Understanding the Corrosion Behavior of Nickel–Aluminum Bronze Induced by Cavitation Corrosion Using Electrochemical Noise: Selective Phase Corrosion and Uniform Corrosion
Abstract
1. Introduction
2. EN Analysis
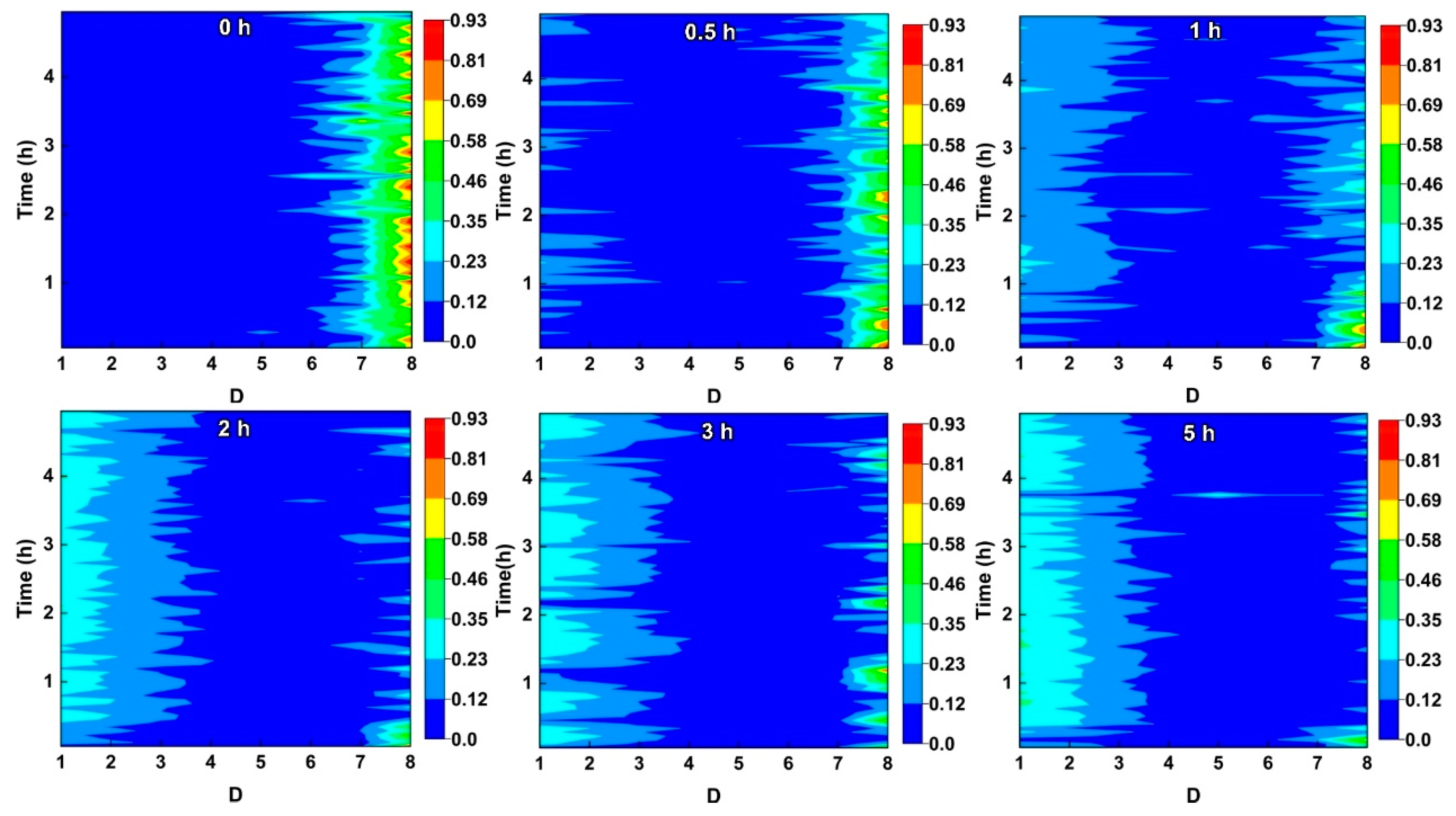
2.1. Wavelet Analysis
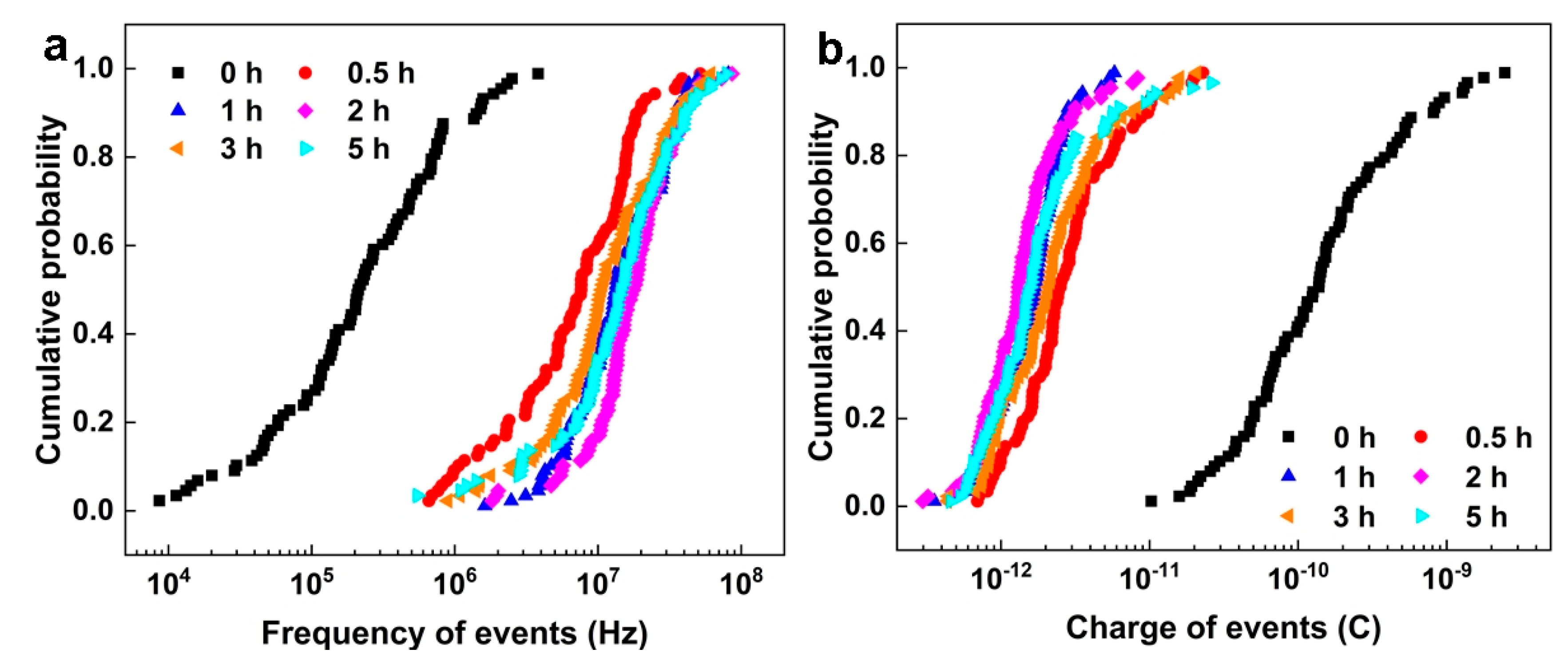
2.2. Shot Noise Theory
2.3. Corrosion Initiation Rate Based on Shot Noise
2.4. Corrosion Growth Probability Based on Shot Noise
3. Experimental Section
4. Results and Discussion
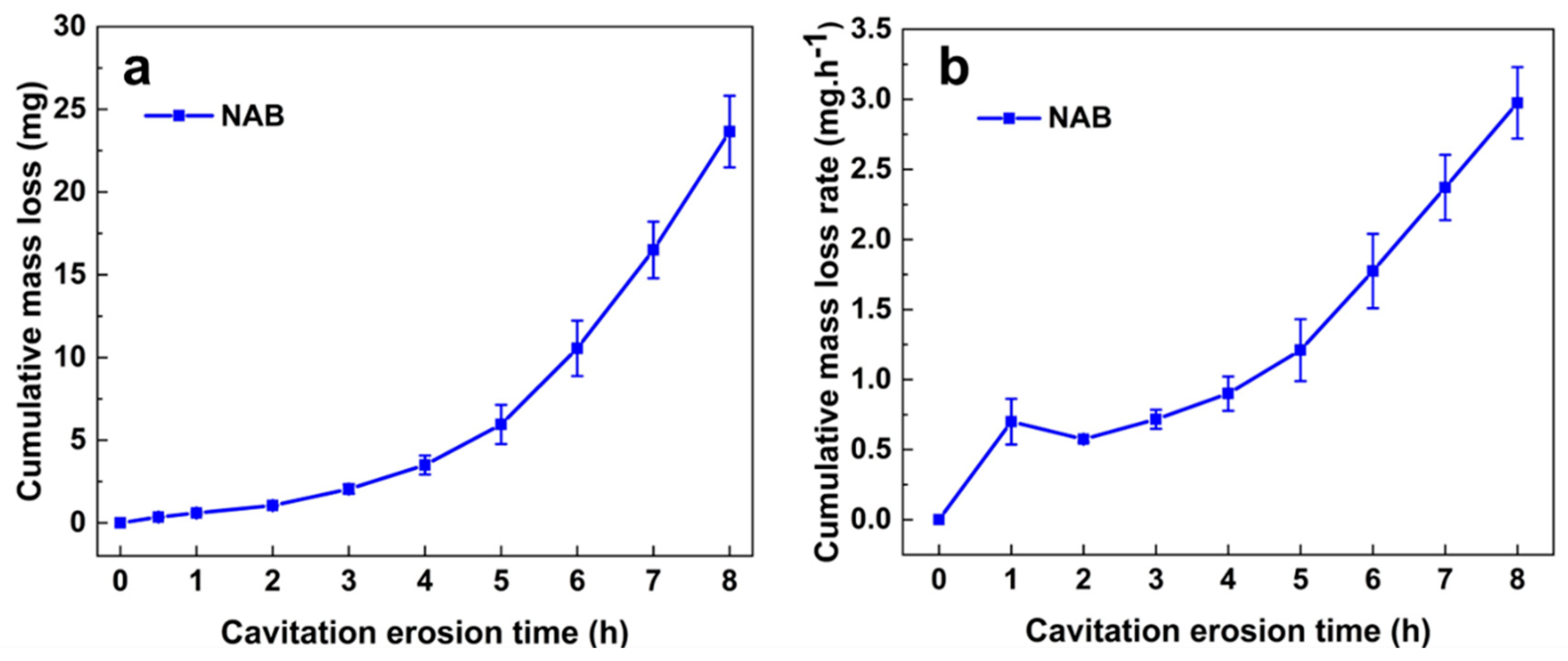
4.1. Mass Loss
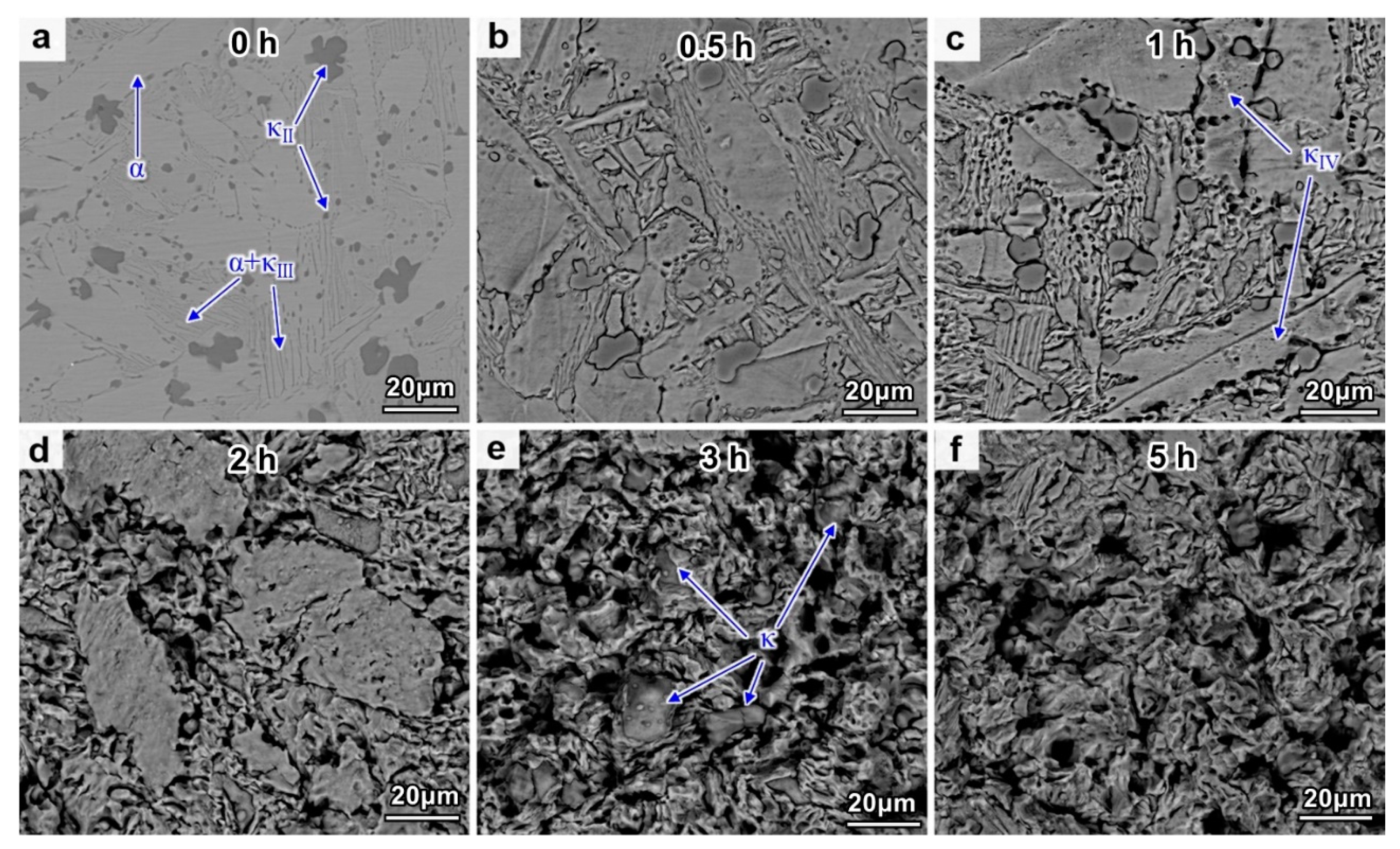
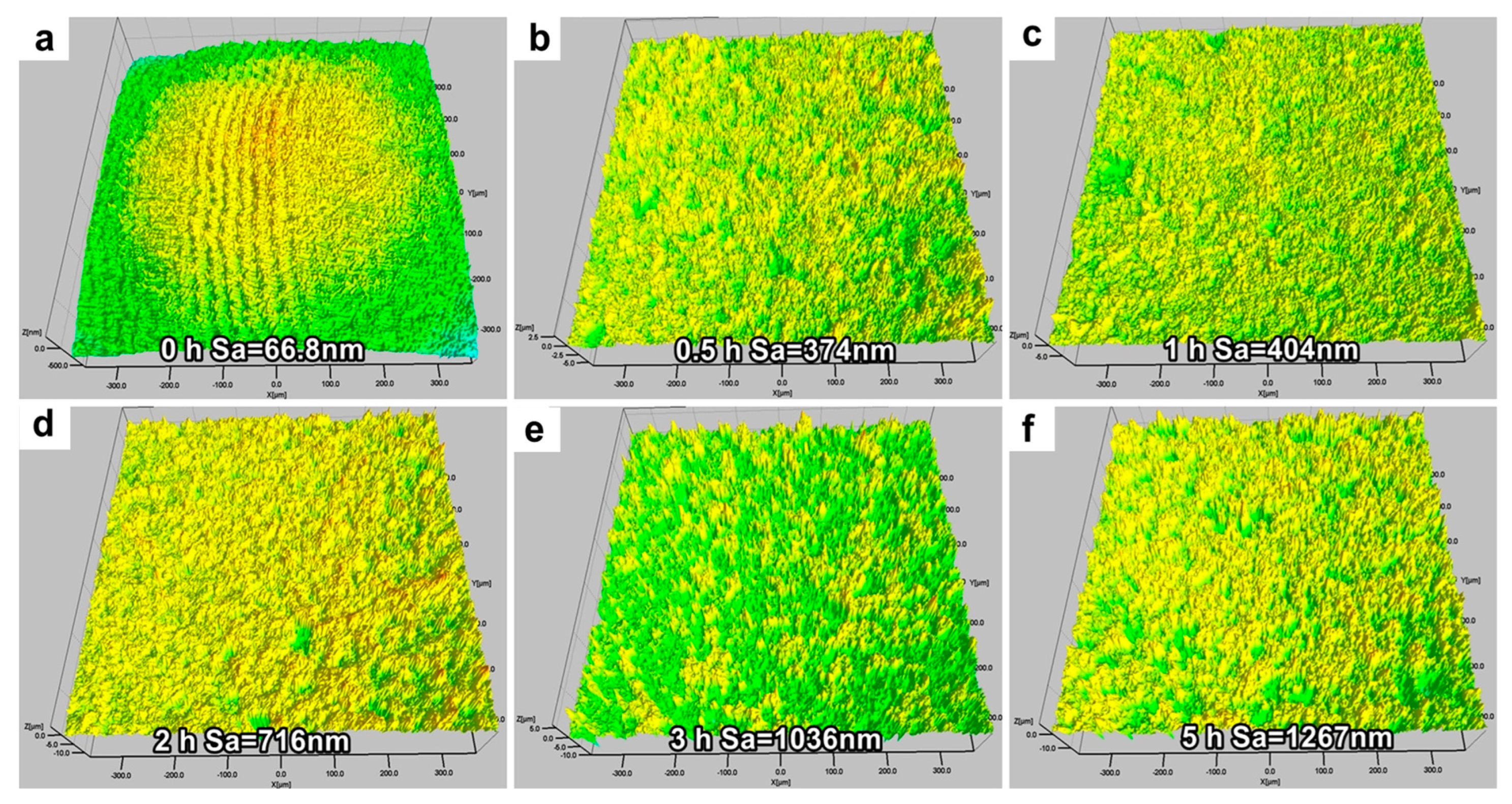
4.2. Evolution of CE Morphology and Surface Roughness
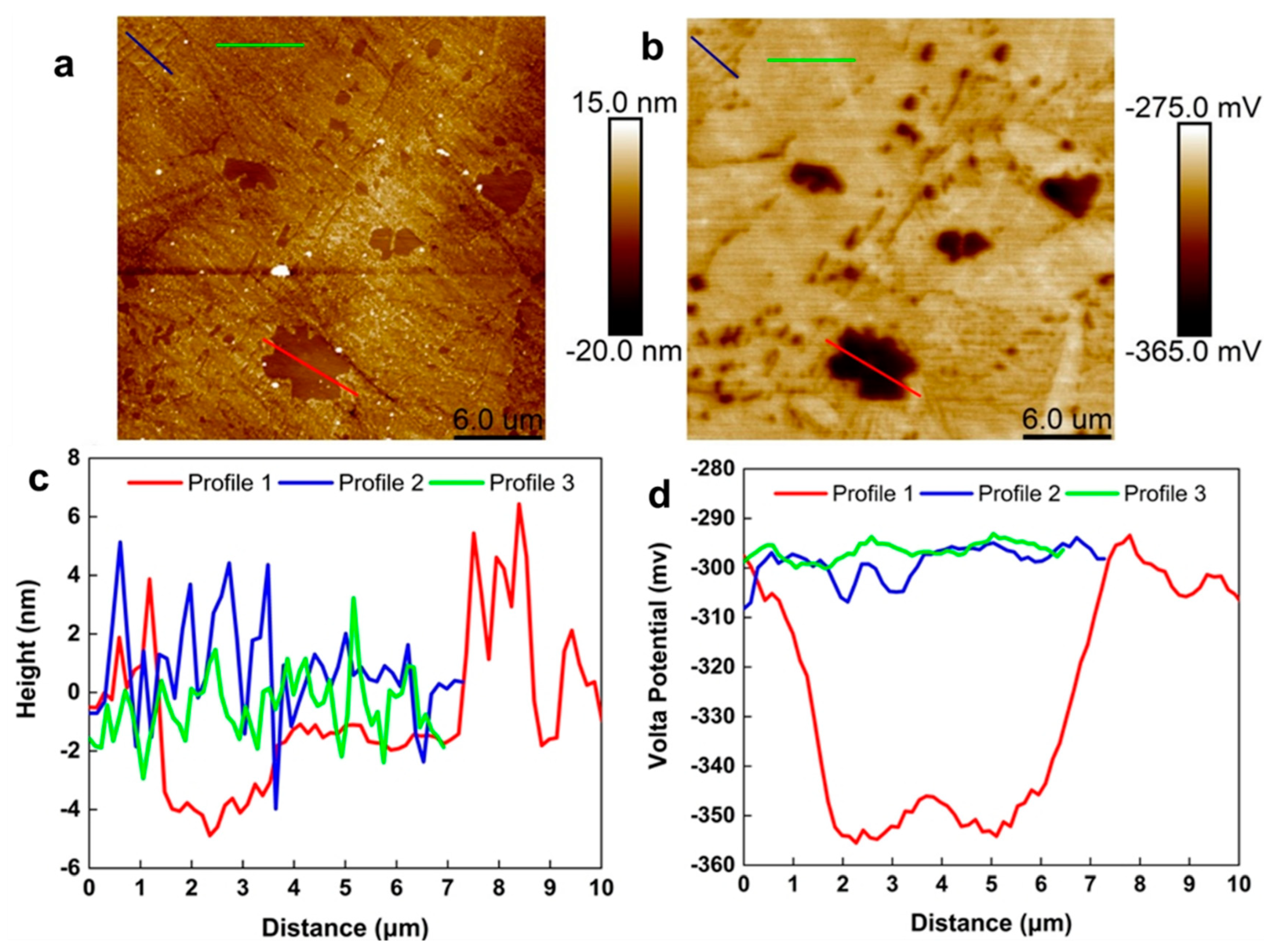
4.3. AFM and SKPFM Analysis
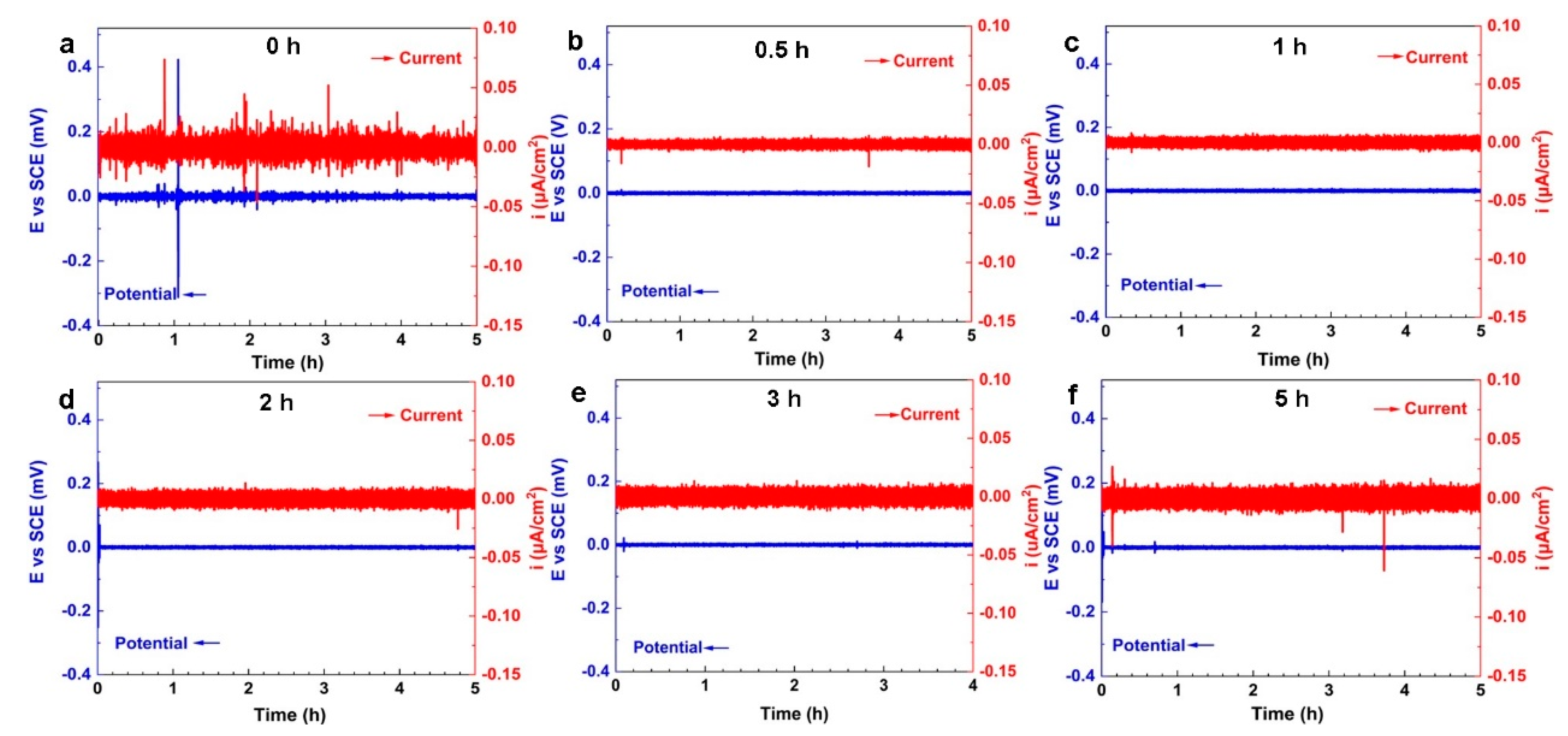
4.4. Electrochemical Noise
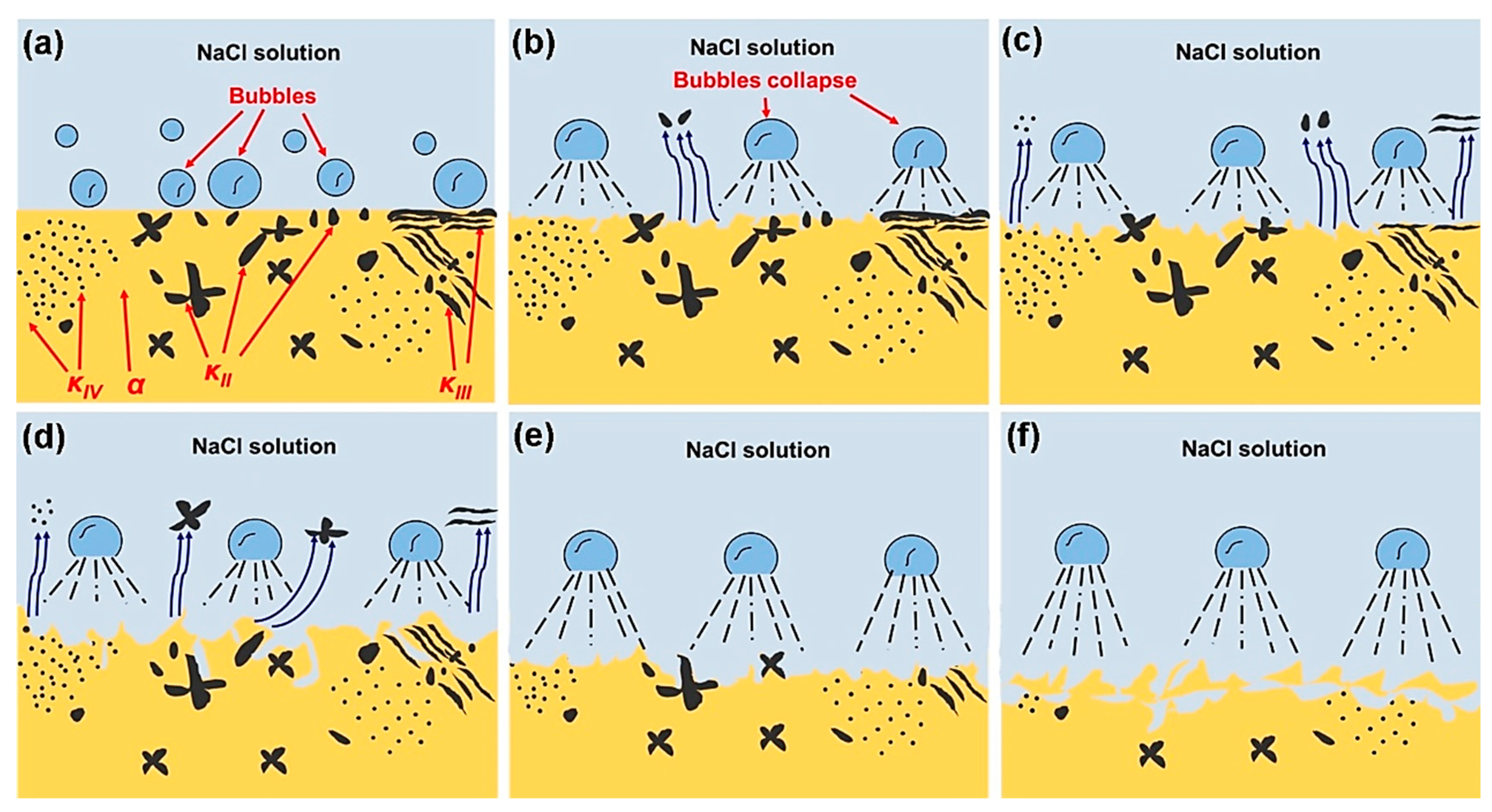
4.5. Corrosion Mechanism of NAB after Different CE Times
5. Conclusions
- The corrosion behavior of the NAB after different CE times was closely related to the change in microstructure. The essence of selective phase corrosion involved the galvanic corrosion between the α phase and the κ phase, which led to the corrosion along the boundary of the α/κ phase in 3.5 wt.% NaCl solution.
- In the CE process, the hard κ phase had a higher resistance to CE impact compared with the α phase. Meanwhile, the κ phase often acted as the cathode and the α phase often acted as the anode, which caused the accelerated corrosion of the α phase when the two kinds of the phases were in contact with one another. Therefore, the damage to the κ phase