A Two-Step Femtosecond Laser-Based Deposition of Robust Corrosion-Resistant Molybdenum Oxide Coating
Abstract
1. Introduction
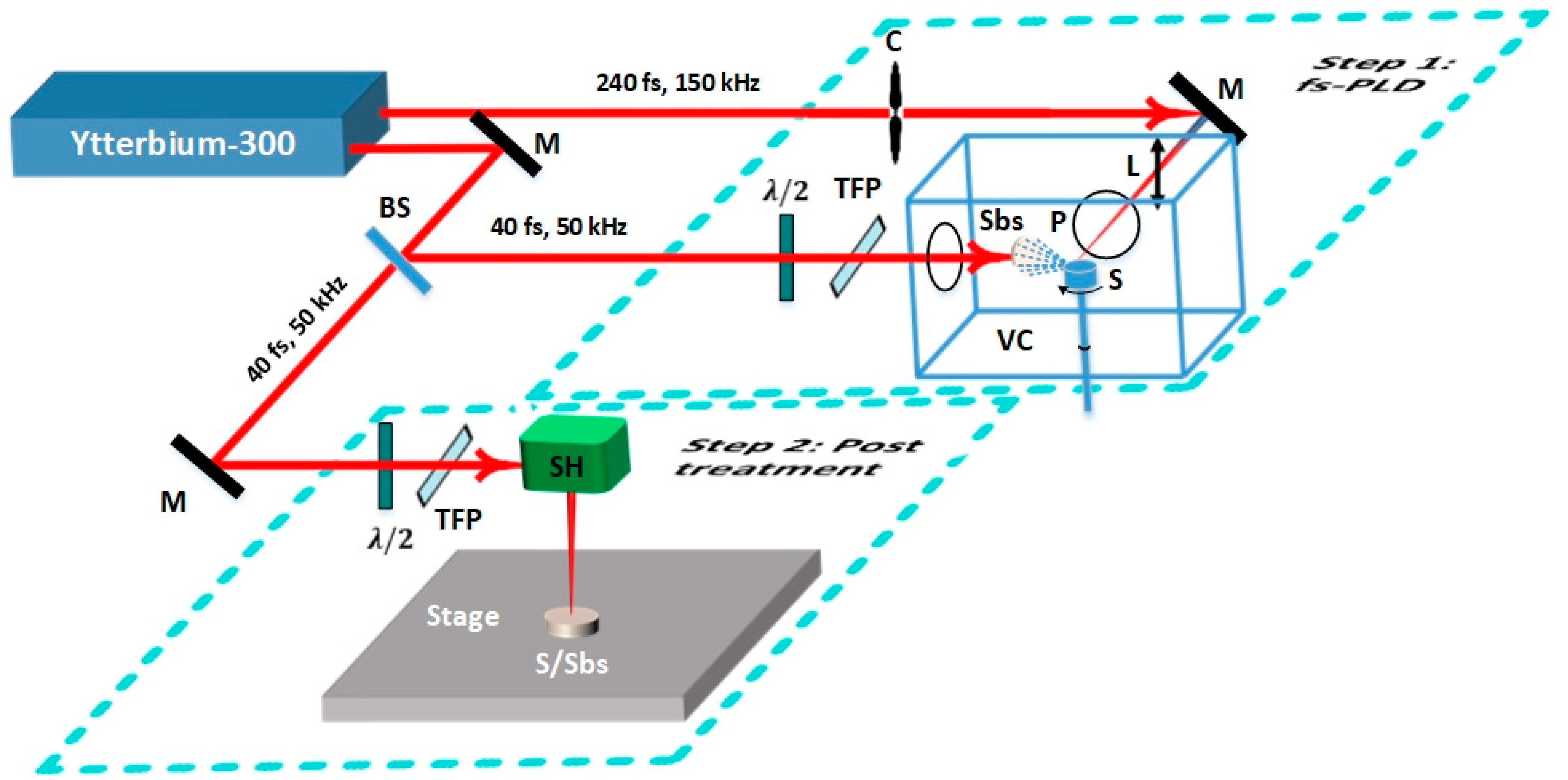
2. Experimental
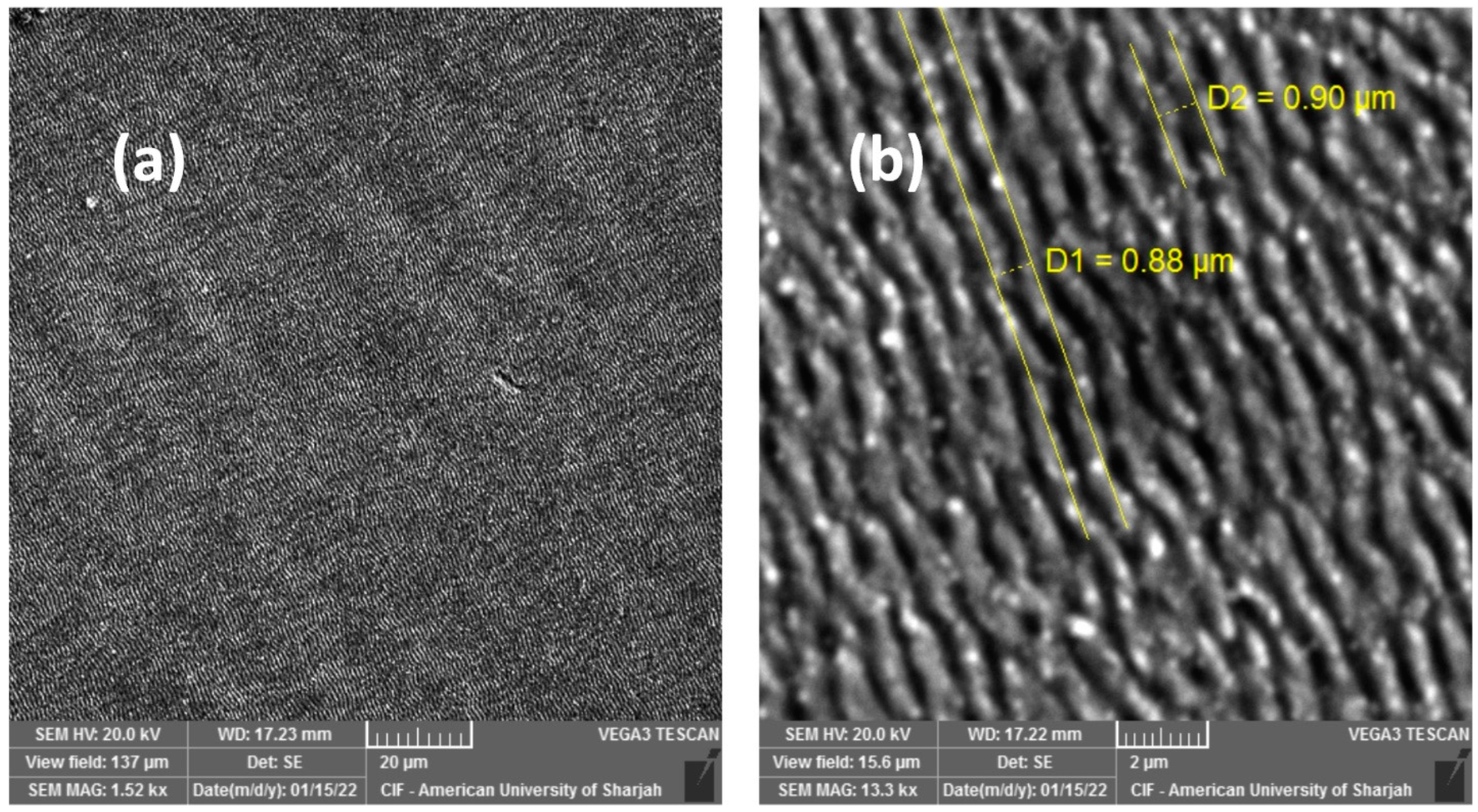
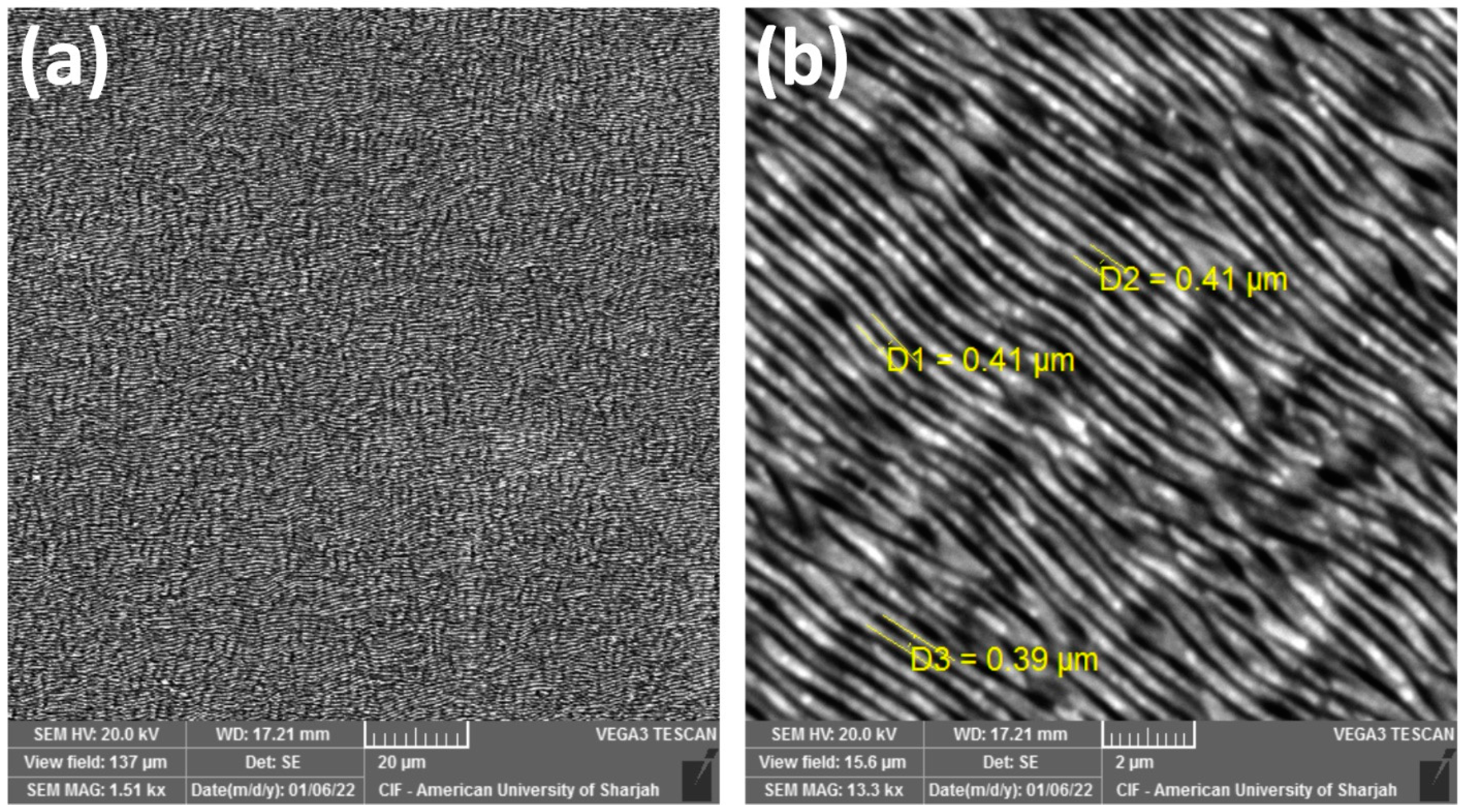
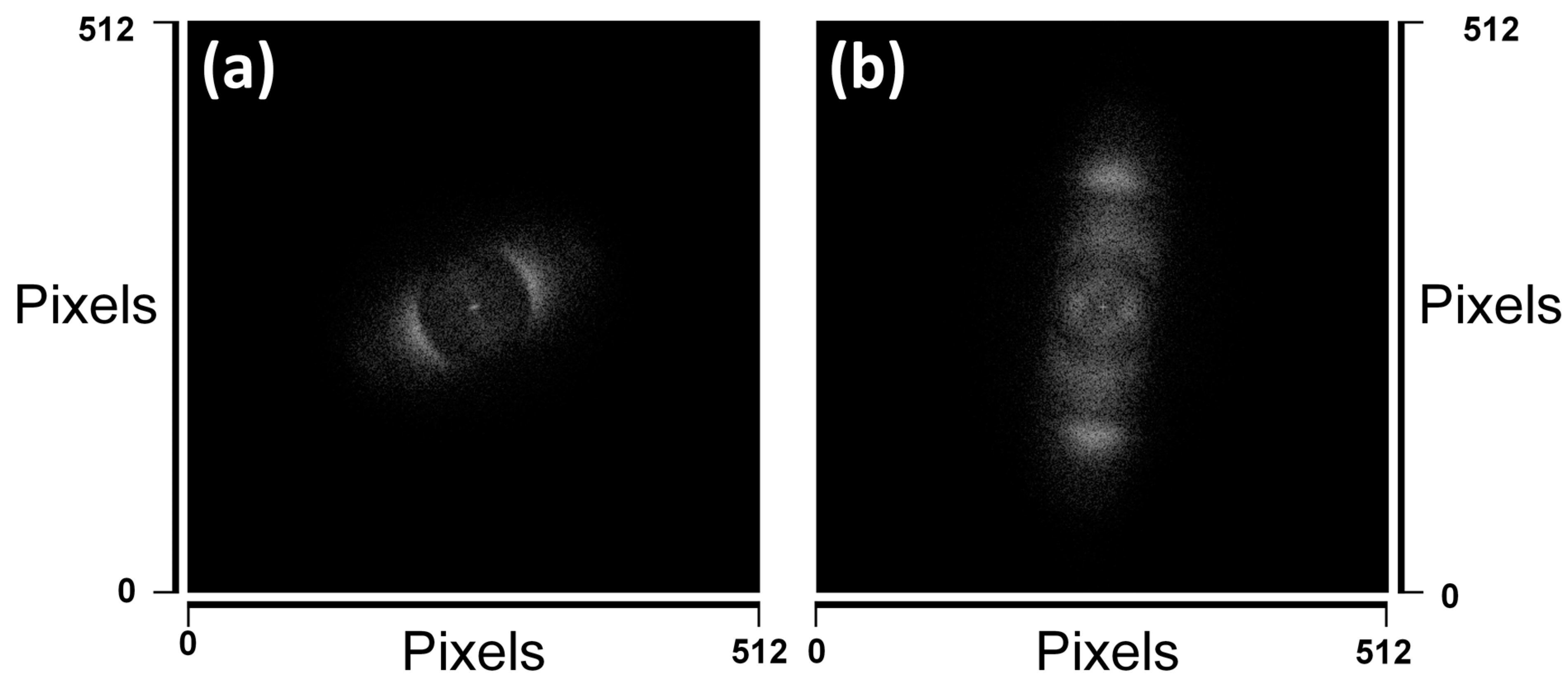
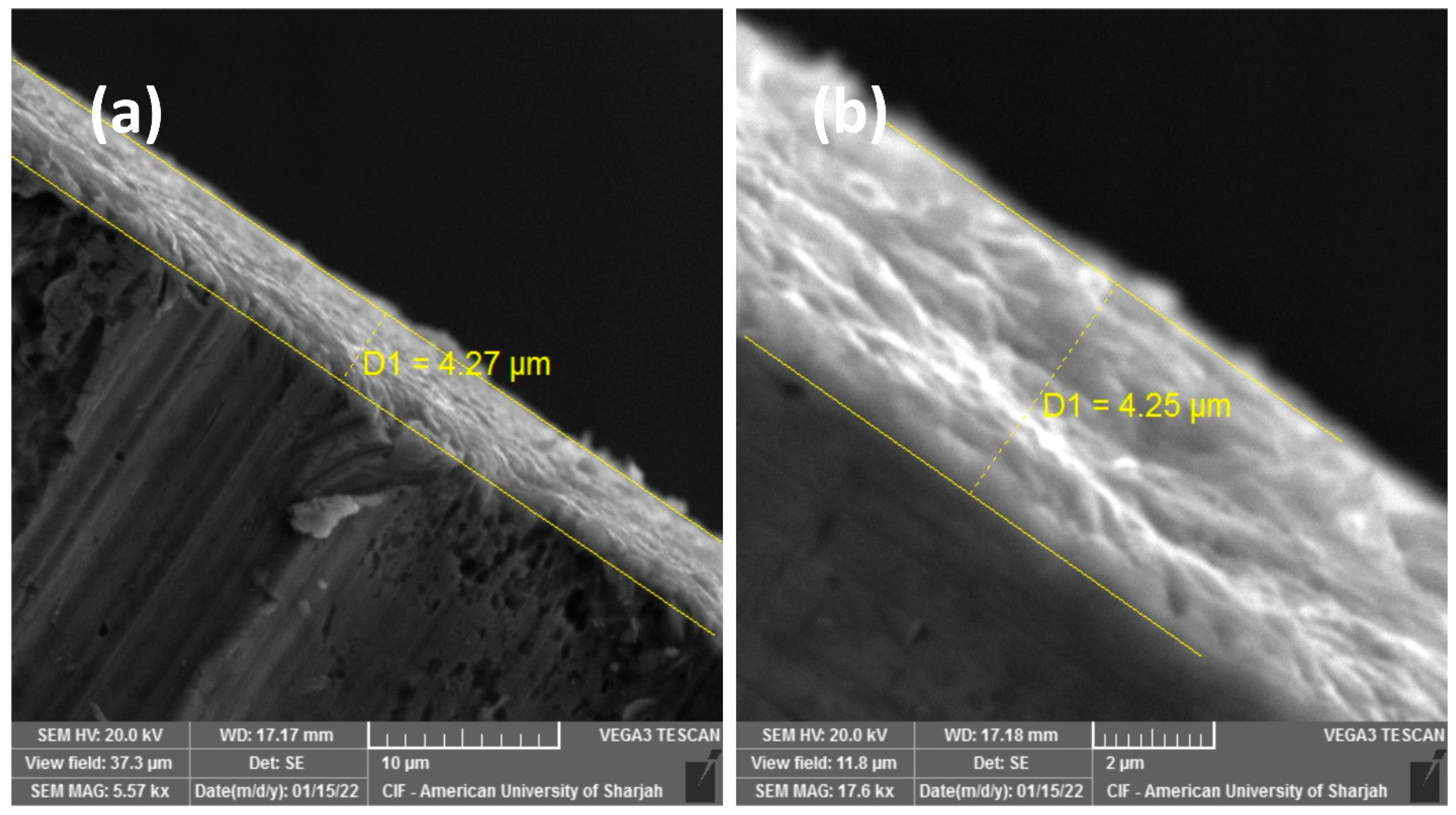
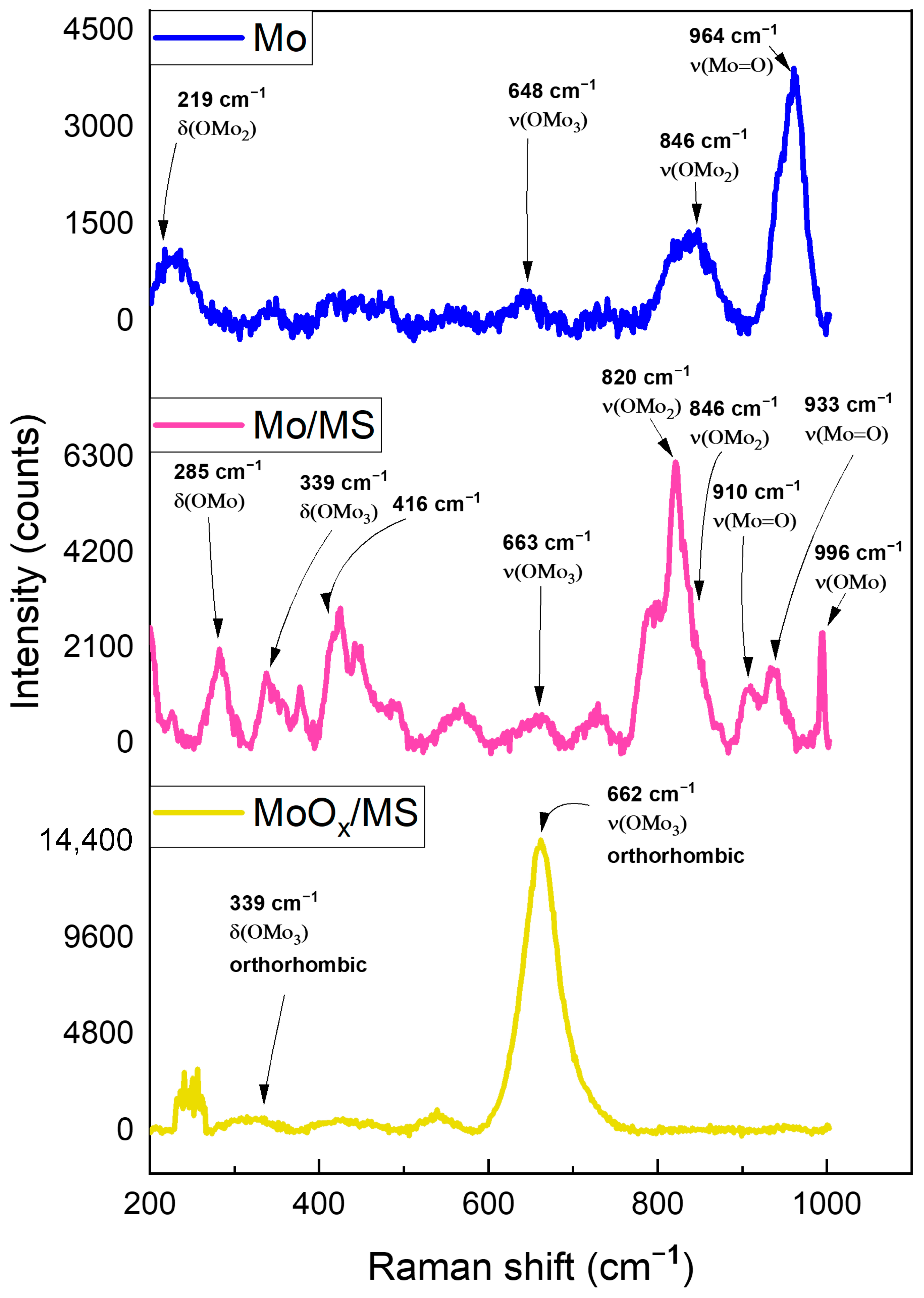
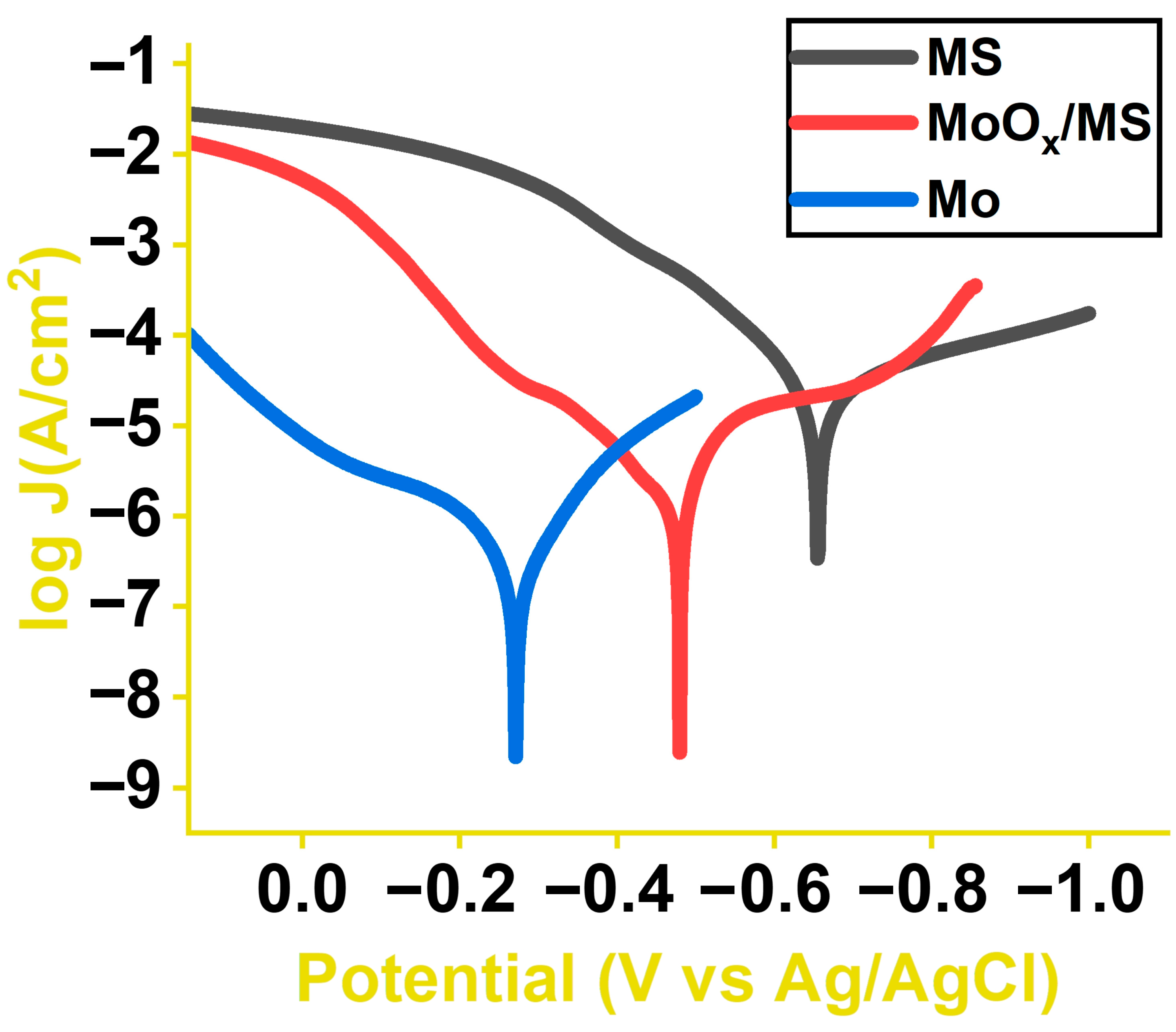
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Bai, S.; Liu, J.; Yang, P.; Huang, H.; Yang, L.-M. Femtosecond fiber laser additive manufacturing of tungsten. Laser 3d Manuf. III 2016, 9738, 96–105. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Ultrafast lasers—Reliable tools for advanced materials processing. Light. Sci. Appl. 2014, 3, e149. [Google Scholar] [CrossRef]
- Vaida, M.E.; Gleitsmann, T.; Tchitnga, R.; Bernhardt, T.M. Femtosecond-Laser Photoemission Spectroscopy of Mo(100) Covered by Ultrathin MgO(100) Films of Variable Thickness. J. Phys. Chem. C 2009, 113, 10264–10268. [Google Scholar] [CrossRef]
- Cerullo, G.; De Silvestri, S. Ultrafast optical parametric amplifiers. Rev. Sci. Instrum. 2003, 74, 1–18. [Google Scholar] [CrossRef]
- Fuchs, S.; Wünsche, M.; Nathanael, J.; Abel, J.J.; Rödel, C.; Biedermann, J.; Reinhard, J.; Hübner, U.; Paulus, G.G. Optical coherence tomography with nanoscale axial resolution using a laser-driven high-harmonic source. Optica 2017, 4, 903–906. [Google Scholar] [CrossRef]
- Shah, J. Ultrafast Spectroscopy of Semiconductors and Semiconductor Nanostructures; Springer: Berlin, Germany, 1999; p. 115. [Google Scholar] [CrossRef]
- Buldt, J.; Mueller, M.; Stark, H.; Jauregui, C.; Limpert, J. Fiber laser-driven gas plasma-based generation of THz radiation with 50-mW average power. Appl. Phys. B Laser Opt. 2019, 126, 1–5. [Google Scholar] [CrossRef]
- Roberts, H.W.; Day, A.; O’Brart, D.P.S. Femtosecond laser–assisted cataract surgery: A review. Eur. J. Ophthalmol. 2019, 30, 417–429. [Google Scholar] [CrossRef]
