Recent Advances in Structured Catalytic Materials Development for Conversion of Liquid Hydrocarbons into Synthesis Gas for Fuel Cell Power Generators
Abstract
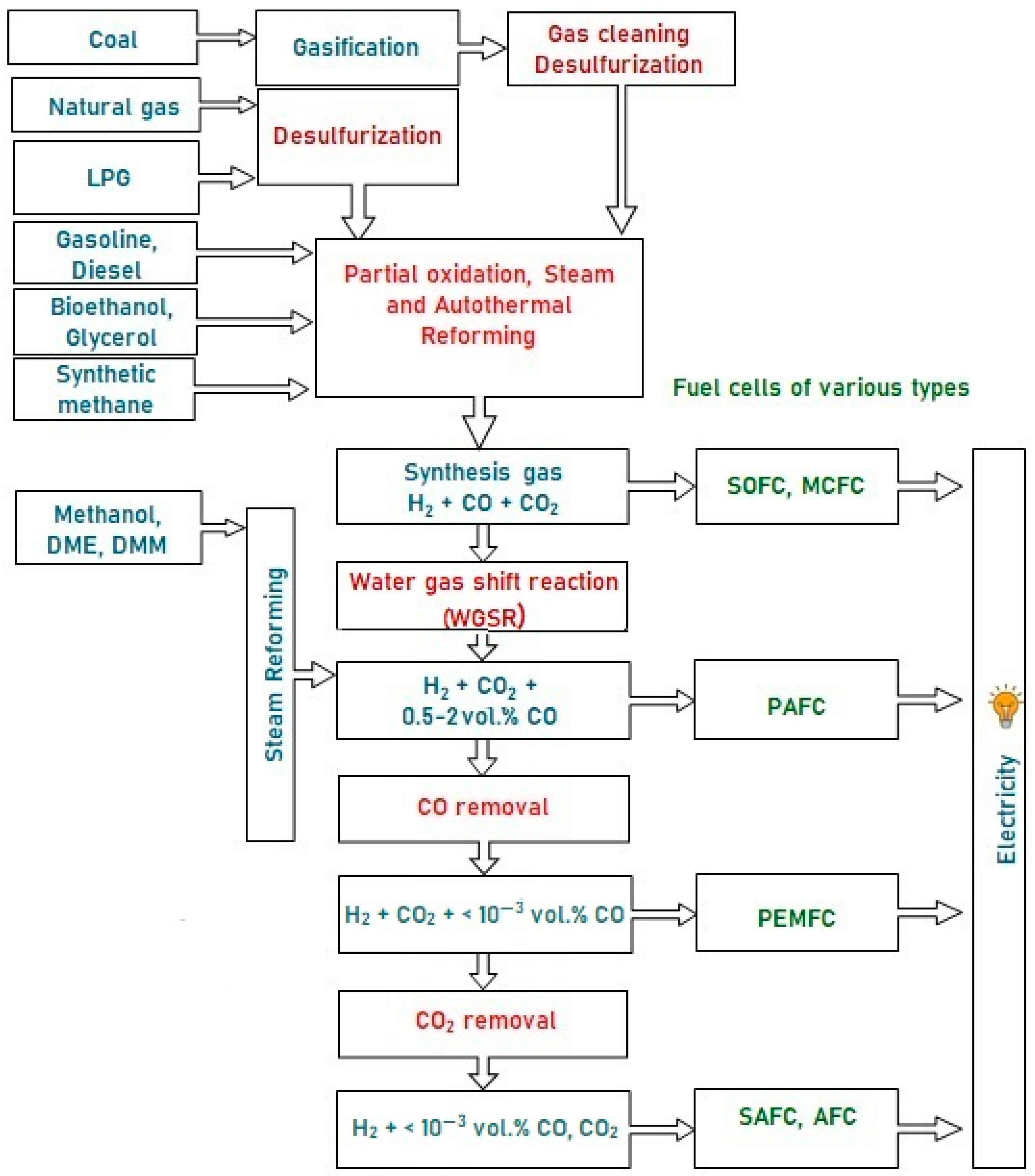
1. Introduction
2. Active Component of Catalysts for the Conversion of Diesel Fuel into Synthesis Gas
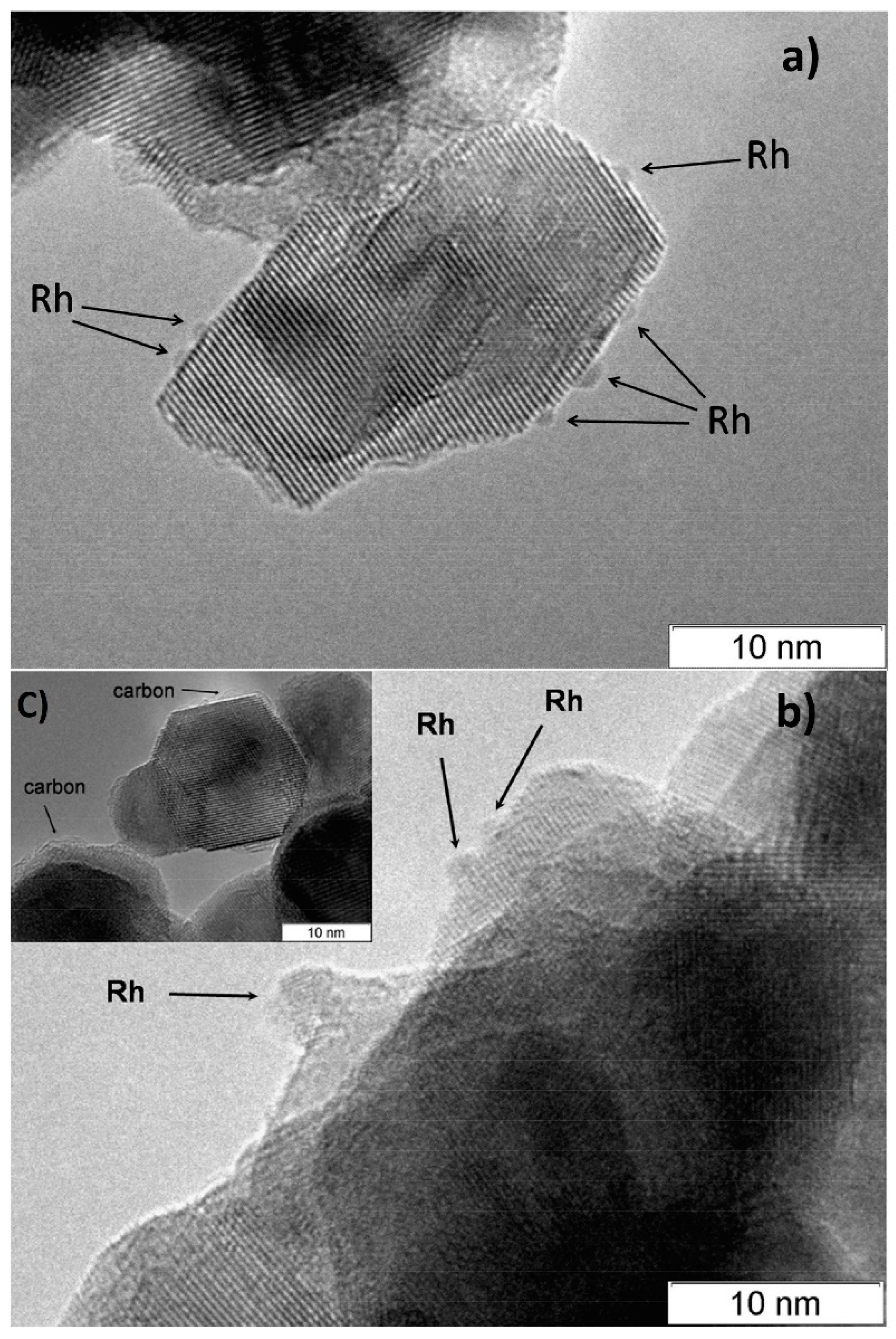
3. Structured Composite Catalysts Supported on FeCrAl Alloy Wire Mesh
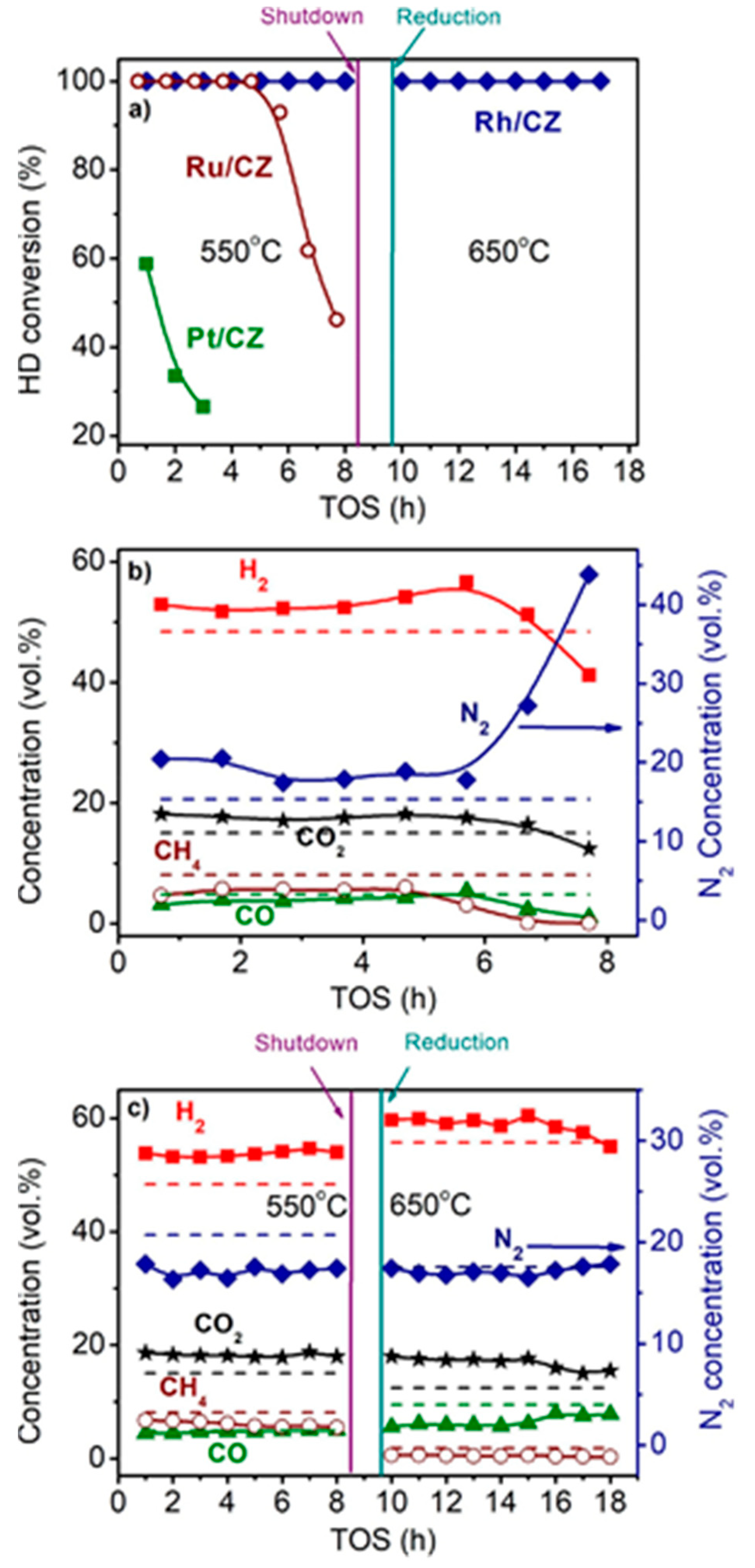
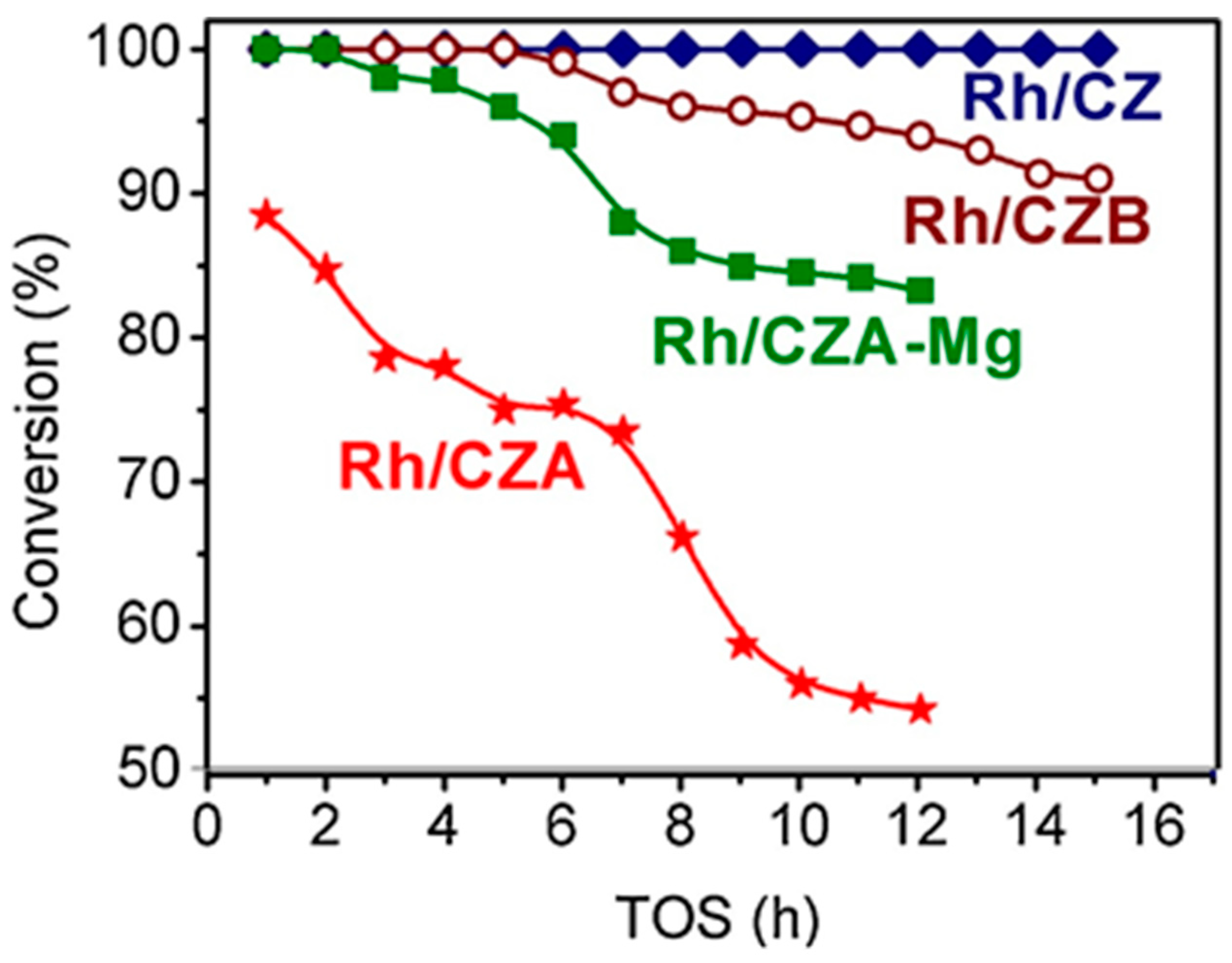
4. Testing of Structured Catalysts
5. Catalyst Coking and Regeneration
6. Testing of Diesel Reformer
7. Development of a Mathematical Model for the Diesel Fuel Conversion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Speight, J.G. Fuels for Fuel Cells. In Fuel Cells: Technologies for Fuel Processing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 29–48. [Google Scholar]
- Peters, R.; Pasel, J.; Samsun, R.C.; Scharf, F.; Tschauder, A.; Stolten, D. Heat Exchanger Design for Autothermal Reforming of Diesel. Int. J. Hydrogen Energy 2018, 43, 11830–11846. [Google Scholar] [CrossRef]
- Samsun, R.C.; Pasel, J.; Peters, R.; Stolten, D. Fuel Cell Systems with Reforming of Petroleum-Based and Synthetic-Based Diesel and Kerosene Fuels for APU Applications. Int. J. Hydrogen Energy 2015, 40, 6405–6421. [Google Scholar] [CrossRef]
- Pasel, J.; Samsun, R.C.; Peters, R.; Stolten, D. Fuel Processing of Diesel and Kerosene for Auxiliary Power Unit Applications. Energy Fuels 2013, 27, 4386–4394. [Google Scholar] [CrossRef]
- Samsun, R.C.; Prawitz, M.; Tschauder, A.; Pasel, J.; Pfeifer, P.; Peters, R.; Stolten, D. An Integrated Diesel Fuel Processing System with Thermal Start-up for Fuel Cells. Appl. Energy 2018, 226, 145–159. [Google Scholar] [CrossRef]
- Samsun, R.C.; Krekel, D.; Pasel, J.; Prawitz, M.; Peters, R.; Stolten, D. A Diesel Fuel Processor for Fuel-Cell-Based Auxiliary Power Unit Applications. J. Power Sources 2017, 355, 44–52. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Christensen, T.S.; Dybkjaer, I. Steam Reforming of Liquid Hydrocarbons. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1998; pp. 81–95. [Google Scholar]
- Bae, J.; Lee, S.; Kim, S.; Oh, J.; Choi, S.; Bae, M.; Kang, I.; Katikaneni, S.P. Liquid Fuel Processing for Hydrogen Production: A Review. Int. J. Hydrogen Energy 2016, 41, 19990–20022. [Google Scholar] [CrossRef]
- Xu, X.; Li, P.; Shen, Y. Small-Scale Reforming of Diesel and Jet Fuels to Make Hydrogen and Syngas for Fuel Cells: A Review. Appl. Energy 2013, 108, 202–217. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Fauteux-Lefebvre, C. Review of Catalytic Syngas Production through Steam or Dry Reforming and Partial Oxidation of Studied Liquid Compounds. Wiley Interdiscip Rev. Energy Environ. 2016, 5, 169–187. [Google Scholar] [CrossRef]
- Pasel, J.; Samsun, R.C.; Peters, R.; Thiele, B.; Stolten, D. Long-Term Stability at Fuel Processing of Diesel and Kerosene. Int. J. Hydrogen Energy 2014, 39, 18027–18036. [Google Scholar] [CrossRef]
- Yoon, S.; Bae, J.; Kim, S.; Yoo, Y.S. Self-Sustained Operation of a KWe-Class Kerosene-Reforming Processor for Solid Oxide Fuel Cells. J. Power Sources 2009, 192, 360–366. [Google Scholar] [CrossRef]
- Samsun, R.C.; Pasel, J.; Janßen, H.; Lehnert, W.; Peters, R.; Stolten, D. Design and Test of a 5 KW High-Temperature Polymer Electrolyte Fuel Cell System Operated with Diesel and Kerosene. Appl. Energy 2014, 114, 238–249. [Google Scholar] [CrossRef]
