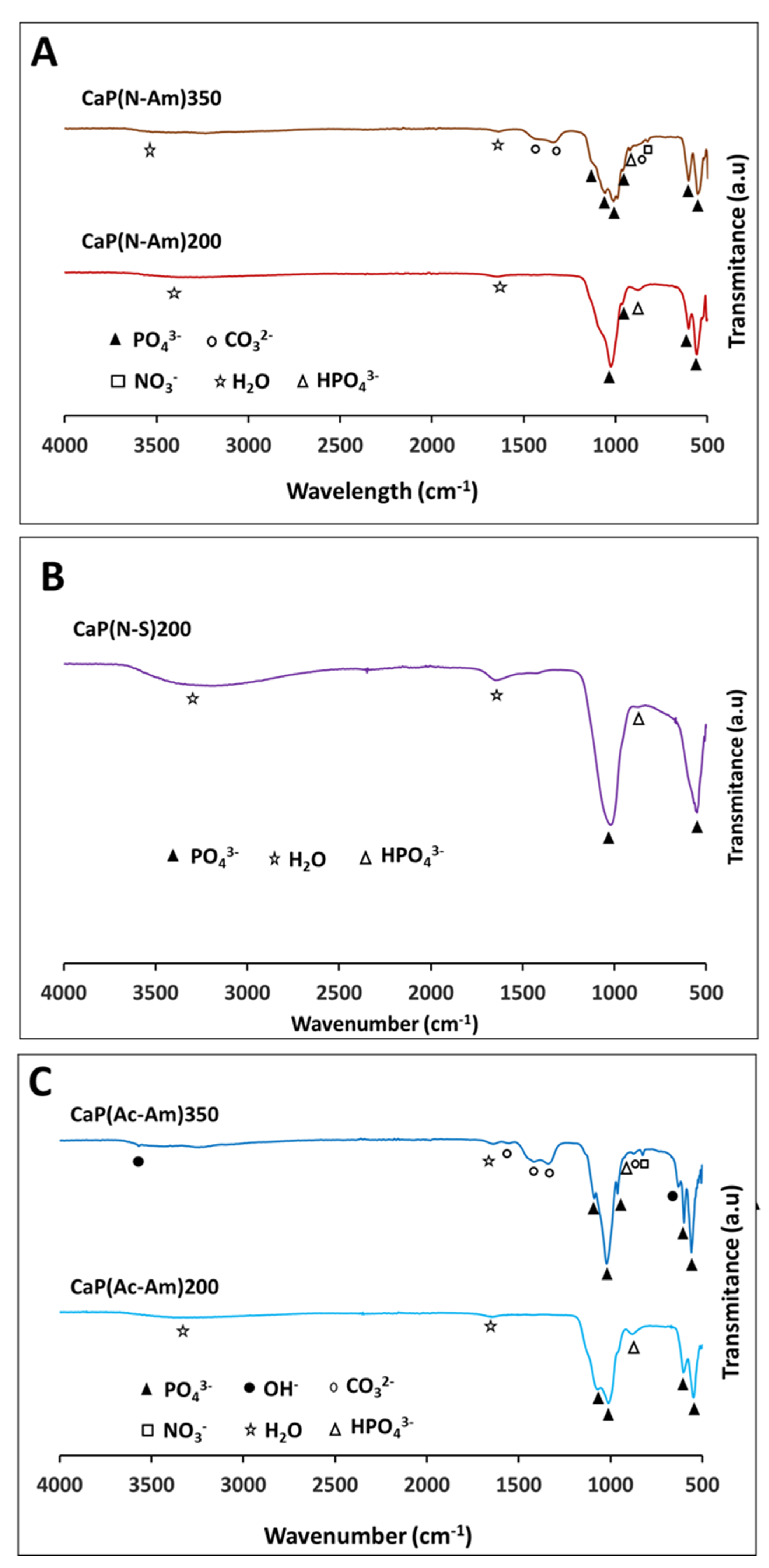
4.2.3. Functional Group Analysis by FTIR
4.3. Hydroxyapatite Synthesis Using Eggshell—Struvite
4.3.1. Investigating Phase Changes Using XRD
4.3.2. Morphology Characterisation Using FEG-SEM-EDX and TEM
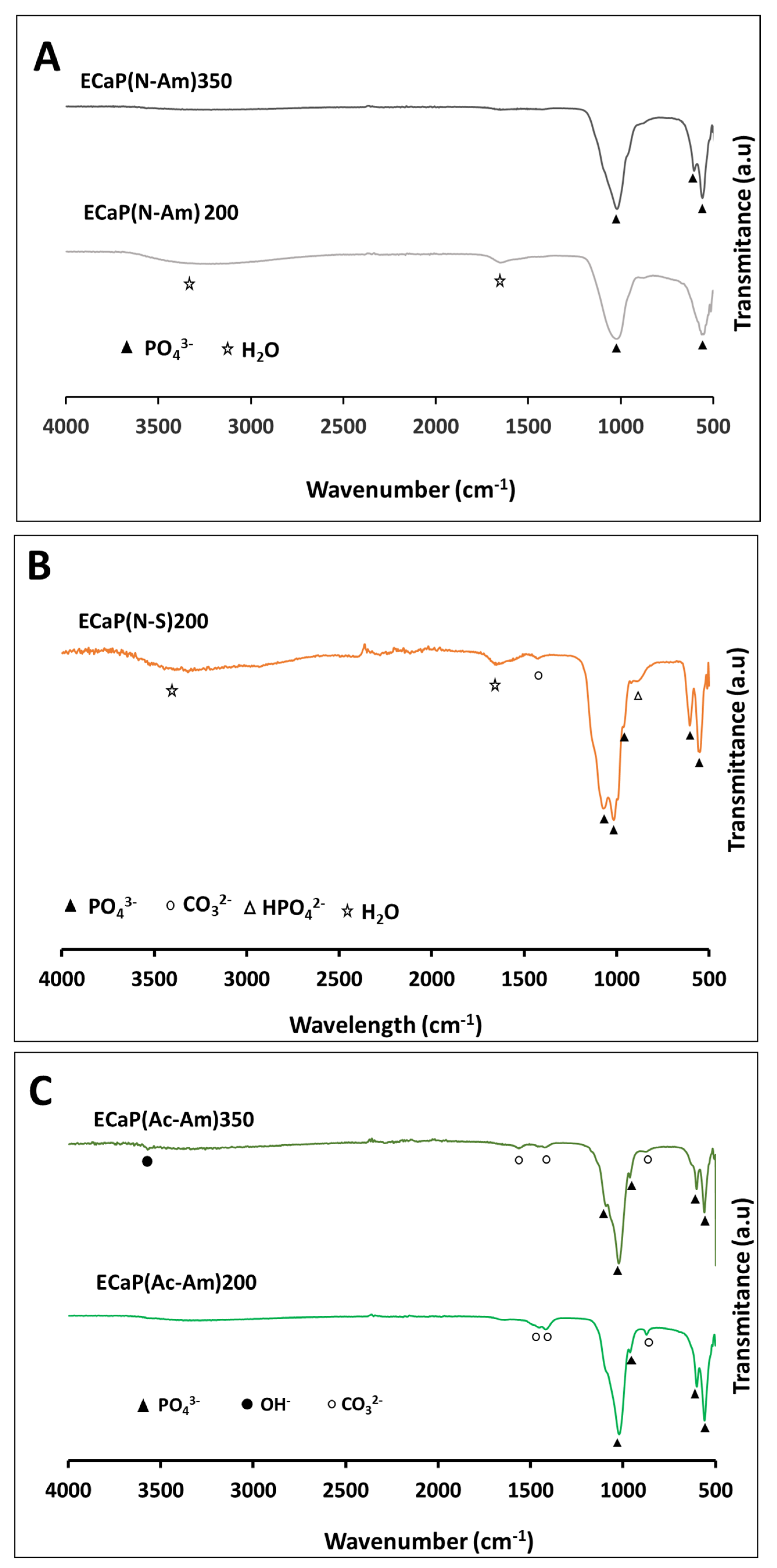
4.3.3. Functional Group Analysis by FTIR
5. Discussion
5.1. Samples Prepared from Calcium Nitrate Tetrahydrate and Struvite Solutions
5.2. Samples Prepared from Eggshell Solution and Struvite Solution
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, A.-Y.; Wang, C.; Hung, W.H.; Zheng, A.; Sen, B. Nano-and biomaterials for sustainable development. J. Nanomater. 2015, 2015, 129894. [Google Scholar] [CrossRef]
- Loffredo, E. Recent advances on innovative materials from biowaste recycling for the removal of environmental estrogens from water and soil. Materials 2022, 15, 1894. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Tarafdar, A.; Kumar, V.; Ganeshan, P.; Rajendran, K.; Giri, B.S.; Gómez-García, R.; Li, H.; Zhang, Z.; Sindhu, R.; et al. Sustainable biorefinery approaches towards circular economy for conversion of biowaste to value added materials and future perspectives. Fuel 2022, 325, 124846. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Romero, A. The use of biowaste for the production of biodegradable superabsorbent materials. Curr. Opin. Food Sci. 2023, 49, 100975. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Sengupta, S.; Gupta, A.; Kumar, S.S.; Vijay, V.; Kumar, V.; Vijay, V.K.; Pant, D. Valorization of agricultural waste for biogas based circular economy in India: A research outlook. Bioresour. Technol. 2020, 304, 123036. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Das, P.; Mukherjee, J.; Banerjee, D.; Ghosh, P.R.; Das, P.K.; Bhattacharya, R.N.; Nandi, S.K. Waste-derived biomaterials as building blocks in the biomedical field. J. Mater. Chem. B 2022, 10, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.F.M.A.; Bahru, R.; Amin, R.; Khan, M.U.A.; Razak, S.I.A.; Abu Hassan, S.; Kadir, M.R.A.; Nayan, N.H.M. Waste to health: A review of waste derived materials for tissue engineering. J. Clean. Prod. 2021, 290, 125792. [Google Scholar] [CrossRef]
- Abdulrahman, I.; Tijani, H.I.; Mohammed, B.A.; Saidu, H.; Yusuf, H.; Ndejiko Jibrin, M.; Mohammed, S. From garbage to biomaterials: An overview on egg shell based hydroxyapatite. J. Mater. 2014, 2014, 802467. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bagnani, M.; Soon, W.L.; Mezzenga, R. Turning food protein waste into Sustainable Technologies. Chem. Rev. 2022. [Google Scholar] [CrossRef]
- Areniello, M.; Matassa, S.; Esposito, G.; Lens, P.N. Biowaste upcycling into second-generation microbial protein through mixed-culture fermentation. Trends Biotechnol. 2023, 41, 197–213. [Google Scholar] [CrossRef]
- de Souza, M.A.; Vilas-Boas, I.T.; Leite-Da-Silva, J.M.; Abrahão, P.D.N.; Teixeira-Costa, B.E.; Veiga-Junior, V.F. Polysaccharides in agro-industrial biomass residues. Polysaccharides 2022, 3, 95–120. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Fiorentino, G.; Nicolaus, B.; Tommonaro, G.; Di Donato, P. Polysaccharides from wastes of vegetable industrial processing: New opportunities for their eco-friendly re-use. Biotechnol. Biopolym. 2011, 33, 56. [Google Scholar]
- Dou, X.; Hasa, I.; Hekmatfar, M.; Diemant, T.; Behm, R.J.; Buchholz, D.; Passerini, S. Pectin, hemicellulose, or lignin? Impact of the biowaste source on the performance of hard carbons for sodium-ion batteries. ChemSusChem 2017, 10, 2668–2676. [Google Scholar] [CrossRef]
