Technological Aspects of Lithium-Titanium Ferrite Synthesis by Electron-Beam Heating
Abstract
1. Introduction
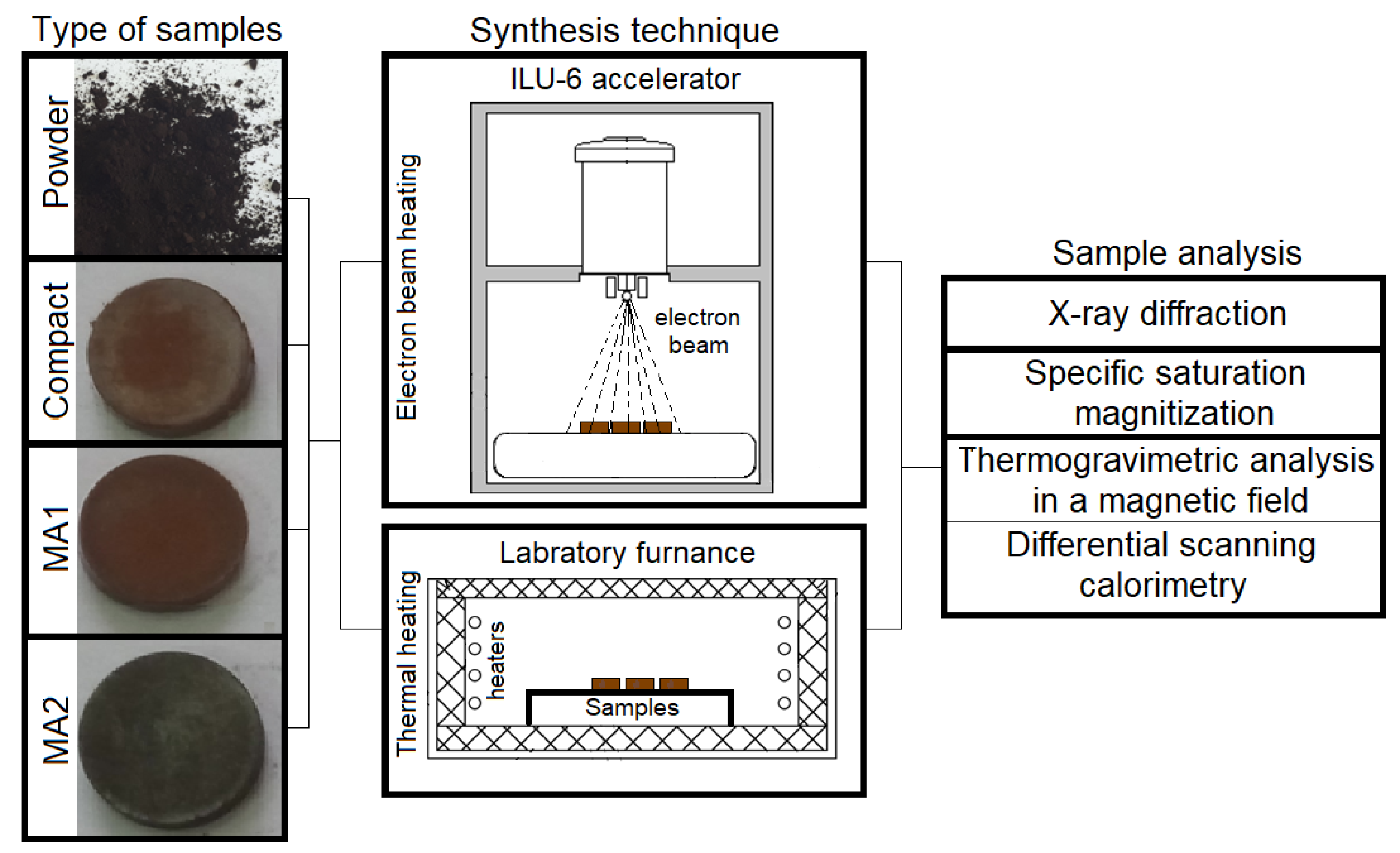
2. Materials and Methods
3. Results and Discussion
3.1. XRD Analysis
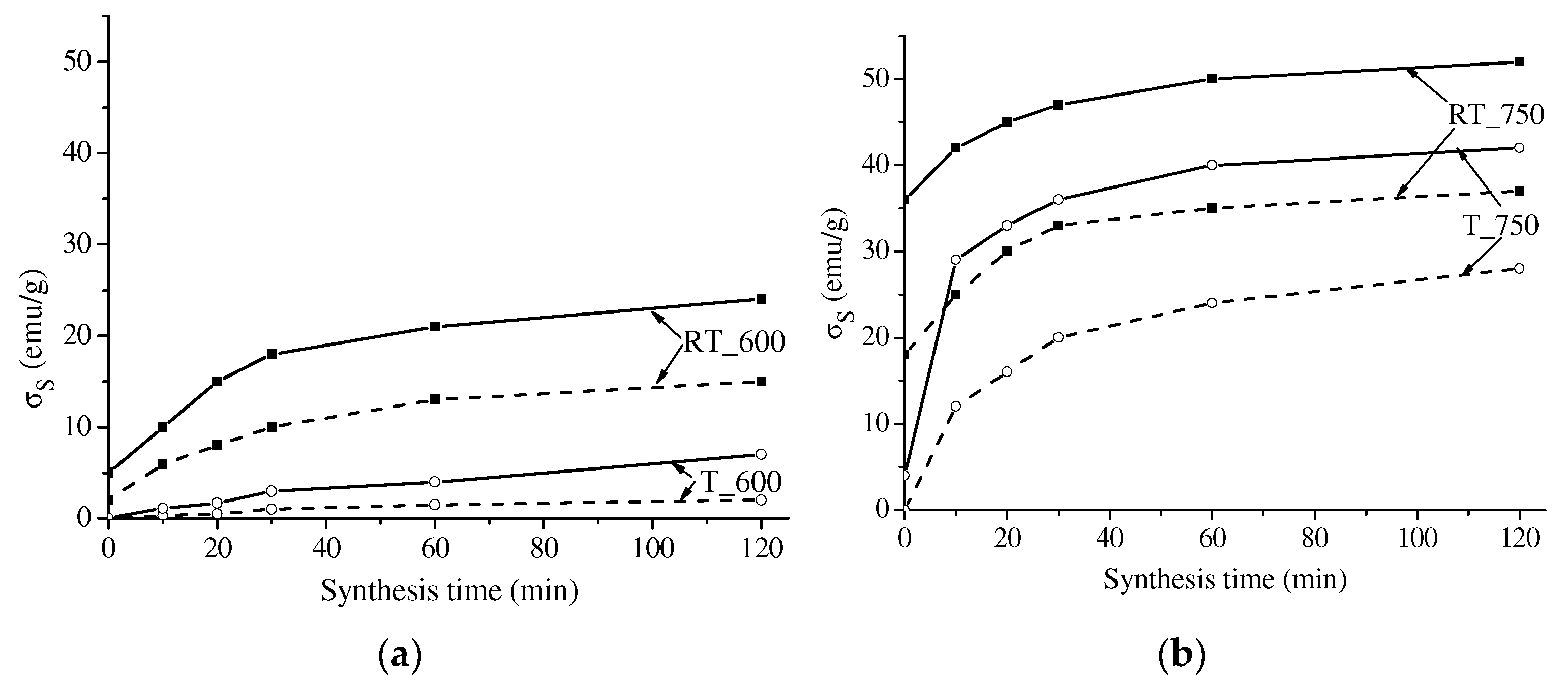
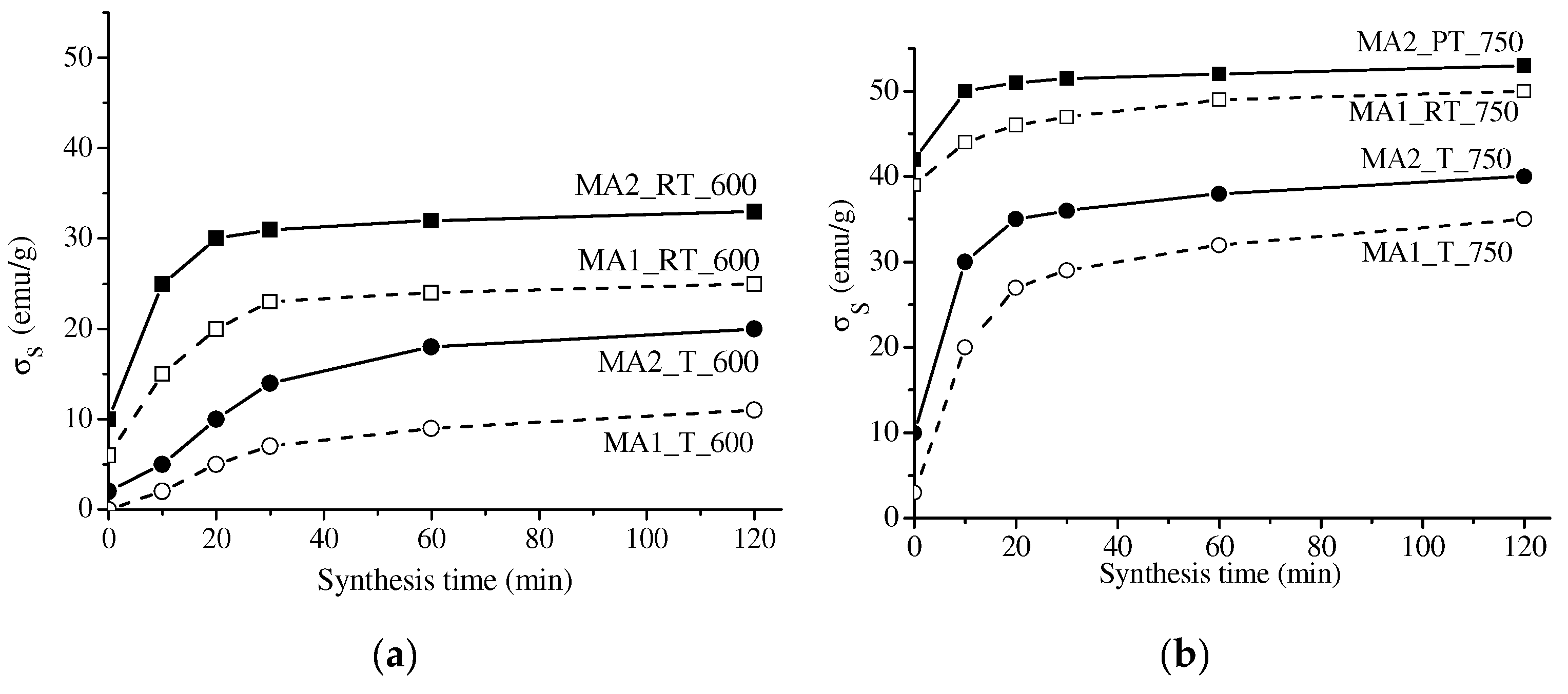
3.2. Study of Specific Saturation Magnetization
3.3. Thermomagnetometric Analysis of Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breton, J.M. Ferrite magnets: Properties and applications. Ref. Mod. Mater. Sci. Mater. Eng. 2021, 3, 206–216. [Google Scholar] [CrossRef]
- Mattei, J.L.; Chevalier, A.; Laur, V. Ferrite Ceramics at Microwave Frequencies: Applications and Characterization. Ref. Mod. Mater. Sci. Mater. Eng. 2021, 3, 183–205. [Google Scholar] [CrossRef]
- Liu, H.; Ji, P.; Han, X. Rheological phase synthesis of nanosized α-LiFeO2 with higher crystallinity degree for cathode material of lithium-ion batteries. Mater. Chem. Phys. 2016, 183, 152–157. [Google Scholar] [CrossRef]
- Rezlescu, N.; Doroftei, C.; Rezlescu, E.; Popa, P.D. Lithium ferrite for gas sensing applications. Sens. Actuat. B 2008, 133, 420–425. [Google Scholar] [CrossRef]
- Sharif, M.; Jacob, J.; Javed, M.; Manzoor, A.; Mahmood, K.; Khan, M.A. Impact of Co and Mn substitution on structural and dielectric properties of lithium soft ferrites. Phys. B 2019, 567, 45–50. [Google Scholar] [CrossRef]
- Wang, X.; Gao, L.; Li, L. Low temperature synthesis of metastable lithium ferrite: Magnetic and electrochemical properties. Nanotechnology 2005, 16, 2677–2680. [Google Scholar] [CrossRef]
- White, G.O.; Patton, C.E. Magnetic properties of lithium ferrite microwave materials. Magn. Magn. Mater. 1978, 9, 299–317. [Google Scholar] [CrossRef]
