The Morphology, Mechanical and Dynamic Properties, Fire Hazard and Toxicity of Chloroprene and Butadiene Rubber Composites Cross-Linked with Zinc
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Sillitin Z86 (Sil) from Hoffmann Mineral GmbH, Neuburg, Germany (pH = 8.5, an average particle size of 12.7 μm);
- -
- Chalcedonite M12 (Chal) from Crusil Co, Inowlodz, Poland (pH = 6–8, an average particle size of 9 μm);
- -
- Fumed silica Aerosil®380 (Aer) from Evonik Industries AG, Essen, Germany (pH = 3.7–4.5, an average particle size of 0.07 μm).
2.2. Formation of the CR/BR Blends and Its Cured Products
2.3. Testing the Properties of the CR/BR Blends and Vulcanizates
2.3.1. Cross-Linking Characteristics
2.3.2. Analysis of the Morphology of Vulcanizates
2.3.3. Mechanical Test
2.3.4. Payne Effect
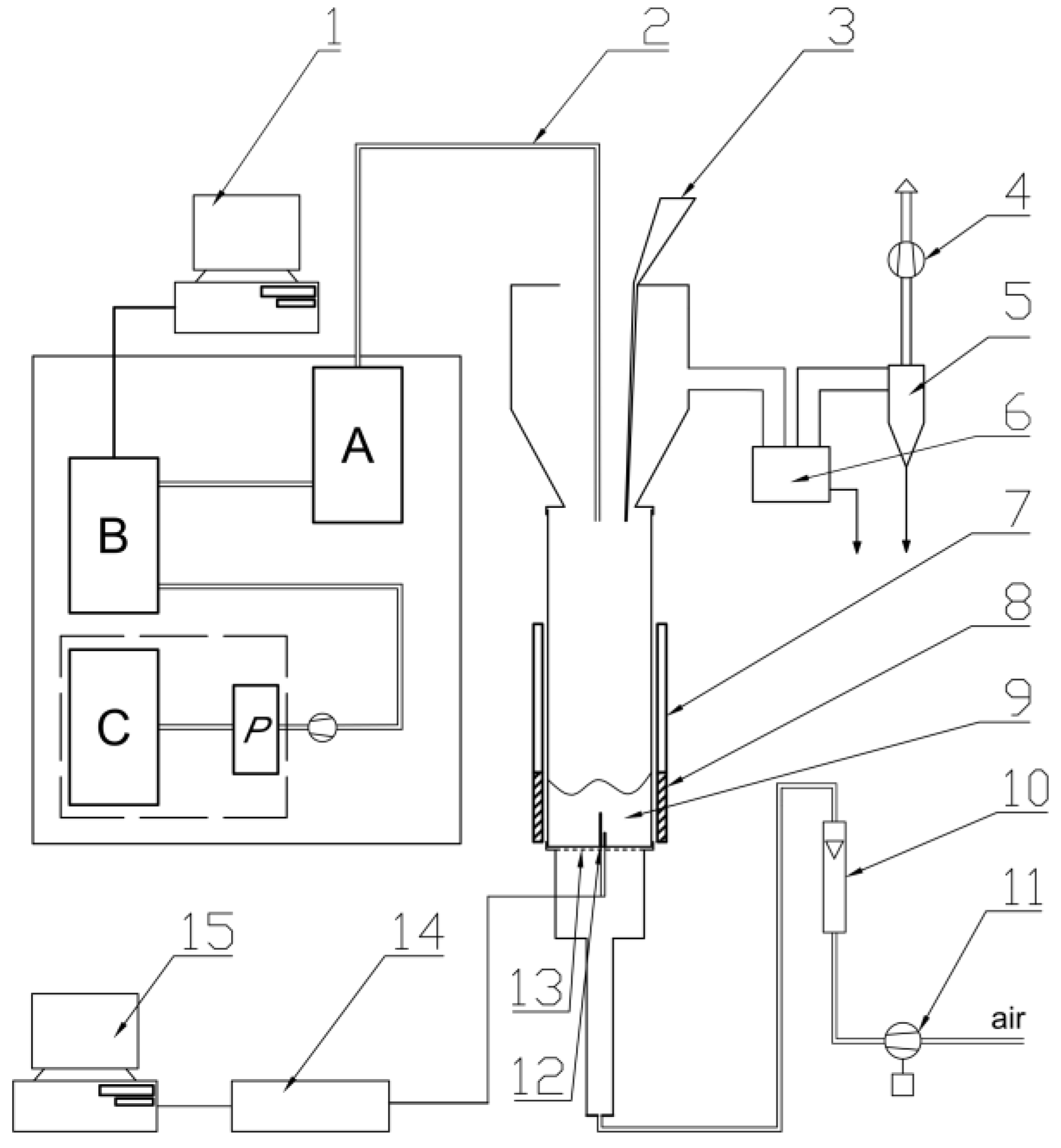
2.3.5. Flammability Tests
2.3.6. Toxicity
3. Results and Discussion
3.1. Cross-Linking Results of Unfilled and Filled CR/BR/Zn Blends
3.2. Mechanical and Dynamic Properties of Unfilled and Filled CR/BR/Zn Vulcanizates
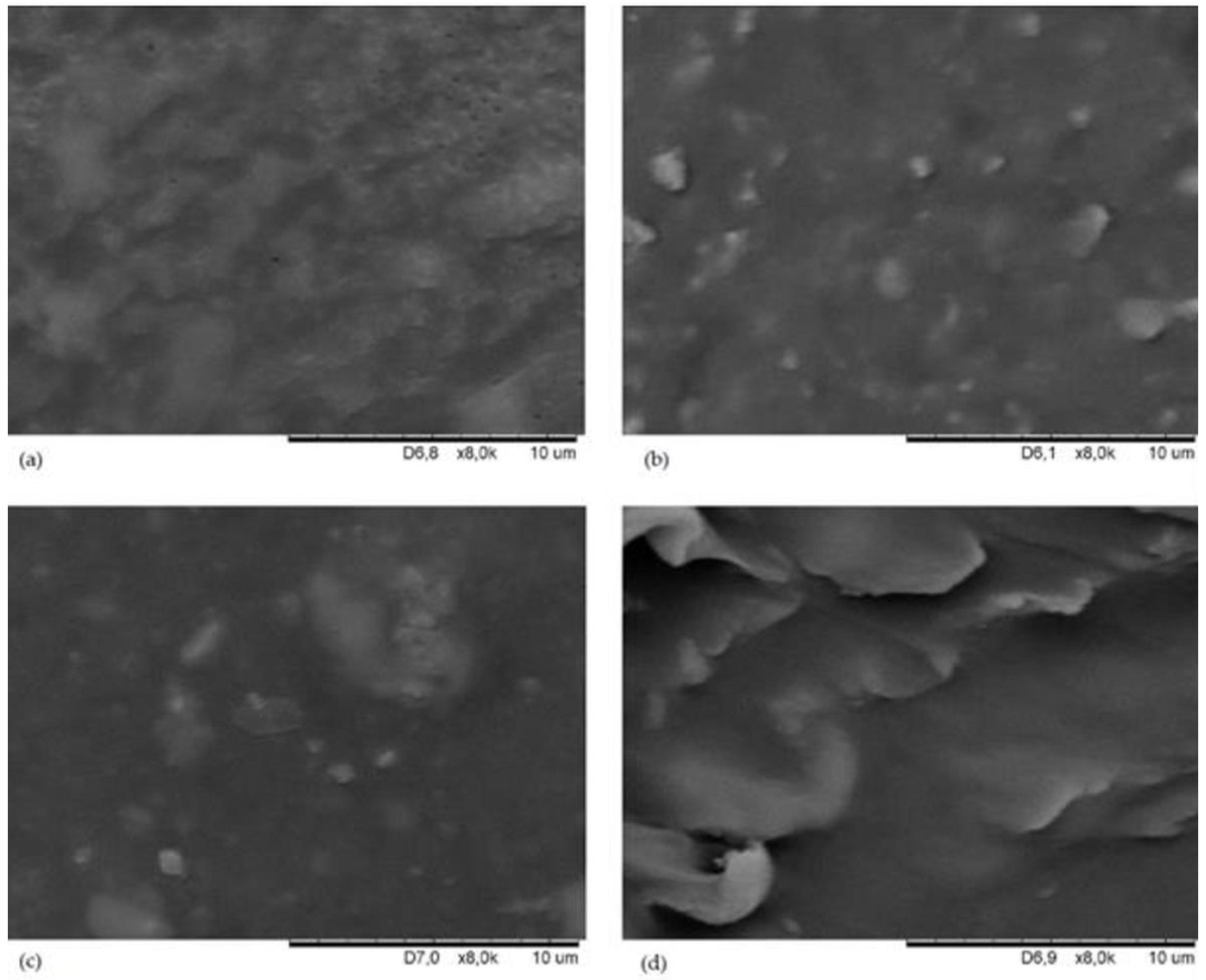
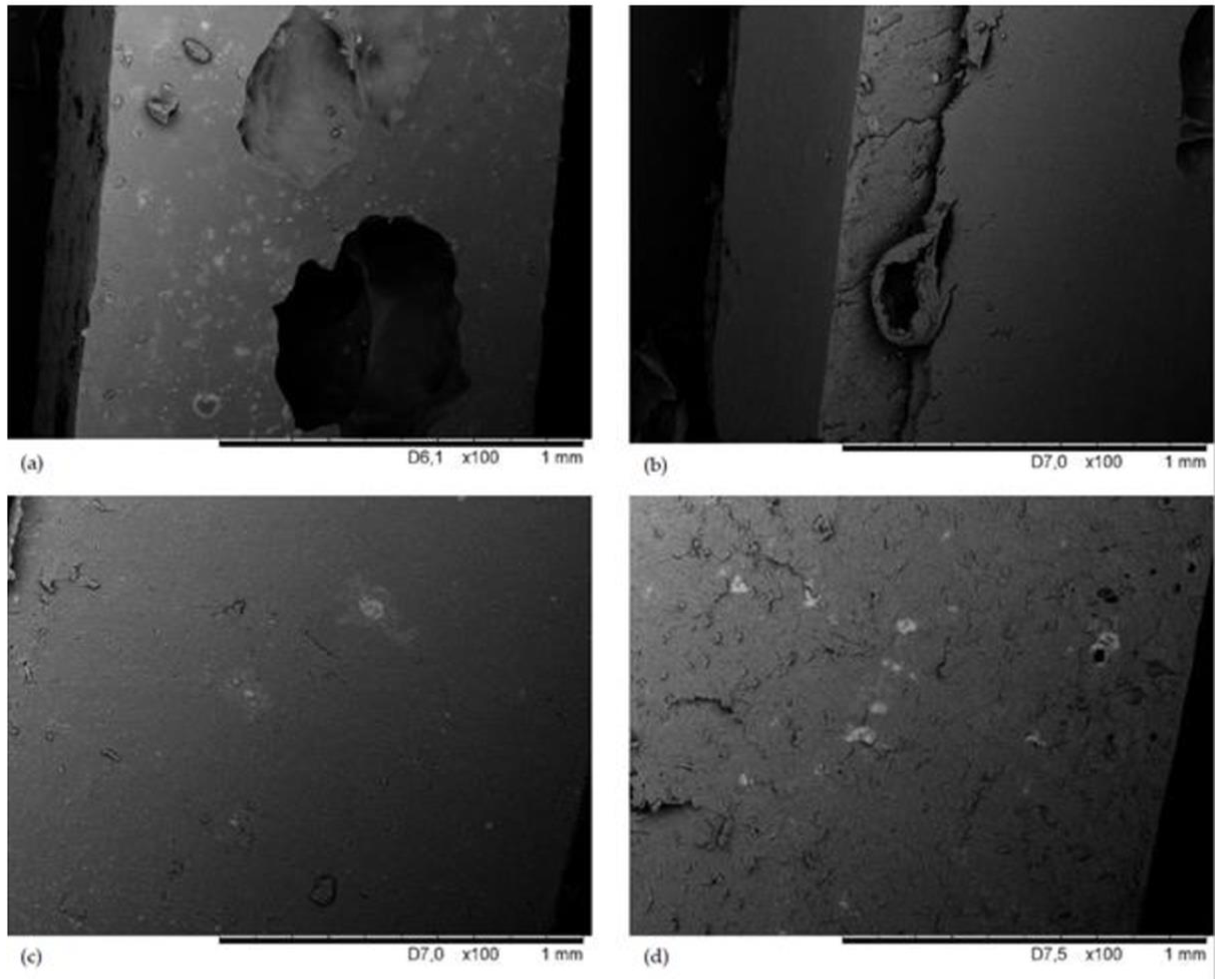
3.3. SEM Analysis of CR/BR/Zn Surface Morphology
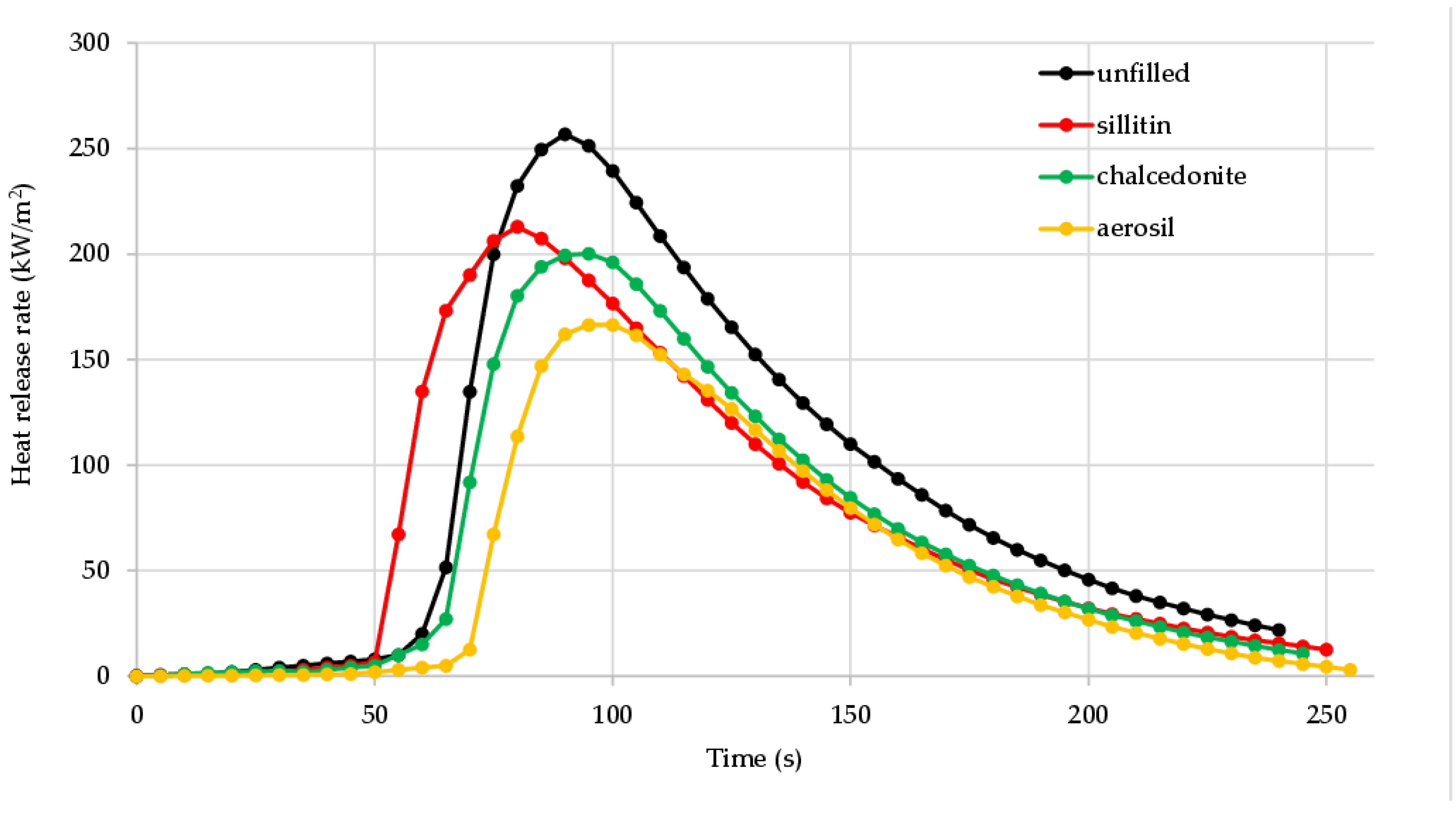