- Ahmad, S.; Egilmez, M.; Iqbal, M.; Ibrahim, T.; Khamis, M.; Alnaser, A.S. Pulsed Laser Deposited Zeolite Coatings on Femtosecond Laser-Nanostructured Steel Meshes for Durable Superhydrophilic/Oleophobic Functionalities. Front. Chem. 2021, 9, 1027. [Google Scholar] [CrossRef]
- Stuke, M. Femtosecond uv excimer laser ablation. Appl. Phys. B Laser Opt. 1987, 44, 199–204. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sutcliffe, E.; Braren, B. Ablation and etching of polymethylmethacrylate by very short (160 fs) ultraviolet (308 nm) laser pulses. Appl. Phys. Lett. 1987, 51, 1285–1287. [Google Scholar] [CrossRef]
- Davis, K.M.; Miura, K.; Sugimoto, N.; Hirao, K. Writing waveguides in glass with a femtosecond laser. Opt. Lett. 1996, 21, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Yeo, J.; Moon, H.; Lim, T.W.; Hong, S.; Nam, K.H.; Yoo, S.; Grigoropoulos, C.P.; Yang, D.-Y.; Ko, S.H. Nanoscale Electronics: Digital Fabrication by Direct Femtosecond Laser Processing of Metal Nanoparticles. Adv. Mater. 2011, 23, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.E., III. Femtosecond-Laser Microstructuring of Silicon for Novel Optoelectronic Devices. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 2004. Available online: https://ui.adsabs.harvard.edu/abs/2004PhDT.......164C/abstract (accessed on 20 December 2022).
