Dispersion and Polishing Mechanism of a Novel CeO2-LaOF-Based Chemical Mechanical Polishing Slurry for Quartz Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Configuration of Polishing Slurry
2.3. CMP Tests
2.4. Characterization
3. Results and Discussion
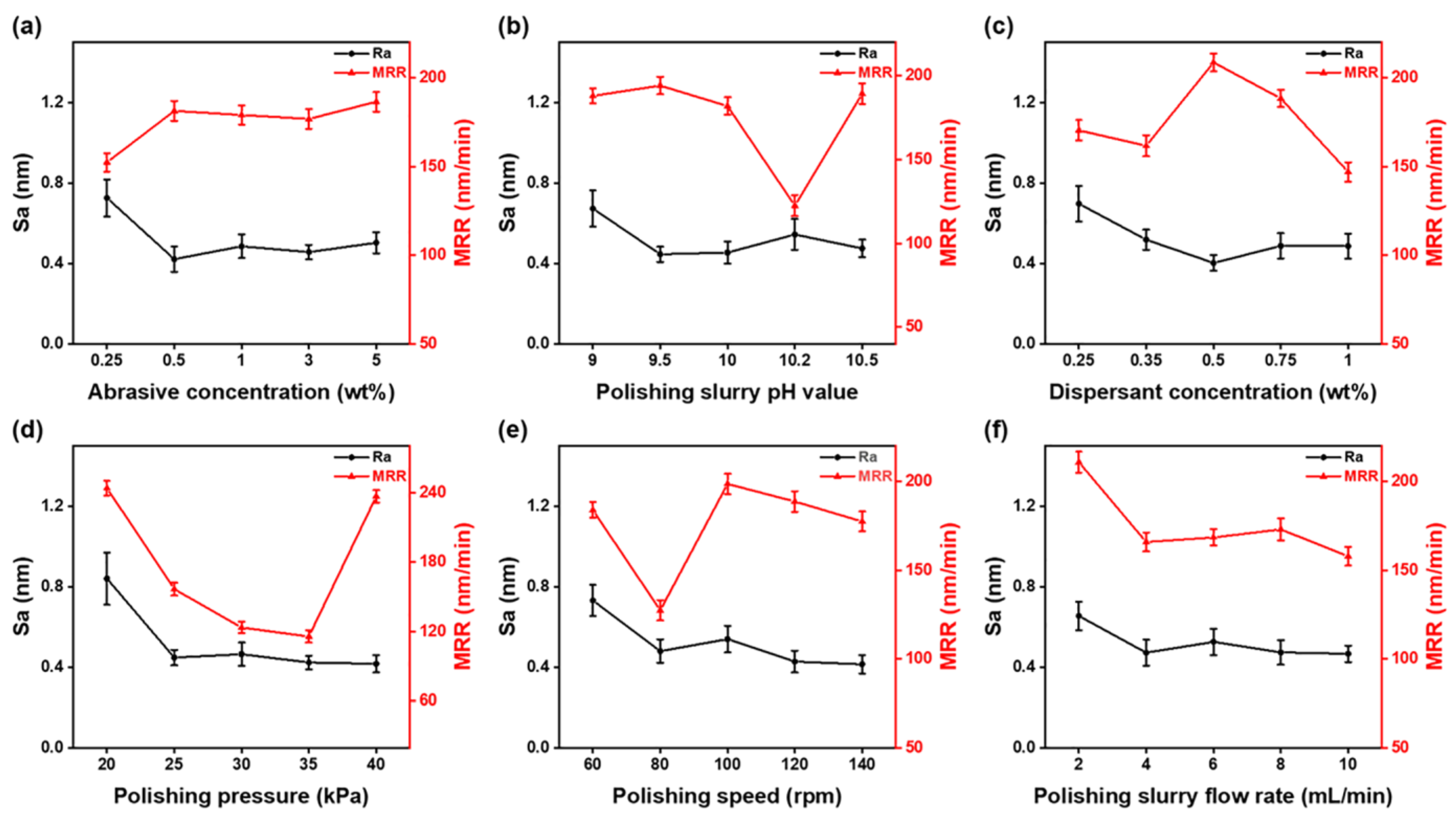
3.1. Quartz Glass Orthogonal Experiments
3.2. Polishing Slurry Dispersibility Experiment
3.3. Investigated the Mechanical Chemical Polishing Mechanism of Quartz Glass
4. Conclusions
- Orthogonal testing was used to achieve the best polishing solution formulation for polishing quartz glass. The MRR of the polishing process was 530.52 nm/min, which was higher than the value reported previously. It was possible to achieve a surface roughness of 0.23 nm in the range of 50 × 50 μm2, which was less than the current 0.9 nm reported for commercial abrasives. All cracks and the subsurface damage layer were removed from the surface, leaving a 4.5 nm densified mesh structure.
- Particle size, zeta potential, UV absorbance, and viscosity were tested and analyzed for the polishing slurry before and after dispersion. The polishing slurry with the optimal combination from the orthogonal test had a more uniform particle size, a larger zeta potential, a higher UV absorbance, and a slightly higher viscosity than the untreated polishing slurry, showing that the dispersion is a very important factor for the polishing slurry.
