The Influence of External Sulfate Attack on the Durability of Reinforced Mortars in the Presence of Calcined River Sediments
Abstract
:1. Introduction
1.1. Materials and Methods
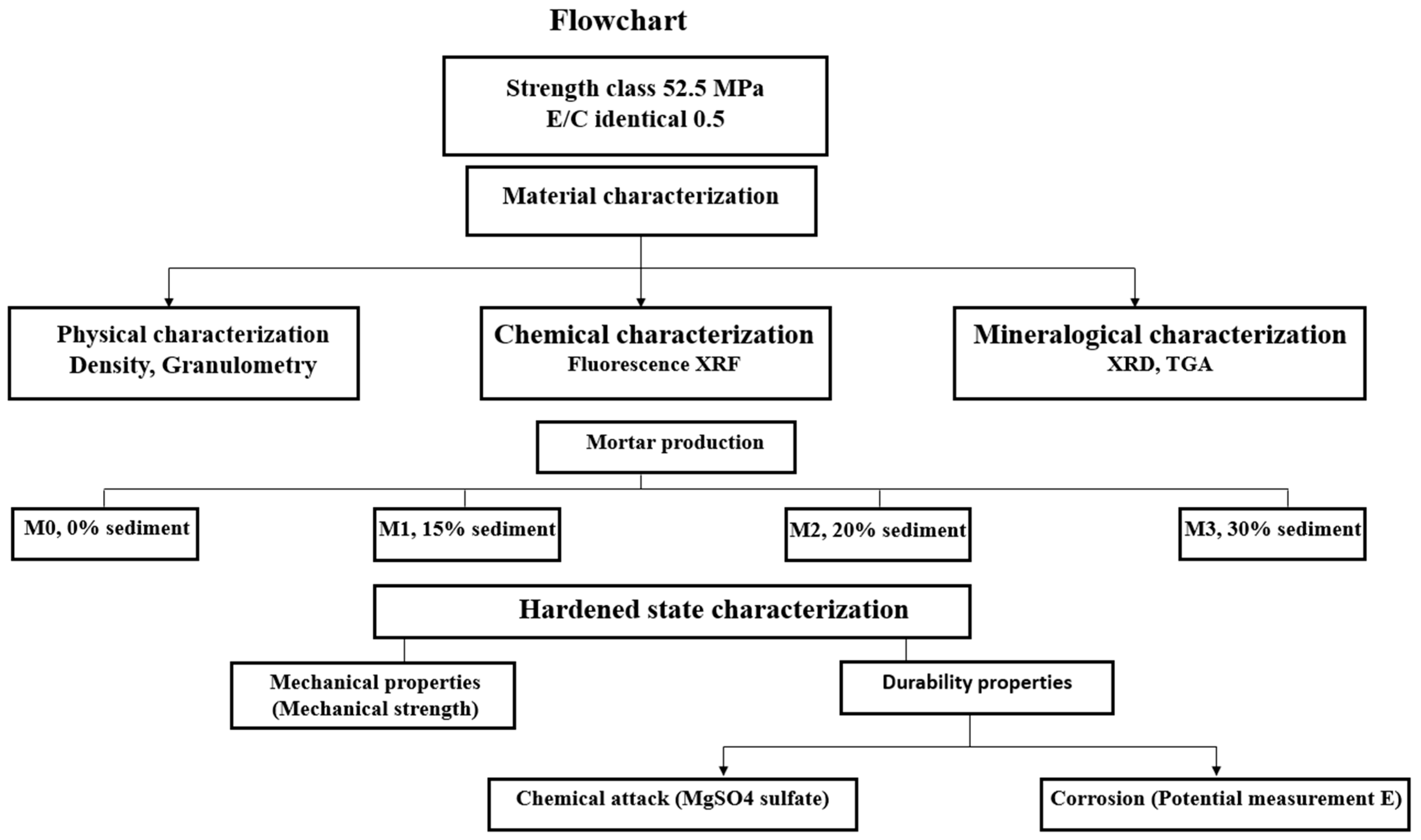
1.2. Flowchart
1.3. Materials
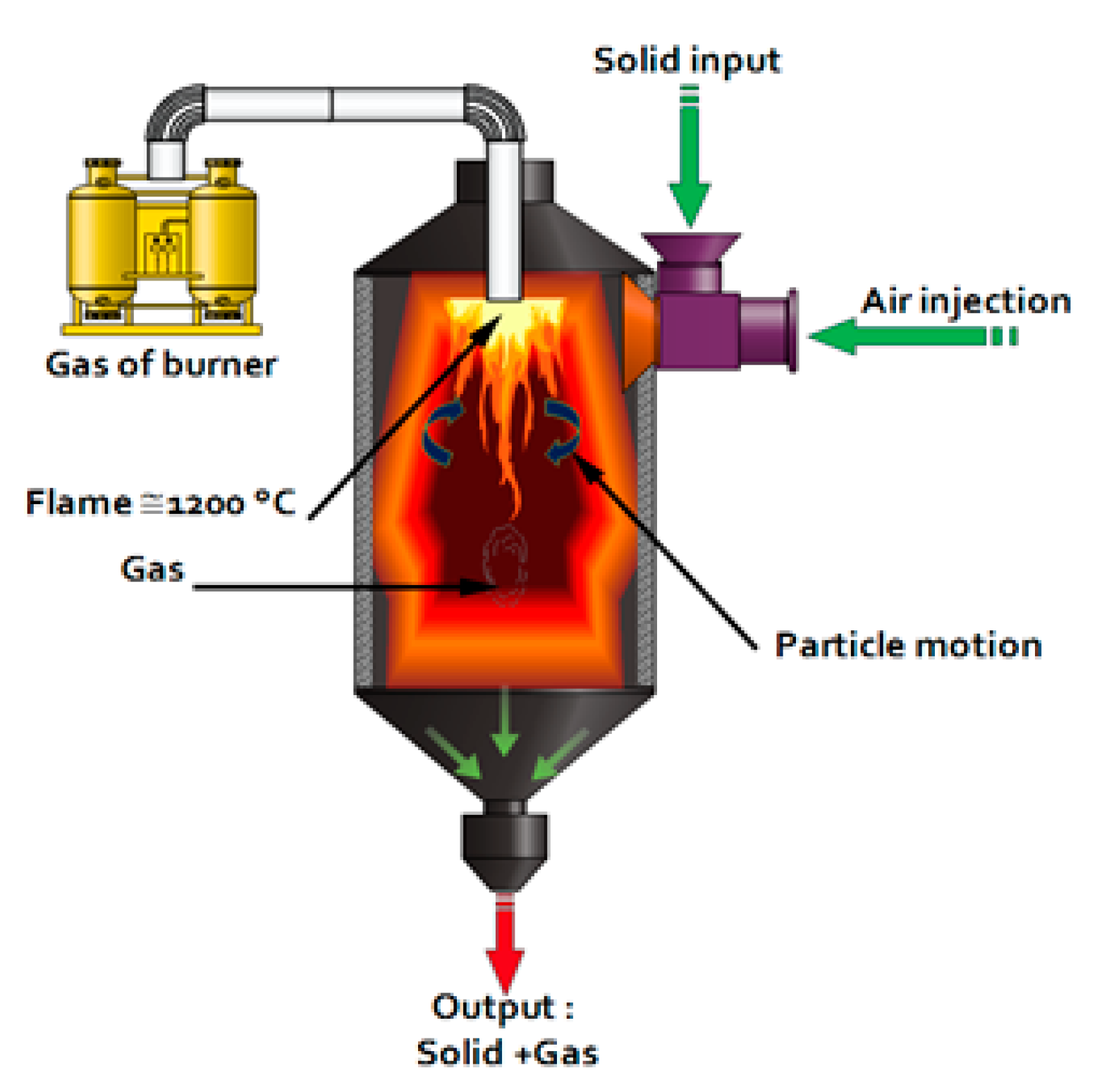
1.4. Calcination of Sediments: “The Process”
1.5. Characterization Tests
2. Results
2.1. Physical Properties
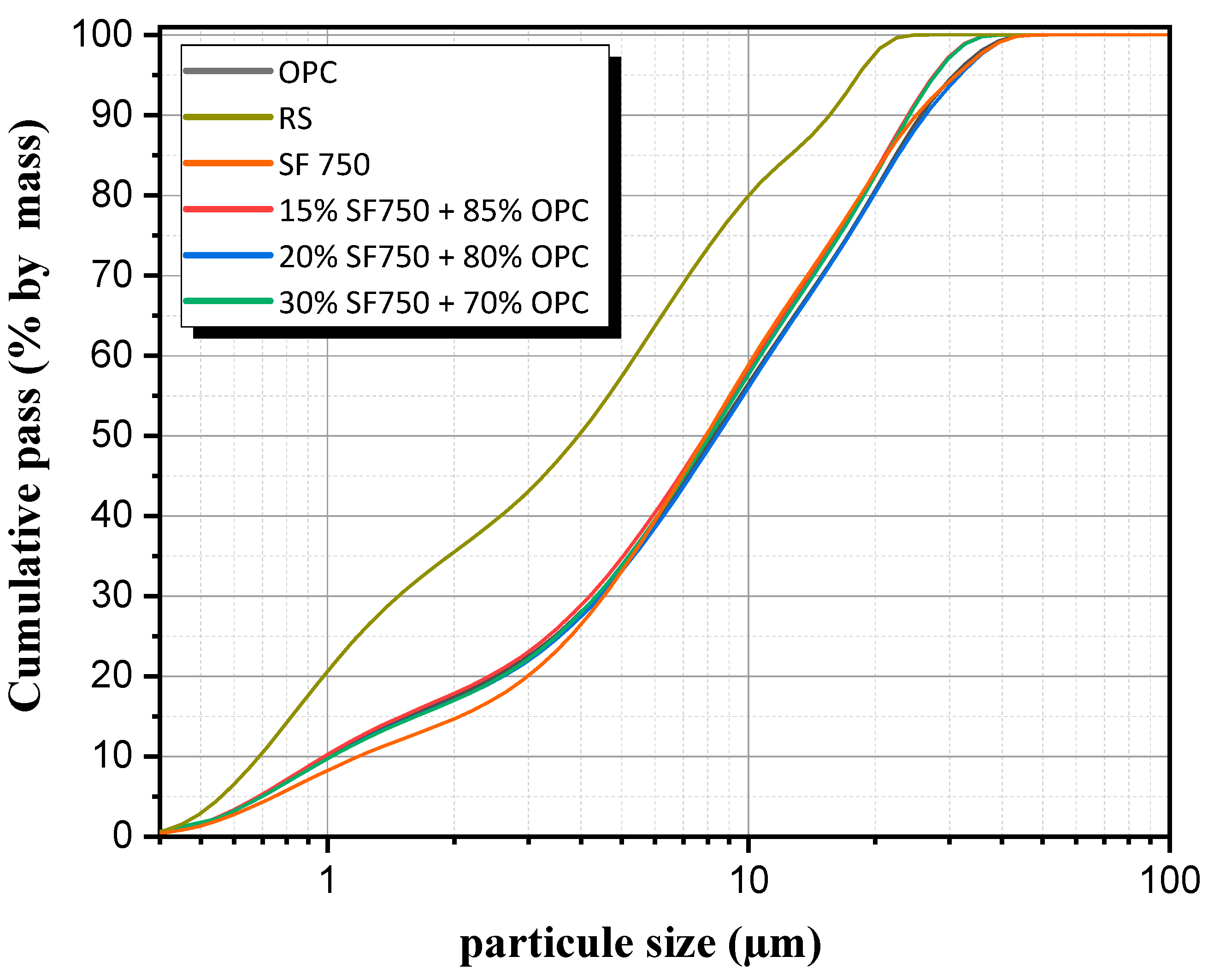
2.2. Granulometry
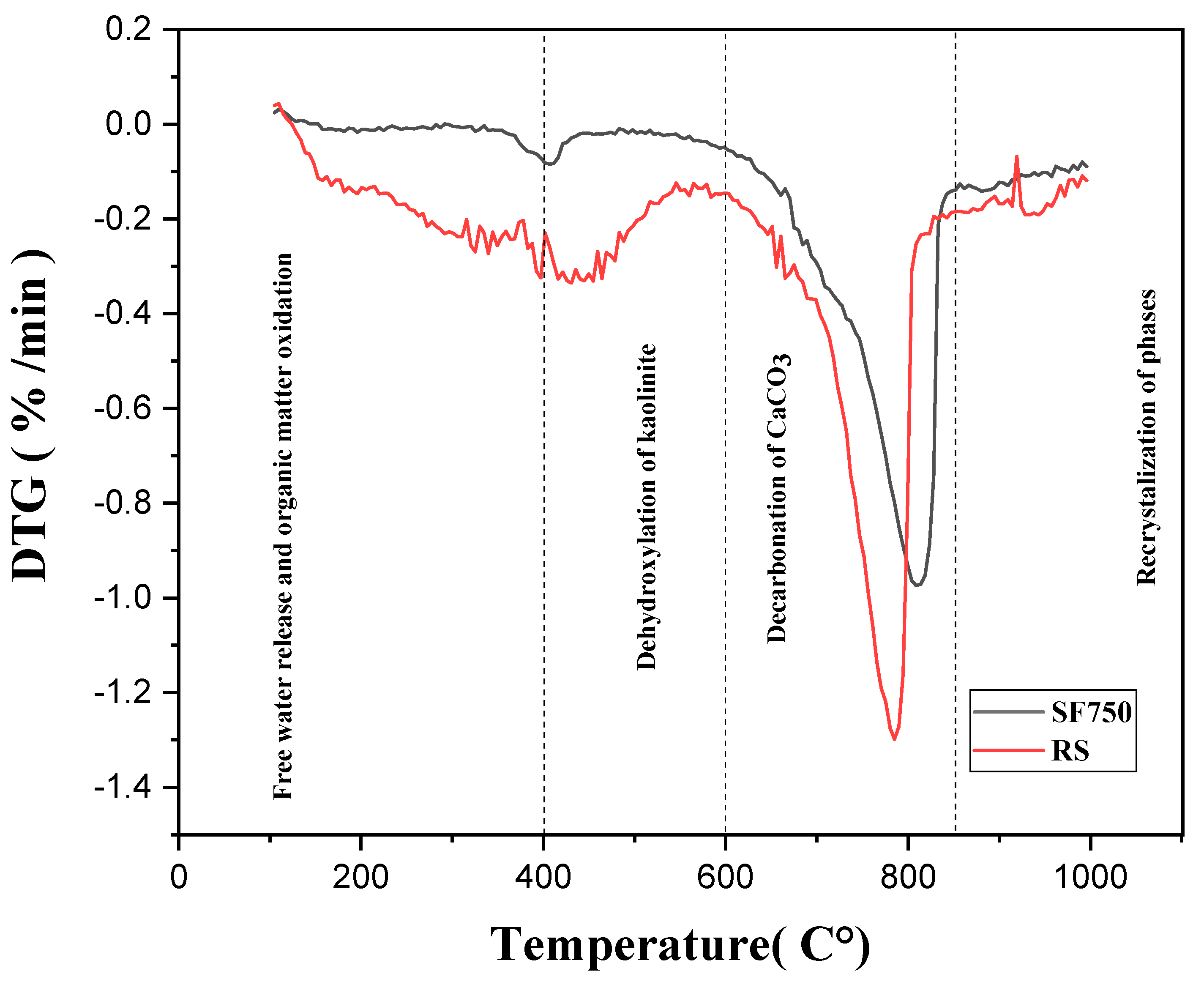
2.3. Thermogravimetric Analysis (TGA)
2.4. Chemical and Mineralogical Analysis
2.4.1. Chemical Composition FX
2.4.2. X-ray Mineralogy
3. Study of Formulation and Characterization of Mortars
3.1. Formulation
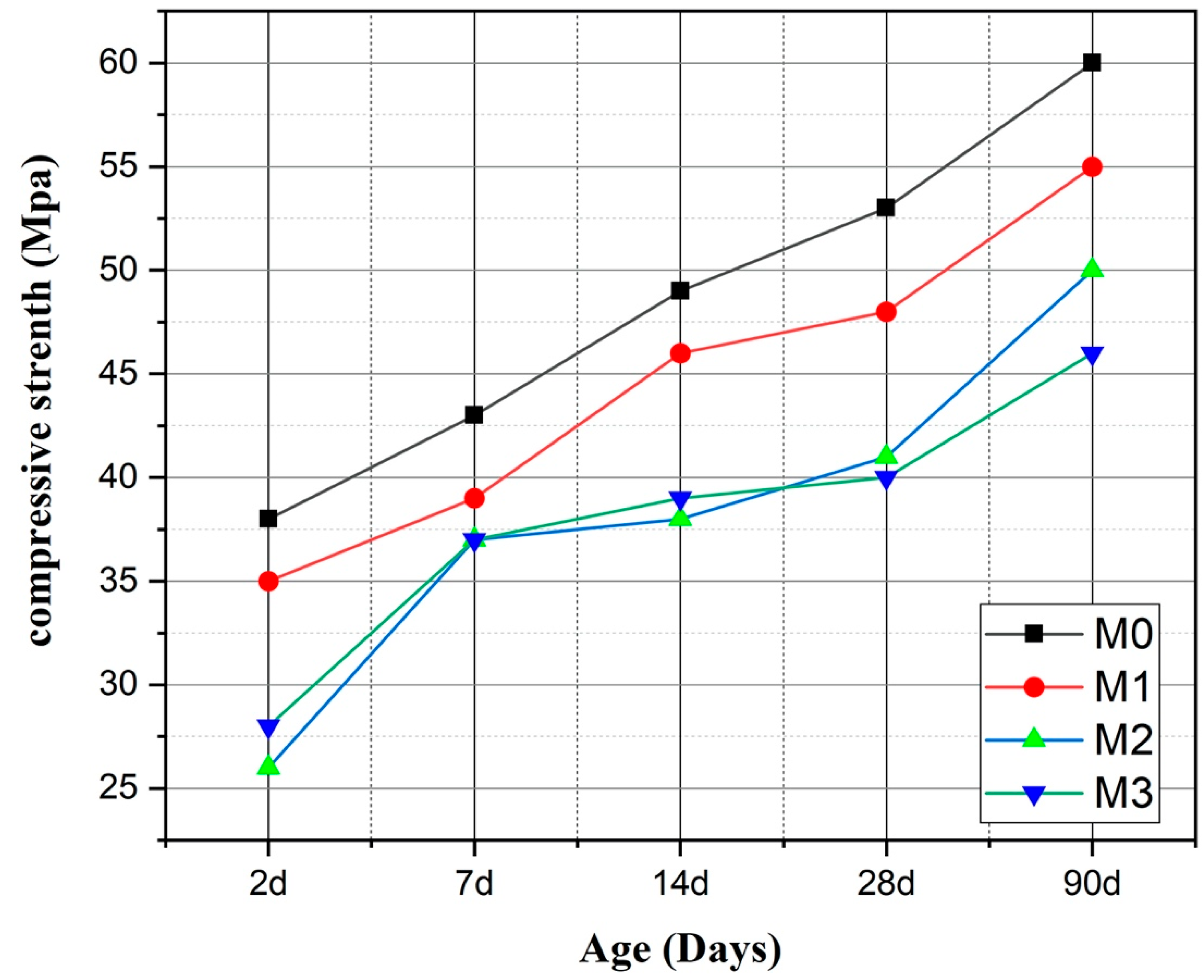
3.2. Compressive Strength of Mortars
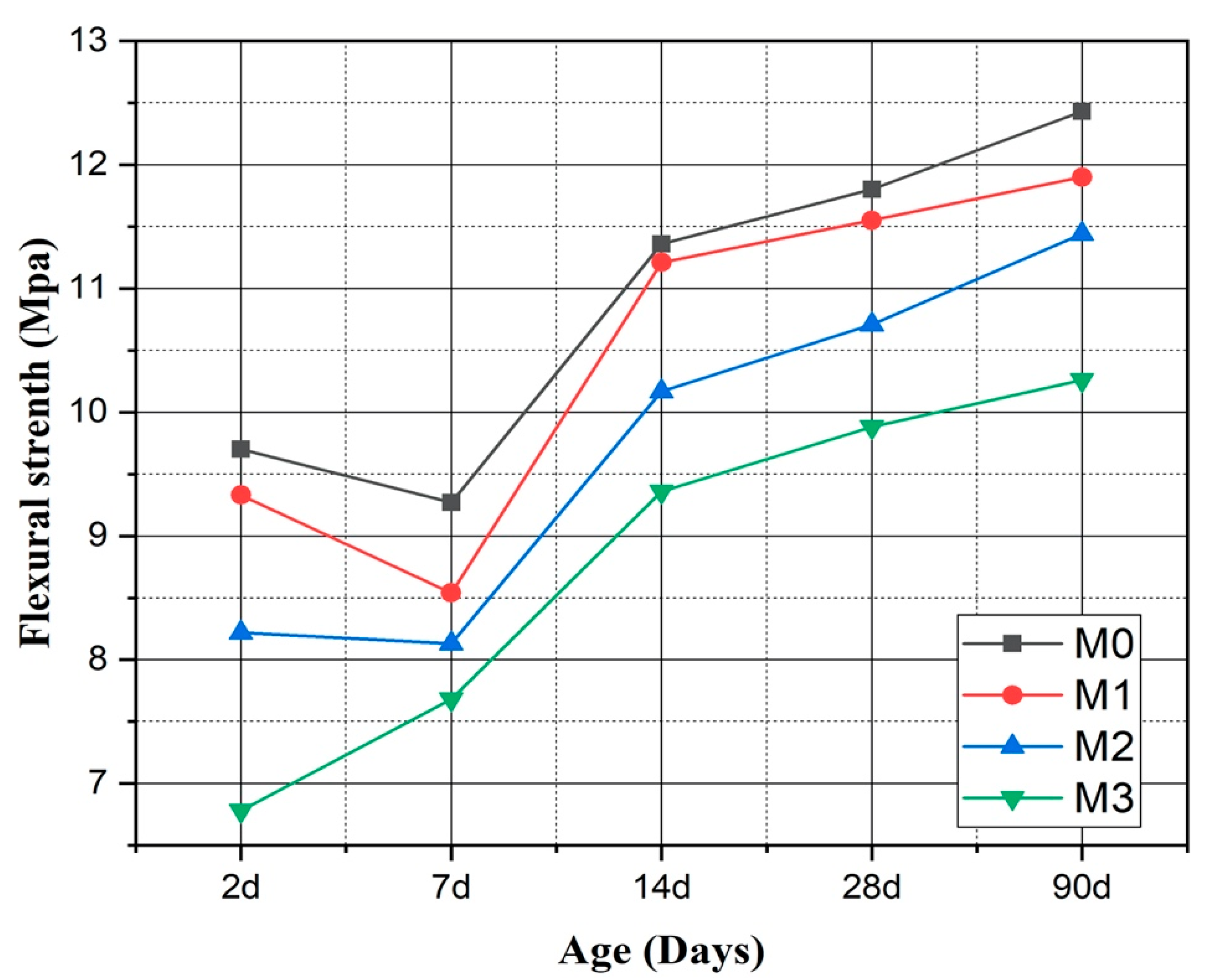
3.3. Flexural Strength of Mortars
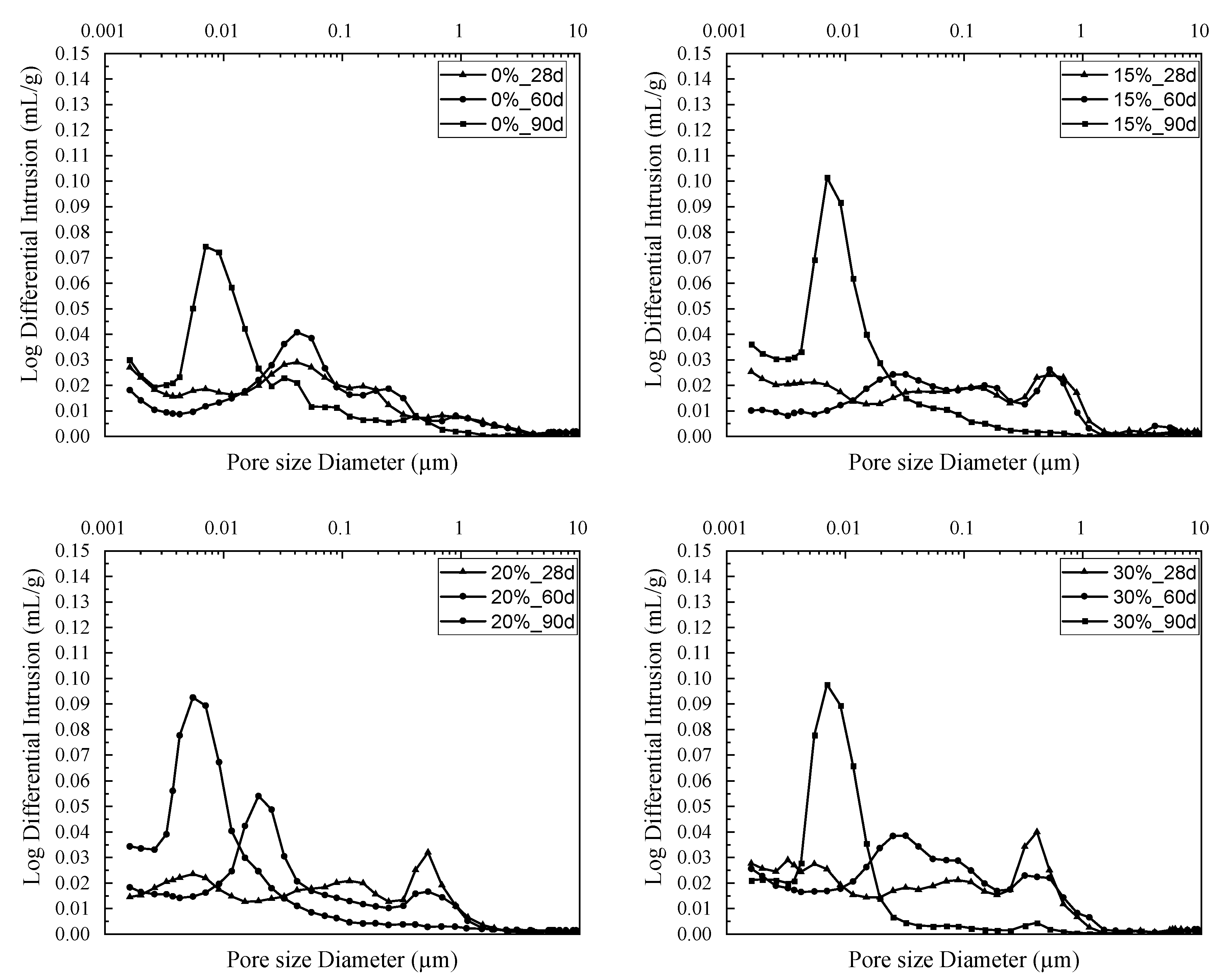
3.4. Microstructural Study
3.5. Resistance to External Sulfate Attack
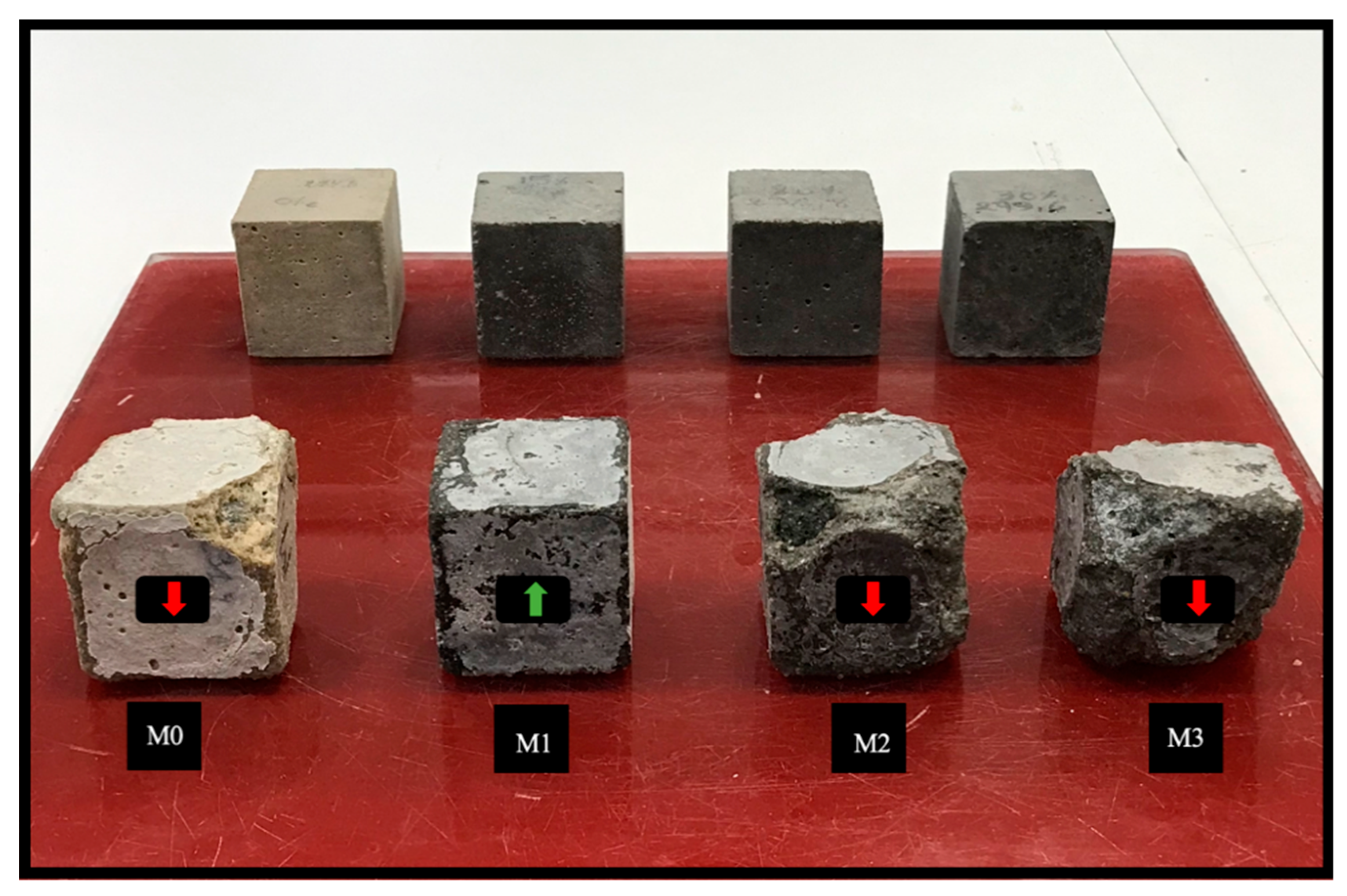
3.5.1. Visual Inspection
3.5.2. The Variation of Mass
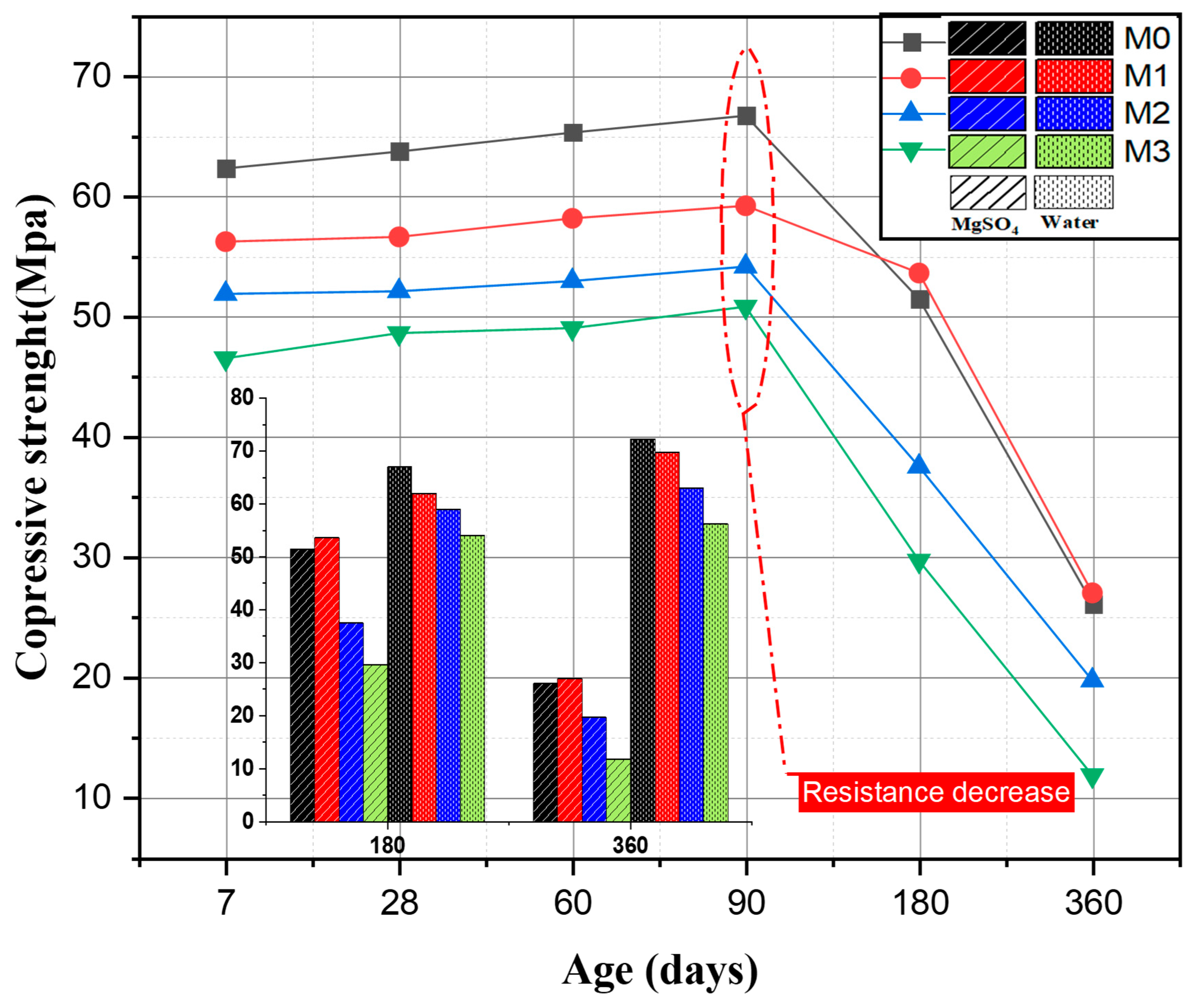
3.5.3. The Evolution of Compressive Strength
