Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks
Abstract
1. Introduction
2. Methods
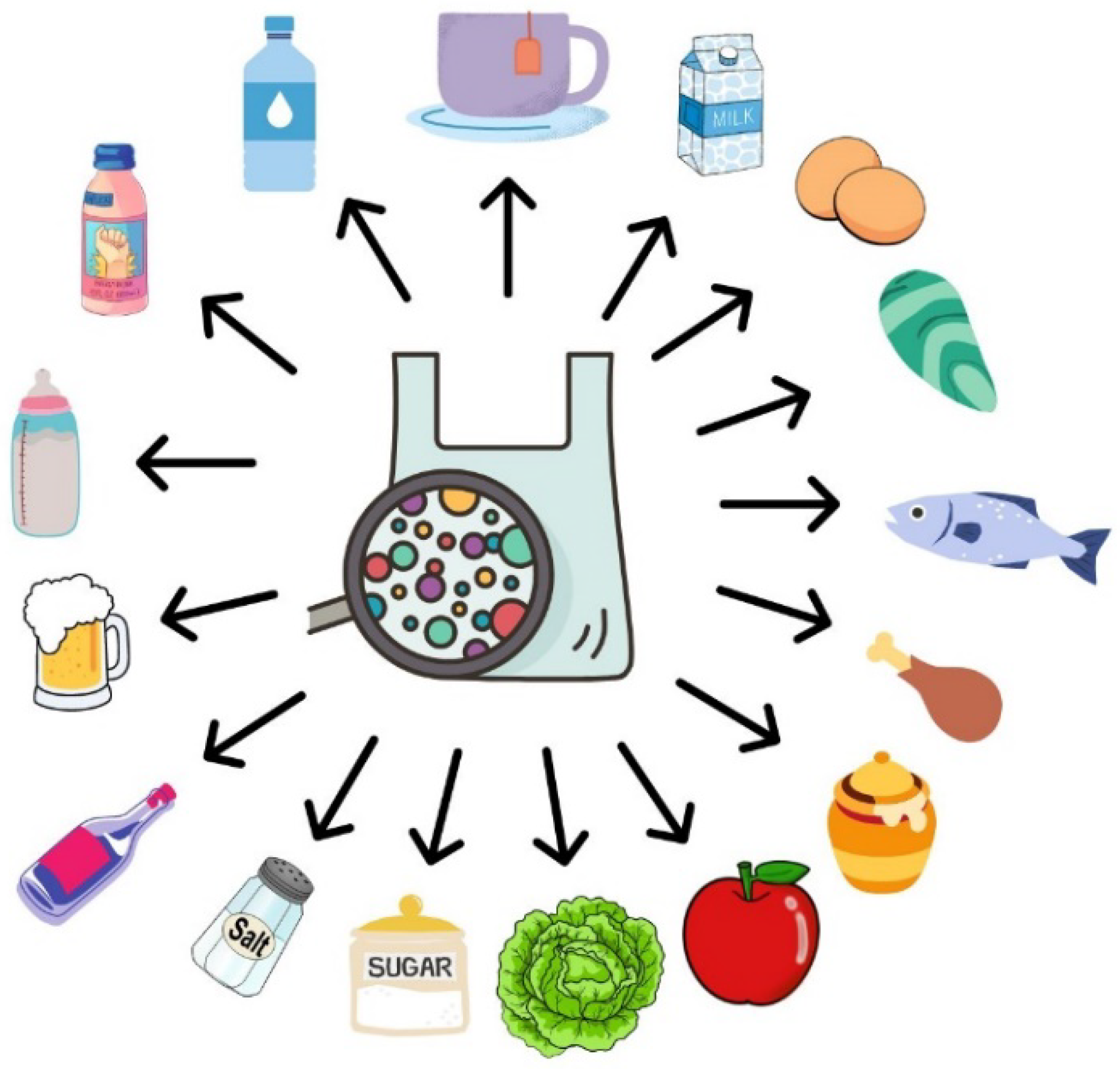
3. Food Packaging
4. Plastics Degradation
4.1. Mechanical Degradation
4.2. Thermal Degradation
4.3. Photodegradation
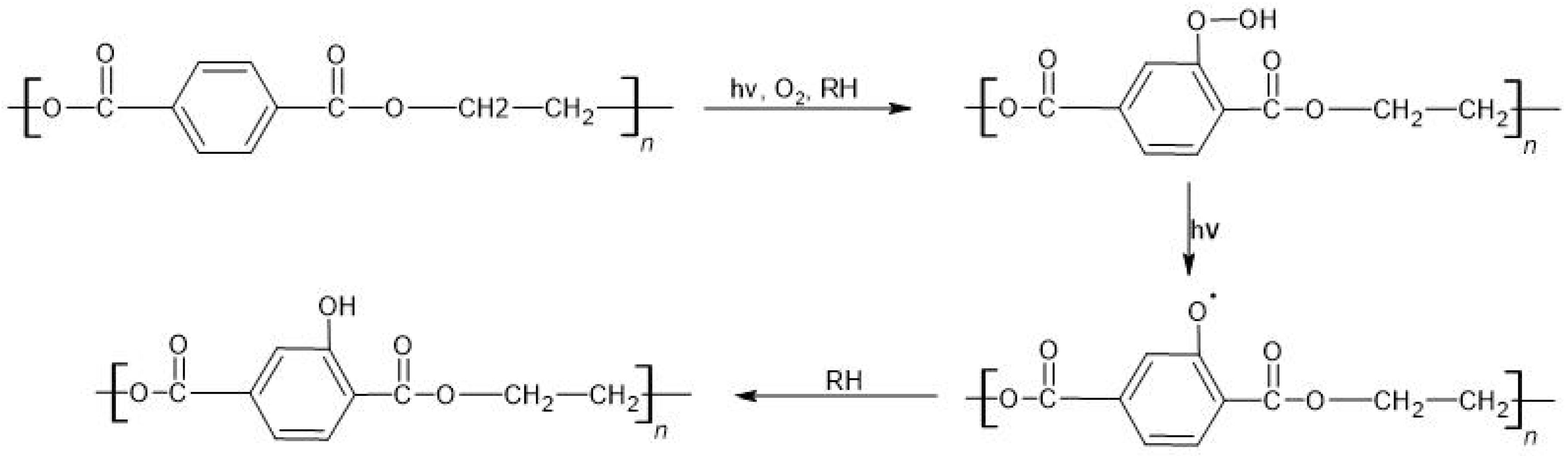
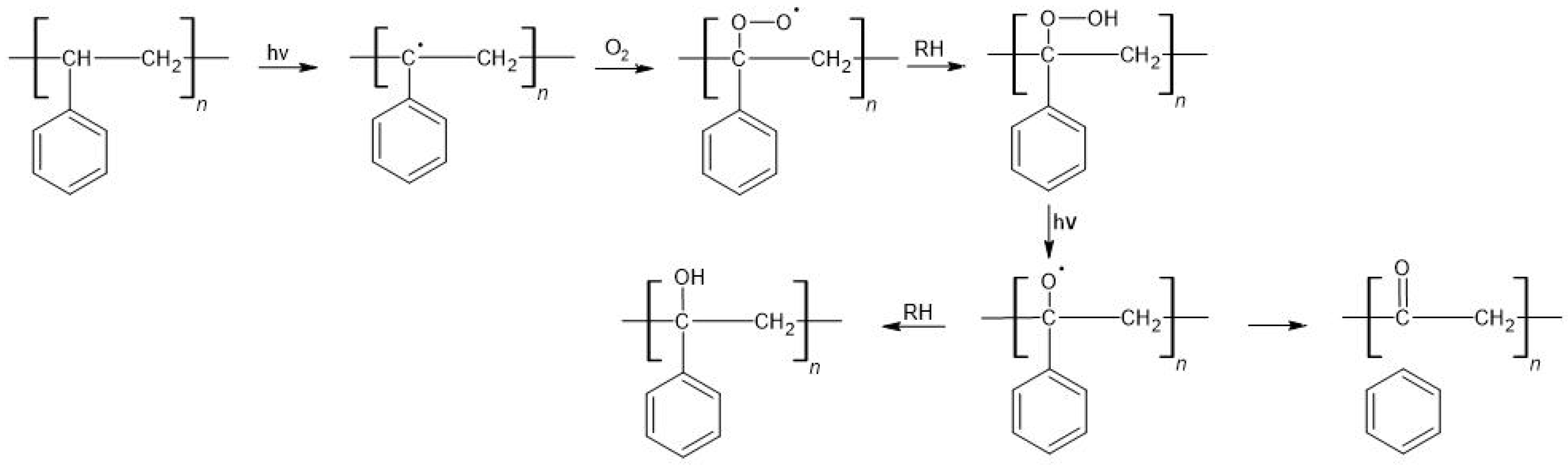
4.3.1. Course of Plastic Degradation
4.3.2. Effect of Various Factors on the Photodegradation of MP
Effect of Radiation
Effect of Oxygen
Effect of Ozone
Effect of Oxides
Effect of Metal Compounds
Effect of Mechanical Factors
4.3.3. Changes in the Properties of MPs
4.3.4. Photodegradation of Selected Polymers
Polyethylene and Polypropylene
Poly(Ethylene Terephthalate)
Polystyrene
4.4. Chemical Degradation
4.5. Biological Degradation
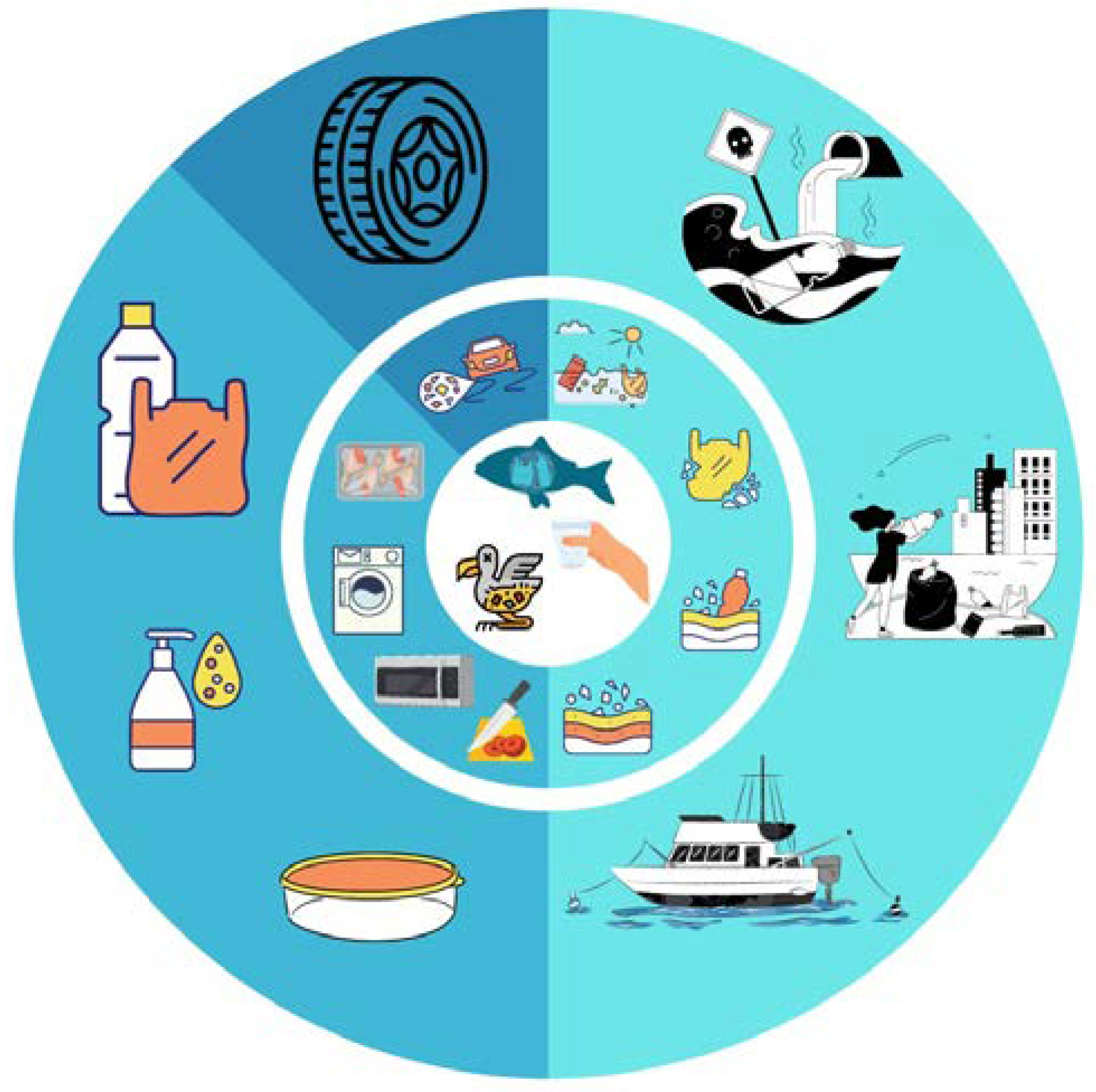
5. Packaging Waste Dump
5.1. Terrestrial Environment
5.1.1. Sources and Transport of Microplastics in the Terrestrial Environment
5.1.2. Impact of Microplastic on the Terrestrial Environment
5.2. Aquatic Environment
5.2.1. Sources and Transport of Microplastics in the Aquatic Environment
5.2.2. Impact of Microplastic on the Aquatic Environment
6. Biodegradable Plastics
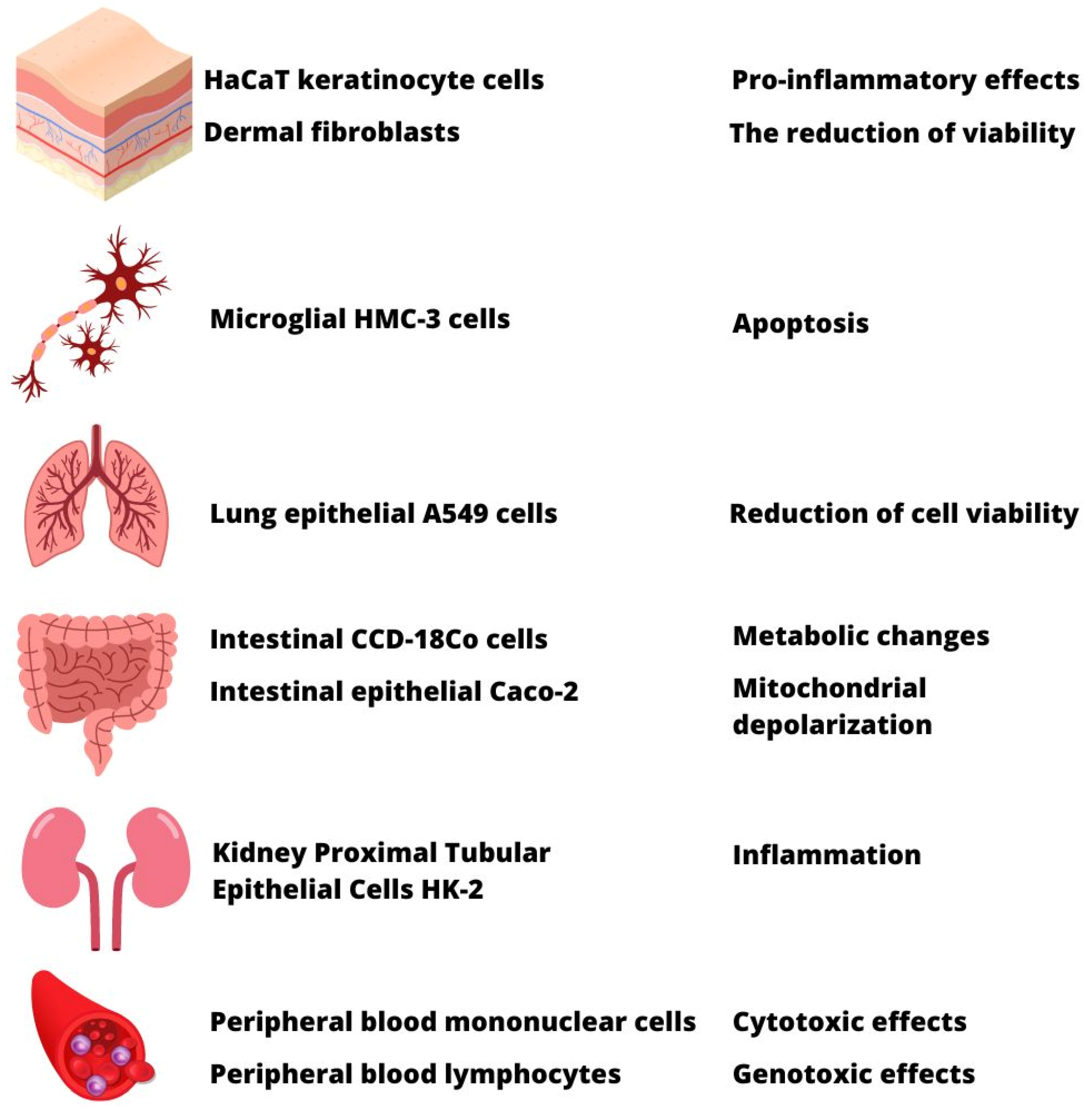
7. Implication of MPs Contamination on Human Health and Toxicological Studies
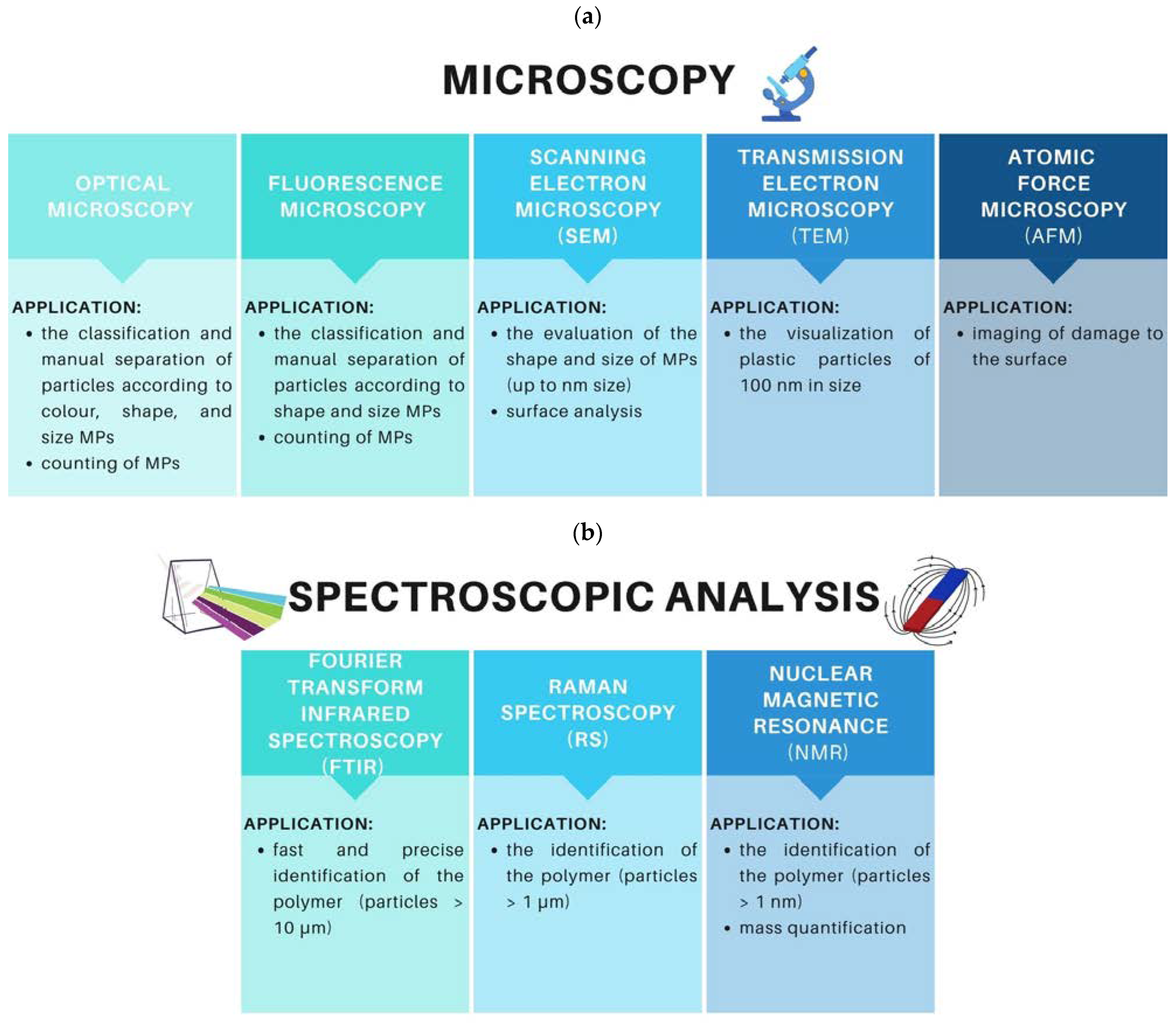
8. Identification Methods of MPs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- García Rellán, A.; Vázquez Ares, D.; Vázquez Brea, C.; Francisco López, A.; Bello Bugallo, P.M. Sources, Sinks and Transformations of Plastics in Our Oceans: Review, Management Strategies and Modelling. Sci. Total Environ. 2023, 854, 158745. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of Microplastics in Water by Micro-Raman Spectroscopy: Release of Plastic Particles from Different Packaging into Mineral Water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Grembecka, M. Food and Human Safety: The Impact of Microplastics. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Mattsson, K.; Jocic, S.; Doverbratt, I.; Hansson, L.-A. Nanoplastics in the Aquatic Environment. In Microplastic Contamination in Aquatic Environments: An Emerging Matter of Environmental Urgency; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 13, pp. 379–399. [Google Scholar] [CrossRef]
- Li, X.; Liang, R.; Li, Y.; Zhang, Y.; Wang, Y.; Li, K. Microplastics in Inland Freshwater Environments with Different Regional Functions: A Case Study on the Chengdu Plain. Sci. Total Environ. 2021, 789, 147938. [Google Scholar] [CrossRef]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Z.; Chen, L.; Cui, Q.; Cui, Y.; Song, D.; Fang, L. Review on Migration, Transformation and Ecological Impacts of Microplastics in Soil. Appl. Soil Ecol. 2022, 176, 104486. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Chen, H.; Yuan, R.; Wang, F. Distribution, Biological Effects and Biofilms of Microplastics in Freshwater Systems—A Review. Chemosphere 2022, 299, 134370. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Peng, X.; Su, Y.; Fu, P.; Ge, C.; Zhao, J.; Yang, L.; Yu, H.; Peng, L. Occurrence and Spatial Distribution of Microplastics, and Their Correlation with Petroleum in Coastal Waters of Hainan Island, China. Environ. Pollut. 2022, 294, 118636. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First Evidence of Microplastics in Antarctic Snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Bao, R.; Wang, Z.; Qi, H.; Mehmood, T.; Cai, M.; Zhang, Y.; Yang, R.; Peng, L.; Liu, F. Occurrence and Distribution of Microplastics in Wastewater Treatment Plant in a Tropical Region of China. J. Clean. Prod. 2022, 349, 131454. [Google Scholar] [CrossRef]
- Šaravanja, A.; Pušić, T.; Dekanić, T. Microplastics in Wastewater by Washing Polyester Fabrics. Materials 2022, 15, 2683. [Google Scholar] [CrossRef]
- Yao, Y.; Glamoclija, M.; Murphy, A.; Gao, Y. Characterization of Microplastics in Indoor and Ambient Air in Northern New Jersey. Environ. Res. 2022, 207, 112142. [Google Scholar] [CrossRef]
- Yu, Z.-f.; Song, S.; Xu, X.-l.; Ma, Q.; Lu, Y. Sources, Migration, Accumulation and Influence of Microplastics in Terrestrial Plant Communities. Environ. Exp. Bot. 2021, 192, 104635. [Google Scholar] [CrossRef]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of Microplastics in Local Marine Organisms Using a Multi-Technology System. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Huber, M.; Archodoulaki, V.M.; Pomakhina, E.; Pukánszky, B.; Zinöcker, E.; Gahleitner, M. Environmental Degradation and Formation of Secondary Microplastics from Packaging Material: A Polypropylene Film Case Study. Polym. Degrad. Stab. 2022, 195, 109794. [Google Scholar] [CrossRef]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic Pollution in Commercial Salt for Human Consumption: A Review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Hou, C.; Wu, Y.; Teng, J.; Zhang, C.; Tan, H.; Shan, E.; Zhang, W.; Zhao, J. Microplastic Uptake in Commercial Fishes from the Bohai Sea, China. Chemosphere 2021, 263, 127962. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N.; Thomson, K.T. Microplastics in the Edible Tissues of Shellfishes Sold for Human Consumption. Chemosphere 2021, 264, 128554. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; He, C.; Zhang, M.; Ju, P.; Ding, N.X. An Examination of the Occurrence and Potential Risks of Microplastics across Various Shellfish. Sci. Total Environ. 2020, 739, 139887. [Google Scholar] [CrossRef]
- Chinfak, N.; Sompongchaiyakul, P.; Charoenpong, C.; Shi, H.; Yeemin, T.; Zhang, J. Abundance, Composition, and Fate of Microplastics in Water, Sediment, and Shellfish in the Tapi-Phumduang River System and Bandon Bay, Thailand. Sci. Total Environ. 2021, 781, 146700. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Lechat, B.; Sire, O.; le Maguer, G.; le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics Contamination in Eggs: Detection, Occurrence and Status. Food Chem. 2022, 397, 133771. [Google Scholar] [CrossRef]
- Kapukotuwa, R.W.M.G.K.; Jayasena, N.; Weerakoon, K.C.; Abayasekara, C.L.; Rajakaruna, R.S. High Levels of Microplastics in Commercial Salt and Industrial Salterns in Sri Lanka. Mar. Pollut. Bull. 2022, 174, 113239. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, N.S.; Kim, J.S.; Kim, S.K. Variation and Uncertainty of Microplastics in Commercial Table Salts: Critical Review and Validation. J. Hazard. Mater. 2021, 402, 123743. [Google Scholar] [CrossRef]
- Manimozhi, N.; Rani, V.; Sudhan, C.; Manimekalai, D.; Shalini, R.; Abarna, K.M. Spatiotemporal Occurrence, Distribution, and Characterization of Microplastics in Salt Pans of the Coastal Region of the Gulf of Mannar, Southeast Coast of India. Reg. Stud. Mar. Sci. 2022, 53, 102350. [Google Scholar] [CrossRef]
- Afrin, S.; Rahman, M.M.; Hossain, M.N.; Uddin, M.K.; Malafaia, G. Are There Plastic Particles in My Sugar? A Pioneering Study on the Characterization of Microplastics in Commercial Sugars and Risk Assessment. Sci. Total Environ. 2022, 837, 155849. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Tympa, L.-E.; Katsara, K.; Moschou, P.N.; Kenanakis, G.; Papadakis, V.M. Do Microplastics Enter Our Food Chain Via Root Vegetables? A Raman Based Spectroscopic Study on Raphanus Sativus. Materials 2021, 14, 2329. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; de France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- al Naggar, Y.; Brinkmann, M.; Sayes, C.M.; Al-Kahtani, S.N.; Dar, S.A.; El-Seedi, H.R.; Grünewald, B.; Giesy, J.P. Are Honey Bees at Risk from Microplastics? Tocics 2021, 9, 109. [Google Scholar] [CrossRef]
- Balzani, P.; Galeotti, G.; Scheggi, S.; Masoni, A.; Santini, G.; Baracchi, D. Acute and Chronic Ingestion of Polyethylene (PE) Microplastics Has Mild Effects on Honey Bee Health and Cognition. Environ. Pollut. 2022, 305, 119318. [Google Scholar] [CrossRef]