- Granlund, M.Z.; Jansson, K.; Nilsson, M.; Dawody, J.; Pettersson, L.J. Evaluation of Co, La, and Mn Promoted Rh Catalysts for Autothermal Reforming of Commercial Diesel: Aging and Characterization. Appl. Catal. B 2015, 172–173, 145–153. [Google Scholar] [CrossRef]
- Cheekatamarla, P.K.; Lane, A.M. Efficient Bimetallic Catalysts for Hydrogen Generation from Diesel Fuel. Int. J. Hydrogen Energy 2005, 30, 1277–1285. [Google Scholar] [CrossRef]
- Lee, S.; Bae, M.; Bae, J.; Katikaneni, S.P. Ni–Me/Ce0.9Gd0.1O2−x (Me: Rh, Pt and Ru) Catalysts for Diesel Pre-Reforming. Int J. Hydrogen Energy 2015, 40, 3207–3216. [Google Scholar] [CrossRef]
- Karatzas, X.; Dawody, J.; Grant, A.; Svensson, E.E.; Pettersson, L.J. Zone-Coated Rh-Based Monolithic Catalyst for Autothermal Reforming of Diesel. Appl. Catal. B 2011, 101, 226–238. [Google Scholar] [CrossRef]
- Shilov, V.A.; Rogozhnikov, V.N.; Ruban, N.V.; Potemkin, D.I.; Simonov, P.A.; Shashkov, M.V.; Sobyanin, V.A.; Snytnikov, P.V. Biodiesel and Hydrodeoxygenated Biodiesel Autothermal Reforming over Rh-Containing Structured Catalyst. Catal. Today 2021, 379, 42–49. [Google Scholar] [CrossRef]
- Martin, S.; Kraaij, G.; Ascher, T.; Baltzopoulou, P.; Karagiannakis, G.; Wails, D.; Wörner, A. Direct Steam Reforming of Diesel and Diesel–Biodiesel Blends for Distributed Hydrogen Generation. Int. J. Hydrogen Energy 2015, 40, 75–84. [Google Scholar] [CrossRef]
- Schwengber, C.A.; Alves, H.J.; Schaffner, R.A.; da Silva, F.A.; Sequinel, R.; Bach, V.R.; Ferracin, R.J. Overview of Glycerol Reforming for Hydrogen Production. Renew. Sustain. Energy Rev. 2016, 58, 259–266. [Google Scholar] [CrossRef]
- Lindermeir, A.; Kah, S.; Kavurucu, S.; Mühlner, M. On-Board Diesel Fuel Processing for an SOFC–APU—Technical Challenges for Catalysis and Reactor Design. Appl. Catal. B 2007, 70, 488–497. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Xu, X.; Li, P. Hydrogen Production via Catalytic Autothermal Reforming of Desulfurized Jet-A Fuel. Int. J. Hydrogen Energy 2017, 42, 1932–1941. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Li, P. Autothermal Reforming of N-Dodecane and Desulfurized Jet-A Fuel for Producing Hydrogen-Rich Syngas. Int. J. Hydrogen Energy 2014, 39, 19593–19602. [Google Scholar] [CrossRef]
- Fabiano, C.; Italiano, C.; Vita, A.; Pino, L.; Laganà, M.; Recupero, V. Performance of 1.5 Nm3/h Hydrogen Generator by Steam Reforming of n-Dodecane for Naval Applications. Int. J. Hydrogen Energy 2016, 41, 19475–19483. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Wang, X.; Li, P. Fuel Adaptability Study of a Lab-Scale 2.5 KWth Autothermal Reformer. Int. J. Hydrogen Energy 2015, 40, 6798–6808. [Google Scholar] [CrossRef]
- Pasel, J.; Samsun, R.C.; Tschauder, A.; Peters, R.; Stolten, D. Advances in Autothermal Reformer Design. Appl. Energy 2017, 198, 88–98. [Google Scholar] [CrossRef]
- Pasel, J.; Samsun, R.C.; Tschauder, A.; Peters, R.; Stolten, D. A Novel Reactor Type for Autothermal Reforming of Diesel Fuel and Kerosene. Appl. Energy 2015, 150, 176–184. [Google Scholar] [CrossRef]
- Krekel, D.; Samsun, R.C.; Pasel, J.; Prawitz, M.; Peters, R.; Stolten, D. Operating Strategies for Fuel Processing Systems with a Focus on Water–Gas Shift Reactor Stability. Appl. Energy 2016, 164, 540–552. [Google Scholar] [CrossRef]