- Kaou, M.H.; Horváth, Z.E.; Balázsi, K.; Balázsi, C. Eco-friendly preparation and structural characterization of calcium silicates derived from eggshell and silica gel. Int. J. Appl. Ceram. Technol. 2023, 20, 689–699. [Google Scholar] [CrossRef]
- Oktar, F.N.; Unal, S.; Gunduz, O.; Nissan, B.B.; Macha, I.J.; Akyol, S.; Duta, L.; Ekren, N.; Altan, E.; Yetmez, M. Marine-derived bioceramics for orthopedic, reconstructive and dental surgery applications. J. Aust. Ceram. Soc. 2023, 59, 57–81. [Google Scholar] [CrossRef]
- Marques, L.A.R.; Biscaia, S.; Massano, A.; Tavares, R.M.; Mateus, A. Sustainable Direct Digital Manufacturing Using Marine Resources. In Marine Organisms: A Solution to Environmental Pollution? Uses in Bioremediation and in Biorefinery; Springer: Cham, Switzerland, 2023; pp. 93–115. [Google Scholar]
- John, M.; Sugunan, A.; SD, R.D. Marine based biomaterials: A Marvel in Periodontal Regeneration–A Review. Adv. Dent. J. 2023, 5, 24–34. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Choudhary, R.; Krishnamurithy, G.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T.; Suresh, A.; Abraham, J.; Praharaj, S.; Swamiappan, S. Comparative investigation on antibacterial, biological and mechanical behaviour of monticellite and diopside derived from biowaste for bone regeneration. Mater. Chem. Phys. 2022, 286, 126157. [Google Scholar] [CrossRef]
- Ressler, A.; Ivanković, T.; Ivanišević, I.; Cvetnić, M.; Antunović, M.; Urlić, I.; Ivanković, H.; Ivanković, M. Multiphase zinc and magnesium mono-substituted calcium phosphates derived from cuttlefish bone: A multifunctional biomaterials. Ceram. Int. 2023, 49, 11005–11017. [Google Scholar] [CrossRef]
- Irwansyah, F.S.; Noviyanti, A.R.; Eddy, D.R.; Risdiana, R. Green Template-Mediated Synthesis of Biowaste Nano-Hydroxyapatite: A Systematic Literature Review. Molecules 2022, 27, 5586. [Google Scholar] [CrossRef]
- ARuffini, A.; Sprio, S.; Preti, L.; Tampieri, A. Synthesis of nanostructured hydroxyapatite via controlled hydrothermal route. In Biomaterial-Supported Tissue Reconstruction or Regeneration; Intechopen: London, UK, 2019; pp. 1–22. [Google Scholar]
- Díaz-Cuenca, A.; Rabadjieva, D.; Sezanova, K.; Gergulova, R.; Ilieva, R.; Tepavitcharova, S. Biocompatible calcium phosphate-based ceramics and composites. Mater. Today Proc. 2022, 61, 1217–1225. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic deposition of hydroxyapatite layer on titanium alloys. Micromachines 2021, 12, 1447. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Felfel, R.M.; Neel, E.A.A.; Grant, D.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Andersson, M. Biomimetic synthesis of nanostructured calcium phosphates. In The World Scientific Encyclopedia of Nanomedicine and Bioengineering II: Bioimplants, Regenerative Medicine, and Nano-Cancer Diagnosis and Phototherapy Volume 3: Design of Bioactive Materials for Bone Repair and Regeneration; World Scientific Publishing: Singapore, 2017; pp. 85–124. [Google Scholar]
- Kumta, P.N.; Sfeir, C.; Lee, D.-H.; Olton, D.; Choi, D. Nanostructured calcium phosphates for biomedical applications: Novel synthesis and characterization. Acta Biomater. 2005, 1, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Macri-Pellizzeri, L.; Hossain, K.M.Z.; Sottile, V.; Ahmed, I. Effect of varying the Mg with Ca content in highly porous phosphate-based glass microspheres. Mater. Sci. Eng. C 2021, 120, 111668. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Nuzulia, N.A.; Macri-Pellizzeri, L.; Nigar, F.; Sari, Y.W.; Ahmed, I. Evolution of silicate bioglass particles as porous microspheres with a view towards orthobiologics. J. Biomater. Appl. 2022, 36, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ahmed, I. Recent Advances in the Development and Applications of Phosphate and Borate Glass Microspheres. In Phosphate and Borate Bioactive Glasses; Royal Society of Chemistry: London, UK, 2022; pp. 227–247. [Google Scholar]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. ® 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Shoeib, M.A.; Abdel-Gawad, S.A. High performance nano hydroxyapatite coating on zinc for biomedical applications. J. Mater. Sci. 2023, 58, 740–756. [Google Scholar] [CrossRef]