- Prata, J.C.; Paço, A.; Reis, V.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of Microplastics in White Wines Capped with Polyethylene Stoppers Using Micro-Raman Spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Afrin, S.; Rahman, M.; Akbor, A.; Siddique, A.B.; Uddin, K.; Malafaia, G. Is There Tea Complemented with the Appealing Flavor of Microplastics? A Pioneering Study on Plastic Pollution in Commercially Available Tea Bags in Bangladesh. Sci. Total Environ. 2022, 837, 155833. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First Study of Its Kind on the Microplastic Contamination of Soft Drinks, Cold Tea and Energy Drinks—Future Research and Environmental Considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of Various Microplastics in Placentas, Meconium, Infant Feces, Breastmilk and Infant Formula: A Pilot Prospective Study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, E.B.; Sankhla, M.S.; Bhat, R.A.; Bhagat, D.S. Microplastics from Food Packaging: An Overview of Human Consumption, Health Threats, and Alternative Solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100608. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. 2017, 56, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.; Thomas, P.; Stuart, B. An Atomic Force Microscopy Investigation of Plastic Wrapping Materials of Forensic Relevance Buried in Soil Environments. Aust. J. Forensic Sci. 2019, 51, 596–605. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and Characterization of Microplastic and Nanoplastic Particles in Marine Environment. Environ. Pollut. 2022, 297, 118773. [Google Scholar] [CrossRef] [PubMed]
- Rydz, J.; Musiol, M.; Zawidlak-Wegrzyńska, B.; Sikorska, W. Present and Future of Biodegradable Polymers for Food Packaging Applications. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 14, pp. 431–467. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food Packaging in the Circular Economy: Overview of Chemical Safety Aspects for Commonly Used Materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- Hahladakis, J.N.; Iacovidou, E. Closing the Loop on Plastic Packaging Materials: What Is Quality and How Does It Affect Their Circularity? Sci. Total Environ. 2018, 630, 1394–1400. [Google Scholar] [CrossRef]
- European Union. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Off. J. Eur. Union 2019, 155, 1–19. [Google Scholar]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Fueser, H.; Mueller, M.T.; Weiss, L.; Höss, S.; Traunspurger, W. Ingestion of Microplastics by Nematodes Depends on Feeding Strategy and Buccal Cavity Size. Environ. Pollut. 2019, 255, 113227. [Google Scholar] [CrossRef]
- He, Y.J.; Qin, Y.; Zhang, T.L.; Zhu, Y.Y.; Wang, Z.J.; Zhou, Z.S.; Xie, T.Z.; Luo, X.D. Migration of (Non-) Intentionally Added Substances and Microplastics from Microwavable Plastic Food Containers. J. Hazard. Mater. 2021, 417, 126074. [Google Scholar] [CrossRef]
- Guan, Y.; Gong, J.; Song, B.; Li, J.; Fang, S.; Tang, S.; Cao, W.; Li, Y.; Chen, Z.; Ye, J.; et al. The Effect of UV Exposure on Conventional and Degradable Microplastics Adsorption for Pb (II) in Sediment. Chemosphere 2022, 286, 131777. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Sheng, Y.; Xiang, Q.; Zhou, Y.; Cizdziel, J.V. Effect of Prothioconazole on the Degradation of Microplastics Derived from Mulching Plastic Film: Apparent Change and Interaction with Heavy Metals in Soil. Environ. Pollut. 2020, 260, 113988. [Google Scholar] [CrossRef]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible Light Photocatalytic Degradation of Polypropylene Microplastics in a Continuous Water Flow System. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef]
- Wróbel, M.; Szymańska, S.; Kowalkowski, T.; Hrynkiewicz, K. Selection of Microorganisms Capable of Polyethylene (PE) and Polypropylene (PP) Degradation. Microbiol. Res. 2023, 267, 127251. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth Kinetics and Biodeterioration of Polypropylene Microplastics by Bacillus sp. and Rhodococcus sp. Isolated from Mangrove Sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Khoironi, A.; Dianratri, I.; Suherman, S.; Muhammad, F.; Vaidyanathan, S. Interactions between Polyethylene and Polypropylene Microplastics and Spirulina sp. Microalgae in Aquatic Systems. Heliyon 2021, 7, e07676. [Google Scholar] [CrossRef]
- Yuan, J.; Cao, J.; Yu, F.; Ma, J. Microbial Degradation of Polystyrene Microplastics by a Novel Isolated Bacterium in Aquatic Ecosystem. Sustain. Chem. Pharm. 2022, 30, 100873. [Google Scholar] [CrossRef]
- Dilara Hatinoglu, M.; Dilek Sanin, F. Fate and Effects of Polyethylene Terephthalate (PET) Microplastics during Anaerobic Digestion of Alkaline-Thermal Pretreated Sludge. Waste Manag. 2022, 153, 376–385. [Google Scholar] [CrossRef]
- Auta, H.S.; Abioye, O.P.; Aransiola, S.A.; Bala, J.D.; Chukwuemeka, V.I.; Hassan, A.; Aziz, A.; Fauziah, S.H. Enhanced Microbial Degradation of PET and PS Microplastics under Natural Conditions in Mangrove Environment. J. Environ. Manag. 2022, 304, 114273. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C.; de Pedro, Z.M.; Casas, J.A. Insights into the Degradation of Microplastics by Fenton Oxidation: From Surface Modification to Mineralization. Chemosphere 2022, 309, 136809. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Dong, J.; Huang, H.; Deng, Y.; Wen, H.; Xu, Z.; Ceylan, S. Interaction between Feedstocks, Absorbers and Catalysts in the Microwave Pyrolysis Process of Waste Plastics. J. Clean. Prod. 2021, 291, 125857. [Google Scholar] [CrossRef]
- Kamalian, P.; Khorasani, S.N.; Abdolmaleki, A.; Karevan, M.; Khalili, S.; Shirani, M.; Neisiany, R.E. Toward the Development of Polyethylene Photocatalytic Degradation. J. Polym. Eng. 2020, 40, 181–191. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible Light Photocatalytic Degradation of Microplastic Residues with Zinc Oxide Nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Amelia, D.; Fathul Karamah, E.; Mahardika, M.; Syafri, E.; Mavinkere Rangappa, S.; Siengchin, S.; Asrofi, M. Effect of Advanced Oxidation Process for Chemical Structure Changes of Polyethylene Microplastics. Mater. Today Proc. 2022, 52, 2501–2504. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Chen, Z.; Yu, Z.; Xue, J.; Luan, T.; Chen, S.; Zhou, S. Mechanisms of Polystyrene Microplastic Degradation by the Microbially Driven Fenton Reaction. Water Res. 2022, 223, 118979. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Jiang, R.; You, J.; Ouyang, G. New Insights into the Photo-Degraded Polystyrene Microplastic: Effect on the Release of Volatile Organic Compounds. J. Hazard. Mater. 2022, 431, 128523. [Google Scholar] [CrossRef]
- Lomonaco, T.; Manco, E.; Corti, A.; la Nasa, J.; Ghimenti, S.; Biagini, D.; di Francesco, F.; Modugno, F.; Ceccarini, A.; Fuoco, R.; et al. Release of Harmful Volatile Organic Compounds (VOCs) from Photo-Degraded Plastic Debris: A Neglected Source of environmental pollution. J. Hazard. Mater. 2020, 394, 122596. [Google Scholar] [CrossRef]
- Kumar, V.; Maitra, S.S.; Singh, R.; Burnwal, D.K. Acclimatization of a Newly Isolated Bacteria in Monomer Tere-Phthalic Acid (TPA) May Enable It to Attack the Polymer Poly-Ethylene Tere-Phthalate(PET). J. Environ. Chem. Eng. 2020, 8, 103977. [Google Scholar] [CrossRef]
- Khairul Anuar, N.F.S.; Huyop, F.; Ur-Rehman, G.; Abdullah, F.; Normi, Y.M.; Sabullah, M.K.; Abdul Wahab, R. An Overview into Polyethylene Terephthalate (PET) Hydrolases and Efforts in Tailoring Enzymes for Improved Plastic Degradation. Int. J. Mol. Sci. 2022, 23, 12644. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current Progress on Plastic/Microplastic Degradation: Fact Influences and Mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef]
- Garnai Hirsch, S.; Barel, B.; Segal, E. Characterization of Surface Phenomena: Probing Early Stage Degradation of Low-Density Polyethylene Films. Polym. Eng. Sci. 2019, 59, E129–E137. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Boyle, D.; Catarino, A.I.; Clark, N.J.; Henry, T.B. Polyvinyl Chloride (PVC) Plastic Fragments Release Pb Additives That Are Bioavailable in Zebrafish. Environ. Pollut. 2020, 263, 114422. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Alomar, C.; Paniagua González, G.; Garcinuño Martínez, R.M.; Soliz Rojas, D.L.; Fernández Hernando, P.; Deudero, S. Assessing Microplastic Ingestion and Occurrence of Bisphenols and Phthalates in Bivalves, Fish and Holothurians from a Mediterranean Marine Protected Area. Environ. Res. 2022, 214, 114034. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Z.; Tang, M.; Yang, X.; Tsang, Y.F. Polycarbonate Microplastics Induce Oxidative Stress in Anaerobic Digestion of Waste Activated Sludge by Leaching Bisphenol A. J. Hazard. Mater. 2023, 443, 130158. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and Its Analogs in Muscle and Liver of Fish from the North East Atlantic Ocean in Relation to Microplastic Contamination. Exposure and Risk to Human Consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef]