- Della Valle, G.; Osellame, R.; LaPorta, P. Micromachining of photonic devices by femtosecond laser pulses. J. Opt. A Pure Appl. Opt. 2008, 11, 013001. [Google Scholar] [CrossRef]
- Halbwax, M.; Sarnet, T.; Delaporte, P.; Sentis, M.; Etienne, H.; Torregrosa, F.; Vervisch, V.; Perichaud, I.; Martinuzzi, S. Micro and nano-structuration of silicon by femtosecond laser: Application to silicon photovoltaic cells fabrication. Thin Solid Films 2008, 516, 6791–6795. [Google Scholar] [CrossRef]
- Chai, N.; Liu, Y.; Yue, Y.; Wei, P.; Wang, X.; Zhao, J.; Zhang, Q.; Huang, F.; Zeng, Z.; Gan, Z.; et al. 3D nonlinear photolithography of Tin oxide ceramics via femtosecond laser. Sci. China Mater. 2021, 64, 1477–1484. [Google Scholar] [CrossRef]
- Cheng, Y.; Sugioka, K.; Midorikawa, K.; Masuda, M.; Toyoda, K.; Kawachi, M.; Shihoyama, K. Control of the cross-sectional shape of a hollow microchannel embedded in photostructurable glass by use of a femtosecond laser. Opt. Lett. 2003, 28, 55–57. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Femtosecond laser processing for optofluidic fabrication. Lab A Chip 2012, 12, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, B.; Xie, W.; Liu, S.; Wei, X.; Cai, Z.; Gu, M.; Tao, Y.; Yang, T.; Zhang, C.; et al. Coupling Among Carriers and Phonons in Femtosecond Laser Pulses Excited SrRuO3: A Promising Candidate for Optomechanical and Optoelectronic Applications. ACS Appl. Nano Mater. 2019, 2, 3882–3888. [Google Scholar] [CrossRef]
- Kaligar, A.B.; Kumar, H.A.; Ali, A.; Abuzaid, W.; Egilmez, M.; Alkhader, M.; Abed, F.; Alnaser, A.S. Femtosecond Laser-Based Additive Manufacturing: Current Status and Perspectives. Quantum Beam Sci. 2022, 6, 5. [Google Scholar] [CrossRef]
- Lu, J.; Huang, T.; Liu, Z.; Zhang, X.; Xiao, R. Long-term wettability of titanium surfaces by combined femtosecond laser micro/nano structuring and chemical treatments. Appl. Surf. Sci. 2018, 459, 257–262. [Google Scholar] [CrossRef]
- Tamaki, T.; Watanabe, W.; Nishii, J.; Itoh, K. Welding of Transparent Materials Using Femtosecond Laser Pulses. Jpn. J. Appl. Phys. 2005, 44, L687. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, W.; Abu Baker, A.; Egilmez, M.; Abuzaid, W.; Orhan, M.F.; Ibrahim, T.; Khamis, M.; Alnaser, A.S. Enhancement of the corrosion resistance of mild steel with femtosecond laser- nanostructuring and CrCoNi medium entropy alloy coating. Appl. Surf. Sci. Adv. 2022, 12. [Google Scholar] [CrossRef]
- Lim, Y.C.; Johnson, J.; Fei, Z.; Wu, Y.; Farson, D.F.; Lannutti, J.J.; Choi, H.W.; Lee, L.J. Micropatterning and characterization of electrospun poly(ε-caprolactone)/gelatin nanofiber tissue scaffolds by femtosecond laser ablation for tissue engineering applications. Biotechnol. Bioeng. 2010, 108, 116–126. [Google Scholar] [CrossRef]
- Zhao, D.; Gierse, N.; Wegner, J.; Pretzler, G.; Oelmann, J.; Brezinsek, S.; Liang, Y.; Neubauer, O.; Rasinski, M.; Linsmeier, C.; et al. Ablation mass features in multi-pulses femtosecond laser ablate molybdenum target. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2018, 418, 54–59. [Google Scholar] [CrossRef]
- Kotsedi, L.; Mthunzi, P.; Nuru, Z.; Eaton, S.; Sechoghela, P.; Mongwaketsi, N.; Ramponi, R.; Maaza, M. Femtosecond laser surface structuring of molybdenum thin films. Appl. Surf. Sci. 2015, 353, 1334–1341. [Google Scholar] [CrossRef]
- Kotsedi, L.; Kaviyarasu, K.; Fuku, X.; Eaton, S.; Amara, E.; Bireche, F.; Ramponi, R.; Maaza, M. Two temperature approach to femtosecond laser oxidation of molybdenum and morphological study. Appl. Surf. Sci. 2017, 421, 213–219. [Google Scholar] [CrossRef]
- Cano-Lara, M.; Camacho-López, S.; Esparza-García, A.; Camacho-López, M. Laser-induced molybdenum oxide formation by low energy (nJ)–high repetition rate (MHz) femtosecond pulses. Opt. Mater. 2011, 33, 1648–1653. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Ganin, D.; Lapshin, K.; Obidin, A.; Vartapetov, S. Single-pulse perforation of thin transparent dielectrics by femtosecond lasers. Appl. Phys. A 2017, 123, 1–7. [Google Scholar] [CrossRef]
- Murray, M.; Jose, G.; Richards, B.; Jha, A. Femtosecond pulsed laser deposition of silicon thin films. Nanoscale Res. Lett. 2013, 8, 272. [Google Scholar] [CrossRef]
- Wasa, K.; Kitabatake, M.; Adachi, H. Thin Film Processes. In Thin Film Materials Technology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 17–69. [Google Scholar] [CrossRef]
- Frey, H.; Khan, H.R. Handbook of Thin-Film Technology. In Handbook of Thin-Film Technology; Springer: Berlin, Germany, 2015; pp. 1–379. [Google Scholar] [CrossRef]
- Vencl, A. Optimisation of the deposition parameters of thick atmospheric plasma spray coatings. J. Balk. Tribol. Assoc. 2012, 18, 405–414. [Google Scholar]