3.4. Flammability, Fire Hazard and Toxicity of Unfilled and Filled CR/BR/Zn Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mark, J.E.; Erman, B. Science and Technology of Rubber, 3rd ed.; Academic Press: Cambridge, MA, USA, 2005; ISBN 0-12-464786-3. [Google Scholar]
- Ghoreishy, M.H.R. A state-of-the-art review on the mathematical modeling and computer simulation of rubber vulcanization process. Iran. Polym. J. 2015, 25, 89–109. [Google Scholar] [CrossRef]
- Tayebi, S.; Pourkazemi, A.; Patino, N.O.; Thibaut, K.; Kamami, O.; Stiens, J. A novel approach to non-destructive rubber vulcanization monitoring by the Transient Radar Method. Sensors 2022, 22, 5010. [Google Scholar] [CrossRef] [PubMed]
- Akiba, M.; Hashim, A.S. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Rzymski, W.M.; Wolska, B. Conventional and unconventional crosslinking of elastomers. Polimery 2003, 48, 520–527. [Google Scholar] [CrossRef]
- Dogadkin, B.A. Chemia Elastomerów; WNT: Warszawa, Poland, 1976; p. 345. [Google Scholar]
- Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.M.; Venkatasubramanian, V.; Walker, K.A. Sulfur vulcanization of natural rubber for benzothiazole accelerated formulations: From reaction mechanisms to a rational kinetic mode. Rubb. Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef]