was far less than that of the α phase. However, the stress concentration and selective phase corrosion indicated that cracks preferentially occurred at the boundary of the α/κ phase, and the κ phase with a small size was preferentially peeled off in the CE process.
- Before CE for 0.5 h, selective phase corrosion played a leading role in the surface damage, which was mainly attributed to the existence of a large number of κ phases, accelerating the corrosion of the boundary of the α/κ phase due to the potential difference. With the prolongation of CE time, the effect of selective phase corrosion on the sample surface gradually declined, while the effect of uniform corrosion gradually increased. Both selective phase corrosion and uniform corrosion presented equal performances after 1 h of CE. The sample surface mainly demonstrated uniform corrosion after CE for 2 h–5 h. However, it is worth noting that the role of selective phase corrosion was enhanced after 3 h of CE, which could be caused by the accelerated selective phase corrosion, due to the exposure of the internal κ phase to the sample surface. A new corrosion mechanism of NAB after different CE times was revealed based on the relevant experimental results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.X.; Zhang, L.M.; Udoh, I.; Ma, A.L.; Zheng, Y.G. Deformation-induced martensite in 304 stainless steel during cavitation erosion: Effect on passive film stability and the interaction between cavitation erosion and corrosion. Tribol. Int. 2022, 167, 107422. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, Y.P.; Hong, S.; Cheng, J. Ultrasonic cavitation erosion mechanism and mathematical model of HVOF sprayed Fe-based amorphous/nanocrystalline coatings. Ultrason. Sonochem. 2019, 52, 142–149. [Google Scholar] [CrossRef]