- Ahmad, S.; Arsalan, A.; Hashmi, A.; Khan, M.A.; Siddiqui, W.A.; Younus, H. A Comparative Study Based on Activity, Conformation and Computational Analysis on the Inhibition of Human Salivary Aldehyde Dehydrogenase by Phthalate Plasticizers: Implications in Assessing the Safety of Packaged Food Items. Toxicology 2021, 462, 152947. [Google Scholar] [CrossRef]
- Sheikh, I.A. Stereoselectivity and the Potential Endocrine Disrupting Activity of Di-(2-Ethylhexyl)Phthalate (DEHP) against Human Progesterone Receptor: A Computational Perspective. J. Appl. Toxicol. 2016, 36, 741–747. [Google Scholar] [CrossRef]
- Duan, C.; Fang, Y.; Sun, J.; Li, Z.; Wang, Q.; Bai, J.; Peng, H.; Liang, J.; Gao, Z. Effects of Fast Food Packaging Plasticizers and Their Metabolites on Steroid Hormone Synthesis in H295R Cells. Sci. Total Environ. 2020, 726, 138500. [Google Scholar] [CrossRef] [PubMed]
- Sree, C.G.; Buddolla, V.; Lakshmi, B.A.; Kim, Y.-J. Phthalate Toxicity Mechanisms: An Update. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2023, 263, 109498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, Y.; Pan, J.; Guo, X.; Han, X.; Zhou, J.; Meng, X. Maternal Exposure to Di-(2-Ethylhexyl) Phthalate (DEHP) Activates the PI3K/Akt/MTOR Signaling Pathway in F1 and F2 Generation Adult Mouse Testis. Exp. Cell Res. 2020, 394, 112151. [Google Scholar] [CrossRef] [PubMed]
- Lucaccioni, L.; Trevisani, V.; Passini, E.; Righi, B.; Plessi, C.; Predieri, B.; Iughetti, L. Perinatal Exposure to Phthalates: From Endocrine to Neurodevelopment Effects. Int. J. Mol. Sci. 2021, 22, 4063. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A.; Beg, M.A. Structural Characterization of Potential Endocrine Disrupting Activity of Alternate Plasticizers Di-(2-Ethylhexyl) Adipate (DEHA), Acetyl Tributyl Citrate (ATBC) and 2,2,4-Trimethyl 1,3-Pentanediol Diisobutyrate (TPIB) with Human Sex Hormone-Binding Globulin. Reprod. Toxicol. 2019, 83, 46–53. [Google Scholar] [CrossRef]
- Rasmussen, L.M.; Sen, N.; Liu, X.; Craig, Z.R. Effects of Oral Exposure to the Phthalate Substitute Acetyl Tributyl Citrate on Female Reproduction in Mice. J. Appl. Toxicol. 2017, 37, 668–675. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.; Kalil, J. Polyethylene Glycol Is a Cause of IgE-Mediated Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2019, 7, 1874–1875. [Google Scholar] [CrossRef]
- Garvey, L.H.; Nasser, S. Anaphylaxis to the First COVID-19 Vaccine: Is Polyethylene Glycol (PEG) the Culprit? Br. J. Anaesth. 2021, 126, e106–e108. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, Y.A.; Shin, C.H.; Hong, Y.C.; Kim, B.N.; Lim, Y.H. Association of Bisphenol A, Bisphenol F, and Bisphenol S with ADHD Symptoms in Children. Environ. Int. 2022, 161, 107093. [Google Scholar] [CrossRef]
- Sirohi, D.; al Ramadhani, R.; Knibbs, L.D. Environmental Exposures to Endocrine Disrupting Chemicals (EDCs) and Their Role in Endometriosis: A Systematic Literature Review. Rev. Environ. Health 2021, 36, 101–115. [Google Scholar] [CrossRef]
- Yoo, M.H.; Lee, S.J.; Kim, W.; Kim, Y.; Kim, Y.B.; Moon, K.S.; Lee, B.S. Bisphenol A Impairs Renal Function by Reducing Na+/K+-ATPase and F-Actin Expression, Kidney Tubule Formation In Vitro and In Vivo. Ecotoxicol. Environ. Saf. 2022, 246, 114141. [Google Scholar] [CrossRef]
- Ďurovcová, I.; Kyzek, S.; Fabová, J.; Makuková, J.; Gálová, E.; Ševčovičová, A. Genotoxic Potential of Bisphenol A: A Review. Environ. Pollut. 2022, 306, 119346. [Google Scholar] [CrossRef]
- Tyner, M.D.W.; Maloney, M.O.; Kelley, B.J.B.; Combelles, C.M.H. Comparing the Effects of Bisphenol A, C, and F on Bovine Theca Cells in Vitro. Reprod. Toxicol. 2022, 111, 27–33. [Google Scholar] [CrossRef]
- Chen, P.-P.; Liu, C.; Zhang, M.; Miao, Y.; Cui, F.-P.; Deng, Y.-L.; Luo, Q.; Zeng, J.-Y.; Shi, T.; Lu, T.-T.; et al. Associations between Urinary Bisphenol A and Its Analogues and Semen Quality: A Cross-Sectional Study among Chinese Men from an Infertility Clinic. Environ. Int. 2022, 161, 107132. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Fan, S.; Zhong, Y.; Li, J.; Zhao, Y.; Ni, S.; Liu, J.; Wu, Y. A Case-Control Study of Urinary Concentrations of Bisphenol A, Bisphenol F, and Bisphenol S and the Risk of Papillary Thyroid Cancer. Chemosphere 2023, 312, 137162. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, Q.; Li, Y.; Chu, S.; He, F.; Li, X.; Sun, N.; Zong, W.; Liu, R. Potential Toxicity of Bisphenol A to α-Chymotrypsin and the Corresponding Mechanisms of Their Binding. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121910. [Google Scholar] [CrossRef]
- Kolli, R.T.; Xu, Z.; Panduri, V.; Taylor, J.A. Differential Gene Expression in Bladder Tumors from Workers Occupationally Exposed to Arylamines. Biomed. Res. Int. 2021, 2021, 2624433. [Google Scholar] [CrossRef]
- Chung, K.-T. Carcinogenicity Allergenicity and Lupus-Inducibility of Arylamines. Front. Biosci. 2016, 8, 748. [Google Scholar] [CrossRef]
- Tipton, D.A.; Lewis, J.W. Effects of a Hindered Amine Light Stabilizer and a UV Light Absorber Used in Maxillofacial Elastomers on Human Gingival Epithelial Cells and Fibroblasts. J. Prosthet. Dent. 2008, 100, 220–231. [Google Scholar] [CrossRef]
- Hirata-Koizumi, M.; Ise, R.; Kato, H.; Matsuyama, T.; Nishimaki-Mogami, T.; Takahashi, M.; Ono, A.; Ema, M.; Hirose, A. Transcriptome Analyses Demonstrate That Peroxisome Proliferator-Activated Receptor α (PPARα) Activity of an Ultraviolet Absorber, 2-(2’-Hydroxy-3’,5’-Di-Tert-Butylphenyl)Benzotriazole, as Possible Mechanism of Their Toxicity and the Gender Differences. J. Toxicol. Sci. 2016, 41, 693–700. [Google Scholar] [CrossRef]
- Denghel, H.; Göen, T. Determination of the UV Absorber 2-(2H-Benzotriazol-2-Yl)-4,6-Di-Tert-Pentylphenol (UV 328) and Its Oxidative Metabolites in Human Urine by Dispersive Liquid-Liquid Microextraction and GC–MS/MS. J. Chromatogr. B 2020, 1144, 122071. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, A.; Tao, J.; Li, J.; Arteel, G.E.; Monga, S.S.; Beier, J.I. Tu1286: Environmental Vinyl Chloride Exposure Aggravates Tumorigenesis in a Murine Model of Hepatocellular Cancer. Gastroenterology 2022, 162, S-1261. [Google Scholar] [CrossRef]
- Lotti, M. Do Occupational Exposures to Vinyl Chloride Cause Hepatocellular Carcinoma and Cirrhosis? Liver Int. 2017, 37, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Frullanti, E.; la Vecchia, C.; Boffetta, P.; Zocchetti, C. Vinyl Chloride Exposure and Cirrhosis: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2012, 44, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, U.; Girardi, P.; Mastrangelo, G. Occupational Exposure to Vinyl Chloride and Liver Diseases. World J. Gastroenterol. 2019, 25, 4885–4891. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, H.; Qiu, Z.; Liu, T.; Yuan, Y.; Guan, R.; Li, N.; Wang, W.; Li, X.; Zhao, C. Effect of Short-Chain Chlorinated Paraffins (SCCPs) on Lipid Membranes: Combination of Molecular Dynamics and Membrane Damage Experiments. Sci. Total Environ. 2021, 775, 144906. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Geng, N.; Zhang, H.; Wang, F.; Gong, Y.; Song, X.; Luo, Y.; Zhang, B.; Chen, J. Comparing the Disrupting Effects of Short-, Medium- and Long-Chain Chlorinated Paraffins on Cell Viability and Metabolism. Sci. Total Environ. 2019, 685, 297–307. [Google Scholar] [CrossRef]
- Li, J.; Giesy, J.P.; Yu, L.; Li, G.; Liu, C. Effects of Tris(1,3-Dichloro-2-Propyl) Phosphate (TDCPP) in Tetrahymena Thermophila: Targeting the Ribosome. Sci. Rep. 2015, 5, 10562. [Google Scholar] [CrossRef]
- Killilea, D.W.; Chow, D.; Xiao, S.Q.; Li, C.; Stoller, M.L. Flame Retardant Tris(1,3-Dichloro-2-Propyl)Phosphate (TDCPP) Toxicity Is Attenuated by N -Acetylcysteine in Human Kidney Cells. Toxicol. Rep. 2017, 4, 260–264. [Google Scholar] [CrossRef]
- el Shanawany, S.; Foda, N.; Hashad, D.I.; Salama, N.; Sobh, Z. The Potential DNA Toxic Changes among Workers Exposed to Antimony Trioxide. Environ. Sci. Pollut. Res. 2017, 24, 12455–12461. [Google Scholar] [CrossRef]
