Shrinkage, Permeation and Freeze–Thaw Characteristics of Ambient Cured High Calcium-Based Alkali-Activated Engineered Composites
Abstract
:1. Introduction
2. Experimental Investigations
2.1. Materials and Mix Designs
2.2. Mixing Protocols
2.3. Test Methods
3. Results and Discussions
3.1. Compressive Strength
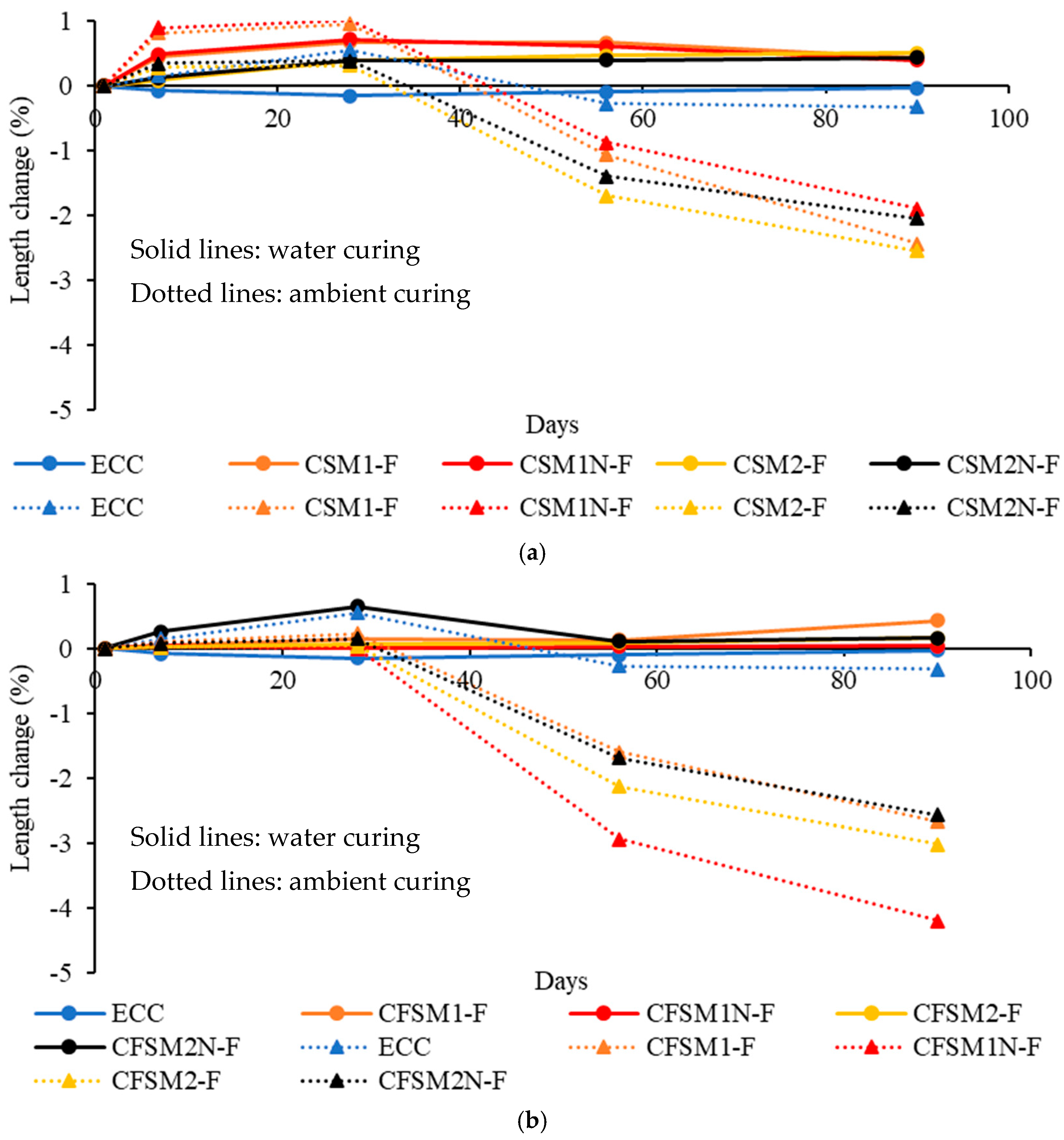
3.2. Shrinkage/Expansion Characteristics
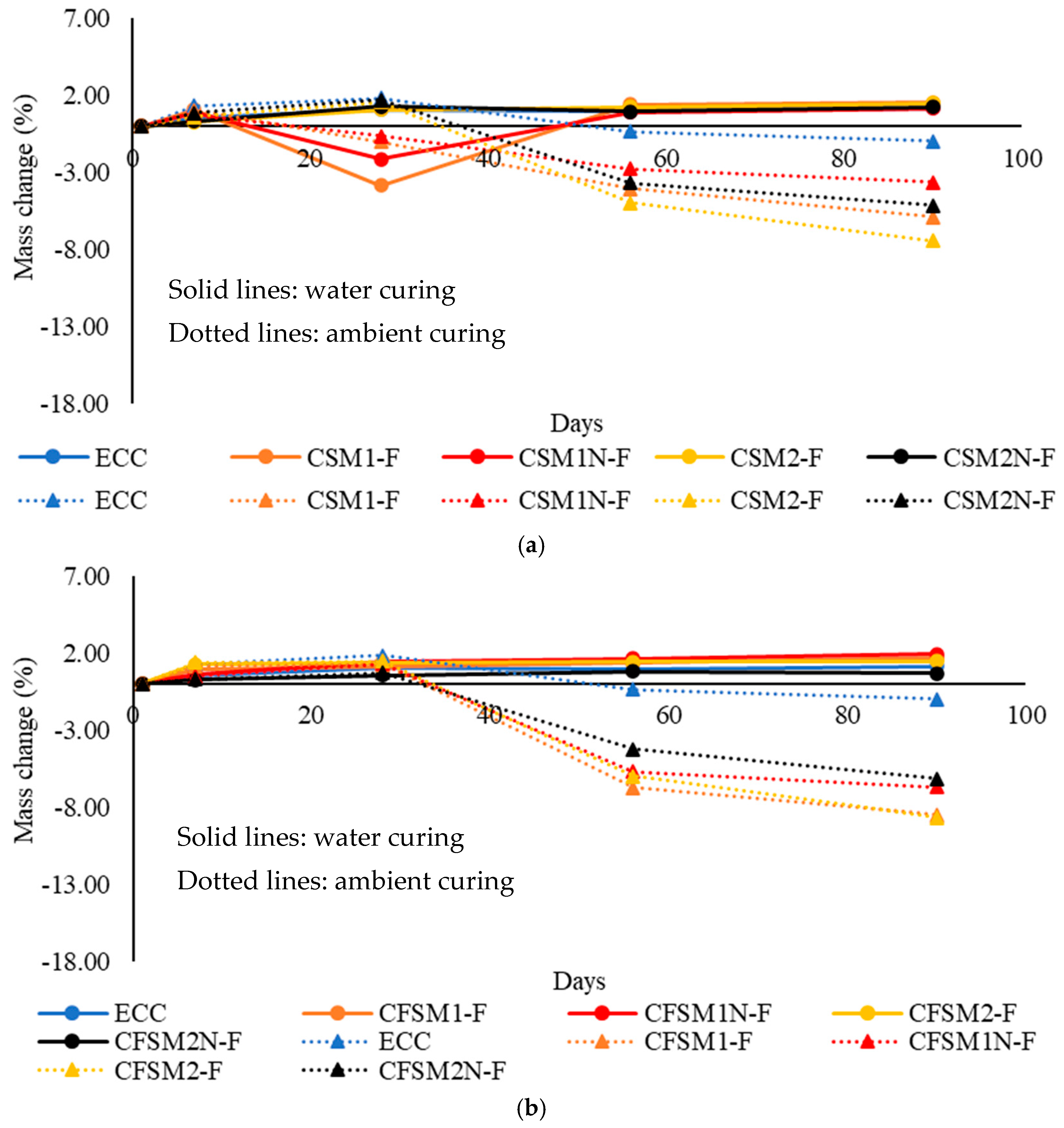
3.3. Mass Change Characteristics
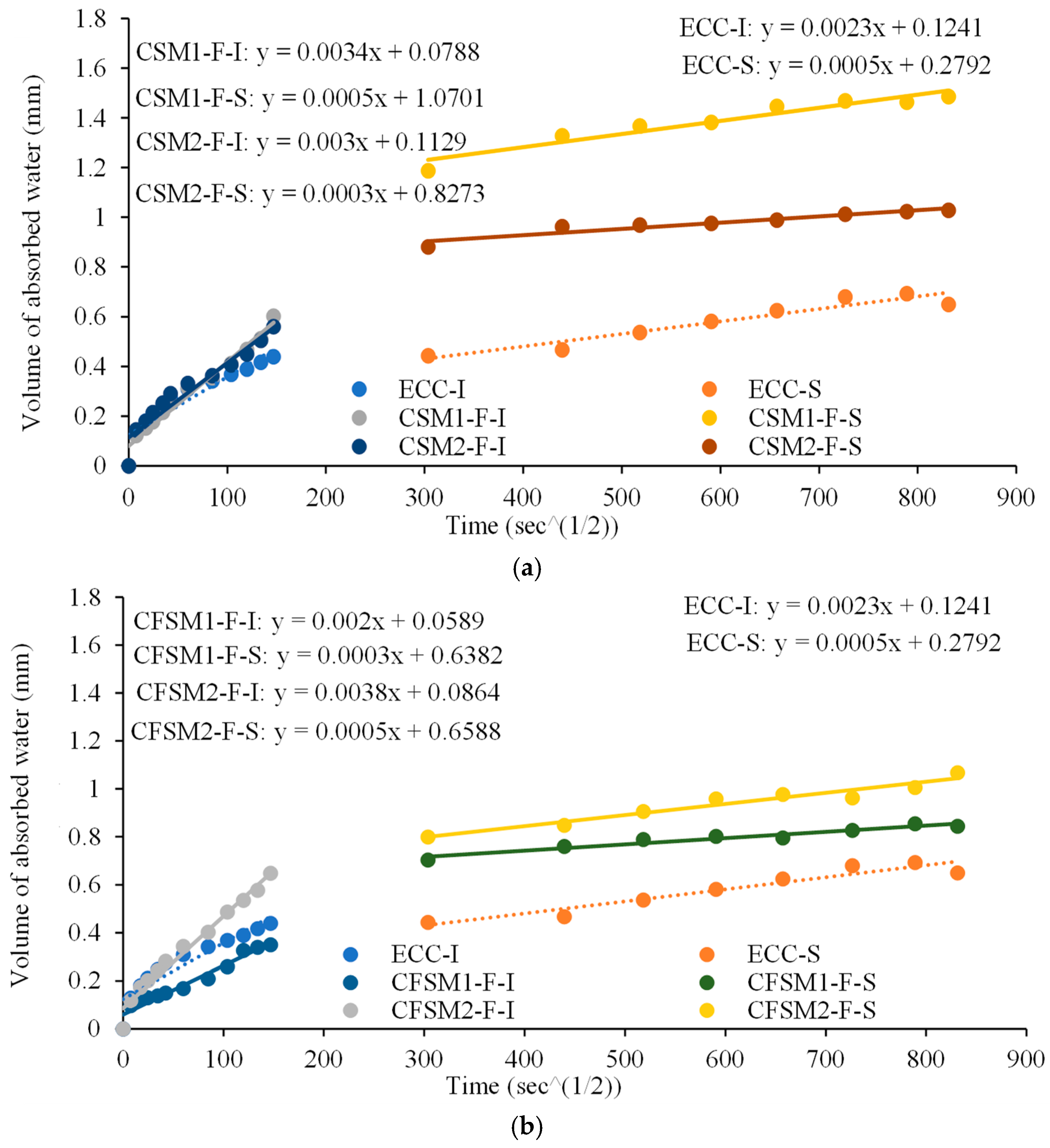
3.4. Permeation (Water Absorption/Sorptivity) Characteristics
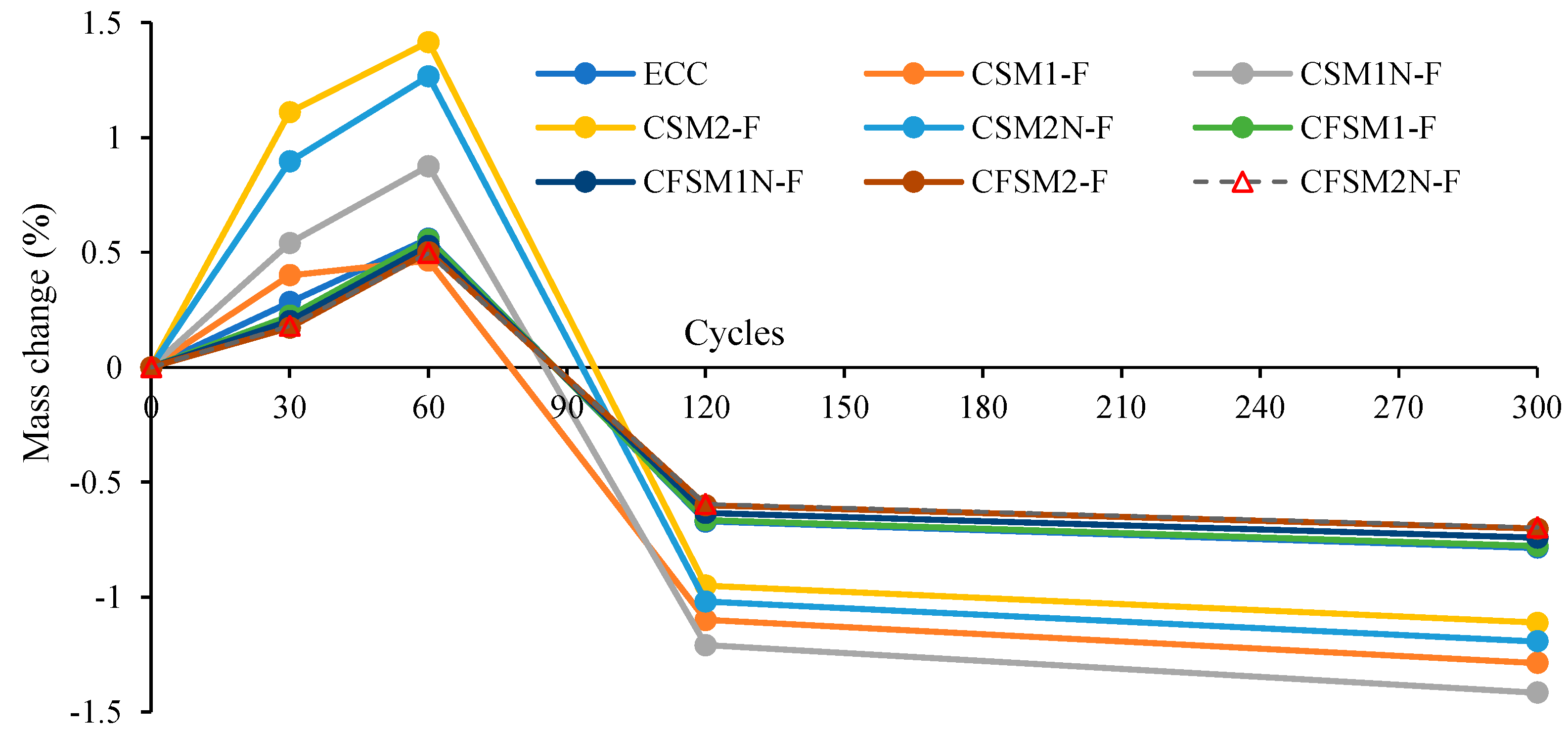
3.5. Resistance to Freezing and Thawing
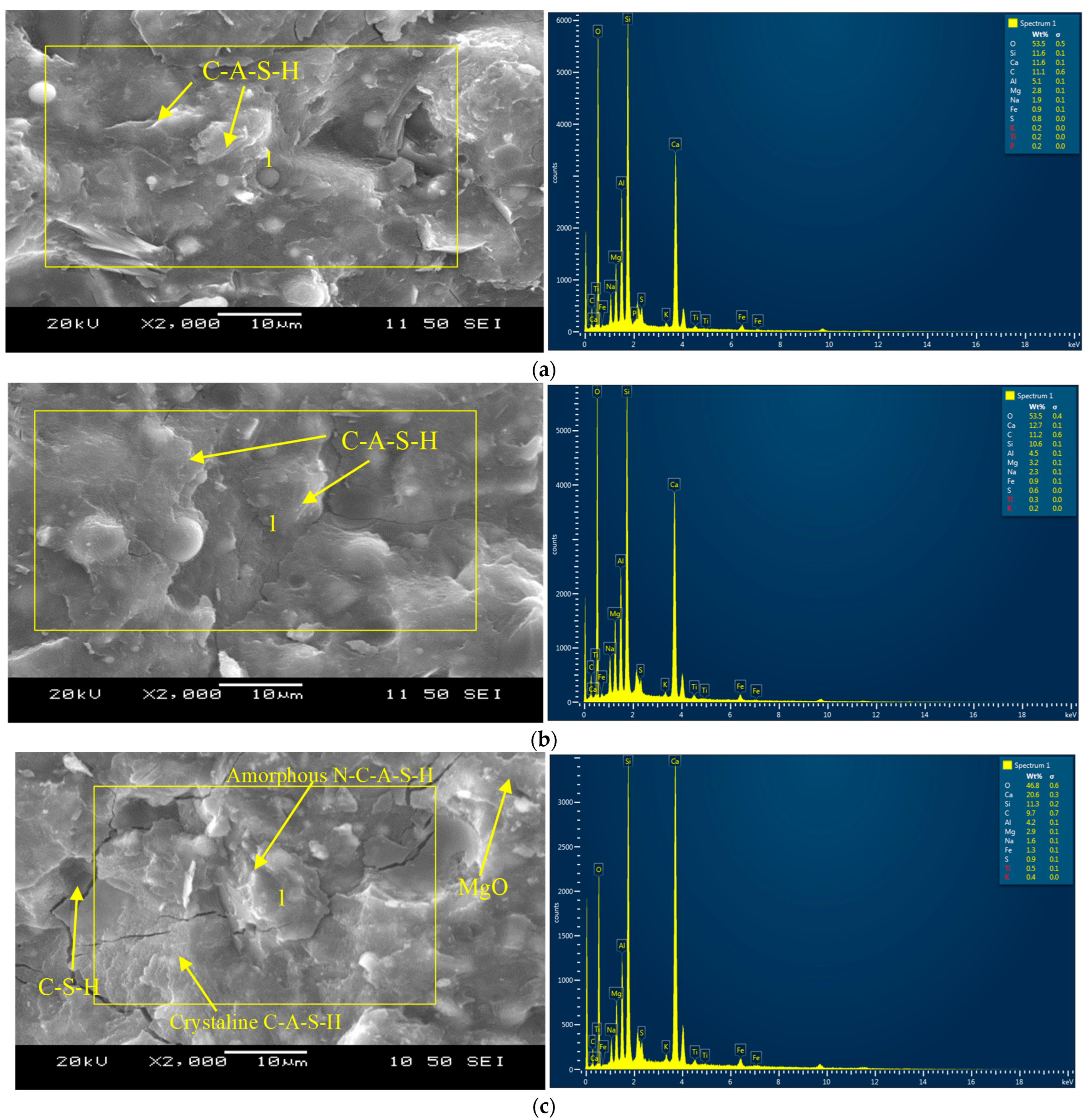
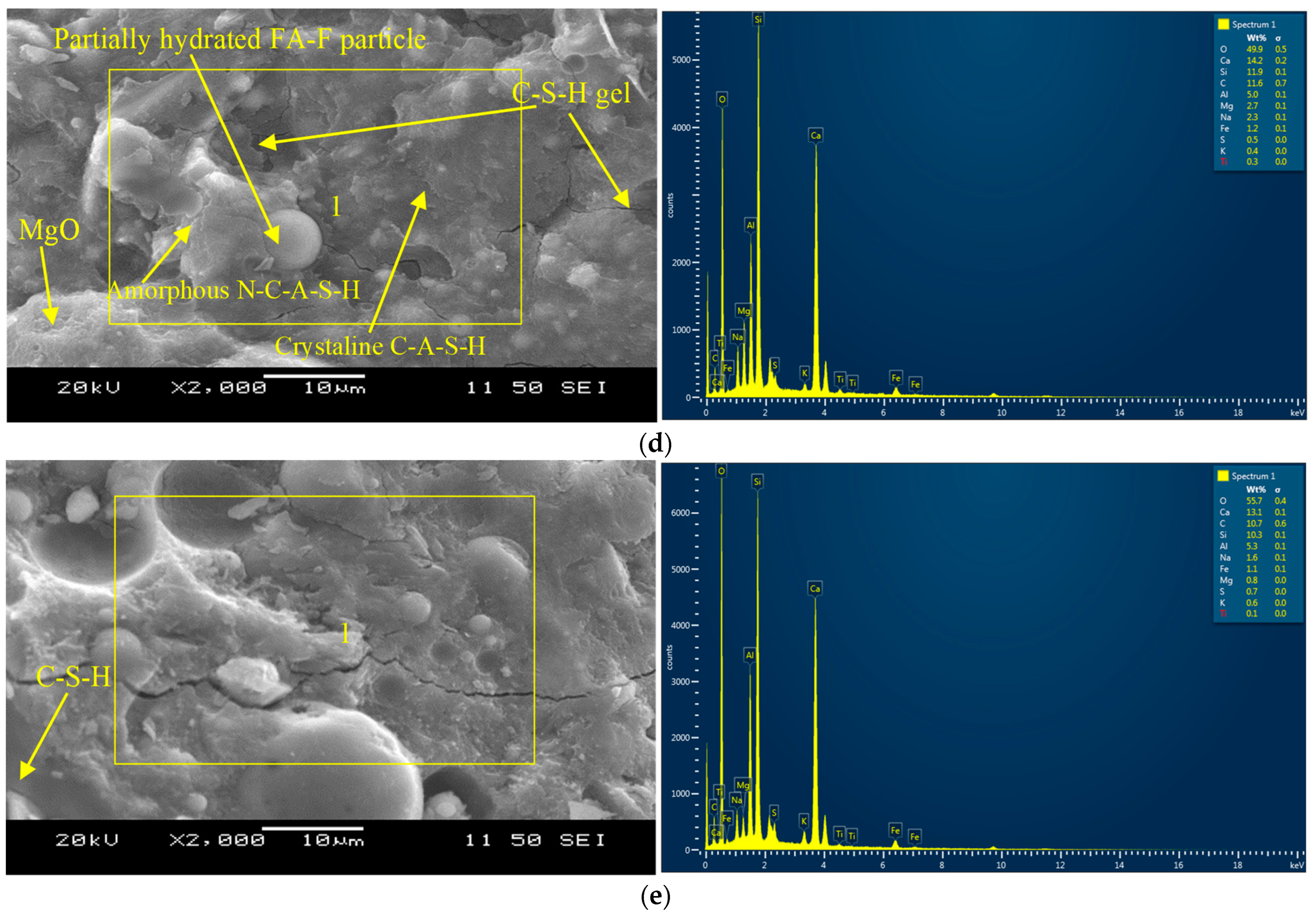
3.6. Microstructural Analysis
4. Conclusions
- The compressive strength of AAECs at 28 days ranged between 34 MPa and 46 MPa (>18 MPa) compared to 52 MPa of conventional cement-based ECC, meeting the criteria for structural applications. PVA fiber addition to AAECs and ECC slightly increased their compressive strength compared to their AAMs without fiber (ranging between 34 and 42 MPa).
- The binary AAECs (made of FA-C and GGBFS) demonstrated lower shrinkage strains (up to −1.77%) and mass decrease (up to −4.98%) in ambient/air curing regime compared to their ternary (made of FA-C, FA-F and GGBFS) counterparts exhibiting shrinkage strains and mass decrease of up to −2.94% and −6.93%, respectively at 56 days. This was attributed to the formation of densified C-A-S-H with additional C-S-H, which led to more compact and denser binary composites than their ternary counterparts. The densification of AAECs due to reaction product development on fibers and fiber-induced micro-confinement leads to lower shrinkage strains and mass change in ambient curing conditions compared to their AAM counterparts.
