Flame-Retardant Foamed Material Based on Modified Corn Straw Using Two Nitrogenous Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Methods
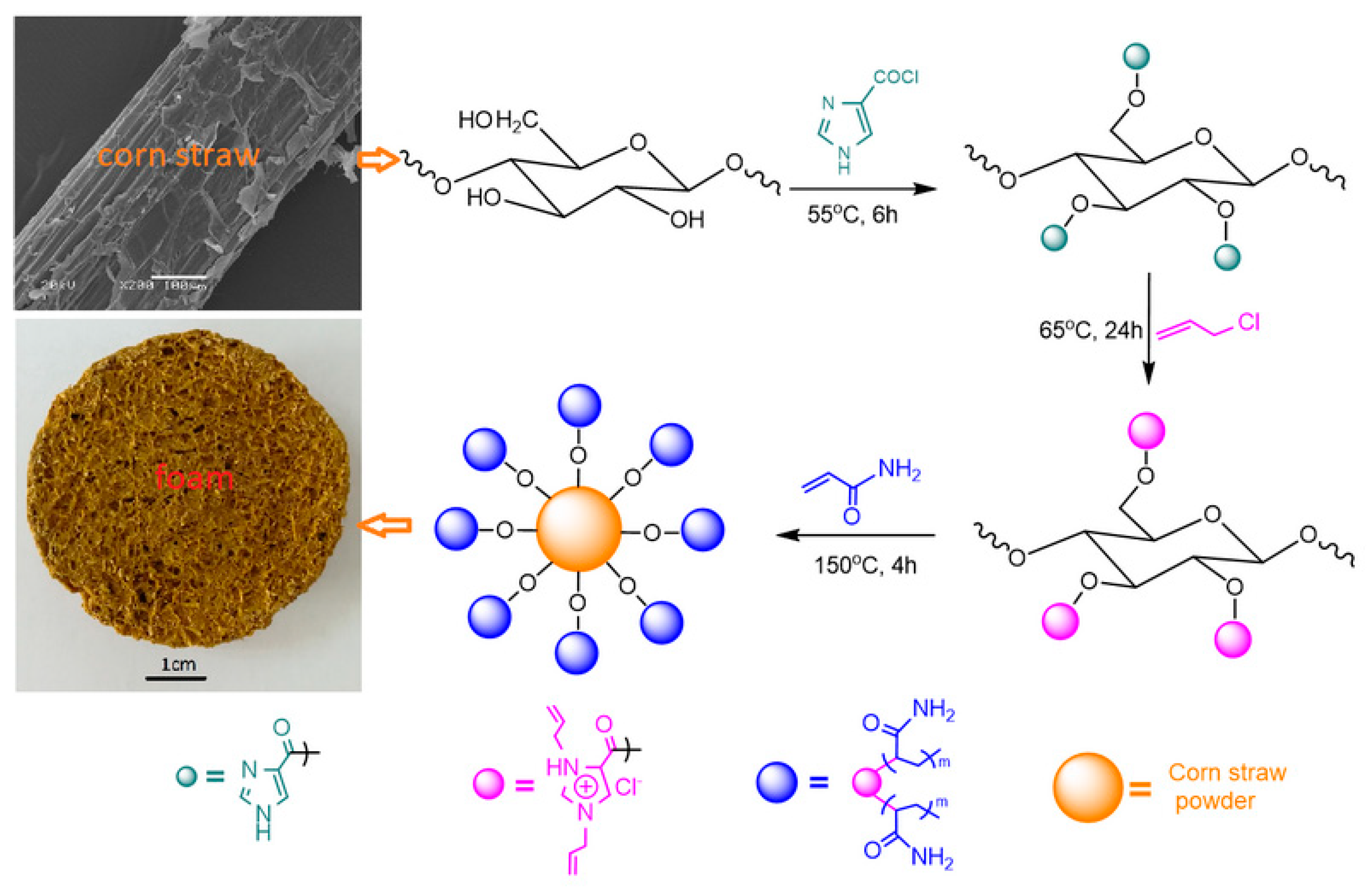
2.3.1. The Preparation and Characterization of the Flame-Retardant Foaming Material Using Modified Corn Straw
2.3.2. The Preparation and Test Processes of the Flame-Retardant Foamed Material
2.4. Analysis of the Materials