- Siemek, K.; Eseev, M.K.; Horodek, P.; Kobets, A.; Kuziv, I. Defects studies of nickel aluminum bronze subjected to cavitation. Appl. Surf. Sci. 2021, 546, 149107. [Google Scholar] [CrossRef]
- Mayer, A.R.; Bertuol, K.; Siqueira, I.B.; Chicoski, A.; Váz, R.F.; de Sousa, M.J.; Pukasiewicz, A.G. Evaluation of cavitation/corrosion synergy of the Cr3C2-25NiCr coating deposited by HVOF process. Ultrason. Sonochem. 2022, 69, 105271. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.K.; Cheng, F.T.; Man, H.C. Synergistic effect of cavitation erosion and corrosion of various engineering alloys in 3.5% NaCl solution. Mater. Sci. Eng. A 2000, 290, 145–154. [Google Scholar] [CrossRef]
- Qin, Z.B.; Li, X.H.; Xia, D.H.; Zhang, Y.; Feng, C.; Wu, Z.; Hu, W. Effect of compressive stress on cavitation erosion-corrosion behavior of nickel-aluminum bronze alloy. Ultrason. Sonochem. 2022, 89, 106143. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Hu, S.B.; Cheng, G.K. Effect of shot peening on surface characterization and cavitation resistance of nickel aluminum bronze. Mater. Today Commun. 2022, 33, 104767. [Google Scholar] [CrossRef]
- Culpan, E.A.; Rose, G. Corrosion behaviour of cast nickel aluminium bronze in sea water. Br. Corros. J. 1979, 14, 160–166. [Google Scholar] [CrossRef]