- Pasel, J.; Samsun, R.C.; Tschauder, A.; Peters, R.; Stolten, D. Water-Gas Shift Reactor for Fuel Cell Systems: Stable Operation for 5000 Hours. Int. J. Hydrogen Energy 2018, 43, 19222–19230. [Google Scholar] [CrossRef]
- Lindström, B.; Karlsson, J.A.J.; Ekdunge, P.; de Verdier, L.; Häggendal, B.; Dawody, J.; Nilsson, M.; Pettersson, L.J. Diesel Fuel Reformer for Automotive Fuel Cell Applications. Int. J. Hydrogen Energy 2009, 34, 3367–3381. [Google Scholar] [CrossRef]
- Karatzas, X.; Nilsson, M.; Dawody, J.; Lindström, B.; Pettersson, L.J. Characterization and Optimization of an Autothermal Diesel and Jet Fuel Reformer for 5kWe Mobile Fuel Cell Applications. Chem. Eng. J. 2010, 156, 366–379. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Gong, J. Catalytic Reforming of Oxygenates: State of the Art and Future Prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef]
- Shafer, L.; Striebich, R.; Gomach, J.; Edwards, T. Chemical Class Composition of Commercial Jet Fuels and other Specialty Kerosene Fuels. In Proceedings of the 14th AIAA/AHI Space Planes and Hypersonic Systems and Technologies Conference, American Institute of Aeronautics and Astronautics, Reston, Virigina, 6 November 2006. [Google Scholar]
- Edwards, T.; Maurice, L.Q. Surrogate Mixtures to Represent Complex Aviation and Rocket Fuels. J. Propuls. Power 2001, 17, 461–466. [Google Scholar] [CrossRef]
- Guggilla, V.S.; Akyurtlu, J.; Akyurtlu, A.; Blankson, I. Steam Reforming of n -Dodecane over Ru−Ni-Based Catalysts. Ind Eng Chem Res 2010, 49, 8164–8173. [Google Scholar] [CrossRef]
- Lee, S.; Bae, J.; Katikaneni, S.P. La0.8Sr0.2Cr0.95Ru0.05O3− and Sm0.8Ba0.2Cr0.95Ru0.05O3 − as Partial Oxidation Catalysts for Diesel. Int J Hydrogen Energy 2014, 39, 4938–4946. [Google Scholar] [CrossRef]
- Zheng, J.; Strohm, J.J.; Song, C. Steam Reforming of Liquid Hydrocarbon Fuels for Micro-Fuel Cells. Pre-Reforming of Model Jet Fuels over Supported Metal Catalysts. Fuel Process. Technol. 2008, 89, 440–448. [Google Scholar] [CrossRef]
- Zheng, Q.; Janke, C.; Farrauto, R. Steam Reforming of Sulfur-Containing Dodecane on a Rh–Pt Catalyst: Influence of Process Parameters on Catalyst Stability and Coke Structure. Appl. Catal. B 2014, 160–161, 525–533. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Fabiano, C.; Pino, L.; Laganà, M.; Recupero, V. Hydrogen-Rich Gas Production by Steam Reforming of n-Dodecane. Appl. Catal. B 2016, 199, 350–360. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Laganà, M.; Recupero, V. Hydrogen-Rich Gas Production by Steam Reforming of n-Dodecane. Part II: Stability, Regenerability and Sulfur Poisoning of Low Loading Rh-Based Catalyst. Appl. Catal. B 2017, 218, 317–326. [Google Scholar] [CrossRef]
- Jung, S.Y.; Ju, D.G.; Lim, E.J.; Lee, S.C.; Hwang, B.W.; Kim, J.C. Study of Sulfur-Resistant Ni–Al-Based Catalysts for Autothermal Reforming of Dodecane. Int. J. Hydrogen Energy 2015, 40, 13412–13422. [Google Scholar] [CrossRef]
- Haynes, D.J.; Berry, D.A.; Shekhawat, D.; Spivey, J.J. Catalytic Partial Oxidation of N-Tetradecane Using Rh and Sr Substituted Pyrochlores: Effects of Sulfur. Catal. Today 2009, 145, 121–126. [Google Scholar] [CrossRef]
- Haynes, D.J.; Campos, A.; Berry, D.A.; Shekhawat, D.; Roy, A.; Spivey, J.J. Catalytic Partial Oxidation of a Diesel Surrogate Fuel Using an Ru-Substituted Pyrochlore. Catal. Today 2010, 155, 84–91. [Google Scholar] [CrossRef]