- Islam, M.T.; Macri-Pellizzeri, L.; Sottile, V.; Ahmed, I. Rapid conversion of highly porous borate glass microspheres into hydroxyapatite. Biomater. Sci. 2021, 9, 1826–1844. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Kim, H.D.; Jang, H.L.; Ahn, H.Y.; Lee, H.K.; Park, J.; Lee, E.S.; Lee, E.A.; Jeong, Y.H.; Kim, D.G.; Nam, K.T.; et al. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials 2017, 112, 31–43. [Google Scholar] [CrossRef]
- Lagier, R.; Baud, C.-A. Magnesium whitlockite, a calcium phosphate crystal of special interest in pathology. Pathol. -Res. Pract. 2003, 199, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Yang, H.; Zhao, Y.; Guo, J.; Yin, X.; Ma, T.; Liu, X.; Li, L. Magnesium-based whitlockite bone mineral promotes neural and osteogenic activities. ACS Biomater. Sci. Eng. 2020, 6, 5785–5796. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Javadpour, J.; Khavandi, A. In situ synthesis and characterization of nano-size hydroxyapatite in poly (vinyl alcohol) matrix. Ceram. Int. 2007, 33, 1579–1583. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Quan, Z.; Yang, P.; Li, C.; Hou, Z.; Lin, J. Hydroxyapatite nano-and microcrystals with multiform morphologies: Controllable synthesis and luminescence properties. Cryst. Growth Des. 2009, 9, 2725–2733. [Google Scholar] [CrossRef]
- Sanosh, K.; Chu, M.-C.; Balakrishnan, A.; Kim, T.; Cho, S.-J. Utilization of biowaste eggshells to synthesize nanocrystalline hydroxyapatite powders. Mater. Lett. 2009, 63, 2100–2102. [Google Scholar] [CrossRef]
- Qiu, Y.; Xia, H.; Jiang, H. Fabrication of nano-hydroxyapatite using a novel ultrasonic atomization precipitation method. J. Nanosci. Nanotechnol. 2010, 10, 2213–2218. [Google Scholar] [CrossRef]
- Gopi, D.; Govindaraju, K.; Victor, C.A.P.; Kavitha, L.; Rajendiran, N. Spectroscopic investigations of nanohydroxyapatite powders synthesized by conventional and ultrasonic coupled sol–gel routes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 1243–1245. [Google Scholar] [CrossRef]
- Lee, S.-J.; Yoon, Y.-S.; Lee, M.-H.; Oh, N.-S. Nanosized hydroxyapatite powder synthesized from eggshell and phosphoric acid. J. Nanosci. Nanotechnol. 2007, 7, 4061–4064. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- ELester, E.; Tang, S.V.; Khlobystov, A.; Rose, V.L.; Buttery, L.; Roberts, C.J. Producing nanotubes of biocompatible hydroxyapatite by continuous hydrothermal synthesis. CrystEngComm 2013, 15, 3256–3260. [Google Scholar] [CrossRef]
- Stieberova, B.; Zilka, M.; Ticha, M.; Freiberg, F.; Caramazana-González, P.; McKechnie, J.; Lester, E. Sustainability assessment of continuous-flow hydrothermal synthesis of nanomaterials in the context of other production technologies. J. Clean. Prod. 2019, 241, 118325. [Google Scholar] [CrossRef]
- Yelten, A.; Yilmaz, S. Comparison of naturally and synthetically derived hydroxyapatite powders. Acta Phys. Pol. A 2017, 131, 55–58. [Google Scholar] [CrossRef]
- Wu, S.-C.; Tsou, H.-K.; Hsu, H.-C.; Hsu, S.-K.; Liou, S.-P.; Ho, W.-F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Adamiano, A.; Scialla, S.; Carella, F.; Casella, M.; Camerini, S.; Quarta, A.; Muntiu, A.; Ferrari, F.; Vitali, A.; Iafisco, M.; et al. Simultaneous extraction of calcium phosphates and proteins from fish bones. Innovative valorisation of food by-products. J. Clean. Prod. 2023, 385, 135656. [Google Scholar] [CrossRef]
