Enhancing the Performance of Quantum Dot Light-Emitting Diodes Using Solution-Processable Highly Conductive Spinel Structure CuCo2O4 Hole Injection Layer
Abstract
1. Introduction
2. Experimental Section
2.1. CuCo2O4 Solution Synthesis
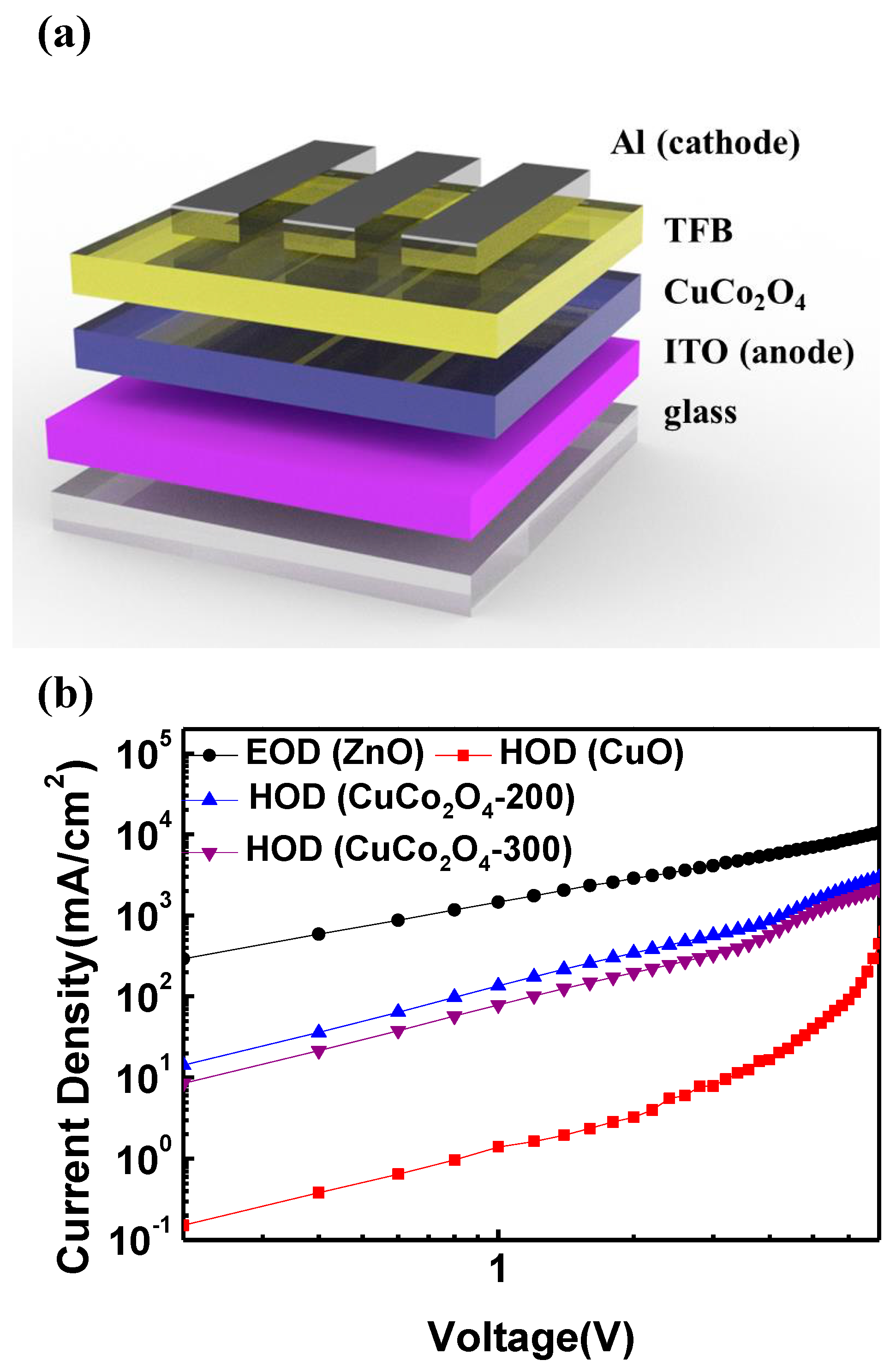
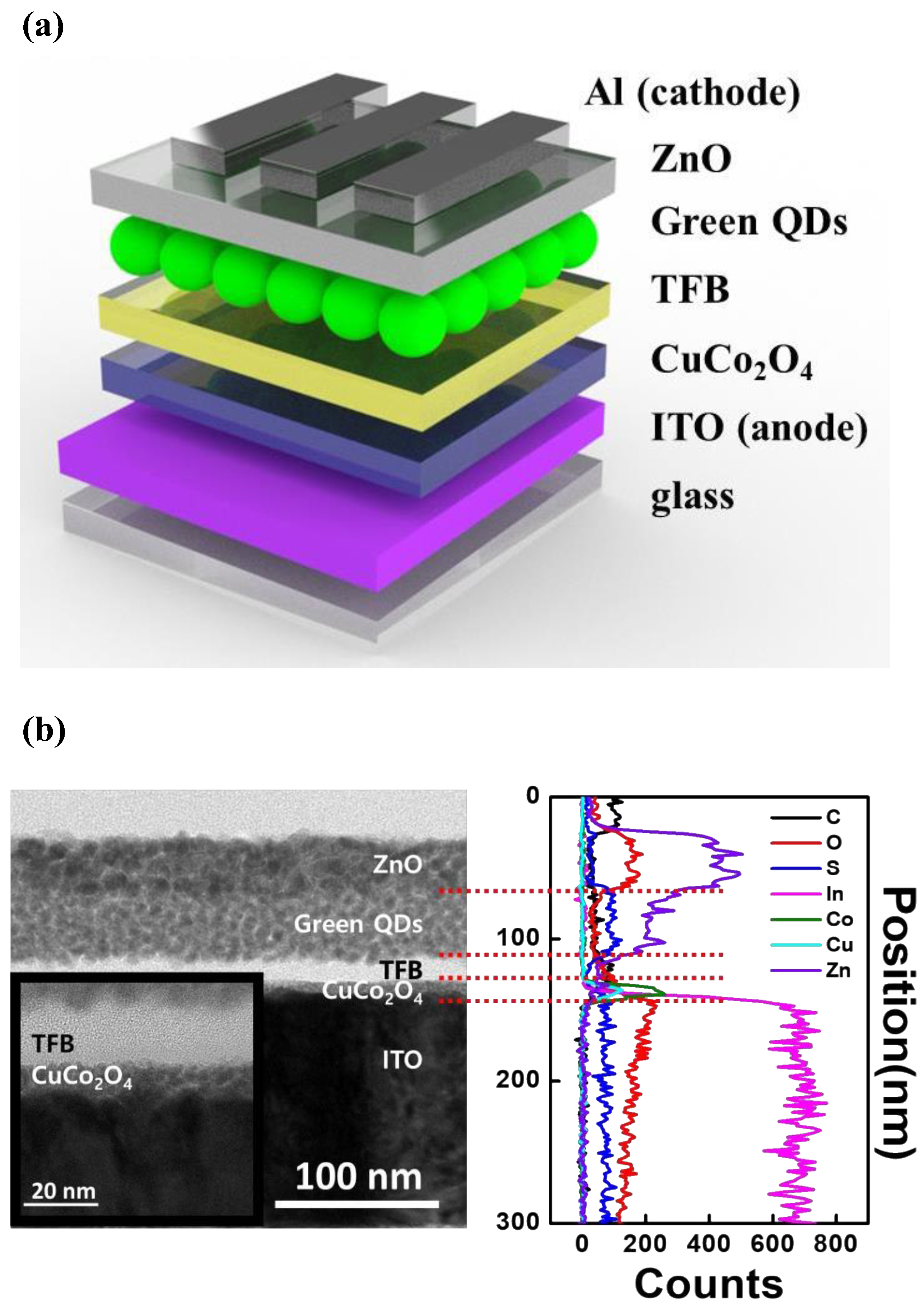
2.2. Device Fabrication
2.3. Characterization
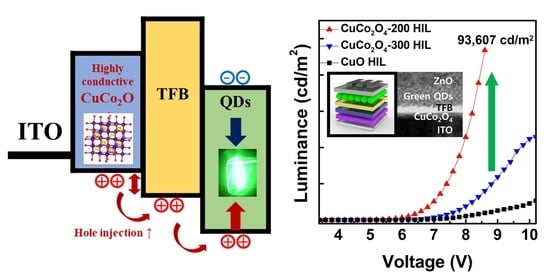
3. Results and Discussion
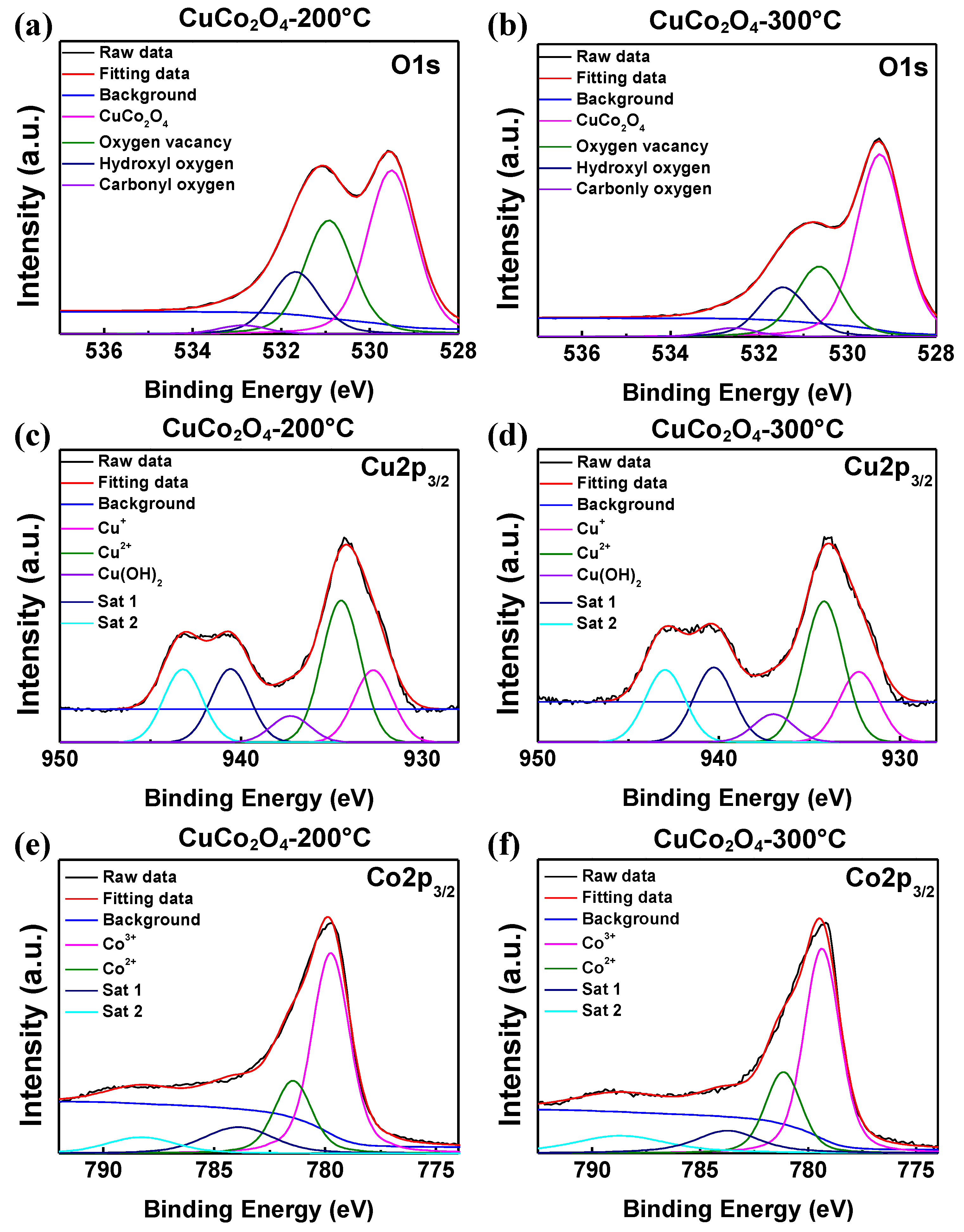
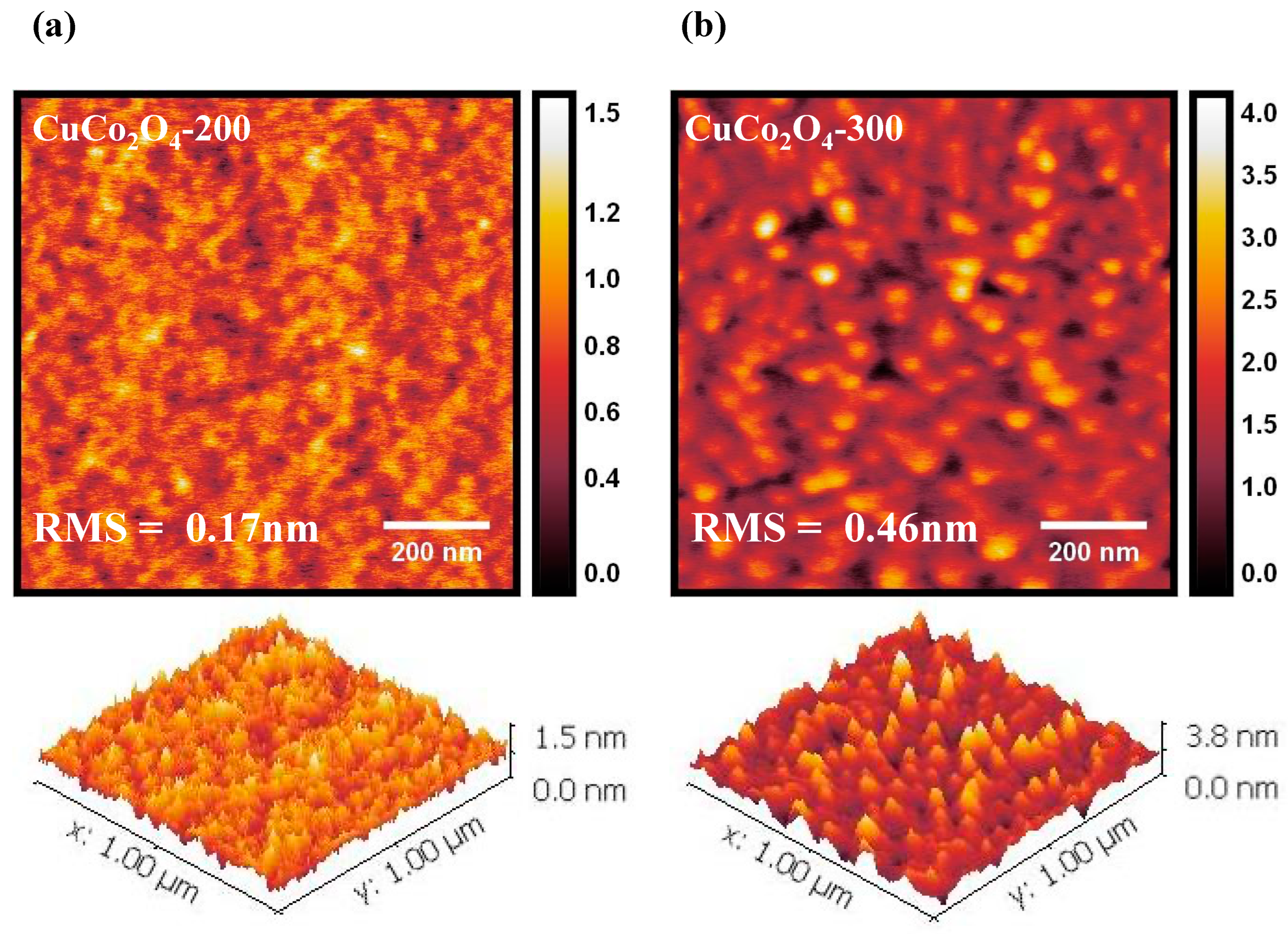
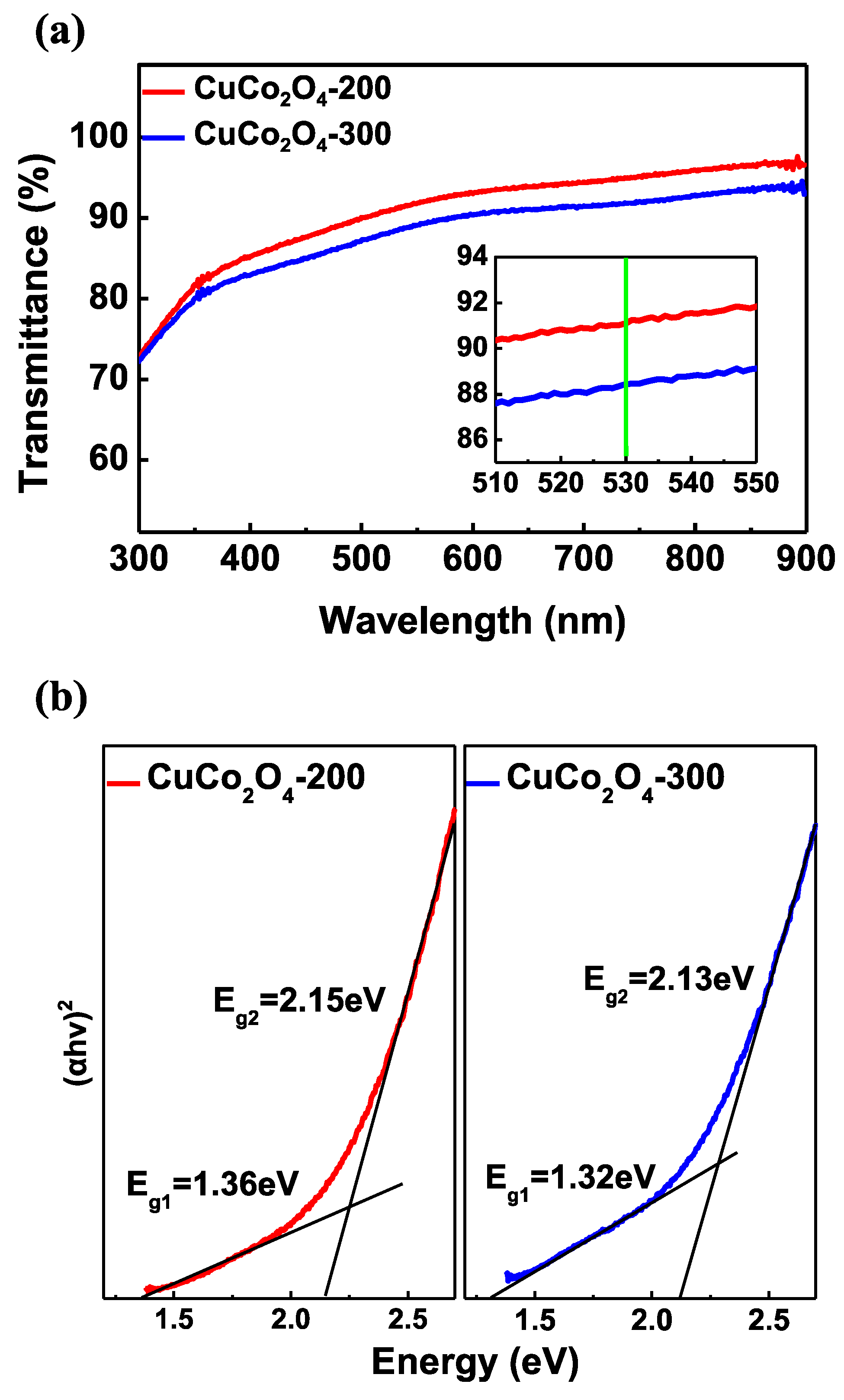
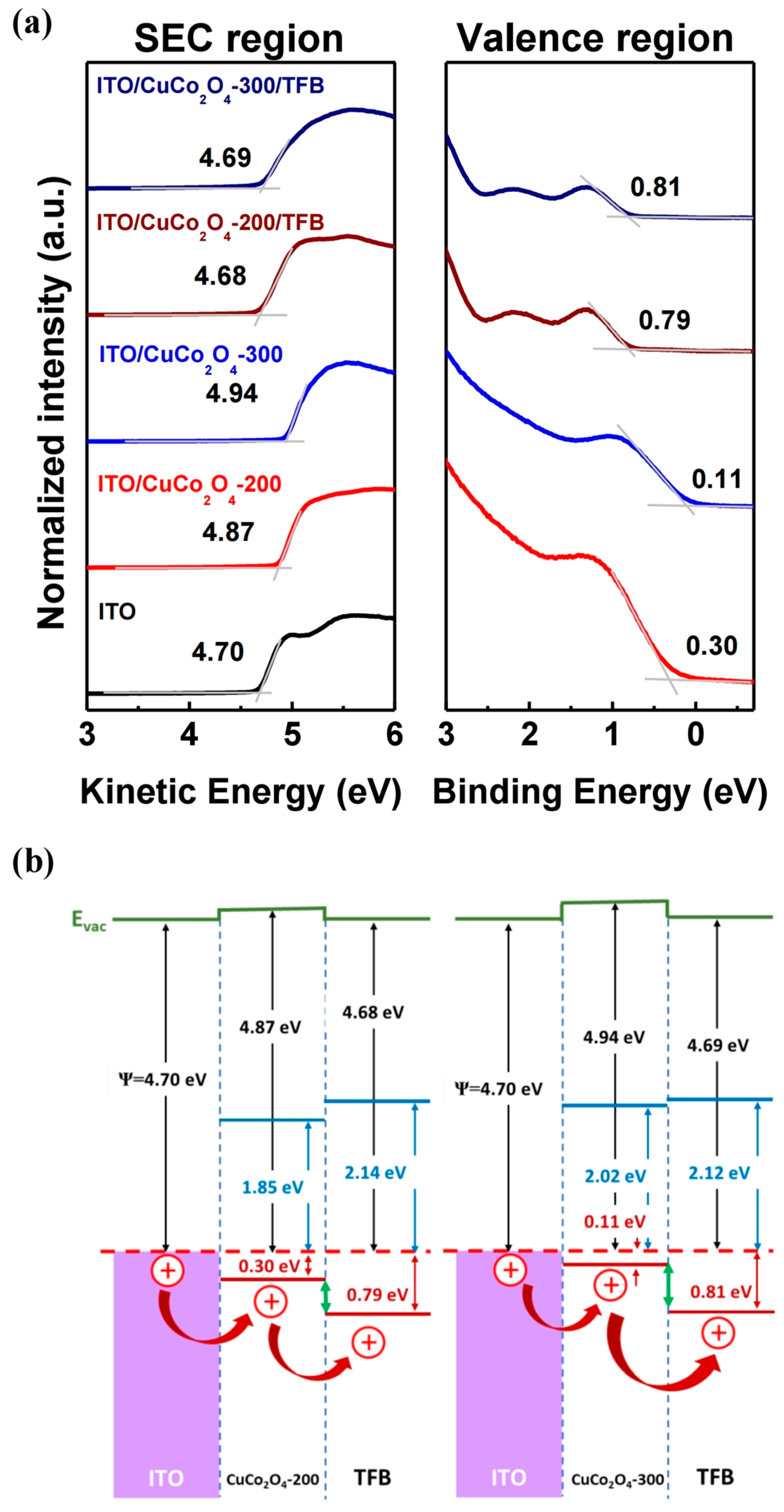
3.1. Characterization of CuCo2O4 Thin Films
3.2. Hole Injection Ability of CuCo2O4 HIL
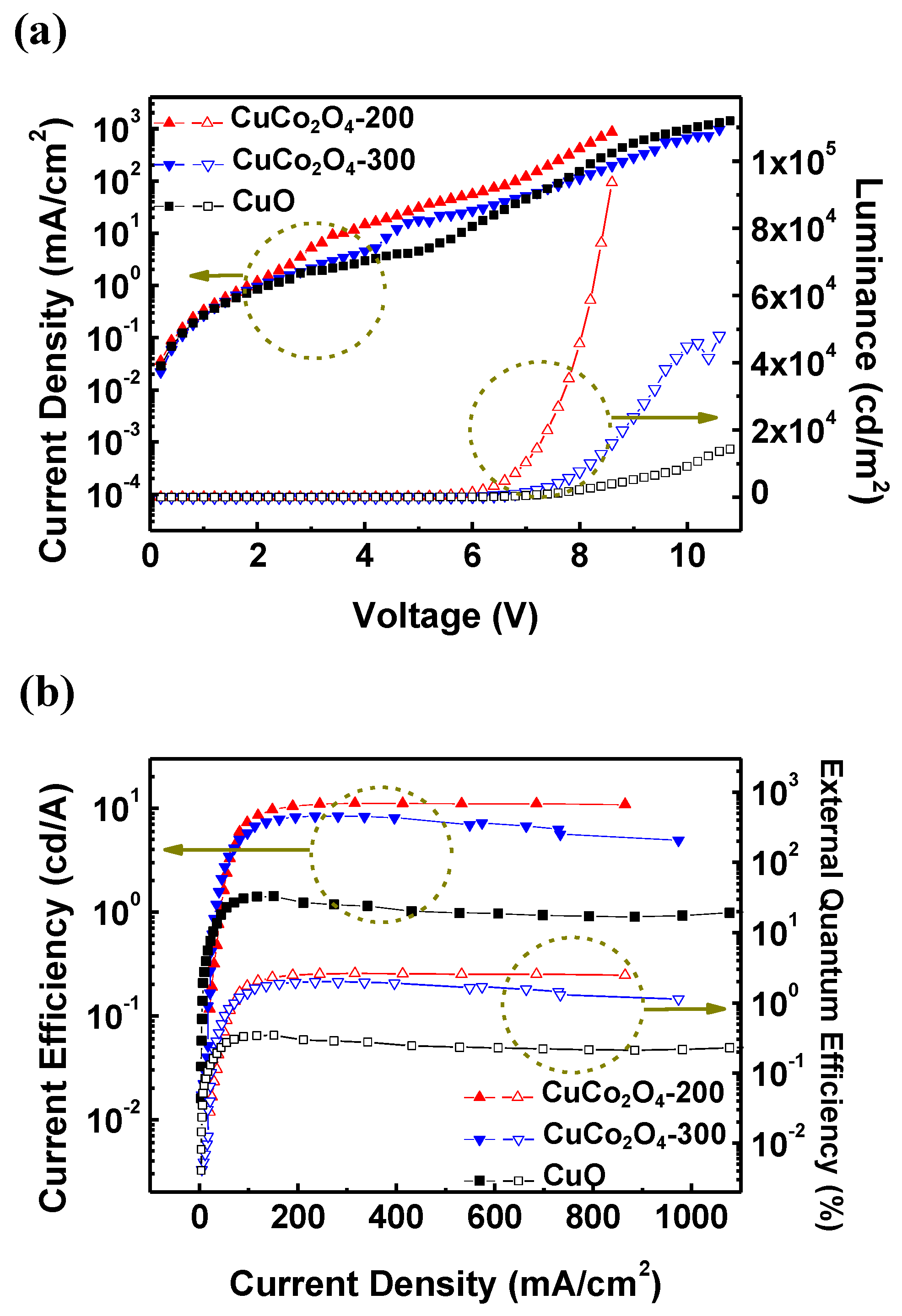
3.3. CuCo2O4 HIL-Based QLED Structure and Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Manna, L.; Yang, W.; Wickham, J.; Scher, E.; Kadavanich, A.; Alivisatos, A.P. Shape control of CdSe nanocrystals. Nature 2000, 404, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe) ZnS core− shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Pietryga, J.M.; Park, Y.-S.; Lim, J.; Fidler, A.F.; Bae, W.K.; Brovelli, S.; Klimov, V.I. Spectroscopic and device aspects of nanocrystal quantum dots. Chem. Rev. 2016, 116, 10513–10622. [Google Scholar] [CrossRef] [PubMed]