- Schildroth, S.; Osborne, G.; Smith, A.R.; Yip, C.; Collins, C.; Smith, M.T.; Sandy, M.S.; Zhang, L. Occupational Exposure to Antimony Trioxide: A Risk Assessment. Occup. Environ. Med. 2021, 78, 413–418. [Google Scholar] [CrossRef]
- Lim, J.-S.; Lee, D.-H.; Jacobs, D.R. Association of Brominated Flame Retardants With Diabetes and Metabolic Syndrome in the U.S. Population, 2003–2004. Diabetes Care 2008, 31, 1802–1807. [Google Scholar] [CrossRef]
- Deziel, N.C.; Alfonso-Garrido, J.; Warren, J.L.; Huang, H.; Sjodin, A.; Zhang, Y. Exposure to Polybrominated Diphenyl Ethers and a Polybrominated Biphenyl and Risk of Thyroid Cancer in Women: Single and Multi-Pollutant Approaches. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1755–1764. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Mora, V.; Gasbarrini, A.; Mele, M.C. Impact of Food Additive Titanium Dioxide on Gut Microbiota Composition, Microbiota-Associated Functions, and Gut Barrier: A Systematic Review of In Vivo Animal Studies. Int. J. Environ. Res. Public Health 2021, 18, 2008. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Yu, J.; Kim, H.-M.; Oh, J.-M.; Choi, S.-J. Food Additive Titanium Dioxide and Its Fate in Commercial Foods. Nanomaterials 2019, 9, 1175. [Google Scholar] [CrossRef]
- Cesa, F.S.; Turra, A.; Checon, H.H.; Leonardi, B.; Baruque-Ramos, J. Laundering and Textile Parameters Influence Fibers Release in Household Washings. Environ. Pollut. 2020, 257, 113553. [Google Scholar] [CrossRef]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire Wear Particles in the Aquatic Environment—A Review on Generation, Analysis, Occurrence, Fate and Effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Sommer, F.; Dietze, V.; Baum, A.; Sauer, J.; Gilge, S.; Maschowski, C.; Gieré, R. Tire Abrasion as a Major Source of Microplastics in the Environment. Aerosol Air Qual. Res. 2018, 18, 2014–2028. [Google Scholar] [CrossRef]
- Pal, P.; Pandey, J.P.; Sen, G. Synthesis and Application as Programmable Water Soluble Adhesive of Polyacrylamide Grafted Gum Tragacanth (GT-g-PAM). In Biopolymer Grafting: Applications; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 4, pp. 153–203. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Physiochemical Properties and Degradation. In Microplastic Pollutants; Crawford, C.B., Quinn, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 4, pp. 57–100. [Google Scholar] [CrossRef]
- Sohma, J. Mechanochemical Degradation. In Comprehensive Polymer Science and Supplements; Allen, G., Bevington, J.C., Eds.; Pergamon: Oxford, UK, 1989; Volume 23, pp. 621–644. [Google Scholar] [CrossRef]
- el Hadri, H.; Gigault, J.; Maxit, B.; Grassl, B.; Reynaud, S. Nanoplastic from Mechanically Degraded Primary and Secondary Microplastics for Environmental Assessments. NanoImpact 2020, 17, 100206. [Google Scholar] [CrossRef]
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An Overview of Degradable and Biodegradable Polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossini, H.; Makhdoumi, P. Review of Microplastic Occurrence and Toxicological Effects in Marine Environment: Experimental Evidence of Inflammation. Process Saf. Environ. Prot. 2020, 142, 1–14. [Google Scholar] [CrossRef]
- Singh, S.; Patil, T.; Tekade, S.P.; Gawande, M.B.; Sawarkar, A.N. Studies on Individual Pyrolysis and Co-Pyrolysis of Corn Cob and Polyethylene: Thermal Degradation Behavior, Possible Synergism, Kinetics, and Thermodynamic Analysis. Sci. Total Environ. 2021, 783, 147004. [Google Scholar] [CrossRef] [PubMed]
- Maubane, L.; Lekalakala, R.; Orasugh, J.T.; Letwaba, J. Effect of Short-Chain Architecture on the Resulting Thermal Properties of Polypropylene. Polymer 2023, 264, 125533. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Hawkins, S.; Mannan, M.S.; Cheng, Z. Study of Thermal and Mechanical Behaviors of Flame Retardant Polystyrene-Based Nanocomposites Prepared Via In-Situ Polymerization Method. J. Loss Prev. Process Ind. 2017, 49, 228–239. [Google Scholar] [CrossRef]
- Martín-Gullón, I.; Esperanza, M.; Font, R. Kinetic Model for the Pyrolysis and Combustion of Poly-(Ethylene Terephthalate) (PET). J. Anal. Appl. Pyrolysis 2001, 58–59, 635–650. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Shim, W.J. The Fragmentation of Nano- and Microplastic Particles from Thermoplastics Accelerated by Simulated-Sunlight-Mediated Photooxidation. Environ. Pollut. 2022, 311, 119847. [Google Scholar] [CrossRef] [PubMed]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging Effects on Low- and High-Density Polyethylene, Polypropylene and Polystyrene under UV Irradiation: An Insight into Decomposition Mechanism by Py-GC/MS for Microplastic Analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Kamweru, P.K.; Ndiritu, F.G.; Kinyanjui, T.K.; Muthui, Z.W.; Ngumbu, R.G.; Odhiambo, P.M. Study of Temperature and UV Wavelength Range Effects on Degradation of Photo-Irradiated Polyethylene Films Using DMA. J. Macromol. Sci. Phys. 2011, 50, 1338–1349. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, H.S.; Torikai, A. Effects of Climate Change and UV-B on Materials. Photochem. Photobiol. Sci. 2003, 2, 68–72. [Google Scholar] [CrossRef]
- Torikai, A.; Takeuchi, A.; Nagaya, S.; Fueki, K. Photodegradation of Polyethylene: Effect of Crosslinking on the Oxygenated Products and Mechanical Properties. Polym. Photochem. 1986, 7, 199–211. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.; Zhang, Y.; Xu, D.; Gao, J.; Ma, Z.; Wang, Y. Vapour-Liquid Equilibrium Measurements and Extractive Distillation Process Design for Separation of Azeotropic Mixture (Dimethyl Carbonate + ethanol). J. Chem. Thermodyn. 2019, 133, 10–18. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- ter Halle, A.; Ladirat, L.; Gendre, X.; Goudouneche, D.; Pusineri, C.; Routaboul, C.; Tenailleau, C.; Duployer, B.; Perez, E. Understanding the Fragmentation Pattern of Marine Plastic Debris. Environ. Sci. Technol. 2016, 50, 5668–5675. [Google Scholar] [CrossRef]
- Lin, C.-C.; Krommenhoek, P.J.; Watson, S.S.; Gu, X. Depth Profiling of Degradation of Multilayer Photovoltaic Backsheets after Accelerated Laboratory Weathering: Cross-Sectional Raman Imaging. Sol. Energy Mater. Sol. Cells 2016, 144, 289–299. [Google Scholar] [CrossRef]
- Zweifel, H. Principles of Oxidative Degradation. In Stabilization of Polymeric Materials. Macromolecular Systems—Materials Approach; Abe, A., Monnerie, L., Shibaev, V., Suter, U.W., Tirrell, D., Ward, I.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 1, pp. 1–40. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P.O. Condensed Encyclopedia of Polymer Engineering Terms, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2001; pp. 193–199. [Google Scholar] [CrossRef]
- McKeen, L.W. Introduction to the Weathering of Plastics. In The Effect of UV Light and Weather on Plastics and Elastomers, 3rd ed.; McKeen, L.W., Ed.; William Andrew: Norwich, CT, USA, 2019; Volume 2, pp. 17–41. [Google Scholar] [CrossRef]
- Bank, M.S.; Hansson, S.V. The Plastic Cycle: A Novel and Holistic Paradigm for the Anthropocene. Environ. Sci. Technol. 2019, 53, 7177–7179. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of Microplastics on the Terrestrial Environment: A Critical Review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.H.; Kirkham, M.B. Particulate Plastics as a Vector for Toxic Trace-Element Uptake by Aquatic and Terrestrial Organisms and Human Health Risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and Nano-Plastics in Marine Environment: Source, Distribution and Threats—A Review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Ojeda, T.; Freitas, A.; Birck, K.; Dalmolin, E.; Jacques, R.; Bento, F.; Camargo, F. Degradability of Linear Polyolefins under Natural Weathering. Polym. Degrad. Stab. 2011, 96, 703–707. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Rånby, B. Basic Reactions in the Photodegradation of Some Important Polymers. J. Macromol. Sci.-Pure Appl. Chem. 1993, 30, 583–594. [Google Scholar] [CrossRef]