- The additional porosity/pore network created by the fibers resulted in higher initial sorptivity indices in AAECs than in AAMs. Reagent 1 (calcium hydroxide:sodium silicate = 2.5:1) based ternary AAECs exhibited the lowest initial sorptivity indices (from 0.002 to 0.0022 mm/) amongst all mixes, as some of the partially/un-hydrated fly ash class-F particles acted as fillers reducing pore sizes and number of connected voids. AAECs and AAMs showed lower or comparable secondary sorptivity indices compared to the control ECC and cementitious mortar (FPCM).
- All AAMs and AAECs exhibited higher shrinkage strains than the control ECC mix but showed comparable shrinkage characteristics to the existing alkali-activated materials in the literature. This was attributed to the different reaction processes occurring in the alkali-activated systems and the cement-based materials, namely conventional control ECC.
- All AAECs exhibited satisfactory freeze–thaw resistance, showing relative dynamic modulus of elasticity (RDME) of greater than 90% at 300 cycles with reagent 2 mixes showing higher RDME values compared to those with reagent 1.
- SEM/EDS analysis revealed possible chemical reaction/bonding between the PVA fibers and the reaction products (cementitious and silica-alumina-rich/alkali-activated gels). Efficient bonding between the fibers and reaction products/gels was evident from the SEM micrographs.
- Developed cement-free AAECs showed comparable or superior performance compared to their control cementitious ECC counterparts in terms of strength and durability characteristics, demonstrating their potential applications in sustainable constructions. However, binary AAECs with reagent 1 are preferable in terms of better compressive strength and shrinkage characteristics, similar other properties and simpler mix compositions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAEC | alkali-activated engineered composite |
| AAM | alkali-activated mortar |
| PVA | polyvinyl alcohol |
| FA-C | fly ash class C |
| FA-F | fly ash class F |
| GGBFS | ground granulated blast furnace slag |
| ECC | engineered cementitious composite |
| Pc | Portland cement |
| FPcM | cement mortar |
| UPV | ultrasonic pulse velocity |
| RDM | relative dynamic modulus of elasticity |
| SEM | scanning electron microcopy |
| EDS | energy dispersive spectroscopy |