- Hussin, M.S.F.; Abdullah, H.Z.; Idris, M.I.; Wahap, M.A.A. Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef]
- Jubier, N.J.; Hassani, R.H.; Hathot, S.F.; Salim, A.A. A new type of carbonate hydroxyapatite nanoparticles made from PMMA and oyster shells: Evaluation of structure, morphology and biocompatible properties. Polym. Bull. 2023, 1–15. [Google Scholar] [CrossRef]
- Sindhya, A.; Jeyakumar, S.J.; Jothibas, M.; Pugalendhi, P.; Vigneshwaran, B. Surface modification of coral skeleton derived nanohydroxyapatite using polymers and its in-vitro studies for bone substitute applications. Vacuum 2023, 210, 111838. [Google Scholar] [CrossRef]
- Hossain, M.S.; Uddin, M.N.; Sarkar, S.; Ahmed, S. Crystallographic dependency of waste cow bone, hydroxyapatite, and β-tricalcium phosphate for biomedical application. J. Saudi Chem. Soc. 2022, 26, 101559. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, Y.; Liang, M.; Zhang, H.; Liu, H. Optimization of the Preparation Conditions for Activated Carbons from Sugarcane Bagasse: An Agricultural Waste. In Proceedings of the 2011 International Conference on Computer Distributed Control and Intelligent Environmental Monitoring, Changsha, China, 19–20 February 2011; pp. 555–559. [Google Scholar]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J. Sustain. Energy Environ. 2011, 2, 77–81. [Google Scholar]
- Rashidi, N.A.; Yusup, S.; Ahmad, M.M.; Mohamed, N.M.; Hameed, B.H. Activated carbon from the renewable agricultural residues using single step physical activation: A preliminary analysis. APCBEE Procedia 2012, 3, 84–92. [Google Scholar] [CrossRef]
- Ha, T.L.B.; Quan, T.M.; Vu, D.N. Naturally derived biomaterials: Preparation and application. In Regenerative Medicine and Tissue Engineering; IntechOpen: London, UK, 2013. [Google Scholar]
- Kim, B.-S.; Kang, H.J.; Yang, S.-S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Ghasemi, M.; Dehpour, A.R. Ethical considerations in animal studies. J. Med. Ethics Hist. Med. 2009, 2, 12. [Google Scholar] [PubMed]
- Lee, S.-W.; Balázsi, C.; Balázsi, K.; Seo, N.-H.; Kim, H.S.; Kim, C.-H.; Kim, S.-G. Comparative Study of hydroxyapatite prepared from seashells and eggshells as a bone graft material. Tissue Eng. Regen. Med. 2014, 11, 113–120. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Hsu, S.-K.; Chang, Y.-C.; Ho, W.-F. Effects of heat treatment on the synthesis of hydroxyapatite from eggshell powders. Ceram. Int. 2015, 41, 10718–10724. [Google Scholar] [CrossRef]
- Goller, G.; Oktar, F.N.; Agathopoulos, S.; Tulyaganov, D.U.; Ferreira JM, F.; Kayali, E.S.; Peker, I. The influence of sintering temperature on mechanical and microstructural properties of bovine hydroxyapatite. Key Eng. Mater. Trans. Tech. Publ. 2005, 284, 325–328. [Google Scholar] [CrossRef]
- Ahmed, S.; KAbir, M.H.; Nigar, F.; Kabir, S.F.; Mustafa, A.I.; Ahsan, M. Structural characterization of pure and doped calcium phosphate bioceramics prepared by simple solid state method. A Sci. J. COMSATS—SCIENCE VISION 2010–2011, 16–17, 81–92. [Google Scholar]
- Wanniarachchi, W.A.C.P.; Janani, V.; Sutharsini, U.; Thanihaichelvan, M.; Ramesh, S.; Ng, C.K. Synthesis and sintering studies of hydroxyapatite derived from biogenic waste materials. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2023; p. 050017. [Google Scholar]