- Song, Q.N.; Tong, Y.; Li, H.L.; Zhang, H.N.; Xu, N. Corrosion and cavitation erosion resistance enhancement of cast Ni–Al bronze by laser surface melting. J. Iron Steel Res. Int. 2022, 29, 359–369. [Google Scholar] [CrossRef]
- Hasan, F.; Jahanafrooz, A.; Lorimer, G.W.; Ridley, N. The morphology, crystallography, and chemistry of phases in as-cast nickel-aluminum bronze. Metall. Trans. A 1982, 13, 1337–1345. [Google Scholar] [CrossRef]
- Li, Y.; Lian, Y.; Sun, Y.J. Cavitation erosion behavior of friction stir processed nickel aluminum bronze. J. Alloys Compd. 2019, 795, 233–240. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, Q.; Qin, Z.; Wu, Z.; Shen, B.; Liu, L.; Hu, W. The synergistic effect of cavitation erosion and corrosion of nickel-aluminum copper surface layer on nickel-aluminum bronze alloy. J. Alloys Compd. 2018, 747, 861–868. [Google Scholar] [CrossRef]
- Zhang, L.M.; Ma, A.L.; Yu, H.; Umoh, A.; Zheng, Y. Correlation of microstructure with cavitation erosion behaviour of a nickel-aluminum bronze in simulated seawater. Tribol. Int. 2019, 136, 250–258. [Google Scholar] [CrossRef]
- Hanke, S.; Fischer, A.; Beyer, M.; dos Santos, J. Cavitation erosion of NiAl-bronze layers generated by friction surfacing. Wear 2011, 273, 32–37. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, H.; Yang, R.; Zhang, H.; Yu, M.; Zhou, P.; Li, H.; Chen, X. Behavior of the hard phases of copper alloys subjected to cavitation erosion investigated by SEM observation. Tribol. Int. 2022, 174, 107771. [Google Scholar] [CrossRef]
- Culpan, E.A.; Foley, A.G. The detection of selective phase corrosion in cast nickel aluminium bronze by acoustic emission techniques. J. Mater. Sci. 1982, 17, 953–964. [Google Scholar] [CrossRef]