References
- Santhosh, K.G.; Subhani, S.M.; Bahurudeen, A. An investigation on the effectiveness of quarry dust and sugarcane bagasse ash in Na-based alkali-activated binder. Mater. Today 2023. [Google Scholar] [CrossRef]
- Yaswanth, K.K.; Revathy, J.; Gajalakshmi, P. Strength, durability and micro-structural assessment of slag-agro blended based alkali activated engineered geopolymer composites. Case Stud. Constr. Mater. 2022, 16, e00920. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvilla, M.; Azevedo, A.R.G.; Franco, J.M.F.; Ribeiro, J.C.L. Application of eco-friendly alternative activators in alkali-activated materials: A review. J. Build. Eng. 2020, 35, 102010. [Google Scholar]
- Çevik, A.; Niş, A. (Eds.) Advanced Fiber-Reinforced Alkali-Activated Composites-Design, Mechanical Properties, and Durability, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780443153013. [Google Scholar]
- Duxson, P.; Ndez-Jimé, A.A.F.; Provis, A.J.L.; Lukey, G.C.; Palomo, A.A.; Van Deventer, A.J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient temperature drying shrinkage and cracking in metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef]
- Puertas, F.; Amat, T.; Fernández-Jiménez, A.; Vázquez, T. Mechanical and durable behaviour of alkaline cement mortars reinforced with polypropylene fibres. Cem. Concr. Res. 2003, 33, 2031–2036. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Yao, Y. Use of slag to improve mechanical properties of engineered cementitious composites (ECCs) with high volumes of fly ash. Constr. Build. Mater. 2012, 36, 1076–1081. [Google Scholar] [CrossRef]
- Adesina, A.; Das, S. Drying shrinkage and permeability properties of fibre reinforced alkali-activated composites. Constr. Build. Mater. 2020, 251, 119076. [Google Scholar] [CrossRef]
- Punurai, W.; Kroehong, W.; Saptamongkol, A.; Chindaprasirt, P. Mechanical properties, microstructure and drying shrinkage of hybrid fly ash-basalt fiber geopolymer paste. Constr. Build. Mater. 2018, 186, 62–70. [Google Scholar] [CrossRef]