- Osypiw, A.R.; Lee, S.; Jung, S.-M.; Leoni, S.; Smowton, P.M.; Hou, B.; Kim, J.M.; Amaratunga, G.A. Solution-processed colloidal quantum dots for light emission. Mater. Adv. 2022, 3, 6773–6790. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, Y.; Sun, X.W.; Zhang, S.; Chen, S. Beyond OLED: Efficient quantum dot light-emitting diodes for display and lighting application. Chem. Rec. 2019, 19, 1729–1752. [Google Scholar] [CrossRef]
- Yu, H.-C.; Zhuo, Q.-H.; Shi, J.-T.; Chu, K.-H. Investigation of quantum dots light emitting diodes with different transition metal oxide as charge injection layers. Org. Electron. 2022, 110, 106646. [Google Scholar] [CrossRef]
- Ding, T.; Yang, X.; Bai, L.; Zhao, Y.; Fong, K.E.; Wang, N.; Demir, H.V.; Sun, X.W. Colloidal quantum-dot LEDs with a solution-processed copper oxide (CuO) hole injection layer. Org. Electron. 2015, 26, 245–250. [Google Scholar] [CrossRef]
- Cao, F.; Wang, H.; Shen, P.; Li, X.; Zheng, Y.; Shang, Y.; Zhang, J.; Ning, Z.; Yang, X. High-efficiency and stable quantum dot light-emitting diodes enabled by a solution-processed metal-doped nickel oxide hole injection interfacial layer. Adv. Funct. Mater. 2017, 27, 1704278. [Google Scholar] [CrossRef]
- Pan, J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size tunable ZnO nanoparticles to enhance electron injection in solution processed QLEDs. ACS Photonics 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Kim, J.-H.; Han, C.-Y.; Lee, K.-H.; An, K.-S.; Song, W.; Kim, J.; Oh, M.S.; Do, Y.R.; Yang, H. Performance improvement of quantum dot-light-emitting diodes enabled by an alloyed ZnMgO nanoparticle electron transport layer. Chem. Mater. 2015, 27, 197–204. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef]