- Wharton, J.A.; Stokes, K.R. The influence of nickel–aluminium bronze microstructure and crevice solution on the initiation of crevice corrosion. Electrochim. Acta 2008, 53, 2463–2473. [Google Scholar] [CrossRef]
- Neodo, S.; Carugo, D.; Wharton, J.A.; Stokes, K. Electrochemical behaviour of nickel–aluminium bronze in chloride media: Influence of pH and benzotriazole. J. Electroanal. Chem. 2013, 695, 38–46. [Google Scholar] [CrossRef]
- Lorimer, G.W.; Hasan, F.; Iqbal, J.; Ridley, N. Observation of microstructure and corrosion behaviour of some aluminium bronzes. Br. Corros. J. 1986, 21, 244–248. [Google Scholar] [CrossRef]
- Gilbert, P.T. Corrosion Resisting Properties of 90/10 Copper–Nickel–Iron Alloy with Particular Reference to Offshore Oil and Gas Applications. Br. Corros. J. 1979, 14, 20–25. [Google Scholar] [CrossRef]
- Peng, S.; Xu, J.; Li, Z.Y.; Jiang, S.; Xie, Z.-H.; Munroe, P. Electrochemical noise analysis of cavitation erosion corrosion resistance of NbC nanocrystalline coating in a 3.5 wt% NaCl solution. Surf. Coat. Tech. 2021, 415, 127113. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, X.L.; Zhao, Z.Y.; Li, X.; Liu, B.; Bai, P. Electrochemical noise comparative study of pitting corrosion of 316L stainless steel fabricated by selective laser melting and wrought. J. Electroanal. Chem. 2021, 894, 115351. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.W.; Meng, G.Z.; Wang, F. Electrochemical noise analysis of the corrosion of AZ91D magnesium alloy in alkaline chloride solution. Electrochim. Acta 2007, 53, 561–568. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.F.; Zhao, Z.Y.; Bai, P.; Liu, B.; Tan, J.; Wu, X. In-situ monitoring of pitting corrosion of Q235 carbon steel by electrochemical noise: Wavelet and recurrence quantification analysis. J. Electroanal. Chem. 2020, 879, 114776. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Galeano, M.; Proverbio, E.; Di Pietro, D.; Cappuccini, F. Identification of damage evolution during SCC on 17-4 PH stainless steel by combining electrochemical noise and acoustic emission techniques. Corros. Sci. 2015, 98, 573–584. [Google Scholar] [CrossRef]
- Li, J.; Du, C.M.; Liu, Z.Y.; Li, X.; Liu, M. Effect of microstructure on the corrosion resistance of 2205 duplex stainless steel. Part 2, Electrochemical noise analysis of corrosion behaviors of different microstructures based on wavelet transform. Constr. Build. Mater. 2018, 189, 1294–1302. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.; Marcos, M. Using wavelets transform in the analysis of electrochemical noise data. Electrochim. Acta 1999, 44, 4805–4816. [Google Scholar] [CrossRef]
- Bahrami, M.J.; Shahidi, M.; Hosseini, S.M.A. Comparison of electrochemical current noise signals arising from symmetrical and asymmetrical electrodes made of Al alloys at different pH values using statistical and wavelet analysis. Part I: Neutral and acidic solutions. Electrochim. Acta 2014, 148, 127–144. [Google Scholar] [CrossRef]
- Naghizade, R.; Shahidi Zandi, M.; Hosseini, S.M.A. Electrochemical noise analysis of corrosion behaviour of asymmetric electrodes made of mild steel in NaHCO3 solutions at different NaCl concentrations. Measurement 2020, 155, 107501. [Google Scholar] [CrossRef]