- Kani, E.N.; Allahverdi, A. Investigating shrinkage changes of natural pozzolan based geopolymer cementpaste. Iran. J. Mater. Sci. Eng. 2011, 8, 50–60. [Google Scholar]
- Cai, Y.; Yu, L.; Yang, Y.; Yang, C. Effect of Early Age-Curing Methods on Drying Shrinkage of Alkali-Activated Slag Concrete Materials. Materials 2019, 12, 1633. [Google Scholar] [CrossRef]
- Li, L.-J.; Liu, Q.-F.; Tang, L.; Hu, Z.; Wen, Y.; Zhang, P. Chloride penetration in freeze–thaw induced cracking concrete: A numerical study. Constr. Build. Mater. 2021, 302, 124291. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, M.; Tang, Q.; Liang, C. Chloride permeability of recycled aggregate concrete under the coupling effect of freezing-thawing, elevated temperature or mechanical damage. Constr. Build. Mater. 2020, 237, 117648. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Mastali, M.; Falah, M.; Shaad, K.M.; Luukkonen, T.; Illikainen, M. Durability of the Reinforced One-Part Alkali-Activated Slag Mortars with Different Fibers. Waste Biomass Valoriz. 2021, 12, 487–501. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Cui, X.; Liu, L. Enhancing Freeze–Thaw Resistance of Alkali-Activated Slag by Metakaolin. ACS Omega 2023, 8, 20869–20880. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, F.; Behfarnia, K. The effects of nano particles on freeze and thaw resistance of alkali-activated slag concrete. Constr. Build. Mater. 2018, 176, 172–178. [Google Scholar] [CrossRef]
- Hossain, M.M.; Karim, M.R.R.; Elahi, M.M.A.; Zain, M.M.F.M. Water absorption and sorptivity of alkali-activated ternary blended composite binder. J. Build. Eng. 2020, 31, 101370. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A. Strength, Fracture and Durability Characteristics of Ambient Cured Alkali—Activated Mortars Incorporating High Calcium. Crystals 2021, 11, 01–30. [Google Scholar]
- Hossain, K.M.A.; Sood, D. The Strength and Fracture Characteristics of One-Part Strain-Hardening Green Alkali-Activated Engineered Composites. Materials 2023, 16, 5077. [Google Scholar] [CrossRef]