- Coran, A.Y. Chemistry of the vulcanization and protection of elastomers: A review of the achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization: Conventional and dynamic. Rubb. Chem. Technol. 1995, 68, 351–375. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds—Overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Nikolova, S.; Mihaylov, M.; Dishovsky, N. Mixed peroxide/sulfur vulcanization of ethylene-propylene terpolymer based composites, curing characteristics, curing kinetics and mechanical properties. J. Chem. Technol. Metall. 2022, 57, 881–894. [Google Scholar]
- Kruželák, J.; Hložeková, K.; Kvasničáková, A.; Tomanová, K.; Hudec, I. Application of sulfur and peroxide curing systems for cross-linking of rubber composites filled with calcium lignosulfonate. Polymers 2022, 14, 1921. [Google Scholar] [CrossRef]
- Rybiński, P.; Janowska, G. Effect of the spatial network and cross-link density of diene rubbers on their thermal stability and fire hazard. J. Therm. Anal. Calorim. 2014, 117, 377–386. [Google Scholar] [CrossRef]
- Datta, R.N.; Flexsys, B.V. Rubber Curing Systems; Rapra Technology: Shrewsbury, UK, 2001; ISBN 9781859573266. [Google Scholar]
- Monti, M.; Giannini, L.; Tadiello, L.; Guerra, S.; Papagni, A.; Vaghi, L. Thermally activable bistetrazoles for elastomers crosslinking. Polymers 2022, 14, 2919. [Google Scholar] [CrossRef]