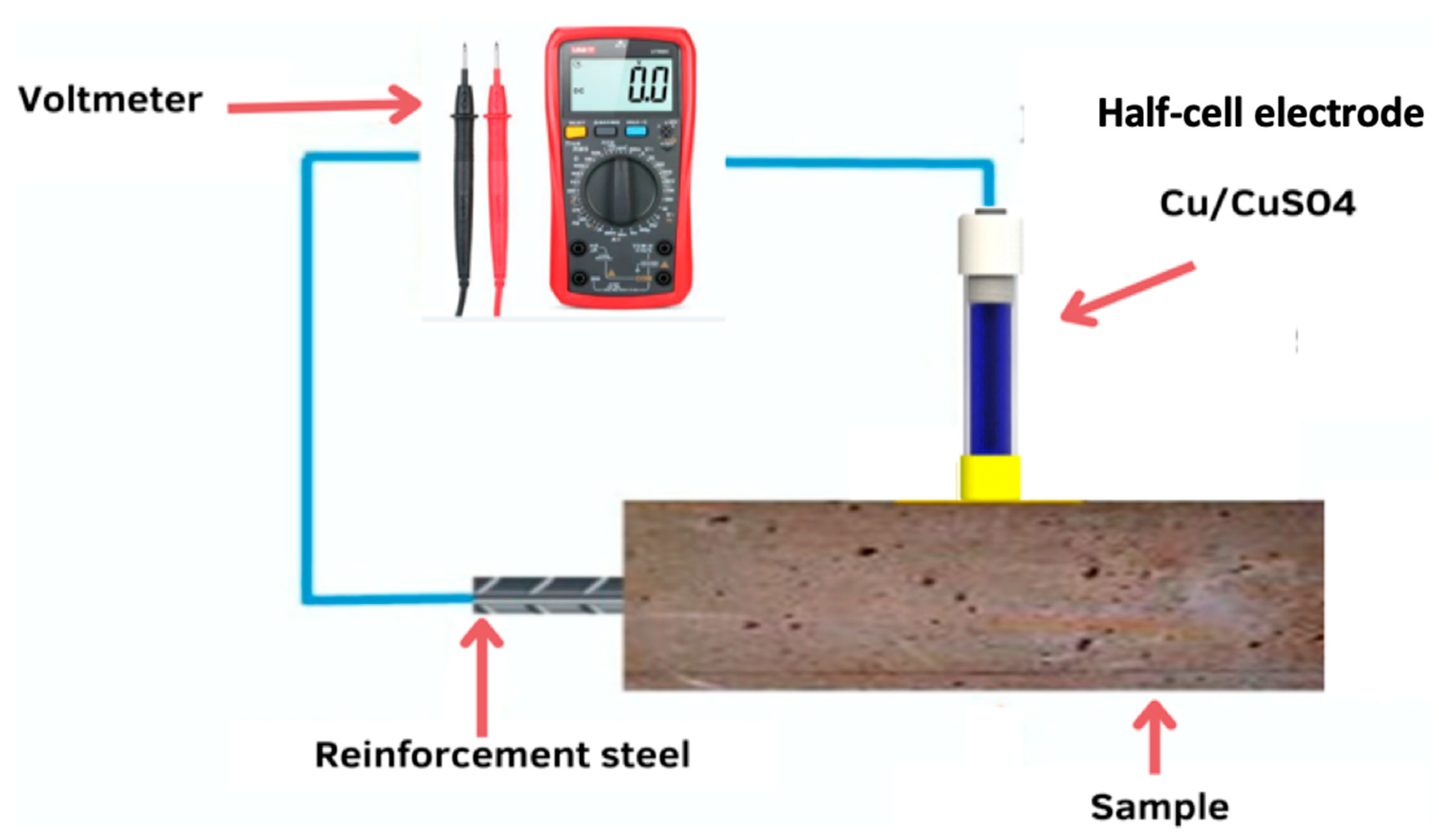
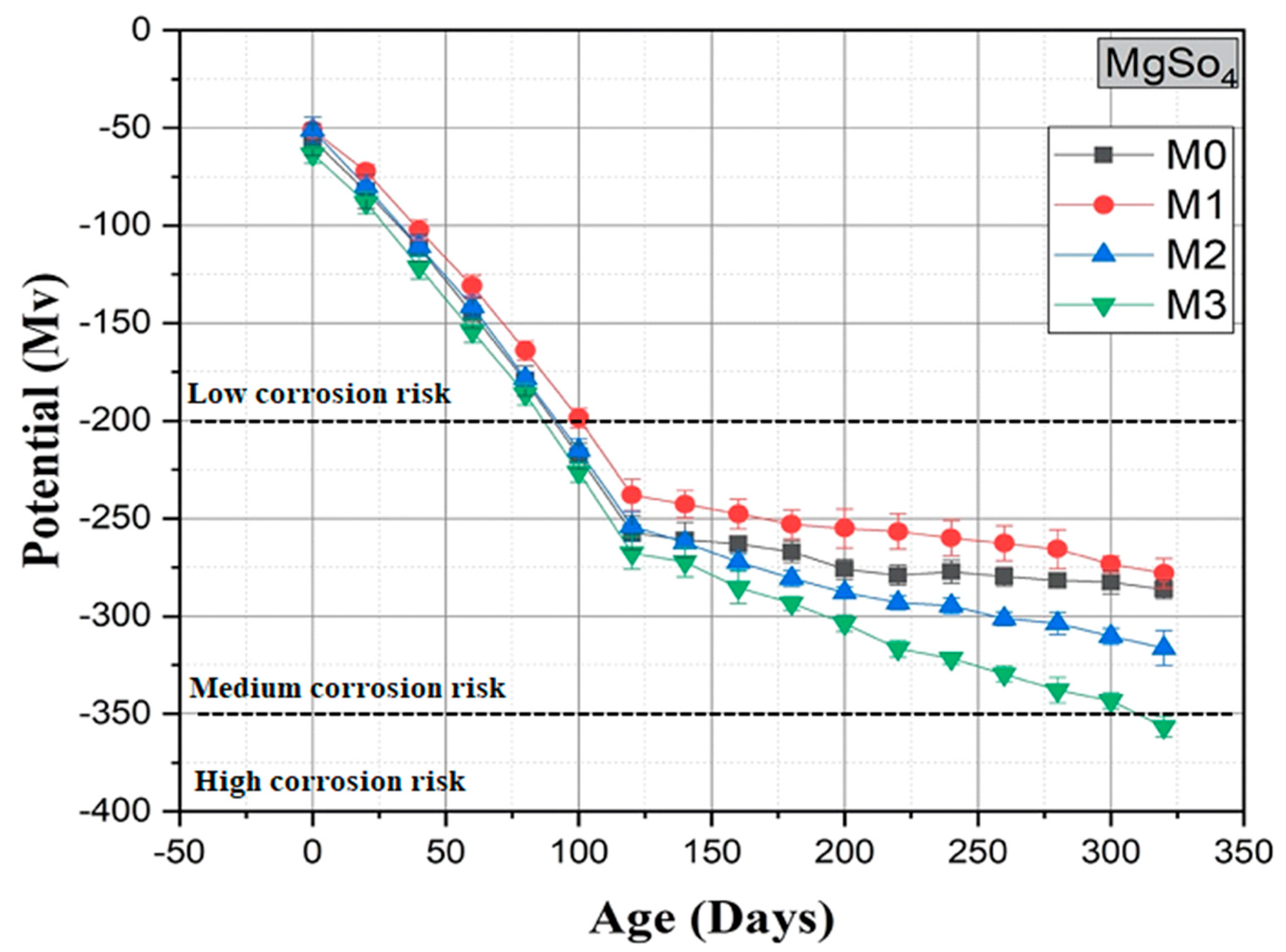
3.6. Measurement of Corrosion Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.unicem.fr/wp-content/uploads/2021/12/snbpe-chiffres-2019-web (accessed on 8 October 2023).
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A.; Ventura, A. LCA allocation procedure used as an incitative method for waste recycling: An application to mineral additions in concrete. Resour. Conserv. Recycl. 2010, 54, 1231–1240. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Hasanbeigi, A. Global Cement Industry’s GHG Emissions. 2021. Available online: https://www.globalefficiencyintel.com/new-blog/2021/global-cement-industry-ghg-emissions (accessed on 17 May 2021).
- LTurner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- CEMBUREAU. Clinker Substitution. 2018. Available online: https://lowcarboneconomy.cembureau.eu/5-parallel-routes/resource-efficiency/clinker-substitution (accessed on 25 July 2019).
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cement. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Juenger, M.C.