- Fairbrother, A.; Hsueh, H.C.; Kim, J.H.; Jacobs, D.; Perry, L.; Goodwin, D.; White, C.; Watson, S.; Sung, L.P. Temperature and Light Intensity Effects on Photodegradation of High-Density Polyethylene. Polym. Degrad. Stab. 2019, 165, 153–160. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Takada, H.; Karapanagioti, H.K. (Eds.) Hazardous Chemicals Associated with Plastics in the Marine Environment, 1st ed.; Springer: Cham, Switzerland, 2019; Volume 78. [Google Scholar] [CrossRef]
- Weber, R.; Watson, A.; Forter, M.; Oliaei, F. Review Article: Persistent Organic Pollutants and Landfills—A Review of Past Experiences and Future Challenges. Waste Manag. Res. 2011, 29, 107–121. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal Solid Waste (MSW) Landfill: A Source of Microplastics?—Evidence of Microplastics in Landfill Leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Wu, D.; Zhan, L.; Shi, H.; Xie, B. Occurrence of Microplastics in Landfill Systems and Their Fate with Landfill Age. Water Res. 2019, 164, 114968. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Hassellöv, M.; Jakubowicz, I. Influence of Thermooxidative Degradation on the in Situ Fate of Polyethylene in Temperate Coastal Waters. Mar. Pollut. Bull. 2018, 135, 187–194. [Google Scholar] [CrossRef]
- Khoironi, A.; Hadiyanto, H.; Anggoro, S.; Sudarno, S. Evaluation of Polypropylene Plastic Degradation and Microplastic Identification in Sediments at Tambak Lorok Coastal Area, Semarang, Indonesia. Mar. Pollut. Bull. 2020, 151, 110868. [Google Scholar] [CrossRef]
- Eubeler, J.P.; Bernhard, M.; Knepper, T.P. Environmental Biodegradation of Synthetic Polymers II. Biodegradation of Different Polymer Groups. Trends Anal. Chem. 2010, 29, 84–100. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.S. Microplastics in the Freshwater and Terrestrial Environments: Prevalence, Fates, Impacts and Sustainable Solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Pico, Y.; Alfarhan, A.; Barcelo, D. Nano- and Microplastic Analysis: Focus on Their Occurrence in Freshwater Ecosystems and Remediation Technologies. Trends Anal. Chem. 2019, 113, 409–425. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as Pollutants in Agricultural Soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Hocker, S.; Rhudy, A.K.; Ginsburg, G.; Kranbuehl, D.E. Polyamide Hydrolysis Accelerated by Small Weak Organic Acids. Polymer 2014, 55, 5057–5064. [Google Scholar] [CrossRef]
- Liu, F.-f.; Liu, G.-z.; Zhu, Z.-l.; Wang, S.-c.; Zhao, F.-f. Interactions between Microplastics and Phthalate Esters as Affected by Microplastics Characteristics and Solution Chemistry. Chemosphere 2019, 214, 688–694. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; O’Halloran, J.; van Pelt, F.N.A.M.; Jansen, M.A.K. Rapid Fragmentation of Microplastics by the Freshwater Amphipod Gammarus Duebeni (Lillj.). Sci. Rep. 2020, 10, 12799. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Cau, A.; Avio, C.G.; Dessì, C.; Moccia, D.; Pusceddu, A.; Regoli, F.; Cannas, R.; Follesa, M.C. Benthic Crustacean Digestion Can Modulate the Environmental Fate of Microplastics in the Deep Sea. Environ. Sci. Technol. 2020, 54, 4886–4892. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective Enrichment of Bacterial Pathogens by Microplastic Biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef] [PubMed]
- Nakamiya, K.; Sakasita, G.; Ooi, T.; Kinoshita, S. Enzymatic Degradation of Polystyrene by Hydroquinone Peroxidase of Azotobacter Beijerinckii HM121. J. Ferment. Bioeng. 1997, 84, 480–482. [Google Scholar] [CrossRef]
- Priya, A.K.; Jalil, A.A.; Dutta, K.; Rajendran, S.; Vasseghian, Y.; Karimi-Maleh, H.; Soto-Moscoso, M. Algal Degradation of Microplastic from the Environment: Mechanism, Challenges, and Future Prospects. Algal Res. 2022, 67, 102848. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, X.; Jiang, X.; Shi, H.; Wu, C. Sinking of Floating Plastic Debris Caused by Biofilm Development in a Freshwater Lake. Chemosphere 2019, 222, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.D. Microbiological Deterioration and Degradation of Synthetic Polymeric Materials: Recent Research Advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Moreira, F.T.; Prantoni, A.L.; Martini, B.; de Abreu, M.A.; Stoiev, S.B.; Turra, A. Small-Scale Temporal and Spatial Variability in the Abundance of Plastic Pellets on Sandy Beaches: Methodological Considerations for Estimating the Input of Microplastics. Mar. Pollut. Bull. 2016, 102, 114–121. [Google Scholar] [CrossRef]
- Turrell, W.R. A Simple Model of Wind-Blown Tidal Strandlines: How Marine Litter Is Deposited on a Mid-Latitude, Macro-Tidal Shelf Sea Beach. Mar. Pollut. Bull. 2018, 137, 315–330. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.; Xiong, X.; Ruan, Y.; Zhou, H.; Wu, C.; Lam, P.K.S. The Hydro-Fluctuation Belt of the Three Gorges Reservoir: Source or Sink of Microplastics in the Water? Environ. Pollut. 2019, 248, 279–285. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic Pollution of Lakeshore Sediments from Remote Lakes in Tibet Plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Liu, P.; Shi, Y.; Wu, X.; Wang, H.; Huang, H.; Guo, X.; Gao, S. Review of the Artificially-Accelerated Aging Technology and Ecological Risk of Microplastics. Sci. Total Environ. 2021, 768, 144969. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, Sources, Human Health Impacts and Mitigation of Microplastic Pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Horton, A.A.; Dixon, S.J. Microplastics: An Introduction to Environmental Transport Processes. WIREs Water 2018, 5, e1268. [Google Scholar] [CrossRef]
- Benítez, A.; Sánchez, J.J.; Arnal, M.L.; Müller, A.J.; Rodríguez, O.; Morales, G. Abiotic Degradation of LDPE and LLDPE Formulated with a Pro-Oxidant Additive. Polym. Degrad. Stab. 2013, 98, 490–501. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the Soil-Groundwater Environment: Aging, Migration, and Co-Transport of Contaminants—A Critical Review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding Plastic Degradation and Microplastic Formation in the Environment: A Review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Li, H.; Liu, L. Short-Term Effects of Polyethene and Polypropylene Microplastics on Soil Phosphorus and Nitrogen Availability. Chemosphere 2022, 291, 132984. [Google Scholar] [CrossRef]
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An Overview of Microplastic and Nanoplastic Pollution in Agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of Plastic Contamination on Water Evaporation and Desiccation Cracking in Soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in Soil: Anal. Methods and Possible Sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. The Input–Output Balance of Microplastics Derived from Coated Fertilizeri Paddy Fields and the Timing of Their Discharge during the Irrigation Season. Chemosphere 2021, 279, 130574. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, S.; Nizzetto, L.; Volta, P. Plastic Sources: A Survey across Scientific and Grey Literature for Their Inventory and Relative Contribution to Microplastics Pollution in Natural Environments, with an Emphasis on Surface Water. Sci. Total Environ. 2019, 693, 133499. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic Fibers in Atmospheric Fallout: A Source of Microplastics in the Environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, L.; Dong, Q.; Zhao, X.; Han, J. Effects of Microplastics on Water Infiltration in Agricultural Soil on the Loess Plateau, China. Agric. Water Manag. 2022, 271, 107818. [Google Scholar] [CrossRef]
- Hu, J.; He, D.; Zhang, X.; Li, X.; Chen, Y.; Wei, G.; Zhang, Y.; Ok, Y.S.; Luo, Y. National-Scale Distribution of Micro(Meso)Plastics in Farmland Soils across China: Implications for Environmental Impacts. J. Hazard. Mater. 2022, 424, 127283. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Wufuer, R.; Duo, J.; Pan, X. Distinct Soil Microplastic Distributions under Various Farmland-Use Types around Urumqi, China. Sci. Total Environ. 2023, 857, 159573. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Keshavarzi, B.; Mohit, F.; Moore, F.; Busquets, R. Microplastic Occurrence in Urban and Industrial Soils of Ahvaz Metropolis: A City with a Sustained Record of Air Pollution. Sci. Total Environ. 2022, 819, 152051. [Google Scholar] [CrossRef]