- Agbeboh, N.I.; Oladele, I.O.; Daramola, O.O.; Akinwekomi, A.D.; Tanimola, M.O.; Olasukanmi, O. Comparing the effects of two wet precipitation methods on the yield of chicken eggshell-derived hydroxyapatite. Futa J. Eng. Eng. Technol. 2022, 16, 95–104. [Google Scholar] [CrossRef]
- Toama, H.Z. World phosphate industry. Iraqi Bull. Geol. Min. 2017, 7, 5–23. [Google Scholar]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D. Phosphorus recovery as struvite from farm, municipal and industrial waste: Feedstock suitability, methods and pre-treatments. Waste Manag. 2016, 49, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, Y.; Hu, Q.; Yu, X.; Qian, F. Effects of three kinds of organic acids on phosphorus recovery by magnesium ammonium phosphate (MAP) crystallization from synthetic swine wastewater. Chemosphere 2014, 101, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, R.; Deng, Y. Phosphorus recovery by struvite crystallization from livestock wastewater and reuse as fertilizer: A review. In Physico-Chemical Wastewater Treatment and Resource Recovery; InTech: London, UK, 2017; pp. 135–152. [Google Scholar]
- Li, B.; Boiarkina, I.; Young, B.; Yu, W.; Singhal, N. Prediction of future phosphate rock: A demand based model. J. Environ. Inf. 2018, 1, 41–53. [Google Scholar] [CrossRef]
- RTaddeo, R.; Honkanen, M.; Kolppo, K.; Lepistö, R. Nutrient management via struvite precipitation and recovery from various agroindustrial wastewaters: Process feasibility and struvite quality. J. Environ. Manag. 2018, 212, 433–439. [Google Scholar]
- Prasad, M.N.V.; Shih, K. Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Academic Press Elsevier: Cambridge, MA, USA, 2016. [Google Scholar]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Shaddel, S.; Grini, T.; Andreassen, J.-P.; Østerhus, S.W.; Ucar, S. Crystallization kinetics and growth of struvite crystals by seawater versus magnesium chloride as magnesium source: Towards enhancing sustainability and economics of struvite crystallization. Chemosphere 2020, 256, 126968. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Dombinov, V.; Robles-Aguilar, A.A.; Jablonowski, N.D.; Ribeiro, C. Struvite-based composites for slow-release fertilization: A case study in sand. Sci. Rep. 2022, 12, 14176. [Google Scholar] [CrossRef]
- Raniro, H.R.; Soares, T.D.M.; Adam, C.; Pavinato, P.S. Waste-derived fertilizers can increase phosphorus uptake by sugarcane and availability in a tropical soil#. J. Plant Nutr. Soil Sci. 2022, 185, 391–402. [Google Scholar]
- Witek-Krowiak, A.; Gorazda, K.; Szopa, D.; Trzaska, K.; Moustakas, K.; Chojnacka, K. Phosphorus recovery from wastewater and bio-based waste: An overview. Bioengineered 2022, 13, 13474–13506. [Google Scholar] [CrossRef] [PubMed]
- Matayeva, A.; Rasmussen, S.R.; Biller, P. Distribution of nutrients and phosphorus recovery in hydrothermal liquefaction of waste streams. Biomass Bioenergy 2022, 156, 106323. [Google Scholar] [CrossRef]
- Ronan, K.; Kannan, M.B. Novel sustainable route for synthesis of hydroxyapatite biomaterial from biowastes. ACS Sustain. Chem. Eng. 2017, 5, 2237–2245. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.-H.; Yao, Q.-Z.; Zhou, G.-T.; Fu, S.-Q. Chemical control of struvite scale by a green inhibitor polyaspartic acid. RSC Adv. 2015, 5, 91601–91608. [Google Scholar] [CrossRef]
- Hallas, J.; Mackowiak, C.; Wilkie, A.; Harris, W. Struvite phosphorus recovery from aerobically digested municipal wastewater. Sustainability 2019, 11, 376. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.; Smit, A. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Niu, D.; Zhao, Y. Acetic acid production from food wastes using yeast and acetic acid bacteria micro-aerobic fermentation. Bioprocess Biosyst. Eng. 2015, 38, 863–869. [Google Scholar] [CrossRef]