- Wang, Z.; Nayak, P.K.; Caraveo-Frescas, J.A.; Alshareef, H.N. Recent developments in p-Type oxide semiconductor materials and devices. Adv. Mater. 2016, 28, 3831–3892. [Google Scholar] [CrossRef] [PubMed]
- Hautier, G.; Miglio, A.; Ceder, G.; Rignanese, G.-M.; Gonze, X. Identification and design principles of low hole effective mass p-type transparent conducting oxides. Nat. Commun. 2013, 4, 2292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, J.; Mahmoudi, B.; Forster, S.; Syrowatka, F.; Maijenburg, A.W.; Scheer, R. Synthesis and characterization of spinel cobaltite (Co3O4) thin films for function as hole transport materials in organometallic halide perovskite solar cells. ACS Appl. Energy Mater. 2020, 3, 3755–3769. [Google Scholar] [CrossRef]
- Kim, M.G.; Shin, J.S.; Ma, J.H.; Jeong, J.H.; Han, D.H.; Kim, B.-S.; Jeon, W.; Park, Y.; Kang, S.J. An Al-doped TiO2 interfacial layer for effective hole injection characteristics of quantum-dot light-emitting diodes. J. Mater. Chem. C 2022, 10, 7294–7303. [Google Scholar] [CrossRef]
- Song, D.-H.; Song, S.-H.; Shen, T.-Z.; Lee, J.-S.; Park, W.-H.; Kim, S.-S.; Song, J.-K. Quantum dot light-emitting diodes using a graphene oxide/PEDOT: PSS bilayer as hole injection layer. Rsc Adv. 2017, 7, 43396–43402. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, W.; Yin, Z.; Huang, L.; Li, J. Synergistic effects of Li-doped NiOx film prepared by low-temperature combustion as hole-injection layer for high performance OLED devices. Org. Electron. 2020, 85, 105823. [Google Scholar] [CrossRef]