- Shekhawat, D.; Gardner, T.H.; Berry, D.A.; Salazar, M.; Haynes, D.J.; Spivey, J.J. Catalytic Partial Oxidation of N-Tetradecane in the Presence of Sulfur or Polynuclear Aromatics: Effects of Support and Metal. Appl. Catal. A Gen 2006, 311, 8–16. [Google Scholar] [CrossRef]
- Haynes, D.J.; Berry, D.A.; Shekhawat, D.; Spivey, J.J. Catalytic Partial Oxidation of N-Tetradecane Using Pyrochlores: Effect of Rh and Sr Substitution. Catal. Today 2008, 136, 206–213. [Google Scholar] [CrossRef]
- Fauteux-Lefebvre, C.; Abatzoglou, N.; Blanchard, J.; Gitzhofer, F. Steam Reforming of Liquid Hydrocarbons over a Nickel–Alumina Spinel Catalyst. J. Power Sources 2010, 195, 3275–3283. [Google Scholar] [CrossRef]
- Lakhapatri, S.L.; Abraham, M.A. Deactivation Due to Sulfur Poisoning and Carbon Deposition on Rh-Ni/Al2O3 Catalyst during Steam Reforming of Sulfur-Doped n-Hexadecane. Appl. Catal. A Gen. 2009, 364, 113–121. [Google Scholar] [CrossRef]
- Shoynkhorova, T.B.; Rogozhnikov, V.N.; Simonov, P.A.; Snytnikov, P.V.; Salanov, A.N.; Kulikov, A.V.; Gerasimov, E.Y.; Belyaev, V.D.; Potemkin, D.I.; Sobyanin, V.A. Highly Dispersed Rh/Ce0.75Zr0.25O2-δ-ƞ-Al2O3/FeCrAl Wire Mesh Catalyst for Autothermal n-Hexadecane Reforming. Mater Lett 2018, 214, 290–292. [Google Scholar] [CrossRef]
- Shoynkhorova, T.B.; Simonov, P.A.; Potemkin, D.I.; Snytnikov, P.V.; Belyaev, V.D.; Ishchenko, A.V.; Svintsitskiy, D.A.; Sobyanin, V.A. Highly Dispersed Rh-, Pt-, Ru/Ce0.75Zr0.25O2–δ Catalysts Prepared by Sorption-Hydrolytic Deposition for Diesel Fuel Reforming to Syngas. Appl. Catal. B 2018, 237, 237–244. [Google Scholar] [CrossRef]
- Shoynkhorova, T.B.; Snytnikov, P.V.; Simonov, P.A.; Potemkin, D.I.; Rogozhnikov, V.N.; Gerasimov, E.Y.; Salanov, A.N.; Belyaev, V.D.; Sobyanin, V.A. From Alumina Modified Rh/Ce0.75Zr0.25O2-δ Catalyst towards Composite Rh/Ce0.75Zr0.25O2-δ-ƞ-Al2O3/FeCrAl Catalytic System for Diesel Conversion to Syngas. Appl Catal B 2019, 245, 40–48. [Google Scholar] [CrossRef]
- Shoynkhorova, T.B.; Rogozhnikov, V.N.; Ruban, N.V.; Shilov, V.A.; Potemkin, D.I.; Simonov, P.A.; Belyaev, V.D.; Snytnikov, P.V.; Sobyanin, V.A. Composite Rh/Ce0.75Zr0.25O2-δ-ƞ-Al2O3/Fecralloy Wire Mesh Honeycomb Module for Natural Gas, LPG and Diesel Catalytic Conversion to Syngas. Int. J. Hydrogen Energy 2019, 44, 9941–9948. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Navarro, R.M.; Rosa, F.; Briceño, Y.; Gordillo Alvarez, F.; Fierro, J.L.G. Performance of La,Ce-Modified Alumina-Supported Pt and Ni Catalysts for the Oxidative Reforming of Diesel Hydrocarbons. Int. J. Hydrogen Energy 2008, 33, 652–663. [Google Scholar] [CrossRef]
- Goud, S.K.; Whittenberger, W.A.; Chattopadhyay, S.; Abraham, M.A. Steam Reforming of N-Hexadecane Using a Pd/ZrO2 Catalyst: Kinetics of Catalyst Deactivation. Int. J. Hydrogen Energy 2007, 32, 2868–2874. [Google Scholar] [CrossRef]
- Liu, L.; Hong, L. Ni/Ce1−xMx Catalyst Generated from Metallo-Organic Network for Autothermal Reforming of Diesel Surrogate. Appl. Catal. A Gen. 2013, 459, 89–96. [Google Scholar] [CrossRef]
- Kang, I.; Bae, J.; Bae, G. Performance Comparison of Autothermal Reforming for Liquid Hydrocarbons, Gasoline and Diesel for Fuel Cell Applications. J. Power Sources 2006, 163, 538–546. [Google Scholar] [CrossRef]
- Kopasz, J.P.; Applegate, D.; Miller, L.; Liao, H.K.; Ahmed, S. Unraveling the Maze: Understanding of Diesel Reforming through the Use of Simplified Fuel Blends. Int. J. Hydrogen Energy 2005, 30, 1243–1250. [Google Scholar] [CrossRef]