- Khatami, K.; Atasoy, M.; Ludtke, M.; Baresel, C.; Eyice, Ö.; Cetecioglu, Z. Bioconversion of food waste to volatile fatty acids: Impact of microbial community, pH and retention time. Chemosphere 2021, 275, 129981. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Watanabe, Y.; Kishita, A.; Enomoto, H.; Kishida, H. Production of acetic acid by hydrothermal two-step process of vegetable wastes for use as a road deicer. In Journal of Physics: Ournal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2008; Volume 121, p. 082005. [Google Scholar]
- Hossain, M.; Mahmud, M.; Mobarak, M.B.; Ahmed, S. Crystallographic analysis of biphasic hydroxyapatite synthesized by different methods: An appraisal between new and existing models. Chem. Pap. 2022, 76, 1593–1605. [Google Scholar] [CrossRef]
- Xu, G.; Aksay, I.A.; Groves, J.T. Continuous crystalline carbonate apatite thin films. A biomimetic approach. J. Am. Chem. Soc. 2001, 123, 2196–2203. [Google Scholar] [CrossRef]
- Landi, E.; Uggeri, J.; Sprio, S.; Tampieri, A.; Guizzardi, S. Human osteoblast behavior on as-synthesized SiO4 and B-CO3 co-substituted apatite. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 94, 59–70. [Google Scholar] [CrossRef]
- Habib, F.; Alam, S.; Zahra, N.; Irfan, M.; Iqbal, W. Synthesis route and characterization of hydroxyapatite powder prepared from waste egg shells. J. Chem. Soc. Pak. 2012, 34. [Google Scholar]
- Krishna, D.S.R.; Siddharthan, A.; Seshadri, S.K.; Kumar, T.S.S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735–1743. [Google Scholar]
- Ariyanto, E.; Ang, H.M.; Sen, T. Effect of initial solution pH on solubility and morphology of struvite crystals. In Proceedings of the CHEMECA Conference, Sydney, Australia, 18–21 September 2011;. 18–21. [Google Scholar]
- Jang, H.L.; Lee, H.K.; Jin, K.; Ahn, H.-Y.; Lee, H.E.; Nam, K.T. Phase transformation from hydroxyapatite to the secondary bone mineral, whitlockite. J. Mater. Chem. B 2015, 3, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Structural Characterisation of Apatite-Like Materials. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2010. [Google Scholar]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Arias, J.; Mayor, M.; Pou, J.; Leng, Y.; León, B.; Amor, M. Micro-and nano-testing of calcium phosphate coatings produced by pulsed laser deposition. Biomaterials 2003, 24, 3403–3408. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Zhou, Y.; Zeng, Y. A rapid way to synthesize magnesium whitlockite microspheres for high efficiency removing heavy metals. Desalin. Water Treat 2019, 162, 220–227. [Google Scholar] [CrossRef]
- Jang, H.L.; Zheng, G.B.; Park, J.; Kim, H.D.; Baek, H.R.; Lee, H.K.; Lee, K.; Han, H.N.; Lee, C.K.; Hwang, N.S. In vitro and in vivo evaluation of whitlockite biocompatibility: Comparative study with hydroxyapatite and β-tricalcium phosphate. Adv. Healthc. Mater. 2016, 5, 128–136. [Google Scholar] [CrossRef]
- Bauer, L.; Ivanković, M.; Ivanković, H. Magneisum substituted hydroxyapatite scaffolds hydrothermally synthesized from Cuttlefish bone. In International Conference MATRIB 21–34 (2018); Croatian Society for Materials and Tribology: Zagreb, Croatia, 2018. [Google Scholar]
- Bayuseno, A.P.; Schmahl, W.W. Crystallization of struvite in a hydrothermal solution with and without calcium and carbonate ions. Chemosphere 2020, 250, 126245. [Google Scholar] [CrossRef]
- Shih, K.; Yan, H. The crystallization of struvite and its analog (K-struvite) from waste streams for nutrient recycling. In Environmental Materials and Waste; Elsevier: Amsterdam, The Netherlands, 2016; pp. 665–686. [Google Scholar]