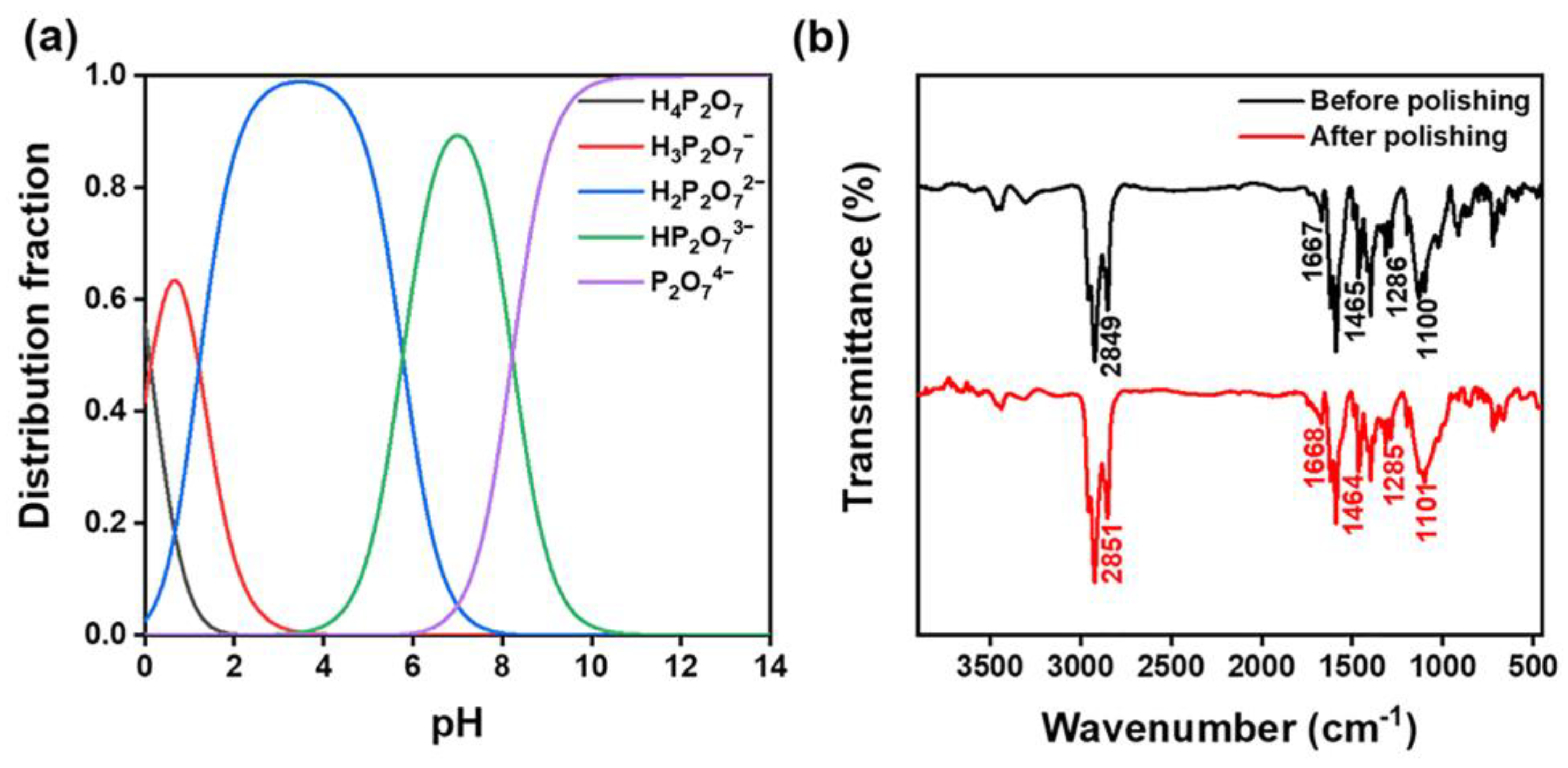
- XPS and FTIR were used to analyze the polishing mechanism. It was discovered that K4P2O7 continually hydrolyzed in water to release the hydroxyl groups, forming an alkali-polishing environment. Then, SiO2 forms an attenuated layer of Si-OH groups. Due to their high electronegativity, anionic dispersants that are added to the SNLS and PAAS will then form chelate products with them via their polar headgroups. Finally, CeO2 and LaOF abrasives removed the softened layer to obtain quartz glass sample pieces with smooth surfaces.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | A | B | C | D | E | F | Sa | MRR |
|---|---|---|---|---|---|---|---|---|
| 1 | 1(0.25) | 1(9) | 1(0.25) | 1(20) | 1(60) | 1(2) | 1.73 | 273.30 |
| 2 | 1 | 2(9.5) | 3 | 4 | 5 | 2(4) | 0.29 | 138.26 |
| 3 | 1 | 3(10) | 5 | 2(25) | 4 | 3(6) | 0.48 | 118.96 |
| 4 | 1 | 4(10.2) | 2(0.35) | 5 | 3 | 4(8) | 0.63 | 168.80 |
| 5 | 1 | 5(10.5) | 4 | 3(30) | 2(80) | 5(10) | 0.51 | 62.70 |
| 6 | 2(0.5) | 1 | 5 | 4(35) | 3(100) | 5 | 0.42 | 112.53 |
| 7 | 2 | 2 | 2 | 2 | 2 | 1 | 0.38 | 155.94 |
| 8 | 2 | 3 | 4 | 5(40) | 1 | 2 | 0.39 | 262.04 |
| 9 | 2 | 4 | 1 | 3 | 5 | 3 | 0.47 | 67.52 |
| 10 | 2 | 5 | 3(0.5) | 1 | 4(120) | 4 | 0.45 | 308.66 |
| 11 | 3(1) | 1 | 4(0.75) | 2 | 5(140) | 4 | 0.39 | 186.48 |
| 12 | 3 | 2 | 1 | 5 | 4 | 5 | 0.35 | 250.79 |
| 13 | 3 | 3 | 3 | 3 | 3 | 1 | 0.41 | 226.67 |
| 14 | 3 | 4 | 5(1) | 1 | 2 | 2 | 0.72 | 109.32 |
| 15 | 3 | 5 | 2 | 4 | 1 | 3 | 0.57 | 122.18 |
| 16 | 4(3) | 1 | 3 | 5 | 2 | 3 | 0.36 | 229.89 |
| 17 | 4 | 2 | 5 | 3 | 1 | 4 | 0.47 | 122.18 |
| 18 | 4 | 3 | 2 | 1 | 5 | 5 | 0.56 | 223.46 |
| 19 | 4 | 4 | 4 | 4 | 4 | 1 | 0.40 | 127.00 |
| 20 | 4 | 5 | 1 | 2 | 3 | 2 | 0.50 | 181.66 |
| 21 | 5(5) | 1 | 2 | 3 | 4 | 2 | 0.47 | 138.26 |
| 22 | 5 | 2 | 4 | 1 | 3 | 3 | 0.75 | 303.84 |
| 23 | 5 | 3 | 1 | 4 | 2 | 4 | 0.44 | 78.77 |
| 24 | 5 | 4 | 3 | 2 | 1 | 5 | 0.50 | 139.86 |
| 25 | 5 | 5 | 5 | 5 | 5 | 1 | 0.36 | 271.69 |
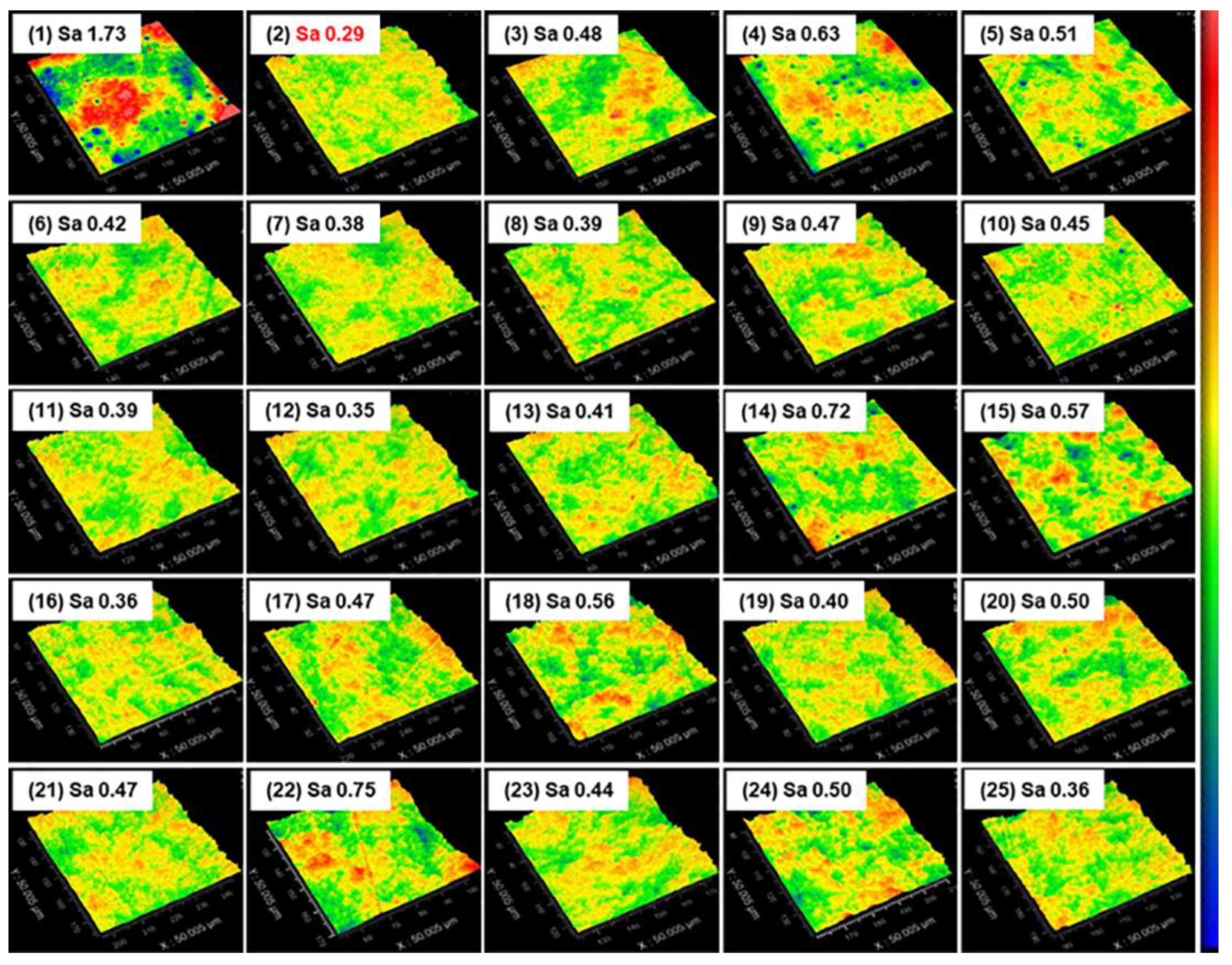
| Factors | Sa (nm) | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| kj1 | 0.73 | 0.67 | 0.70 | 0.84 | 0.73 | 0.65 |
| kj2 | 0.42 | 0.45 | 0.52 | 0.45 | 0.48 | 0.47 |
| kj3 | 0.49 | 0.45 | 0.40 | 0.46 | 0.54 | 0.53 |
| kj4 | 0.46 | 0.54 | 0.49 | 0.42 | 0.43 | 0.47 |