- Alam, M.N.; Debnath, S.C.; Choi, J. Nitrosoamine-safe thiuram disulfide and benzothiazole sulfenamide as a synergistic pair of accelerators for the vulcanization of rubber. J. Polym. Res. 2021, 28, 317–326. [Google Scholar] [CrossRef]
- Jurkowski, B.; Jurkowska, B. Sporządzanie Kompozycji Polimerowych. Elementy Teorii i Praktyki; WNT: Warszawa, Poland, 1995. [Google Scholar]
- Rzymski, W.M. The mechano-chemical reactions in elastomers. Polimery 1997, 42, 450–457. [Google Scholar] [CrossRef]
- Rzymski, W.M.; Radusch, H.J. Thermoplastic elastomers manufactured of polymer blends. Polimery 2002, 47, 229–233. [Google Scholar] [CrossRef]
- Halasa, A.F.; Massie, J.M.; Ceresa, R.J. The chemical modification of polymers. In Science and Technology of Rubber, 2nd ed.; Mark, J.R., Erman, B., Eirich, F.R., Eds.; Academic Press Inc.: Cambridge, MA, USA, 1994; p. 513. [Google Scholar]
- ASTM D5289-19a; Standard Test Method for Rubber Property–Vulcanization Using Rotorless Cure Meters. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- ASTM D 471; Standard Test Method for Rubber Property. Effect of Liquids. American National Standards Institute (ANSI): Washington, DC, USA, 2016.
