In Situ Modification of Multi-Walled Carbon Nanotubes with Polythiophene-Based Conjugated Polymer for Information Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurements and Instrument
2.2. Synthesis of PDHT
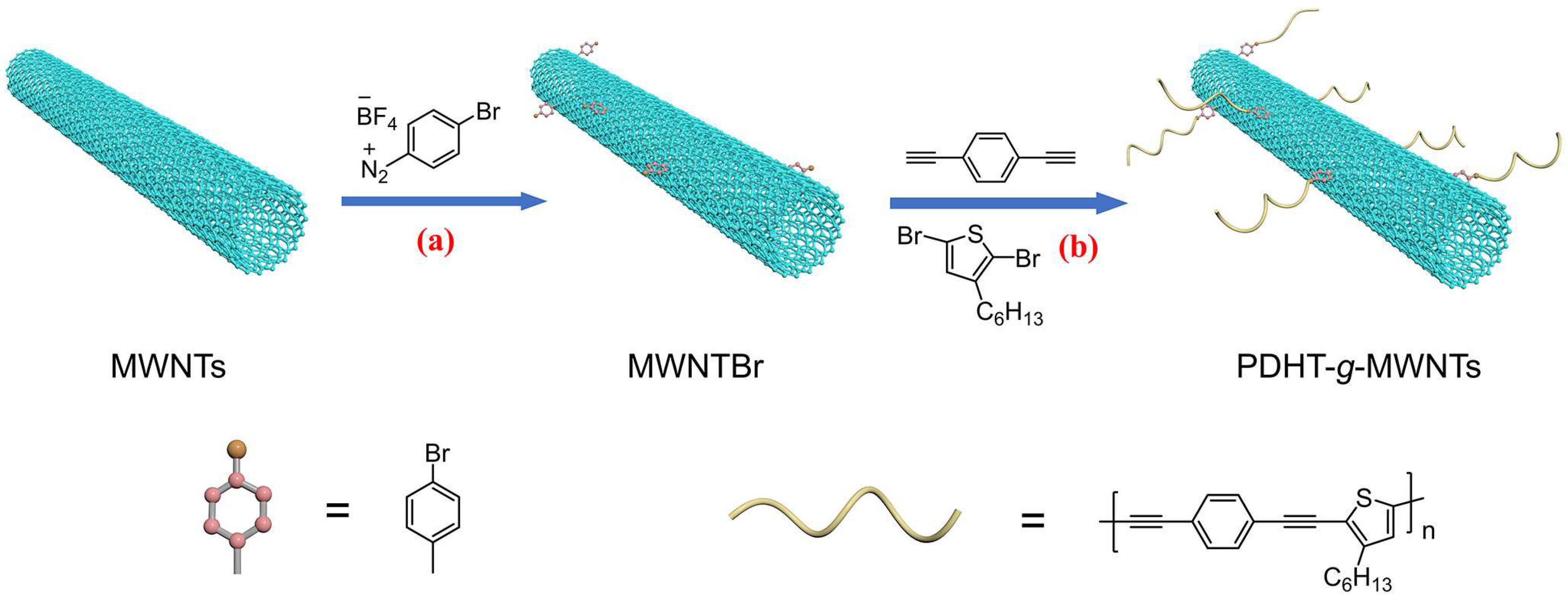
2.3. Synthesis of MWNTBr
2.4. Synthesis of PDHT-g-MWNTs
2.5. Device Preparation and Characterization
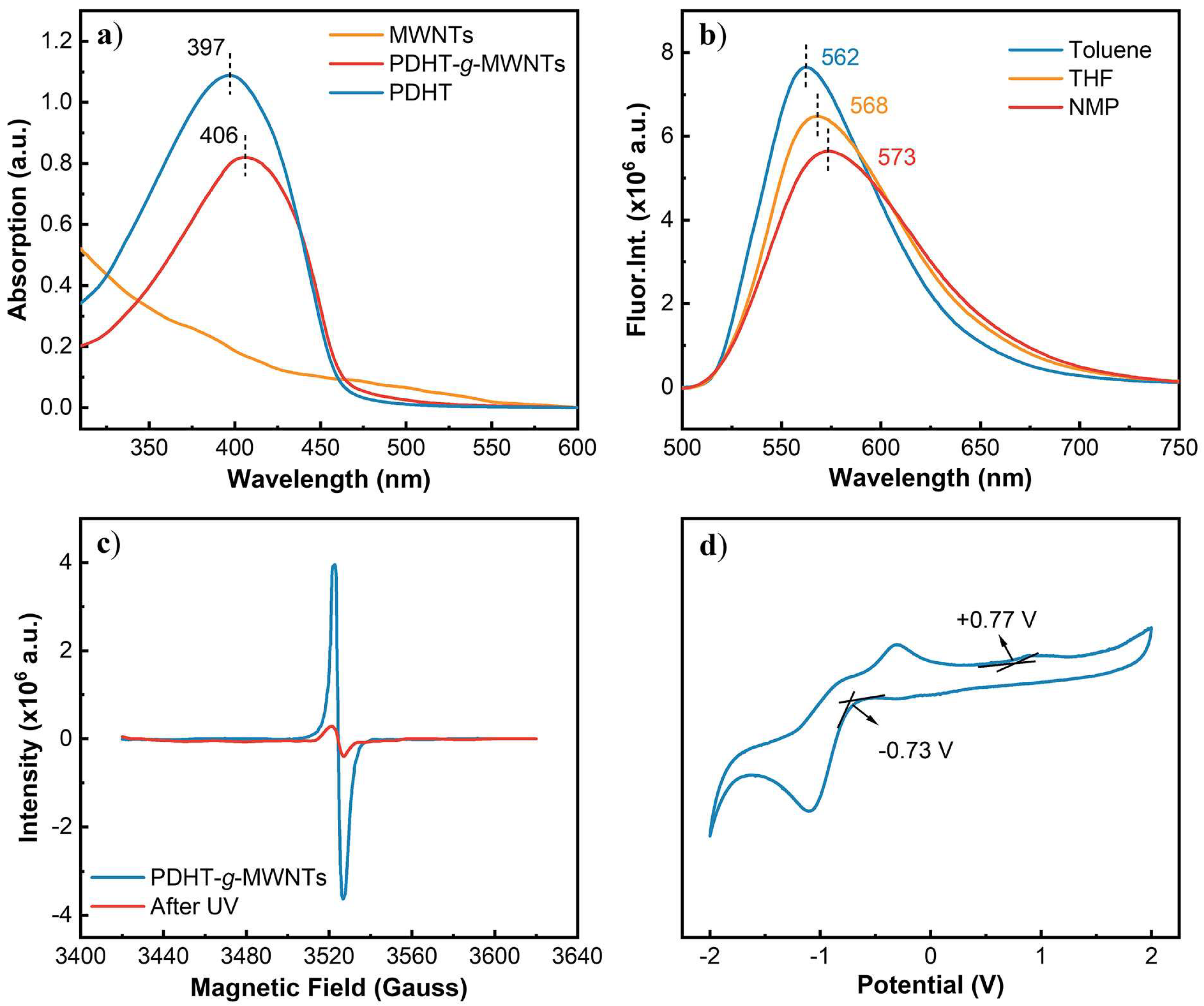
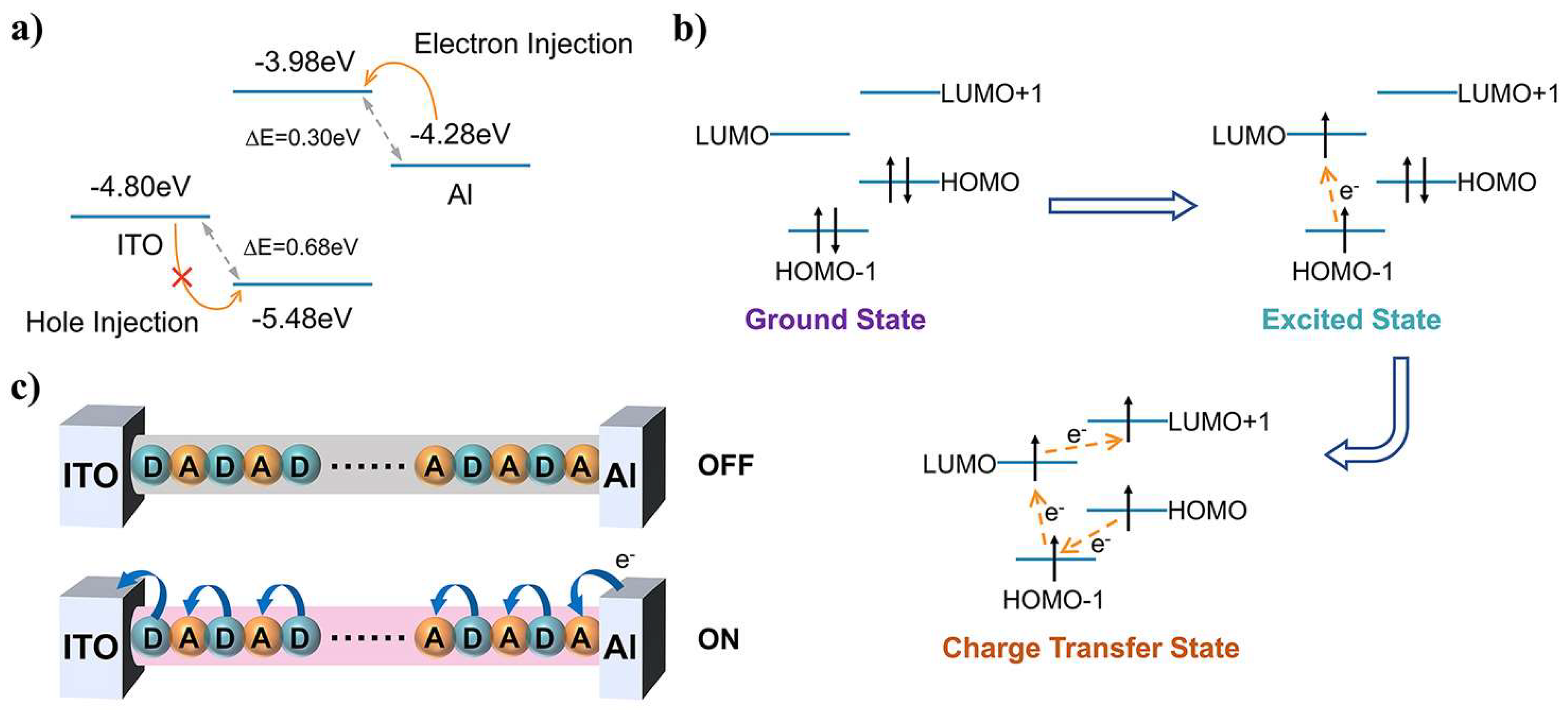
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, S.; Sharma, S. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Paul, R.; Dai, Q.; Hu, C.; Dai, L. Ten years of carbon-based metal-free electrocatalysts. Carbon Energy 2019, 1, 19–31. [Google Scholar] [CrossRef]
- Rao, C.; Satishkumar, B.; Govindaraj, A.; Nath, M. Nanotubes. ChemPhysChem 2001, 2, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, W.; Zhang, Z. Macroscopic Carbon Nanotube Assemblies: Preparation, Properties, and Potential Applications. Small 2011, 7, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Gun’ko, Y. Recent Advances in Research on Carbon Nanotube-Polymer Composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef]
- Dyke, C.; Tour, J. Overcoming the Insolubility of Carbon Nanotubes through High Degrees of Sidewall Functionalization. Chem. Eur. J. 2004, 10, 812–817. [Google Scholar] [CrossRef]
- Grady, B. Recent Developments Concerning the Dispersion of Carbon Nanotubes in Polymers. Macromol. Rapid Commun. 2010, 31, 247–257. [Google Scholar] [CrossRef]
- Singh, P.; Campidelli, S.; Giordani, S.; Bonifazi, D.; Bianco, A.; Prato, M. Organic functionalisation and characterisation of single-walled carbon nanotubes. Chem. Soc. Rev. 2009, 38, 2214–2230. [Google Scholar] [CrossRef]