| kj5 | 0.50 | 0.47 | 0.49 | 0.42 | 0.41 | 0.47 |
| Rj | 0.31 | 0.23 | 0.29 | 0.42 | 0.32 | 0.19 |
| Factor Priorities | D > E > A > C > B > F | |||||
| Superior level | A2 B2 C3 D5 E5 F5 | |||||
| Factors | MRR (nm/min) | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| kj1 | 152.40 | 188.09 | 170.41 | 243.72 | 183.91 | 210.92 |
| kj2 | 181.34 | 194.20 | 161.73 | 156.58 | 127.32 | 165.91 |
| kj3 | 179.09 | 181.98 | 208.67 | 123.47 | 198.70 | 168.48 |
| kj4 | 176.84 | 122.50 | 188.41 | 115.75 | 188.73 | 172.98 |
| kj5 | 186.48 | 189.38 | 146.94 | 236.64 | 177.48 | 157.87 |
| Rj | 34.08 | 71.70 | 61.73 | 127.97 | 71.38 | 53.05 |
| Factor Priorities | D > B > E > C > F > A | |||||
| Superior level | A5 B2 C3 D1 E3 F1 | |||||
References
- Kumar, A.; Marcolli, C.; Peter, T. Ice nucleation activity of silicates and aluminosilicates in pure water and aqueous solutions–Part 2: Quartz and amorphous silica. Atmos. Chem. Phys. 2019, 19, 6035–6058. [Google Scholar] [CrossRef]
- Krishnan, M.; Nalaskowski, J.W.; Cook, L.M. Chemical Mechanical Planarization: Slurry Chemistry, Materials, and Mechanisms. Chem. Rev. 2010, 110, 178–204. [Google Scholar] [CrossRef]
- Rampf, M.; Fisch, M.; Helsch, G.; Deubener, J.; Ritzberger, C.; Hland, W.; Dittmer, M. Quartz-containing glass-ceramics in the SiO2-Li2O-K2O-MgO-CaO-Al2O3-P2O5 system. Int. J. Appl. Glass Sci. 2019, 10, 330–338. [Google Scholar] [CrossRef]
- Suratwala, T.; Steele, R.; Feit, M.D.; Wong, L.; Miller, P.; Menapace, J.; Davis, P. Effect of rogue particles on the sub-surface damage of fused silica during grinding/polishing. J. Non-Cryst. Solids 2008, 354, 2023–2037. [Google Scholar] [CrossRef]
- Yang, X.-l.; Su, J. Progress of magnetorheological finishing. J. Wuhan Univ. Technol. 2010, 32, 136–139. [Google Scholar]
- Xiu, S.C.; Wang, R.S.; Sun, B.W.; Ma, L.; Song, W.L. Preparation and experiment of magnetorheological polishing fluid in reciprocating magnetorheological polishing process. J. Intell. Mater. Syst. Struct. 2018, 29, 125–136. [Google Scholar] [CrossRef]
- Fang, H.; Guo, p.; Yu, J. Research on material removal mechanism of fluid jet polishing. Opt. Technol. 2004, 30, 248–250. [Google Scholar]
- Lu, Y.; Xie, X.H.; Zhou, L. Design and performance analysis of an ultraprecision ion beam polishing tool. Appl. Opt. 2016, 55, 1544–1550. [Google Scholar] [CrossRef]