- Cheekatamarla, P.K.; Finnerty, C.M. Synthesis Gas Production via Catalytic Partial Oxidation Reforming of Liquid Fuels. Int. J. Hydrogen Energy 2008, 33, 5012–5019. [Google Scholar] [CrossRef]
- Shamsi, A.; Baltrus, J.P.; Spivey, J.J. Characterization of Coke Deposited on Pt/Alumina Catalyst during Reforming of Liquid Hydrocarbons. Appl Catal A Gen 2005, 293, 145–152. [Google Scholar] [CrossRef]
- Qi, A.; Wang, S.; Ni, C.; Wu, D. Autothermal Reforming of Gasoline on Rh-Based Monolithic Catalysts. Int. J. Hydrogen Energy 2007, 32, 981–991. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, I.; Bae, J. Suppression of Ethylene-Induced Carbon Deposition in Diesel Autothermal Reforming. Int. J. Hydrogen Energy 2009, 34, 1844–1851. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Y.; Li, Y.; Wang, X.; Song, C. Sulfur Poisoning of CeO2–Al2O3-Supported Mono- and Bi-Metallic Ni and Rh Catalysts in Steam Reforming of Liquid Hydrocarbons at Low and High Temperatures. Appl. Catal. A Gen. 2010, 390, 210–218. [Google Scholar] [CrossRef]
- Granlund, M.Z.; Jansson, K.; Nilsson, M.; Dawody, J.; Pettersson, L.J. Evaluation of Co, La, and Mn Promoted Rh Catalysts for Autothermal Reforming of Commercial Diesel. Appl. Catal. B 2014, 154–155, 386–394. [Google Scholar] [CrossRef]
- Meißner, J.; Pasel, J.; Peters, R.; Samsun, R.C.; Tschauder, A.; Stolten, D. Elimination of By-Products of Autothermal Diesel Reforming. Chem. Eng. J. 2016, 306, 107–116. [Google Scholar] [CrossRef]
- Karatzas, X.; Jansson, K.; Dawody, J.; Lanza, R.; Pettersson, L.J. Microemulsion and Incipient Wetness Prepared Rh-Based Catalyst for Diesel Reforming. Catal. Today 2011, 175, 515–523. [Google Scholar] [CrossRef]
- Karatzas, X.; Creaser, D.; Grant, A.; Dawody, J.; Pettersson, L.J. Hydrogen Generation from N-Tetradecane, Low-Sulfur and Fischer–Tropsch Diesel over Rh Supported on Alumina Doped with Ceria/Lanthana. Catal. Today 2011, 164, 190–197. [Google Scholar] [CrossRef]
- Peters, R.; Pasel, J.; Samsun, R.C.; Scharf, F.; Tschauder, A.; Müller, M.; Müller, A.; Beer, M.; Stolten, D. Spray Formation of Middle Distillates for Autothermal Reforming. Int. J. Hydrogen Energy 2017, 42, 16946–16960. [Google Scholar] [CrossRef]
- Porš, Z.; Pasel, J.; Tschauder, A.; Dahl, R.; Peters, R.; Stolten, D. Optimised Mixture Formation for Diesel Fuel Processing. Fuel Cells 2008, 8, 129–137. [Google Scholar] [CrossRef]
- Kang, I.; Bae, J.; Yoon, S.; Yoo, Y. Performance Improvement of Diesel Autothermal Reformer by Applying Ultrasonic Injector for Effective Fuel Delivery. J. Power Sources 2007, 172, 845–852. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Kyriakidou, E.A.; Choi, J.-S.; Toops, T.J.; Binder, A.J.; Thomas, C.; Parks, J.E.; Schwartz, V.; Chen, J.; Hensley, D.K. Enhancing Low-Temperature Activity and Durability of Pd-Based Diesel Oxidation Catalysts Using ZrO2 Supports. Appl. Catal. B 2016, 187, 181–194. [Google Scholar] [CrossRef]
- Wong, A.P.; Kyriakidou, E.A.; Toops, T.J.; Regalbuto, J.R. The Catalytic Behavior of Precisely Synthesized Pt–Pd Bimetallic Catalysts for Use as Diesel Oxidation Catalysts. Catal. Today 2016, 267, 145–156. [Google Scholar] [CrossRef]
- Xiong, H.; Peterson, E.; Qi, G.; Datye, A.K. Trapping Mobile Pt Species by PdO in Diesel Oxidation Catalysts: Smaller Is Better. Catal. Today 2016, 272, 80–86. [Google Scholar] [CrossRef]