- Jang, M.-H.; Hwang, Y. Effects of functionalized multi-walled carbon nanotubes on toxicity and bioaccumulation of lead in Daphnia magna. PLoS ONE 2018, 13, e0194935. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, P.; Abousalman-Rezvani, Z.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Polymer-functionalization of carbon nanotube by in situ conventional and controlled radical polymerizations. Adv. Colloid Interface Sci. 2021, 294, 102471. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- González-Grandío, E.; Demirer, G.; Jackson, C.; Yang, D.; Ebert, S.; Molawi, K.; Keller, H.; Landry, M. Carbon nanotube biocompatibility in plants is determined by their surface chemistry. J. Nanobiotechnol. 2021, 19, 431. [Google Scholar] [CrossRef]
- Wu, T.-M.; Chang, H.-L.; Lin, Y.-W. Synthesis and characterization of conductive polypyrrole/multi-walled carbon nanotubes composites with improved solubility and conductivity. Compos. Sci. Technol. 2009, 69, 639–644. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef]
- Ou, H.; Chen, X.; Lin, L.; Fang, Y.; Wang, X. Biomimetic Donor-Acceptor Motifs in Conjugated Polymers for Promoting Exciton Splitting and Charge Separation. Angew. Chem. Int. Ed. 2018, 57, 8729–8733. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Shao, J.; Zhao, X.; Yang, B. Non-Conjugated Polymer Dots with Crosslink-Enhanced Emission in the Absence of Fluorophore Units. Angew. Chem. Int. Ed. 2015, 54, 14626–14637. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Wang, S. Conjugated Polymer Nanoparticles for Imaging, Cell Activity Regulation, and Therapy. Adv. Funct. Mater. 2019, 29, 1806818. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Gan, Q.; Cao, Y.; Yuan, H.; Hua, D. Donor–Acceptor-Type Conjugated Polymer-Based Multicolored Drug Carriers with Tunable Aggregation-Induced Emission Behavior for Self-Illuminating Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41853–41861. [Google Scholar] [CrossRef]