- Zheng, G.C.; Chen, Q.C.; Nie, J.W.; Li, M.J.; Chen, M.Y. Characterization of plasma polished quartz glass. Vacuum. 2020, 57, 72–76. [Google Scholar]
- Zhang, K.-L.; Song, Z.-T.; Zhang, J.-X.; Tan, B.-M.; Liu, Y.-L. Study on preparation and application of silica sol nano-abrasive with large particle for CMP of dielectric in ULSI. Chin. J. Electron Devices 2004, 27, 556–563. [Google Scholar]
- Xiaolan, S.; Yukun, L.I.; Nan, J.; Yixin, Q.U.; Guanzhou, O.I.U. Recent development of chemical mechanical polishing. Chem. Ind. Eng. Prog. 2008, 27, 26–31. [Google Scholar]
- Seo, E.B.; Park, J.G.; Bae, J.Y.; Park, J.H. Highly Selective Polishing Rate Between a Tungsten Film and a Silicon-Dioxide Film by Using a Malic-Acid Selectivity Agent in Tungsten-Film Chemical-Mechanical Planarization. J. Korean Phys. Soc. 2020, 76, 1127–1132. [Google Scholar] [CrossRef]
- Hoshino, T.; Kurata, Y.; Terasaki, Y.; Susa, K. Mechanism of polishing of SiO2 films by CeO2 particles. J. Non-Cryst. Solids 2001, 283, 129–136. [Google Scholar] [CrossRef]
- Evans, C.J.; Paul, E.; Dornfeld, D.; Lucca, D.A.; Byrne, G.; Tricard, M.; Klocke, F.; Dambon, O.; Mullany, B.A. Material Removal Mechanisms in Lapping and Polishing. CIRP Ann. 2003, 52, 611–633. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Kurokawa, S.; Toyama, T.; Hayashi, T. CMP characteristics of quartz glass substrate by aggregated colloidal ceria slurry. Precis. Eng.-J. Int. Soc. Precis. Eng. Nanotechnol. 2019, 60, 458–464. [Google Scholar] [CrossRef]
- Yuan, X.C.; Li, Q.Z.; Yang, S.Y. Development of polishing solution for ultrasonic fine atomization polishing of quartz glass. Lubr. Seal. 2021, 46, 103–108. [Google Scholar]
- Shi, X.L.; Chen, G.P.; Xu, L.; Kang, C.X.; Luo, G.H.; Luo, H.M.; Zhou, Y.; Dargusch, M.S.; Pan, G.S. Achieving ultralow surface roughness and high material removal rate in fused silica via a novel acid SiO2 slurry and its chemical-mechanical polishing mechanism. Appl. Surf. Sci. 2020, 500, 8. [Google Scholar] [CrossRef]
- Tang, Y.T.; Yang, C.C. Study of chemical polishing process of quartz glass sheet. Opt. Optoelectron. Technol. 2022, 20, 159–164. [Google Scholar]
- Guang-Lin, C.; De-Fu, L.; Tao, C.; Yi-Xi, S.J.S.T. Dispersion Stability of CeO2 Nano Particles Polishing Agent and Its Properties in Chemical Mechanical Polishing Process. Surf. Technol. 2016, 45, 187–193. [Google Scholar]