- Sherir, M.A.A.; Hossain, K.M.A.; Lachemi, M. Structural performance of polymer fiber reinforced engineered cementitious composites subjected to static and fatigue flexural loading. Polymers 2015, 7, 1299–1330. [Google Scholar] [CrossRef]
- ASTM C109/C109M-16; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- ASTM C157/C157M-17; Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM C1585-13; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2013. [CrossRef]
- ASTM C666-15; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Thomas, R.J.; Lezama, D.; Peethamparan, S. On drying shrinkage in alkali-activated concrete: Improving dimensional stability by aging or heat-curing. Cem. Concr. Res. 2017, 91, 13–23. [Google Scholar] [CrossRef]
- Dolado, J.S.; Griebel, M.; Hamaekers, J. A molecular dynamic study of cementitious calcium silicate hydrate (C-S-H) gels. J. Am. Ceram. Soc. 2007, 90, 3938–3942. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Liu, Q.-F.; Cai, Y.; Peng, H.; Meng, Z.; Mundra, S.; Castel, A. A numerical study on chloride transport in alkali-activated fly ash/slag concretes. Cem. Concr. Res. 2023, 166, 107094. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Wang, Y.; Dai, J.; Xu, Q. Effect of superplasticizers on properties of one-part Ca(OH)2/Na2SO4 activated geopolymer pastes. Constr. Build. Mater. 2020, 241, 117990. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. The durability of one-part alkali activated slag-based mortars in different environments. Sustainablity 2020, 12, 3561. [Google Scholar] [CrossRef]


| Mix ID (Mortars, Composites) | Total SCMs (Binder *) | Portland Cement (Pc) | FA-C | FA-F | GGBFS | Reagent Type | Reagent/ Binder Ratio | Silica Sand | Water-to-Binder Ratio |
|---|---|---|---|---|---|---|---|---|---|
| AAM and PVA fiber incorporated AAEC (with end letter F in mix ID) mixes; CS: Binary and CFS: Ternary mixes | |||||||||
| CSM1, CSM1-F | 1 | 0 | 0.55 | 0 | 0.45 | 1 | 0.09 | 0.3 | 0.35 |
| CSM1N, CSM1N-F | 1 | 0 | 0.50 | 0 | 0.50 | 1 | 0.09 | 0.3 | 0.35 |
| CFSM1, CFSM1-F | 1 | 0 | 0.25 | 0.35 | 0.40 | 1 | 0.09 | 0.3 | 0.35 |
| CFSM1N, CFSM1N-F | 1 | 0 | 0.25 | 0.25 | 0.50 | 1 | 0.09 | 0.3 | 0.35 |
