Ultra-High-Molecular-Weight Polyethylene in Hip and Knee Arthroplasties
Abstract
1. Introduction
2. Conventional UHMWPE
3. Polyethylene Wear, Osteolysis, and Loosening
4. First-Generation HXLPE
5. Second-Generation HXLPE
6. Creep and Wear of UHMWPE
7. Surface Damage of UHMWPE
8. Registry Data
8.1. Hip
8.2. Knee
9. Clinical Results with Second-Generation HXLPE
10. Effects of Femoral Head Material in THA and Femoral Component Material in TKA on UHMWPE Wear
11. Microorganism Adhesion on UHMWPE
12. Future Direction of UHMWPE
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, J.T.; Evans, J.P.; Walker, R.W.; Blom, A.W.; Whitehouse, M.R.; Sayers, A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Sabatini, L.; Aprato, A.; Bracco, P.; Bellare, A. Ultra-high molecular weight polyethylene (UHMWPE) for hip and knee arthroplasty: The present and the future. J. Orthop. 2021, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Currier, B.H.; Mayor, M.B.; Collier, J.P. Gamma-irradiation sterilization in an inert environment: A partial solution. Clin. Orthop. Relat. Res. 2012, 470, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- McKellop, H.A.; Shen, F.W.; Campbell, P.; Ota, T. Effect of molecular weight, calcium stearate, and sterilization methods on the wear of ultra high molecular weight polyethylene acetabular cups in a hip joint simulator. J. Orthop. Res. 1999, 17, 329–339. [Google Scholar] [CrossRef]
- Langlois, J.; Hamadouche, M. What have we learned from 20 years of using highly crosslinked PE in total hip arthroplasty? Orthop. Traumatol. Surg. Res. 2022, 109, 103457. [Google Scholar] [CrossRef]
- Muratoglu, O.; Bragdon, C.R. Highly cross-linked and melted UHMWPE. In UHMWPE Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2016; pp. 264–273. [Google Scholar]
- Oral, E.; Greenbaum, E.S.; Malhi, A.S.; Harris, W.H.; Muratoglu, O.K. Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials 2005, 26, 6657–6663. [Google Scholar] [CrossRef]
- Oral, E.; Muratoglu, O.K. Vitamin E diffused, highly crosslinked UHMWPE: A review. Int. Orthop. 2011, 35, 215–223. [Google Scholar] [CrossRef]
- Kurtz, S.M. A Primer on UHMWPE. In UHMWPE Biomaterial Handbook; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–6. [Google Scholar]
- Bracco, P.; Oral, E. Vitamin E-stabilized UHMWPE for total joint implants: A review. Clin. Orthop. Relat. Res. 2011, 469, 2286–2293. [Google Scholar] [CrossRef]
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef]
- Kyi, M.S.; Holton, J.; Ridgway, G.L. Assessment of the efficacy of a low temperature hydrogen peroxide gas plasma sterilization system. J. Hosp. Infect. 1995, 31, 275–284. [Google Scholar] [CrossRef]
- Bracco, P.; Bellare, A.; Bistolfi, A.; Affatato, S. Ultra-High Molecular Weight Polyethylene: Influence of the Chemical, Physical and Mechanical Properties on the Wear Behavior. A Review. Materials 2017, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Tone, S.; Hasegawa, M.; Pezzotti, G.; Puppulin, L.; Sudo, A. Effect of e-beam sterilization on the in vivo performance of conventional UHMWPE tibial plates for total knee arthroplasty. Acta. Biomater. 2017, 55, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Dannenmaier, W.C.; Haynes, D.W.; Nelson, C.L. Granulomatous reaction and cystic bony destruction associated with high wear rate in a total knee prosthesis. Clin. Orthop. Relat. Res. 1985, 198, 224–230. [Google Scholar] [CrossRef]
- Pryor, G.A.; Villar, R.N.; Coleman, N. Tissue reaction and loosening of carbon-reinforced polyethylene arthroplasties. J. Bone Joint Surg. Br. 1992, 74, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Willert, H.G.; Semlitsch, M. Tissue reactions to plastic and metallic wear products of joint endoprostheses. Clin. Orthop. Relat. Res. 1996, 333, 4–14. [Google Scholar] [CrossRef]
- Athanasou, N.A.; Quinn, J.; Bulstrode, C.J. Resorption of bone by inflammatory cells derived from the joint capsule of hip arthroplasties. J. Bone Joint Surg. Br. 1992, 4, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.J.; Anoushiravani, A.A.; Sayeed, Z.; Chambers, M.C.; El-Othmani, M.M.; Saleh, K.J. Osteolysis Complicating Total Knee Arthroplasty. JBJS Rev. 2016, 4, e1. [Google Scholar] [CrossRef]