- Guma i Kauczuk Termoplastyczny—OZNACZANIE Właściwości Wytrzymałościowych Przy Rozciąganiu; Polski Komitet Normalizacyjny: Warsaw, Poland, 2007. Available online: https://sklep.pkn.pl/pn-iso-37-2007p.html (accessed on 14 September 2021).
- Rybiński, P.; Janowska, G.; Jóźwiak, M.; Pająk, A. Thermal properties and flammability of nanocomposites based on diene rubbers and naturally occurring and activated halloysite nanotubes. J. Therm. Anal. Calorim. 2012, 107, 1243–1249. [Google Scholar] [CrossRef]
- Janowska, G.; Kucharska-Jastrząbek, A.; Rybiński, P.; Wesołek, D.; Wójcik, I. Flammability of diene rubbers. J. Therm. Anal. Calorim. 2010, 102, 1043–1049. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Marzec, A.; Szadkowski, B.; Kuśmierek, M.; Śliwka-Kaszyńska, M.; Zakirovich Mirkhodjaev, U. Effect of basalt and carbon fillers on fire hazard, thermal and mechanical properties of EPDM rubber composites. Materials 2021, 14, 5245. [Google Scholar] [CrossRef]
- Rybiński, P.; Bradło, D.; Żukowski, W.; Syrek, B. Determination of toxic products emissions of polymers thermal decomposition using fluidised bed reactor and FTIR analysis. Polym. Test. 2019, 79, 106040. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Żukowski, W.; Bradło, D. Impact of basalt filler and ceramizable additives on the toxicity of gaseous products emitted from thermal decomposition of silicone rubber composites. Materials 2019, 12, 3478. [Google Scholar] [CrossRef]
- Rybiński, P.; Żukowski, W.; Bradło, D. Effect of cenospheric fillers on the flammability and fire hazard of silicone rubber composites. J. Therm. Anal. Calorim. 2016, 125, 1373–1386. [Google Scholar] [CrossRef]
- Juma, M.; Bafrnec, M. Experimental determination of rubber curing reaction heat using the transient heat conduction equation. Chem. Pap. 2004, 58, 29–32. [Google Scholar]
- Lopes, H.; Silva, S.S.; Carvalho, J.P.; Machado, J. A new modelling approach for predicting process evolution of cork-rubber composites slabs vulcanization. Sci. Rep. 2022, 12, 8002–8015. [Google Scholar] [CrossRef] [PubMed]