| CSM2, CSM2-F | 1 | 0 | 0.55 | 0 | 0.45 | 2 | 0.12 | 0.3 | 0.375 |
| CSM2N, CSM2N-F | 1 | 0 | 0.50 | 0 | 0.50 | 2 | 0.12 | 0.3 | 0.375 |
| CFSM2, CFSM2-F | 1 | 0 | 0.25 | 0.35 | 0.40 | 2 | 0.12 | 0.3 | 0.375 |
| CFSM2N, CFSM2N-F | 1 | 0 | 0.25 | 0.25 | 0.50 | 2 | 0.12 | 0.3 | 0.375 |
| Control mortar and PVA fiber incorporated engineered cementitious composite (ECC) mixes | |||||||||
| FPCM/ECC | 1 | 0.45 | 0 | 0.55 | 0 | - | - | 0.36 | 0.27 |
| Mix ID (Mortars, Composites) | Reagent | Chemical Ratios (Precursors + Reagents) | ||||
|---|---|---|---|---|---|---|
| Type | Component Ratio | SiO2/Al2O3 | Na2O/SiO2 | CaO/SiO2 | Na2O/Al2O3 | |
| CSM1, CSM1-F | 1 | 1:2.5 | 2.62 | 0.09 | 0.84 | 0.23 |
| CSM1N, CSM1N-F | 1 | 1:2.5 | 2.71 | 0.08 | 0.87 | 0.23 |
| CFSM1, CFSM1-F | 1 | 1:2.5 | 2.75 | 0.08 | 0.59 | 0.22 |
| CFSM1N, CFSM1N-F | 1 | 1:2.5 | 2.86 | 0.07 | 0.69 | 0.21 |
| CSM2, CSM2-F | 2 | 2.5:1 | 2.56 | 0.14 | 1.02 | 0.35 |
| CSM2N, CSM2N-F | 2 | 2.5:1 | 2.64 | 0.13 | 1.02 | 0.35 |
| CFSM2, CFSM2-F | 2 | 2.5:1 | 2.69 | 0.12 | 0.73 | 0.32 |
| CFSM2N, CFSM2N-F | 2 | 2.5:1 | 2.80 | 0.12 | 0.84 | 0.33 |
| FPCM, ECC | - | - | 2.70 | 0.06 | 0.82 | 0.16 |
| Control Mortar/ AAMs | Comp. Strength (MPa) | Length Change (%) | Mass Change (%) | ECC/AAECs | Comp. Strength (MPa) | Length Change (%) | Mass Change (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 d | 56 d | 90 d | 56 d | 90 d | 28 d | 56 d | 90 d | 56 d | 90 d | ||
| FPCM | 43.5 | 0.59 | 0.55 | 1.69 | 1.75 | ECC | 52.5 | −0.10 | −0.03 | 0.94 | 1.16 |
| CSM1 | 42.6 | −0.58 | −0.41 | 0.22 | 0.48 | CSM1-F | 46.5 | 0.67 | 0.43 | 1.42 | 1.52 |
| CSM1N | 35.0 | −0.56 | −0.35 | 0.23 | 0.54 | CSM1N-F | 34.1 | 0.61 | 0.40 | 0.92 | 1.15 |
| CSM2 | 41.2 | 0.35 | 0.45 | 1.86 | 2.41 | CSM2-F | 41.2 | 0.47 | 0.51 | 1.23 | 1.48 |
| CSM2N | 35.8 | −0.15 | −0.15 | 0.71 | 0.80 | CSM2N-F | 40.8 | 0.40 | 0.44 | 0.96 | 1.24 |
| CFSM1 | 40.4 | −0.39 | −0.22 | 0.69 | 1.24 | CFSM1-F | 44.3 | 0.14 | 0.43 | 1.37 | 1.70 |
| CFSM1N | 34 | −0.26 | −0.18 | 0.99 | 0.85 | CFSM1N-F | 36.5 | 0.04 | 0.05 | 1.65 | 1.92 |