- Merkel, K.D.; Erdmann, J.M.; McHugh, K.P.; Abu-Amer, Y.; Ross, F.P.; Teitelbaum, S.L. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am. J. Pathol. 1999, 154, 203–210. [Google Scholar] [CrossRef]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef]
- Tian, Y.; Terkawi, M.A.; Onodera, T.; Alhasan, H.; Matsumae, G.; Takahashi, D.; Hamasaki, M.; Ebata, T.; Aly, M.K.; Kida, H.; et al. Blockade of XCL1/Lymphotactin Ameliorates Severity of Periprosthetic Osteolysis Triggered by Polyethylene-Particles. Front. Immunol. 2020, 11, 1720. [Google Scholar] [CrossRef]
- Dyskova, T.; Gallo, J.; Kriegova, E. The Role of the Chemokine System in Tissue Response to Prosthetic By-products Leading to Periprosthetic Osteolysis and Aseptic Loosening. Front. Immunol. 2017, 8, 1026. [Google Scholar] [CrossRef] [PubMed]
- Diamond, P.; Labrinidis, A.; Martin, S.K.; Farrugia, A.N.; Gronthos, S.; To, L.B.; Fujii, N.; O’Loughlin, P.D.; Evdokiou, A.; Zannettino, A.C. Targeted disruption of the CXCL12/CXCR4 axis inhibits osteolysis in a murine model of myeloma-associated bone loss. J. Bone Miner. Res. 2009, 24, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sato, T.; Yao, Z.; Keeney, M.; Pajarinen, J.; Lin, T.H.; Loi, F.; Egashira, K.; Goodman, S.; Yang, F. Local delivery of mutant CCL2 protein-reduced orthopaedic implant wear particle-induced osteolysis and inflammation in vivo. J. Orthop. Res. 2016, 34, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, C.; Morony, S.; Warmington, K.; Adamu, S.; Lacey, D.; Dunstan, C.R.; Stouch, B.; Martin, S.; Kostenuik, P.J. Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): A pharmacodynamic and pharmacokinetic analysis in rats. J. Bone Miner. Res. 2003, 18, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Vinther, M.; Carmody, E.E.; Goaterm, J.J.; Søballe, S.K.; O’Keefe, R.J.; Schwarz, E.M. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J. Bone Joint Surg. Am. 2002, 84, 1405–1412. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, H.; Gong, W.; Zhang, L.; Jia, T.; Wooley, P.H.; Yang, S.Y. The effect of osteoprotegerin gene modification on wear debris-induced osteolysis in a murine model of knee prosthesis failure. Biomaterials 2009, 30, 6102–6108. [Google Scholar] [CrossRef]
- Sköldenberg, O.; Rysinska, A.; Eisler, T.; Salemyr, M.; Bodén, H.; Muren, O. Denosumab for treating periprosthetic osteolysis; study protocol for a randomized, double-blind, placebo-controlled trial. BMC Musculoskelet. Disord. 2016, 17, 174. [Google Scholar] [CrossRef]
- Prock-Gibbs, H.; Pumilia, C.A.; Meckmongkol, T.; Lovejoy, J.; Mumith, A.; Coathup, M. Incidence of Osteolysis and Aseptic Loosening Following Metal-on-Highly Cross-Linked Polyethylene Hip Arthroplasty: A Systematic Review of Studies with Up to 15-Year Follow-up. J. Bone Joint Surg. Am. 2021, 103, 728–740. [Google Scholar] [CrossRef]
- Sheridan, G.A.; Clesham, K.; Garbuz, D.S.; Masri, B.A. Highly cross-linked polyethylene (HXLPE) is equivalent to conventional polyethylene (CPE) in total knee arthroplasty: A systematic review and meta-analysis. Knee 2021, 33, 318–326. [Google Scholar] [CrossRef]
- Muratoglu, O.K.; Bragdon, C.R.; O’Connor, D.O.; Jasty, M.; Harris, W.H. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J. Arthroplast. 2001, 16, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.D.; Weaver, K.; Rose, R.M.; Gunther, J.; Sauer, W.; Beals, N. Fatigue strength of polyethylene after sterilization by gamma irradiation or ethylene oxide. Clin. Orthop. Relat. Res. 1996, 333, 87–95. [Google Scholar] [CrossRef]
- Rimnac, C.; Pruitt, L. Implant Wear Symposium 2007 Engineering Work Group. How do material properties influence wear and fracture mechanisms? J. Am. Acad. Orthop. Surg. 2008, 16 (Suppl. S1), S94–S100. [Google Scholar] [CrossRef]
- Baker, D.A.; Bellare, A.; Pruitt, L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J. Biomed. Mater. Res. A 2003, 66, 146–154. [Google Scholar] [CrossRef]
- Akagi, M.; Asano, T.; Clarke, I.C.; Niiyama, N.; Kyomoto, M.; Nakamura, T.; Hamanishi, C. Wear and toughness of crosslinked polyethylene for total knee replacements: A study using a simulator and small-punch testing. J. Orthop. Res. 2006, 24, 2021–2027. [Google Scholar] [CrossRef]