- Chkalov, R.V.; Chkalova, D.G. Pulsed Laser Deposition of Thin-Film Coatings in an Electrostatic Field. Defect Diffus. Forum 2021, 410, 753–757. [Google Scholar] [CrossRef]
- Chkalov, R.V.; Chkalova, D.G. Collection of Laser Ablation Products by Means of an Electrostatic Field. Defect Diffus. Forum 2021, 410, 748–752. [Google Scholar] [CrossRef]
- Kumi-Barimah, E.; Penhale-Jones, R.; Salimian, A.; Upadhyaya, H.; Hasnath, A.; Jose, G. Phase evolution, morphological, optical and electrical properties of femtosecond pulsed laser deposited TiO2 thin films. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, M.; Higashikawa, K.; Hanabusa, M. Pulsed laser deposition of ZnO thin films using a femtosecond laser. Appl. Surf. Sci. 2000, 154–155, 424–427. [Google Scholar] [CrossRef]
- Varacalle, D.J.; Guillen, D.; Deason, D.M.; Rhodaberger, W.; Sampson, E. Effect of Grit-Blasting on Substrate Roughness and Coating Adhesion. J. Therm. Spray Technol. 2006, 15, 348–355. [Google Scholar] [CrossRef]
- El-Shabasy, M. Perspective of adhesion of thin films. Period. Polytech. Electr. Eng. (Arch.) 1981, 25, 123–134. [Google Scholar]
- Lunk, H.-J.; Hartl, H. Discovery, properties and applications of molybdenum and its compounds. Chemtexts 2017, 3, 13. [Google Scholar] [CrossRef]
- Thirunaukkarasu, K.; Taher, A.; Chaudhary, N.; Rajput, V.K.; Naik, C.A.; Gautam, J.P.; Naraharisetty, S.R.G. Mechanically and thermally stable thin sheets of broadband antireflection surfaces fabricated by femtosecond lasers. Opt. Laser Technol. 2022, 150, 107935. [Google Scholar] [CrossRef]
- Sanz, M.; Walczak, M.; de Nalda, R.; Oujja, M.; Marco, J.F.; Rodriguez, J.; Izquierdo, J.G.; Bañares, L.; Castillejo, M. Femtosecond pulsed laser deposition of nanostructured TiO2 films. Appl. Surf. Sci. 2008, 255, 5206–5210. [Google Scholar] [CrossRef]
- Kihlborg, L. Studies on molybdenum oxides. Acta. Chem. Scand 1959, 13, 962. Available online: http://actachemscand.org/pdf/acta_vol_13_p0954-0962.pdf (accessed on 20 December 2022). [CrossRef]
- Cuando-Espitia, N.; Redenius, J.; Mensink, K.; Camacho-López, M.; Camacho-López, S.; Aguilar, G. Influence of oxygen pressure on the fs laser-induced oxidation of molybdenum thin films. Opt. Mater. Express 2018, 8, 581–596. [Google Scholar] [CrossRef]
- Girolami, M.; Bellucci, A.; Mastellone, M.; Orlando, S.; Valentini, V.; Montereali, R.; Vincenti, M.; Polini, R.; Trucchi, D.M. Optical characterization of double-nanotextured black diamond films. Carbon 2018, 138, 384–389. [Google Scholar] [CrossRef]
- Prada-Rodrigo, J.; Rodríguez-Beltrán, R.I.; Ezquerra, T.A.; Moreno, P.; Rebollar, E. Influence of film thickness and substrate roughness on the formation of laser induced periodic surface structures in poly(ethylene terephthalate) films deposited over gold substrates. Opt. Laser Technol. 2023, 159, 109007. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell Meets Marangoni—A Review of Theories on Laser-Induced Periodic Surface Structures. Laser Photon- Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- He, S.; Nivas, J.J.; Anoop, K.; Vecchione, A.; Hu, M.; Bruzzese, R.; Amoruso, S. Surface structures induced by ultrashort laser pulses: Formation mechanisms of ripples and grooves. Appl. Surf. Sci. 2015, 353, 1214–1222. [Google Scholar] [CrossRef]
- He, S.; Nivas, J.J.; Vecchione, A.; Hu, M.; Amoruso, S. On the generation of grooves on crystalline silicon irradiated by femtosecond laser pulses. Opt. Express 2016, 24, 3238–3247. [Google Scholar] [CrossRef]
- Nivas, J.; Amoruso, S. Generation of Supra-Wavelength Grooves in Femtosecond Laser Surface Structuring of Silicon. Nanomaterials 2021, 11, 174. [Google Scholar] [CrossRef]
- Shi, X.; Xu, X. Laser fluence dependence of ripple formation on fused silica by femtosecond laser irradiation. Appl. Phys. A 2019, 125, 256. [Google Scholar] [CrossRef]
- Abere, M.J.; Zhong, M.; Krüger, J.; Bonse, J. Ultrafast laser-induced morphological transformations. MRS Bull. 2016, 41, 969–974. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Zhang, Y.; Xie, C.; Liu, K.; Zhou, Q. Periodic structures on germanium induced by high repetition rate femtosecond laser. Opt. Laser Technol. 2017, 101, 291–297. [Google Scholar] [CrossRef]
- Preuss, S.; Demchuk, A.; Stuke, M. Sub-picosecond UV laser ablation of metals. Appl. Phys. A 1995, 61, 33–37. [Google Scholar] [CrossRef][Green Version]
- Shirk, M.D.; Molian, P.A. A review of ultrashort pulsed laser ablation of materials. J. Laser Appl. 1998, 10, 18–28. [Google Scholar] [CrossRef]
- Hermann, J.; Benfarah, M.; Coustillier, G.; Bruneau, S.; Axente, E.; Guillemoles, J.-F.; Sentis, M.; Alloncle, P.; Itina, T. Selective ablation of thin films with short and ultrashort laser pulses. Appl. Surf. Sci. 2006, 252, 4814–4818. [Google Scholar] [CrossRef]