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cement Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An eco material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Sabir, B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Dang, T.A.; Kamali-Bernard, S.; Prince, W.A. Design of new blended cement based on marine dredged sediment. Constr. Build. Mater. 2013, 41, 602–611. [Google Scholar] [CrossRef]
- Rabehi, B.; Ghernouti, Y.; Boumchedda, K. Strength and compressive behaviour of ultra high-performance fibre-reinforced concrete (UHPFRC) incorporating Algerian calcined clays as pozzolanic materials and silica fume. Eur. J. Environ. Civ. Eng. 2013, 17, 599–615. [Google Scholar] [CrossRef]
- Chikouche, M.A.; Ghorbel, E.; Bibi, M. The possibility of using dredging sludge in manufacturing cements: Optimization of heat treatment cycle and ratio replacement. Constr. Build. Mater. 2016, 106, 330–341. [Google Scholar] [CrossRef]
- Bouhamou, N.-E.; Mostefa, F.; Mebrouki, A.; Bendani, K.; Belas, N. Influence of dredged sediment on the shrinkage behavior of self-compacting concrete. Mater. Technol. 2016, 50, 127–135. [Google Scholar]
- Snellings, R.; Cizer, Ö.; Horckmans, L.; Durdzinski, P.T.; Dierckx, P.; Nielsen, P.; Van Balen, K.; Vandewalle, L. Properties and pozzolanic reactivity of flash calcined dredging sediments. Appl. Clay Sci. 2016, 129, 35–39. [Google Scholar] [CrossRef]
- Faure, A.; Smith, A.; Coudray, C.; Anger, B.; Colina, H.; Moulin, I.; Thery, F. Ability of Two Dam Fine-Grained Sediments to be Used in Cement Industry as Raw Material for Clinker Production and as Pozzolanic Additional Constituent of Portland-Composite Cement. Waste Biomass-Valorization 2017, 8, 2141–2163. [Google Scholar] [CrossRef]
- Snellings, R.; Horckmans, L.; Van Bunderen, C.; Vandewalle, L.; Cizer, Ö. Flash-calcined dredging sediment blended cements: Effect on cement hydration and properties. Mater. Struct. 2017, 50, 241. [Google Scholar] [CrossRef]
- CVan Bunderen, C.; Snellings, R.; Vandewalle, L.; Cizer, Ö. Early-age hydration and autogenous deformation of cement paste containing flash calcined dredging sediments. Constr. Build. Mater. 2019, 200, 104–115. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N.-E. From dredged sediment to supplementary cementitious material: Characterization, treatment, and reuse. Int. J. Sediment Res. 2021, 36, 92–109. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Abriak, N.-E. Designing Efficient Flash-Calcined Sediment-Based Ecobinders. Materials 2022, 15, 7107. [Google Scholar] [CrossRef]
- Snellings, R.; Horckmans, L.; Durdzinski, P.; Van Bunderen, C.; Vandewalle, L.; Van Balen, K.; Dockx, J.; Vandekeybus, J.; Cizer, Ö. Calcined dredged sediments as supplementary cementitious materials: Properties and pozzolanic reactivity. In Proceedings of the International RILEM Conference on Materials, Systems and Structures in Civil Engineering (Segment on Concrete with Supplementary Cementitious Materials); Jensen, O.M., Kovler, K., De Belie, N., Eds.; RILEM: Champs-sur-Marne, France, 2016; pp. 205–212. [Google Scholar]
- Azrar, H.; Zentar, R.; Abriak, N.-E. The Effect of Granulation Time of the Pan Granulation on the Characteristics of the Aggregates Containing Dunkirk Sediments. Procedia Eng. 2016, 143, 10–17. [Google Scholar] [CrossRef]
- Dalton, J.L.; Gardner, K.H.; Seager, T.P.; Weimer, M.L.; Spear, J.C.; Magee, B.J. Properties of Portland cement made from contaminated sediments. Resour. Conserv. Recycl. 2004, 41, 227–241. [Google Scholar] [CrossRef]