- Li, Y.-H.; Lu, X.; Wang, R.; Ma, Y.-Y.; Duhm, S.; Fung, M.-K. Cu-Doped nickel oxide prepared using a low-temperature combustion method as a hole-injection layer for high-performance OLEDs. J. Mater. Chem. C 2017, 5, 11751–11757. [Google Scholar] [CrossRef]
- Lee, J.H.; Jin, I.S.; Noh, Y.W.; Park, S.H.; Jung, J.W. A solution-processed spinel CuCo2O4 as an effective hole transport layer for efficient perovskite solar cells with negligible hysteresis. ACS Sustain. Chem. Eng. 2019, 7, 17661–17670. [Google Scholar] [CrossRef]
- Kang, W.; Tang, Y.; Li, W.; Li, Z.; Yang, X.; Xu, J.; Lee, C.-S. Porous CuCo2O4 nanocubes wrapped by reduced graphene oxide as high-performance lithium-ion battery anodes. Nanoscale 2014, 6, 6551–6556. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; Pal, U.; Villanueva, M.S. Structural, magnetic, and catalytic evaluation of spinel Co, Ni, and Co–Ni ferrite nanoparticles fabricated by low-temperature solution combustion process. ACS Omega 2018, 3, 14986–15001. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ouyang, D.; Shih, C.J.; Yang, B.; Choy, W.C. Solution-Processed Ternary Oxides as Carrier Transport/Injection Layers in Optoelectronics. Adv. Energy Mater. 2020, 10, 1900903. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Kaverlavani, S.K.; Moosavifard, S.; Bakouei, A. Designing graphene-wrapped nanoporous CuCo2O4 hollow spheres electrodes for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 14301–14309. [Google Scholar] [CrossRef]
- Gautier, J.; Trollund, E.; Ríos, E.; Nkeng, P.; Poillerat, G. Characterization of thin CuCo2O4 films prepared by chemical spray pyrolysis. Study of their electrochemical stability by ex situ spectroscopic analysis. J. Electroanal. Chem. 1997, 428, 47–56. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Liao, M.; Hu, X.; Fang, X. Electrical transport properties of large, individual NiCo2O4 nanoplates. Adv. Funct. Mater. 2012, 22, 998–1004. [Google Scholar] [CrossRef]
- Sadegh, F.; Akin, S.; Moghadam, M.; Mirkhani, V.; Ruiz-Preciado, M.A.; Wang, Z.; Tavakoli, M.M.; Graetzel, M.; Hagfeldt, A.; Tress, W. Highly efficient, stable and hysteresis-less planar perovskite solar cell based on chemical bath treated Zn2SnO4 electron transport layer. Nano Energy 2020, 75, 105038. [Google Scholar] [CrossRef]
- Ogawa, H.; Nishikawa, M.; Abe, A. Hall measurement studies and an electrical conduction model of tin oxide ultrafine particle films. J. Appl. Phys. 1982, 53, 4448–4455. [Google Scholar] [CrossRef]
- Jiang, F.; Choy, W.C.; Li, X.; Zhang, D.; Cheng, J. Post-treatment-free solution-processed non-stoichiometric NiOx nanoparticles for efficient hole-transport layers of organic optoelectronic devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Qin, Y.; Wan, L.; Su, J.; Lin, Z.; Zhang, J.; Chang, J.; Wu, J.; Hao, Y. Metal oxide heterojunctions for high performance solution grown oxide thin film transistors. Appl. Surf. Sci. 2020, 527, 146774. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.-H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wu, C.-K.; Lou, Y.; Burda, C.; Koberstein, J.T.; Zhu, Y.; O’Brien, S. Copper oxide nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Matienzo, J.; Yin, L.I.; Grim, S.O.; Swartz, W.E., Jr. X-ray photoelectron spectroscopy of nickel compounds. Inorg. Chem. 1973, 12, 2762–2769. [Google Scholar] [CrossRef]
- Van der Laan, G.; Westra, C.; Haas, C.; Sawatzky, G. Satellite structure in photoelectron and Auger spectra of copper dihalides. Phys. Rev. B 1981, 23, 4369. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Khassin, A.A.; Yurieva, T.M.; Kaichev, V.V.; Bukhtiyarov, V.I.; Budneva, A.A.; Paukshtis, E.A.; Parmon, V.N. Metal–support interactions in cobalt-aluminum co-precipitated catalysts: XPS and CO adsorption studies. J. Mol. Catal. A Chem. 2001, 175, 189–204. [Google Scholar] [CrossRef]
- Zhang, H.; Zhan, Z.; Liu, X. Electrophoretic deposition of (Mn, Co)3O4 spinel coating for solid oxide fuel cell interconnects. J. Power Sources 2011, 196, 8041–8047. [Google Scholar] [CrossRef]
- Raebiger, H.; Lany, S.; Zunger, A. Origins of the p-type nature and cation deficiency in Cu2O and related materials. Phys. Rev. B 2007, 76, 045209. [Google Scholar] [CrossRef]