- Akbay, I.K.; Güngör, A.; Özdemir, T.T. Optymization of the vulcanization parameters for ethylene-propylene-diene termonomer (EPDM)/ground waste tyre composite using response surface methodology. Polym. Bull. 2017, 74, 5095–5109. [Google Scholar] [CrossRef]
- Janowska, G.; Rybiński, P.; Krauze, S. The effect of the curing agent type on the flammability of butadiene-acrylonitrile rubbers. Polimery 2006, 51, 735–741. [Google Scholar] [CrossRef]
- Babrauskas, V. Describing product fire performance—Manufacturers versus modelers needs. Fire Mater. 1994, 18, 289–296. [Google Scholar] [CrossRef]
| Ingredient | Ingredient Content (phr) | |||
|---|---|---|---|---|
| CR/BR | 80/20 | 80/20 | 80/20 | 80/20 |
| Stearic acid | 1 | 1 | 1 | 1 |
| Zn | 2.5 | 2.5 | 2.5 | 2.5 |
| Sillitin (Sil) | - | 30 | - | - |
| Chalcedonite (Chal) | - | - | 30 | - |
| Aerosil (Aer) | - | - | - | 30 |
| Parameter | Filler | |||
|---|---|---|---|---|
| - | Sil | Chal | Aer | |
| t02 (min) | 5.54 | 4.38 | 4.42 | 1.10 |
| t90 (min) | 31.36 | 29.26 | 29.19 | 29.18 |
| Mmin (dN·m) | 0.55 | 0.74 | 0.70 | 4.81 |
| ∆M30 (dN·m) | 6.25 | 5.86 | 6.70 | 9.19 |
| CRI (min−1) | 3.87 | 4.02 | 4.04 | 3.56 |
| Qv (mL/mL) | 8.48 | 9.21 | 8.33 | 8.15 |
| αc | 0.12 | 0.11 | 0.12 | 0.13 |
| Parameter | Filler | |||
|---|---|---|---|---|
| - | Sil | Chal | Aer | |
| Se100 (MPa) | 0.66 ± 0.01 | 0.81 ± 0.02 | 0.75 ± 0.03 | 2.72 ± 0.18 |
| Se200 (MPa) | 1.02 ± 0.02 | 1.14 ± 0.03 | 1.10 ± 0.07 | 4.04 ± 0.24 |
| Se300 (MPa) | 1.41 ± 0.04 | 1.50 ± 0.03 | 1.45 ± 0.11 | 5.23 ± 0.28 |
| TSb (MPa) | 8.56 ± 0.41 | 8.94 ± 0.33 | 9.00 ± 0.48 | 10.97 ± 0.72 |
| Eb (%) | 750 ± 50 | 762 ± 44 | 756 ± 70 | 647 ± 69 |
| HA (°Sh A) | 30.4 ± 1.2 | 39.1 ± 0.8 | 35.7 ± 0.5 | 65.3 ± 0.9 |
| Tτw (%) | 10.25 ± 0.13 | 19.88 ± 0.09 | 21.27 ± 2.43 | 29.40 ± 1.07 |
| G’max (MPa) | 0.215 | 0.223 | 0.268 | 0.423 |
| G”max (MPa) | 0.041 | 0.047 | 0.098 | 0.114 |
| ΔG’ (MPa) | 0.182 | 0.185 | 0.193 | 0.392 |
| Parameter | Filler | |||
|---|---|---|---|---|
| - | Sil | Chal | Aer | |
| tb (s) | 5 | 5 | 5 | 5 |
| OI (%) | 30.1 | 37.5 | 37.5 | 37.5 |
| TTI (s) | 55 | 39 | 52 | 61 |
| THR (MJ/m2) | 17.8 | 13.6 | 12.5 | 10.0 |
| HRR (kW/m2) | 150.73 | 132.81 | 130.33 | 117.06 |