| CFSM2 | 42 | 0.13 | 0.32 | 0.64 | 0.55 | CFSM2-F | 42 | 0.10 | 0.16 | 1.45 | 1.48 |
| CFSM2N | 38.1 | 0.13 | 0.29 | 1.70 | 1.35 | CFSM2N-F | 39.2 | 0.11 | 0.16 | 0.79 | 0.66 |
| Control Mortar/AAMs | Length Change (%) | Mass Change (%) | ECC/AAECs | Length Change (%) | Mass Change (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 56 d | 90 d | 56 d | 90 d | 56 d | 90 d | 56 d | 90 d | ||
| FPCM | −0.04 | −0.22 | −0.08 | −0.52 | ECC | −0.27 | −0.32 | −0.36 | −0.99 |
| CSM1 | −1.33 | −1.34 | −7.51 | −11.28 | CSM1-F | −1.06 | −2.44 | −4.06 | −5.90 |
| CSM1N | −0.96 | −0.90 | −5.56 | −7.36 | CSM1N-F | −0.87 | −1.89 | −2.76 | −3.65 |
| CSM2 | −1.98 | −2.65 | −6.15 | −8.21 | CSM2-F | −1.70 | −2.55 | −4.98 | −7.44 |
| CSM2N | −1.58 | −2.14 | −6.71 | −8.67 | CSM2N-F | −1.40 | −2.04 | −3.69 | −5.16 |
| CFSM1 | −2.82 | −3.46 | −9.56 | −10.60 | CFSM1-F | −1.60 | −2.66 | −6.73 | −8.49 |
| CFSM1N | −2.61 | −4.14 | −6.66 | −8.38 | CFSM1N-F | −2.94 | −4.20 | −5.70 | −6.69 |
| CFSM2 | −1.62 | −2.82 | −5.73 | −9.60 | CFSM2-F | −2.13 | −3.02 | −6.01 | −8.67 |
| CFSM2N | −0.03 | −1.66 | −0.86 | −3.51 | CFSM2N-F | −1.69 | −2.57 | −4.24 | −6.15 |
| Mix. Des. | Mortars | Composites | ||||
|---|---|---|---|---|---|---|
| Mortars, Composites | Initial (I) Sorptivity (mm/) | Secondary (S) Sorptivity (mm/) | Decrease from I to S Sorptivity (%) | Initial Sorptivity (mm/) | Secondary Sorptivity (mm/) | Decrease from I to S Sorptivity (%) |
| FPCM, ECC | 1.9 × 10−3 | 5.2 × 10−4 | 72.6 | 2.3 × 10−3 | 5.0 × 10−4 | 78.3 |
| CSM1, CSM1-F | 2.0 × 10−3 | 5.1 × 10−4 | 74.5 | 3.4 × 10−3 | 5.3 × 10−4 | 84.4 |
| CSM1N, CSM1N-F | 2.3 × 10−3 | 5.3 × 10−4 | 77.0 | 3.6 × 10−3 | 4.9 × 10−4 | 86.4 |
| CSM2, CSM2-F | 4.9 × 10−3 | 4.4 × 10−4 | 91.0 | 3.0 × 10−3 | 2.5 × 10−4 | 91.7 |
| CSM2N, CSM2N-F | 5.1 × 10−3 | 4.5 × 10−4 | 91.2 | 3.2 × 10−3 | 3.2 × 10−4 | 90.0 |
| CFSM1, CFSM1-F | 1.0 × 10−3 | 3.1 × 10−4 | 69.0 | 2 × 10−3 | 2.6 × 10−4 | 87.0 |
| CFSM1N, CFSM1N-F | 1.1 × 10−3 | 3.4 × 10−4 | 69.1 | 2.2 × 10−3 | 3.3 × 10−4 | 85.0 |
| CFSM2, CFSM2-F | 3.0 × 10−3 | 1.9 × 10−4 | 93.7 | 3.8 × 10−3 | 4.6 × 10−4 | 87.9 |