- Achouri, I.E.; Abatzoglou, N.; Fauteux-Lefebvre, C.; Braidy, N. Diesel Steam Reforming: Comparison of Two Nickel Aluminate Catalysts Prepared by Wet-Impregnation and Co-Precipitation. Catal. Today 2013, 207, 13–20. [Google Scholar] [CrossRef]
- Koo, K.Y.; Park, M.G.; Jung, U.H.; Kim, S.H.; Yoon, W.L. Diesel Pre-Reforming over Highly Dispersed Nano-Sized Ni Catalysts Supported on MgO–Al2O3 Mixed Oxides. Int. J. Hydrogen Energy 2014, 39, 10941–10950. [Google Scholar] [CrossRef]
- Fauteux-Lefebvre, C.; Abatzoglou, N.; Braidy, N.; Achouri, I.E. Diesel Steam Reforming with a Nickel–Alumina Spinel Catalyst for Solid Oxide Fuel Cell Application. J. Power Sources 2011, 196, 7673–7680. [Google Scholar] [CrossRef]
- Krumpelt, M. Fuel Processing for Fuel Cell Systems in Transportation and Portable Power Applications. Catal Today 2002, 77, 3–16. [Google Scholar] [CrossRef]
- Smith, M.W.; Shekhawat, D.; Berry, D.A.; Haynes, D.J.; Floyd, D.L.; Spivey, J.J.; Ranasingha, O. Carbon Formation on Rh-Substituted Pyrochlore Catalysts during Partial Oxidation of Liquid Hydrocarbons. Appl. Catal. A Gen. 2015, 502, 96–104. [Google Scholar] [CrossRef]
- Villoria, J.A.; Alvarez-Galvan, M.C.; Al-Zahrani, S.M.; Palmisano, P.; Specchia, S.; Specchia, V.; Fierro, J.L.G.; Navarro, R.M. Oxidative Reforming of Diesel Fuel over LaCoO3 Perovskite Derived Catalysts: Influence of Perovskite Synthesis Method on Catalyst Properties and Performance. Appl. Catal. B 2011, 105, 276–288. [Google Scholar] [CrossRef]
- Mota, N.; Álvarez-Galván, M.C.; Al-Zahrani, S.M.; Navarro, R.M.; Fierro, J.L.G. Diesel Fuel Reforming over Catalysts Derived from LaCo1−xRuxO3 Perovskites with High Ru Loading. Int. J. Hydrogen Energy 2012, 37, 7056–7066. [Google Scholar] [CrossRef]
- Kondakindi, R.R.; Kundu, A.; Karan, K.; Peppley, B.A.; Qi, A.; Thurgood, C.; Schurer, P. Characterization and Activity of Perovskite Catalysts for Autothermal Reforming of Dodecane. Appl. Catal. A Gen. 2010, 390, 271–280. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Álvarez-Galván, M.C.; Mota, N.; Villoria de la Mano, J.A.; Al-Zahrani, S.M.; Fierro, J.L.G. Catalysts for Hydrogen Production from Heavy Hydrocarbons. ChemCatChem 2011, 3, 440–457. [Google Scholar] [CrossRef]
- Xue, Q.; Gao, L.; Lu, Y. Sulfur-Tolerant Pt/Gd2O3–CeO2–Al2O3 Catalyst for High Efficiency H2 Production from Autothermal Reforming of Retail Gasoline. Catal. Today 2009, 146, 103–109. [Google Scholar] [CrossRef]
- Mota, N.; Álvarez-Galván, M.C.; Villoria, J.A.; Rosa, F.; Fierro, J.L.G.; Navarro, R.M. Reforming of Diesel Fuel for Hydrogen Production over Catalysts Derived from LaCo1−x M x O3 (M = Ru, Fe). Top. Catal. 2009, 52, 1995–2000. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Xu, B. A Survey of Nickel-Based Catalysts and Monolithic Reformers of the Onboard Fuel Reforming System for Fuel Cell APU Applications. Int. J. Energy Res 2016, 40, 1157–1177. [Google Scholar] [CrossRef]
- Xu, L.; Mi, W.; Su, Q. Hydrogen Production through Diesel Steam Reforming over Rare-Earth Promoted Ni/γ-Al2O3 Catalysts. J. Nat. Gas Chem. 2011, 20, 287–293. [Google Scholar] [CrossRef]
- Sugisawa, M.; Takanabe, K.; Harada, M.; Kubota, J.; Domen, K. Effects of La Addition to Ni/Al2O3 Catalysts on Rates and Carbon Deposition during Steam Reforming of n-Dodecane. Fuel Process. Technol. 2011, 92, 21–25. [Google Scholar] [CrossRef]
- Kim, T.; Song, K.H.; Yoon, H.; Chung, J.S. Steam Reforming of N-Dodecane over K2Ti2O5-Added Ni-Alumina and Ni-Zirconia (YSZ) Catalysts. Int. J. Hydrogen Energy 2016, 41, 17922–17932. [Google Scholar] [CrossRef]