- Pei, W.; Zhao, D.; Chen, X.; Wang, X.; Yang, X.; Wang, J.; Li, Z.; Zhou, L. Evolution of the phases and the polishing performance of ceria-based compounds synthesized by a facile calcination method. RSC Adv. 2019, 9, 26996–27001. [Google Scholar] [CrossRef]
- Shinn, D.B.; Eick, H.A. Phase analyses of lanthanide oxide fluorides. Inorg. Chem. 1969, 8, 232–235. [Google Scholar] [CrossRef]
- Niihara, K.; Yajima, S. Studies of Rare Earth Oxyfluorides in the High-temperature Region. Bull. Chem. Soc. Jpn. 1972, 45, 20–23. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, S.; Huang, J.; Chen, C.; Kang, R.; Jin, Z.; Guo, D. Effects of pressure and slurry on removal mechanism during the chemical mechanical polishing of quartz glass using ReaxFF MD. Appl. Surf. Sci. 2020, 505, 144610. [Google Scholar] [CrossRef]
- Basim, G.B.; Adler, J.J. Effect of Particle Size of Chemical Mechanical Polishing Slurries for Enhanced Polishing with Minimal Defects. J. Electrochem. Soc. 2000, 147, 3523. [Google Scholar] [CrossRef]
- Ramarajan, S.; Li, Y.; Hariharaputhiran, M.; Her, Y.; Babu, S.V. Effect of pH and Ionic Strength on Chemical Mechanical Polishing of Tantalum. J. Gerontol. 2000, 56, M328–M340. [Google Scholar] [CrossRef]
- Lin, Z.C.; Ho, C.Y. Analysis and application of grey relation and ANOVA in chemical-mechanical polishing process parameters. Int. J. Adv. Manuf. Technol. 2003, 21, 10–14. [Google Scholar]
- Luo, J.F.; Dornfeld, D.A. Effects of abrasive size distribution in chemical mechanical planarization: Modeling and verification. IEEE Trans. Semicond. Manuf. 2003, 16, 469–476. [Google Scholar]
- Park, K.H.; Kim, H.J.; Chang, O.M.; Jeong, H.D. Effects of pad properties on material removal in chemical mechanical polishing. J. Mater. Process. Technol. 2007, 187, 73–76. [Google Scholar] [CrossRef]
- Guo, X.G.; Huang, J.X.; Yuan, S.; Chen, C.; Jin, Z.J.; Kang, R.K.; Guo, D.M. Effect of surface hydroxylation on ultra-precision machining of quartz glass. Appl. Surf. Sci. 2020, 501, 10. [Google Scholar] [CrossRef]
- Zhai, J.; Ni, Z.F.; Li, Q.Z. An Alkaline SiO2 Slurry for Fine Atomizing CMP. In Proceedings of the International Conference on Mechanics, Solid State and Engineering Materials (ICMSSEM), Hangzhou, China, 1–2 September 2011; Trans Tech Publications Ltd.: Hangzhou, China, 2011; pp. 258–261. [Google Scholar]