- Ors-Unsal, A.; Archodoulaki, V.M. Comparison of In-Vivo Performance Characteristics of First-Generation and Second-Generation Cross-Linked and Conventional Explants. J. Arthroplast. 2020, 35, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.H.; Currier, J.H.; Mayor, M.B.; Lyford., K.A.; Van Citters, D.W.; Collier, J.P. In vivo oxidation of gamma-barrier-sterilized ultra-high-molecular-weight polyethylene bearings. J. Arthroplast. 2007, 22, 721–731. [Google Scholar] [CrossRef]
- Miura, Y.; Hasegawa, M.; Sudo, A.; Pezzotti, G.; Puppulin, L. In-vivo degradation of middle-term highly cross-linked and remelted polyethylene cups: Modification induced by creep, wear and oxidation. J. Mech. Behav. Biomed. Mater. 2015, 51, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tower, S.S.; Currier, J.H.; Currier, B.H.; Lyford, K.A.; Van Citters, D.W.; Mayor, M.B. Rim cracking of the cross-linked longevity polyethylene acetabular liner after total hip arthroplasty. J. Bone Joint Surg. Am. 2007, 89, 2212–2217. [Google Scholar] [CrossRef]
- Moore, K.D.; Beck, P.R.; Petersen, D.W.; Cuckler, J.M.; Lemons, J.E.; Eberhardt, A.W. Early failure of a cross-linked polyethylene acetabular liner. A case report. J. Bone Joint Surg. Am. 2008, 90, 2499–2504. [Google Scholar] [CrossRef]
- Ast, M.P.; John, T.K.; Labbisiere, A.; Robador, N.; Valle, A.G. Fractures of a single design of highly cross-linked polyethylene acetabular liners: An analysis of voluntary reports to the United States Food and Drug Administration. J. Arthroplast. 2014, 29, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Takatori, Y.; Ishihara, K.; Nakamura, K.; Kawaguchi, H. 2006 Frank Stinchfield Award: Grafting of biocompatible polymer for longevity of artificial hip joints. Clin. Orthop. Relat. Res. 2006, 453, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Kawaguchi, H.; Ishihara, K.; Kyomoto, M.; Karita, T.; Ito, H.; Nakamura, K.; Takatori, Y. Wear resistance of artificial hip joints with poly(2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: Comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials 2009, 30, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Yamane, S.; Kyomoto, M.; Moro, T.; Watanabe, K.; Hashimoto, M.; Takatori, Y.; Tanaka, S.; Ishihara, K. Effects of extra irradiation on surface and bulk properties of PMPC-grafted cross-linked polyethylene. J. Biomed. Mater. Res. A 2016, 104, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Takatori, Y.; Tanaka, S.; Ishihara, K.; Oda, H.; Kim, Y.T.; Umeyama, T.; Fukatani, E.; Ito, H.; Kyomoto, M.; et al. Clinical safety and wear resistance of the phospholipid polymer-grafted highly cross-linked polyethylene liner. J. Orthop. Res. 2017, 35, 2007–2016. [Google Scholar] [CrossRef]
- Moro, T.; Takatori, Y.; Kyomoto, M.; Ishihara, K.; Hashimoto, M.; Ito, H.; Tanaka, T.; Oshima, H.; Tanaka, S.; Kawaguchi, H. Long-term hip simulator testing of the artificial hip joint bearing surface grafted with biocompatible phospholipid polymer. J. Orthop. Res. 2014, 32, 369–376. [Google Scholar] [CrossRef]
- Tone, S.; Hasegawa, M.; Puppulin, L.; Pezzotti, G.; Sudo, A. Surface modifications and oxidative degradation in MPC-grafted highly cross-linked polyethylene liners retrieved from short-term total hip arthroplasty. Acta Biomater. 2018, 66, 157–165, Erratum in Acta Biomater. 2019, 83, 487–488. [Google Scholar] [CrossRef]
- Hosoi, T.; Hasegawa, M.; Tone, S.; Nakasone, S.; Kishida, N.; Marin, E.; Zhu, W.; Pezzotti, G.; Sudo, A. MPC-grafted highly cross-linked polyethylene liners retrieved from short-term total hip arthroplasty: Further evidences for the unsuitability of the MPC method. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2857–2867. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Patel, J.D. The Clinical Performance of Highly Cross-linked UHMWPE in Hip Replacements. In UHMWPE Biomaterial Handbook; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–71. [Google Scholar]
- Dumbleton, J.H.; D’Antonio, J.A.; Manley, M.T.; Capello, W.N.; Wang, A. The basis for a second-generation highly cross-linked UHMWPE. Clin. Orthop. Relat. Res. 2006, 453, 265–271. [Google Scholar] [CrossRef]