- Islam, T.; Hossain, K.M.Z.; Sharmin, N.; Parsons, A.J.; Ahmed, I. Effect of magnesium content on bioactivity of near invert phosphate-based glasses. Int. J. Appl. Glass Sci. 2017, 8, 391–402. [Google Scholar] [CrossRef]
- Kim, H.-K.; Han, H.-S.; Lee, K.-S.; Lee, D.-H.; Lee, J.W.; Jeon, H.; Cho, S.-Y.; Roh, H.-J.; Kim, Y.-C.; Seok, H.-K. Comprehensive study on the roles of released ions from biodegradable Mg–5 wt% Ca–1 wt% Zn alloy in bone regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 2710–2724. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 1991, 15, 109–111. [Google Scholar]
- Tas, A.C. Synthesis of biomimetic Ca-hydroxyapatite powders at 37 C in synthetic body fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar]
- Batool, S.; Liaqat, U.; Hussain, Z.; Sohail, M. Synthesis, characterization and process optimization of bone whitlockite. Nanomater. 2020, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphates using Fourier transform infrared spectroscopy. Infrared Spectrosc. -Mater. Sci. Eng. Technol. 2012, 12, 251–263. [Google Scholar]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Yang, X.; Jiang, T. Are different crystallinity-index-calculating methods of hydroxyapatite efficient and consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]
- Theoret, A.; Sandorfy, C. Infrared spectra and crystalline phase transitions of ammonium nitrate. Can. J. Chem. 1964, 42, 57–62. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahsan, M. Synthesis of Ca-hydroxyapatite bioceramic from egg shell and its characterization. Bangladesh J. Sci. Ind. Res. 2008, 43, 501–512. [Google Scholar] [CrossRef]
- Kumar, G.S.; Thamizhavel, A.; Girija, E. Microwave conversion of eggshells into flower-like hydroxyapatite nanostructure for biomedical applications. Mater. Lett. 2012, 76, 198–200. [Google Scholar] [CrossRef]
- Ramesh, S.; Natasha, A.; Tan, C.; Bang, L.T.; Ching, C.; Chandran, H. Direct conversion of eggshell to hydroxyapatite ceramic by a sintering method. Ceram. Int. 2016, 42, 7824–7829. [Google Scholar] [CrossRef]
- Kamalanathan, P.; Ramesh, S.; Bang, L.; Niakan, A.; Tan, C.; Purbolaksono, J.; Chandran, H.; Teng, W. Synthesis and sintering of hydroxyapatite derived from eggshells as a calcium precursor. Ceram. Int. 2014, 40, 16349–16359. [Google Scholar] [CrossRef]
- Jang, H.L.; Jin, K.; Lee, J.; Kim, Y.; Nahm, S.H.; Hong, K.S.; Nam, K.T. Revisiting whitlockite, the second most abundant biomineral in bone: Nanocrystal synthesis in physiologically relevant conditions and biocompatibility evaluation. ACS Nano 2014, 8, 634–641. [Google Scholar] [CrossRef]
- Diallo-Garcia, S.; Osman, M.B.; Krafft, J.-M.; Boujday, S.; Guylène, C. Discrimination of infrared fingerprints of bulk and surface POH and OH of hydroxyapatites. Catal. Today 2014, 226, 81–88. [Google Scholar] [CrossRef]


| Sample Names | Acid to Dissolve Struvite | pH Buffer | Temperature |
|---|---|---|---|
| CaP(N-Am)200 | HNO3 (N) | NH4OH (Am) | 200 °C |
| CaP(N-Am)350 | 350 °C | ||
| CaP(N-S)200 | NaOH (S) | 200 °C | |
| CaP(Ac-Am)200 | CH3COOH (Ac) | NH4OH (Am) | 200 °C |
| CaP(Ac-Am)350 | 350 °C |
| Sample Names | Acid to Dissolve Struvite | pH Buffer | Temperature |
|---|---|---|---|
| ECaP(N-Am)200 | HNO3 (N) | NH4OH (Am) | 200 °C |
| ECaP(N-Am)350 | 350 °C | ||
| ECaP(N-S)200 | NaOH (S) | 200 °C | |
| ECaP(Ac-Am)200 | CH3COOH (Ac) | NH4OH (Am) | 200 °C |
| ECaP(Ac-Am)350 | 350 °C |
| Components | Mol% | Wt% |
|---|---|---|
| Ca | 97.11 ± 0.23 | 98.13 ± 0.14 |
| Na | 0.56 ± 0.08 | 0.32 ± 0.04 |
| Mg | 1.89 ± 0.08 | 1.16 ± 0.05 |
| P | 0.40 ± 0.08 | 0.31 ± 0.06 |
| Sr | 0.02 ± 0.01 | 0.04 ± 0.01 |
| Zn | 0.02 ± 0.01 | 0.03 ± 0.01 |