- Cao, M.C.; Zhao, H.Y.; Xie, R.Q.; Zhao, L.Y.; Zhao, S.J.; Bai, J.F. Multiparameter optimization design of chemical mechanical polishing for planar optics. Int. J. Adv. Manuf. Technol. 2021, 113, 2153–2162. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Yuan, J.L.; Lv, B.H.; Zheng, J.J. Study on Pad Conditioning Parameters in Silicon Wafer CMP process. In Proceedings of the 14th Conference of Abrasive Technology in China, Nanjing, China, 26–28 October 2007; Trans Tech Publications Ltd.: Nanjing, China, 2008; pp. 309–313. [Google Scholar]
- Lv, W.; Wu, Y.S.; Shi, Y.C.; Li, J.G. Effect of ammonium polyacrylate adsorption on the stability and rheology of TiO2 suspensions. J. Shandong Univ. 2003, 4, 353–356. [Google Scholar]
- Tsai, M.S. The study of formation colloidal silica via sodium silicate. Mater. Sci. Eng. B 2004, 106, 52–55. [Google Scholar] [CrossRef]
- Li, D.H.; Guo, L.C. Study on the dispersion stability of ultrafine alumina aqueous suspensions. Silic. Bull. 2005, 1, 36–40. [Google Scholar]
- Song, X.L.; Wu, X.L.; Qu, P.; Wang, H.B.; Qiu, G.Z. Study on the factors influencing the dispersion stability performance of nano-SiO2 and the mechanism of action. Silic. Bull. 2005, 1, 3–7. [Google Scholar]
- Zhou, X.M.; Li, B.W.; Li, Y.X.; Xu, X.L. Study on surface electrical properties and suspension dispersion stability of high cerium polishing powder. Rare Earths 2007, 1, 12–16. [Google Scholar]
- Hua, C.X. Study on the Flow Characteristics of Polishing Solution in Chemical Mechanical Polishing. Master’s Thesis, Nanjing University of Aeronautics and Astronautics, Nanjing, China, 2017. [Google Scholar]
- Li, H.; Li, S.F. Synthesis study of sodium N-lauroyl sarcosinate. J. Xiamen Univ. 2005, 3, 386–389. [Google Scholar]
- Li, S.F.; Li, H. Corrosion inhibition properties of sodium N-lauroyl sarcosinate and its complexes. Corros. Sci. Prot. Technol. 2008, 4, 289–291. [Google Scholar]
- Cui, J. Synthesis and Properties of Sodium N-Lauroyl Sarcosinate. Master’s Thesis, Jiangnan University, Wuxi, China, 2018. [Google Scholar]
- Tao, L.M.; Wang, J.J.; Zhang, W.J.; Jiang, Z.Y.; Sun, W.; Gao, Z.Y.; Wang, C.; Wu, S.H.; Hu, Y. Selective separation of fluorite and calcite by sodium lauroyl sarcosinate and its mechanism of action. Chin. J. Nonferrous Met. 2022, 32, 1810–1820. [Google Scholar]