- Deckard, E.R.; Meneghini, R.M. Femoral Head Penetration Rates of Second-Generation Sequentially Annealed Highly Cross-Linked Polyethylene at Minimum Five Years. J. Arthroplast. 2019, 34, 781–788. [Google Scholar] [CrossRef]
- Chen, Y.; Hallab, N.J.; Liao, Y.S.; Narayan, V.; Schwarz, E.M.; Xie, C. Antioxidant impregnated ultra-high molecular weight polyethylene wear debris particles display increased bone remodeling and a superior osteogenic:osteolytic profile vs. conventional UHMWPE particles in a murine calvaria model. J. Orthop. Res. 2016, 34, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Neut, D.; van der Veen, H.C.; Bulstra, S.K. Effects of vitamin E incorporation in polyethylene on oxidative degradation, wear rates, immune response, and infections in total joint arthroplasty: A review of the current literature. Int. Orthop. 2019, 43, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G. Raman spectroscopy of biomedical polyethylenes. Acta Biomater. 2017, 55, 28–99. [Google Scholar] [CrossRef]
- Estok, D.M., 2nd; Bragdon, C.R.; Plank, G.R.; Huang, A.; Muratoglu, O.K.; Harris, W.H. The measurement of creep in ultrahigh molecular weight polyethylene: A comparison of conventional versus highly cross-linked polyethylene. J. Arthroplast. 2005, 20, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Hasegawa, M.; Tone, S.; Wakabayashi, H.; Sudo, A. Minimum 10-Year Follow-Up of Cementless Total Hip Arthroplasty with a 32-mm Cobalt-Chromium Head on Highly Cross-Linked Polyethylene and a Tapered, Fiber Metal Proximally Coated Femoral Stem. J. Arthroplast. 2021, 36, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, C.R.; Barrett, S.; Martell, J.M.; Greene, M.E.; Malchau, H.; Harris, W.H. Steady-state penetration rates of electron beam-irradiated, highly cross-linked polyethylene at an average 45-month follow-up. J. Arthroplast. 2006, 21, 935–943. [Google Scholar] [CrossRef]
- Geller, J.A.; Malchau, H.; Bragdon, C.; Greene, M.; Harris, W.H.; Freiberg, A.A. Large diameter femoral heads on highly cross-linked polyethylene: Minimum 3-year results. Clin. Orthop. Relat. Res. 2006, 447, 53–59. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sudo, A. In vivo wear performance of highly cross-linked polyethylene vs. yttria stabilized zirconia and alumina stabilized zirconia at a mean seven-year follow-up. BMC Musculoskelet. Disord. 2013, 14, 154. [Google Scholar] [CrossRef]
- Okita, S.; Hasegawa, M.; Takahashi, Y.; Puppulin, L.; Sudo, A.; Pezzotti, G. Failure analysis of sandwich-type ceramic-on-ceramic hip joints: A spectroscopic investigation into the role of the polyethylene shell component. J. Mech. Behav. Biomed. Mater. 2014, 31, 55–67. [Google Scholar] [CrossRef]
- Tone, S.; Hasegawa, M.; Naito, Y.; Pezzotti, G.; Sudo, A. Raman spectroscopy reveals creep and wear rate of e-beam-sterilized conventional UHMWPE tibial inserts. J. Mech. Behav. Biomed. Mater. 2020, 110, 103902. [Google Scholar] [CrossRef]
- Hood, R.W.; Wright, T.M.; Burstein, A.H. Retrieval analysis of total knee prostheses: A method and its application to 48 total condylar prostheses. J. Biomed. Mater. Res. 1983, 17, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M. The Clinical Performance of UHMWPE in Knee Replacements. In UHMWPE Biomaterial Handbook; Elsevier: Amsterdam, The Netherlands, 2016; pp. 123–144. [Google Scholar]
- Manescu Paltanea, V.; Antoniac, I.; Antoniac, A.; Paltanea, G.; Miculescu, M.; Bita, A.I.; Laptoiu, S.; Niculescu, M.; Stere, A.; Paun, C.; et al. Failure Analysis of Ultra-High Molecular Weight Polyethylene Tibial Insert in Total Knee Arthroplasty. Materials 2022, 15, 7102. [Google Scholar] [CrossRef] [PubMed]
- Łapaj, Ł.; Mróz, A.; Kokoszka, P.; Markuszewski, J.; Wendland, J.; Helak-Łapaj, C.; Kruczyński, J. Peripheral snap-fit locking mechanisms and smooth surface finish of tibial trays reduce backside wear in fixed-bearing total knee arthroplasty. Acta Orthop. 2017, 88, 62–69. [Google Scholar] [CrossRef]
- Schroder, D.T.; Kelly, N.H.; Wright, T.M.; Parks, M.L. Retrieved highly crosslinked UHMWPE acetabular liners have similar wear damage as conventional UHMWPE. Clin. Orthop. Relat. Res. 2011, 469, 387–394. [Google Scholar] [CrossRef]