- Ridgley, D.H.; Lessoff, H.; Childress, J.D. Effects of lithium and oxygen losses on magnetic and crystallographic properties of spinel lithium ferrite. J. Am. Ceram. Soc. 1970, 53, 304–311. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Z.; Shi, R.; Zhang, H.; Zhou, X. Electromagnetic and microwave absorption properties of Ti doped Li-Zn ferrites. J. Alloys Compd. 2019, 805, 934–941. [Google Scholar] [CrossRef]
- Manjula, R.; Murthy, V.R.K.; Sobhanadri, J. Electrical conductivity and thermoelectric power measurements of some lithium-titanium ferrites. J. Appl. Phys. 1986, 59, 2929–2932. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, Y.; Liu, Q.; Wang, Y.; Chen, J.; Wang, H.; Wu, C.; Zhang, H. Study of the microstructure and microwave magnetic characteristics of Ti-doped Li-Zn ferrite. J. Mater. Sci. Mater. Electron. 2019, 30, 5430–5437. [Google Scholar] [CrossRef]
- Ali, M.A.; Khan, M.N.I.; Chowdhury, F.-U.-Z.; Hossain, M.M.; Hoque, S.M.; Uddin, M.M. Impact of Sn4+ substitution in Mg–Zn ferrites: Deciphering the structural, morphological, dielectric, electrical and magnetic properties. Mater. Chem. Phys. 2021, 263, 124357. [Google Scholar] [CrossRef]
- Hreščak, J.; Malič, B.; Cilenšek, J.; Benčan, A. Solid-state synthesis of undoped and Sr-doped K0.5Na0.5NbO3. J. Therm. Anal. Calorim. 2017, 127, 129–136. [Google Scholar] [CrossRef]
- Sharma, P.; Diwan, P.K.; Pandey, O.P. Impact of environment on the kinetics involved in the solid-state synthesis of bismuth ferrite. Mater. Chem. Phys. 2019, 233, 171–179. [Google Scholar] [CrossRef]
- Rathod, V.; Anupama, A.V.; Vijaya Kumar, R.; Jali, V.M.; Sahoo, B. Correlated vibrations of the tetrahedral and octahedral complexes and splitting of the absorption bands in FTIR spectra of Li-Zn ferrites. Vib. Spectrosc. 2017, 92, 267–272. [Google Scholar] [CrossRef]