- Niu, Y.X.; Wang, Y.; Wang, E.D.; Fu, J.F. Effect of sodium polyacrylate on the stability performance of nano-SiO2 dispersion. J. Northeast. Univ. 2008, 11, 1641–1644. [Google Scholar]
- Liu, Y.L. Effect of dispersant on the stability of ultrafine CeO2 polishing solution. Diam. Abras. Eng. 2011, 31, 59–62. [Google Scholar]
- Wang, W.; Zhang, B.; Shi, Y.; Zhou, D.; Wang, R. Improvement in dispersion stability of alumina suspensions and corresponding chemical mechanical polishing performance. Appl. Surf. Sci. 2022, 597, 153703. [Google Scholar] [CrossRef]
- Chu, Y. Production and analytical studies of potassium pyrophosphate for foodstuffs. China Water Transp. 2007, 12, 85–87. [Google Scholar]
- Wang, M.M.; Lv, W.Y.; Yao, K.; Ding, L.; Wang, Y.H.; Wang, P.P. Leaching remediation of copper-contaminated soil with potassium pyrophosphate. J. Environ. Eng. 2017, 11, 1211–1216. [Google Scholar]
- de Levie, R. The Henderson-Hasselbalch equation: Its history and limitations. J. Chem. Educ. 2003, 80, 146. [Google Scholar] [CrossRef]
- Morgan, W.E.; Van Wazer, J.R. Binding energy shifts in the x-ray photoelectron spectra of a series of related Group IVa compounds. J. Phys. Chem. 1973, 77, 964–969. [Google Scholar] [CrossRef]
- Andersson, S.L.T.; Scurrell, M.S. Infrared And Esca Studies Of A Heterogenized Rhodium Carbonylation Catalyst. J. Catal. 1979, 59, 340–356. [Google Scholar]
- Chen, J.R.; Chao, H.Y.; Lin, Y.L.; Yang, I.J.; Oung, J.C.; Pan, F.M. Studies on Carbon-Steel Corrosion in Molybdate and Silicate Solutions as Corrosion-Inhibitors. Surf. Sci. 1991, 247, 352–359. [Google Scholar] [CrossRef]
- Williams, C.T.; Takoudis, C.G.; Weaver, M.J. Methanol oxidation on rhodium as probed by surface-enhanced Raman and mass spectroscopies: Adsorbate stability, reactivity, and catalytic relevance. J. Phys. Chem. B 1998, 102, 406–416. [Google Scholar] [CrossRef]
- Cardinaud, C.; Turban, G. Mechanistic Studies of the Initial-Stages of Etching of Si and SiO2 in A Chf3 Plasma. Appl. Surf. Sci. 1990, 45, 109–120. [Google Scholar] [CrossRef]