| HRRmax (kW/m2) | 256.79 | 212.89 | 200.20 | 166.46 |
| tHRRmax (s) | 90 | 80 | 95 | 95 |
| THR (MJ/m2) | 17.8 | 13.6 | 12.5 | 10.0 |
| EHC (MJ/kg) | 8.92 | 8.06 | 7.17 | 6.59 |
| EHCmax (MJ/kg) | 79.76 | 72.01 | 66.15 | 71.77 |
| MLR (g/s) | 0.149 | 0.146 | 0.159 | 0.154 |
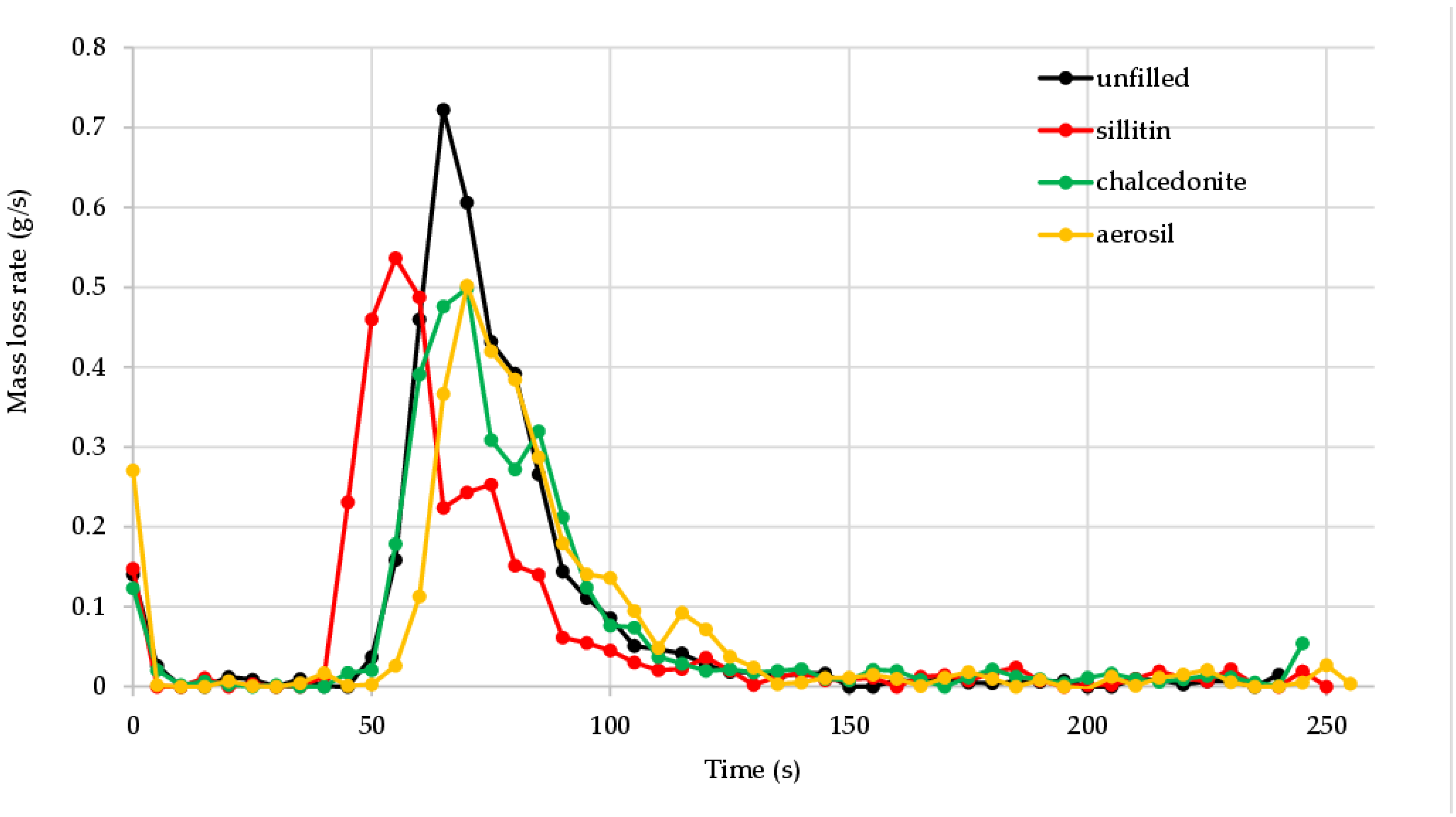
| MLRmax (g/s) | 0.722 | 0.536 | 0.498 | 0.502 |
| AMLR (g/m2·s) | 53.90 | 39.44 | 40.29 | 32.57 |
| 1/tflashover (kW/m2·s) | 4.67 | 5.46 | 3.85 | 2.73 |
| FIGRA (kW/m2·s) | 2.85 | 2.66 | 2.10 | 1.75 |
| MARHE (kW/m2) | 106.1 | 97.5 | 83.6 | 67.1 |
| Filler | T (°C) | Emission (g/g) | |||||
|---|---|---|---|---|---|---|---|
| CO2 | CO | NO2 | SO2 | HCl | HCN | ||
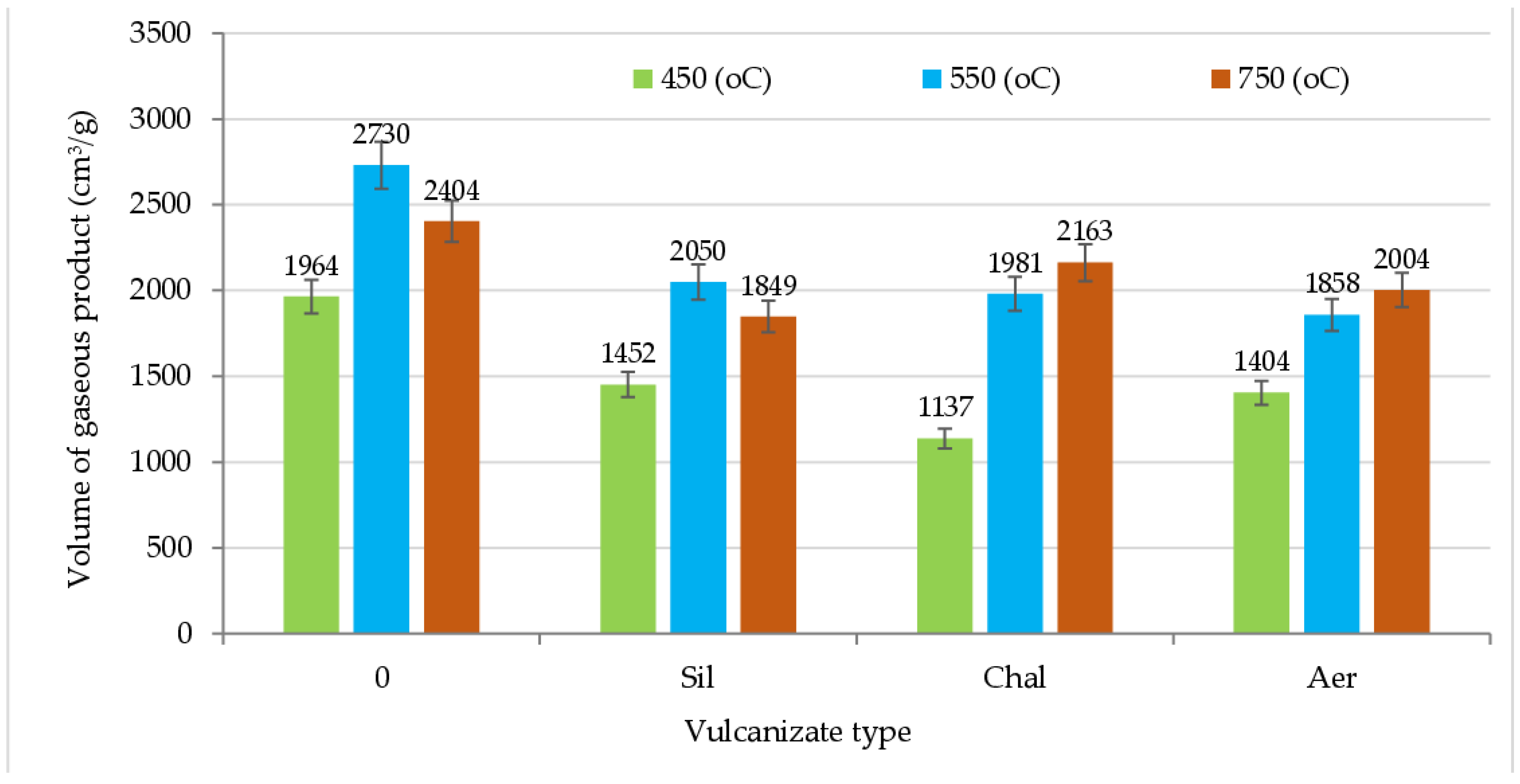
| - | 450 | 1.88 ± 0.16 | 0.27 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.15 ± 0.04 | 0.00 ± 0.00 |
| 550 | 2.49 ± 0.27 | 0.40 ± 0.08 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.20 ± 0.02 | 0.00 ± 0.00 | |
| 750 | 2.84 ± 0.23 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.16 ± 0.01 | 0.00 ± 0.00 | |
| Sil | 450 | 1.28 ± 0.20 | 0.26 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.13 ± 0.03 | 0.00 ± 0.00 |
| 550 | 1.78 ± 0.17 | 0.36 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.02 | 0.00 ± 0.00 | |
| 750 | 2.24 ± 0.31 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.03 | 0.00 ± 0.00 | |
| Chal | 450 | 1.11 ± 0.25 | 0.20 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.10 ± 0.02 | 0.00 ± 0.00 |
| 550 | 1.75 ± 0.12 | 0.36 ± 0.04 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.01 | 0.00 ± 0.00 | |
| 750 | 2.53 ± 0.10 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.15 ± 0.01 | 0.00 ± 0.00 | |
| Aer | 450 | 1.26 ± 0.16 | 0.27 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.12 ± 0.01 | 0.00 ± 0.00 |
| 550 | 1.50 ± 0.15 | 0.39 ± 0.07 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.13 ± 0.02 | 0.00 ± 0.00 | |
| 750 | 2.36 ± 0.17 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.01 | 0.00 ± 0.00 | |
| Filler | T (°C) | WLC50 (g/m3) | WLC50M | WLC50SM | |||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 | CO | NO2 | SO2 | HCl | HCN | ||||