- Aouad, G.; Laboudigue, A.; Gineys, N.; Abriak, N. Dredged sediments used as novel supply of raw material to produce Portland cement clinker. Cem. Concr. Compos. 2012, 34, 788–793. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Abriak, N.-E. Towards the establishment of formulation laws for sediment-based mortars. J. Build. Eng. 2018, 16, 106–117. [Google Scholar] [CrossRef]
- Achour, R.; Zentar, R.; Abriak, N.-E.; Rivard, P.; Gregoire, P. Durability study of concrete incorporating dredged sediments. Case Stud. Constr. Mater. 2019, 11, e00244. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Abriak, N.-E.; Mamindy-Pajany, Y. Study of the pozzolanic activity of a dredged sediment from Dunkirk harbour. Powder Technol. 2017, 320, 748–764. [Google Scholar] [CrossRef]
- Chu, D.C.; Amar, M.; Kleib, J.; Benzerzour, M.; Betrancourt, D.; Abriak, N.-E.; Nadah, J. The Pozzolanic Activity of the Sediment Treated by the Flash Calcination Method. 2021. Available online: https://www.researchgate.net/publication/356093231_The_Pozzolanic_Activity_of_the_Sediment_Treated_by_the_Flash_Calcination_Method (accessed on 8 October 2023).