- da Silva Paes, E.; Gloaguen, T.V.; Silva, H. dos A. da C.; Duarte, T.S.; de Almeida, M. da C.; Costa, O.D.A.V.; Bomfim, M.R.; Santos, J.A.G. Widespread Microplastic Pollution in Mangrove Soils of Todos Os Santos Bay, Northern Brazil. Environ. Res. 2022, 210, 112952. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; González-Sálamo, J.; Hernández-Sánchez, C.; González-Pleiter, M.; Hernández-Borges, J.; Díaz-Peña, F.J. Recycled Wastewater as a Potential Source of Microplastics in Irrigated Soils from an Arid-Insular Territory (Fuerteventura, Spain). Sci. Total Environ. 2022, 817, 152830. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics Research—From Sink to Source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic Transport in Soil by Earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Kumar, S.; Hatha, A.A.M.; Christi, K.S. Diversity and Effectiveness of Tropical Mangrove Soil Microflora on the Degradation of Polythene Carry Bags. Rev. Biol. Trop. 2007, 55, 777–786. [Google Scholar] [CrossRef]
- Otake, Y.; Kobayashi, T.; Asabe, H.; Murakami, N.; Ono, K. Biodegradation of Low-Density Polyethylene, Polystyrene, Polyvinyl Chloride, and Urea Formaldehyde Resin Buried under Soil for over 32 Years. J. Appl. Polym. Sci. 1995, 56, 1789–1796. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in Surface Waters and Sediments of the Wei River, in the Northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Liu, F.; Olesen, K.B.; Borregaard, A.R.; Vollertsen, J. Microplastics in Urban and Highway Stormwater Retention Ponds. Sci. Total Environ. 2019, 671, 992–1000. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of Microplastics from Litter into Burrows of Lumbricus Terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Zhang, C.; Zhou, Y.; Qin, W. Soil Microplastic Characteristics and the Effects on Soil Properties and Biota: A Systematic Review and Meta-Analysis. Environ. Pollut. 2022, 313, 120183. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic Pollution in the Surface Waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics Profile along the Rhine River. Sci. Rep. 2016, 5, 17988. [Google Scholar] [CrossRef]
- Rowley, K.H.; Cucknell, A.C.; Smith, B.D.; Clark, P.F.; Morritt, D. London’s River of Plastic: High Levels of Microplastics in the Thames Water Column. Sci. Total Environ. 2020, 740, 140018. [Google Scholar] [CrossRef]
- Ivar Do Sul, J.A.; Costa, M.F. The Present and Future of Microplastic Pollution in the Marine Environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.S.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine Litter Distribution and Density in European Seas, from the Shelves to Deep Basins. PLoS ONE 2014, 9, e95839. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.H.E.; Not, C. Selective Accumulation of Plastic Debris at the Breaking Wave Area of Coastal Waters. Environ. Pollut. 2019, 245, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Pabortsava, K.; Lampitt, R.S. High Concentrations of Plastic Hidden beneath the Surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef] [PubMed]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic Sea Ice Is an Important Temporal Sink and Means of Transport for Microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef]
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring Polymer Degradation under Different Conditions in the Marine Environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, X.; Wu, C.; Xia, Y.; Li, J.; Wu, Y. Influence of Light and Temperature on the Development and Denitrification Potential of Periphytic Biofilms. Sci. Total Environ. 2018, 613–614, 1430–1437. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Recyclability of Four Types of Plastics Exposed to UV Irradiation in a Marine Environment. Waste Manag. 2018, 79, 339–345. [Google Scholar] [CrossRef]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C.; Papathanassiou, E.; Galgani, F.; Papatheodorou, G. The Degradation Potential of PET Bottles in the Marine Environment: An ATR-FTIR Based Approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef]
- Williams, A.T.; Simmons, S.L. The Degradation of Plastic Litter in Rivers: Implications for Beaches. J. Coast. Conserv. 1996, 2, 63–72. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X. How Long for Plastics to Decompose in the Deep Sea? Geochem. Perspect. Lett. 2022, 22, 20–25. [Google Scholar] [CrossRef]
- Collard, F.; Gasperi, J.; Gabrielsen, G.W.; Tassin, B. Plastic Particle Ingestion by Wild Freshwater Fish: A Critical Review. Environ. Sci. Technol. 2019, 53, 12974–12988. [Google Scholar] [CrossRef]
- Koongolla, J.B.; Lin, L.; Pan, Y.F.; Yang, C.P.; Sun, D.R.; Liu, S.; Xu, X.R.; Maharana, D.; Huang, J.S.; Li, H.X. Occurrence of Microplastics in Gastrointestinal Tracts and Gills of Fish from Beibu Gulf, South China Sea. Environ. Pollut. 2020, 258, 113734. [Google Scholar] [CrossRef]
- Nanninga, G.B.; Scott, A.; Manica, A. Microplastic Ingestion Rates Are Phenotype-Dependent in Juvenile Anemonefish. Environ. Pollut. 2020, 259, 113855. [Google Scholar] [CrossRef]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic Ingestion by Riverine Macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef]
- Li, B.; Su, L.; Zhang, H.; Deng, H.; Chen, Q.; Shi, H. Microplastics in Fishes and Their Living Environments Surrounding a Plastic Production Area. Sci. Total Environ. 2020, 727, 138662. [Google Scholar] [CrossRef]
- Prata, J.C.; Venâncio, C.; Girão, A.V.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of Virgin and Weathered Polystyrene and Polypropylene Microplastics on Raphidocelis Subcapitata and Embryos of Danio Rerio under Environmental Concentrations. Sci. Total Environ. 2022, 816, 151642. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, M.-N. Isolation of Bacteria Degrading Poly(Butylene Succinate-Co-Butylene Adipate) and Their Lip A Gene. Int. Biodeterior. Biodegrad. 2010, 64, 184–190. [Google Scholar] [CrossRef]
- al Hosni, A.S.; Pittman, J.K.; Robson, G.D. Microbial Degradation of Four Biodegradable Polymers in Soil and Compost Demonstrating Polycaprolactone as an Ideal Compostable Plastic. Waste Manag. 2019, 97, 105–114. [Google Scholar] [CrossRef]
- Goto, T.; Kishita, M.; Sun, Y.; Sako, T.; Okajima, I. Degradation of Polylactic Acid Using Sub-Critical Water for Compost. Polymers 2020, 12, 2434. [Google Scholar] [CrossRef] [PubMed]
- Itävaara, M.; Karjomaa, S.; Selin, J.-F. Biodegradation of Polylactide in Aerobic and Anaerobic Thermophilic Conditions. Chemosphere 2002, 46, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Cheng, Z.; Hou, Y.; Xie, C.; Pu, J.; Peng, L.; Gao, L.; Chen, W.; Su, Y. Secondary Microplastics Formation and Colonized Microorganisms on the Surface of Conventional and Degradable Plastic Granules during Long-Term UV Aging in Various Environmental Media. J. Hazard. Mater. 2022, 439, 129686. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cheng, Z.; Hou, Y.; Lin, S.; Gao, L.; Wang, Z.; Bao, R.; Peng, L. Biodegradable and Conventional Microplastics Posed Similar Toxicity to Marine Algae Chlorella Vulgaris. Aquat. Toxicol. 2022, 244, 106097. [Google Scholar] [CrossRef]
- Fan, X.; Zou, Y.; Geng, N.; Liu, J.; Hou, J.; Li, D.; Yang, C.; Li, Y. Investigation on the Adsorption and Desorption Behaviors of Antibiotics by Degradable MPs with or without UV Ageing Process. J. Hazard. Mater. 2021, 401, 123363. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the Mass of Microplastics Ingested—A Pivotal First Step Towards Human Health Risk Assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.J.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in Take-Out Food Containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- Chen, H.; Xu, L.; Yu, K.; Wei, F.; Zhang, M. Release of Microplastics from Disposable Cups in Daily Use. Sci. Total Environ. 2023, 854, 158606. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic Release from the Degradation of Polypropylene Feeding Bottles During Infant Formula Preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Luo, Y.; Chuah, C.; Amin, A.; Khoshyan, A.; Gibson, C.T.; Tang, Y.; Naidu, R.; Fang, C. Assessment of Microplastics and Nanoplastics Released from a Chopping Board Using Raman Imaging in Combination with Three Algorithms. J. Hazard. Mater. 2022, 431, 128636. [Google Scholar] [CrossRef]