| - | 450 | 105 ± 9 | 14 ± 1 | 0 ± 0 | 92 ± 15 | 7 ± 2 | 321 ± 44 | 4.2 ± 0.8 | 4.15 |
| 550 | 79 ± 9 | 10 ± 2 | 0 ± 0 | 66 ± 8 | 5 ± 0 | 0 ± 0 | 3.0 ± 0.3 | ||
| 750 | 69 ± 6 | 645 ± 618 | 0 ± 0 | 83 ± 16 | 6 ± 0 | 0 ± 0 | 5.2 ± 0.3 | ||
| Sil | 450 | 155 ± 23 | 14 ± 1 | 0 ± 0 | 123 ± 29 | 8 ± 2 | 343 ± 61 | 4.7 ± 0.8 | 4.89 |
| 550 | 111 ± 11 | 10 ± 0 | 0 ± 0 | 95 ± 16 | 7 ± 1 | 0 ± 0 | 3.8 ± 0.3 | ||
| 750 | 89 ± 13 | 1087 ± 640 | 0 ± 0 | 115 ± 17 | 7 ± 1 | 0 ± 0 | 6.2 ± 1.2 | ||
| Chal | 450 | 179 ± 42 | 19 ± 3 | 0 ± 0 | 156 ± 12 | 10 ± 2 | 962 ± 108 | 6.0 ± 0.9 | 5.16 |
| 550 | 113 ± 8 | 11 ± 1 | 0 ± 0 | 88 ± 20 | 7 ± 0 | 0 ± 0 | 3.8 ± 0.3 | ||
| 750 | 78 ± 3 | 1919 ± 2044 | 0 ± 0 | 118 ± 12 | 6 ± 0 | 0 ± 0 | 5.6 ± 0.4 | ||
| Aer | 450 | 156 ± 19 | 14 ± 1 | 0 ± 0 | 124 ± 17 | 8 ± 1 | 540 ± 73 | 4.8 ± 0.2 | 4.96 |
| 550 | 132 ± 14 | 10 ± 2 | 0 ± 0 | 97 ± 25 | 8 ± 1 | 0 ± 0 | 4.0 ± 0.7 | ||
| 750 | 84 ± 6 | 1195 ± 1296 | 0 ± 0 | 117 ± 19 | 7 ± 0 | 0 ± 0 | 6.1 ± 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smejda-Krzewicka, A.; Rybiński, P.; Bradło, D.; Żukowski, W. The Morphology, Mechanical and Dynamic Properties, Fire Hazard and Toxicity of Chloroprene and Butadiene Rubber Composites Cross-Linked with Zinc. Materials 2023, 16, 1240. https://doi.org/10.3390/ma16031240
Smejda-Krzewicka A, Rybiński P, Bradło D, Żukowski W. The Morphology, Mechanical and Dynamic Properties, Fire Hazard and Toxicity of Chloroprene and Butadiene Rubber Composites Cross-Linked with Zinc. Materials. 2023; 16(3):1240. https://doi.org/10.3390/ma16031240
Chicago/Turabian StyleSmejda-Krzewicka, Aleksandra, Przemysław Rybiński, Dariusz Bradło, and Witold Żukowski. 2023. "The Morphology, Mechanical and Dynamic Properties, Fire Hazard and Toxicity of Chloroprene and Butadiene Rubber Composites Cross-Linked with Zinc" Materials 16, no. 3: 1240. https://doi.org/10.3390/ma16031240
APA StyleSmejda-Krzewicka, A., Rybiński, P., Bradło, D., & Żukowski, W. (2023). The Morphology, Mechanical and Dynamic Properties, Fire Hazard and Toxicity of Chloroprene and Butadiene Rubber Composites Cross-Linked with Zinc. Materials, 16(3), 1240. https://doi.org/10.3390/ma16031240