- Rathod, V.; Anupama, A.V.; Jali, V.M.; Hiremath, V.A.; Sahoo, B. Combustion synthesis, structure and magnetic properties of Li-Zn ferrite ceramic powders. Ceram. Int. 2017, 43, 14431–14440. [Google Scholar] [CrossRef]
- Kotsikau, D.; Ivanovskaya, M.; Pankov, V.; Fedotova, Y. Structure and magnetic properties of manganese-zinc-ferrite prepared by spray pyrolysis method. Solid State Sci. 2015, 39, 69–73. [Google Scholar] [CrossRef]
- Berezhnaya, M.V.; Mittova, I.Y.; Perov, N.S.; Al’myasheva, O.V.; Nguyen, A.T.; Mittova, V.O.; Bessalova, V.V.; Viryutina, E.L. Production of zinc-doped yttrium ferrite nanopowders by the sol-gel method. Russ. J. Inorgan. Chem. 2018, 63, 742–746. [Google Scholar] [CrossRef]
- Saikia, P.; Blaise Allou, N.; Borah, A.; Goswamee, R.L. Iso-conversional kinetics study on thermal degradation of Ni-Al layered double hydroxide synthesized by ‘soft chemical’ sol-gel method. Mater. Chem. Phys. 2017, 186, 52–60. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, N.; Rana, D.S.; Kumar, P.; Arora, M.; Pant, R.P. Structural and magnetic studies of the nickel doped CoFe2O4 ferrite nanoparticles synthesized by the chemical co-precipitation method. J. Magn. Magn. Mater. 2015, 394, 379–384. [Google Scholar] [CrossRef]
- Surzhikov, A.P.; Frangulyan, T.S.; Ghyngazov, S.A.; Lysenko, E.N. Investigation of structural states and oxidation processes in Li0.5Fe2.5O4−δ using TG analysis. J. Therm. Anal. Calorim. 2012, 108, 1207–1212. [Google Scholar] [CrossRef]
- Berbenni, V.; Marini, A.; Matteazzi, P.; Ricceri, R.; Welham, N.J. Solid-state formation of lithium ferrites from mechanically activated Li2CO3–Fe2O3 mixtures. J. Eur. Ceram. Soc. 2003, 23, 527–536. [Google Scholar] [CrossRef]
- Widatallah, H.M.; Johnson, C.; Berry, F.J. The influence of ball milling and subsequent calcination on the formation of LiFeO2. J. Mater. Sci. 2002, 37, 4621–4625. [Google Scholar] [CrossRef]