| CFSM2N, CFSM2N-F | 3.3 × 10−3 | 2.0 × 10−4 | 93.9 | 4.1 × 10−3 | 4.7 × 10−4 | 88.5 |
| Composites | Ultrasonic Pulse Velocity (UPV) in Longitudinal Direction (355.6 mm) | Ultrasonic Pulse Velocity (UPV) in Transverse Direction (50.8 mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Freeze–Thaw Cycles | ||||||||||
| 0 | 30 | 60 | 120 | 300 | 0 | 30 | 60 | 120 | 300 | |
| ECC | 3977 | 4022 | 4000 | 3800 | 3640 | 4047 | 4080 | 4047 | 3804 | 3642 |
| CSM1-F | 3358 | 3243 | 3600 | 3420 | 3276 | 3227 | 2318 | 3493 | 3283 | 3144 |
| CSM1N-F | 3532 | 3330 | 3471 | 3297 | 3159 | 3517 | 2849 | 3541 | 3329 | 3187 |
| CSM2-F | 3614 | 3515 | 3571 | 3392 | 3250 | 3617 | 3617 | 3493 | 3283 | 3144 |
| CSM2N-F | 3947 | 3887 | 3912 | 3716 | 3560 | 3984 | 4014 | 3922 | 3687 | 3530 |
| CFSM1-F | 3389 | 3368 | 3441 | 3269 | 3131 | 2428 | 2428 | 2550 | 2397 | 2295 |
| CFSM1N-F | 3425 | 3409 | 3351 | 3183 | 3049 | 3493 | 3517 | 3493 | 3283 | 3144 |
| CFSM2-F | 3228 | 3214 | 3138 | 2981 | 2856 | 2361 | 2318 | 2394 | 2250 | 2155 |
| CFSM2N-F | 3636 | 3600 | 3448 | 3276 | 3138 | 3777 | 3722 | 3750 | 3525 | 3375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, K.M.A.; Sood, D. Shrinkage, Permeation and Freeze–Thaw Characteristics of Ambient Cured High Calcium-Based Alkali-Activated Engineered Composites. Materials 2023, 16, 7101. https://doi.org/10.3390/ma16227101
Hossain KMA, Sood D. Shrinkage, Permeation and Freeze–Thaw Characteristics of Ambient Cured High Calcium-Based Alkali-Activated Engineered Composites. Materials. 2023; 16(22):7101. https://doi.org/10.3390/ma16227101
Chicago/Turabian StyleHossain, Khandaker M. A., and Dhruv Sood. 2023. "Shrinkage, Permeation and Freeze–Thaw Characteristics of Ambient Cured High Calcium-Based Alkali-Activated Engineered Composites" Materials 16, no. 22: 7101. https://doi.org/10.3390/ma16227101
APA StyleHossain, K. M. A., & Sood, D. (2023). Shrinkage, Permeation and Freeze–Thaw Characteristics of Ambient Cured High Calcium-Based Alkali-Activated Engineered Composites. Materials, 16(22), 7101. https://doi.org/10.3390/ma16227101