- Dudenko, D.; Kiersnowski, A.; Shu, J.; Pisula, W.; Sebastiani, D.; Spiess, H.; Hansen, M. A Strategy for Revealing the Packing in Semicrystalline π-Conjugated Polymers: Crystal Structure of Bulk Poly-3-hexyl-thiophene (P3HT). Angew. Chem. 2012, 124, 11230–11234. [Google Scholar] [CrossRef]
- Freudenberg, J.; Jänsch, D.; Hinkel, F.; Bunz, U. Immobilization Strategies for Organic Semiconducting Conjugated Polymers. Chem. Rev. 2018, 118, 5598–5689. [Google Scholar] [CrossRef] [PubMed]
- Scanga, R.; Reuther, J. Helical polymer self-assembly and chiral nanostructure formation. Polym. Chem. 2021, 12, 1857–1897. [Google Scholar] [CrossRef]
- Lim, B.; Baeg, K.-J.; Jeong, H.-G.; Jo, J.; Kim, H.; Park, J.-W.; Noh, Y.-Y.; Vak, D.; Park, J.-H.; Park, J.-W.; et al. A New Poly(thienylenevinylene) Derivative with High Mobility and Oxidative Stability for Organic Thin-Film Transistors and Solar Cells. Adv. Mater. 2009, 21, 2808–2814. [Google Scholar] [CrossRef]
- Leclerc, M.; Faid, K. Electrical and optical properties of Processable Polythiophene Derivatives: Structure-Property relationships. Adv. Mater. 1997, 9, 1087–1094. [Google Scholar] [CrossRef]
- Yu, C.; Choi, K.; Yin, L.; Grunlan, J. Light-Weight Flexible Carbon Nanotube Based Organic Composites with Large Thermoelectric Power Factors. ACS Nano 2011, 5, 7885–7892. [Google Scholar] [CrossRef] [PubMed]