- Liu, L.; Hong, L. Interactions between CeO2 and Ni P for Enhancing Coking and Sulfur Resistance in Autothermal Reforming of Liquid Hydrocarbons. Fuel 2012, 96, 348–354. [Google Scholar] [CrossRef]
- Pengpanich, S.; Meeyoo, V.; Rirksomboon, T.; Schwank, J. Iso-Octane Partial Oxidation over Ni-Sn/Ce0.75Zr0.25O2 Catalysts. Catal Today 2008, 136, 214–221. [Google Scholar] [CrossRef]
- Pauletto, G.; Vaccari, A.; Groppi, G.; Bricaud, L.; Benito, P.; Boffito, D.C.; Lercher, J.A.; Patience, G.S. FeCrAl as a Catalyst Support. Chem. Rev. 2020, 120, 7516–7550. [Google Scholar] [CrossRef]
- Porsin, A.V.; Rogoznikov, V.N.; Kulikov, A.V.; Salanov, A.N.; Serkova, A.N. Crystallization of Aluminum Hydroxide in a Sodium Aluminate Solution on a Heterogeneous Surface. Cryst. Growth Des. 2017, 17, 4730–4738. [Google Scholar] [CrossRef]
- Rogozhnikov, V.N.; Kuzin, N.A.; Snytnikov, P.V.; Potemkin, D.I.; Shoynkhorova, T.B.; Simonov, P.A.; Shilov, V.A.; Ruban, N.V.; Kulikov, A.V.; Sobyanin, V.A. Design, Scale-up, and Operation of a Rh/Ce0.75Zr0.25O2-δ-ƞ-Al2O3/FeCrAl Alloy Wire Mesh Honeycomb Catalytic Module in Diesel Autothermal Reforming. Chem. Eng. J. 2019, 374, 511–519. [Google Scholar] [CrossRef]
- Potemkin, D.I.; Rogozhnikov, V.N.; Ruban, N.V.; Shilov, V.A.; Simonov, P.A.; Shashkov, M.V.; Sobyanin, V.A.; Snytnikov, P.V. Comparative Study of Gasoline, Diesel and Biodiesel Autothermal Reforming over Rh-Based FeCrAl-Supported Composite Catalyst. Int. J. Hydrogen Energy 2020, 45, 26197–26205. [Google Scholar] [CrossRef]
- Rogozhnikov, V.N.; Potemkin, D.I.; Ruban, N.V.; Shilov, V.A.; Salanov, A.N.; Kulikov, A.V.; Simonov, P.A.; Gerasimov, E.Y.; Sobyanin, V.A.; Snytnikov, P.V. Post-Mortem Characterization of Rh/Ce0.75Zr0.25O2/Al2O3/FeCrAl Wire Mesh Composite Catalyst for Diesel Autothermal Reforming. Mater. Lett. 2019, 257, 126715. [Google Scholar] [CrossRef]
- Zazhigalov, S.V.; Rogozhnikov, V.N.; Snytnikov, P.V.; Potemkin, D.I.; Simonov, P.A.; Shilov, V.A.; Ruban, N.V.; Kulikov, A.V.; Zagoruiko, A.N.; Sobyanin, V.A. Simulation of Diesel Autothermal Reforming over Rh/Ce0.75Zr0.25O2-δ-η-Al2O3/FeCrAl Wire Mesh Honeycomb Catalytic Module. Chem. Eng. Process.-Process Intensif. 2020, 150, 107876. [Google Scholar] [CrossRef]
- Zazhigalov, S.V.; Shilov, V.A.; Rogozhnikov, V.N.; Potemkin, D.I.; Sobyanin, V.A.; Zagoruiko, A.N.; Snytnikov, P.V. Modeling of Hydrogen Production by Diesel Reforming over Rh/Ce0.75Zr0.25O2-δ-ƞ-Al2O3/FeCrAl Wire Mesh Honeycomb Catalytic Module. Catal. Today 2021, 378, 240–248. [Google Scholar] [CrossRef]
- Shilov, V.A.; Rogozhnikov, V.N.; Zazhigalov, S.V.; Potemkin, D.I.; Belyaev, V.D.; Shashkov, M.V.; Zagoruiko, A.N.; Sobyanin, V.A.; Snytnikov, P.V. Operation of Rh/Ce0.75Zr0.25O2-δ-η-Al2O3/FeCrAl Wire Mesh Honeycomb Catalytic Modules in Diesel Steam and Autothermal Reforming. Int. J. Hydrogen Energy 2021, 46, 35866–35876. [Google Scholar] [CrossRef]
- Shilov, V.A.; Rogozhnikov, V.N.; Potemkin, D.I.; Belyaev, V.D.; Shashkov, M.V.; Sobyanin, V.A.; Snytnikov, P.V. The Influence of Aromatic Compounds on the Rh-Containing Structured Catalyst Performance in Steam and Autothermal Reforming of Diesel Fuel. Int. J. Hydrogen Energy 2022, 47, 11316–11325. [Google Scholar] [CrossRef]
- Zazhigalov, S.V.; Shilov, V.A.; Rogozhnikov, V.N.; Potemkin, D.I.; Sobyanin, V.A.; Zagoruiko, A.N.; Snytnikov, P.V. Mathematical Modeling of Diesel Autothermal Reformer Geometry Modifications. Chem. Eng. J. 2022, 442, 136160. [Google Scholar] [CrossRef]