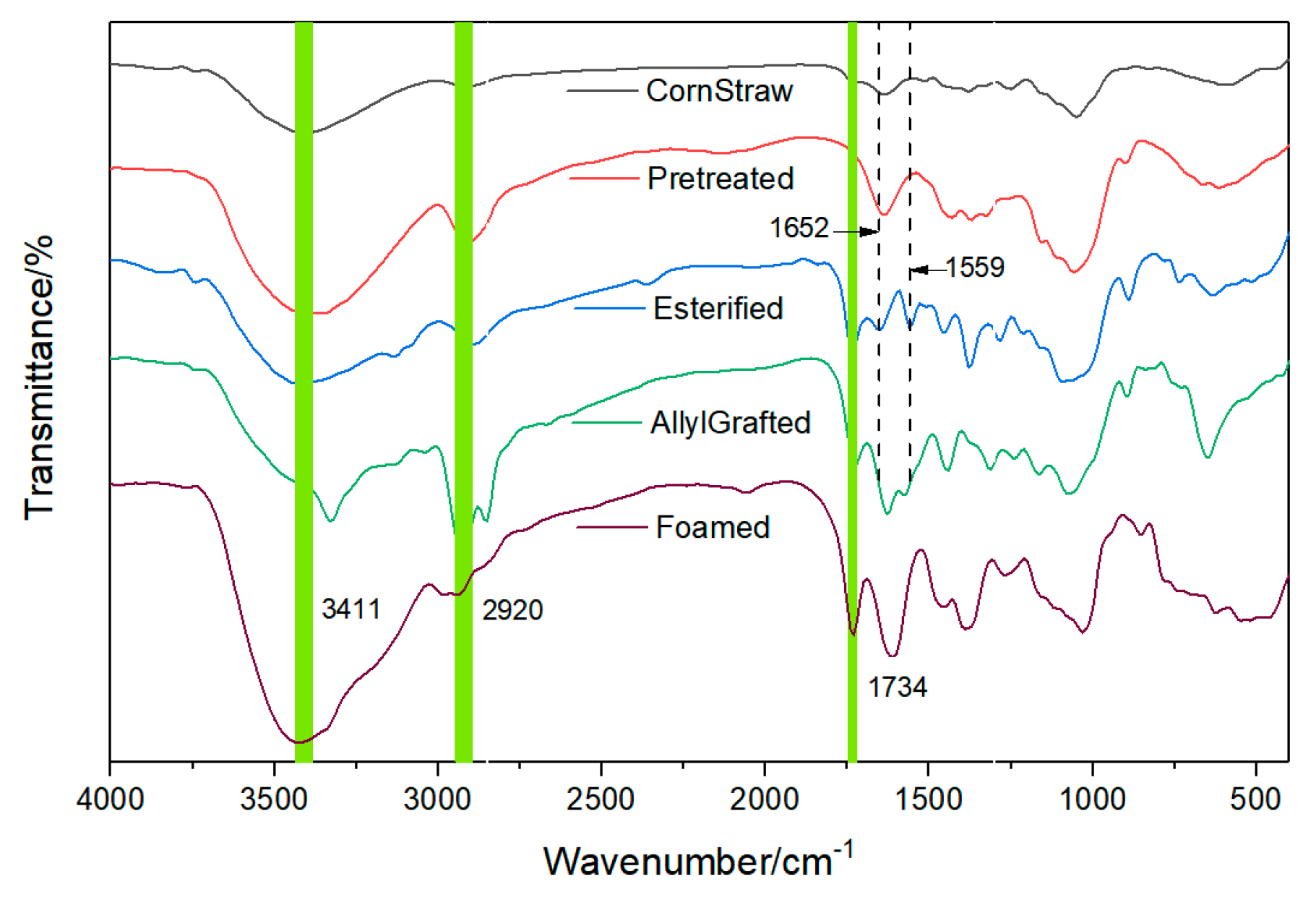
2.4.1. Fourier Transform-Infrared Spectroscopy (FT-IR) Characterization
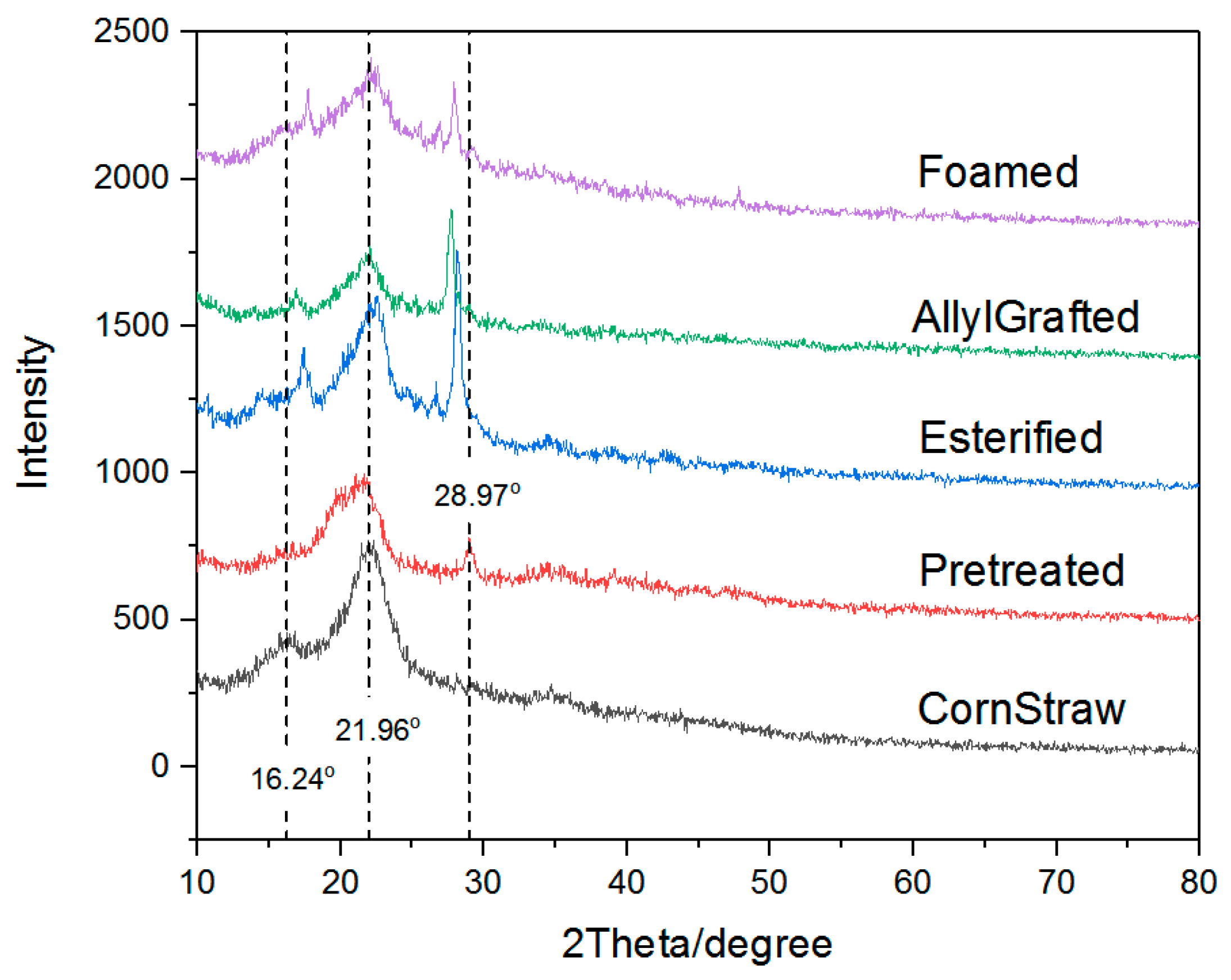
2.4.2. X-ray Diffraction (XRD) Characterization
2.4.3. Scanning Electron Microscopy (SEM) Analysis
2.4.4. Apparent Density
2.4.5. Foaming Ratio
2.4.6. Mechanical Strength Test
2.4.7. Flammability Test
3. Results and Discussion
3.1. The First Step: The Pretreatment of Corn Straw