- Munoz-Garcia, C.; Canteli, D.; Lauzurica, S.; Morales, M.; Molpeceres, C.; Ros, E.; Ortega, P.; López-González, J.; Voz, C. Influence of wavelength and pulse duration on the selective laser ablation of WOx, VOx and MoOx thin films. Surf. Interfaces 2021, 28, 101613. [Google Scholar] [CrossRef]
- Backus, S.; Durfee, C.G.; Murnane, M.M.; Kapteyn, H. High power ultrafast lasers. Rev. Sci. Instrum. 1998, 69, 1207–1223. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A Mater. Sci. Process 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Komatsu, T.; Iordanova, R.; Dimitriev, Y. Structure study of MoO3–ZnO–B2O3 glasses by Raman spectroscopy and formation of α-ZnMoO4 nanocrystals. Opt. Mater. 2011, 33, 839–845. [Google Scholar] [CrossRef]
- Velvarská, R.; Tišler, Z.; Raichlová, V.; Hidalgo-Herrador, J.M. Raman Spectroscopy as Molybdenum and Tungsten Content Analysis Tool for Mesoporous Silica and Beta Zeolite Catalysts. Molecules 2020, 25, 4918. [Google Scholar] [CrossRef]
- Chae, B.; Jung, Y.M.; Wu, X.; Bin Kim, S. Characterization of a series of sodium molybdate structures by two-dimensional Raman correlation analysis. J. Raman Spectrosc. 2003, 34, 451–458. [Google Scholar] [CrossRef]
- Das, R.; Deo, M.; Mukherjee, J.; Rao, M.R. Strain induced FCC to BCC structural change in sputtered molybdenum thin films. Surf. Coat. Technol. 2018, 353, 292–299. [Google Scholar] [CrossRef]
- Creighton, J.; Withnall, R. The Raman spectrum of gallium metal. Chem. Phys. Lett. 2000, 326, 311–313. [Google Scholar] [CrossRef]
- Geng, C.; Li, J.; Weiske, T.; Schwarz, H. Complete cleavage of the N≡N triple bond by Ta2N+ via degenerate ligand exchange at ambient temperature: A perfect catalytic cycle. Proc. Natl. Acad. Sci. USA 2019, 116, 21416–21420. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Phyical Data; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Dongil, A.B.; Conesa, J.M.; Pastor-Pérez, L.; Sepúlveda-Escribano, A.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Carbothermally generated copper–molybdenum carbide supported on graphite for the CO2 hydrogenation to methanol. Catal. Sci. Technol. 2021, 11, 4051–4059. [Google Scholar] [CrossRef]
- Ali, A.; Piatkowski, P.A.; Alawadhi, H.; Alnaser, A.S. Reducing the Cut-In Voltage of a Silicon Carbide/p-Silicon Heterojunction Diode Using Femtosecond Laser Ablation. ACS Appl. Electron. Mater. 2022, 4, 6076–6086. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; He, M.; Jiang, Y.; Zhang, B.; Hang, W.; Huang, B. Laser-induced plasma temperature. Spectrochim. Acta Part B At. Spectrosc. 2014, 97, 13–33. [Google Scholar] [CrossRef]
- Sun, X.; Ehrhardt, M.; Lotnyk, A.; Lorenz, P.; Thelander, E.; Gerlach, J.W.; Smausz, T.; Decker, U.; Rauschenbach, B. Crystallization of Ge2Sb2Te5 thin films by nano- and femtosecond single laser pulse irradiation. Sci. Rep. 2016, 6, 28246. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Oh, J.Y.; Kim, S.K.; Choi, Y.J.; Yoon, S.Y.; Kim, C.O. Electric-field-enhanced crystallization of amorphous silicon. Nature 1998, 395, 481–483. [Google Scholar] [CrossRef]
- Oh, M.S.; Yang, B.S.; Lee, J.H.; Oh, S.H.; Lee, U.S.; Kim, Y.J.; Kim, H.J.; Huh, M.S. Improvement of electrical and optical properties of molybdenum oxide thin films by ultralow pressure sputtering method. J. Vac. Sci. Technol. A 2012, 30, 031501. [Google Scholar] [CrossRef]
- Kc, S.; Longo, R.C.; Addou, R.; Wallace, R.M.; Cho, K. Electronic properties of MoS2/MoOx interfaces: Implications in Tunnel Field Effect Transistors and Hole Contacts. Sci. Rep. 2016, 6, 33562. [Google Scholar] [CrossRef]

| Nomenclature | |||
|---|---|---|---|
| Mild steel (MS) | 1312 | ||
| MoOx/MS | 10,953 | ||
| Mo foil | 80,445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Piatkowski, P.; Nawaz, T.; Ahmad, S.; Ibrahim, T.; Khamis, M.; Alnaser, A.S. A Two-Step Femtosecond Laser-Based Deposition of Robust Corrosion-Resistant Molybdenum Oxide Coating. Materials 2023, 16, 909. https://doi.org/10.3390/ma16030909
Ali A, Piatkowski P, Nawaz T, Ahmad S, Ibrahim T, Khamis M, Alnaser AS. A Two-Step Femtosecond Laser-Based Deposition of Robust Corrosion-Resistant Molybdenum Oxide Coating. Materials. 2023; 16(3):909. https://doi.org/10.3390/ma16030909
Chicago/Turabian StyleAli, Asghar, Piotr Piatkowski, Tahir Nawaz, Shahbaz Ahmad, Taleb Ibrahim, Mustafa Khamis, and Ali S. Alnaser. 2023. "A Two-Step Femtosecond Laser-Based Deposition of Robust Corrosion-Resistant Molybdenum Oxide Coating" Materials 16, no. 3: 909. https://doi.org/10.3390/ma16030909
APA StyleAli, A., Piatkowski, P., Nawaz, T., Ahmad, S., Ibrahim, T., Khamis, M., & Alnaser, A. S. (2023). A Two-Step Femtosecond Laser-Based Deposition of Robust Corrosion-Resistant Molybdenum Oxide Coating. Materials, 16(3), 909. https://doi.org/10.3390/ma16030909