- Basheer, B.; George, J.; Siengchin, S.; Parameswaranpillai, J. Polymer grafted carbon nanotubes—Synthesis, properties, and applications: A review. Nano-Struct. Nano-Objects 2020, 22, 100429. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.-L.; Chen, Y.; Neoh, K.-G.; Li, Y.-X.; Zhu, C.-X.; Tok, E.-S.; Kang, E.-T. Nonvolatile Rewritable Memory Effects in Graphene Oxide Functionalized by Conjugated Polymer Containing Fluorene and Carbazole Units. Chem. Eur. J. 2011, 17, 10304–10311. [Google Scholar] [CrossRef]
- Zhuang, X.; Chen, Y.; Wang, L.; Neoh, K.-G.; Kang, E.-T.; Wang, C. A solution-processable polymer-grafted graphene oxide derivative for nonvolatile rewritable memory. Polym. Chem. 2014, 5, 2010–2017. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Liu, G.; Xu, L.-Q.; Chen, J.; Zhu, C.-X.; Neoh, K.-G.; Kang, E.-T. Push–Pull archetype of reduced graphene oxide functionalized with polyfluorene for nonvolatile rewritable memory. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 378–387. [Google Scholar] [CrossRef]
- Sun, S.; Zhuang, X.; Liu, B.; Wang, L.; Gu, L.; Song, S.; Zhang, B.; Chen, Y. In Situ Synthesis and Characterization of Poly(aryleneethynylene)-Grafted Reduced Graphene Oxide. Chem. Eur. J. 2016, 22, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, M.; Il’in, O.; Osotova, O.; Smirnov, V.; Ageev, O. Memristors based on strained multi-walled carbon nanotubes. Diam. Relat. Mater. 2022, 123, 108858. [Google Scholar] [CrossRef]
- Liu, G.; Ling, Q.-D.; Teo, E.; Zhu, C.-X.; Chan, D.-H.; Neoh, K.-G.; Kang, E.-T. Electrical Conductance Tuning and Bistable Switching in Poly(N-vinylcarbazole)–Carbon Nanotube Composite Films. ACS Nano 2009, 3, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Zhao, X.; Zhang, H.; Wang, S.; Wang, C.; Ma, D.; Yan, P. Bistable electrical switching and nonvolatile memory effect in poly (9,9-dioctylfluorene-2,7-diyl) and multiple-walled carbon nanotubes. Org. Electron. 2019, 74, 110–117. [Google Scholar] [CrossRef]