- Sadok, R.H.; Belaribi, N.B.; Mazouzi, R.; Sadok, F.H. Life cycle assessment of cementitious materials based on calcined sediments from Chorfa II dam for low carbon binders as sustainable building materials. Sci. Total. Environ. 2022, 826, 154077. [Google Scholar] [CrossRef]
- Safer, O.; Belas, N.; Belaribi, O.; Belguesmia, K.; Bouhamou, N.-E.; Mebrouki, A. Valorization of Dredged Sediments as a Component of Vibrated Concrete: Durability of These Concretes Against Sulfuric Acid Attack. Int. J. Concr. Struct. Mater. 2018, 12, 44. [Google Scholar] [CrossRef]
- Aoual-Benslafa, F.K.; Kerdal, D.; Ameur, M.; Mekerta, B.; Semcha, A. Durability of Mortars Made with Dredged Sediments. Procedia Eng. 2015, 118, 240–250. [Google Scholar] [CrossRef]
- Zhao, Z.; Benzerzour, M.; Abriak, N.-E.; Damidot, D.; Courard, L.; Wang, D. Use of uncontaminated marine sediments in mortar and concrete by partial substitution of cement. Cem. Concr. Compos. 2018, 93, 155–162. [Google Scholar] [CrossRef]
- NF EN 196-1; Méthode d’Essai des Ciments—Partie 1: Détermination des Résistances. AFNOR: Paris, France, 2016.
- Salvador, S.; Pons, O. A semi-mobile flash dryer/calciner unit to manufacture pozzolana from raw clay soils—Application to soil stabilisation. Constr. Build. Mater. 2000, 14, 109–117. [Google Scholar] [CrossRef]
- Teklay, A.; Yin, C.; Rosendahl, L.; Køhler, L.L. Experimental and modeling study of flash calcination of kaolinite rich clay particles in a gas suspension calciner. Appl. Clay Sci. 2015, 103, 10–19. [Google Scholar] [CrossRef]
- Sperinck, S.; Raiteri, P.; Marks, N.; Wright, K. Dehydroxylation of Kaolinite to Metakaolin—A Molecular Dynamics Study. Mater. Chem. 2011, 21, 2118–2125. Available online: http://pubs.rsc.org/en/content/articlehtml/2011/jm/c0jm01748e (accessed on 23 August 2016). [CrossRef]
- Bich, C.; Ambroise, J.; Péra, J. Influence of degree of dehydroxylation on the pozzolanic activity of metakaolin. Appl. Clay Sci. 2009, 44, 194–200. [Google Scholar] [CrossRef]
- NF EN 196-6; Méthodes d’essai des Ciments—Détermination de la Finesse. Afnor EDITIONS: Paris, France, 2018.
- NF EN ISO 18757; Céramiques Techniques—Détermination de la Surface Spécifique (Aire Massique) des Poudres Céramiques par Adsorption de Gaz à L’aide de la Méthode BET. Afnor EDITIONS: Paris, France, 2006.
- NF EN 1097-7; Tests for Mechanical and Physical Properties of Aggregates—Part 7: Determination of the Particle Density of Filler—Pyknometer Methodz. Afnor EDITIONS: Paris, France, 2008.
- NF P 94-410-3; Roches—Essais Pour Déterminer les Propriétés Physiques des Roches—Partie 3: Détermination de la Porosité. Afnor EDITIONS: Paris, France, 2001.
- Siham, K.; Fabrice, B.; Edine, A.N.; Patrick, D. Marine dredged sediments as new materials resource for road construction. Waste Manag. 2008, 28, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, O.; Kacimi, L.; López-Delgado, A.; Frías, M.; Guerrero, A. Characterization of Algerian reservoir sludges for use as active additions in cement: New pozzolans for eco-cement manufacture. Constr. Build. Mater. 2013, 40, 275–279. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, C.; Xu, B.; Ruan, X.; Wei, Z. The use of urban river sediments as a primary raw material in the production of highly insulating brick. Ceram. Int. 2014, 40, 8833–8840. [Google Scholar] [CrossRef]
- ASTM C1012-04; Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2004.
- ASTM C 618; Standard Specification for Coal Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2023.
- Caré, Y.C.S.; Linder, R.; Baroghel-Bouny, V.; de Larrard, F. Effet des additions minérales sur les propriétés d’usage des bétons, Plans d’expériences et analyse statistique. In Etudes et Recherches des Laboratoires des Ponts et Chaussees—Serie Ouvrages ‘Art; Laboratoire Central des Ponts et Chausees (LCPC): Paris, France, 2000; p. 115. [Google Scholar]
- Feldman, R.; Ramachandran, V. Influence of CaCO3 on the Hydration of 3CaO·Al2O3. Am. Ceram. 1965, 48, 25–30. Available online: http://onlinelibrary.wiley.com/doi/10.1111/j.1151-2916.1965.tb11787.x/full (accessed on 21 August 2016). [CrossRef]
- ASTM C470/C470M-15; Standard Specification for Molds for Forming Concrete Test Cylinders Vertically. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2023.
- Nielsen, L.F. Strength development in hardened cement paste: Examination of some empirical equations. Mater. Struct. 1993, 26, 255–260. [Google Scholar] [CrossRef]
- Kourtaa, S.; Chabannes, M.; Becquart, F.; Abriak, N.-E. Evaluation of Marine Dredged Sediment as Reactive Powder Compared to Ground Basaltic Pyroclastic Materials for the Development of Eco-Friendly Lime-Pozzolan Binders. Available online: https://www.sciencedirect.com/science/article/pii/S0958946522001469 (accessed on 8 October 2023).
- Pereira Silva, L.H.; Nehring, V.; Guedes de Paiva, F.F.; Tamashiro, J.R.; Galvín, A.P.; López-Uceda, A.; Kinoshita, A. Use of Blast Furnace Slag in Cementitious Materials for Pavements—Systematic Literature Review and Eco-Efficiency. Available online: https://www.sciencedirect.com/science/article/pii/S2352554123000645 (accessed on 8 October 2023).
- Nayak, D.K.; Abhilash, P.P.; Singh, R.; Kumar, R.; Kumar, V. FLY ash for Sustainable Construction: A Review of Fly Ash Concrete and Its Beneficial Use Case Studies. Available online: https://www.sciencedirect.com/science/article/pii/S2772397622001034 (accessed on 8 October 2023).