- Barreca, D.; Massignan, C.; Daolio, S.; Fabrizio, M.; Piccirillo, C.; Armelao, L.; Tondello, E. Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt (II) precursor by chemical vapor deposition. Chem. Mater. 2001, 13, 588–593. [Google Scholar] [CrossRef]
- Manickam, M.; Ponnuswamy, V.; Sankar, C.; Suresh, R. Cobalt oxide thin films prepared by NSP technique: Impact of molar concentration on the structural, optical, morphological and electrical properties. Optik 2016, 127, 5278–5284. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chen, J.-H. Preparation of p-type CuCo2O4 thin films by sol-gel processing. Mater. Lett. 2017, 188, 63–65. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Liu, Y.-Z.; Li, J.-B.; Sun, P.; Zhao, X.-C.; Liu, C.-J. Photophysical and photocatalytic properties of core-ring structured NiCo2O4 nanoplatelets. J. Phys. Chem. C 2009, 113, 14083–14087. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Wang, Y.; Duan, Y. Solubility-controlled synthesis of high-quality Co3O4 nanocrystals. Chem. Mater. 2005, 17, 4023–4030. [Google Scholar] [CrossRef]
| Sample | Resistivity (Ω·cm) | Mobility (cm2/V·s) | Hole concentration (cm−3) |
|---|---|---|---|
| CuCo2O4-200 | 2.295 | 0.96 | +2.834 × 1018 |
| CuCo2O4-300 | 0.3917 | 0.72 | +2.214 × 1019 |
| CuO | N/A | N/A | N/A |
| Sample | Lmax (cd/m2) | CEmax (cd/A) | EQEmax (%) | FWHM (nm) | CIE 1931 (x, y) |
|---|---|---|---|---|---|