- Hajji, S.; Ben-Haddad, M.; Abelouah, M.R.; De-la-Torre, G.E.; Alla, A.A. Occurrence, Characteristics, and Removal of Microplastics in Wastewater Treatment Plants Located on the Moroccan Atlantic: The Case of Agadir Metropolis. Sci. Total Environ. 2023, 862, 160815. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Rauert, C.; Thomas, K.V. Mass Quantification of Microplastic at Wastewater Treatment Plants by Pyrolysis-Gas Chromatography–Mass Spectrometry. Sci. Total Environ. 2023, 856, 159251. [Google Scholar] [CrossRef]
- Tian, L.; Skoczynska, E.; van Putten, R.-J.; Leslie, H.A.; Gruter, G.-J.M. Quantification of Polyethylene Terephthalate Micro- and Nanoplastics in Domestic Wastewater Using a Simple Three-Step Method. Sci. Total Environ. 2023, 857, 159209. [Google Scholar] [CrossRef]
- Carlos Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as Active Samplers for Microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef]
- Napper, I.E.; Wright, L.S.; Barrett, A.C.; Parker-Jurd, F.N.F.; Thompson, R.C. Potential Microplastic Release from the Maritime Industry: Abrasion of Rope. Sci. Total Environ. 2022, 804, 150155. [Google Scholar] [CrossRef]
- Gautam, R.; Jo, J.-H.; Acharya, M.; Maharjan, A.; Lee, D.-E.; Bahadur, P.; Kim, C.-Y.; Kim, K.; Kim, H.-A.; Heo, Y. Evaluation of Potential Toxicity of Polyethylene Microplastics on Human Derived Cell Lines. Sci. Total Environ. 2022, 838, 156089. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Olga, V.; Xue, Y.; Lv, S.; Diao, X.; Zhang, Y.; Han, Q.; Zhou, H. The Potential Effects of Microplastic Pollution on Human Digestive Tract Cells. Chemosphere 2022, 291, 132714. [Google Scholar] [CrossRef]
- Bonanomi, M.; Salmistraro, N.; Porro, D.; Pinsino, A.; Colangelo, A.M.; Gaglio, D. Polystyrene Micro and Nano-Particles Induce Metabolic Rewiring in Normal Human Colon Cells: A Risk Factor for Human Health. Chemosphere 2022, 303, 134947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Lee, Y.-H.; Hsu, Y.-H.; Chiu, I.-J.; Huang, C.C.-Y.; Huang, C.-C.; Chia, Z.-C.; Lee, C.-P.; Lin, Y.-F.; Chiu, H.-W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 2021, 129, 57003. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An Assessment of the Toxicity of Polypropylene Microplastics in Human Derived Cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential Toxicity of Polystyrene Microplastic Particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Amarakoon, D.; Wei, C.-I.; Choi, K.Y.; Smolensky, D.; Lee, S.-H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021, 154, 112356. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution In Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and Cytotoxic Effects of Polyethylene Microplastics on Human Peripheral Blood Lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E.; et al. Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis In Vitro and In Vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 Cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Luqman, A.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Putri, B.Q.; Amalludin, F.I.; Rofiqa, E.A.; Götz, F.; et al. Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments 2021, 8, 138. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Cho, Y.M.; Choi, K.H. The Current Status of Studies of Human Exposure Assessment of Microplastics and Their Health Effects: A Rapid Systematic Review. Environ. Anal. Health Toxicol. 2021, 36, e2021004. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, J.; Zuo, R.; Xu, Q.; Qian, Y.; Lihui, A.N. Identification of Microplastics in Human Placenta Using Laser Direct Infrared Spectroscopy. Sci. Total Environ. 2023, 856, 159060. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Li, M.; Cai, Z.; Gong, H.; Yan, M. Microplastics as an aquatic pollutant affect gut microbiota within aquatic animals. J. Hazard. Mater. 2022, 423, 127094. [Google Scholar] [CrossRef]
- Dusza, H.M.; Katrukha, E.A.; Nijmeijer, S.M.; Akhmanova, A.; Vethaak, A.D.; Walker, D.I.; Legler, J. Uptake, Transport, and Toxicity of Pristine and Weathered Micro- and Nanoplastics in Human Placenta Cells. Environ. Health Perspect. 2022, 130, 097006. [Google Scholar] [CrossRef]
- Udovicki, B.; Andjelkovic, M.; Cirkovic-Velickovic, T.; Rajkovic, A. Microplastics in Food: Scoping Review on Health Effects, Occurrence, and Human Exposure. Int. J. Food Contam. 2022, 9, 7. [Google Scholar] [CrossRef]
- Santana, M.F.M.; Moreira, F.T.; Turra, A. Trophic Transference of Microplastics under a Low Exposure Scenario: Insights on the Likelihood of Particle Cascading along Marine Food-Webs. Mar. Pollut. Bull. 2017, 121, 154–159. [Google Scholar] [CrossRef]
- Boháčková, J.; Havlíčková, L.; Semerád, J.; Titov, I.; Trhlíková, O.; Beneš, H.; Cajthaml, T. In Vitro Toxicity Assessment of Polyethylene Terephthalate and Polyvinyl Chloride Microplastics Using Three Cell Lines From Rainbow Trout (Oncorhynchus Mykiss). Chemosphere 2023, 312, 136996. [Google Scholar] [CrossRef]
- Bai, C.L.; Liu, L.Y.; Hu, Y.-B.; Zeng, E.Y.; Guo, Y. Microplastics: A Review of Analytical Methods, Occurrence and Characteristics in Food, and Potential Toxicities to Biota. Sci. Total Environ. 2022, 806, 150263. [Google Scholar] [CrossRef] [PubMed]
- Lievens, S.; Slegers, T.; Mees, M.A.; Thielemans, W.; Poma, G.; Covaci, A.; Van Der Borght, M. A Simple, Rapid and Accurate Method for the Sample Preparation and Quantification of Meso- and Microplastics in Food and Food Waste Streams. Environ. Pollut. 2022, 307, 119511. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, T.; Nakajima, R.; Fujiwara, A.; Onodera, J.; Itoh, M.; Toyoshima, J.; Watanabe, E.; Murata, A.; Nishino, S.; Kikuchi, T. Horizontal Distribution of Surface Microplastic Concentrations and Water-Column Microplastic Inventories in the Chukchi Sea, Western Arctic Ocean. Sci. Total Environ. 2023, 855, 159564. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, L.; Scibetta, L.; Markuszewski, P.; Mazurkiewicz, M.; Drozdowska, V.; Makuch, P.; Jutrzenka-Trzebiatowska, P.; Zaleska-Medynska, A.; Andò, S.; Saliu, F.; et al. Airborne and Marine Microplastics from an Oceanographic Survey at the Baltic Sea: An Emerging Role of Air-Sea Interaction? Sci. Total Environ. 2022, 824, 153709. [Google Scholar] [CrossRef] [PubMed]
- Gunaalan, K.; Almeda, R.; Lorenz, C.; Vianello, A.; Iordachescu, L.; Papacharalampos, K.; Kiær, K.M.R.; Vollertsen, J.; Nielsen, T.G. Abundance and Distribution of Microplastics in Surface Waters of the Kattegat/Skagerrak (Denmark). Environ. Pollut. 2023, 318, 120853. [Google Scholar] [CrossRef]
- Ragoobur, D.; Amode, N.S.; Somaroo, G.D.; Nazurally, N. Microplastics in Estuarine Water and Sediment in Mauritius. Reg. Stud. Mar. Sci. 2023, 57, 102766. [Google Scholar] [CrossRef]
- Matjašič, T.; Mori, N.; Hostnik, I.; Bajt, O.; Viršek, M.K. Microplastic Pollution in Small Rivers Along Rural–Urban Gradients: Variations Across Catchments and Between Water Column and Sediments. Sci. Total Environ. 2023, 858, 160043. [Google Scholar] [CrossRef]
- Cha, J.; Lee, J.-Y.; Chia, R.W. Microplastics Contamination and Characteristics of Agricultural Groundwater in Haean Basin of Korea. Sci. Total Environ. 2023, 864, 161027. [Google Scholar] [CrossRef]
- Kernchen, S.; Löder, M.G.J.; Fischer, F.; Fischer, D.; Moses, S.R.; Georgi, C.; Nölscher, A.C.; Held, A.; Laforsch, C. Airborne Microplastic Concentrations and Deposition Across the Weser River Catchment. Sci. Total Environ. 2022, 818, 151812. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Zheng, L.; Dai, D.; Li, F. Atmospheric Microplastics in the Northwestern Pacific Ocean: Distribution, Source, and Deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef]
- Leitão, I.A.; van Schaik, L.; Ferreira, A.J.D.; Alexandre, N.; Geissen, V. The Spatial Distribution of Microplastics in Topsoils of an Urban Environment—Coimbra City Case-Study. Environ. Res. 2023, 218, 114961. [Google Scholar] [CrossRef]
- Singh, S.; Chakma, S.; Alawa, B.; Kalyanasundaram, M.; Diwan, V. Identification, Characterization, and Implications of Microplastics in Soil—A Case Study of Bhopal, Central India. J. Hazard. Mater. Adv. 2023, 9, 100225. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persistence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]