- Gua, M.; Zhao, Z.; Lie, J.; Liu, G.; Zhang, B.; El-Khouly, M.; Chen, Y. Conjugated polymer covalently modified multi-walled carbon nanotubes for flexible nonvolatile RRAM devices. Eur. Polym. J. 2021, 142, 110153. [Google Scholar] [CrossRef]
- Xu, G.; Wu, W.-T.; Wang, Y.; Pang, W.; Zhu, Q.; Wang, P. Functionalized carbon nanotubes with polystyrene-block-poly (N-isopropylacrylamide) by in situ RAFT polymerization. Nanotechnology 2007, 18, 145606. [Google Scholar] [CrossRef]
- Wróbel, D.; Graja, A. Photoinduced electron transfer processes in fullerene–organic chromophore systems. Coord. Chem. Rev. 2011, 255, 2555–2577. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, X.; Zhang, B.; Chen, Y. In Situ Modification of Multi-Walled Carbon Nanotubes with Polythiophene-Based Conjugated Polymer for Information Storage. Materials 2023, 16, 908. https://doi.org/10.3390/ma16030908
Li W, Wang X, Zhang B, Chen Y. In Situ Modification of Multi-Walled Carbon Nanotubes with Polythiophene-Based Conjugated Polymer for Information Storage. Materials. 2023; 16(3):908. https://doi.org/10.3390/ma16030908
Chicago/Turabian StyleLi, Wei, Xiaoyang Wang, Bin Zhang, and Yu Chen. 2023. "In Situ Modification of Multi-Walled Carbon Nanotubes with Polythiophene-Based Conjugated Polymer for Information Storage" Materials 16, no. 3: 908. https://doi.org/10.3390/ma16030908
APA StyleLi, W., Wang, X., Zhang, B., & Chen, Y. (2023). In Situ Modification of Multi-Walled Carbon Nanotubes with Polythiophene-Based Conjugated Polymer for Information Storage. Materials, 16(3), 908. https://doi.org/10.3390/ma16030908