- Widatallah, H.M.; Ren, X.-L.; Al-Omari, I.A. The influence of TiO2 polymorph, mechanical milling and subsequent sintering on the formation of Ti-substituted spinel-related Li0.5Fe2.5O4. J. Mater. Sci. 2006, 41, 6333–6338. [Google Scholar] [CrossRef]
- Mazen, S.A.; Abu-Elsaad, N.I. Dielectric properties and impedance analysis of polycrystalline Li-Si ferrite prepared by high energy ball milling technique. J. Magn. Magn. Mat. 2017, 442, 72–79. [Google Scholar] [CrossRef]
- González-Angeles, A.; Lipka, J.; Grusková, A.; Jančárik, V.; Tóth, I.; Sláma, J. (Ni, Zn, Sn) Ru and (Ni, Sn) Sn substituted barium ferrite prepared by mechanical alloying. Hyperfine Interact. 2008, 184, 135–141. [Google Scholar] [CrossRef]
- Arab, A.; Mardaneh, M.R.; Yousefi, M.H. Investigation of magnetic properties of MnZn-substituted strontium ferrite nanopowders prepared via conventional ceramic technique followed by a high energy ball milling. J. Magn. Magn. Mater. 2015, 374, 80–84. [Google Scholar] [CrossRef]
- Chen, D.; Harward, I.; Baptist, J.; Goldman, S.; Celinski, Z. Curie temperature and magnetic properties of aluminum doped barium ferrite particles prepared by ball mill method. J. Magn. Magn. Mater. 2015, 395, 350–353. [Google Scholar] [CrossRef]
- Šepelák, V.; Wibmann, S.; Becker, K.D. Magnetism of nanostructured mechanically activated and mechanosynthesized spinel ferrites. J. Magn. Magn. Mat. 1999, 203, 135–137. [Google Scholar] [CrossRef]
- Vasoya, N.H.; Vanpariya, L.H.; Sakariya, P.N.; Timbadiya, M.D.; Pathak, T.K.; Lakhani, V.K.; Modi, K.B. Synthesis of nanostructured material by mechanical milling and study on structural property modifications in Ni0.5Zn0.5Fe2O4. Ceram. Int. 2010, 36, 947–954. [Google Scholar] [CrossRef]