- Cros, A.; Saoudi, R.; Hollinger, G.; Hewett, C.A.; Lau, S.S. An x-ray photoemission spectroscopy investigation of oxides grown on Au x Si 1 x layers. J. Appl. Phys. 1990, 67, 1826–1830. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, C.W.; Qu, Q.; Tong, J.Y.; Cao, C.N. Study on the protection of TiO2-K2SiO3 inorganic coatings for Ag used in space. Acta Chim. Sin. 2003, 61, 1369–1374. [Google Scholar]
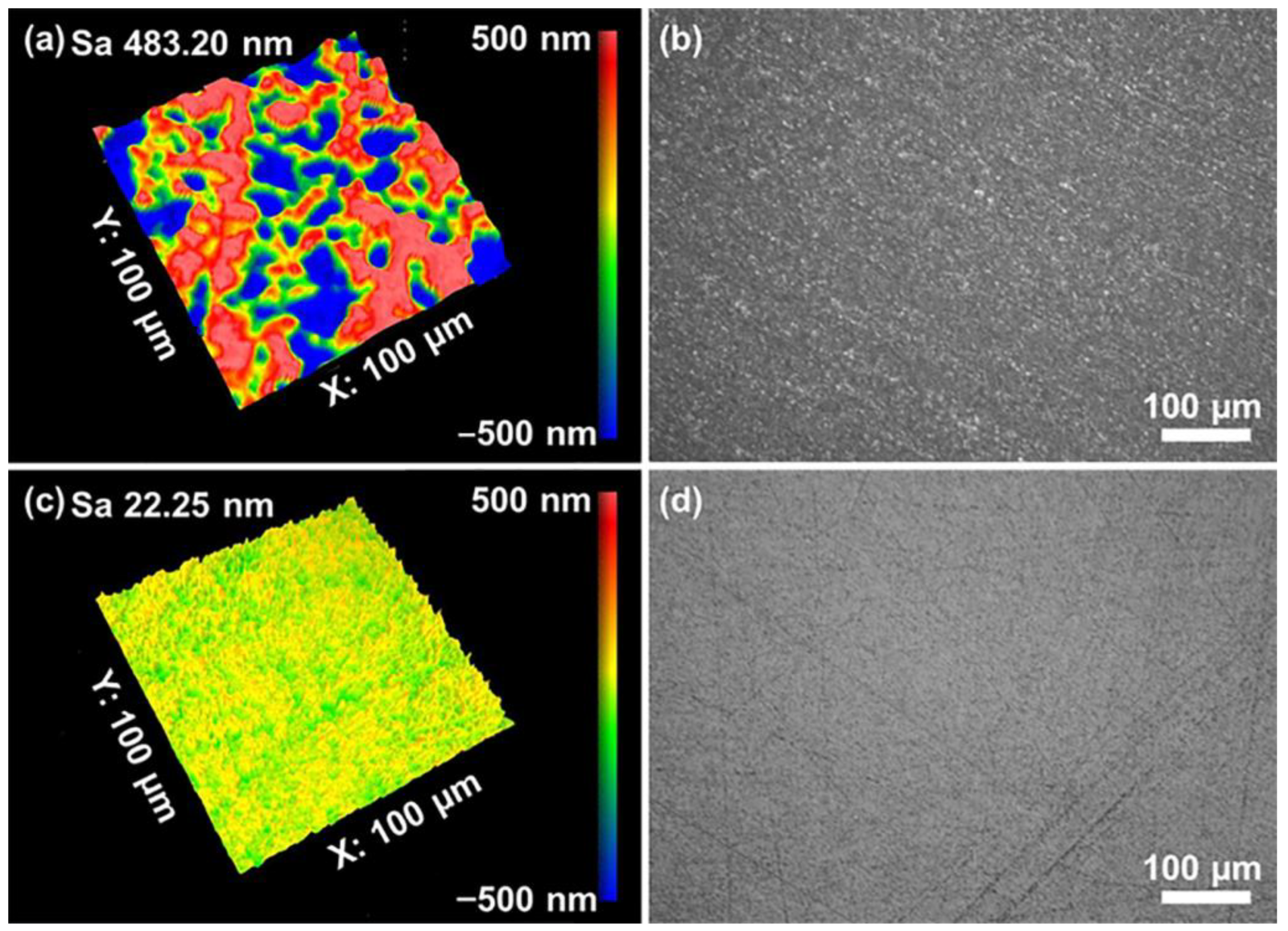


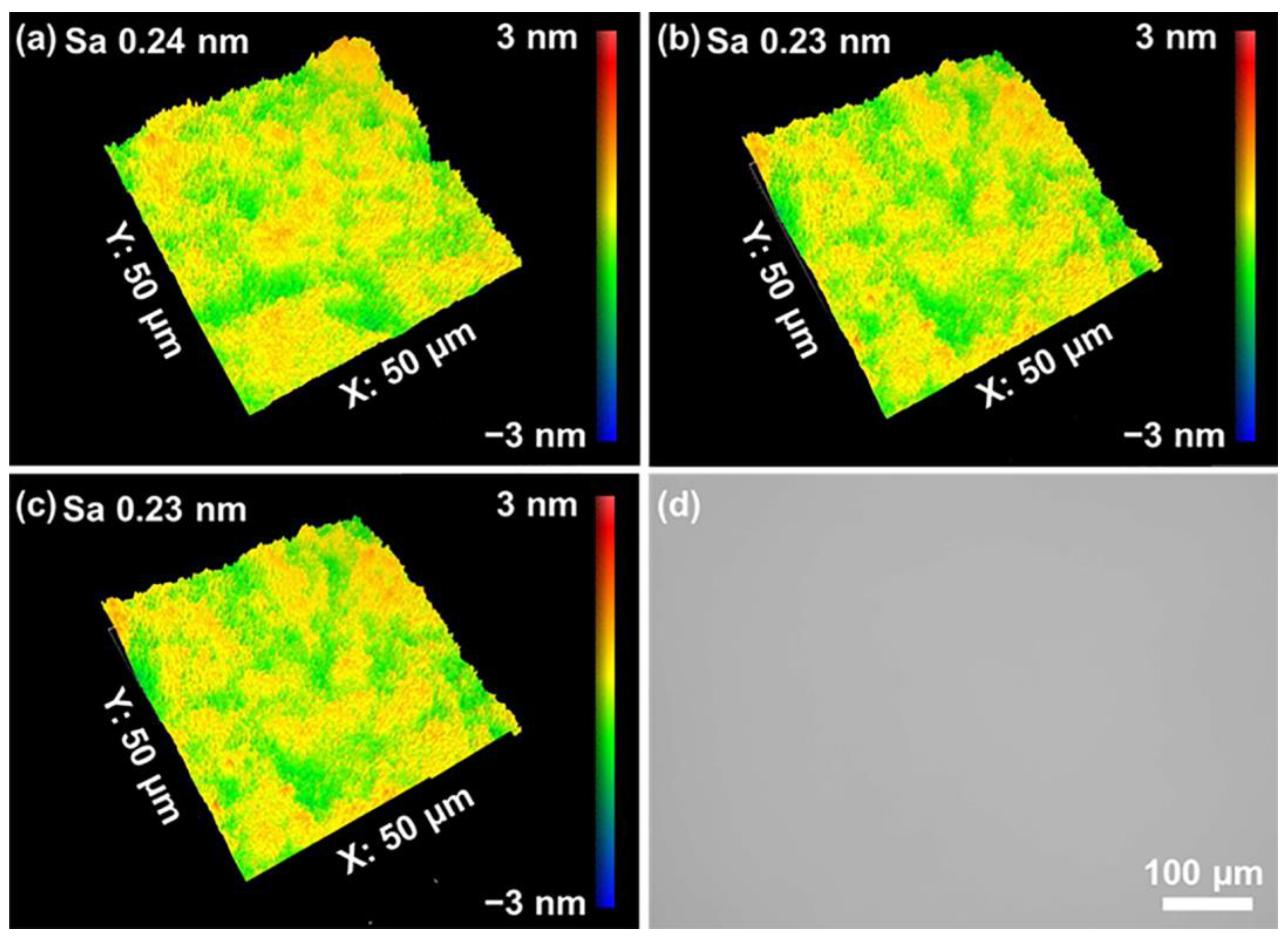
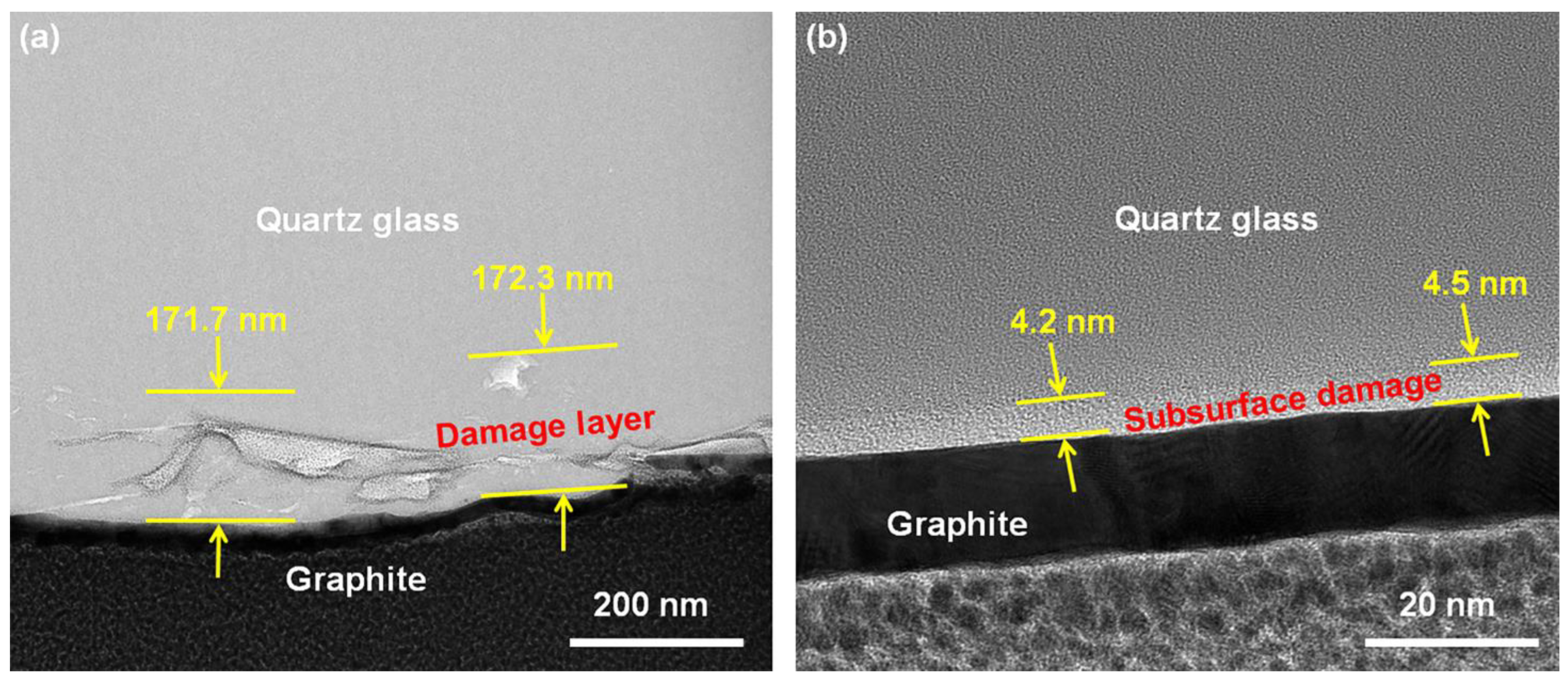
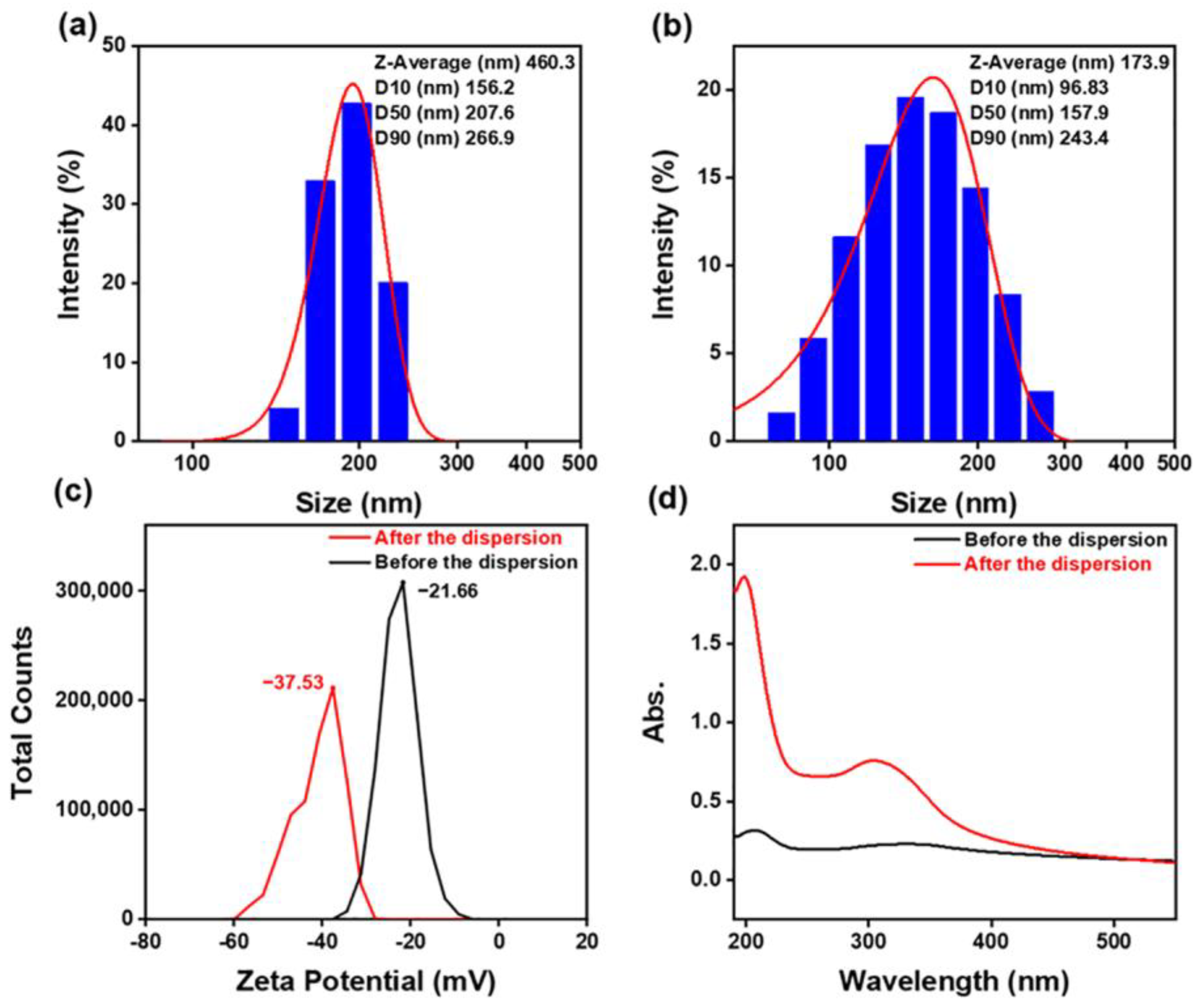
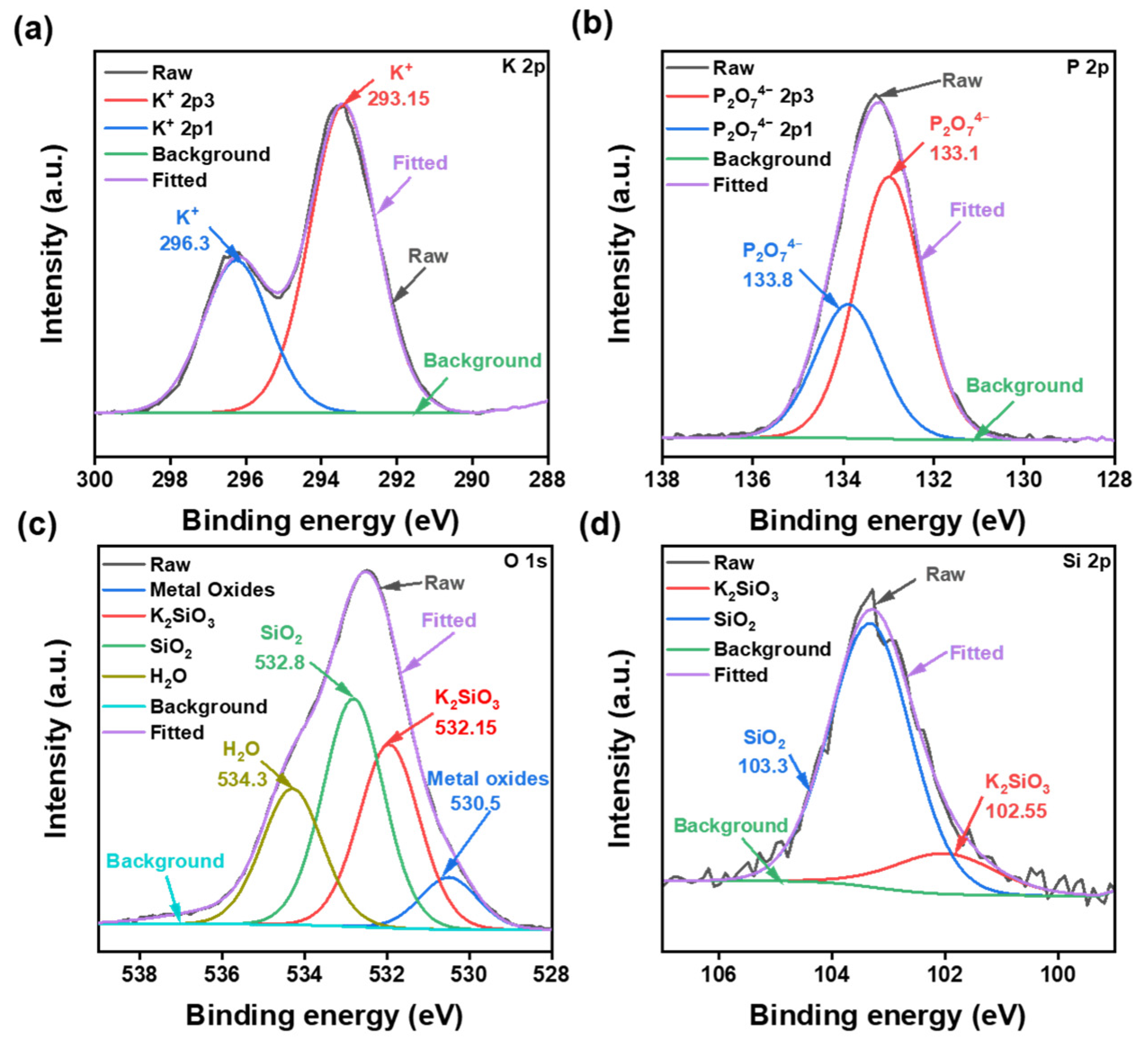


| No. | Factors | Levels | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 3 | 5 | ||
| A | Abrasive concentration (wt%) | 0.25 | 0.5 | 1 | 3 | 5 |
| B | Polishing solution pH value | 9 | 9.5 | 10 | 10.2 | 10.5 |
| C | Dispersant concentration (wt%) | 0.25 | 0.35 | 0.5 | 0.75 | 1 |
| D | Polishing pressure (kPa) | 20 | 25 | 30 | 35 | 40 |
| E | Polishing speed (rpm) | 60 | 80 | 100 | 120 | 140 |
| F | Polishing fluid flow rate (mL/min) | 2 | 4 | 6 | 8 | 10 |
| Index | Before the Dispersion | After the Dispersion |
|---|---|---|
| Viscosity (mPa·S) | 1.13 | 5.06 |
| Rotor (#) | 0 | 0 |
| Rotational speed (rpm) | 60 | 60 |
| Torque (%) | 11.3 | 50.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhang, Z.; Shi, C.; Feng, J.; Zhuang, X.; Li, L.; Meng, F.; Li, H.; Xue, Z.; Liu, D. Dispersion and Polishing Mechanism of a Novel CeO2-LaOF-Based Chemical Mechanical Polishing Slurry for Quartz Glass. Materials 2023, 16, 1148. https://doi.org/10.3390/ma16031148
Zhao Z, Zhang Z, Shi C, Feng J, Zhuang X, Li L, Meng F, Li H, Xue Z, Liu D. Dispersion and Polishing Mechanism of a Novel CeO2-LaOF-Based Chemical Mechanical Polishing Slurry for Quartz Glass. Materials. 2023; 16(3):1148. https://doi.org/10.3390/ma16031148
Chicago/Turabian StyleZhao, Zifeng, Zhenyu Zhang, Chunjing Shi, Junyuan Feng, Xuye Zhuang, Li Li, Fanning Meng, Haodong Li, Zihang Xue, and Dongdong Liu. 2023. "Dispersion and Polishing Mechanism of a Novel CeO2-LaOF-Based Chemical Mechanical Polishing Slurry for Quartz Glass" Materials 16, no. 3: 1148. https://doi.org/10.3390/ma16031148
APA StyleZhao, Z., Zhang, Z., Shi, C., Feng, J., Zhuang, X., Li, L., Meng, F., Li, H., Xue, Z., & Liu, D. (2023). Dispersion and Polishing Mechanism of a Novel CeO2-LaOF-Based Chemical Mechanical Polishing Slurry for Quartz Glass. Materials, 16(3), 1148. https://doi.org/10.3390/ma16031148