| CuCo2O4-200 | 93,607 | 11.14 | 2.62 | 24 | (0.214, 0.751) |
| CuCo2O4-300 | 47,935 | 8.42 | 2.00 | 24 | (0.219, 0.747) |
| CuO | 14,268 | 1.42 | 0.34 | 26 | (0.249, 0.725) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.H.; Kim, M.G.; Ma, J.H.; Jeong, J.H.; Ha, H.J.; Kim, W.; Park, S.; Kang, S.J. Enhancing the Performance of Quantum Dot Light-Emitting Diodes Using Solution-Processable Highly Conductive Spinel Structure CuCo2O4 Hole Injection Layer. Materials 2023, 16, 972. https://doi.org/10.3390/ma16030972
Park MH, Kim MG, Ma JH, Jeong JH, Ha HJ, Kim W, Park S, Kang SJ. Enhancing the Performance of Quantum Dot Light-Emitting Diodes Using Solution-Processable Highly Conductive Spinel Structure CuCo2O4 Hole Injection Layer. Materials. 2023; 16(3):972. https://doi.org/10.3390/ma16030972
Chicago/Turabian StylePark, Min Ho, Min Gye Kim, Jin Hyun Ma, Jun Hyung Jeong, Hyoun Ji Ha, Wonsik Kim, Soohyung Park, and Seong Jun Kang. 2023. "Enhancing the Performance of Quantum Dot Light-Emitting Diodes Using Solution-Processable Highly Conductive Spinel Structure CuCo2O4 Hole Injection Layer" Materials 16, no. 3: 972. https://doi.org/10.3390/ma16030972
APA StylePark, M. H., Kim, M. G., Ma, J. H., Jeong, J. H., Ha, H. J., Kim, W., Park, S., & Kang, S. J. (2023). Enhancing the Performance of Quantum Dot Light-Emitting Diodes Using Solution-Processable Highly Conductive Spinel Structure CuCo2O4 Hole Injection Layer. Materials, 16(3), 972. https://doi.org/10.3390/ma16030972