- Cottis, R.A. Interpretation of Electrochemical Noise Data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Sanchez-Amaya, J.M.; Cottis, R.A.; Botana, F.J. Shot noise and statistical parameters for the estimation of corrosion mechanisms. Corros. Sci. 2005, 47, 3280–3299. [Google Scholar] [CrossRef]
- Meng, F.D.; Liu, Y.S.; Liu, L.; Cui, Y.; Wang, F. Effect of Asymmetric Epoxy Coating/Metal Electrodes on the Electrochemical Noise Measurements Under Marine Alternating Hydrostatic Pressure. Coatings 2019, 9, 852. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.Q. Interpreting electrochemical noise signal arising from stress corrosion cracking of 304 stainless steel in simulated PWR primary water environment by coupling acoustic emission. J. Mater. Res. Technol. 2022, 20, 3807–3817. [Google Scholar] [CrossRef]
- Park, J.-J.; Pyun, S.-I. Stochastic approach to the pit growth kinetics of Inconel alloy 600 in Cl− ion-containing thiosulphate solution at temperatures 25–150 °C by analysis of the potentiostatic current transients. Corros. Sci. 2004, 46, 285–296. [Google Scholar] [CrossRef]
- Engelhardt, G.; Macdonald, D.D. Unification of the deterministic and statistical approaches for predicting localized corrosion damage. I. Theoretical foundation. Corros. Sci. 2004, 46, 2755–2780. [Google Scholar] [CrossRef]
- G32-16 A; Standard Test Method for Cavitation Erosion Using Vibratory Apparatus. Annual Book of ASTM Standards. ASTM: West Conshohochen, PA, USA, 2016.