3.2. The Second Step: The Esterification of the Pretreated Corn Straw Using a Nitrogen-Containing Compound
3.3. The Third Step: The Graft of the Esters Using Allyl Chloride
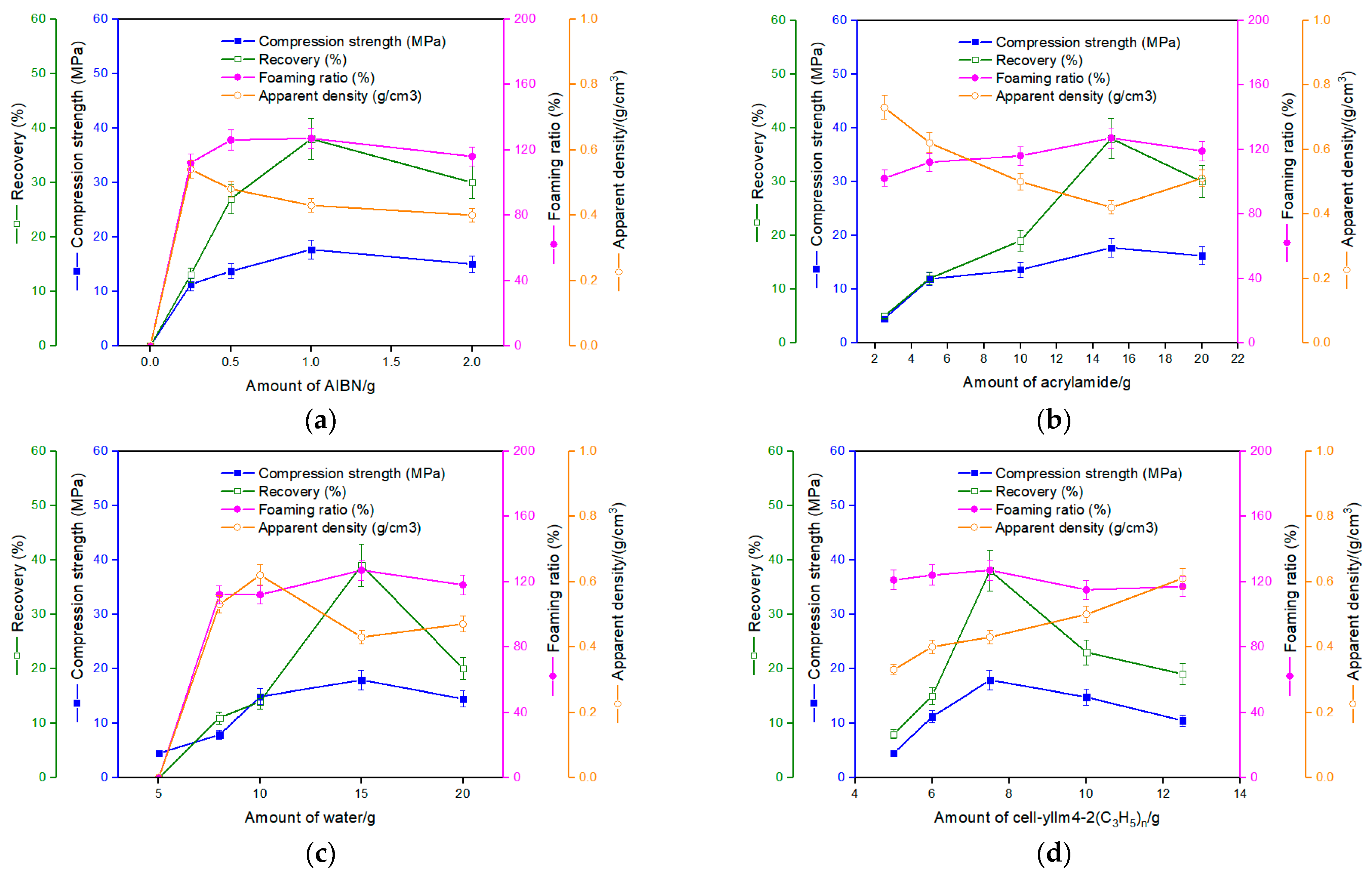
3.4. The Fourth Step: The Foaming Process Accompanying a Grafting through Acrylamide
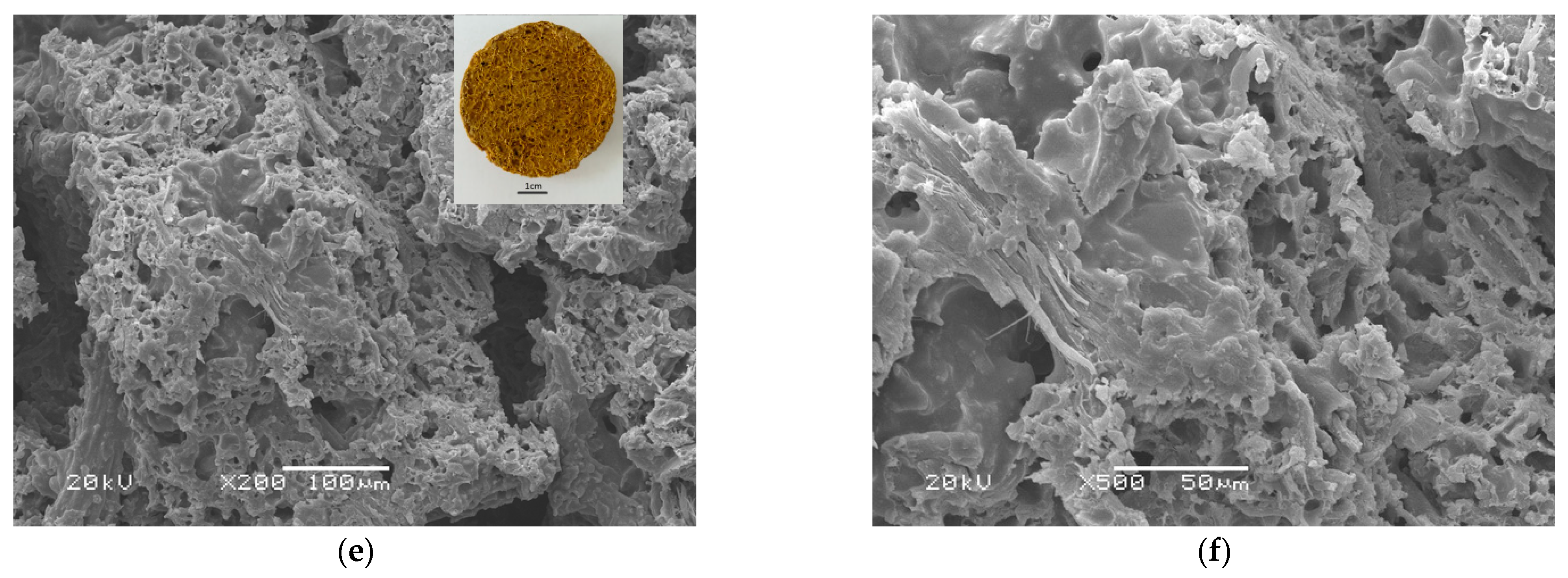
3.5. Observation of the Intermediate Products and the Foamed Material
3.6. Flammability Test Result
4. Conclusions
- The modified corn straw powder had a core/shell structure, in which rigid cellulose fiber bundles acted as the core and the long soft chains acted as the shell. The long soft chains contained two nitrogenous layers. The inner thin nitrogenous layer consisted of imidazole rings, which came from an esterification reaction of the corn powder with 1H-imidazole-4-carbonyl chloride, and the outer thick nitrogenous layer was prepared by grafting acrylamide onto an intermediate layer through a free-radical polymerization. The excellent flame-retardancy was ascribed to the two nitrogenous layers, which provided a sufficient nitrogen source for non-combustible gases. These gases, along with the char crust, effectively prevented the heat transfer and retarded the flame.