- Currier, B.H.; Currier, J.H.; Franklin, K.J.; Mayor, M.B.; Reinitz, S.D.; Van Citters, D.W. Comparison of Wear and Oxidation in Retrieved Conventional and Highly Cross-Linked UHMWPE Tibial Inserts. J. Arthroplast. 2015, 30, 2349–2353. [Google Scholar] [CrossRef]
- Mathis, D.T.; Schmidli, J.; Hirschmann, M.T.; Amsler, F.; Henckel, J.; Hothi, H.; Hart, A. Comparative retrieval analysis of antioxidant polyethylene: Bonding of vitamin-E does not reduce in-vivo surface damage. BMC Musculoskelet. Disord. 2021, 22, 1003. [Google Scholar] [CrossRef]
- Cerquiglini, A.; Henckel, J.; Hothi, H.; Moser, L.B.; Eskelinen, A.; Hirschmann, M.T.; Hart, A.J. Retrieval analysis of contemporary antioxidant polyethylene: Multiple material and design changes may decrease implant performance. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- de Steiger, R.; Lorimer, M.; Graves, S.E. Cross-Linked Polyethylene for Total Hip Arthroplasty Markedly Reduces Revision Surgery at 16 Years. J. Bone Joint Surg. Am. 2018, 100, 1281–1288. [Google Scholar] [CrossRef]
- Davis, E.T.; Pagkalos, J.; Kopjar, B. Polyethylene manufacturing characteristics have a major effect on the risk of revision surgery in cementless and hybrid total hip arthroplasties. Bone Joint J. 2020, 102-B, 90–101. [Google Scholar] [CrossRef]
- Kjærgaard, K.; Varnum, C.; Ding, M.; Overgaard, S. Revision Risk of Total Hip Arthroplasty With Vitamin E Doped Liners: Results From the Danish Hip Arthroplasty Register. J. Arthroplast. 2022, 37, 1136–1142. [Google Scholar] [CrossRef]
- Hemmilä, M.; Laaksonen, I.; Matilainen, M.; Eskelinen, A.; Haapakoski, J.; Puhto, A.P.; Kettunen, J.; Pamilo, K.; Mäkelä, K.T. Implant survival of 2723 vitamin E-infused highly crosslinked polyethylene liners in total hip arthroplasty: Data from the Finnish Arthroplasty Register. Acta Orthop. 2021, 92, 316–322. [Google Scholar] [CrossRef] [PubMed]
- de Steiger, R.N.; Muratoglu, O.; Lorimer, M.; Cuthbert, A.R.; Graves, S.E. Lower prosthesis-specific 10-year revision rate with crosslinked than with non-crosslinked polyethylene in primary total knee arthroplasty. Acta Orthop. 2015, 86, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Partridge, T.C.J.; Baker, P.N.; Jameson, S.S.; Mason, J.; Reed, M.R.; Deehan, D.J. Conventional Versus Highly Cross-Linked Polyethylene in Primary Total Knee Replacement: A Comparison of Revision Rates Using Data from the National Joint Registry for England, Wales, and Northern Ireland. J. Bone Joint Surg. Am. 2020, 102, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kendall, J.; Pelt, C.E.; Imlay, B.; Yep, P.; Mullen, K.; Kagan, R. No Reduction in Revision Risk Associated With Highly Cross-linked Polyethylene With or Without Antioxidants Over Conventional Polyetheylene in TKA: An Analysis From the American Joint Replacement Registry. Clin. Orthop. Relat. Res. 2022, 480, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Sax, O.C.; Douglas, S.J.; Chen, Z.; Mont, M.A.; Nace, J.; Delanois, R.E. Low Wear at 10-Year Follow-Up of a Second-Generation Highly Cross-Linked Polyethylene in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, S592–S597. [Google Scholar] [CrossRef]
- Fransen, B.L.; Bengoa, F.J.; Neufeld, M.E.; Sheridan, G.A.; Garbuz, D.S.; Howard, L.C. Thin highly cross-linked polyethylene liners combined with large femoral heads in primary total hip arthroplasty show excellent survival and low wear rates at a mean follow-up of 12.8 years. Bone Joint J. 2023, 105-B, 29–34. [Google Scholar] [CrossRef]
- Kurtz, S.M.; MacDonald, D.W.; Mont, M.A.; Parvizi, J.; Malkani, A.L.; Hozack, W. Retrieval analysis of sequentially annealed highly crosslinked polyethylene used in total hip arthroplasty. Clin. Orthop. Relat. Res. 2015, 473, 962–971. [Google Scholar] [CrossRef]
- MacDonald, D.W.; Higgs, G.B.; Chen, A.F.; Malkani, A.L.; Mont, M.A.; Kurtz, S.M. Oxidation, Damage Mechanisms, and Reasons for Revision of Sequentially Annealed Highly Crosslinked Polyethylene in Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 1235–1241. [Google Scholar] [CrossRef]
- Shareghi, B.; Johanson, P.E.; Kärrholm, J. Wear of Vitamin E-Infused Highly Cross-Linked Polyethylene at Five Years. J. Bone Joint Surg. Am. 2017, 99, 1447–1452. [Google Scholar] [CrossRef]