- Zhang, L.M.; Li, Z.X.; Hu, J.X.; Ma, A.; Zhang, S.; Daniel, E.; Umoh, A.; Hu, H.; Zheng, Y. Understanding the roles of deformation-induced martensite of 304 stainless steel in different stages of cavitation erosion. Tribol. Int. 2021, 155, 106752. [Google Scholar] [CrossRef]
- Muster, T.H.; Hughes, A.E. Applications and limitations of scanning kelvin probe force microscopy for the surface analysis of aluminum alloys. J. Electrochem. Soc. 2006, 153, B474. [Google Scholar] [CrossRef]
- Song, Q.N.; Zheng, Y.G.; Ni, D.R.; Ma, Z. Studies of the nobility of phases using scanning Kelvin probe microscopy and its relationship to corrosion behaviour of Ni-Al bronze in chloride media. Corros. Sci. 2015, 92, 95–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.Y.; Bai, P.K.; Li, X.; Liu, B.; Tan, J.; Wu, X. In-situ monitoring of pitting corrosion of AZ31 magnesium alloy by combining electrochemical noise and acoustic emission techniques. J. Alloys Compd. 2021, 878, 160334. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, Y.; Song, S.Z.; Behnamian, Y.; Xu, L.; Wu, Z.; Qin, Z.; Gao, Z.; Hu, W. Identifying defect levels in organic coatings with electrochemical noise (EN) measured in Single Cell (SC) mode. Prog. Org. Coat. 2019, 126, 53–61. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Behnamian, Y. Detection of corrosion degradation using electrochemical noise (EN): Review of signal processing methods for identifying corrosion forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Cottis, R.; Al-Awadhi, M.A.A.; Al-Mazeedi, H.; Turgoose, S. Measures for the detection of localized corrosion with electrochemical noise. Electrochim. Acta 2001, 46, 3665–3674. [Google Scholar] [CrossRef]
- Al-Mazeedi, H.A.A.; Cottis, R.A. A practical evaluation of electrochemical noise parameters as indicators of corrosion type. Electrochim. Acta 2004, 49, 2787–2793. [Google Scholar] [CrossRef]
- Moradi, M.; Rezaei, M. Electrochemical noise analysis to evaluate the localized anti-corrosion properties of PP/graphene oxide nanocomposite coatings. J. Electroanal. Chem. 2022, 921, 116665. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Bezaatpour, A.; Asadpour Joghani, R. Shot noise analysis to investigate the corrosion inhibition of AZ91D magnesium alloy in sulfuric acid solution. Prot. Met. Phys. Chem. Surf. 2016, 52, 329–338. [Google Scholar] [CrossRef]
- Cheng, Q.L.; Tao, B.; Song, L.Y.; Zhang, W.; Liu, X.; Li, W.; Hou, B.; Liu, Q. Corrosion behaviour of Q235B carbon steel in sediment water from crude oil. Corros. Sci. 2016, 111, 61–71. [Google Scholar] [CrossRef]



| Brand | Al | Ni | Fe | Mn | C | Si | Cu |
|---|---|---|---|---|---|---|---|
| ZCuAl9Fe4Ni4Mn2 | 9.66 | 4.66 | 4.45 | 2.19 | 0.05 | 0.10 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Qiao, Y.; Zhang, L.; Ma, A.; Ma, R.; Zheng, Y. Understanding the Corrosion Behavior of Nickel–Aluminum Bronze Induced by Cavitation Corrosion Using Electrochemical Noise: Selective Phase Corrosion and Uniform Corrosion. Materials 2023, 16, 669. https://doi.org/10.3390/ma16020669
Li L, Qiao Y, Zhang L, Ma A, Ma R, Zheng Y. Understanding the Corrosion Behavior of Nickel–Aluminum Bronze Induced by Cavitation Corrosion Using Electrochemical Noise: Selective Phase Corrosion and Uniform Corrosion. Materials. 2023; 16(2):669. https://doi.org/10.3390/ma16020669
Chicago/Turabian StyleLi, Liang, Yanxin Qiao, Lianmin Zhang, Aili Ma, Rongyao Ma, and Yugui Zheng. 2023. "Understanding the Corrosion Behavior of Nickel–Aluminum Bronze Induced by Cavitation Corrosion Using Electrochemical Noise: Selective Phase Corrosion and Uniform Corrosion" Materials 16, no. 2: 669. https://doi.org/10.3390/ma16020669
APA StyleLi, L., Qiao, Y., Zhang, L., Ma, A., Ma, R., & Zheng, Y. (2023). Understanding the Corrosion Behavior of Nickel–Aluminum Bronze Induced by Cavitation Corrosion Using Electrochemical Noise: Selective Phase Corrosion and Uniform Corrosion. Materials, 16(2), 669. https://doi.org/10.3390/ma16020669