| Sample Names | Products (Percentage of Phases) | Crystallite Size (nm) | Crystallinity Index | ||
|---|---|---|---|---|---|
| at the plane (002) for HA | at the plane (0210) for Mg-WH | at the plane (002) for HA | at the plane (0210) for Mg-WH | ||
| CaP(N-Am)200 | HA (51%) + Mg-WH (49%) | 16.8 | 81.6 | 0.1 | 13.8 |
| CaP(N-Am)350 | Mg-WH | - | 81.6 | - | 13.8 |
| CaP(N-S)200 | Mg-WH | - | 81.6 | - | 13.8 |
| CaP(Ac-Am)200 | Mg-WH | - | 62 | - | 5.8 |
| CaP(Ac-Am)350 | HA (88%) + Mg-WH (12%) | 79.8 | 81.6 | 13.4 | 13.8 |
| Samples | Morphology (Particle Size) | Ca/P | (Ca + Mg)/P |
|---|---|---|---|
| CaP(N-Am)200 | Irregular along with plate shape | 1.42–1.65 (Avg. 1.51 ± 0.25) | 1.47–1.83 (Avg. 1.66 ± 0.19) |
| CaP(N-Am)350 | Rhombohedral | 1.3 ± 0.05 | 1.4 ± 0.04 |
| CaP(N-S)200 | Rhombohedral | 1.23 ± 0.01 | 1.39 ± 0.00 |
| CaP(N-Am)200 | Platelet | 1.3 ± 0.01 | 1.4 ± 0.03 |
| CaP(Ac-Am)350 | Hexagonal tube (50–160 nm diameter with 20–40 nm thickness) + Rhombohedral (23–720 nm) | 1.45–1.77 (Avg. 1.67 ± 0.16) | 1.5–1.74 (Avg. 1.72 ± 0.13) |
| Sample Names | Product (Percentage of Phases) | Crystallite Size (nm) | Crystallinity Index | ||
|---|---|---|---|---|---|
| at the plane (002) for HA | at the plane (0210) for Mg-WH | at the plane (002) for HA | at the plane (0210) for Mg-WH | ||
| ECaP(N-Am)200 | - | - | - | - | - |
| ECaP(N-Am)350 | HA (51%) + Mg-WH (49%) | - | 81.6 | - | 13.8 |
| ECaP(N-S)200 | Mg-WH | 80.6 | - | 13.8 | - |
| ECaP(Ac-Am)200 | HA | 80.6 | 81.7 | 13.8 | 13.8 |
| ECaP(Ac-Am)350 | HA (81%) + Mg-WH (19%) | 80.6 | 81.6 | 13.8 | 13.8 |
| Samples | Crystal Morphology (Particle Size) | Ca/P | (Ca + Mg)/P |
|---|---|---|---|
| ECaP(N-Am)200 | Spherical (28–196 nm) | 0.78 ± 0.04 | 1.45 ± 0.05 |
| ECaP(N-Am)350 | Ellipsoidal (width 273–522 nm) Tube (inner diameter 99–290 thickness 28–71 nm) Rhombohedral (238–278 nm) | 1.23–1.5 (Avg. 1.24 ± 0.12) | 1.6–1.7 (Avg. 1.6 ± 0.08) |
| ECaP(N-S)200 | Irregular-shaped along with rhombohedral | 1.3 ± 0.03 | 1.4 ± 0.00 |
| ECaP(Ac-Am)200 | Ellipsoidal (96–273 nm) | 1.6 ± 0.03 | 1.7 ± 0.03 |
| ECaP(Ac-Am)350 | Rod, Tube (37–266 nm diameter) Rhombohedral (96–214 nm) | 1.3–1.5 (Avg. 1.46 ± 0.04) 1.48 | 1.6–1.7 (Avg. 1.68 ± 0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigar, F.; Johnston, A.-L.; Smith, J.; Oakley, W.; Islam, M.T.; Felfel, R.; Grant, D.; Lester, E.; Ahmed, I. Production of Nano Hydroxyapatite and Mg-Whitlockite from Biowaste-Derived products via Continuous Flow Hydrothermal Synthesis: A Step towards Circular Economy. Materials 2023, 16, 2138. https://doi.org/10.3390/ma16062138
Nigar F, Johnston A-L, Smith J, Oakley W, Islam MT, Felfel R, Grant D, Lester E, Ahmed I. Production of Nano Hydroxyapatite and Mg-Whitlockite from Biowaste-Derived products via Continuous Flow Hydrothermal Synthesis: A Step towards Circular Economy. Materials. 2023; 16(6):2138. https://doi.org/10.3390/ma16062138
Chicago/Turabian StyleNigar, Farah, Amy-Louise Johnston, Jacob Smith, William Oakley, Md Towhidul Islam, Reda Felfel, David Grant, Edward Lester, and Ifty Ahmed. 2023. "Production of Nano Hydroxyapatite and Mg-Whitlockite from Biowaste-Derived products via Continuous Flow Hydrothermal Synthesis: A Step towards Circular Economy" Materials 16, no. 6: 2138. https://doi.org/10.3390/ma16062138
APA StyleNigar, F., Johnston, A.-L., Smith, J., Oakley, W., Islam, M. T., Felfel, R., Grant, D., Lester, E., & Ahmed, I. (2023). Production of Nano Hydroxyapatite and Mg-Whitlockite from Biowaste-Derived products via Continuous Flow Hydrothermal Synthesis: A Step towards Circular Economy. Materials, 16(6), 2138. https://doi.org/10.3390/ma16062138