- Hajalilou, A.; Hashim, M.; Taghi Masoudi, M. A comparative study of in-situ mechanochemically synthesized Mn0.5Zn0.5Fe2O4 ferrite nanoparticles in the MnO/ZnO/Fe2O3 and MnO2/Zn/Fe2O3 systems. Ceram. Int. 2015, 41, 8070–8079. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Nikolaev, E.V.; Surzhikov, A.P.; Nikolaeva, S.A.; Plotnikova, I.V. The influence of reagents ball milling on the lithium ferrite formation. J. Therm. Anal. Calorim. 2019, 138, 2005–2013. [Google Scholar] [CrossRef]
- Auslender, V.L.; Bochkarev, I.G.; Boldyrev, V.V.; Lyakhov, N.Z.; Voronin, A.P. Electron beam induced diffusion controlled reaction in solids. Sol. St. Ion. 1997, 101–103, 489–493. [Google Scholar] [CrossRef]
- Neronov, V.A.; Voronin, A.P.; Tatarintseva, M.I.; Melekhova, T.E.; Auslender, V.L. Sintering under a high-power electron beam. J. Less-Common Met. 1986, 117, 391–394. [Google Scholar] [CrossRef]
- Ancharova, U.V.; Mikhailenko, M.A.; Tolochko, B.P.; Lyakhov, N.Z.; Korobeinikov, M.V.; Bryazgin, A.A.; Bezuglov, V.V.; Shtarklev, E.A. Synthesis and Staging of the Phase Formation for Strontium Ferrites in Thermal and Radiation Thermal Reactions. IOP Conf. Ser. Mater. Sci. Eng. 2015, 81, 012122. [Google Scholar] [CrossRef]
- Boldyrev, V.V.; Voronin, A.P.; Gribkov, O.S.; Tkachenko, E.V.; Karagedov, G.R.; Yakobson, B.I.; Auslender, V.L. Radiation-thermal synthesis. Current achievement and outlook. Solid State Ion. 1989, 36, 1–6. [Google Scholar] [CrossRef]
- Lyakhov, N.Z.; Boldyrev, V.V.; Voronin, A.P.; Gribkov, O.S.; Bochkarev, I.G.; Rusakov, S.V.; Auslender, V.L. Electron beam stimulated chemical reaction in solids. J. Therm. Anal. Calorim. 1995, 43, 21–31. [Google Scholar] [CrossRef]
- Zhuravlev, V.A.; Naiden, E.P.; Minin, R.V.; Itin, V.I.; Suslyaev, V.I.; Korovin, E.Y. Radiation-thermal synthesis of W-type hexaferrites. IOP Conf. Ser. Mater. Sci. Eng. 2015, 81, 012003. [Google Scholar] [CrossRef]