- Peez, N.; Rinesch, T.; Kolz, J.; Imhof, W. Applicable and cost-efficient microplastic analysis by quantitative 1H-NMR spectroscopy using benchtop NMR and NoD methods. Magn. Reson. Chem. 2022, 60, 172–183. [Google Scholar] [CrossRef]
- Mauel, A.; Pötzschner, B.; Meides, N.; Siegel, R.; Strohriegl, P.; Senker, J. Quantification of photooxidative defects in weathered microplastics using 13C multiCP NMR spectroscopy. RSC Adv. 2022, 12, 10875–10885. [Google Scholar] [CrossRef]
- Meides, N.; Mauel, A.; Menzel, T.; Altstädt, V.; Ruckdäschel, H.; Senker, J.; Strohriegl, P. Quantifying the fragmentation of polypropylene upon exposure to accelerated weathering. Micropl. Nanopl. 2022, 2, 23. [Google Scholar] [CrossRef]

| Polymer Type | Degradation Method | Effect | References |
|---|---|---|---|
| PE | Photodegradation | Oxygen functional groups on the surface; the increase of specific surface area | [55] |
| Chemical degradation (prothioconazole) | Cracks | [56] | |
| PP | Photodegradation | Reduction in microplastic particle volume | [57] |
| Biological degradation (Serratia marcescens and Enterobacter spp.) | Surface changes (microcracks and corrugations) | [58] | |
| Biological degradation (Rhodococcus sp. And Bacillus sp.) | The reduction of the polymer mass; structural and morphological changes in PP | [59] | |
| PE, PP | Biological degradation (Spirulina sp.) | Changes of functional groups; a decrease in carbon in PE and PP | [60] |
| PS | Biological degradation (Bacillus cereus CH6) | The surface morphology changes | [61] |
| PET | Chemical and thermal degradation | Changes in surface morphology, crystallinity, and carbonyl index | [62] |
| PS, PET | Biological degradation (Bacillus sp.) | Structural and surface changes; weight loss; a decrease in the carbon content | [63] |
| PE, PP, PS, PET | Chemical degradation (Fenton’s reagent) | Wrinkles, voids, and holes on the surface; oxygen functional groups on the surface; increased hydrophilicity and acidity of the surface; reduced MP size | [64] |
| Polymer Type | Degradation Method | Degradation Products | References |
|---|---|---|---|
| PE | Thermal degradation | H2, CH4, C2H4, and C3H6 | [65] |
| Photodegradation | CO2, H2O | [66] | |
| Photodegradation | C2H6, CO2, H2O, and formaldehyde | [67] | |
| Chemical degradation (O3 and H2O2) | 3-pentanol, 3-pentanone | [68] | |
| PP | Photodegradation | Formaldehyde, acetaldehyde, 2-propynyl, hydroxypropyl, acetone, 2-propenyl, butanal, 4-pentyn-1-olate, 4-pentyn-1-olate, (2-ethoxyethyl)oxonium, and acetylacetonate | [57] |
| PS | Biological degradation (the microbially driven Fenton reaction) | 2-isopropyl-5-methyl-1-heptanol, nonahexacontanoic acid | [69] |
| Photodegradation | Benzene, toluene, phenol, styrene, and 2-propenylbenzene | [70] | |
| Photodegradation | Acrolein, benzene, propanal, methyl vinyl ketone, and methyl propenyl ketone | [71] | |
| PET | Biological degradation (Rhococcus sp. SSM1) | Monomer—terephthalic acid (TPA) | [72] |
| Biological degradation (petase) | Mono-(2-hydroxyethyl) terephthalate, bis-(2-hydroxyethyl) terephthalate and ethylene glycol | [73] | |
| PE, PP, PET, PS | Chemical degradation (Fenton oxidation) | CO2 | [74] |
| Additive Type | Example of the Chemical Compound | Health Effects | References |
|---|---|---|---|
| Plasticizers | Phthalates [di(2-ethylhexyl)phthalate (DEHP), diethyl phthalate (DEP), and dibutyl phthalate (DBP)] | Increased oxidative stress and inflammation:
| [81,82,83,84,85,86] |
| Acetyl tributyl citrate (ATBC) | Risk of interaction with drugs:
| [87,88] | |
| Poly(ethylene glycol) (PEG) | Allergy:
| [89,90] | |
| Bisphenol A | Endocrine effects:
| [91,92,93,94,95,96,97,98] | |
| Antioxidants | Arylamines | Pro-cancer activity:
| [99,100] |
| Light stabilizers and ultraviolet (UV) absorbents | Hindered amines light stabilizers (e.g., bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate) | Cytotoxic effect:
| [101] |
| Benzotriazole UV stabilizers [e.g., UV-328 (2-(2H-benzotriazol-2-yl)-4,6-di-tert-pentylphenol)] | Inflammation:
| [102,103] | |
| Heat stabilizers | Vinyl chloride | Liver diseases:
| [104,105,106,107] |
| Flame retardants | Short, medium, and long chlorinated paraffins (SCCP/MCCP/LCCP) | Cytotoxic effect:
| [108,109] |
| Tris(1,3-dichloro-2-propyl) phosphate (TDCPP) | Cytotoxic effect:
| [110,111] | |
| Sb2O3 | Pro-cancer activity:
| [112,113] | |
| Polybrominated diphenyl (PBB) and polybrominated diphenyl ethers (PBDES) | Pro-cancer activity:
| [114,115] | |
| Pigments | TiO2 | Inflammation:
| [116,117] |
| Sources of MPs | Quantity of MPs | Polymer Types | References |
|---|---|---|---|
| Effluents | 50–86 MPs/dm3 | PE, PP, and PS | [243] |
| 840–3116 μg/dm3 | PE, PP, PET, PVC, and PMMA | [244] | |
| 1.2–23.1 μg/ dm3 | PET | [245] | |
| Bees | – | Polyester, PE, PVC, PU, epoxy resin, PAN, POM, PP, PS, PSU, PTFE, and PA | [246] |
| Boat ropes | 11–822 MPs/m | PP, polysteel (a blend of PE and PP) | [247] |
| Take-out food containers | 3–29 MPs/container | PS, polyester, rayon, acrylic, nylon, PE, PP, and PET (depending on the container type) | [239] |
| Disposable cups (pe-coated paper cups) | 675–5984 MPs/dm3 | PE | [240] |
| Disposable cups (PP cups) | 781–4951 MPs/dm3 | PP | |
| Disposable cups (PS cups) | 838–5215 MPs/dm3 | PS | |
| Plastic bottles for children | 16.2 million MPs/dm3 | PP (bottle material) | [241] |
| Cutting board | 100–300 MPs/mm per cut | – | [242] |
| Occurrence of MPs | Methods of MPs Analysis | Abundance of MPs | References |
|---|---|---|---|
| The Chukchi Sea, western Arctic Ocean | FTIR | 0–18,815 MPs/km2; 0–445 g/km2 | [272] |
| The open Baltic Sea | Optical microscope, FTIR | 79 ± 18 MPs/m3 | [273] |
| Surface waters of the Kattegat/Skagerrak, Denmark | μ-FTIR | 11–87 MPs/m3 | [274] |
| Estuarine surface water in Mauritius | Optical microscope, FTIR | 249–412 MPs/dm3 | [275] |
| The water columns of catchments in Kamniška Bistrica, Slovenia | Optical microscope, FTIR | 59 ± 16 MPs/m3 | [276] |
| The water columns of catchments in Ljubljanica, Slovenia | Optical microscope, FTIR, μ-FTIR | 31 ± 14 MPs/m3 | |
| Groundwater in the Haean Basin of Korea | μ-FTIR | 0.02–3.48 MPs/dm3 | [277] |
| Estuarine sediments in Mauritius | Optical microscope, FTIR | 74–235 MPs/kg | [275] |
| The sediments of catchments in Kamniška Bistrica, Slovenia | Optical microscope, FTIR | 22 ± 20 MPs/kg | [276] |
| The sediments of catchments in Ljubljanica, Slovenia | Optical microscope, FTIR, μ-FTIR | 23 ± 25 MPs/kg | |
| The sediments of the Weser River catchment, Germany | μ-FTIR | 99 ± 85 MPs/m2 | [278] |
| The atmosphere of the Northwestern Pacific Ocean | Optical microscope, μ-FTIR | 0.0046–0.064 MPs/m3 | [279] |
| Air in the Gdańsk harbour | Optical microscope, µ-Raman | 161 ± 75 MPs/m3 | [273] |
| Air of Baltic Sea | Optical microscope, µ-Raman | 24 ± 9 MPs/m3 | |
| Air of the Gotland Island | Optical microscope, µ-Raman | 45 ± 20 MPs/m3 | |
| Air in the Weser River catchment, Germany | RS | 91 ± 47 MPs/m3 | [278] |
| Soil of the green park in Coimbra, Portugal | Optical microscope, μ-FTIR | 158,000 MPs/kg | [280] |
| Soil of the landfill in Coimbra, Portugal | Optical microscope, μ-FTIR | 150,000 MPs/kg | |
| Soil of industrial area in Coimbra, Portugal | Optical microscope, μ-FTIR | 127,000 MPs/kg | |
| Soil of dump in Coimbra, Portugal | Optical microscope, μ-FTIR | 126,000 MPs/kg | |
| Soil of the forest in Coimbra, Portugal | Optical microscope, μ-FTIR | 55,000 MPs/kg | |
| Soil of Bhopal, India | Optical microscope, FTIR | 2.5 ± 0.71–180 ± 13.44 MPs/kg | [281] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674
Kadac-Czapska K, Knez E, Gierszewska M, Olewnik-Kruszkowska E, Grembecka M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials. 2023; 16(2):674. https://doi.org/10.3390/ma16020674
Chicago/Turabian StyleKadac-Czapska, Kornelia, Eliza Knez, Magdalena Gierszewska, Ewa Olewnik-Kruszkowska, and Małgorzata Grembecka. 2023. "Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks" Materials 16, no. 2: 674. https://doi.org/10.3390/ma16020674
APA StyleKadac-Czapska, K., Knez, E., Gierszewska, M., Olewnik-Kruszkowska, E., & Grembecka, M. (2023). Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials, 16(2), 674. https://doi.org/10.3390/ma16020674