- Roziere, E. Etude de la Durabilité des Bétons par Une Approche Performantielle. Ph.D. Thesis, Ecole Centrale de Nantes, Nantes, France, 2007. [Google Scholar]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B. Attack on plain and blended cements exposed to aggressive sulfate environments. Cem. Concr. Compos. 2002, 24, 305–316. [Google Scholar] [CrossRef]
- Al Amoudi, O.S.B. Performance of 15 reinforced concrete mixtures in magnesium-sodium sulphate environments. Constr. Build. Mater. 1995, 9, 149–158. [Google Scholar] [CrossRef]
- Tittelboom, K.; Belie, N.; Hooton, R.D. Test Methods for Resistance of Concrete to Sulfate Attack—A Critical Review. In Performance of Cement-Based Materials in Aggressive Aqueous Environments: State-of-the-Art Report, RILEM TC 211-PAE; Alexander, M., Bertron, A., De Belie, N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 251–288. [Google Scholar]
- Crammond, N.J. The thaumasite form of sulfate attack in the UK. Cem. Concr. Compos. 2003, 25, 809–818. [Google Scholar] [CrossRef]
- Türker, F.; Aköz, F.; Koral, S.; Yüzer, N. Effects of magnesium sulfate concentration on the sulfate resistance of mortars with and without silica fume. Cem. Concr. Res. 1997, 27, 205–214. [Google Scholar] [CrossRef]
- Mehta, P.K. Evaluation of sulphate resisting cements by a new test method. J. Proc. 1975, 72, 573–575. [Google Scholar]
- Makhloufi, Z.; Aggoun, S.; Benabed, B.; Kadri, E.; Bederina, M. Effect of magnesium sulfate on the durability of limestone mortars based on quaternary blended cements. Cem. Concr. Compos. 2016, 65, 186–199. [Google Scholar] [CrossRef]
- Nielsen, P.; Nicolai, S.; Darimont, A.; Kestemont, X. Influence of cement and aggregate type on thaumasite formation in concrete. Cem. Concr. Compos. 2014, 53, 115–126. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Physical and chemical characteristics affecting the durability of concrete. ACI Mater. J. 1991, 88, 186–196. [Google Scholar]
- Brown, P.W. An evaluation of sulfate resistance of cements in a controlled environment. Cem. Concr. Res. 1981, 11, 719–727. [Google Scholar] [CrossRef]
- Binici, H.; Aksoğan, O. Sulfate resistance of plain and blended cement. Cem. Concr. Compos. 2006, 28, 39–46. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Chen, H.; Zhou, Y. Influence of the initial moist curing time on the sulfate attack resistance of concretes with different binders. Constr. Build. Mater. 2017, 144, 541–551. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Zeng, Q.; Yang, C.; Luo, C.; Yang, K. Deterioration of mortars exposed to sulfate attack under electrical field. Constr. Build. Mater. 2016, 117, 121–128. [Google Scholar] [CrossRef]
- Lee, S.T.; Moon, H.Y.; Swamy, R.N. Sulfate attack and role of silica fume in resisting strength loss. Cem. Concr. Compos. 2005, 27, 65–76. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Sharif, A.M.; Shameem, M.; Ibrahim, M. Sulfate resistance of plain and blended cements exposed to varying concentrations of sodium sulfate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- Lee, S.-T. Performance deterioration of Portland cement matrix due to magnesium sulfate attack. KSCE J. Civ. Eng. 2007, 11, 157–163. [Google Scholar] [CrossRef]
- Sezer, G.İ. Compressive strength and sulfate resistance of limestone and/or silica fume mortars. Constr. Build. Mater. 2012, 26, 613–618. [Google Scholar] [CrossRef]
- Sumer, M. Compressive strength and sulfate resistance properties of concretes containing Class F and Class C fly ashes. Constr. Build. Mater. 2012, 34, 531–536. [Google Scholar] [CrossRef]
- Tosun-Felekoglu, K. The effect of C3A content on sulfate durability of Portland limestone cement mortars. Constr. Build. Mater. 2012, 36, 437–447. [Google Scholar] [CrossRef]
- Pipilikaki, P.; Papageorgiou, D.; Teas, C.; Chaniotakis, E.; Katsioti, M. The effect of temperature on thaumasite formation. Cem. Concr. Compos. 2008, 30, 964–969. [Google Scholar] [CrossRef]
- Jaubertie, R.; Laquerbe, M.; Rendell, F. Dgradations de Mortiers sous Différents Milieux Sulfatiques; ESKA EDITIONS: Paris, France, 1999. [Google Scholar]
- Yu, Q.; Sawayama, K.; Sugita, S.; Shoya, M.; Sojima, Y. The reaction between rice husk ash and CA(OH)2 solution and the nature of its product. Cem. Concr. Res. 1999, 29, 37–43. [Google Scholar] [CrossRef]
- ASTM C876-91; Standard Test Method for Half-Cell Potentials of Uncoated Reinforcing Steel in Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 1991.
- ASTM C876-15; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2015.
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Gadve, S.; Mukherjee, A.; Malhotra, S.N. Corrosion of Steel Reinforcements Embedded in FRP Wrapped Concrete. Constr. Build. Mater. 2009, 23, 153–161. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhattacharjee, B. Half-Cell Potential as an Indicator of Chloride-Induced Rebar Corrosion Initiation in RC. J. Mater. Civ. Eng. 2009, 21, 543–552. [Google Scholar] [CrossRef]



| Materials | Nomenclature |
|---|---|
| Ordinary Portland cement CEM I 52.5 N | OPC |
| Raw sediment | RS |
| Sediment calcined flash at 750 °C | SF 750 |
| Witness mortar | M0 |
| Mortar with 15% sediment | M1 |
| Mortar with 20% sediment | M2 |
| Mortar with 30% sediment | M3 |
| Materials | CEM I 52.5 N (OPC) | Raw Sediment (RS) | Flash Calcined Sediment (SF 750) | Natural Sand (NS) |
|---|---|---|---|---|
| Density (g/cm3) | 3.15 | 2.48 | 2.65 | — |
| BET (m2/g) | 1.06 | 7.32 | 27.49 | — |
| LOI (%) | 1.90 | 16.10 | 4.35 | — |
| D10 (μm) | 1.02 | 0.69 | 1.22 | — |
| D50 (μm) | 8.62 | 3.99 | 8.03 | — |
| D90 (μm) | 27.00 | 15.84 | 25.39 | — |
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | Fe2O3 | ZnO | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RS (%) | 0.45 | 0.75 | 8.65 | 33.25 | 1.36 | 0.26 | 1.49 | 13.10 | 0.42 | 3.89 | 0.17 |
| SF750 (%) | 0.63 | 0.91 | 10.30 | 43.45 | 1.56 | 0.32 | 1.84 | 15.40 | 0.54 | 4.95 | 0.25 |
| OPC (%) | 0.79 | 0.90 | 5.30 | 19.54 | — | 3.61 | 0.83 | 64.02 | — | 3.15 | — |
| Materials | M0 | M1 | M2 | M3 |
|---|---|---|---|---|
| Sand | 1350 | 1350 | 1350 | 1350 |
| OPC | 450 | 391.44 | 371.31 | 330.09 |
| SF750 | — | 58.55 | 78.68 | 119.91 |
| Water | 225 | 225 | 225 | 225 |
| Corrosion Risk | Half-Cell Potential (Versus Cu/CuSO4) |
|---|---|
| Severe corrosion | Less than −500 mV |
| High corrosion risk (90% probability) | Between −500 mV & −350 mV |
| Medium corrosion risk (50% probability) | Between −500 mV & −350 mV |
| Low corrosion risk (10% probability) | Higher than −200 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkabouche, A.; Amar, M.; Benzerzour, M.; Abriak, N.-E.; T’kint, M.; Mouli, M. The Influence of External Sulfate Attack on the Durability of Reinforced Mortars in the Presence of Calcined River Sediments. Materials 2023, 16, 6684. https://doi.org/10.3390/ma16206684
Benkabouche A, Amar M, Benzerzour M, Abriak N-E, T’kint M, Mouli M. The Influence of External Sulfate Attack on the Durability of Reinforced Mortars in the Presence of Calcined River Sediments. Materials. 2023; 16(20):6684. https://doi.org/10.3390/ma16206684
Chicago/Turabian StyleBenkabouche, Ali, Mouhamadou Amar, Mahfoud Benzerzour, Nor-Edine Abriak, Michèle T’kint, and Mohamed Mouli. 2023. "The Influence of External Sulfate Attack on the Durability of Reinforced Mortars in the Presence of Calcined River Sediments" Materials 16, no. 20: 6684. https://doi.org/10.3390/ma16206684
APA StyleBenkabouche, A., Amar, M., Benzerzour, M., Abriak, N.-E., T’kint, M., & Mouli, M. (2023). The Influence of External Sulfate Attack on the Durability of Reinforced Mortars in the Presence of Calcined River Sediments. Materials, 16(20), 6684. https://doi.org/10.3390/ma16206684