- Naiden, E.P.; Zhuravlev, V.A.; Minin, R.V.; Suslyaev, V.I.; Itin, V.I.; Korovin, E.Y. Structural and magnetic properties of SHS-produced multiphase W-type hexaferrites: Influence of radiation-thermal treatment. Int. J. Self-Propag. High-Temp. Synth. 2015, 24, 148–151. [Google Scholar] [CrossRef]
- Kostishin, V.G.; Andreev, V.G.; Korovushkin, V.V.; Chitanov, D.N.; Yudanov, N.A.; Morchenko, A.T.; Komlev, A.S.; Adamtsov, A.Y.; Nikolaev, A.N. Preparation of 2000NN ferrite ceramics by a complete and a short radiation-enhanced thermal sintering process. Inorg. Mat. 2014, 50, 1317. [Google Scholar] [CrossRef]
- Kostishyn, V.; Isaev, I.; Scherbakov, S.; Nalogin, A.; Belokon, E.; Bryazgin, A. Obtaining anisotropic hexaferrites for the base layers of microstrip SHF devices by the radiation-thermal sintering. East.-Eur. J. Enterp. Technol. 2016, 5, 32–39. [Google Scholar] [CrossRef]
- Kounsalye, J.S.; Kharat, P.B.; Bhoyar, D.N.; Jadhav, K.M. Radiation-induced modification in structural, electrical and dielectric properties of Ti4+ ions substituted Li0.5Fe2.5O4 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 8601–8609. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Vlasov, V.A.; Surzhikov, A.P. Investigation of kinetics of lithium ferrite formation under electron beam treatment. Nucl. Instr. Meth. Phys. Res. Sect. B 2020, 466, 31–36. [Google Scholar] [CrossRef]
- Surzhikov, A.P.; Lysenko, E.N.; Vlasov, V.A.; Malyshev, A.V.; Vasendina, E.A. Magnetization study in solid state formation of lithium-titanium ferrites synthesized by electron beam heating. Mater. Chem. Phys. 2016, 176, 110–114. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Nikolaev, E.V.; Vlasov, V.A.; Surzhikov, A.P. Technological scheme for lithium-substituted ferrite production under complex high-energy impact. Nucl. Instr. Meth. Phys. Res. Sect. B 2020, 474, 49–56. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Nikolaev, E.V.; Vlasov, V.A.; Surzhikov, A.P. Microstructure and reactivity of Fe2O3-Li2CO3-ZnO ferrite system ball-milled in a planetary mill. Thermochim. Acta 2018, 664, 100–107. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Astafyev, A.L.; Vlasov, V.A.; Surzhikov, A.P. Analysis of phase composition of LiZn and LiTi ferrites by XRD and thermomagnetometric analysis. J. Magn. Magn. Mater. 2018, 465, 457–461. [Google Scholar] [CrossRef]
- Ahniyaz, A.; Fujiwara, T.; Song, S.-W.; Yoshimura, M. Low temperature preparation of β-LiFe5O8 fine particles by hydrothermal ball milling. J. Solid State Ion. 2002, 151, 419–423. [Google Scholar] [CrossRef]
- An, S.Y.; Shim, I.-B.; Kim, C.S. Synthesis and magnetic properties of LiFe5O8 powders by a sol-gel process. J. Magn. Magn. Mater. 2005, 290, 1551–1554. [Google Scholar] [CrossRef]
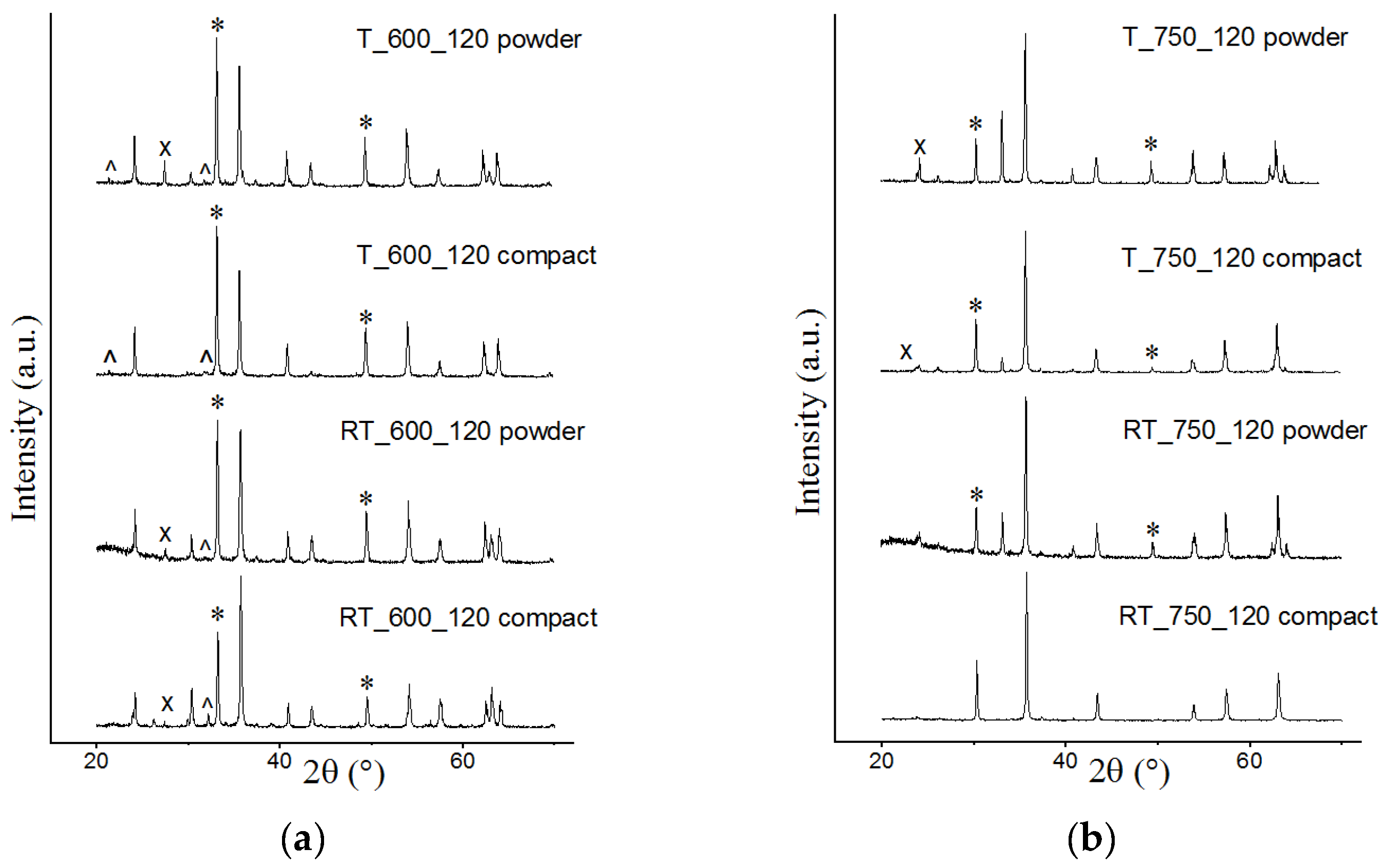

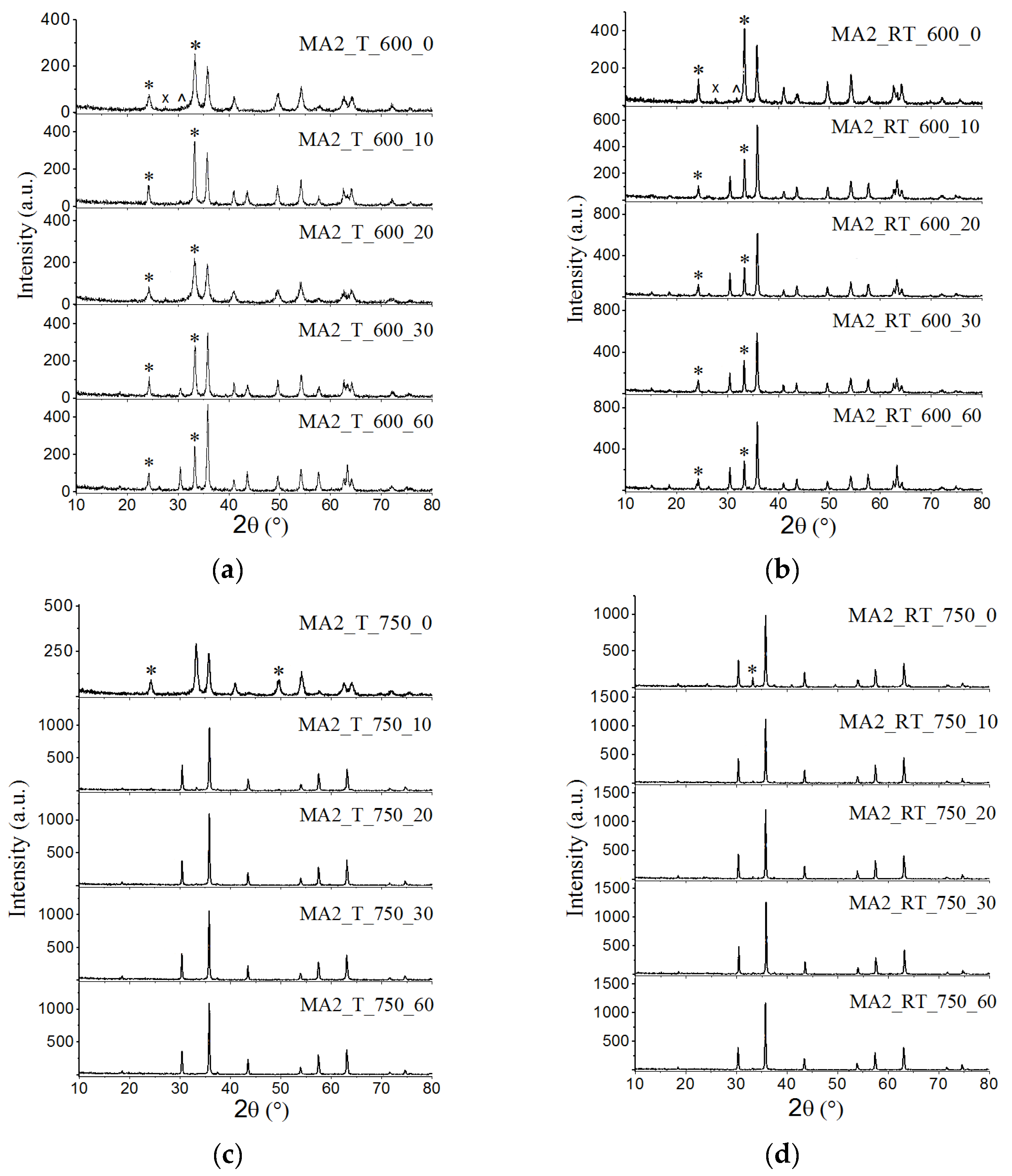
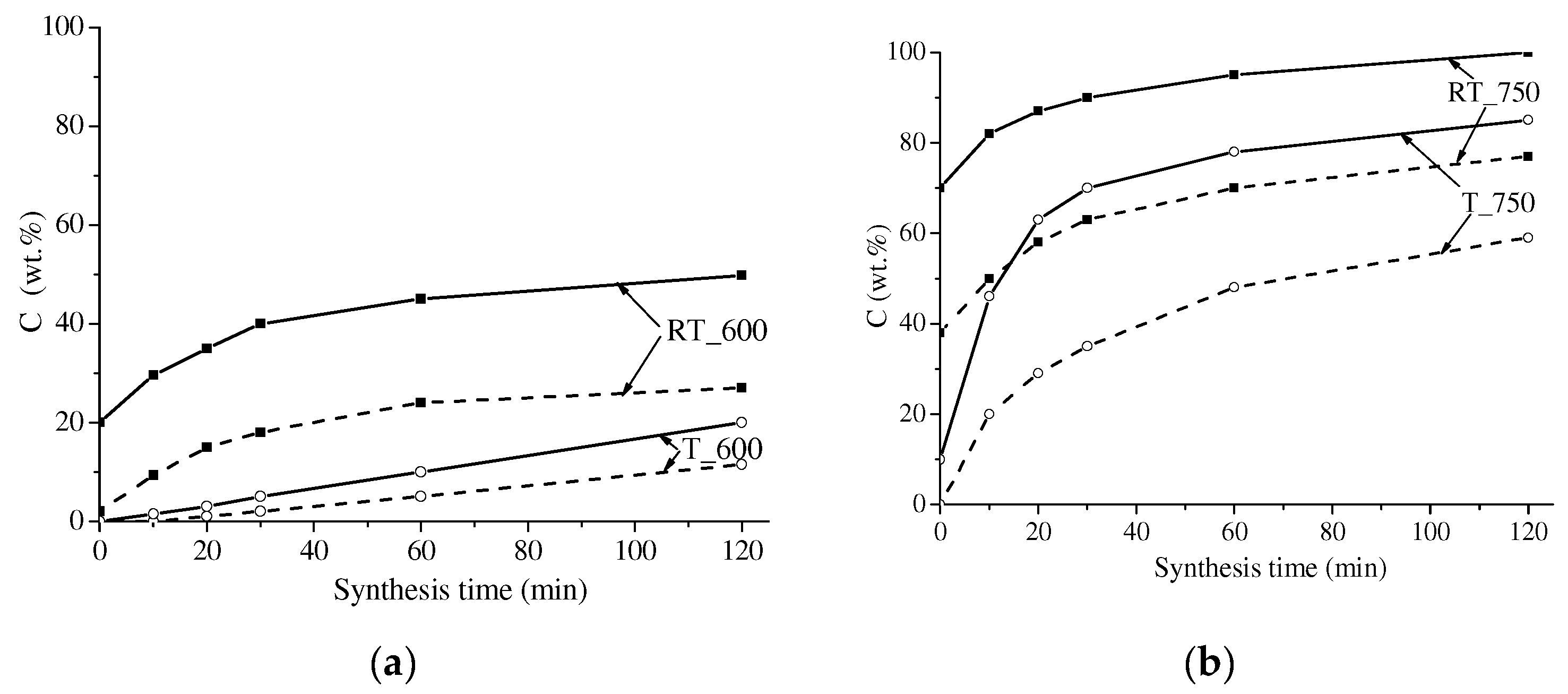
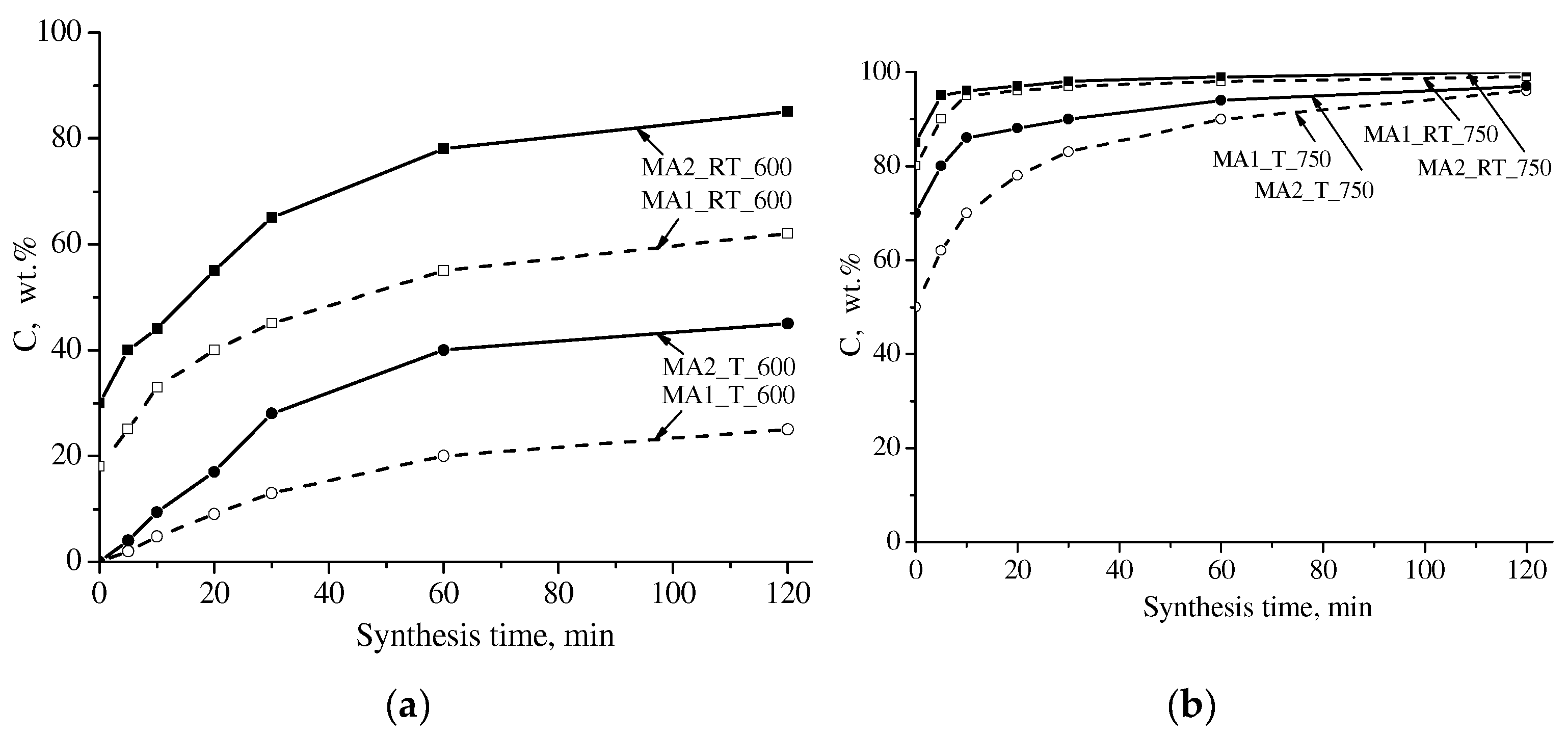


| Sample | Cspinel (wt.%) | Lattice Parameter (Ǻ) | Crystallite Size (nm) | Microstrain Δd/d·103 | Tc (°C) | σS (emu/g) |
|---|---|---|---|---|---|---|
| T_600_120 powder | 11.5 | 8.325 | 53 | 0.6 | - | 1.3 |
| T_600_120 compact | 20.0 | 8.330 | 77 | 0.6 | 629 | 6.6 |
| RT_600_120 powder | 28.4 | 8.327 | 70 | 0.4 | 629 | 14.9 |
| RT_600_120 compact | 49.8 | 8.329 | 88 | 0.7 | 632 | 24.0 |
| Sample | Cspinel (wt.%) | Lattice Parameter (Ǻ) | Crystallite Size (nm) | Microstrain Δd/d·103 | Tc (°C) | σS (emu/g) |
|---|---|---|---|---|---|---|
| T_750_120 powder | 59.0 | 8.327 | 120 | 0.5 | 628 | 27.9 |
| T_750_120 compact | 83.0 | 8.331 | 130 | 1.1 | 631 | 42.0 |