- Ruban, N.; Rogozhnikov, V.; Zazhigalov, S.; Zagoruiko, A.; Emelyanov, V.; Snytnikov, P.; Sobyanin, V.; Potemkin, D. Composite Structured M/Ce0.75Zr0.25O2/Al2O3/FeCrAl (M = Pt, Rh, and Ru) Catalysts for Propane and n-Butane Reforming to Syngas. Materials 2022, 15, 7336. [Google Scholar] [CrossRef] [PubMed]

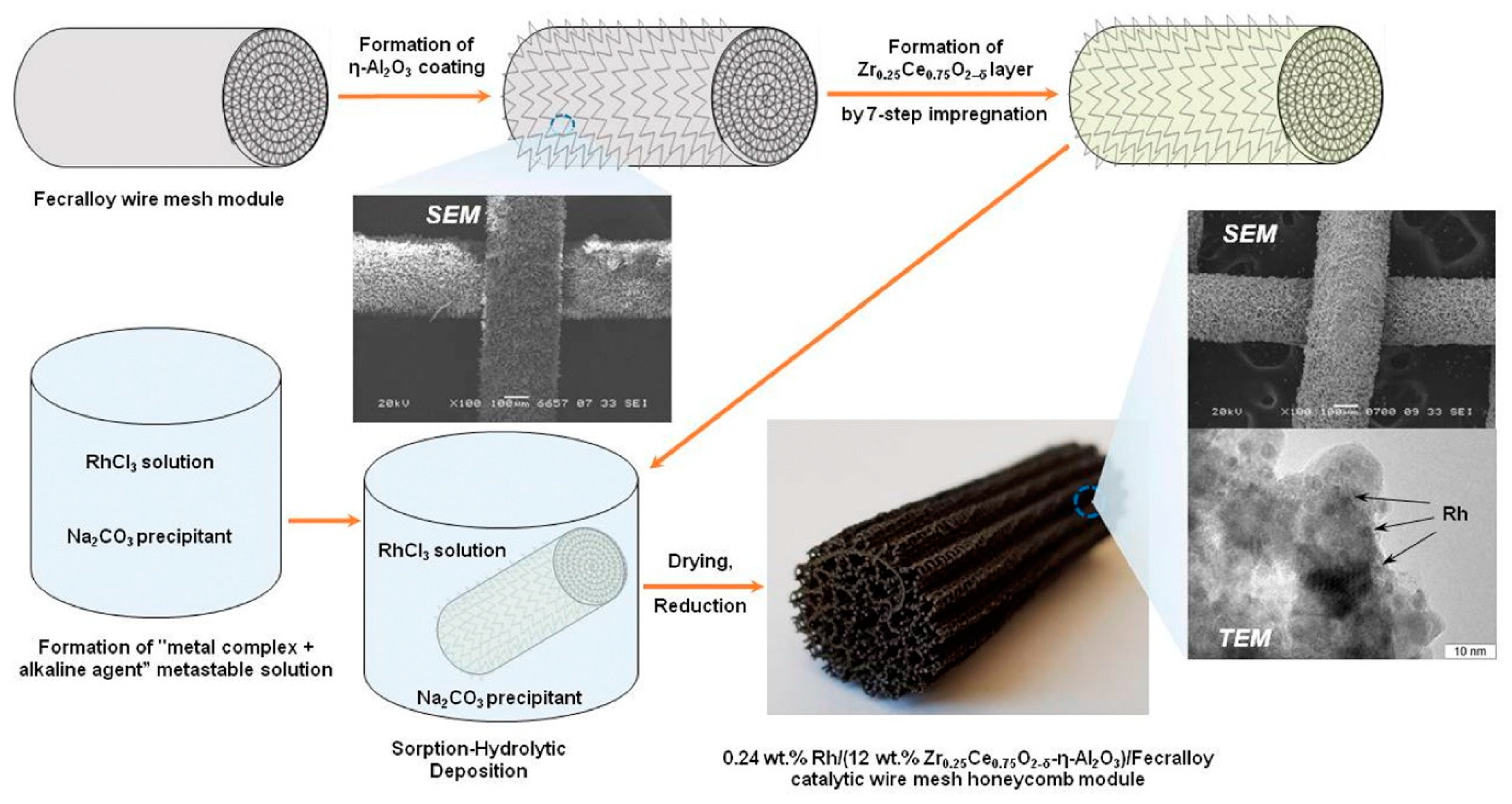
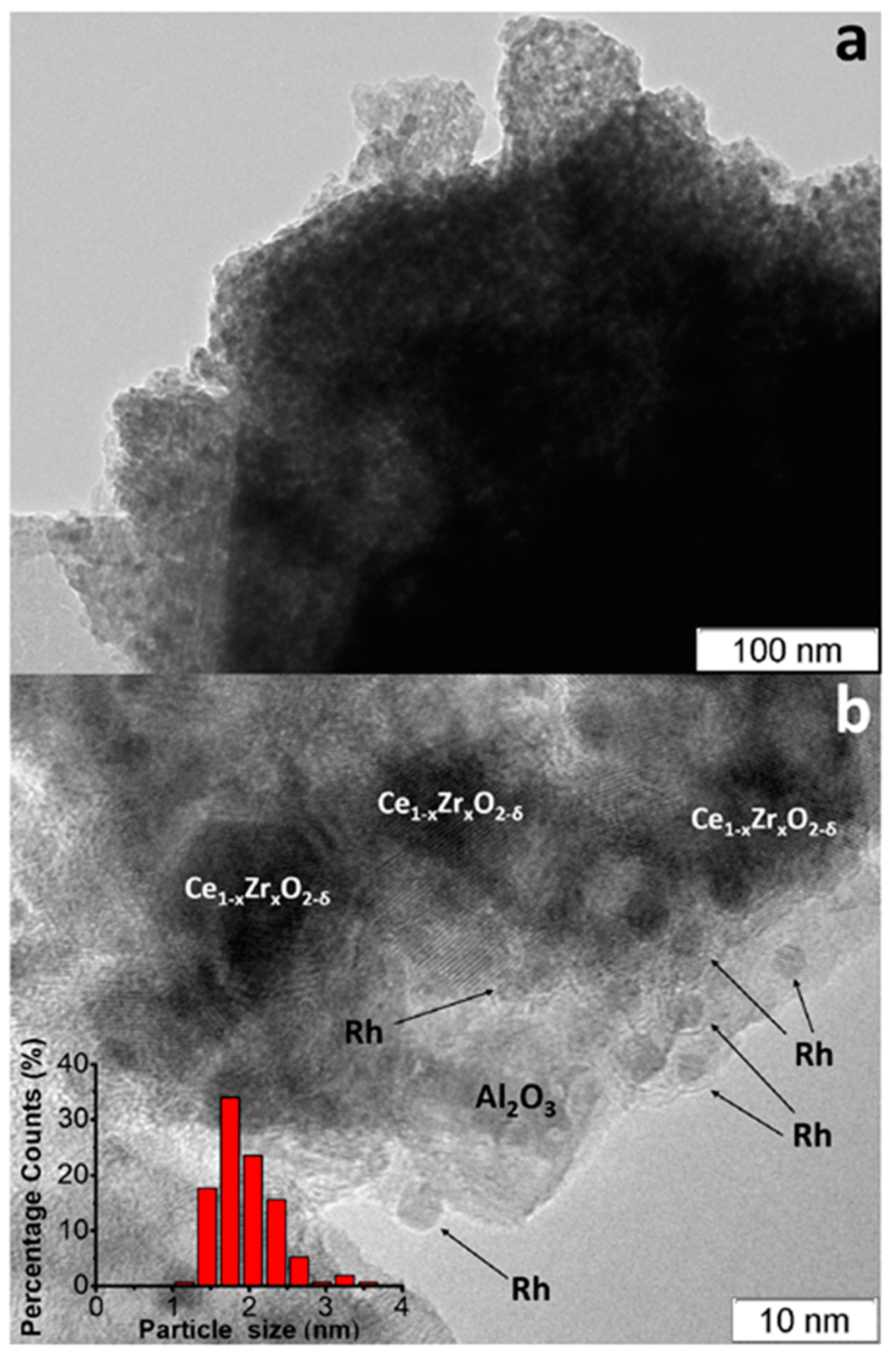
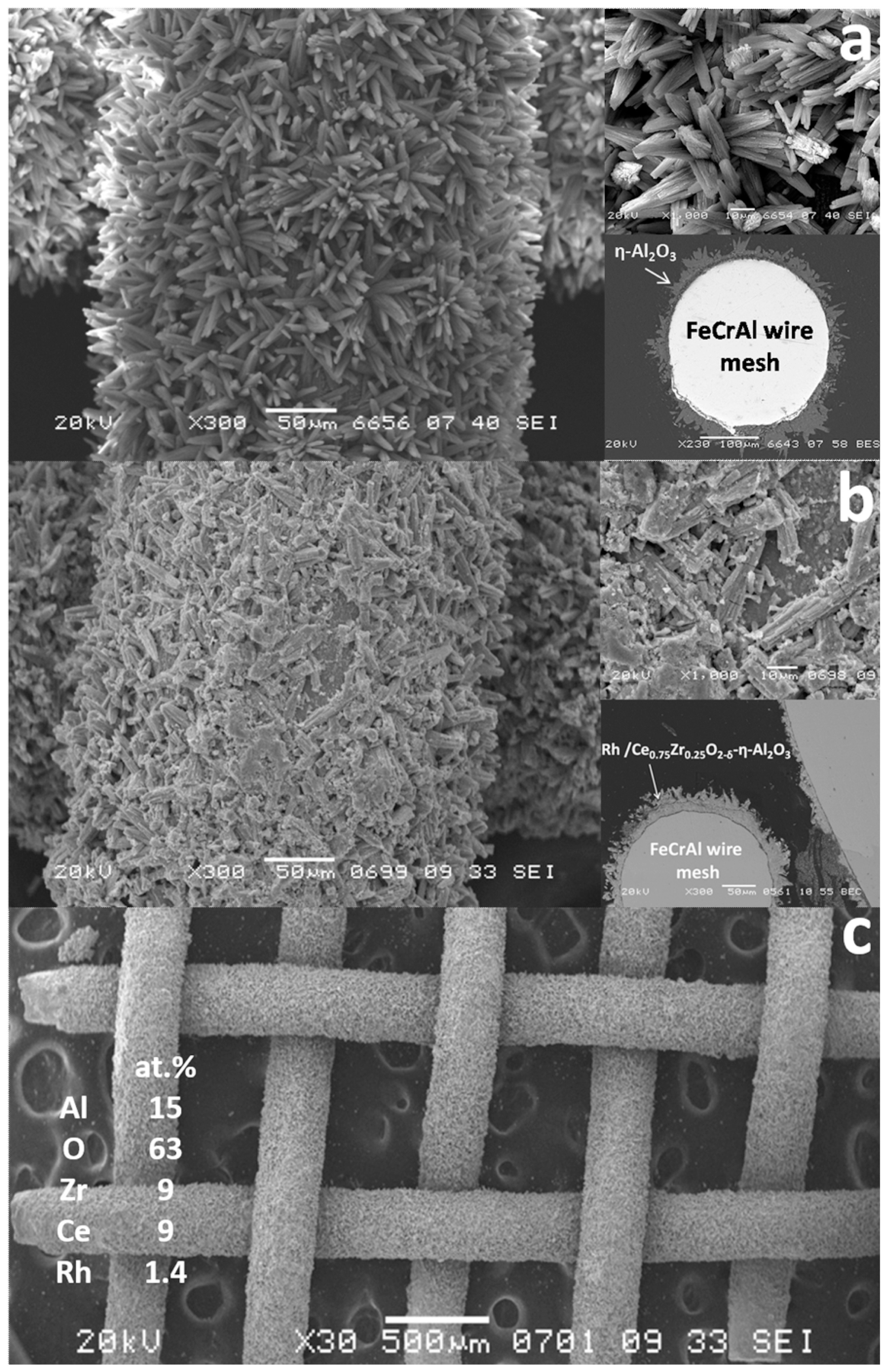
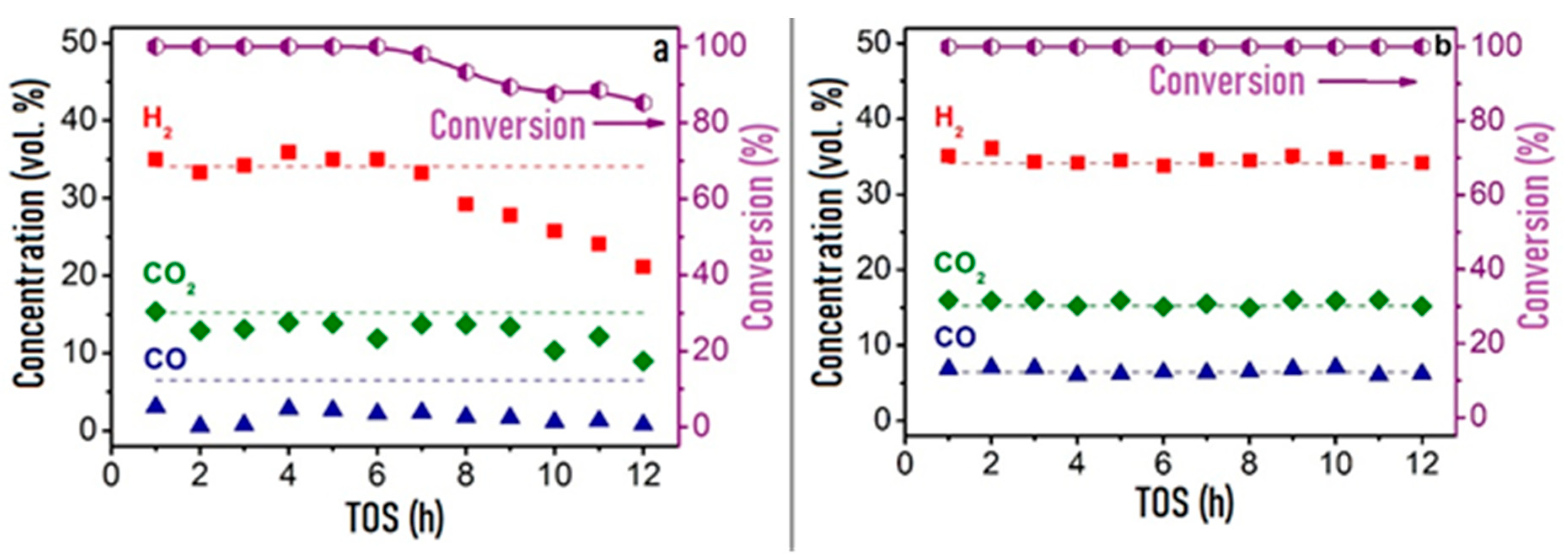
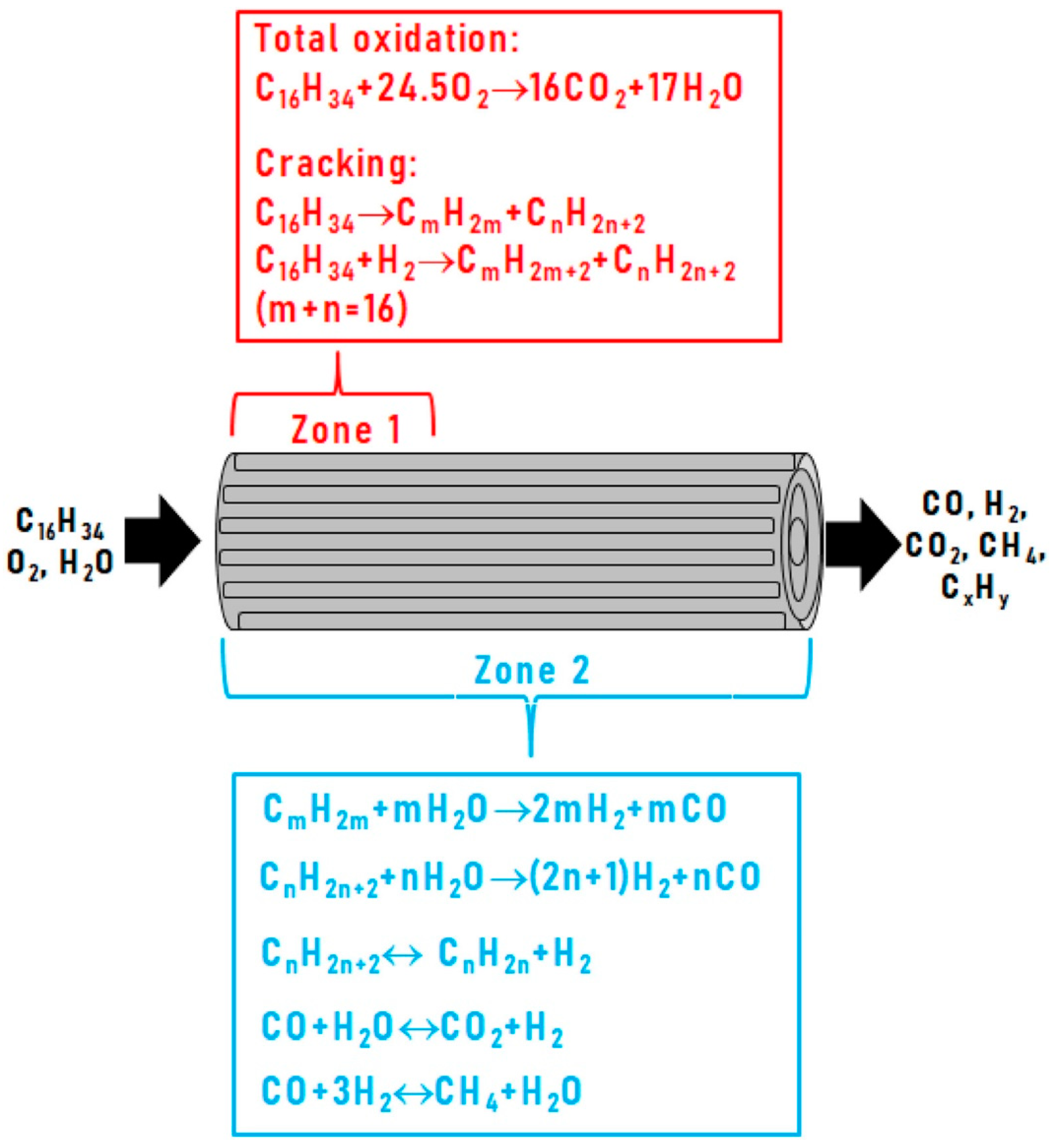
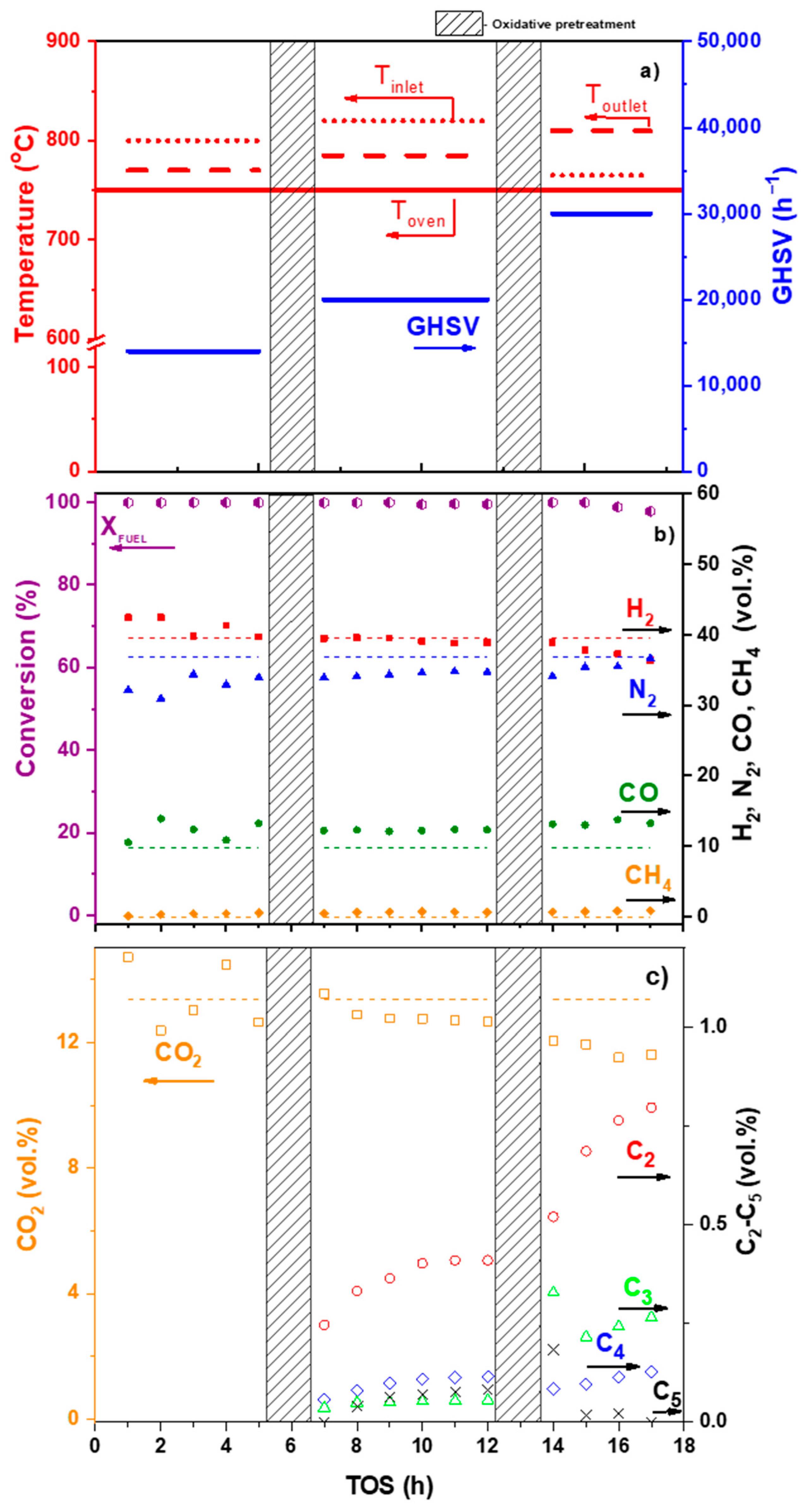
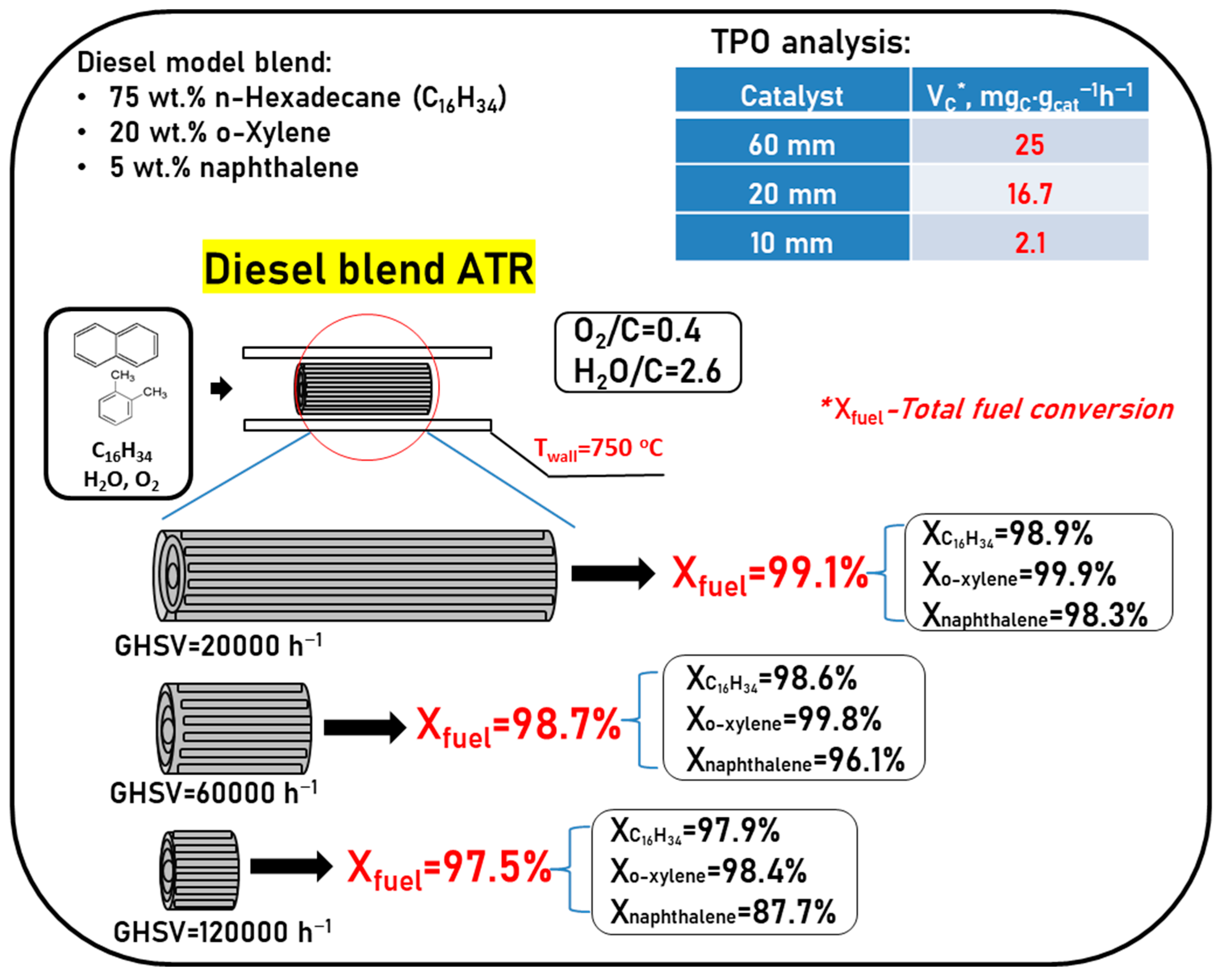
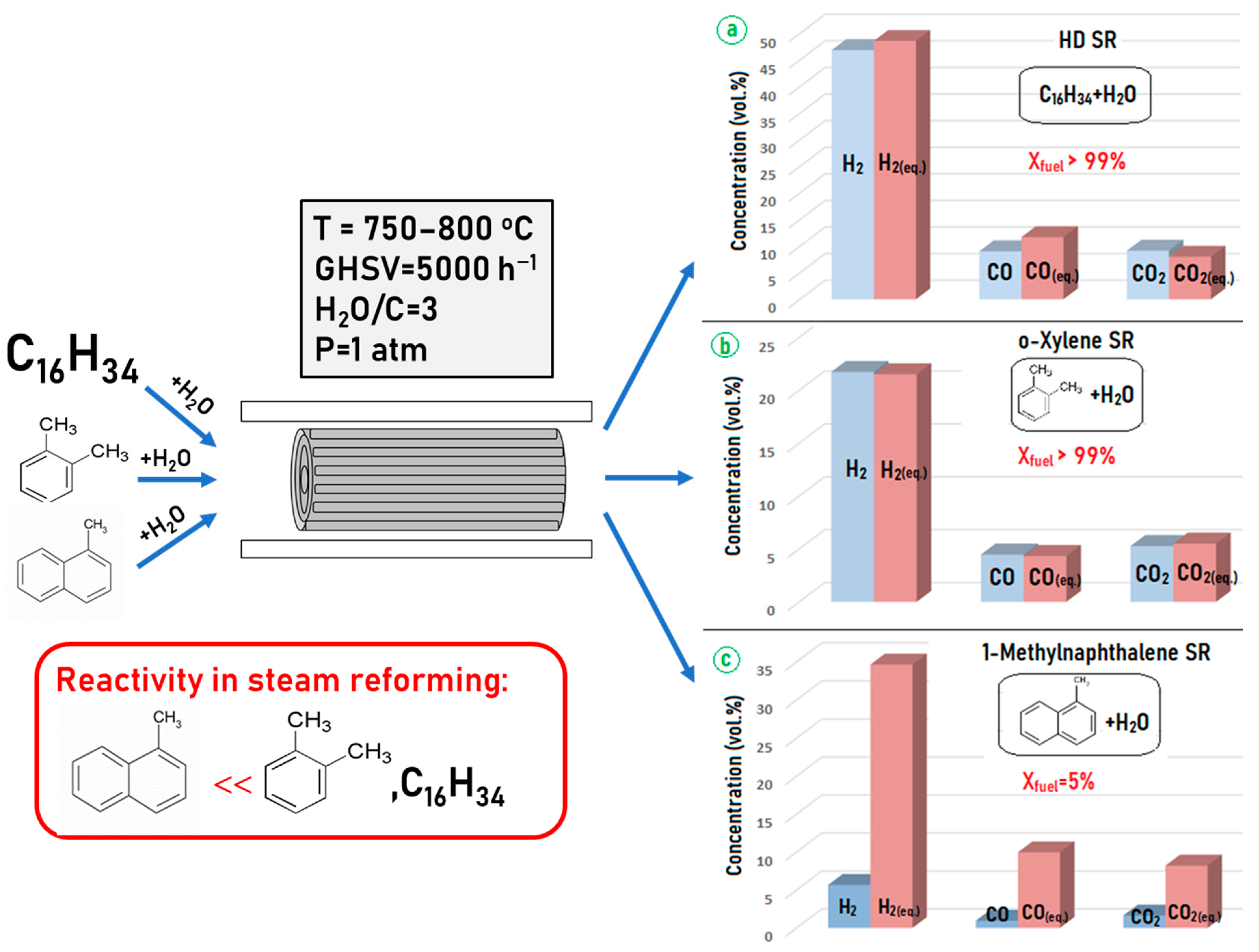


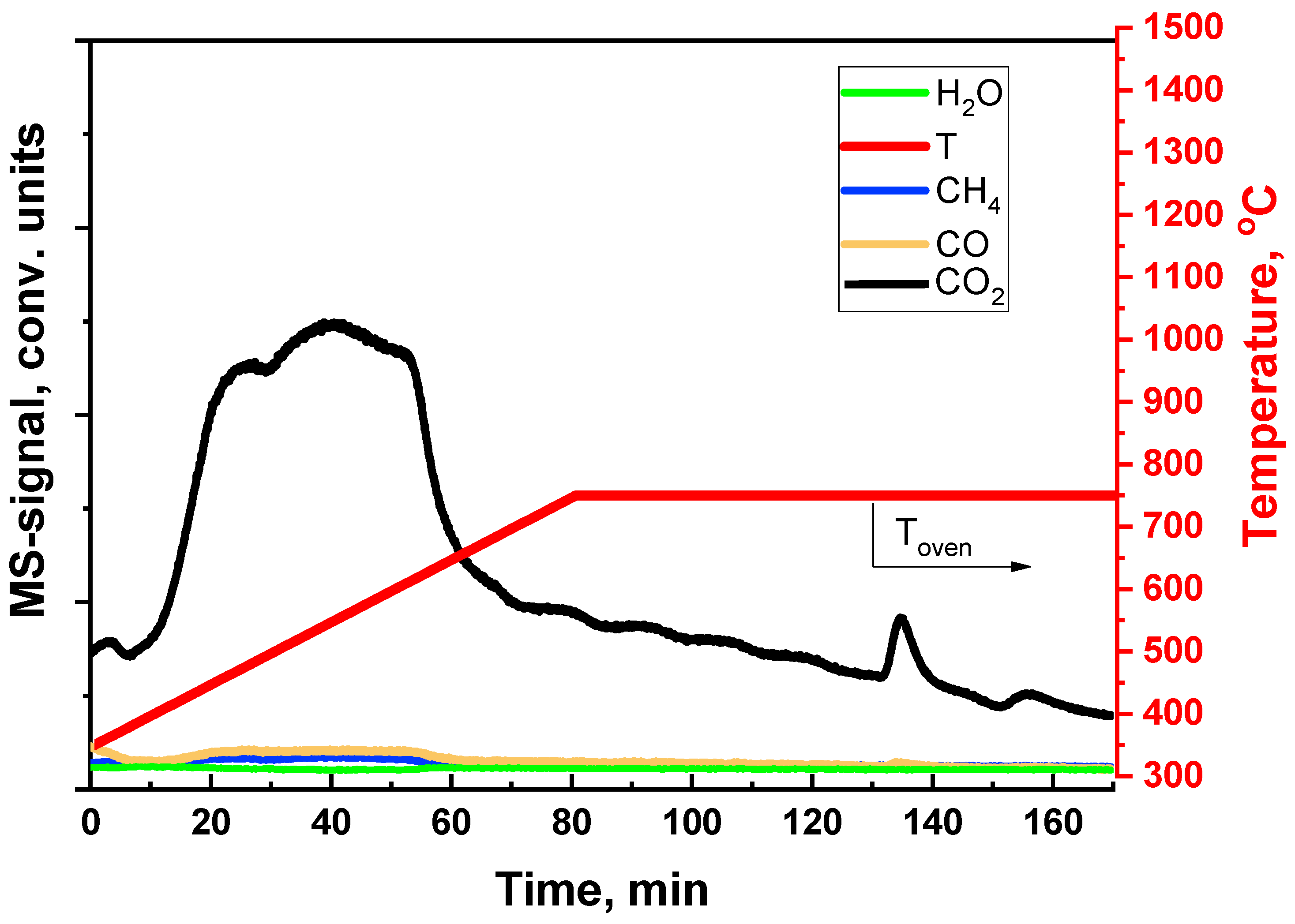
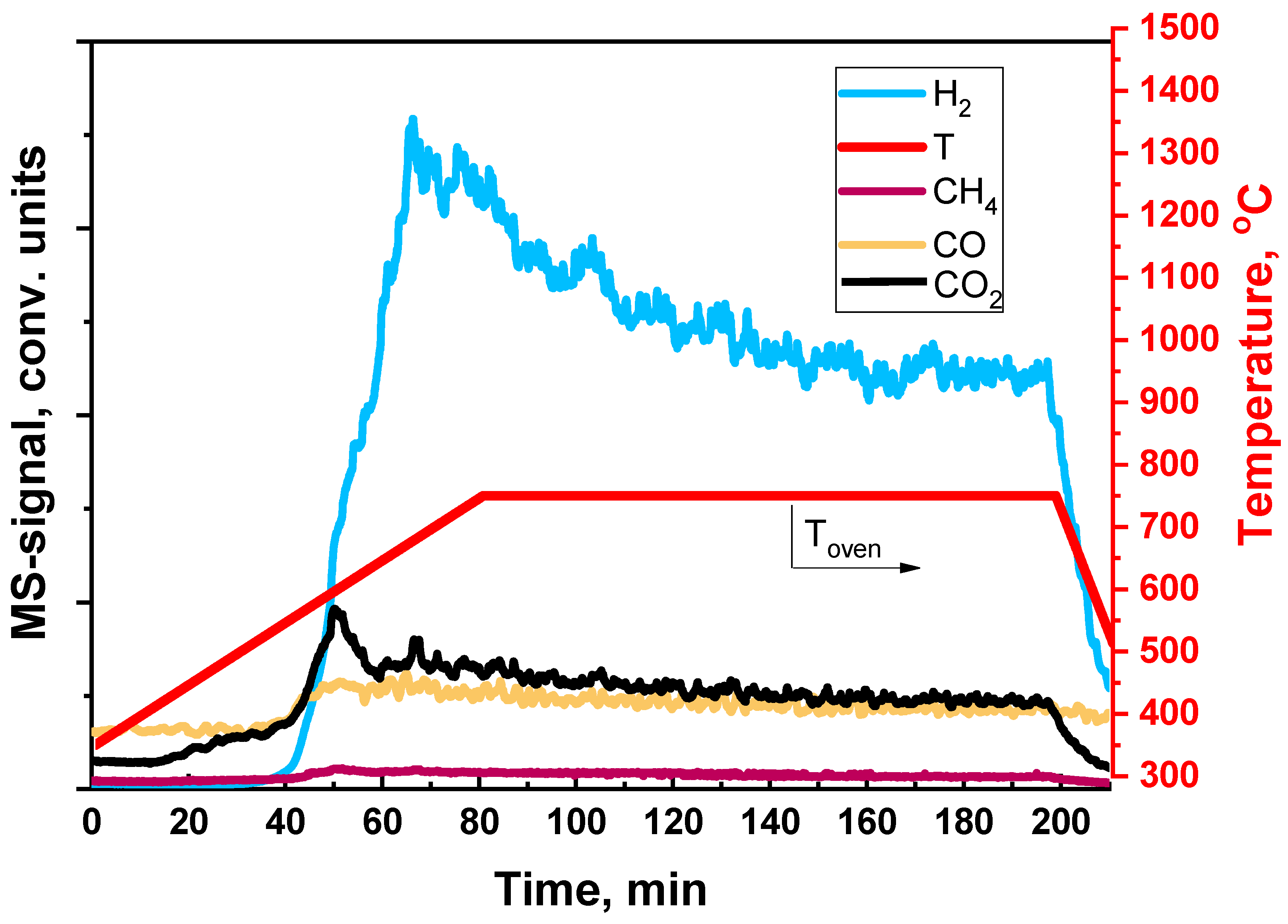
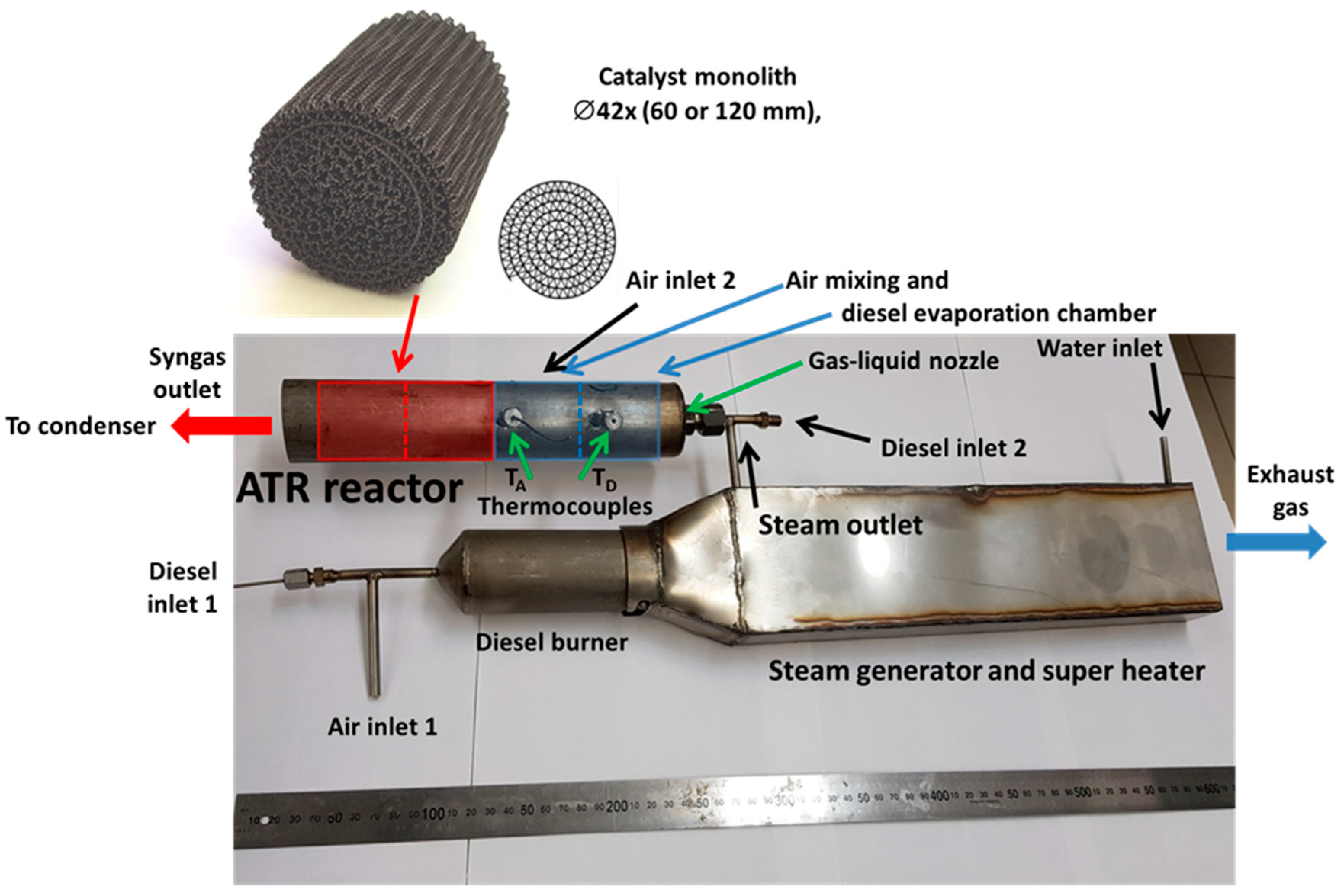
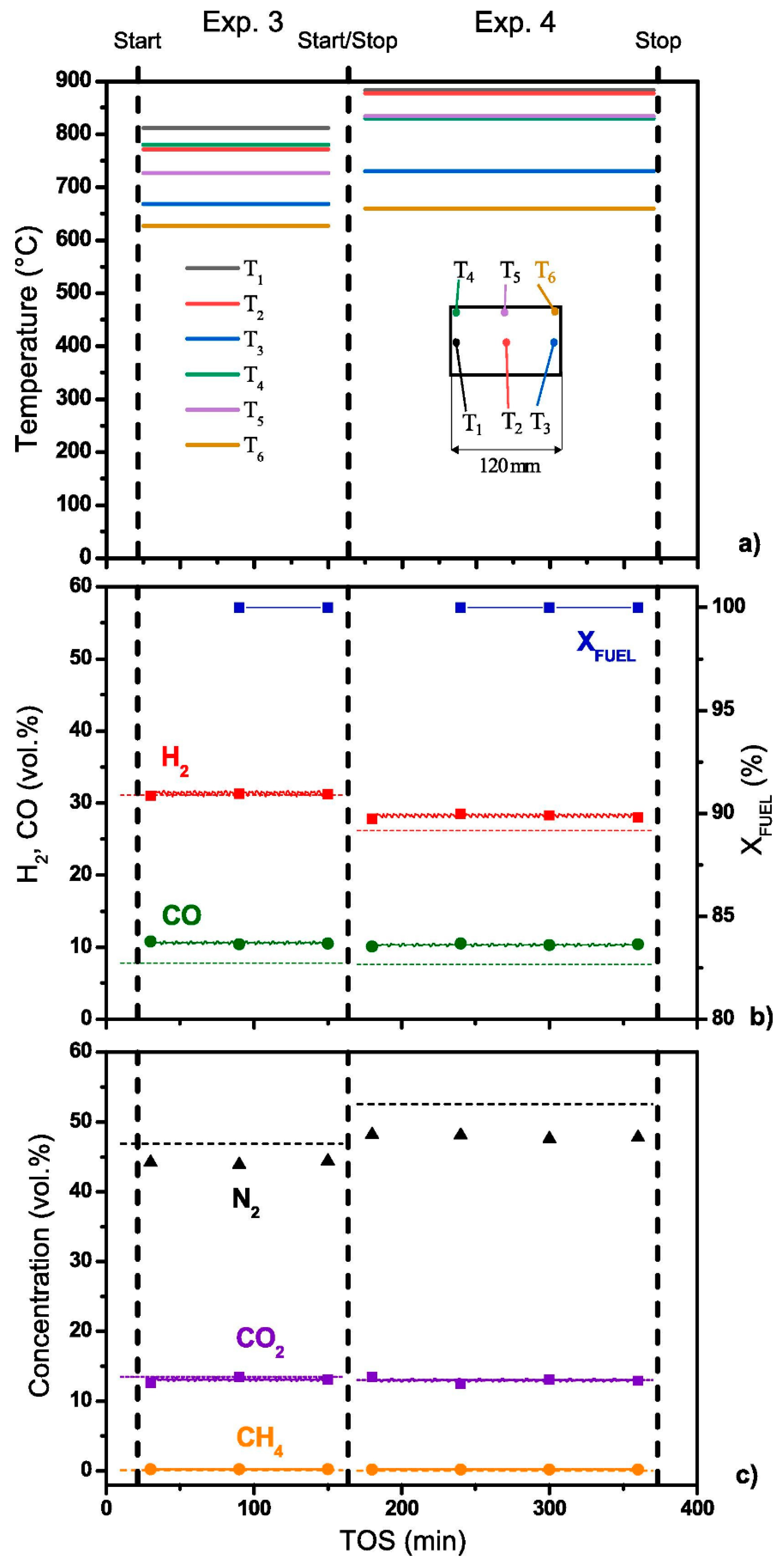
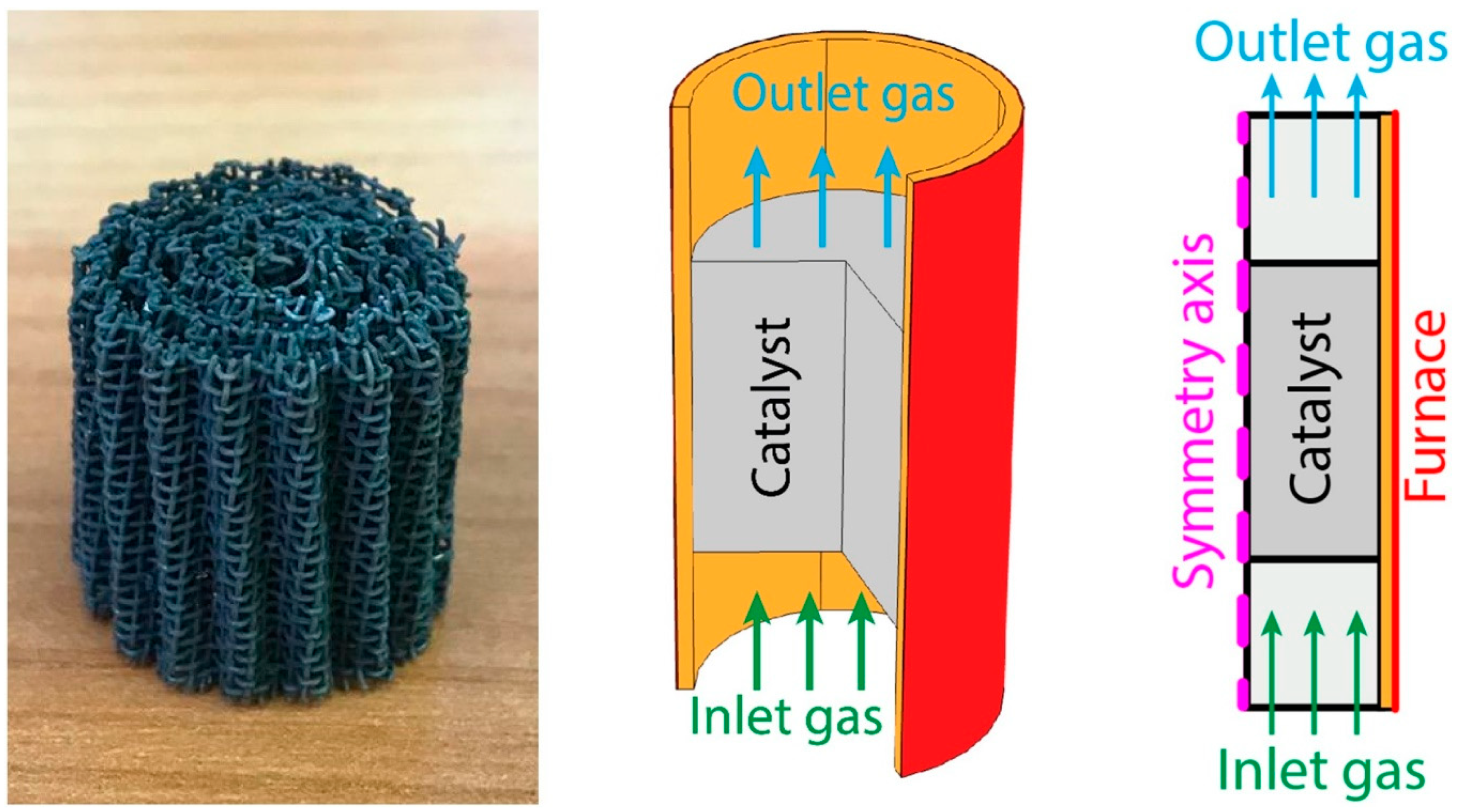

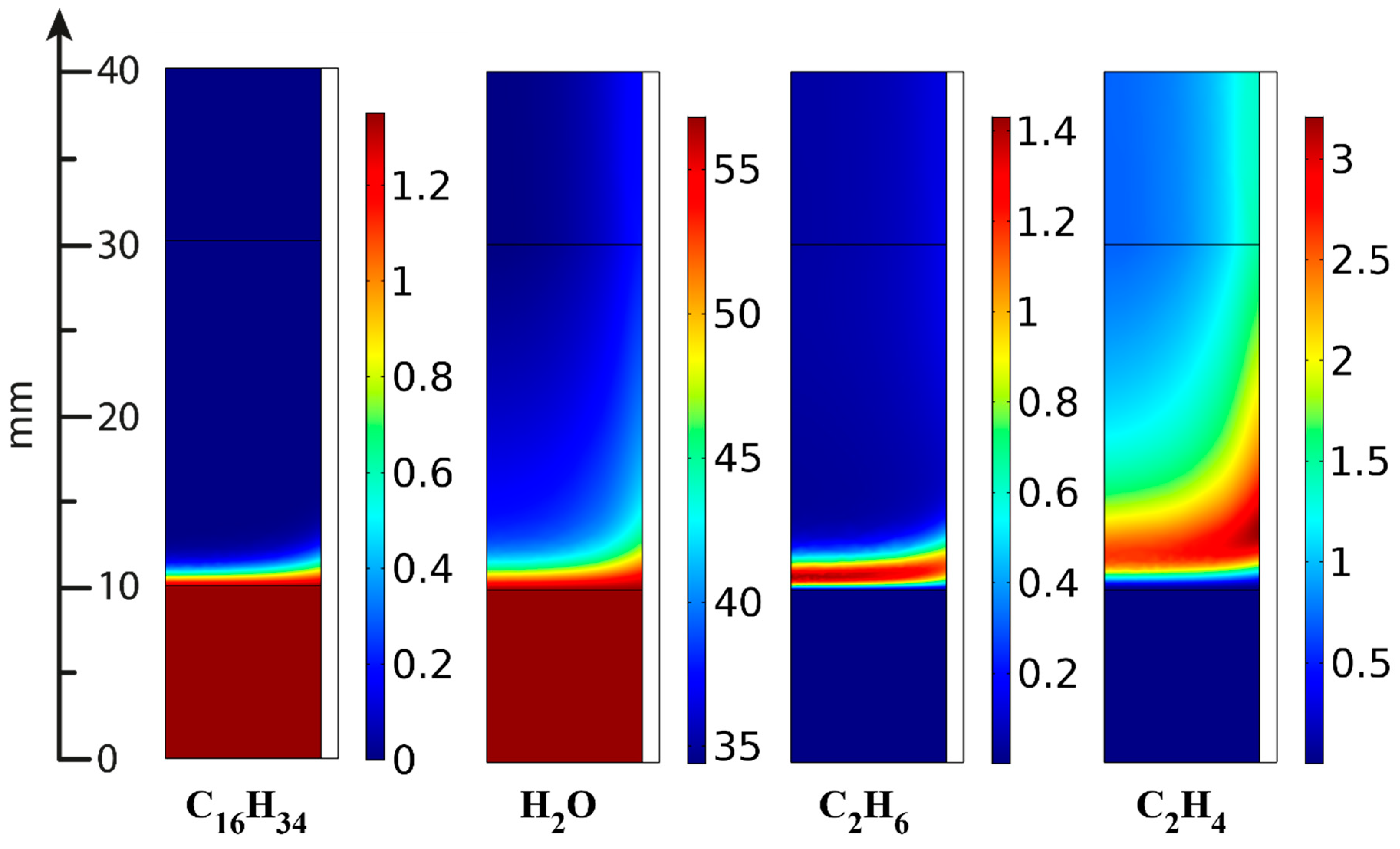
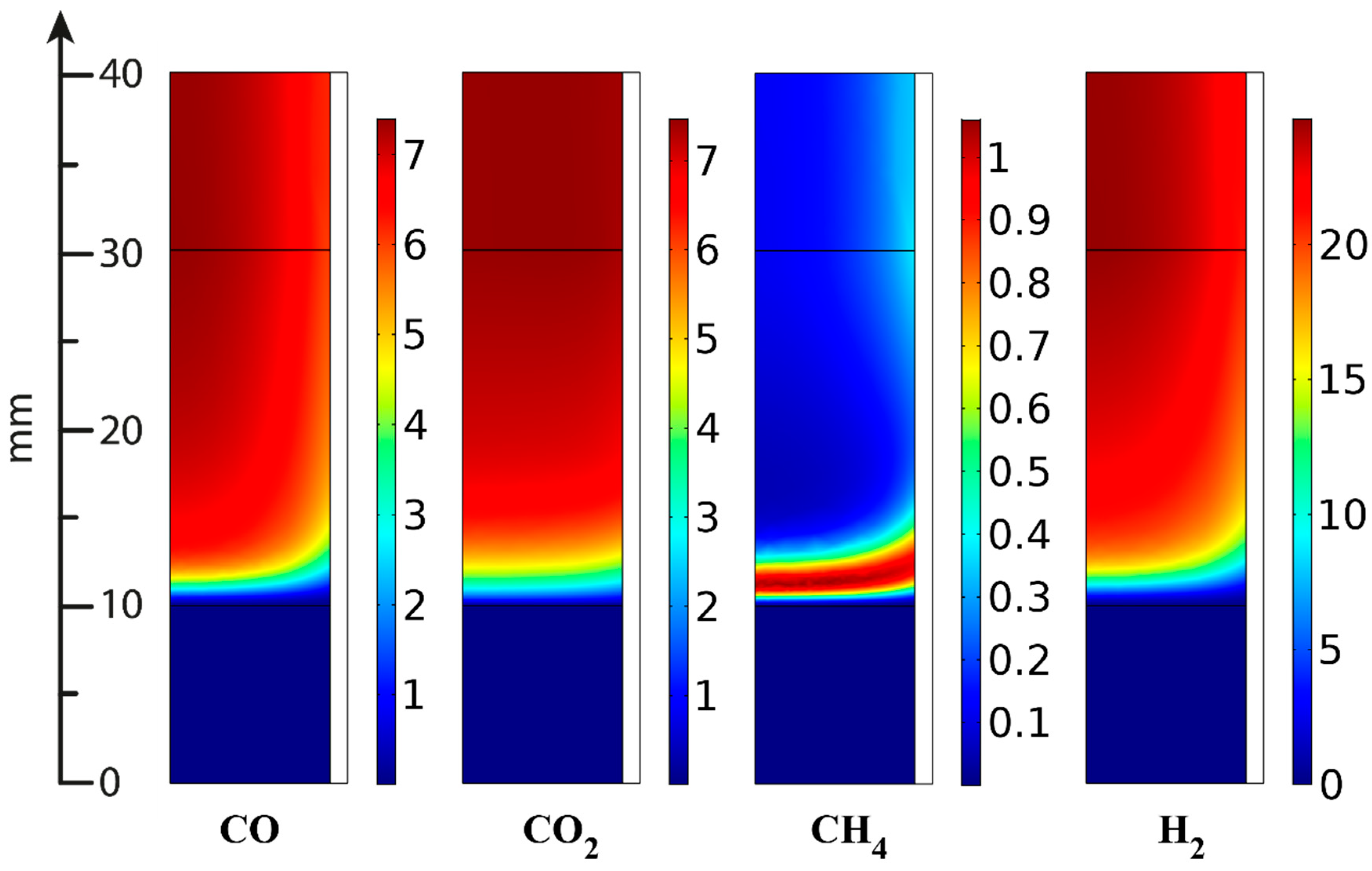
| Component | Content (vol.%) |
|---|---|
| n-Paraffins | 20 |
| iso-paraffins | 15–20 |
| Cycloparaffins | 35 |
| Alkylbenzenes | 20–23 |
| Diaromatic hydrocarbons | 5 |
| Polycyclic aromatics | <2 |
| Sulfur compounds | 0.0005–0.0008 (5–8 ppm) |
| Composition | Winter DF | Oily Residue | Dimension |
|---|---|---|---|
| Monoaromatics | 25 | 23 | wt.% |
| Diaromatics | 5 | 46 | wt.% |
| Polyaromatics | 1 | 9 | wt.% |
| Sulfur | 8 | - | ppm |
| H/C | 1.94 | - | - |
| No. | Kinetic Equation * | Pre-exponential Factor | Activation Energy Ei, kJ/mol |
|---|---|---|---|
| 1. | 1.9 × 106 | 47.3 | |
| 2. | 5 × 1012 | 208 | |
| 3. | 3 × 108 | 125 | |
| 4. | 2 × 102 | 10 | |
| 5. | 8 × 108 | 80 | |
| 6. | 2 × 105 | 40 | |
| 7. | 3 × 1010 | 124.7 | |
| 8. | 7 × 104 | 8.4 | |
| 9. | 1011 | 149 | |
| 10. | 7 × 1013 | 208 | |
| 11. | 1.5 × 1013 | 208 | |
| 12. | 2 × 106 | 53 | |
| 13. | 106 | 47 |
| HD Conversion, % | Outlet Product Distribution (vol.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| CO | CO2 | CH4 | H2 | N2 | H2O | C2+ | ||
| Model | 99.9 | 6.7 | 7.4 | 0.23 | 23 | 25.5 | 35.9 | 1.1 |
| Experiment (averaged data) | 99.2 | 5.3 | 8.5 | 0.25 | 23 | 25.8 | 36.1 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilov, V.; Potemkin, D.; Rogozhnikov, V.; Snytnikov, P. Recent Advances in Structured Catalytic Materials Development for Conversion of Liquid Hydrocarbons into Synthesis Gas for Fuel Cell Power Generators. Materials 2023, 16, 599. https://doi.org/10.3390/ma16020599
Shilov V, Potemkin D, Rogozhnikov V, Snytnikov P. Recent Advances in Structured Catalytic Materials Development for Conversion of Liquid Hydrocarbons into Synthesis Gas for Fuel Cell Power Generators. Materials. 2023; 16(2):599. https://doi.org/10.3390/ma16020599
Chicago/Turabian StyleShilov, Vladislav, Dmitriy Potemkin, Vladimir Rogozhnikov, and Pavel Snytnikov. 2023. "Recent Advances in Structured Catalytic Materials Development for Conversion of Liquid Hydrocarbons into Synthesis Gas for Fuel Cell Power Generators" Materials 16, no. 2: 599. https://doi.org/10.3390/ma16020599
APA StyleShilov, V., Potemkin, D., Rogozhnikov, V., & Snytnikov, P. (2023). Recent Advances in Structured Catalytic Materials Development for Conversion of Liquid Hydrocarbons into Synthesis Gas for Fuel Cell Power Generators. Materials, 16(2), 599. https://doi.org/10.3390/ma16020599