- Nebergall, A.K.; Greene, M.E.; Laursen, M.B.; Nielsen, P.T.; Malchau, H.; Troelsen, A. Vitamin E diffused highly cross-linked polyethylene in total hip arthroplasty at five years: A randomised controlled trial using radiostereometric analysis. Bone Joint J. 2017, 99-B, 577–584. [Google Scholar] [CrossRef]
- Galea, V.P.; Connelly, J.W.; Shareghi, B.; Kärrholm, J.; Sköldenberg, O.; Salemyr, M.; Laursen, M.B.; Muratoglu, O.; Bragdon, C.; Malchau, H. Evaluation of in vivo wear of vitamin E-diffused highly crosslinked polyethylene at five years: A multicentre radiostereometric analysis study. Bone Joint J. 2018, 100-B, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Galea, V.P.; Rojanasopondist, P.; Laursen, M.; Muratoglu, O.K.; Malchau, H.; Bragdon, C. Evaluation of vitamin E-diffused highly crosslinked polyethylene wear and porous titanium-coated shell stability: A seven-year randomized control trial using radiostereometric analysis. Bone Joint J. 2019, 101-B, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Thoen, P.S.; Nordsletten, L.; Pripp, A.H.; Röhrl, S.M. Results of a randomized controlled trial with five-year radiostereometric analysis results of vitamin E-infused highly crosslinked versus moderately crosslinked polyethylene in reverse total hip arthroplasty. Bone Joint J. 2020, 102-B, 1646–1653. [Google Scholar] [CrossRef]
- Kjærgaard, K.; Ding, M.; Jensen, C.; Bragdon, C.; Malchau, H.; Andreasen, C.M.; Ovesen, O.; Hofbauer, C.; Overgaard, S. Vitamin E-doped total hip arthroplasty liners show similar head penetration to highly cross-linked polyethylene at five years: A multi-arm randomized controlled trial. Bone Joint J. 2020, 102-B, 1303–1310. [Google Scholar] [CrossRef]
- Baghdadi, J.; Alkhateeb, S.; Roth, A.; VITAS-Group; Jäger, M. Cup positioning and its effect on polyethylene wear of vitamin E- and non-vitamin E-supplemented liners in total hip arthroplasty: Radiographic outcome at 5-year follow-up. Arch. Orthop. Trauma. Surg. 2022; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Massier, J.R.A.; Van Erp, J.H.J.; Snijders, T.E.; Gast, A. A vitamin E blended highly cross-linked polyethylene acetabular cup results in less wear: 6-year results of a randomized controlled trial in 199 patients. Acta Orthop. 2020, 91, 705–710. [Google Scholar] [CrossRef]
- Spece, H.; Schachtner, J.T.; MacDonald, D.W.; Klein, G.R.; Mont, M.A.; Lee, G.C.; Kurtz, S.M. Reasons for Revision, Oxidation, and Damage Mechanisms of Retrieved Vitamin E-Stabilized Highly Crosslinked Polyethylene in Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 3088–3093. [Google Scholar] [CrossRef]
- Barrack, R.L. Retrieval Analysis of an Early Fracture of a Vitamin E-Stabilized Tibial Liner in Total Knee Arthroplasty: A Case Report. JBJS Case Connect. 2013, 3 (Suppl. S2), e44. [Google Scholar] [CrossRef]
- Bates, M.D.; Mauerhan, D.R. Early Fracture of a Vitamin-E-Infused, Highly Cross-Linked Polyethylene Liner After Total Hip Arthroplasty: A Case Report. JBJS Case Connect. 2015, 5, e65. [Google Scholar] [CrossRef]
- Kim, K.B.; Lee, S.M.; Moon, N.H.; Do, M.U.; Shin, W.C. Early unexpected failure of a vitamin E-infused highly cross-linked polyethylene liner: A case report. Medicine 2021, 100, e27454. [Google Scholar] [CrossRef]
- Mertz, K.C.; Yang, J.; Chung, B.C.; Chen, X.; Mayfield, C.K.; Heckmann, N.D. Ceramic Femoral Heads Exhibit Lower Wear Rates Compared to Cobalt Chrome: A Meta-Analysis. J. Arthroplast. 2023, 38, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Bergvinsson, H.; Sundberg, M.; Flivik, G. Polyethylene Wear With Ceramic and Metal Femoral Heads at 5 Years: A Randomized Controlled Trial With Radiostereometric Analysis. J. Arthroplast. 2020, 35, 3769–3776. [Google Scholar] [CrossRef] [PubMed]
- Gosling, O.B.; Ferreri, T.G.; Khoshbin, A.; Whitehouse, M.R.; Atrey, A. A systematic review and meta-analysis of survivorship and wear rates of metal and ceramic heads articulating with polyethylene liners in total hip arthroplasty. Hip Int. 2020, 30, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Malahias, M.A.; Atrey, A.; Gu, A.; Chytas, D.; Nikolaou, V.S.; Waddell, J.P. Is Oxidized Zirconium Femoral Head Superior to Other Bearing Types in Total Hip Arthroplasty? A Systematic Review and Meta-Analysis. J. Arthroplast. 2019, 34, 1844–1852. [Google Scholar] [CrossRef]