- Azodiisobutyronitrile (AIBN) acted as an initiator and the main foaming agent, and deionized water as a plasticizing agent and an auxiliary foaming agent. This effectively simplified the formula.
- Two levels of foam cavities, one of several microns and generally unconnected, and the other of ten to one hundred microns and connected, were formed. It is believed the nonuniformity was caused by the heterogeneous precursor, which was composed of rigid cores of fiber bundles and soft shells of long carbon chains.
- The strength of the final foamed material was relatively high, which was favorable to applications at some conditions with heavy loads. It was believed the high strength partly came from maintaining the fiber form of the corn straw. In addition, a large part of the crystal region of the cellulose remained after the modification reactions and the foaming process, which was also helpful for obtaining foamed material with high strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.Q.; Zhao, C.S.; Yu, D.M. Study on Foamed Buffer Material with the Residues of Wheat Straw Pulping. Adv. Mat. Res. 2011, 236–238, 1317–1321. [Google Scholar] [CrossRef]
- Tugiman, N.; Mohamad, Z.; Rahman, W.A.W.A. Thermal Properties Determination of Polypropylene/Rice Straw Biocomposite Foam via TGA. Appl. Mech. Mater. 2014, 695, 216–219. [Google Scholar] [CrossRef]
- Mengeloglu, F.; Kilic, I.; Ozdemir, T.; Seker, B.; Karakus, K. Manufacture of Natural Fiber Filled Polystyrene Composites and Their Microcellular Foams. Proligno 2013, 9, 525–531. [Google Scholar]
- Grigoriou, A.H. Straw-wood composites bonded with various adhesive systems. Wood Sci. Technol. 2000, 34, 355–365. [Google Scholar] [CrossRef]
- Darnerud, P.O. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9. [Google Scholar] [CrossRef]
- Gilman, J.W.; Takashi, K.; Joseph, D.L. Nanocomposites: A revolutionary new flame retardant approach. Sampe J. 1997, 33, 40–46. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Duan, W.; Xia, L.; Zheng, X. A facile method to flame-retard epoxy resin with maintained mechanical properties through a novel P/N/S-containing flame retardant of tautomerization. Mat. Lett. 2017, 204, 77–80. [Google Scholar] [CrossRef]
- Uddin, K.M.A.; Ago, M.; Rojas, O.J. Hybrid films of chitosan, cellulose nanofibrils and boric acid: Flame retardancy, optical and thermo-mechanical properties. Carbohydr. Polym. 2017, 177, 13–21. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Harris, R.H.; Zhang, X.; Briber, R.M.; Cipriano, B.H.; Raghavan, S.R.; Awad, W.H.; Shields, J.R. Flame retardant mechanism of polyamide 6–clay nanocomposites. Polymer 2004, 45, 881–891. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Klosterhaus, S.; Eagle, S.; Fuh, J.; Meeker, J.D.; Blum, A.; Webster, T.F. Detection of organophosphate flame retardants in furniture foam and us house dust. Environ. Sci. Technol. 2009, 43, 7490. [Google Scholar] [CrossRef]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin to flame retard acrylonitrile butadiene styrene (ABS). Polym. Degrad. Stab. 2016, 127, 32–43. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Li, L.; Cai, Z. Flame-retardant properties and mechanisms of epoxy thermosets modified with two phosphorus-containing phenolic amines. J. Appl. Polym. Sci. 2016, 133, 43953. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Kandola, B.K.; Horrocks, A.R.; Price, D.; Coleman, G.V. Flame-Retardant Treatments of Cellulose and Their Influence on the Mechanism of Cellulose Pyrolysis. J. Macromol. Sci. C 1996, 36, 721–794. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Deng, C.-L.; Chen, L.; Wang, D.-Y.; Wang, Y.-Z. Flame-Retardant Effect of Sepiolite on an Intumescent Flame-Retardant Polypropylene System. Ind. Eng. Chem. Res. 2011, 50, 2047–2054. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, B. Synthesis and properties of phosphorus and nitrogen containing intumescent flame-retardant curing agent for epoxy resin. Plast. Rubber Compos. 2018, 47, 306–314. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W.; Klemm, D. Comprehensive Cellulose Chemistry; Fundamentals and Analytical Methods; Wiley-VCH: Weinheim, Germany, 1998; Volume 1. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic liquids: Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Oudiani, A.E.; Chaabouni, Y.; Msahli, S.; Sakli, F. Crystal transition from cellulose I to cellulose II in NaOH treated Agave americana L. fibre. Carbohydr. Polym. 2011, 86, 1221–1229. [Google Scholar] [CrossRef]
- Meyer, K.H.; Misch, L. Positions des atomes dans le nouveau modèle spatial de la cellulose. Helv. Chim. Acta 1937, 20, 232–244. [Google Scholar] [CrossRef]
- Gardner, K.H.; Blackwell, J. The structure of native cellulose. J. Pep. Sci. 1974, 13, 1975–2001. [Google Scholar] [CrossRef]
- Sarko, A.; Muggli, R. Packing Analysis of Carbohydrates and Polysaccharides. III. Valonia Cellulose and Cellulose II. Macromolecules 1974, 7, 486–494. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2011, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Increment in evolution of cellulose crystallinity analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Yao, W.; Weng, Y.; Catchmark, J.M. Improved cellulose X-ray diffraction analysis using Fourier series modeling. Cellulose 2020, 27, 5563–5579. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. Int. Union Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Horacek, H.; Grabner, R. Advantages of flame retardants based on nitrogen compounds. Polym. Degrad. Stab. 1996, 54, 205–215. [Google Scholar] [CrossRef]
- Lu, S.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Nazir, R.; Gaan, S. Recent developments in P(O/S)–N containing flame retardants. J. Appl. Polym. Sci. 2019, 137, 47910. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Wang, H.; Wang, Y.; Liang, S.; Pang, S.; Zhao, X.; Sun, X.; Shi, X.; Zhao, J. Flame-Retardant Foamed Material Based on Modified Corn Straw Using Two Nitrogenous Layers. Materials 2023, 16, 952. https://doi.org/10.3390/ma16030952
Su Q, Wang H, Wang Y, Liang S, Pang S, Zhao X, Sun X, Shi X, Zhao J. Flame-Retardant Foamed Material Based on Modified Corn Straw Using Two Nitrogenous Layers. Materials. 2023; 16(3):952. https://doi.org/10.3390/ma16030952
Chicago/Turabian StyleSu, Qiong, Hongling Wang, Yanbin Wang, Shuang Liang, Shaofeng Pang, Xiangfei Zhao, Xiyang Sun, Xiaoqin Shi, and Jun Zhao. 2023. "Flame-Retardant Foamed Material Based on Modified Corn Straw Using Two Nitrogenous Layers" Materials 16, no. 3: 952. https://doi.org/10.3390/ma16030952
APA StyleSu, Q., Wang, H., Wang, Y., Liang, S., Pang, S., Zhao, X., Sun, X., Shi, X., & Zhao, J. (2023). Flame-Retardant Foamed Material Based on Modified Corn Straw Using Two Nitrogenous Layers. Materials, 16(3), 952. https://doi.org/10.3390/ma16030952