| RT_750_120 powder | 77.0 | 8.330 | 141 | 0.3 | 630 | 37.6 |
| RT_750_120 compact | 100 | 8.334 | 127 | 1.1 | 634 | 51.5 |
| Sample | Cspinel (wt.%) | Lattice Parameter (Ǻ) | Crystallite Size (nm) | Microstrain Δd/d·103 | Tc (°C) | σS (emu/g) |
|---|---|---|---|---|---|---|
| MA1_T_600 | 18.9 | 8.321 | 45 | 1.0 | - | 8.1 |
| MA1_T_750 | 98.0 | 8.337 | 84 | 0.8 | 581 | 34.0 |
| MA1_RT_600 | 54.5 | 8.328 | 88 | 0.9 | 618 | 27.0 |
| MA1_RT_750 | 100 | 8.338 | 142 | 0.6 | 539 | 49.7 |
| Sample | Cspinel (wt.%) | Lattice Parameter (Ǻ) | Crystallite Size (nm) | Microstrain Δd/d·103 | Tc (°C) | σS (emu/g) |
|---|---|---|---|---|---|---|
| MA2_T_600 | 37.1 | 8.322 | 42 | 1.0 | 593 | 18.3 |
| MA2_T_750 | 99.0 | 8.335 | 80 | 0.6 | 555 | 38.5 |
| MA2_RT_600 | 78.9 | 8.333 | 63 | 0.4 | 605 | 32.1 |
| MA2_RT_750 | 100 | 8.337 | 49 | 0.8 | 534 | 51.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lysenko, E.; Vlasov, V.; Nikolaev, E.; Surzhikov, A.; Ghyngazov, S. Technological Aspects of Lithium-Titanium Ferrite Synthesis by Electron-Beam Heating. Materials 2023, 16, 604. https://doi.org/10.3390/ma16020604
Lysenko E, Vlasov V, Nikolaev E, Surzhikov A, Ghyngazov S. Technological Aspects of Lithium-Titanium Ferrite Synthesis by Electron-Beam Heating. Materials. 2023; 16(2):604. https://doi.org/10.3390/ma16020604
Chicago/Turabian StyleLysenko, Elena, Vitaly Vlasov, Evgeniy Nikolaev, Anatoliy Surzhikov, and Sergei Ghyngazov. 2023. "Technological Aspects of Lithium-Titanium Ferrite Synthesis by Electron-Beam Heating" Materials 16, no. 2: 604. https://doi.org/10.3390/ma16020604
APA StyleLysenko, E., Vlasov, V., Nikolaev, E., Surzhikov, A., & Ghyngazov, S. (2023). Technological Aspects of Lithium-Titanium Ferrite Synthesis by Electron-Beam Heating. Materials, 16(2), 604. https://doi.org/10.3390/ma16020604