- Salipas, A.; Poole, A.S.; Teeter, M.G.; Somerville, L.E.; Naudie, D.D.; McCalden, R.W. A Ten-Year Radiostereometric Analysis of Polyethylene Wear Between Oxidized Zirconium and Cobalt Chrome Articulations in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, S692–S696. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Minoda, Y.; Nakagawa, S.; Kadoya, Y.; Takemura, S.; Kobayashi, A.; Mizokawa, S.; Ohta, Y.; Takahashi, S.; Yamamura, K.; et al. Clinical results of alumina medial pivot total knee arthroplasty at a minimum follow-up of 10 years. Knee 2017, 24, 434–438. [Google Scholar] [CrossRef]
- Bergschmidt, P.; Ellenrieder, M.; Bader, R.; Kluess, D.; Finze, S.; Schwemmer, B.; Mittelmeier, W. Prospective comparative clinical study of ceramic and metallic femoral components for total knee arthroplasty over a five-year follow-up period. Knee 2016, 23, 871–876. [Google Scholar] [CrossRef]
- Xiang, S.; Zhao, Y.; Li, Z.; Feng, B.; Weng, X. Clinical outcomes of ceramic femoral prosthesis in total knee arthroplasty: A systematic review. J. Orthop. Surg. Res. 2019, 14, 57. [Google Scholar] [CrossRef]
- Vertullo, C.J.; Lewis, P.L.; Graves, S.; Kelly, L.; Lorimer, M.; Myers, P. Twelve-Year Outcomes of an Oxinium Total Knee Replacement Compared with the Same Cobalt-Chromium Design: An Analysis of 17,577 Prostheses from the Australian Orthopaedic Association National Joint Replacement Registry. J. Bone Joint Surg. Am. 2017, 99, 275–283. [Google Scholar] [CrossRef]
- Banche, G.; Bracco, P.; Allizond, V.; Bistolfi, A.; Boffano, M.; Cimino, A.; Brach del Prever, E.M.; Cuffini, A.M. Do crosslinking and vitamin E stabilization influence microbial adhesions on UHMWPE-based biomaterials? Clin. Orthop. Relat. Res. 2015, 473, 974–986. [Google Scholar] [CrossRef]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Faccenda, C.; Viotto, M.; Sabatini, L.; Berchialla, P.; Sciannameo, V.; Graziano, E.; Massè, A. Comparable results between crosslinked polyethylene and conventional ultra-high molecular weight polyethylene implanted in total knee arthroplasty: Systematic review and meta-analysis of randomised clinical trials. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3120–3130. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Bracco, P.; Bistolfi, A.; Allizond, V.; Boffano, M.; Costa, L.; Cimino, A.; Cuffini, A.M.; Del Prever, E.M. Vitamin E blended UHMWPE may have the potential to reduce bacterial adhesive ability. J. Orthop. Res. 2011, 29, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Allizond, V.; Bracco, P.; Bistolfi, A.; Boffano, M.; Cimino, A.; Brach del Prever, E.M.; Cuffini, A.M. Interplay between surface properties of standard, vitamin E blended and oxidised ultra high molecular weight polyethylene used in total joint replacement and adhesion of Staphylococcus aureus and Escherichia coli. Bone Joint J. 2014, 96-B, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Chikkali, S.H.; Sivaram, S. Ultrahigh molecular weight polyethylene: Catalysis, structure, properties, processing and applications. Prog. Polym. Sci. 2020, 109, 101290. [Google Scholar] [CrossRef]
- Suñer, S.; Gowland, N.; Craven, R.; Joffe, R.; Emami, N.; Tipper, J.L. Ultrahigh molecular weight polyethylene/graphene oxide nanocomposites: Wear characterization and biological response to wear particles. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 183–190. [Google Scholar] [CrossRef]
- Sahu, S.K.; Badgayan, N.D.; Sreekanth, P.S.R. Understanding the influence of contact pressure on the wear performance of HDPE/multi-dimensional carbon filler based hybrid polymer nanocomposites. Wear 2019, 438–439, 102824. [Google Scholar] [CrossRef]
- Sreekanth, P.S.R.; Kanagaraj, S. Influence of multi walled carbon nanotubes reinforcement and gamma irradiation on the wear behaviour of UHMWPE. Wear 2015, 334–335, 82–90. [Google Scholar] [CrossRef]
- Dalai, N.; Sreekanth, P.S.R. UHMWPE/nanodiamond nanocomposites for orthopaedic applications: A novel sandwich configuration based approach. J. Mech. Behav. Biomed. Mater. 2021, 116, 104327. [Google Scholar] [CrossRef]
- Danilova, S.N.; Yarusova, S.B.; Kulchin, Y.N.; Zhevtun, I.G.; Buravlev, I.Y.; Okhlopkova, A.A.; Gordienko, P.S.; Subbotin, E.P. UHMWPE/CaSiO3 Nanocomposite: Mechanical and Tribological Properties. Polymers 2021, 13, 570. [Google Scholar] [CrossRef]
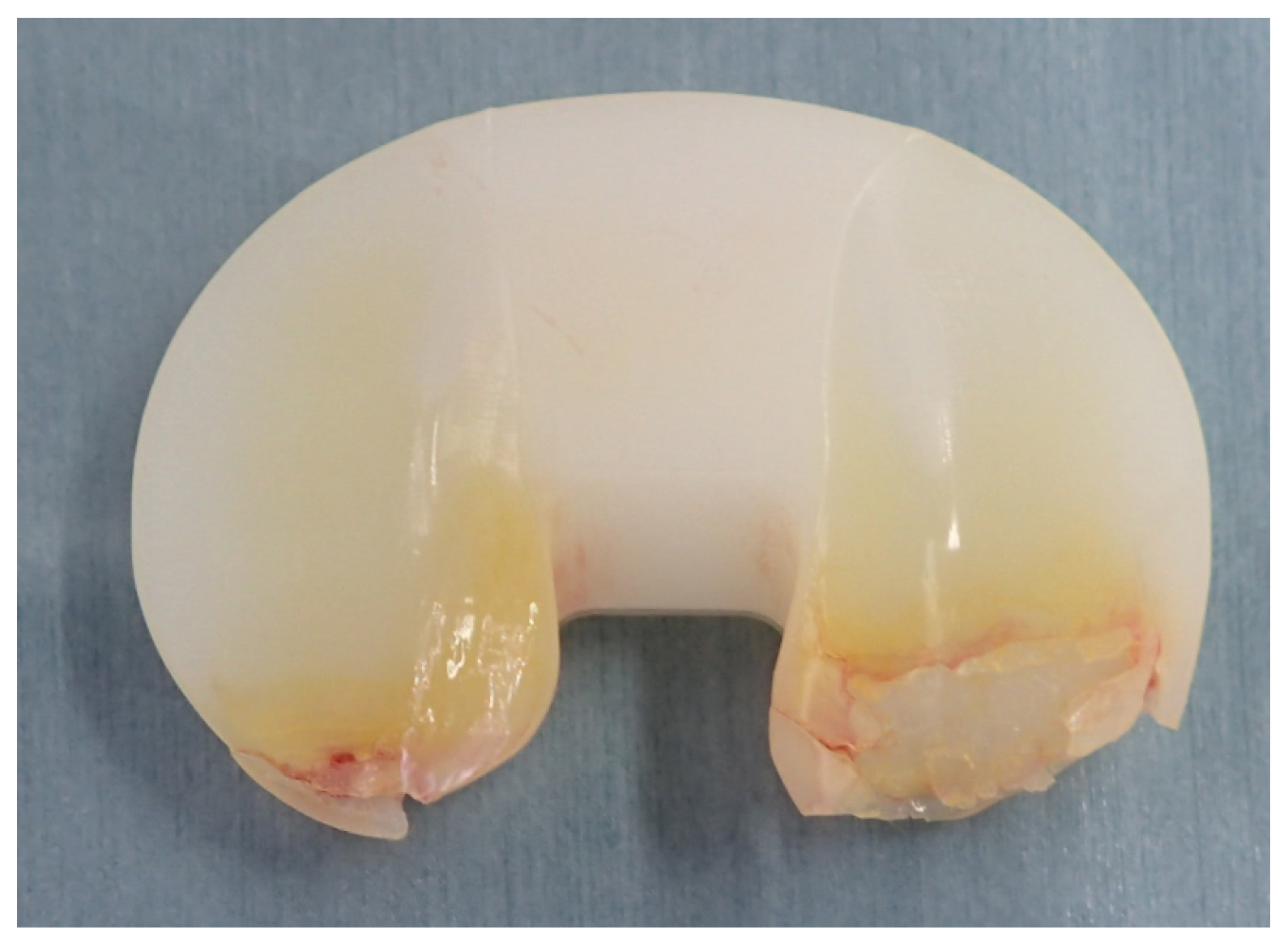
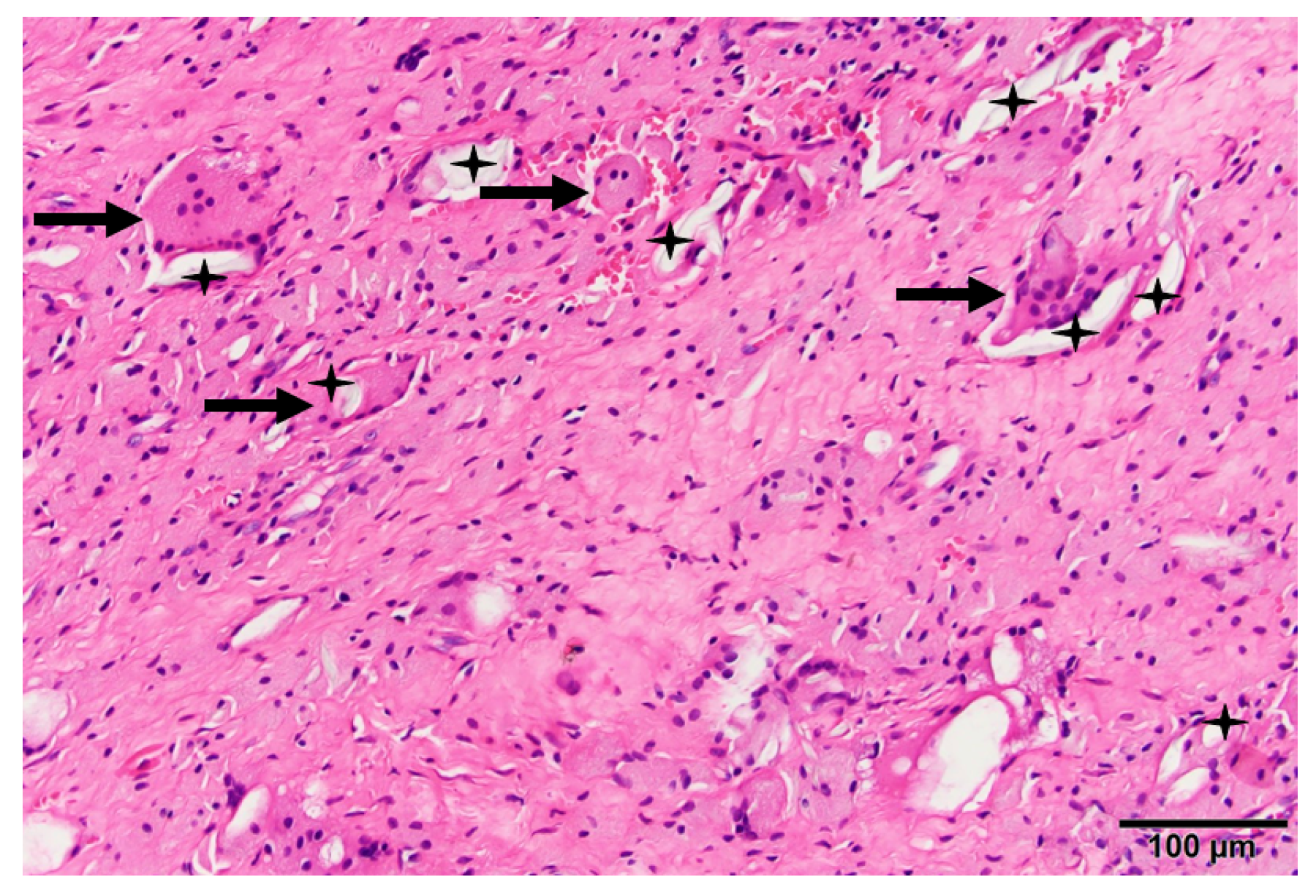
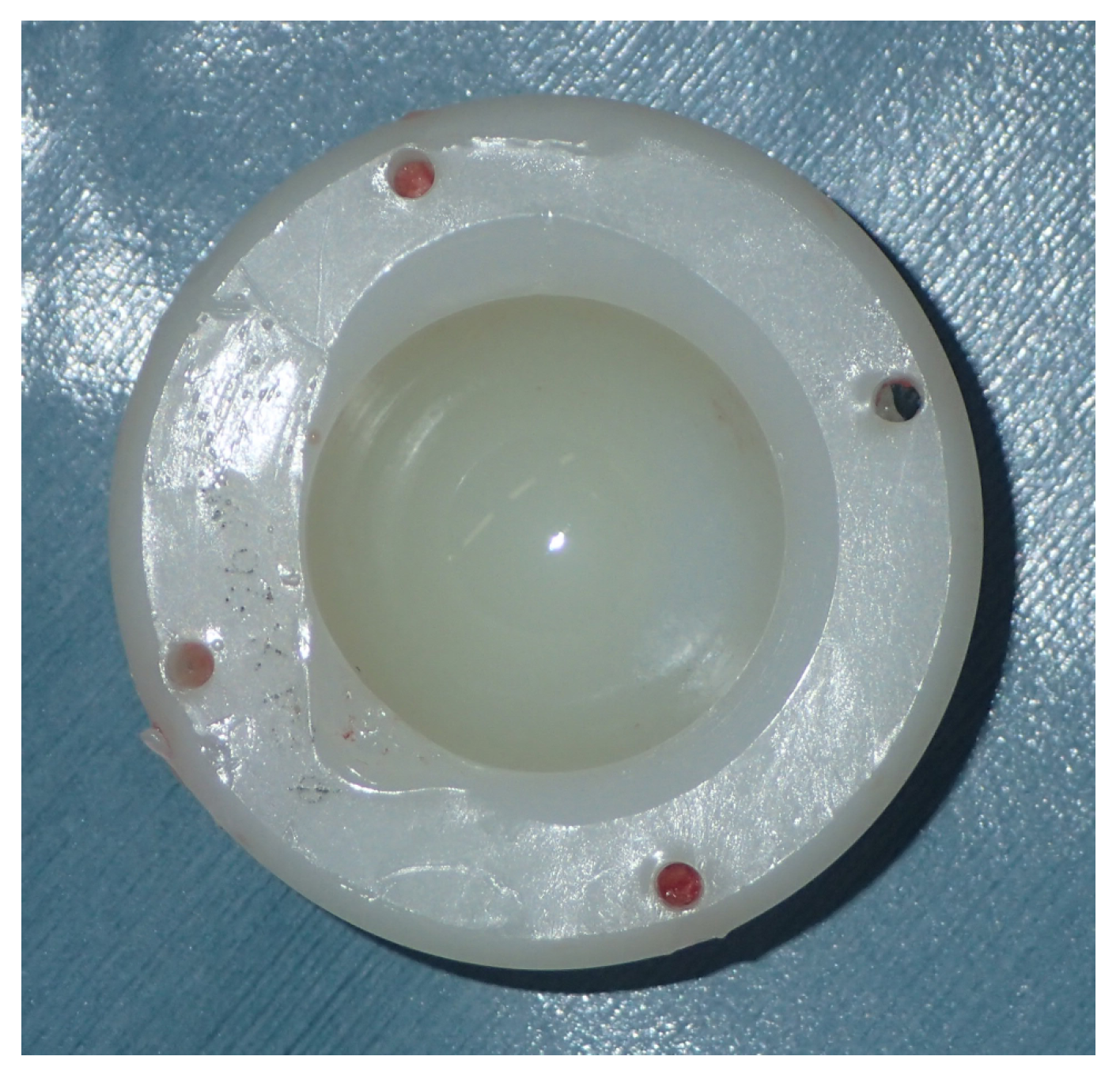


| Type | Brand (Manufacturer) | Irradiation, Dose (kGy) | Sterilization | Total Irradiation Dose (kGy) |
|---|---|---|---|---|
| Annealed | Crossfire (Stryker) | Gamma, 75 | Gamma in nitrogen, 30 kGy | 105 |
| Annealed | ArCom XL (Zimmer Biomet) | Gamma, 50 | Gas plasma | 50 |
| Annealed | Aeonian (Kyocera) | Gamma, 35 | Gamma in nitrogen, 25 kGy | 60 |
| Annealed | Excellink (Kyocera) | Gamma, 50 | Gamma in nitrogen, 25 kGy | 75 |
| Annealed | Aquala (Kyocera) | Gamma, 50 | Gamma in nitrogen, 25 kGy | 75 |
| Remelted | Longevity (Zimmer Biomet) | Electron beam, 100 | Gas plasma | 100 |
| Remelted | Prolong (Zimmer Biomet) | Electron beam, 65 | Gas plasma or ethylene oxide | 65 |
| Remelted | Durasul (Zimmer Biomet) | Electron beam, 95 | Ethylene oxide | 95 |
| Remelted | Marathon (DePuy Synthes) | Gamma, 50 | Gas plasma | 50 |
| Remelted | XLPE (Smith & Nephew) | Gamma, 100 | Ethylene oxide | 100 |
| Radiation Dose (kGy) | Crystallinity (%) | Elastic Modulus (Mpa) | Yield Strength (Mpa) | True Stress at Break (Mpa) | ΔKincep (MPa√m) |
|---|---|---|---|---|---|
| 0 | 50.1 | 495 | 20.2 | 315.5 | 1.41 |
| 50 | 45.6 | 412 | 19.9 | 237.6 | 0.91 |
| 100 | 46.3 | 386 | 18.9 | 185.7 | 0.69 |
| Type | Brand (Manufacturer) | Irradiation, Dose (kGy) | Sterilization | Total Irradiation Dose (kGy) |
|---|---|---|---|---|
| Sequentially annealed | X3 (Stryker) | Gamma, 30 in 3 steps | Gas plasma | 90 |
| Vitamin E-diffused | E1 (Zimmer Biomet) | Gamma, 100 | Gamma, 30 kGy | 130 |
| Vitamin E-blended | Vivacit-E (Zimmer Biomet) | Electron beam, not available | Ethylene oxide | Not available |
| Vitamin E-blended | Vitelene (Aesculap) | Electron beam, 80 | Ethylene oxide | 80 |
| Vitamin E-blended | Vitamys (Mathys) | Gamma, 100 | Gas plasma | 100 |
| Vitamin E-blended | ECiMa (Corin) | Gamma, 120 | Ethylene oxide | 120 |
| Vitamin E-blended | Blend-E XL (Nakashima) | Electron beam, 300 | Ethylene oxide | 300 |
| Vitamin E-blended | Aquala VE (Kyocera) | Gamma, 100 | Gamma, 25 kGy | 125 |
| COVERNOX antioxidant-blended | AOX (DePuy Synthes) | Gamma, 85 | Gamma, 30 kGy | 115 |
| Study | Shareghi et al. [83] | Nebergall et al. [84] | Thoen et al. [87] | |
| Follow-up | (years) | 5 | 5 | 5 |
| Design | RCT | RCT | RCT | |
| Material | Vitamin E | E1 | E1 | E1 |
| Non-vitamin E | ArComXL | ArComXL | Marathon | |
| Wear (mm) | Vitamin E | 0.13 | −0.05 | 0.17 |
| Non-vitamin E | 0.2 | 0.07 | 0.2 | |
| Wear rate (mm/year) * | Vitamin E | 0.02 | ||
| Non-vitamin E | 0.04 | |||
| Results | Significantly lower wear of vitamin E liner | Significantly lower wear of vitamin E liner | Significantly lower wear of vitamin E liner | |
| Study | Kjærgaard et al. [88] | Galea et al. [85] | Galea et al. [86] | |
| Follow-up | (years) | 5 | 5 | 7 |
| Design | RCT | Prospective study | RCT | |
| Material | Vitamin E | E1 | E1 | E1 |
| Non-vitamin E | ArComXL | ArComXL | ArComXL | |
| Wear (mm) | Vitamin E | 0.006 | 0.06 | −0.07 |
| Non-vitamin E | 0.09 | 0.13 | 0 | |
| Wear rate (mm/year) * | Vitamin E | −0.006 | ||
| Non-vitamin E | 0.005 | |||
| Results | Not significant | Significantly lower wear of vitamin E liner | Not significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, M.; Tone, S.; Naito, Y.; Sudo, A. Ultra-High-Molecular-Weight Polyethylene in Hip and Knee Arthroplasties. Materials 2023, 16, 2140. https://doi.org/10.3390/ma16062140
Hasegawa M, Tone S, Naito Y, Sudo A. Ultra-High-Molecular-Weight Polyethylene in Hip and Knee Arthroplasties. Materials. 2023; 16(6):2140. https://doi.org/10.3390/ma16062140
Chicago/Turabian StyleHasegawa, Masahiro, Shine Tone, Yohei Naito, and Akihiro Sudo. 2023. "Ultra-High-Molecular-Weight Polyethylene in Hip and Knee Arthroplasties" Materials 16, no. 6: 2140. https://doi.org/10.3390/ma16062140
APA StyleHasegawa, M., Tone, S., Naito, Y., & Sudo, A. (2023). Ultra-High-Molecular-Weight Polyethylene in Hip and Knee Arthroplasties. Materials, 16(6), 2140. https://doi.org/10.3390/ma16062140






